Abstract
Antitumour treatments are evolving, including bacteria-mediated cancer therapy which is concurrently an ancient and cutting-edge approach. Salmonella typhimurium is a widely studied bacterial species that colonizes tumor tissues, showing oncolytic and immune system-regulating properties. It can be used as a delivery vector for genes and drugs, supporting conventional treatments that lack tumor-targeting abilities. This article summarizes recent evidence on the anticancer mechanisms of S. typhimurium alone and in combination with other anticancer treatments, suggesting that it may be a suitable approach to disease management.
Keywords: bacteria-mediated cancer therapy (BMCT), Salmonella, tumor therapy, bactofection, bacterial delivery vector, combination therapy
Introduction
Bacteria-mediated cancer therapy (BMCT) is concurrently an ancient and cutting-edge approach to cancer treatment. The therapeutic effect of some infectious diseases on malignancies was reported in the 1700s [1]. Over a century ago, Coley, an orthopedic surgeon, injected heat-inactivated Streptococcus pyogenes into unresectable bone and soft tissue sarcomas (STS) and observed tumor regression, with some of the treated patients experiencing symptom relief and prolonged survival associated with what became known as Coley’s toxins [ 2, 3] . However, without a clear understanding of the mechanisms of BMCT at that time, disappointing results in clinical practice followed. However, with the development of biological, medical, and especially genetic engineering, many mechanisms of BMCT have been clarified. BMCT can not only destroy cancer tissue by bacteria itself but also trigger antitumour immune responses and even overcome some of the limitations of traditional cancer therapy. The number of papers about BMCT has increased since the mid-1990s, and an increasing number of studies suggest that BMCT may be a suitable approach to cancer treatment.
Salmonella typhimurium ( ST) is a parthenogenic anaerobic bacterium that is most commonly used in BMCT studies. This review aims to summarize the evidence on ST’s unique antitumour mechanisms, including tumor-targeting colonization, intrinsic oncolytic capacity, immune response regulation, and susceptibility to genetic modification, which make it an ideal vector for drug and gene delivery. Studies combining ST with traditional therapies are also summarized. We hope our work can provide a foundation for further research and clinical application.
Main Advantages of S. typhimurium in Cancer Treatment
As the most-studied bacterial strain of BMCT, ST is obviously favoured for a reason. Compared to other bacteria, Salmonella has several special antitumour properties, which is summarized in Table 1.
Table 1 Examples of BMCT combined with traditional anti-cancer therapy
|
Traditional therapy |
Combined with |
Tumor |
|
Chemotherapy |
CHOP |
B-NHL |
|
Doxorubicin |
Breast cancer |
|
|
Cisplatin |
Primary and metastatic hepatocellular and lung cancers |
|
|
Gemcitabine |
Pancreatic cancer |
|
|
Cyclophosphamide |
Melanoma |
|
|
OMV-coated paclitaxel |
Colon, breast, and liver cancer |
|
|
OMV-coated tegafur |
Melanoma |
|
|
Radiotherapy |
Bacteria carrying gold nanoparticles |
Colon cancer |
|
Bacteria carrying PDA |
Melanoma |
|
|
Immunomodulatory factors |
IL-2 |
Unresectable hepatocellular carcinoma, osteosarcoma, |
|
malignant melanoma |
||
|
IFN-γ |
Malignant melanoma |
|
|
IL-18 |
Colon cancer |
|
|
CCL-21 |
Colon cancer, and melanoma |
|
|
LIGHT |
Breast cancer, and colon cancer |
|
|
Immune checkpoint inhibitors |
shIDO-ST |
Non-small cell lung cancer, colon cancer |
|
Anti PD-L1 antibody |
Melanoma |
|
|
IDO-shRNA, anti-CTLA-4, PD-1 antibodies |
Lung cancer |
|
|
siRNA-PD-1, pimozide |
Melanoma |
|
|
siRNA-PD-1, nifuroxazide |
Colon cancer |
|
|
siRNA-PD-1, chloroquine |
Colon cancer |
|
|
siRNA-PD-1, CpG ODN |
Malignant melanoma |
Tumor-targeting colonization
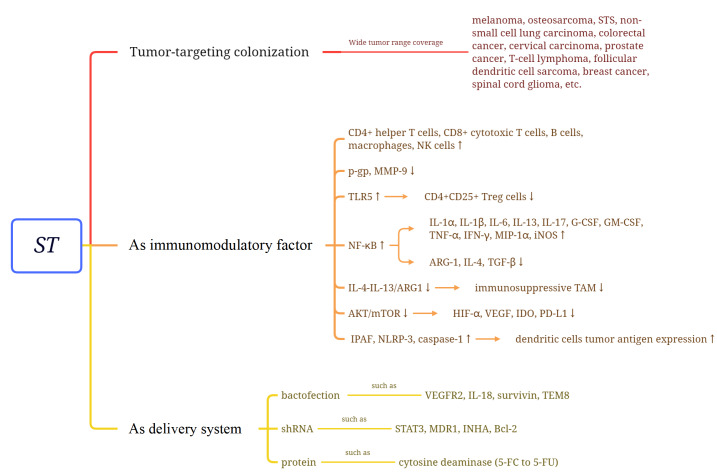
S. typhimurium colonizes tumor tissues at a rate that is over 1000-fold higher than that at which it colonizes normal tissues [ 4, 5] . It was believed that a hypoxic and poorly vascularized environment within tumor tissue is preferred by ST [6]. Additionally, no bacterial accumulation was observed in nontumor hypoxic tissues, supporting the role of other factors in ST tumor selectivity [7]. Several studies have shown that chemotaxis and motility are the key factors [ 8– 12] , but Stritzker et al. [13] pointed out that it is the tumor microenvironment (TME), bacterial metabolism, and host reticuloendothelial system that cause this tendency [13]. However, regardless of the exact mechanism involved, ST tumor-targeting can be used to support drug delivery in conventional anticancer therapy, helping minimize dosing frequency and toxicity.
Wide tumor range coverage
ST cancer treatment has been proven effective in a variety of murine tumor studies, including melanoma [ 14, 15] , osteosarcoma [16], STS [17], non-small cell lung carcinoma [18], colorectal cancer [19], cervical carcinoma [20], prostate cancer [21], T-cell lymphoma [22], and follicular dendritic cell sarcoma [23]. Metastasis inhibition by ST was also observed in osteosarcoma [24], breast cancer [25], and spinal cord glioma [26].
Oncolytic capacity
ST achieves tumor lysis by inducing apoptosis in tumor cells. Nutritional deficiency and bacterial toxin release may promote apoptosis [ 27, 28] . ST can induce autophagy by downregulating the AKT/mTOR pathway in a time- and dose-dependent manner in melanoma cells [29]. The downregulation of this pathway inhibits the expressions of HIF-α and vascular endothelial growth factor (VEGF), interfering with intratumor angiogenesis, which slows tumor growth [ 30– 32] . In addition, ST lysis releases nitrate reductase, which converts nitrite and nitrate to nitric oxide, thereby inducing tumor cell apoptosis [ 33, 34] . Additionally, the expression of some oncoproteins is downregulated by ST in tumor cells, such as p-glycoprotein (p-gp) [35] which causes drug resistance, and matrix metalloproteinase 9 (MMP-9) [36] which promotes tumor metastasis.
Immunomodulatory effects
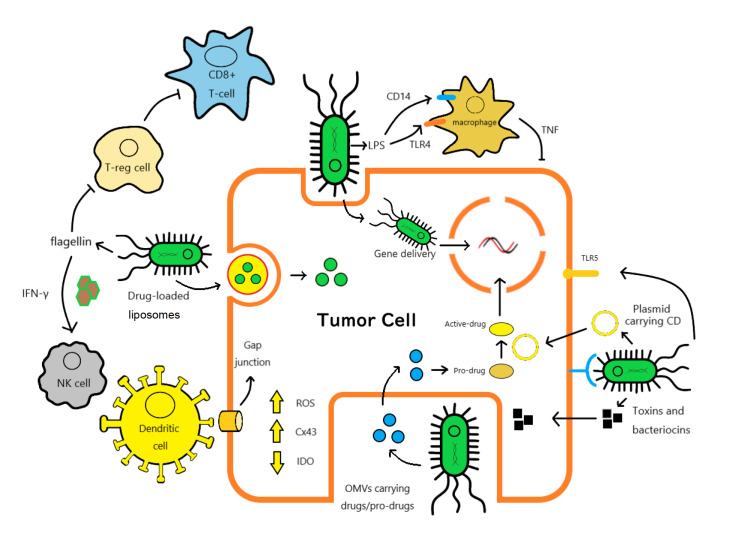
Salmonella infection induces a series of immune responses ( Figure 1). Therefore, since ST tends to colonize tumor tissues, the immune status in the TME will be changed. The TME contains immune cells, cancer-associated fibroblasts, and structural elements, such as collagen and hyaluronic acid, which form the extracellular matrix. They directly surround tumor tissue, promoting tumor growth, survival, immune escape, and metastasis [37]. Changes in the TME can affect the proliferation and metastatic capacity of tumors, limiting or promoting disease progression [38]. The immunosuppressive TME may limit the efficacy of conventional cancer therapies. Poor prognosis of cancer patients is associated with an immunosuppressive TME [ 39– 41] .
