Abstract
Hypoxia plays a crucial role in pulmonary vascular remodelling at the early stage of chronic obstructive pulmonary disease (COPD). Circle RNA (circRNA) has been identified to play a critical role in multiple diseases. However, the role of circRNAs in pulmonary vascular remodelling in COPD remains unclear. In this study, we aim to investigate the role of circRNAs in pulmonary arterial smooth muscle cell proliferation and pulmonary vascular remodelling in COPD. COPD patients show lower partial pressure of arterial oxygen and pulmonary arterial remodeling as compared with controls. circRNA microarray and real-time PCR analyses show significantly higher level of circ-BPTF and lower miR-486-5p level in the pulmonary arteries of COPD patients as compared with controls. Hypoxia suppresses miR-486-5p expression but promotes expressions of circ-BPTF and cell migration inducing protein (CEMIP) in human pulmonary arterial smooth muscle cells (PASMCs) in vitro. Loss- and gain-of-function experiments show that circ-BPTF promotes PASMC proliferation in vitro. Moreover, luciferase reporter assay results indicate that circ-BPTF regulates PASMC proliferation by acting as an miR-486-5p sponge. CEMIP is identified as a candidate target gene of miR-486-5p by luciferase reporter assay. Overall, our study shows that circ-BPTF serves as a miR-486-5p sponge to regulate CEMIP and promote hypoxic PASMC proliferation in pulmonary vascular remodelling in COPD.
Keywords: pulmonary vascular remodelling, hypoxia, chronic obstructive pulmonary disease (COPD), circ-BPTF, miR-486-5p, CEMIP
Introduction
As a serious and progressive pulmonary vascular disease, pulmonary hypertension (PH) is a complication of multiple chronic lung diseases [1]. PH is characterized by elevated pulmonary arterial pressure and pulmonary vascular resistance, which are mainly caused by pulmonary vasoconstriction and pulmonary vascular remodelling [2]. Chronic obstructive pulmonary disease (COPD) is one of the most common disorders of the respiratory system and eventually leads to hypoxic PH [3]. Previous reports, including our studies, have proven that pulmonary vascular remodelling occurs in the early stage of COPD [ 4– 7] . We also revealed that cyclin D1 and NR4A3 are involved in pulmonary vascular remodelling of COPD [ 5– 9] .
Although an increasing number of studies have demonstrated the role of multiple growth factors or pathways (such as prostacyclin, nitric oxide and endothelin, etc.) in pulmonary vascular remodelling, drugs targeting these pathways have little benefit to COPD patients with PH [ 10, 11] . As a critical feature of pulmonary vascular remodelling, hypertrophy and proliferation of pulmonary artery smooth muscle cells (PASMCs) contribute greatly to the sustained increase in pulmonary vascular resistance and pulmonary artery pressure in PH [ 12, 13] . Therefore, it is essential to explore the molecular mechanisms responsible for PASMC proliferation to ameliorate pulmonary vascular remodelling.
Circle RNAs (circRNAs) are RNA molecules with covalently joined 3′- and 5′-ends formed by back-splicing events [14]. circRNAs widely exist in mammalian cells and exert biological effects at the posttranscriptional level by sponging miRNAs at response elements or miRNA-binding sites [15]. Recent reports indicated that circRNAs are involved in PH [ 16– 20] . Circ-calm4 has been proven to promote PASMC proliferation by sponging miR-337-3p. hsa_circ_0016070 and mmu_circ_0000790 are also involved in PH by regulating PASMC proliferation. However, the function of circRNAs in pulmonary vascular remodelling of COPD has not been clearly reported.
In this study, we screened a novel circRNA, circ-BPTF, which showed increased expression in pulmonary arteries of COPD. We also explored its roles in PASMC proliferation and pulmonary vascular remodelling in COPD.
Materials and Methods
Lung tissue collection
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Soochow University and Dushu Lake Hospital Affiliated to Soochow University, and written informed consents were obtained from all subjects. Human peripheral lung samples were collected from patients who underwent pneumonectomy for lung volume reduction or pulmonary nodules from January 2016 to December 2019 in our hospital. Lung tissue samples were obtained from normal areas as far as possible from the pulmonary nodules (at least 2 cm). Peripheral lung samples or pulmonary arterial explants were inflated with 4% (w/v) paraformaldehyde for embedding in paraffin or stored at –80°C for subsequent protein extraction. Pulmonary function tests were performed before surgery in all patients. COPD was determined by the standard of pulmonary function as FEV1/FVC less than 0.7. Arterial blood samples were collected before the operation and patients were not receiving oxygen before blood sampling.
Pulmonary vascular morphometry
Paraffin-embedded peripheral lung samples were cut into multiple 5-μm-thick sections. Sections were deparaffinized and stained with hematoxylin and eosin (Beyotime, Shanghai, China) for morphometric measurements under an optical microscope (Olympus, Tokyo, Japan) as described in our previous reports [ 5– 7] . The vessel wall thickness was expressed as a percentage of the external diameter [(external diameter−internal diameter)/external diameter×100%]. The assessment was limited to medium and small arteries (≤500 μm diameter) with a complete circumferential smooth muscle layer. At least ten vessels were measured in each section.
Immunohistochemical analysis
Immunohistochemistry (IHC) analysis was performed according to the following procedure. The primary segment-specific antibody for α-smooth muscle actin (1:1000; Abcam) was applied to the tissue sections and incubated overnight at 4°C. The slides were incubated with goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:1000; Abcam) for 30 min at 22°C, followed by washing and incubation with diaminobenzidine (DAB; Beyotime). A light hematoxylin counterstain was performed,dehydrated, cleared, and mounted. Stained slides were photographed by an immunofluorescence microscope (Olympus, Japan, Tokyo). All these arteries were classified into three groups: nonmuscularized (25% circumference with α-actin staining), partially muscularized (25% to 75% circumference with α-actin staining) and fully muscularized (75% circumference with α-actin staining). The fully muscularized vessels were calculated and expressed as the percentage of total medium and small arteries. The assessment was limited to medium and small arteries (≤ 500 μm diameter) with a complete circumferential smooth muscle layer. At least five vessels were measured in each section.
circRNA microarray scan
Pulmonary arterial explants of lung samples were collected from the COPD group and the non-COPD group (three samples from each group). Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. RNA concentration determination, RNA quality control and microarray hybridization were performed according to the manufacturer’s instructions of the Arraystar Super RNA Labeling kit (Arraystar, Rockville, USA). Linear RNAs were eliminated using RNase R (Epicenter, New York, USA). The enriched circRNAs were subsequently amplified and transcribed into fluorescent complementary RNAs (cRNAs) with a random priming method of the Arraystar Super RNA Labelling Kit. Labelled cRNAs were then purified using the RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). Labelled cRNA quality and quantity were determined with the NanoDrop ND-1000 spectrophotometer (Agilent Technologies, Santa Clara, USA). Subsequently, the labelled cRNA was fragmented and hybridized onto the Arraystar human circRNA microarray (circular RNA array V2.0 RNA; Arraystar). The hybridized arrays were scanned using an Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, USA).
