Abstract
Arcobacter spp. is an emerging pathogen that is increasingly recognized as a cause of human infections. Gastrointestinal manifestations are most described in the case report literature. We present a case of the first documented case of Arcobacter spp. isolated in pericardial fluid in an immunocompromised patient with worsening cardiac tamponade that was successfully managed with an urgent pericardiocentesis and ensuing steroids, antibiotics, and a pericardial drain. The patient had a past medical history of HIV, latent syphilis, PCP pneumonia, ESRD, and hypertension, and presented with worsening dyspnea, subjective fever, myalgias, cough, pleuritic chest pain, and pericardial rub. Diagnostic workup revealed a positive COVID-19 PCR test, elevated high-sensitive cardiac troponins, elevated CRP, elevated D-dimer, and elevated creatinine. An ECG revealed diffuse ST-segment elevation, and imaging showed cardiomegaly with pulmonary vascular congestion and diffuse interstitial edema. Urgent TTE showed a large circumferential pericardial effusion with tamponade physiology present. Culture on aerobic blood agar grew Arcobacter spp. of unknown specific species, and blood cultures were also positive for Arcobacter spp. Treatment involved intravenous meropenem for five days, followed by oral ciprofloxacin, low-dose colchicine, and a tapered dose of ibuprofen. Repeat laboratory data and TTE showed complete resolution of the pericardial effusion and improved left ventricular function. This case highlights the potential for Arcobacter spp. to cause severe infections and the importance of considering it as a possible pathogen in patients with atypical presentations.
Keywords: Arcobacter, Pericardial effusion, Cardiac tamponade, COVID-19, COVID-19 vaccine, Case report, HIV/AIDS
Introduction
Bacteria of the genus Arcobacter are a group of emerging pathogens that are increasingly recognized as a cause of human infections. Previously included in the genus Campylobacter, Arcobacter spp. is an aerotolerant, gram-negative bacteria that can grow at temperatures < 30 °C [1], [2]. Of the more than 30 known species of Arcobacter, three have been reported to infect humans: Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii [3], [4], [5]. Among case report literature, persistent watery diarrhea is commonly the main symptom associated with Arcobacter spp., in contrast to bloody diarrhea produced by Campylobacter jejuni [2]. In addition, Arcobacter spp. has been known to cause a wide range of clinical syndromes, including gastroenteritis, bacteremia, and septicemia [2]. The incidence of Arcobacter infections in humans is not well established, but it is thought to be relatively low compared to other bacterial pathogens, and of such infections, most are primarily associated with gastroenteritis involving mild to moderate diarrhea. Herein, we describe to our knowledge the first documented case of Arcobacter spp. isolated in pericardial fluid in an immunocompromised patient with worsening cardiac tamponade that was successfully managed with an urgent pericardiocentesis and ensuing steroids, antibiotics, and a pericardial drain.
Case presentation
A 32-year-old male with a past medical history of well-controlled Human Immunodeficiency Virus (HIV) on antiviral therapy, a history of latent syphilis, and Pneumocystis pneumonia (PCP) pneumonia infection, end-stage renal disease (ESRD) on maintenance hemodialysis, and hypertension presented to the Emergency Department (ED) with worsening dyspnea that started sometime within the past week. The patient reported that about a month ago he had several episodes of diarrhea after consuming chicken from a local restaurant and despite symptoms of loss of smell and taste a Coronavirus 2019 (COVID-19) polymerase chain reaction (PCR) test returned negative. Fourteen days later, and with his diarrhea resolved, he received the first dose of the Moderna COVID-19 vaccine. However, three days post-vaccination he began experiencing symptoms of subjective fever, myalgias, cough, pleuritic chest pain, and worsening dyspnea which prompted his decision to come to the ED. On initial examination, his vitals were noted as blood pressure of 89/54 mmHg, pulse rate of 109 bpm, 89% oxygen saturation on ambient air, respiratory rate of 22, and a 100.9°F temperature. Cardiac auscultation was significant for a pericardial rub with normal heart sounds and without jugular venous distension (JVD). The remainder of his physical examination was unremarkable.
Initial diagnostic workup was significant for a positive COVID-19 PCR test, elevated high-sensitive cardiac troponins without a delta change, elevated CRP, elevated D-dimer, and elevated creatinine (Table 1). An electrocardiogram (ECG) revealed diffuse ST-segment elevation with PR segment depression in all leads except lead aVR (Fig. 1). Imaging included a chest x-ray showing cardiomegaly with pulmonary vascular congestion with diffuse interstitial edema. Given the elevated D-dimer, a follow-up computed tomography (CT) pulmonary angiogram was performed showing diffuse bilateral opacities consistent with multifocal pneumonia, a right upper lobe bullae suggestive of a small mycetoma, and a small pericardial effusion (Fig. 2). The patient was admitted to internal medicine for suspected COVID-19 pneumonia infection.
Table 1.