Figure 1 .
Main advantages in cancer treatment of Salmonella typhimurium
ST tends to colonize in multiple tumor tissues and can induce a series of anti-cancer immune responses. ST is also an ideal tumor-targeting vector which can deliver almost everything to tumor tissues.
The formation of an immunosuppressive TME can be attributed to tumor molecular heterogeneity and tumor-intrinsic dysregulated signaling pathways. By activating Wnt/β-catenin proteins in tumor cells, tumor-specific T cells can be removed from the TME [42]. By activating STAT3 signaling, the expression of tumor-secreted proinflammatory mediators can be significantly reduced [43]. Activation of the PI3K/PTEN/AKT pathway may negatively affect immune responses; for example, mutations such as PIK3CA activation or PTEN loss of function can activate the PI3K/AKT signaling, which increases the level of immunosuppressive tumor-associated macrophage (TAM) infiltration [44]. In addition, activating the PI3K/AKT signaling axis can upregulate indoleamine-2,3-dioxygenase (IDO), a tryptophan-degrading enzyme, in tumor cells and the TME [ 45, 46] . With high IDO activity, the availability of tryptophan to effector immune cells is deprived, which traps T cells in the stationary cell cycle (G1 phase). T cells sensing tryptophan deficiency trigger general control nonderepressible 2 (GCN2) kinase release, which mediates proliferative arrest and increases Treg populations [ 47, 48] . Moreover, kynurenine, which is produced in tryptophan catabolism, induces apoptosis of T cells [49]. It is believed that abnormal and high expression of IDO leads to poor clinical prognosis [ 50, 51] .
ST modulation of the immune system can convert the TME from immunosuppressive to immunogenic by increasing multiple innate and adaptive immune cells, such as CD4+ helper T cells [ 52– 54] , CD8+ cytotoxic T cells [ 53– 55] , B cells [56], macrophages [ 52, 54] , and NK cells [53], enhancing the anticancer immune response. Meanwhile, through TLR5 signaling, ST flagellin can reduce the number of CD4+CD25+ Treg cells in the TME, thus promoting immunosuppression disruption in the TME [ 57, 58] . By activating the NF-κB pathway, the expression of proinflammatory cytokines and chemokines, such as IL-1α, IL-1β, IL-6, IL-13, IL-17, G-CSF, GM-CSF, TNF-α, IFN-γ, MIP-1α, and iNOS [ 15, 59– 61] , is upregulated [ 62– 65] ; at the same time, immunosuppressive factors, such as ARG-1, IL-4, and TGF-β, are downregulated [ 15, 66] . In addition, phenotypic and functional activation of intratumor myeloid cells may be induced, reducing the immunosuppressive effect of the TME. The significant reduction in IL-4-IL-13/ARG1 axis activity may explain the phenomenon, which is a marker of the activity of immunosuppressive tumor-associated macrophages [15]. The activation of inflammatory vesicle pathways accompanied by increased levels of IPAF, NLRP-3, and caspase-1 has also been observed, alongside the enhancement of tumor antigen expression by dendritic cells [67]. Reduced IDO expression and activity, associated with inhibited AKT/mTOR/p70S6K signaling, have been reported [46]. The inhibition of this pathway also downregulates the expression of programmed death ligand 1 (PD-L1), which is a transmembrane receptor for PD-1 [68]. High PD-L1 expression on the tumor surface inhibits the activation of effector T cells, the production of cytokines, and TCR-mediated proliferation [69], which can be reversed by ST [68]. ST can also alter immune check-point expression (seen in “Immune checkpoint inhibitors”).
Deliver everything: DNA, RNA, and protein
Because of its cancer-targeting property, ST has become an ideal delivery system that can carry DNA, RNA, and protein to cancer tissues ( Figure 2). Compared with viral vectors, ST is a cheaper, safer and more accurate way to deliver therapeutic genes [70]. Some concepts and efforts are shown as follows.
Figure 2 .
Examples of different mechanisms followed by Salmonella typhimurium for cancer therapy
ST can increase multiple innate and adaptive immune cells like CD8+ T cells, NK cells and macrophages, it also reduces Treg cells in TME. Upregulation and activation of Cx43 expression by ST enhances antigen delivery in dendritic cells and facilitates the delivery of chemotherapeutic drugs and apoptotic signals within cancer cells. Using gene-modified strains, drug-loaded liposomes or OMVs carrying drugs/pro-drugs, anti-tumor genes/drugs can be delivered directly into tumor tissues by ST. Plasmid carrying cytosine deaminase can convert non-toxic 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU). This approach limits the toxicity of chemotherapeutic drugs to the tumor area.
DNA vaccines are a method of delivering plasmids encoding tumor-expressed antigens into tumor cells to induce a host immune response. By the control of a eukaryotic promoter, bacteria deliver plasmids encoding therapeutic genes into tumor cells. Once entered, bacteria release the plasmids into the cytoplasm and transfer them to the nucleus, where the therapeutic genes are expressed by the transcriptional and translational systems of the host cell. This process is called bactofection. Several trials have reported the inhibition of tumor growth and metastasis using oral attenuated ST bactofection of vascular endothelial growth factor receptor 2 (VEGFR2) into tumor cells, thus inhibiting intratumoral vascular growth [ 71– 73] . Similar oncogenic effects have been observed with IL-18 [74], survivin [75], and tumor endothelial marker 8 (TEM8) [76].
RNA interference (RNAi) can regulate mRNA stability and translation in almost all human cells. Short hairpin RNA (shRNA) molecules can effectively silence specific genes [77]. ST is a safe, effective, and inexpensive vector for delivering shRNAs. ShRNAs targeting STAT3 [78], multidrug resistance protein 1 (MDR1) [79], INHA [ 80, 81] , and Bcl-2 [82] have been tested in various tumor-bearing mouse models using attenuated ST, resulting in tumor growth inhibition and prolonged survival.
The use of bacteria to deliver proteins is a promising approach to cancer treatment, which uses bacteria to express therapeutic proteins that have therapeutic effects. Unlike bactofection, this treatment requires the continuous and stable presence of bacteria in the target tissue, e.g., the enzyme-prodrug approach. Attenuated ST can deliver cytosine deaminase to tumor cells, which converts nontoxic 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU), associated with antitumour effects. This approach limits the toxicity of chemotherapeutic drugs to the tumor area [ 83, 84] .
Most Commonly Used Attenuated Salmonella Strains
As mentioned above, ST is an ideal living anticancer nanomachine. However, ST is also the most common pathogen of food poisoning. It is obviously unwise and unfeasible to directly use the wild-type strains of ST for cancer treatment. Thus, gene-edited attenuated Salmonella strains were born. Here are the most-used attenuated Salmonella strains.
VNP20009
VNP20009 is derived from the ST ATCC14028 wild-type (WT) strain. It is a genetically attenuated strain with an excellent safety profile due to the absence of the purl and msbB genes, which reduces its bacterial virulence and potential to induce infectious shock while maintaining its antibiotic sensitivity. The strain is also genetically stable, and after multiple generations of in vitro and in vivo propagation, it still maintains its genetic and phenotypic stability.
In vivo experiments in a monkey model showed that the strain appeared in the bloodstream at 24 h after infection and at day 41 and was totally cleared from all organs [5]. VNP20009 exhibited significant tumor-targeting properties and colonized transplanted mouse tumors and various patient-derived organoid-based xenografts (PDOX) at a ratio higher than 1000:1 in tumor-bearing mice [5].