Cell culture and proliferation assay
As described previously [ 5– 7] , primary hPASMCs were separated from control subjects using distal pulmonary arteries and cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM; Biological Industries, Cromwell, USA) containing 10% fetal bovine serum (FBS; Biological Industries), 1% penicillin and streptomycin (Beyotime, shanghai, China) at 37°C. Then, cells at passages 3–8 were used for the in vitro experiments.
Cell count kit-8 (CCK-8; Beyotime) was used to evaluate cell proliferation. Briefly, sells were seeded into 24-well plates at a concentration of 1×10 4 cells/well. After treatment, 10 μL CCK-8 solution was added to each well and incubated at 37°C for 2 h. The absorbance at 450 nm was determined with an iMark TM microplate reader (Bio-Rad, Hercules, USA). Cell viability was assessed by MTT assay. Briefly, cells were cultured in 96-well plates and treated with different vectors for 24 h to 72 h. After treatment, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Beyotime) was add to each well and incubated for 4 h at 37°C. After addition of 100 μL DMSO to each well, the absorbance was measured at 570 nm with the iMark TM microplate reader. All experiments were performed in triplicate.
Cell transfection
All small interfering RNA (circ-BPTF-siRNA-1, circ-BPTF-siRNA-2, NC-siRNA and NC-mimics) sequences were purchased from (Genepharma, Shanghai, China). According to the manufacturer’s instructions, a total of 2×10 5 hPASMCs were seeded in a 6-well plate overnight at 37°C. The cells were then cultured in serum-free DMEM medium for 2 h prior to transfection. The cells were subsequently transiently transfected with small interfering RNA and cells were transfected with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA). All interference sequences are listed in Table 1.
Table 1 Sequences of the primers and miRNAs used in this study
|
Name |
Sequence (5′→3′) |
|
hsa_circ_0004893 (F) |
GTTGCAGGATTGGGAAGGTT |
|
hsa_circ_0004893 (R) |
CAGTCTCGGTGTCCAGGAGT |
|
hsa_circ_0002970 (F) |
AGACGAAAGGGGAGGTTATGA |
|
hsa_circ_0002970 (R) |
TGCAGGTACATTGGTTTCCC |
|
hsa_circ_0008199 (F) |
AGAATAAAGATAACCAAGACAAGAG |
|
hsa_circ_0008199 (R) |
TTGCGAAGACACCTGAAGC |
|
hsa_circ_0045462 (circ-BPTF) (F) |
CCTGTGATGGTACAGCCAGAAA |
|
hsa_circ_0045462 (circ-BPTF) (R) |
TGGTGCTGGTTGTACTGCTTGT |
|
hsa_circ_0045462 siRNA-1 (circ-BPTF-siRNA-1) sense |
GAUGGUACAGCCAGAAACGTT |
|
hsa_circ_0045462 siRNA-1 (circ-BPTF-siRNA-1) antisense |
CGUUUCUGGCUGUACCAUCTT |
|
hsa_circ_0045462 siRNA-2 (circ-BPTF-siRNA-2) sense |
CAGCCAGAAACGACUGGAGTT |
|
hsa_circ_0045462 siRNA-2 (circ-BPTF-siRNA-2) antisense |
CUCCAGUCGUUUCUGGCUGTT |
|
Negative control siRNA (NC-siRNA) (sense) |
UUCUCCGAACGUGUCACGUTT |
|
Negative control siRNA (NC-siRNA) (antisense) |
ACGUGACACGUUCGGAGAATT |
|
Negative control mimics (NC-mimics) (sense) |
UUCUCCGAACGUGUCACGUTT |
|
Negative control mimics (NC-mimics) (antisense) |
ACGUGACACGUUCGGAGAATT |
|
miR-486-5p sense |
GATGCACATCCTGTACTGAGCTG |
|
miR-486-5p antisense |
TATGGTTGTTCACGACTCCTTCAC |
|
U6 sense |
CTCGCTTCGGCAGCACA |
|
U6 antisense |
CTCGCTTCGGCAGCACA |
|
CEMIP sense |
CCAGGAATGTTGAATGTCT |
|
CEMIP antisense |
ATTGGCTCTTGGTGAATG |
|
GAPDH (F) |
AATCCCATCACCATCTTC |
|
GAPDH(R) |
AGGCTGTTGTCATACTTC |
qRT-PCR
Total RNA was isolated using the TRIzol reagent. cDNA was synthesized using a First-Strand cDNA Synthesis Kit (Invitrogen). The relative mRNA expression was analysed via RT-PCR using SYBR Green qPCR Mix (TransGen Biotech, Beijing, China). PCR conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 60 s, 95°C for 15 s, and 60°C for 15 s. A Roche LightCycler 480 Instrument II (Roche, Indianapolis, USA) was used for real-time quantitative fluorescence analysis with a two-step method. GAPDH and U6 nuclear RNA were used as internal controls. The primers used in this study are listed in Table 1.
Luciferase reporter assay
circ-BPTF and CEMIP containing miR-486-5p binding sites were amplified through PCR and cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Genepharma). Conditions for the PCR were: 95°C, 3 min, 94°C, 30 s, 65°C, 30 s, 72°C, 1 min, and 30 cycles, 72°C, 5 min. The mutation fragment of the 3′-UTR of CEMIP or circ-BPTF was inserted into the same sites in the Dual-Luciferase miRNA Target Expression Vector. Cells were cotransfected with miR-486-5p mimics and pmirGLO vector carrying the desired fragment using the Lipofectamine 2000 reagent for 48 h. Luciferase activities were measured by the dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions.
Immunocytochemistry staining
After different treatments, cells in 24-well plates were fixed with 75% alcohol for 5 min and permeabilized with 0.5% Triton X-100 for 5 min. Cells were incubated with primary antibodies against α-smooth muscle actin (1:1000) or PCNA for (1:4000; Abcam) 2 h and subsequent with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Abcam, Cambridge, USA) for 2 h at room temperature. Immunoreactivity was visualized by using diaminobenzidine (DAB; Beyotime). A negative control was performed using PBS instead of the primary antibodies. Images were obtained using a fluorescence inverted microscope (Olympus, Tokyo, Japan). The relative PCNA level was expressed as the ratio of PCNA-positive cells (brown stained) to the total cell number.
TUNEL assay
After different treatments, cells in 24-well plates were fixed with 75% alcohol. Apoptosis of human PASMCs in vitro was evaluated using a TUNEL staining kit (AB66110; Abcam, Cambridge, UK) according to the manufacturer’s protocol, and observed under the fluorescence inverted microscope. For the evaluation of TUNEL staining, the number of TUNEL-positive nuclei was counted in 5 randomly selected fields, averaged and expressed as a percentage of labeled nuclei within the fields.
Western blot analysis
Total protein of cells was extracted and determined using Bradford reagent (Bio-Rad). Then, 50 μg of protein was subject to 10% SDS-PAGE. Protein expression were detected by immunoblotting using primary antibodies against CEMIP (1:300; Abcam), cyclin D1 (1:1000; Abcam), and β-actin (1:5000; Santa Cruz). The secondary antibody was the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. A commercial enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, USA) was used for immunoreactivity detection. The optical density of the visualized blots was analysed using a gel imaging analysis system (Bio-Rad). The relative density values of each target protein were normalized to GAPDH.
Statistical analysis
Data are expressed as the mean±standard deviation (SD). For the intergroup comparisons, an unpaired two-tailed Student’s t-test was applied. For the multigroup comparisons, a one-way ANOVA followed by Tukey’s test as the post hoc test was applied. P<0.05 was considered statistically significant.
Results
Clinical data
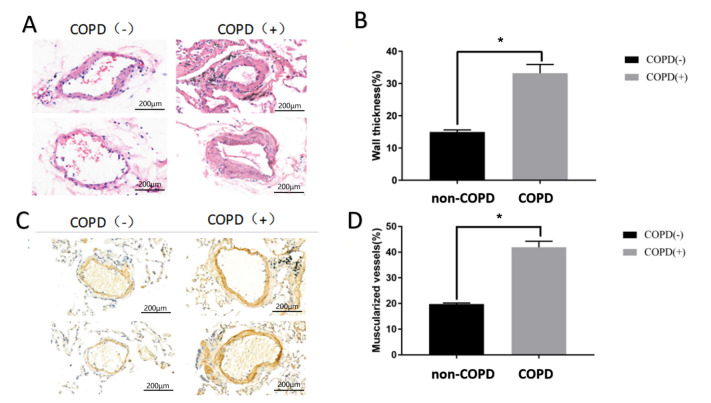
The baseline characteristics of all subjects are shown in Table 2. There were no differences between the two groups with regard to age and smoking history. As expected from the selected criteria, the forced expiratory volume in 1 second/fixed vital capacity ratio (FEV1/FVC) and forced expiratory volume in 1 second (FEV1% predicted) in COPD patients were significantly lower than those in the control group (FEV1/FVC: 73.65±11.59 vs 94.87±11.13, P<0.01; FEV1% predicted: 60.52±8.75 vs 79.13±7.04, P<0.01). Although no hypoxemia existed in any of the patients who underwent surgery, the partial pressure of arterial oxygen in COPD patients was markedly lower than that in non-COPD patients (69.3±6.2 mmHg vs 82.6±5.2 mmHg, P<0.01). Previous reports and our recent studies demonstrated pulmonary vascular remodelling in early-stage COPD patients [ 4– 7] . In this study, we first evaluated pulmonary vascular remodelling in COPD patients. As shown in Figure 1A,B, the lung tissue that was HE stained in each patient was focused on intermediate vessels (50–500 μm) for evaluating pulmonary vascular remodelling. The pulmonary vascular wall thickness and muscularized vessel ratio in COPD patients were both significantly higher than those in non-COPD patients ( Figure 1C,D). These data indicated that COPD patients with the lower partial pressure of arterial oxygen are more likely to have pulmonary arterial remodeling.
Table 2 Baseline characteristics of all patients
|
COPD ( n=18) |
non-COPD ( n=13) |
P value |
|
|
M/F ( n) |
13/5 |
9/4 |
0.85 |
|
Age (years) |
64.20±9.82 |
57.07±12.18 |
0.09 |
|
Smoker ( n) |
4 |
2 |
0.65 |
|
FEV1/pred (%) |
73.65±11.59 |
94.87±11.13 |
<0.01 |
|
FEV1/FVC (%) |
60.52±8.75 |
79.13±7.04 |
<0.01 |
|
PaO 2 (mmHg) |
69.3±6.2 |
82.6±5.2 |
<0.01 |
Figure 1 .
Pulmonary vascular remodelling in COPD patients
Lung tissues were collected from COPD or non-COPD patients, and pulmonary vascular remodelling was estimated. (A,B) HE staining showed the wall thickness of pulmonary vessels in patients. (C,D) Immunohistochemistry staining (α-SMA) showed the muscularized vessels of pulmonary vessels in patients. * P<0.05.
circ-BPTF is upregulated in the lungs of COPD patients
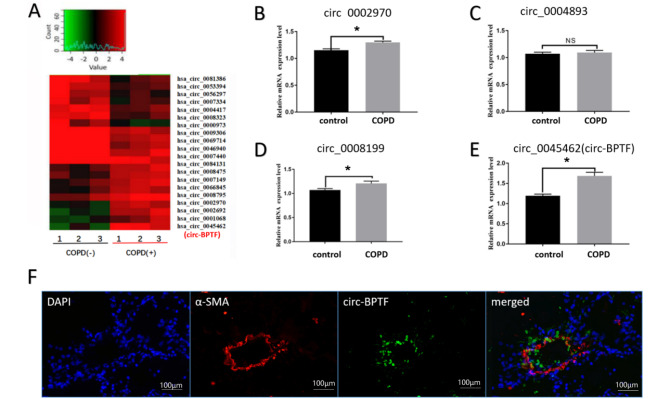
To determine the involvement of circRNAs in the progression of COPD, we screened 3610 expressed circRNAs by high-throughput transcriptome sequencing of pulmonary arterial explants in lung tissues from COPD patients or controls and found 48 differentially expressed circRNAs with changes of more than 2 folds. Overall, 26 of these circRNAs were significantly upregulated, and 22 were significantly downregulated. We selected 4 significantly upregulated circRNAs and further verified the expressions of these circRNAs by quantitative polymerase chain reaction (qPCR) using pulmonary arterial smooth muscle strips from lung tissues of COPD patients or controls ( Figure 2A–E). Our results showed that circ-BPTF was the most significantly elevated circRNA, consistent with the sequencing results. We then chose circ-BPTF for subsequent analyses. Additionally, we performed fluorescence in situ hybridization analysis to determine the distribution of circ-BPTF and found that circ-BPTF was mainly localized in pulmonary arteries ( Figure 2F). These data indicated that circ-BPTF may play vital roles in the regulation of COPD .