However, VNP20009 shows unsatisfactory efficacy in clinical trials [85]. In a trial of 25 patients (24 metastatic melanoma and 1 metastatic renal cell carcinoma) treated with VNP20009 (1×10 6 to 1×10 9 cfu/m 2) intravenously, the maximum tolerated dose was 3×10 8 cfu/m 2. In this trial, one patient (dose of 1×10 9 cfu/m 2) presented with toxic reactions (dose-limited), including anemia, thrombocytopenia, persistent bacteremia, hyperbilirubinemia, nausea, vomiting, diarrhea, hypophosphatemia, and alkaline phosphatase elevation. In addition, VNP20009 increased the blood levels of multiple proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-12. In three patients, VNP20009 tumor colonization was detected; however, no significant tumor regression was observed in any patient. Although intratumoral colonization was observed, VNP20009 did not show significant anticancer effects. The authors suggested that further studies may focus on reducing dose-related toxic effects or increasing tumor localization for therapeutic purposes [85]. However, excessive toxicity reduction of VNP20009 may result in its unsatisfactory efficacy. Some studies have shown that the addition of VNP20009 to LPS reduces its toxicity while decreasing its anticancer effects [86]. Therefore, VNP20009 may be more suitable as a delivery system. Some studies have attempted to enhance the anticancer properties of the strain. Cheng et al. [87] deleted phoP and phoQ genes from VNP20009 and obtained a strain characterized by reduced colonization in normal liver and spleen and increased tumor localization ability. The VNP20009 genome has been sequenced; in addition to the genes identified in knock-out studies, purM deletions, 108-kbp Suwwan deletions, and 50 nonsynonymous SNPs have been identified [88].
A1/A1-R
Strain A1 is derived from ATCC14028wt, which is a nutrient-deficient strain (leucine/arginine-dependent). This strain may grow aggressively in tumor xenografts, likely due to amino acid availability in tumor tissue that supports bacterial growth. It can also colonize normal tissues but can be cleared even in immunodeficient mice (excised thymic tissue). In vitro, A1 grows in PC-3 human prostate cancer cells and eventually destroys the nucleus. In vivo, intravenous injection of A1 into a murine model of PC-3 human prostate cancer cells showed that bacteria invaded tumor cells and sustained replication, arresting tumor growth. The tumor/liver bacterial ratio was approximately 2000–10,000:1 on day 4, and the bacteria could not be detected in normal tissues such as lung, liver, spleen, or kidney by day 15 after the injection. In contrast, the tumor regressed completely by day 20 after a direct injection of A1 into the tumor. Neither route of administration caused adverse host effects. Safety testing showed that under the administration of the 1×10 7 cfu/m 2 A1 strain, no mouse deaths were observed, whereas mice receiving ATCC14028wt all died within 3 days [89]. A1-R is a modified A1 strain with high antitumour virulence. Compared with A1, the adhesion ability is approximately 6 times stronger for A1-R to HT-29 human colon cancer cells [90]. In vitro, within 200 min, all A1-R-infected PC-3 cells were ablated; meanwhile, in vivo, A1-R colonized PC-3 tumor cells at 100-fold higher concentrations than A1 [91]. Studies involving PDOX models have shown that this strain performs well in malignant and metastatic tumors, such as prostate cancers, breast cancers, spinal tumors, pancreatic cancers, malignant melanoma, glioma, STS, and cervical and ovarian cancers [ 92– 96] . Meanwhile, A1-R may induce the differentiation of cell cycle quiescent tumor cells to the S/G2/M phase to enhance their response to chemotherapy [97].
ÄppGpp
The pathogenicity of Salmonella is associated with certain genes known as Salmonella Pathogenicity Island (SPI) [98]. At least five SPIs have been discovered. One of the most studied SPIs, SPI-1, which encodes type III secretion system 1 (T3SS-1), is related to host cell infection. The transcription of SPI-1 is regulated by various factors and is especially regulated by a transcription regulator, hilA, which is also encoded within SPI-1 [ 99, 100] . It was found that the expression of genes encoded by SPI-1 and hilA depends on a stringent signal molecule, ppGpp [ 101, 102] . ppGpp is synthesized by two enzymes, RelA and spoT [101]. Thus, when RelA and spoT are deleted, attenuated ÄppGpp strains are generated. All SPI-1 genes, including hilA, will not be expressed in this attenuated strain. Studies have shown that in female BALB/c mice, the LD 50 of the attenuated strain is approximately 10,000-fold higher than that of the WT strain (oral, 3×10 9 CFU/m 2 vs 4×10 4 CFU/m 2; intraperitoneal, 3×10 6 CFU/m 2 vs<10 CFU/m 2), regardless of the route of administration. At the same time, the attenuated strain can also effectively induce cellular and humoral immunity [61]. All of these factors make ÄppGpp an excellent vector for targeting tumors. Tan et al. [103] used engineered ÄppGpp ST expressing cytolysin A (ClyA) to treat pancreatic cancer-bearing nude mice and observed that ST markedly accumulated and proliferated in tumor tissues and that tumor growth was successfully inhibited. A previous study compared ÄppGpp and VNP20009 in MC38 xenograft mice. They showed similar effects in cancer suppression, while the ÄppGpp group had a higher rate of complete tumor eradication and longer survival time; and compared with the VNP20009 group, the levels of inflammatory cytokines of the ÄppGpp group were much higher in the TME, while those in circulation were lower [104]. Therefore, ÄppGpp may promote the selection of BMCT.
Combination with Traditional Anticancer Therapy
Some studies suggest that the antitumour effect of Salmonella is related to its toxicity [ 105, 106] , and the results of several clinical trials support this view [ 85, 107] . How to balance the antitumour efficacy and toxicity of Salmonella is a topic that needs further research. In addition to modifying the strain itself, combining existing anticancer treatments seems to be a more convenient and feasible approach. Traditional cancer treatment methods have been proven to be effective in many clinical applications but at the same time bring serious and even fatal toxic side effects to patients. Poor tumor targeting is one of the main reasons. The tumor-targeting property of ST can compensate for this shortcoming. At present, a considerable number of studies have combined ST with traditional anticancer treatments to evaluate their anticancer effects ( Table 1). The results are inspiring. Combined treatment not only exerts the advantages of ST and traditional treatment methods but also has a sensitizing effect on each other.
Chemotherapy
Currently, chemotherapy is still the first-line anticancer therapy for most cancers. However, the associated systemic side effects and tumor resistance limit its efficacy. Many studies have examined the use of ST in combination with chemotherapeutic agents. Bascuas et al. [108] used attenuated ST combined with CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone), which is the first-line chemotherapy regimen for B-cell non-Hodgkin’s lymphoma (B-NHL), and observed tumor growth-slowing and longer survival time in mice. This study also mentioned that the combination treatment is more advantageous than any single treatment, given that the bacterial vector acts as an immunotherapeutic agent, and fewer systemic toxicities of chemotherapy were observed with intratumoral administration. This work successfully demonstrated that combination chemotherapy with ST is a safe and effective cancer treatment [108]. In a study of breast cancer, mice receiving intravenous attenuated ST and low-dose chemotherapy (doxorubicin, 1.25 mg/kg) showed an acceptable rate of tumor growth (<3-fold volume at day 35, superior to low-dose doxorubicin monotherapy) and 5% weight loss. Although mice given an extreme dose of doxorubicin intravenously (5 mg/kg) showed a more pronounced slowing of tumor growth (1.4-fold volume), toxicities were significant in this group (25% weight loss) [109]. A separate study demonstrated the efficacy of attenuated ST carrying an anti-vascular growth gene in combination with cisplatin in primary and metastatic hepatocellular and lung cancers [110]. ST A1-R may improve gemcitabine efficacy in pancreatic cancer [111]. ST VNP20009 in combination with cyclophosphamide achieved good results in mice with B16F10 melanoma [112].