Figure 2 .
circ-BPTF is upregulated in the lung and smooth muscle of COPD patients
Lung tissues were collected from COPD or control patients. Total RNA was isolated, and circRNA microarray hybridization was performed based on Arraystar’s standard protocols. (A) Heatmap and volcano map analysis of circular RNAs in lung tissues from COPD patients and controls. (B-E) qPCR analysis of the expressions of four circRNAs in lung tissues from COPD patients and controls (n=6). (F) Fluorescence in situ hybridization of circ-BPTF in lung tissues from COPD patients. * P<0.05.
circ-BPTF promotes hPASMC proliferation
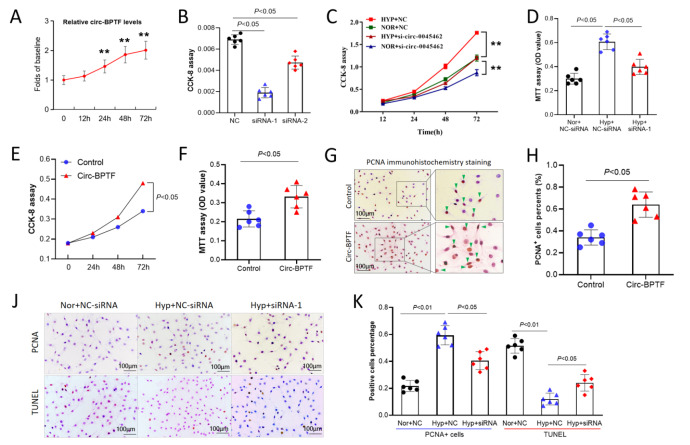
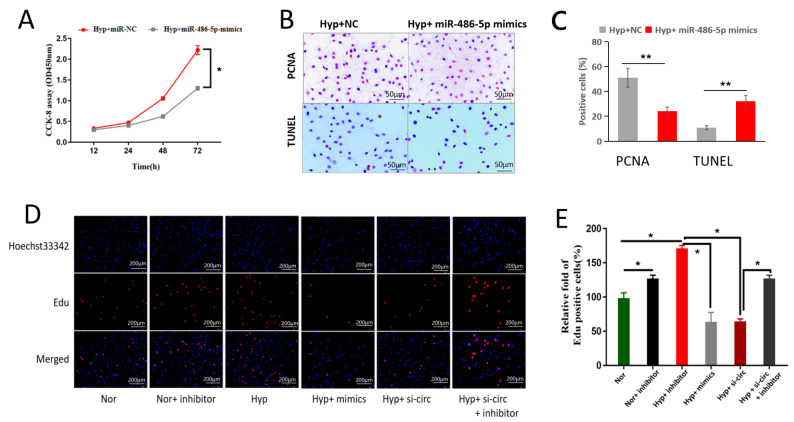
In order to investigated the function of circ-BPTF in the pathogenesis of COPD, we analyzed the role of circ-BPTF in pulmonary arterial smooth muscle cell proliferation under hypoxic conditions. Hypoxia induced circ-BPTF upregulation in hPASMCs in vitro in a time-dependent manner ( Figure 3A). Specific siRNA targeting circ-BPTF significantly inhibited the mRNA level of circ-BPTF in hPASMCs exposed to hypoxia, and we found that siRNA-1 had the most obvious effects ( Figure 3B). Therefore, we transfected hPASMCs with siRNA-1 to study the function of circ-BPTF. As shown in Figure 3C, the CCK-8 results indicated that hypoxia induced cell proliferation in a time-dependent manner. However, siRNA targeting circ-BPTF significantly inhibited proliferation in hypoxia-exposed cells. These results were also confirmed by MTT assay ( Figure 3D). Next, we transfected PASMCs with a circ-BPTF-overexpressing vector. As shown in Figure 3E,F, circ-BPTF overexpression significantly promoted cell proliferation. Overexpression of circ-BPTF also increased the percentage of PCNA-positive PASMCs ( Figure 3G,H). The immunocytochemistry staining showed that hypoxia-induced PCNA overexpression was reversed by knockdown of circ-BPTF following transfection of PASMCs with circ-BPTF siRNA ( Figure 3J,K). Additionally, hypoxia decreased the percentage of TUNEL-positive cells, which was also reversed by knockdown of circ-BPTF ( Figure 3J,K). These data suggested that circ-BPTF promotes hPASMC proliferation.
Figure 3 .
circ-BPTF promotes PASMC proliferation
Human PASMCs were cultured in vitro under hypoxia or normoxia. Specific siRNA was used to knockdown circ-BPTF. An overexpression vector was used for overexpression of circ-BPTF. (A) qPCR analysis of circ-BPTF levels after exposure to hypoxia for different time. (B) Specific siRNA knockdown of circ-BPTF in hypoxia-exposed PASMCs. (C–D) CCK-8 and MTT assays showed that circ-BPTF-specific siRNA suppressed cell proliferation in hypoxia-exposed PASMCs. (E–F) CCK-8 and MTT assays showed that circ-BPTF overexpression promoted PASMC proliferation. (G–H) circ-BPTF overexpression increased the percentage of PCNA-positive cells. (J–K) Immunohistochemistry staining and the TUNEL assay showed that circ-BPTF-specific siRNA suppressed PCNA-positive cells and increased TUNEL-positive cells in hypoxia-exposed PASMCs. ** P<0.01.
circ-BPTF is a molecular sponge for miR-486-5p
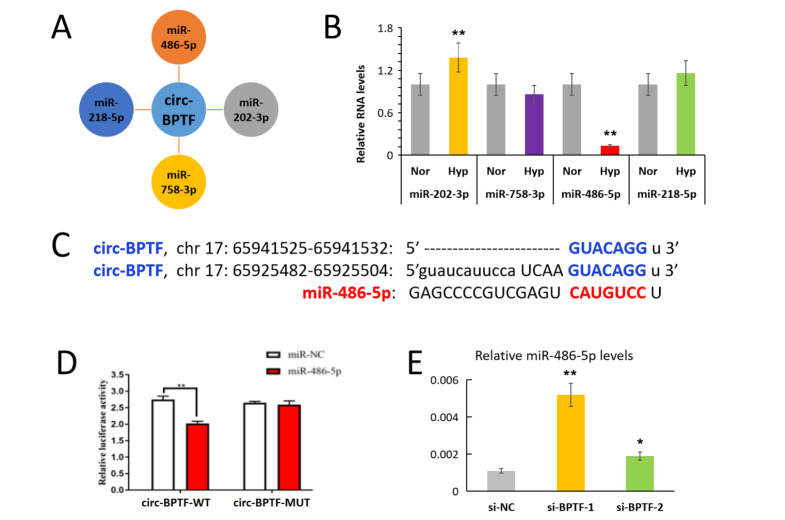
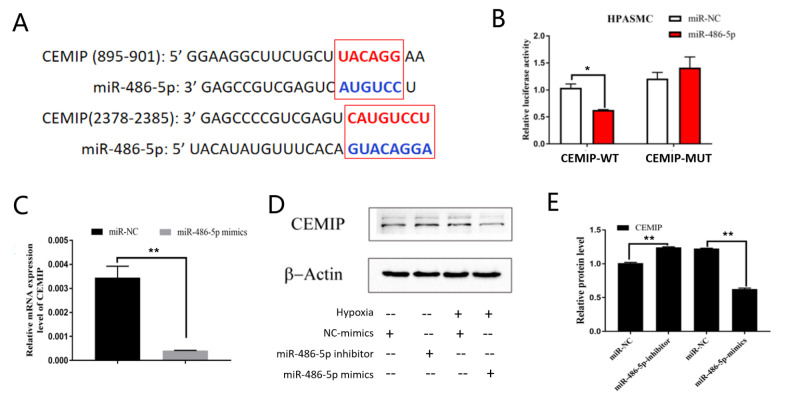
circRNAs act as a competitive endogenous sponges for miRNAs. We speculated that circ-BPTF might serve as a molecular sponge to promote PASMC proliferation. Using the bioinformatics software miRanda and RNAhybrid, we predicted that circ-BPTF may sponge miR-202-3p, miR-758-3p, miR-486-5p and miR-218-5p ( Figure 4A). qPCR results showed that miR-486-5p level was downregulated, but miR-202-3p level were upregulated after exposure to hypoxia, ( Figure 4B). There were no significant differences in miR-218-5p and miR-758-3p levels between normoxic cells and hypoxic cells. Moreover, a recent report demonstrated the downregulation of miR-486-5p in PASMCs [21]. Therefore, we focused on miR-486-5p in subsequent analyses. We predicted that there are 2 binding sites between circ-BPTF and miR-486-5p by TargetScan analysis ( Figure 4C). Next, we used a dual luciferase vector plasmid system to verify whether circ-BPTF binds to miR-486-5p. We found that the ratio of luciferase for wild-type circ-BPTF was significantly decreased when compared with that of the negative control after miR-486-5p exposure ( Figure 4D). However, the ratio of luciferase for mutant circ-BPTF did not change compared with the negative control after miR-486-5p exposure. Moreover, circ-BPTF-specific siRNA significantly reversed the suppression of circ-BPTF on miR-486-5p expression ( Figure 4E). These results indicated that circ-BPTF is a molecular sponge for miR-486-5p.
Figure 4 .
circ-BPTF sponges miR-486-5p
(A) Bioinformatics software (miRanda and RNAhybrid) predicted miRNAs that could bind to circ-BPTF. (B) Human PASMCs were exposed to hypoxia for 24 h, and qPCR was used to detect miR-202-3p, miR-758-3p, miR-486-5p and miR-218-5p expression levels. (C) The predicted binding sites of circ-BPTF and miR-486-5p by TargetScan. (D) Dual luciferase reporter assays demonstrated the interactions between circ-BPTF and miR-486-5p. (E) Human PASMCs were transfected with circ-BPTF-specific siRNA (si-BPTF) or negative control siRNA (si-NC) before exposure to hypoxia for 24 h. qPCR was used to detect miR-486-5p level. * P<0.05, ** P<0.01.
circ-BPTF regulates cell proliferation by sponging miR-486-5p
To identify the role of miR-486-5p in cell proliferation, PASMCs were transfected with miR-486-5p mimics and exposed to hypoxia for 24-72 hours. The CCK-8 results showed that miR-486-5p mimics significantly inhibited hypoxia-induced cell proliferation ( Figure 5A). The PCNA-positive cells were also significantly decreased by miR-486-5p transfection, as shown by immunocytochemistry staining ( Figure 5B, C). Additionally, miR-486-5p mimics increased the percentage of TUNEL-positive cells ( Figure 5B,C). Next, we used rescue experiments to identify whether circ-BPTF promotes cell proliferation by sponging miR-486-5p. We cotransfected PASCMCs with circ-BPTF siRNA and miR-486-5p inhibitors before exposure to hypoxia. The results of the EdU assays showed that both circ-BPTF siRNA and miR-486-5p mimics inhibited hypoxia-induced cell proliferation, whereas the miR-486-5p inhibitor reversed the inhibitory effect of circ-BPTF siRNA on cell proliferation ( Figure 5D,E). These results demonstrated that circ-BPTF could regulate PASCMC proliferation by sponging miR-486-5p.
Figure 5 .
circ-BPTF regulates cell proliferation by sponging miR-486-5p
Human PASMCs were cultured in vitro under hypoxia or normoxia. Cells were transfected with miR-486-5p mimic, miR-486-5p inhibitor or circ-BPTF-specific siRNA. (A) CCK-8 assay showed that miR-486-5p mimic inhibited proliferation of hypoxic cells. (B,C) Immunohistochemistry staining and the TUNEL assay showed that miR-486-5p mimic suppressed the percentage of PCNA-positive cells but increased the percentage of TUNEL-positive cells. (D,E) Immunofluorescence staining for the EdU assay showed that both circ-BPTF siRNA and miR-486-5p mimic decreased the percentage of hypoxia-induced Edu-positive cells, whereas the miR-486-5p inhibitor reversed this inhibitory effect. Inhibitor: miR-486-5p inhibitor; mimic: miR-486-5p mimic; sic-circ: circ-BPTF specific siRNA. * P<0.05, ** P<0.01.
miR-486-5p targets CEMIP in PASMCs exposed to hypoxia
Previous reports have demonstrated that cell migration-inducing protein (CEMIP) is a downstream target protein of miR-486-5p [ 22, 23] . We next attempted to identify the regulatory role of miR-486-5p in CEMIP expression in PASMCs. Using the bioinformatics software miRanda and TargetScanHuman, we predicted two putative binding sites for miR-486-5p on the 3′-UTR of CEMIP mRNA ( Figure 6A). The results of dual luciferase reporter assay showed that the ratio of luciferase for wild-type CEMIP was significantly decreased compared with that of the negative control after miR-486-5p mimic exposure ( Figure 6B). However, the ratio of luciferase for mutant CEMIP did not change compared with the negative control after miR-486-5p mimic exposure. These results indicated that miR-486-5p could regulate CEMIP expression. Next, we explored the regulatory role of miR-486-5p in CEMIP expression. As shown in Figure 6C, miR-486-5p mimics markedly suppressed the mRNA level of CEMIP in human PASMCs after hypoxia exposure. Furthermore, miR-486-5p mimics significantly inhibited CEMIP protein level, while miR-486-5p inhibitor increased CEMIP protein level ( Figure 6D,E). These results indicated that CEMIP might be a target of miR-486-5p in hypoxia-induced PASMC proliferation.