Several possible mechanisms may account for these efficacy-enhancing effects of the bacterial-chemotherapy combination, including ST anticancer and immune-mediating properties. Tumor-targeting and colonization abilities of bacteria-chemotherapy combinations that are greater than those of each agent alone may be attributed to the disruption of tumor microcirculation and creation of a local hypoxic tumor environment associated with the combination therapy [ 59, 110, 112] . Chang et al. [113] suggested that the upregulation of connexin 43 (Cx43) expression by ST may increase treatment efficacy. Reduced Cx43 expression in a variety of cancer cells, which attenuates gap junction intracellular communication, makes tumor cells less responsive to treatment [114]. In contrast, upregulation and activation of Cx43 expression by ST enhances antigen delivery in dendritic cells and facilitates the delivery of chemotherapeutic drugs and apoptotic signals within cancer cells [ 67, 113] . Yang et al. [115] found that Salmonella cholerae inhibited the expression of p-gp on tumor cells, which is also known as MDR1, associated with removing foreign substances from the cell. Therefore, decreased expression of p-gp decreases the pumping ability of tumor cells, thus reducing tumor cell chemoresistance. In addition, the expressions of p-p70S6K, p-AKT, and p-mTOR were reduced, while high expression of p-AKT reversed S. cholerae-mediated downregulation of p-gp. Overall, S. cholerae may enhance the sensitivity of tumor cells to chemotherapeutic drugs by downregulating p-gp expression levels [35]. It can also push quiescent tumor cells, which remain in G0/G1 phase, into the S/G2/M phase to enhance tumor cell drug sensitivity [ 116, 117] . Studies have shown that most cancer cells (nearly 80%) are in the stationary cell cycle phase (G0/G1 phase), and even more (up to 90%) are in tumor centers, while most current drugs effectively kill proliferating cancer cells but not stationary cancer cells [118]. Using CDDP-resistant osteosarcoma lung metastasis PDOX model mice, Hoffman et al. [119] compared the efficacy of CDDP alone, the ST A1-R+CDDP combination, and the ST A1-R+rMETase+CDDP combination. Among them, rMETae, a recombinant methionine enzyme, selectively traps S/G2 phase tumor cells and causes cell cycle arrest. Significant tumor suppression was observed in all conditions, except single CDDP, creating a novel approach to tumor therapy called “induce, trap, and kill” [ 16, 116] .
Bacterial outer membrane vesicles (OMVs) brought new ideas to this combination. OMVs are released from the membrane of gram-negative bacteria during their natural growth [ 120– 122] . They contain most of the immunogenic components of their parent bacteria, so OMVs have the capacity to stimulate anticancer immune responses [123]. Additionally, like their parent bacteria, they prefer to colonize tumor tissues, so they seem to be ideal carriers for tumor-targeting drug delivery [124]. Because they cannot self-replicate, it seems safer to use attenuated bacteria directly. Rasha et al. [125] demonstrated the safety of ST-OMVs. Additionally, they used OMVs combined with paclitaxel to treat HTC116, MCF-7 and HepG2 tumor-bearing mice and observed a significant decrease in tumor volume, a significant increase in the tumor growth inhibition rate, and a series of tumor-suppression immune responses. Chen et al. [126] coated tegafur (prodrug of 5-FU) in OMVs, prepared engineered OMV-coated polymeric nanoparticles, and used them to treat melanoma-bearing mice, successfully inhibiting tumor growth and prolonging survival. In addition, they found that the nanoparticles effectively inhibited the lung metastasis of melanoma. These attempts provide a new approach to tumor-targeted chemotherapy.
Radiotherapy
Radiation therapy is an anticancer modality prescribed to approximately 50% of cancer patients [127]. However, radiotherapy does not target the tumor and may cause tumor resistance, limiting its efficacy. A cumulative therapeutic effect of attenuated ST combined with radiotherapy has been reported in various tumor cells; bacteria may also increase the radiosensitivity of tumor cells [ 128– 130] . The increased radiosensitivity of tumors may be caused by changes to the immune components of the TME, including increased count and activity of dendritic cells, recruitment and activation of effector T cells, suppression of T-cell depletion, elimination of inhibitory signals, and release of cytokines (IL-2, GM-CSF, and IL-12) and chemokines (CCL3 and CCL5) [131]. Meanwhile, Kefayat et al. [132] used attenuated ST Ty21a as a vector for delivering gold nanoparticles to the central hypoxic radiation-resistant region of CT26 colon cancer. Gold nanoparticles are an excellent radiosensitizer. The bacteria-sensitizer combination enhances the efficacy of radiotherapy in the central hypoxic region of the tumor [133]. Chen et al. [134] used ST VNP20009 covered with polydopamine (PDA), a photothermal agent that converts near-infrared light into heat, to enhance the radiotherapy efficacy in mice bearing melanoma. VNP20009 delivers PDA to the hypoxic part of the tumor before radiotherapy. The additional heat generated by PDA locally raises the temperature and helps kill the surrounding cancer cells. This combination treatment helped eliminate melanoma without evidence of recurrence or distant metastasis [134].
Immunomodulatory factors
Immunomodulatory factors have been used in cancer treatment. The United States Food and Drug Administration (FDA) approved IL-2 for the treatment of metastatic kidney cancer and metastatic melanoma [135]; meanwhile, in Europe, IFN-α has been used in hairy cell leukemia [136]. However, due to limited efficacy and severe toxicity associated with systemic administration, immunomodulatory factors have been withdrawn from clinical use [ 137, 138] . ST can be used as a vector for targeted delivery of immunomodulatory factors to tumor tissues, helping maximize therapeutic effects and minimize toxicity. In a study using ST carrying the IL-2 gene to treat mice with unresectable hepatocellular carcinoma, the combination therapy showed antitumour and antimetastatic effects superior to those of ST monotherapy. This study also demonstrated that CD8+ T cells and NK cells have anticancer activity [139]. Similar results were reported in trials of this combination in osteosarcoma [140] and malignant melanoma [32]. In a clinical trial (phase I) in 2020, patients bearing metastatic gastrointestinal tumors treated with attenuated ST carrying the IL-2 gene showed no significant toxicity alongside a significant NK cell and NK-T-cell increase in the circulatory system. However, patient prognosis and survival were not improved [107]. Yoon et al. [141] used ST carrying the IFN-γ gene to treat mice with B16F10 malignant melanoma cells and successfully inhibited tumor development and prolonged survival by activating NK cells. However, the authors concluded that the oncogenic effect of ST was due to direct killing of tumor cells rather providing a stable anticancer immune response. Another study showed that ST carrying TNF-α had similar anticancer effects and could enhance the efficacy of other treatments ( e.g., chemotherapy) [142]. Several other studies using bacteria carrying other cytokines (e.g., CCL21, LIGHT, IL-18, among others) yielded good results [ 74, 143, 144] . Overall, this evidence suggests that ST is an effective and safe carrier of immunomodulatory factors.
Immune checkpoint inhibitors
Immune checkpoint inhibitors changed cancer immunotherapy. By inhibiting negative regulators of T-cell function and restoring T-cell activity, agents such as CTLA-4, PD-1, and PD-L1 kill tumor cells. Great efficacy has been observed by using immune checkpoint inhibitors in the treatment of different cancer types [ 145– 148] . However, severe, even fatal, immune-related adverse reactions have been observed in some patients [ 149, 150] .
A study by Ebelt et al. [151] on attenuated ST containing an IDO-targeted shRNA plasmid (shIDO- ST) in suppressing the expression of CTLA-4, PD-1, and PD-L1 in different splenic immune cells highlighted the ability of ST to change the immune components of the TME. By using ST A1-R carrying an anti-PD-L1 antibody, Binder et al. [152] successfully rescued the function of tumor-specific CD8+ T cells against melanoma and enhanced cancer rejection. Phan et al. [153] used attenuated shIDO- ST to treat two murine models of colon cancer (CT26 and MC38), showing delayed tumor growth; however, a combination with anti-PD-1 did not show additional tumor-suppressive effects. A study using IDO-shRNA- ST in combination with anti-CTLA-4 and PD-1 antibodies to treat lung cancer-bearing mice reported a delay in tumor growth associated with the combination treatment. The increased infiltration of CD4+ and CD8+ T cells may be the reason [151]. Zhao et al. [154] used attenuated ST carrying siRNA-PD-1 plasmids (PD-1-siRNA- ST) in combination with pimozide to treat B16 melanoma mice and observed tumor suppression and prolonged survival of the mice, suggesting that this combination stimulates the immune response and enhances the antimelanoma effect of pimozide. Similar results were obtained using PD-1-siRNA- ST in combination with nifuroxazide (Stat3 inhibitor) to treat CT26 colon cancer mice [155], combination regimens with chloroquine for colon tumors [156] and with CpG ODN for malignant melanoma [157] have been used with good results. Overall, this evidence suggests that an ST-immune checkpoint inhibitor combination may effectively treat tumors, while ST may be a high-quality tumor-targeting delivery vector.