Figure 6 .
miR-486-5p targets CEMIP
(A) TargetScan predicted the binding sites between miR-486-5p and CEMIP. (B) Dual luciferase reporter assay was used to identify the interaction between miR-486-5p and CEMIP. (C) qPCR showed that miR-486-5p mimic inhibited CEMIP expression in hypoxic PASMCs. (D,E) Western blot analysis results verified that the miR-486-5p inhibitor upregulated CEMIP protein level, but the miR-486-5p mimic downregulated CEMIP protein level. * P<0.05, ** P<0.01.
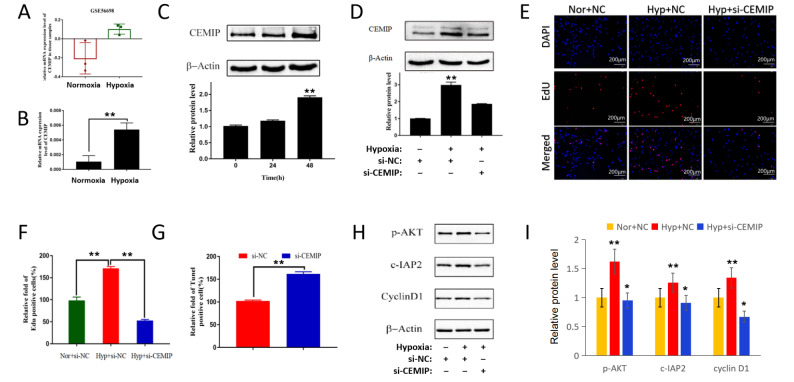
CEMIP promotes cell proliferation in PASMCs exposed to hypoxia
Subsequently, we tried to uncover the role of CEMIP in hypoxia-induced PASMC proliferation. Figure 7A shows the data from the GEO database (GSE56698). A microarray study of lung homogenates revealed that CEMIP is highly upregulated in hypoxic mice compared with that in control mice. Next, we examined CEMIP expression in human PASMCs. As shown in Figure 7B, C, hypoxia exposure not only increased CEMIP mRNA level but also increased CEMIP protein level. Moreover, hypoxia increased CEMIP expression in a time-dependent manner ( Figure 7C). Then, we used CEMIP-specific siRNA to knockdown its expression. In PASMCs exposed to hypoxia, specific siRNA significantly inhibited CEMIP expression ( Figure 7D). Although hypoxia increased the Edu-positive cell percentage, CEMIP-specific siRNA significantly reversed this increase compared with the negative control siRNA ( Figure 7E,F). In contrast, CEMIP-specific siRNA increased the number of TUNEL-positive cells in hypoxia-exposed PASMCs ( Figure 7G). Additionally, CEMIP-specific siRNA inhibited the protein expressions of p-AKT, cyclin D1 and cIAP2 ( Figure 7H,I). These results showed that CEMIP might promote PASMC proliferation via the p-AKT, cyclin D1 and cIAP2 pathways after hypoxia exposure.
Figure 7 .
CEMIP promotes the proliferation of PASMCs exposed to hypoxia
Human PASMCs were cultured in vitro under hypoxia or normoxia. Cells were transfected with CEMIP-specific siRNA or negative control siRNA. (A) A microarray study of lung homogenates from the GEO database (GSE56698) showed the upregulation of CEMIP in hypoxic mice. (B) qPCR was used to detect the CEMIP mRNA level in hypoxic PASMCs. (C) Western blot analysis results showed a time-dependent upregulation of CEMIP protein level in hypoxic PASMCs. (D) Specific siRNA (si-CEMIP) suppressed CEMIP protein expression in hypoxic PASMCs. (E,F) Immunofluorescence staining for the EdU assay showed that si-CEMIP inhibited the percentage of EdU-positive cells in hypoxic PASMCs. (G) TUNNEL assay showed that si-CEMIP increased the percentage of TUNNEL-positive cells in hypoxic PASMCs. (H,I) si-CEMIP inhibited the downstream targets in hypoxic PASMCs. * P<0.05, ** P<0.01.
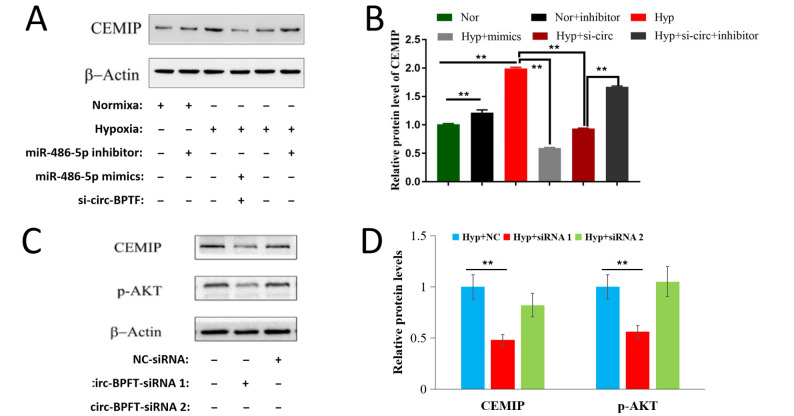
circ-BPTF regulates the miR-486-5p/CEMIP axis
To identify the regulatory effect of circ-BPTF on the miR-486-5p/CEMIP axis, we cotransfected PASMCs with circ-BPTF siRNA and miR-486-5p mimic or inhibitor before exposure to hypoxia in the rescue experiments. Western blot analysis results showed that contransfection with circ-BPTF-specific siRNA and miR-486-5p mimic inhibited hypoxia-induced CEMIP protein upregulation. However, miR-486-5p inhibitor reversed the inhibitory effect of circ-BPTF-specific siRNA on CEMIP protein expression ( Figure 8A,B). circ-BPTF-specific siRNA suppressed not only CEMIP expression but also the expression of its downstream target, p-AKT ( Figure 8C,D). These results demonstrated the role of the circ-BPTF/miR-486-5p/CEMIP axis in regulating hypoxia-induced PASMC proliferation.
Figure 8 .
circ-BPTF regulates the miR-486-5p/CEMIP axis
Human PASMCs were cultured in vitro under hypoxia or normoxia. Cells were cotransfected with miR-486-5p mimic or miR-486-5p inhibitor and circ-BPTF-specific siRNA. (A,B) Western blot analysis results showed the effect of miR-486-5p mimic, miR-486-5p inhibitor and circ-BPTF-specific siRNA on CEMIP protein level under hypoxia or normoxia. circ-BPTF-specific siRNA and miR-486-5p mimic inhibited CEMIP protein level in hypoxic PASMCs, but miR-486-5p inhibitor reversed this inhibitory effect. (C,D) Western blot analysis results showed the inhibitory effect of circ-BPTF-specific siRNA on CEMIP protein level and its downstream targets in hypoxic PASMCs. ** P<0.01.
Discussion
In this study, we identified a novel circRNA, circ-BPTF, and demonstrated the role of the circ-BPTF/miR-486-5p/CEMIP axis in regulating hypoxia-induced PASMC proliferation. Although circRNAs have been implicated in cancers, only a few reports focused on the role of circRNAs in COPD. This study provided more evidence about the role of circRNAs in PASMC proliferation and pulmonary vascular remodelling in COPD.
Although some reports have proven the role of circRNAs in cardiovascular disorders and pulmonary hypertension, few studies focused on the role of circRNAs in COPD-associated pulmonary vascular remodelling [ 24– 26] . In this study, we used microarray analysis to identify changes in circRNA profiles in lung tissues of COPD patients and in PASMCs exposed to hypoxia. We indentified a novel circRNA, circ-BPTF, and demonstrated the potential mechanism of circ-BPTF in pulmonary vascular remodelling of COPD by promoting PASMC proliferation via regulating target miRNAs and downstream proteins. As a new class of noncoding RNA molecules, circRNAs are protected from degradation and have greater stability than linear RNAs. Numerous circRNAs are expressed in a disease-specific manner. Furthermore, due to other features of circRNAs (including stability, conservation and high abundance in body fluids), they are believed to be potential biomarkers for various diseases. Our study proved a novel circRNA which is specific to COPD-associated pulmonary vascular remodelling. This might provide evidence that circRNAs may be potential biomarkers.
As a molecular sponges of miRNAs, circRNAs act by securing the target miRNA-mediated suppression of downstream proteins [ 27, 28] . In this study, we used bioinformatics analysis to predict miRNAs that may bind to circ-BPTF and verified that miR-486-5p could be sponged by circ-BPTF via binding sites at positions 525-532 and 485-504 of circ-BPTF. We further revealed that circ-BPTF promoted PASMC proliferation by sponging miR-486-5p. This result is consistent with other reports on the roles of circRNAs in pulmonary hypertension [ 16– 20] , and suggests a circRNA/miRNA pathway underlying PASMC proliferation and pulmonary vascular remodelling in COPD patients.
Many previous studies have indicated that miR-486-5p regulates the proliferation of many kinds of cancer cells [ 21, 29, 30, 22] . However, no reports focused on the role of miR-486-5p in regulating hypoxic PASMC proliferation. Our reports first revealed that miR-486-5p inhibits cell proliferation in hypoxia-exposed PASMCs. Moreover, we identified that the circ-BPTF/miR-486-5p pathway is involved in promoting PASMC proliferation under hypoxic conditions. miRNAs are small noncoding RNAs involved in the regulation of posttranscriptional gene expression [31]. miRNA dysregulation has been reported to promote the development of various diseases, including cancers [32]. Indeed, impairment of miRNA expression has been shown to be involved in vascular cell remodelling processes, such as adventitial fibroblast migration, PASMC proliferation and pulmonary arterial endothelial cell dysfunction [33]. This study demonstrated the mechanisms through which a novel circRNA (circ-BPTF) regulates hypoxic PASMC proliferation and pulmonary vascular remodelling in COPD by sponging miR-486-5p. This might enrich our understanding of the mechanism underlying COPD-associated pulmonary vascular remodelling or pulmonary hypertension.
By inhibiting downstream target genes, miRNAs exert pathophysiological functions in multiple physiological and pathological processes [34]. Bioinformatics analysis in this study showed that CEMIP is a target of miR-486-5p. Although other reports indicated that CEMIP plays a critical role in tumor development and cell proliferation, its role in PASMC proliferation and vascular remodelling in COPD remains unclear [ 23, 35] . This study first showed that the miR-486-5p/CEMIP axis regulates PASMC proliferation under hypoxic conditions, providing a new potential diagnostic and therapeutic target for this disease. Our previous studies demonstrated that cyclin D1 participates in pulmonary vascular remodelling in COPD and pulmonary hypertension [ 5– 7] . Although some pathways might be involved in this process, such as cyclin D1 and p-AKT, it is unclear whether this mechanism participates in pulmonary vascular remodelling in COPD or pulmonary hypertension. In this report, we preliminarily showed that cyclin D1, p-AKT and c-IAP2 might be the downstream targets of CEMIP in PASMC proliferation. Further studies are needed to determine the roles of CEMIP and its downstream mechanism in pulmonary vascular remodelling.
In summary, our findings indicated that hypoxia upregulates circ-BPTF. This change results in the promotion of PASMC proliferation by sponging miR-486-5p, which targets CEMIP. Thus, we identified a novel circRNA and elucidated the function and mechanisms of this circRNA in PASMCs and pulmonary vascular remodelling in COPD. Future studies are needed to confirm these findings and to provide additional insights into the promising applications of this circRNA.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Suzhou Municipal Application Basic Research Project (SYS2019047), the Program of Key Talents of Medical Science in Jiangsu Province (QNRC2016745), and the National Nature Science Foundation of China (No. 81970051).