Conclusions and Prospects
ST’s anticancer effects are associated with its oncolytic properties and abilities to change the immunosuppressive TME and to trigger an immune response. ST can also colonize and proliferate in tumor tissues and can be genetically modified to enhance its colonization ability and reduce bacterial virulence, making it an ideal delivery vector for anticancer compounds, which have been used in studies on combinations with chemotherapy, radiotherapy, and immunotherapy.
However, clinical trials have yielded less favorable results, including reports of tumor progression and metastasis [ 85, 107] , suggesting that ST technology needs to be improved before it can be safely and effectively used in BMCT. Some of the clinical trials of ST-based cancer therapy are listed in Table 2. ST is a pathogenic bacterium, and excessive attenuation may compromise its oncolytic and immune system-stimulating properties. Identifying the clinically optimal strain dose requires further investigation. As with conventional anticancer agents, the balance between toxicity and therapeutic efficacy is critical and challenging in BMCT. Oral administration tends to be safe and convenient; however, it may reduce treatment efficacy due to exposure to the acidic environment of the gastrointestinal tract, which kills most bacteria. Meanwhile, intravenous administration may cause toxicity and infection, while direct intratumoral injection seems to be the most ideal method but still requires more evidence. Further studies are required to identify the optimum administration route. Overall, ST is a promising potential contributor to cancer therapy; however, further evidence is required before it can be used in the clinic.
Table 2 Examples of clinical trials for Salmonella-based cancer therapy
|
Strain |
Cargo |
Cancer type |
Phase |
Status |
Enrollment |
NCT No. |
Website |
|
Bacteria alone |
Neoplasm metastasis |
I |
Completed |
45 |
https://clinicaltrials.gov/ct2/show/NCT00004988 |
||
|
Bacteria alone |
Unspecified solid tumor |
I |
Completed |
45 |
https://clinicaltrials.gov/ct2/show/NCT00006254 |
||
|
Bacteria alone |
Unspecified solid tumor |
I |
Completed |
40 |
https://clinicaltrials.gov/ct2/show/NCT00004216 |
||
|
x4550 |
IL-2 |
Liver cancer, biliary cancer |
I |
Completed |
22 |
https://clinicaltrials.gov/ct2/show/NCT01099631 |
|
|
VXM01 |
VEGFR-2 |
Stage IV pancreatic cancer |
I |
Completed |
72 |
https://clinicaltrials.gov/ct2/show/NCT01486329 |
|
|
TXSVN |
Survivin |
Multiple myeloma |
I |
Recruiting |
18 |
https://clinicaltrials.gov/ct2/show/NCT03762291 |
|
|
SS2017 |
Tyrosine hydroxylase Phox2B, Survivin, MAGEA1, MAGEA3, and PRAME |
Relapsed Neuroblastoma |
I |
Recruiting |
12 |
https://clinicaltrials.gov/ct2/show/NCT04049864 |
|
|
Saltikva |
IL2 |
Metastatic pancreatic cancer |
II |
Recruiting |
60 |
https://clinicaltrials.gov/ct2/show/NCT04589234 |
|
|
SGN1 |
L-Methioninase |
Advanced solid tumor |
I |
Not yet recruiting |
50 |
https://clinicaltrials.gov/ct2/show/NCT05038150 |
|
|
SGN1 |
L-Methioninase |
Advanced solid tumor |
I |
Not yet recruiting |
50 |
https://clinicaltrials.gov/ct2/show/NCT05103345 |
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grant from RuiJin Hospital Luwan Branch, Shanghai Jiaotong University School of Medicine.
References
- 1.Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. . 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 2.Coley WB. Contribution to the knowledge of Sarcoma . Ann Surg. . 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991, 262: 3–11 . [PubMed]
- 4.Al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Attoub S, Xu D, Chouaib S. Attenuated bacteria as effectors in cancer immunotherapy. Ann New York Acad Sci. . 2008;1138:351–357. doi: 10.1196/annals.1414.036. [DOI] [PubMed] [Google Scholar]
- 5.Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium . J Infect Dis. . 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 6.Liang K, Liu Q, Li P, Luo H, Wang H, Kong Q. Genetically engineered Salmonella typhimurium: recent advances in cancer therapy . Cancer Lett. . 2019;448:168–181. doi: 10.1016/j.canlet.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Yu YA, Zhang Q, Szalay AA. Establishment and characterization of conditions required for tumor colonization by intravenously delivered bacteria. Biotechnol Bioeng. . 2008;100:567–578. doi: 10.1002/bit.21785. [DOI] [PubMed] [Google Scholar]
- 8.Kasinskas RW, Forbes NS. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro . Biotechnol Bioeng. . 2006;94:710–721. doi: 10.1002/bit.20883. [DOI] [PubMed] [Google Scholar]
- 9.Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis . Cancer Res. . 2007;67:3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CJ, Clark DE, Adli M, Kendall MM. Ethanolamine signaling promotes Salmonella niche recognition and adaptation during infection. PLoS Pathog. 2015, 11: e1005278 . [DOI] [PMC free article] [PubMed]
- 11.Silva-Valenzuela CA, Desai PT, Molina-Quiroz RC, Pezoa D, Zhang Y, Porwollik S, Zhao M, et al. Solid tumors provide niche-specific conditions that lead to preferential growth of Salmonella . Oncotarget. . 2016;7:35169–35180. doi: 10.18632/oncotarget.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toley BJ, Forbes NS. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integrative Biol. . 2012;4:165–176. doi: 10.1039/c2ib00091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stritzker J, Weibel S, Seubert C, Götz A, Tresch A, van Rooijen N, Oelschlaeger TA, et al. Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int J Med Microbiol. . 2010;300:449–456. doi: 10.1016/j.ijmm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Chen T, Wan L, Lu J, Wei H, Deng KY, Wei J, et al. Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo . Appl Microbiol Biotechnol. . 2020;104:3517–3528. doi: 10.1007/s00253-020-10485-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaimala S, Mohamed YA, Nader N, Issac J, Elkord E, Chouaib S, Fernandez-Cabezudo MJ, et al. Salmonella-mediated tumor regression involves targeting of tumor myeloid suppressor cells causing a shift to M1-like phenotype and reduction in suppressive capacity . Cancer Immunol Immunother. . 2014;63:587–599. doi: 10.1007/s00262-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Miyake M, Li S, Han Q, et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic . Cell Cycle. . 2018;17:801–809. doi: 10.1080/15384101.2018.1431596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model . Oncotarget. . 2016;7:12783–12790. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, He J, Cheng H, Zhu Z, Xu H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct . J Exp Clin Cancer Res. . 2016;35:107. doi: 10.1186/s13046-016-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami T, Hiroshima Y, Zhao M, Zhang Y, Chishima T, Tanaka K, Bouvet M, et al. Therapeutic efficacy of tumor-targeting Salmonella typhimurium A1-R on human colorectal cancer liver metastasis in orthotopic nude-mouse models . Oncotarget. . 2015;6:31368–31377. doi: 10.18632/oncotarget.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models . PLoS One. . 2015;10:e0120358. doi: 10.1371/journal.pone.0120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe E, Kazmierczak RA, Eisenstark A. Phenotypic evolution of therapeutic Salmonella enterica serovar typhimurium after invasion of TRAMP mouse prostate tumor. mBio. 2014, 5: e01182–14 . [DOI] [PMC free article] [PubMed]
- 22.Vendrell A, Gravisaco MJ, Goin JC, Pasetti MF, Herschllik L, Toro JD, Rodríguez C, et al. Therapeutic effects of Salmonella typhi in a mouse model of T-cell lymphoma . J ImmunoTher. . 2013;36:171–180. doi: 10.1097/CJI.0b013e3182886d95. [DOI] [PubMed] [Google Scholar]
- 23.Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model . Oncotarget. . 2016;7:33046–33054. doi: 10.18632/oncotarget.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Kishimoto H, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhymurium . Cell Cycle. . 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 25.Miwa S, Yano S, Zhang Y, Matsumoto Y, Uehara F, Yamamoto M, Hiroshima Y, et al. Tumor-targeting Salmonella typhimurium A1-R prevents experimental human breast cancer bone metastasis in nude mice . Oncotarget. . 2014;5:7119–7125. doi: 10.18632/oncotarget.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, Bouvet M, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium . Cell Proliferation. . 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenstark A, Kazmierczak RA, Dino A, Khreis R, Newman D, Schatten H. Development of Salmonella strains as cancer therapy agents and testing in tumor cell lines. Methods Mol Biol. 2007, 394: p. 323–54 . [DOI] [PubMed]