References
- 1.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. . 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei Y, Yang Q, Nie Y, Wan J, Deng M. Small-molecule inhibitor LF3 restrains the development of pulmonary hypertension through the Wnt/β-catenin pathway. Acta Biochim Biophys Sin. . 2021;53:1277–1289. doi: 10.1093/abbs/gmab103. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Liu P, Song R, Zhang Y, Lei S, Wu S. Immune cells and autoantibodies in pulmonary arterial hypertension. Acta Biochim Biophys Sin. . 2017;49:1047–1057. doi: 10.1093/abbs/gmx095. [DOI] [PubMed] [Google Scholar]
- 4.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. . 2008;294:L612–L631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 5.Zeng D, Liu X, Xu Y, Wang R, Xiang M, Xiong W, Ni W, et al. Plasmid-based short hairpin RNA against cyclin D1 attenuated pulmonary vascular remodeling in smoking rats. Microvascular Res. . 2010;80:116–122. doi: 10.1016/j.mvr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Li C, Lei W, Jiang JH, Huang J, Zeng D. The association of neuron-derived orphan receptor 1 with pulmonary vascular remodeling in COPD patients. COPD. . 2018;13:1177–1186. doi: 10.2147/COPD.S151820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhang W, Wang C, Huang J, Jiang J, Zeng D. Resveratrol prevented experimental pulmonary vascular remodeling via miR-638 regulating NR4A3/cyclin D1 pathway. Microvascular Res. . 2020;130:103988. doi: 10.1016/j.mvr.2020.103988. [DOI] [PubMed] [Google Scholar]
- 8.Zeng D, Xu G, Lei W, Wang R, Wang C, Huang J. Suppression of cyclin D1 by plasmid-based short hairpin RNA ameliorated experimental pulmonary vascular remodeling. Microvascular Res. . 2013;90:144–149. doi: 10.1016/j.mvr.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Wang CG, Lei W, Li C, Zeng DX, Huang JA. Neuron-derived orphan receptor 1 promoted human pulmonary artery smooth muscle cells proliferation. Exp Lung Res. . 2015;41:208–215. doi: 10.3109/01902148.2014.993776. [DOI] [PubMed] [Google Scholar]
- 10.Bunel V, Guyard A, Dauriat G, Danel C, Montani D, Gauvain C, Thabut G, et al. Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest. . 2019;156:33–44. doi: 10.1016/j.chest.2019.02.333. [DOI] [PubMed] [Google Scholar]
- 11.Pichl A, Sommer N, Bednorz M, Seimetz M, Hadzic S, Kuhnert S, Kraut S, et al. Riociguat for treatment of pulmonary hypertension in COPD: a translational study. Eur Respir J. . 2019;53:1802445. doi: 10.1183/13993003.02445-2018. [DOI] [PubMed] [Google Scholar]
- 12.Blanco I, Piccari L, Barberà JA. Pulmonary vasculature in COPD: The silent component. Respirology. . 2016;21:984–994. doi: 10.1111/resp.12772. [DOI] [PubMed] [Google Scholar]
- 13.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev. . 2016;21:239–257. doi: 10.1007/s10741-015-9519-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zhu M, Pan J, Chen C, Xia S, Song Y. Circular RNAs: a rising star in respiratory diseases. Respir Res. . 2019;20:3. doi: 10.1186/s12931-018-0962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnet S, Boucherat O, Paulin R, Wu D, Hindmarch CCT, Archer SL, Song R, et al. Clinical value of non-coding RNAs in cardiovascular, pulmonary, and muscle diseases. Am J Physiol Cell Physiol. . 2020;318:C1–C28. doi: 10.1152/ajpcell.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Li Y, Qi J, Yu X, Ren H, Zhao X, Xin W, et al. Circ- calm4 Serves as an miR-337-3p sponge to regulate Myo10 (Myosin 10) and promote pulmonary artery smooth muscle proliferation . Hypertension. . 2020;75:668–679. doi: 10.1161/HYPERTENSIONAHA.119.13715. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Jiang H, Li M, Wu P, Sun L, Liu Y, Zhu K, et al. Circular RNA hsa_circ_0016070 is associated with pulmonary arterial hypertension by promoting PASMC proliferation. Mol Ther Nucleic Acids. . 2019;18:275–284. doi: 10.1016/j.omtn.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, Gu R, Wang X, He S, Bai J, Zhang L, Zhang J, et al. mmu_circ_0000790 is involved in pulmonary vascular remodelling in mice with HPH via microRNA-374c-mediated FOXC1. Mol Ther Nucleic Acids. . 2020;22:530–541. doi: 10.1016/j.omtn.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin X, Xu Y, Guo M, Sun Y, Ding J, Li L, Zheng X, et al. hsa_circNFXL1_009 modulates apoptosis, proliferation, migration, and potassium channel activation in pulmonary hypertension. Mol Ther Nucleic Acids. . 2020;23:1007–1019. doi: 10.1016/j.omtn.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Liu Z. Up‐regulation of circRNA_0068481 promotes right ventricular hypertrophy in PAH patients via regulating miR‐646/miR‐570/miR‐885. J Cell Mol Med. . 2021;25:3735–3743. doi: 10.1111/jcmm.16164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo J, Yang HC, Rhee WJ, Kang H. Vascular smooth muscle cell-derived exosomal microRNAs regulate endothelial cell migration Under PDGF stimulation. Cells. . 2020;9:639. doi: 10.3390/cells9030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Tian X, Han R, Zhang X, Wang X, Shen H, Xue L, et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene. . 2014;33:1181–1189. doi: 10.1038/onc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deroyer C, Charlier E, Neuville S, Malaise O, Gillet P, Kurth W, Chariot A, et al. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. . 2019;10:103. doi: 10.1038/s41419-019-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Liang H, Meng X, Shen L, Guan Z, Hei B, Yu H, et al. mmu_circ_0000790 is involved in pulmonary vascular remodeling in mice with HPH via microRNA-374c-mediated FOXC1. Mol Ther Nucleic Acids. . 2020;20:292–307. doi: 10.1016/j.omtn.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Liu H, Yu H, Zhou Y, Zhang J, Xin W, Li Y, et al. Circular RNA calm4 regulates hypoxia-induced pulmonary arterial smooth muscle cells pyroptosis via the circ-calm4/miR-124-3p/PDCD6 axis. Arterioscler Thromb Vasc Biol. . 2021;41:1675–1693. doi: 10.1161/ATVBAHA.120.315525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aufiero S, Reckman YJ, Pinto YM, Creemers EE. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol. . 2019;16:503–514. doi: 10.1038/s41569-019-0185-2. [DOI] [PubMed] [Google Scholar]
- 27.Boon RA, Jaé N, Holdt L, Dimmeler S. Long noncoding RNAs. J Am College Cardiol. . 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Jaé N, Dimmeler S. Noncoding RNAs in vascular diseases. Circ Res. . 2020;126:1127–1145. doi: 10.1161/CIRCRESAHA.119.315938. [DOI] [PubMed] [Google Scholar]
- 29.Kong Y, Li Y, Luo Y, Zhu J, Zheng H, Gao B, Guo X, et al. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol Cancer. . 2020;19:82. doi: 10.1186/s12943-020-01205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna JA, Garcia MR, Lardennois A, Leavey PJ, Maglic D, Fagnan A, Go JC, et al. PAX3-FOXO1 drives miR-486-5p and represses miR-221 contributing to pathogenesis of alveolar rhabdomyosarcoma. Oncogene. . 2018;37:1991–2007. doi: 10.1038/s41388-017-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negi V, Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight. . 2017;2:e91327. doi: 10.1172/jci.insight.91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boateng E, Krauss-Etschmann S. miRNAs in lung development and diseases. Int J Mol Sci. . 2020;21:2765. doi: 10.3390/ijms21082765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. . 2015;52:139–151. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jusic A, Devaux Y. Noncoding RNAs in hypertension. Hypertension. . 2019;74:477–492. doi: 10.1161/HYPERTENSIONAHA.119.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, Kim HS, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. . 2019;21:1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.