- 28.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. . 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Lin ST, Liu JJ, Chang WW, Hsieh JL, Wang WK. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway . Gene Ther. . 2014;21:309–316. doi: 10.1038/gt.2013.86. [DOI] [PubMed] [Google Scholar]
- 30.Tu DG, Chang WW, Lin ST, Kuo CY, Tsao YT, Lee CH. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor . Oncotarget. . 2016;7:37513–37523. doi: 10.18632/oncotarget.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity . Cell Cycle. . 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Bashir G, Chouaib S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin Immunol. . 2009;130:89–97. doi: 10.1016/j.clim.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Spector MP, Garcia del Portillo F, Bearson SMD, Mahmud A, Magut M, Finlay BB, Dougan G, et al. The rpoS-dependent starvation-stress response locus stiA encodes a nitrate reductase (narZYWV) required for carbon-starvation-inducible thermotolerance and acid tolerance in Salmonella typhimurium . Microbiology. . 1999;145:3035–3045. doi: 10.1099/00221287-145-11-3035. [DOI] [PubMed] [Google Scholar]
- 34.Barak Y, Schreiber F, Thorne SH, Contag CH, deBeer D, Matin A. Role of nitric oxide in Salmonella typhimurium-mediated cancer cell killing . BMC Cancer. . 2010;10:146. doi: 10.1186/1471-2407-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang CJ, Chang WW, Lin ST, Chen MC, Lee CH. Salmonella overcomes drug resistance in tumor through P-glycoprotein downregulation . Int J Med Sci. . 2018;15:574–579. doi: 10.7150/ijms.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsao YT, Kuo CY, Cheng SP, Lee CH. Downregulations of AKT/mTOR Signaling pathway for Salmonella-mediated suppression of matrix metalloproteinases-9 expression in mouse tumor models . Int J Mol Sci. . 2018;19:1630. doi: 10.3390/ijms19061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neophytou CM, Panagi M, Stylianopoulos T, Papageorgis P. The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers. . 2021;13:2053. doi: 10.3390/cancers13092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badie F, Ghandali M, Tabatabaei SA, Safari M, Khorshidi A, Shayestehpour M, Mahjoubin-Tehran M, et al. Use of Salmonella bacteria in cancer therapy: direct, drug delivery and combination approaches . Front Oncol. . 2021;11:624759. doi: 10.3389/fonc.2021.624759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorge NAN, Cruz JGV, Pretti MAM, Bonamino MH, Possik PA, Boroni M. Poor clinical outcome in metastatic melanoma is associated with a microRNA-modulated immunosuppressive tumor microenvironment. J Transl Med. . 2020;18:56. doi: 10.1186/s12967-020-02235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. . 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, Validire P, et al. The clinical role of the TME in solid cancer. Br J Cancer. . 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. . 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. . 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 44.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3 kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. . 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, Ciorba MA. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Res. . 2019;79:1138–1150. doi: 10.1158/0008-5472.CAN-18-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuan YD, Lee CH. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2,3-dioxygenase 1 expression . Oncotarget. . 2016;7:374–385. doi: 10.18632/oncotarget.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. . 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Lin HC, Yang CJ, Kuan YD, Wang WK, Chang WW, Lee CH. The inhibition of indoleamine 2,3-dioxygenase 1 by connexin 43. Int J Med Sci. . 2017;14:1181–1188. doi: 10.7150/ijms.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. . 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H, Shen Z, Wang Z, Wang X, Zhang H, Qin J, Qin X, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep. . 2016;6:21319. doi: 10.1038/srep21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Wu J, Shen H, Wang J. The prognostic value of IDO expression in solid tumors: a systematic review and meta-analysis. BMC Cancer. . 2020;20:471. doi: 10.1186/s12885-020-06956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis . Clin Cancer Res. . 2008;14:1905–1912. doi: 10.1158/1078-0432.CCR-07-2050. [DOI] [PubMed] [Google Scholar]
- 53.Grille S, Moreno M, Bascuas T, Marqués JM, Muñoz N, Lens D, Chabalgoity JA. Salmonella enterica serovar typhimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect . Immunology. . 2014;143:428–437. doi: 10.1111/imm.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaimala S, Al-Sbiei A, Cabral-Marques O, Fernandez-Cabezudo MJ, Al-Ramadi BK. Attenuated bacteria as immunotherapeutic tools for cancer treatment. Front Oncol. . 2018;8:136. doi: 10.3389/fonc.2018.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CH, Hsieh JL, Wu CL, Hsu PY, Shiau AL. T cell augments the antitumor activity of tumor-targeting Salmonella . Appl Microbiol Biotechnol. . 2011;90:1381–1388. doi: 10.1007/s00253-011-3180-z. [DOI] [PubMed] [Google Scholar]
- 56.Lee CH, Hsieh JL, Wu CL, Hsu HC, Shiau AL. B cells are required for tumor-targeting Salmonella in host . Appl Microbiol Biotechnol. . 2011;92:1251–1260. doi: 10.1007/s00253-011-3386-0. [DOI] [PubMed] [Google Scholar]
- 57.Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. . 2011;71:2466–2475. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng JH, Nguyen V, Jiang SN, Park SH, Tan W, Hong SH, Shin MG, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017, 9: eaak9537 . [DOI] [PubMed]
- 59.Chen J, Qiao Y, Tang B, Chen G, Liu X, Yang B, Wei J, et al. Modulation of Salmonella tumor-colonization and intratumoral anti-angiogenesis by triptolide and its mechanism . Theranostics. . 2017;7:2250–2260. doi: 10.7150/thno.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kocijancic D, Felgner S, Schauer T, Frahm M, Heise U, Zimmermann K, Erhardt M, et al. Local application of bacteria improves safety of Salmonella-mediated tumor therapy and retains advantages of systemic infection . Oncotarget. . 2017;8:49988–50001. doi: 10.18632/oncotarget.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Na HS, Kim HJ, Lee HC, Hong Y, Rhee JH, Choy HE. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis . Vaccine. . 2006;24:2027–2034. doi: 10.1016/j.vaccine.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 62.Simon R, Samuel CE. Activation of NF-κB-dependent gene expression by Salmonella flagellins FliC and FljB . Biochem Biophys Res Commun. . 2007;355:280–285. doi: 10.1016/j.bbrc.2007.01.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johannessen M, Askarian F, Sangvik M, Sollid JE. Bacterial interference with canonical NFκB signalling. Microbiology. . 2013;159:2001–2013. doi: 10.1099/mic.0.069369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Souvannavong V, Saidji N, Chaby R. Lipopolysaccharide from Salmonella enterica activates NF-κB through both classical and alternative pathways in primary B lymphocytes . Infect Immun. . 2007;75:4998–5003. doi: 10.1128/IAI.00545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janeway Jr. CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. . 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez-Luna MA, Luria-Perez R. Cancer immunotherapy: priming the host immune response with live attenuated Salmonella enterica. J Immunol Res. 2018, 2018: 2984247 . [DOI] [PMC free article] [PubMed]
- 67.Saccheri F, Pozzi C, Avogadri F, Barozzi S, Faretta M, Fusi P, Rescigno M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med. . 2010;2:44ra57. doi: 10.1126/scitranslmed.3000739. [DOI] [PubMed] [Google Scholar]
- 68.Chen MC, Pangilinan CR, Lee CH. Salmonella breaks tumor immune tolerance by downregulating tumor programmed death-ligand 1 expression . Cancers. . 2020;12:57. doi: 10.3390/cancers12010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. . 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardlik R, Fruchauf JH. Bacterial vectors and delivery systems in cancer therapy. IDrug. 2010, 13: 701–706 . [PubMed]
- 71.Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, Chu PK, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. . 2015;15:2732–2739. doi: 10.1021/acs.nanolett.5b00570. [DOI] [PubMed] [Google Scholar]
- 72.Feng KK, Zhao HY, Qiu H, Chen J. Specific anti-glioma angiogenesis immune response induced by attenuated Salmonella typhimurium vaccine expressing vascular endothelial growth factor receptor-2. Ai Zheng. 2005. 24: 548–553 . [PubMed]
- 73.Kim B, Suvas S, Sarangi PP, Lee S, Reisfeld RA, Rouse BT. Vascular endothelial growth factor receptor 2-based DNA immunization delays development of herpetic stromal keratitis by antiangiogenic effects. J Immunol. . 2006;177:4122–4131. doi: 10.4049/jimmunol.177.6.4122. [DOI] [PubMed] [Google Scholar]
- 74.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. IL-18-producing Salmonella inhibit tumor growth . Cancer Gene Ther. . 2008;15:787–794. doi: 10.1038/cgt.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stegantseva MV, Shinkevich VA, Tumar EM, Meleshko AN. Multi-antigen DNA vaccine delivered by polyethylenimine and Salmonella enterica in neuroblastoma mouse model . Cancer Immunol Immunother. . 2020;69:2613–2622. doi: 10.1007/s00262-020-02652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, et al. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J ImmunoTher. . 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 77.Setten RL, Rossi JJ, Han S. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. . 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 78.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JDI, Hensel M, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors . Cancer Res. . 2011;71:4183–4191. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng J, Guo Y, Jiang Z, Yang M, Li H, Wang J. Enhancement of ovarian cancer chemotherapy by delivery of multidrug-resistance gene small interfering RNA using tumor targeting Salmonella . J Obstet Gynaecol Res. . 2015;41:615–622. doi: 10.1111/jog.12598. [DOI] [PubMed] [Google Scholar]
- 80.Yoon W, Park Y, Kim S, Park Y, Kim CY. Combined therapy with microRNA-expressing Salmonella and irradiation in melanoma . Microorganisms. . 2021;9:2408. doi: 10.3390/microorganisms9112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon W, Yoo Y, Chae YS, Kee SH, Kim BM. Therapeutic advantage of genetically engineered Salmonella typhimurium carrying short hairpin RNA against inhibin alpha subunit in cancer treatment . Ann Oncol. . 2018;29:2010–2017. doi: 10.1093/annonc/mdy240. [DOI] [PubMed] [Google Scholar]
- 82.Yang N, Zhu X, Chen L, Li S, Ren D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic . Cancer Biol Ther. . 2008;7:145–151. doi: 10.4161/cbt.7.1.5195. [DOI] [PubMed] [Google Scholar]
- 83.Mesa‐Pereira B, Medina C, Camacho EM, Flores A, Santero E. Improved cytotoxic effects of Salmonella‐producing cytosine deaminase in tumour cells . Microb Biotechnol. . 2015;8:169–176. doi: 10.1111/1751-7915.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, Le T, et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent . Hum Gene Ther. . 2002;13:1225–1233. doi: 10.1089/104303402320139005. [DOI] [PubMed] [Google Scholar]
- 85.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma . J Clin Oncol. . 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frahm M, Felgner S, Kocijancic D, Rohde M, Hensel M, Curtiss R, Erhardt M, et al. Efficiency of conditionally attenuated Salmonella enterica serovar typhimurium in bacterium-mediated tumor therapy. mBio. 2015, 6: e00254-15 . [DOI] [PMC free article] [PubMed]
- 87.Cheng X, Zhang X, Zhou Y, Zhang C, Hua ZC. A Salmonella typhimurium mutant strain capable of RNAi delivery . Cancer Biol Ther. . 2014;15:1068–1076. doi: 10.4161/cbt.29185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Broadway KM, Modise T, Jensen RV, Scharf BE. Complete genome sequence of Salmonella enterica serovar typhimurium VNP20009, a strain engineered for tumor targeting . J Biotechnol. . 2014;192:177–178. doi: 10.1016/j.jbiotec.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 89.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium . Proc Natl Acad Sci USA. . 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice . Cancer Res. . 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 91.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer . Proc Natl Acad Sci USA. . 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffman RM. Tumor-seeking Salmonella amino acid auxotrophs . Curr Opin Biotechnol. . 2011;22:917–923. doi: 10.1016/j.copbio.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 93.Hoffman RM. Back to the future: are tumor-targeting bacteria the next-generation cancer therapy? Methods Mol Biol. 2015, 1317: 239–260 . [DOI] [PubMed]
- 94.Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) . J Cell Biochem. . 2014;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- 95.Murakami T, Hiroshima Y, Miyake K, Kiyuna T, Endo I, Zhao M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R against malignancies in patient-derived orthotopic xenograft (PDOX) murine models . Cells. . 2019;8:599. doi: 10.3390/cells8060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, et al. Efficacy of tumor-targeting Salmonella A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model . PLoS One. . 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiroshima Y, Zhang Y, Murakami T, Maawy A, Miwa S, Yamamoto M, Yano S, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models . Oncotarget. . 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. Salmonella pathogenicity islands: big virulence in small packages . Microbes Infect. . 2000;2:145–156. doi: 10.1016/S1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 99.Bajaj V, Lucas RL, Hwang C, Lee CA. Co‐ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression . Mol Microbiol. . 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 100.Bajaj V, Hwang C, Lee CA. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes . Mol Microbiol. . 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 101.Pizarro-Cerdá J, Tedin K. The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression . Mol Microbiol. . 2004;52:1827–1844. doi: 10.1111/j.1365-2958.2004.04122.x. [DOI] [PubMed] [Google Scholar]
- 102.Song M, Kim HJ, Kim EY, Shin M, Lee HC, Hong Y, Rhee JH, et al. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1 . J Biol Chem. . 2004;279:34183–34190. doi: 10.1074/jbc.M313491200. [DOI] [PubMed] [Google Scholar]
- 103.Tan W, Duong MTQ, Zuo C, Qin Y, Zhang Y, Guo Y, Hong Y, et al. Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium . Mol Ther. . 2022;30:662–671. doi: 10.1016/j.ymthe.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Guo Y, Sun Y, Chen Y, Tan W, Min JJ, Zheng JH. Comparison of anticancer activities and biosafety between Salmonella enterica serovar typhimurium deltappGpp and VNP20009 in a murine cancer model . Front Microbiol. . 2022;13:914575. doi: 10.3389/fmicb.2022.914575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang K, Zhang R, Luo H, Zhang J, Tian Z, Zhang X, Zhang Y, et al. Optimized attenuated Salmonella typhimurium suppressed tumor growth and improved survival in mice . Front Microbiol. . 2021;12:774490. doi: 10.3389/fmicb.2021.774490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Felgner S, Kocijancic D, Frahm M, Curtiss Iii R, Erhardt M, Weiss S. Optimizing Salmonella enterica serovar typhimurium for bacteria-mediated tumor therapy . Gut Microbes. . 2016;7:171–177. doi: 10.1080/19490976.2016.1155021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gniadek TJ, Augustin L, Schottel J, Leonard A, Saltzman D, Greeno E, Batist G. A phase I, dose escalation, single dose trial of oral attenuated Salmonella typhimurium containing human IL-2 in patients with metastatic gastrointestinal cancers . J ImmunoTher. . 2020;43:217–221. doi: 10.1097/CJI.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bascuas T, Moreno M, Grille S, Chabalgoity JA. Salmonella immunotherapy improves the outcome of CHOP chemotherapy in non-Hodgkin lymphoma-bearing mice . Front Immunol. . 2018;9:7. doi: 10.3389/fimmu.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saltzman D, Augustin L, Leonard A, Mertensotto M, Schottel J. Low dose chemotherapy combined with attenuated Salmonella decreases tumor burden and is less toxic than high dose chemotherapy in an autochthonous murine model of breast cancer . Surgery. . 2018;163:509–514. doi: 10.1016/j.surg.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 110.Lee CH, Wu CL, Tai YS, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol Ther. . 2005;11:707–716. doi: 10.1016/j.ymthe.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Kawaguchi K, Miyake K, Zhao M, Kiyuna T, Igarashi K, Miyake M, Higuchi T, et al. Tumor targeting Salmonella typhimurium A1-R in combination with gemcitabine (GEM) regresses partially GEM-resistant pancreatic cancer patient-derived orthotopic xenograft (PDOX) nude mouse models . Cell Cycle. . 2018;17:2019–2026. doi: 10.1080/15384101.2018.1480223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jia LJ, Wei DP, Sun QM, Jin GH, Li SF, Huang Y, Hua ZC. Tumor-targetingSalmonella typhimurium improves cyclophosphamide chemotherapy at maximum tolerated dose and low-dose metronomic regimens in a murine melanoma model. Int J Cancer. . 2007;121:666–674. doi: 10.1002/ijc.22688. [DOI] [PubMed] [Google Scholar]
- 113.Chang WW, Lai CH, Chen MC, Liu CF, Kuan YD, Lin ST, Lee CH. Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation . Int J Cancer. . 2013;133:1926–1935. doi: 10.1002/ijc.28155. [DOI] [PubMed] [Google Scholar]
- 114.Wang M, Berthoud VM, Beyer EC. Connexin43 increases the sensitivity of prostate cancer cells to TNFα-induced apoptosis. J Cell Sci. . 2007;120:320–329. doi: 10.1242/jcs.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nanayakkara AK, Follit CA, Chen G, Williams NS, Vogel PD, Wise JG. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci Rep. . 2018;8:967. doi: 10.1038/s41598-018-19325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yano S, Takehara K, Zhao M, Tan Y, Han Q, Li S, Bouvet M, et al. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging . Cell Cycle. . 2016;15:1715–1723. doi: 10.1080/15384101.2016.1181240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy . Cell Cycle. . 2014;13:3958–3963. doi: 10.4161/15384101.2014.964115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yano S, Zhang Y, Miwa S, Tome Y, Hiroshima Y, Uehara F, Yamamoto M, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle. . 2014;13:2110–2119. doi: 10.4161/cc.29156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffman RM, Yano S, Igarashi K. Methioninase cell-cycle trap cancer chemotherapy. Methods Mol Biol. 2019, 1866: 133–148 . [DOI] [PubMed]
- 120.Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. . 2014;8:1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 121.Pierson T, Matrakas D, Taylor YU, Manyam G, Morozov VN, Zhou W, van Hoek ML. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine . J Proteome Res. . 2011;10:954–967. doi: 10.1021/pr1009756. [DOI] [PubMed] [Google Scholar]
- 122.Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, et al. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol. 2014, 21: 747–754 . [DOI] [PMC free article] [PubMed]
- 123.Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. . 2017;8:626. doi: 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. . 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 125.Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS. Antineoplastic activity of Salmonella typhimurium outer membrane nanovesicles . Exp Cell Res. . 2021;399:112423. doi: 10.1016/j.yexcr.2020.112423. [DOI] [PubMed] [Google Scholar]
- 126.Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, et al. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. . 2020;20:11–21. doi: 10.1021/acs.nanolett.9b02182. [DOI] [PubMed] [Google Scholar]
- 127.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment. Cancer. . 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 128.Yoon WS, Kim S, Seo S, Park Y. Salmonella typhimurium with γ-radiation induced H2AX phosphorylation and apoptosis in melanoma . Biosci Biotechnol Biochem. . 2014;78:1082–1085. doi: 10.1080/09168451.2014.905173. [DOI] [PubMed] [Google Scholar]
- 129.Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low KB, Pawelek J. Antitumour effects of genetically engineered Salmonella in combination with radiation . Eur J Cancer. . 2000;36:2397–2402. doi: 10.1016/S0959-8049(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 130.Liu X, Jiang S, Piao L, Yuan F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer . Exp Anim. . 2016;65:413–418. doi: 10.1538/expanim.16-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. . 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kefayat A, Ghahremani F, Motaghi H, Rostami S, Mehrgardi MA. Alive attenuated Salmonella as a cargo shuttle for smart carrying of gold nanoparticles to tumour hypoxic regions . J Drug Targeting. . 2019;27:315–324. doi: 10.1080/1061186X.2018.1523417. [DOI] [PubMed] [Google Scholar]
- 133.Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C. Radiosensitization by gold nanoparticles: will they ever make it to the clinic? RadioTher Oncol. . 2017;124:344–356. doi: 10.1016/j.radonc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 134.Chen W, Wang Y, Qin M, Zhang X, Zhang Z, Sun X, Gu Z. Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy. ACS Nano. . 2018;12:5995–6005. doi: 10.1021/acsnano.8b02235. [DOI] [PubMed] [Google Scholar]
- 135.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. . 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quesada JR, Hersh EM, Manning J, Reuben J, Keating M, Schnipper E, Itri L, et al. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. . 1986;68:493–497. doi: 10.1182/blood.V68.2.493.493. [DOI] [PubMed] [Google Scholar]
- 137.Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. . 2019;35:150923. doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Z, Zheng L, Chen W, Weng W, Song J, Ji J. Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. . 2019;12:126. doi: 10.1186/s13045-019-0817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saltzman DA, Katsanis E, Heise CP, Hasz DE, Vigdorovich V, Kelly SM, Curtiss Iii R, et al. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? . J Pediatr Surg. . 1997;32:301–306. doi: 10.1016/s0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 140.Sorenson BS, Banton KL, Frykman NL, Leonard AS, Saltzman DA. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma . Clin Orthopaedics Relat Res. . 2008;466:1285–1291. doi: 10.1007/s11999-008-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoon W, Park YC, Kim J, Chae YS, Byeon JH, Min SH, Park S, et al. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma . Eur J Cancer. . 2017;70:48–61. doi: 10.1016/j.ejca.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 142.Yoon WS, Chae YS, Hong J, Park YK. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-α in mice . Appl Microbiol Biotechnol. . 2011;89:1807–1819. doi: 10.1007/s00253-010-3006-4. [DOI] [PubMed] [Google Scholar]
- 143.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother. . 2009;58:769–775. doi: 10.1007/s00262-008-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loeffler M, Le′Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth . Proc Natl Acad Sci USA. . 2007;104:12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. . 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, et al. Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med. . 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 147.Hodi FS, O′Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. . 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Robert C, Thomas L, Bondarenko I, O′Day S, Weber J, Garbe C, Lebbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. . 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 149.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, et al. Fatal toxic effects associated with immune checkpoint inhibitors. JAMA Oncol. . 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res. . 2013;19:3977–3986. doi: 10.1158/1078-0432.CCR-12-3243. [DOI] [PubMed] [Google Scholar]
- 151.Ebelt ND, Zuniga E, Marzagalli M, Zamloot V, Blazar BR, Salgia R, Manuel ER. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase restructures the immune contexture to improve checkpoint blockade efficacy . Biomedicines. . 2020;8:617. doi: 10.3390/biomedicines8120617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, Karrison T, et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res. . 2013;1:123–133. doi: 10.1158/2326-6066.CIR-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Phan T, Nguyen VH, D’Alincourt MS, Manuel ER, Kaltcheva T, Tsai W, Blazar BR, et al. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth . Cancer Gene Ther. . 2020;27:235–245. doi: 10.1038/s41417-019-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao T, Wei T, Guo J, Wang Y, Shi X, Guo S, Jia X, et al. PD-1-siRNA delivered by attenuated Salmonella enhances the antimelanoma effect of pimozide . Cell Death Dis. . 2019;10:164. doi: 10.1038/s41419-019-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao T, Feng Y, Guo M, Zhang C, Wu Q, Chen J, Guo S, et al. Combination of attenuated Salmonella carrying PD‐1 siRNA with nifuroxazide for colon cancer therapy . J Cell Biochem. . 2020;121:1973–1985. doi: 10.1002/jcb.29432. [DOI] [PubMed] [Google Scholar]
- 156.Lu S, Gao J, Jia H, Li Y, Duan Y, Song F, Liu Z, et al. PD-1-siRNA delivered by attenuated Salmonella enhances the antitumor effect of chloroquine in colon cancer . Front Immunol. . 2021;12:707991. doi: 10.3389/fimmu.2021.707991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jia X, Guo J, Guo S, Zhao T, Liu X, Cheng C, Wang L, et al. Antitumor effects and mechanisms of CpG ODN combined with attenuated Salmonella-delivered siRNAs against PD-1 . Int Immunopharmacol. . 2021;90:107052. doi: 10.1016/j.intimp.2020.107052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.