Initial laboratory investigation.
| Lab Parameter | On admission | Normal value (units) |
|---|---|---|
| Hemoglobin (Hb) | 8.8 | 14–18 gm/dl |
| Hematocrit | 28 | 40–54% |
| TLC | 7500 | 4000–11000 |
| Neutrophils | 69 | |
| Lymphocytes | 18 | 25–45% |
| Platelet count | 137000 | 140000–440000/microliter |
| ESR | 112 | 0–15 mm/hr |
| CRP | 344 | 0–5 mg/lt |
| CD4 count | 415 | |
| HIV viral load | 50.3 | 0 copies/ml |
| BUN | 44 | 6–22 mg/dl |
| High sensitivity Troponin T | 104 | < 22 ng/lt |
| High sensitivity delta troponin T | 0 | < 7 ng/lt |
| D-dimer | 1680 | 0–500 ng/ml |
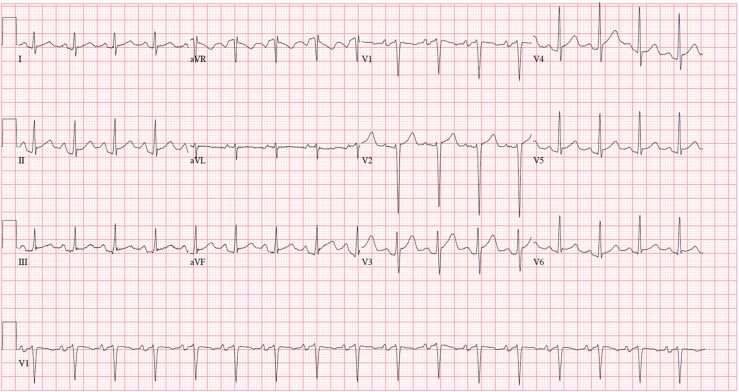
Fig. 1.
ECG illustrating diffuse ST-elevations with diffuse PR depressions; sinus tachycardia with heart rate 102; biatrial enlargement present.
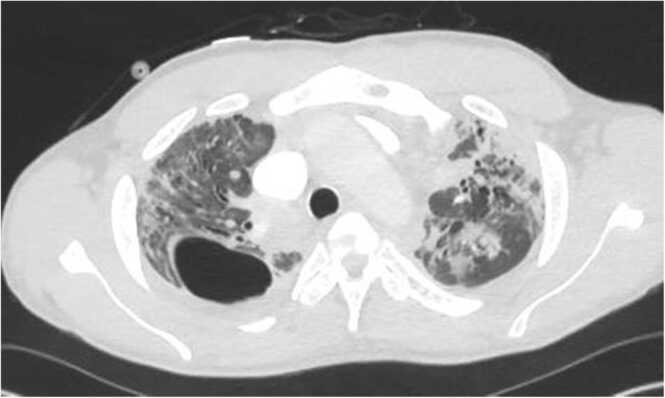
Fig. 2.
CT pulmonary angiogram showing diffuse opacities suggestive of an infectious etiology along with a right upper lobe bullae consistent with a mycetoma was also noted.
Given the concerning ECG and imaging studies, an urgent transthoracic echocardiogram (TTE) was ordered that showed a large circumferential pericardial effusion characterized by an inflammatory, fibrinous deposition with tamponade physiology present including diastolic collapse of the right ventricle (Fig. 3).
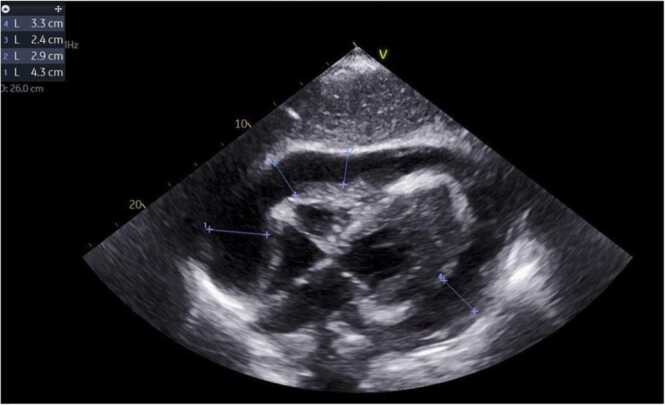
Fig. 3.
Subcostal TTE view depicting a large pericardial effusion with tamponade physiology and diastole collapse of the right ventricle.
Further, left ventricular dysfunction was noted with an ejection fraction of 45–50% without evidence of vegetations or infective endocarditis. Subsequently, an urgent pericardiocentesis was performed by interventional cardiology. Approximately 2000 cc of purulent fluid was drained with immediate improvement in the patient’s hemodynamics. Analysis of the pericardial fluid was significant for elevated total leukocytes, low glucose, elevated albumin/protein, and elevated LDH. Cultures on aerobic blood agar grew grey shiny colonies of gram-negative rods (GNR) within 24 h with the GNR isolate was morphologically like Campylobacter jejuni, and follow-up subculture on MacConkey Agar revealed non-lactose fermenters via VITEK 2 identified a bacterial isolate belonging to the Arcobacter genus. In addition, blood cultures also returned positive for Arcobacter spp. Of note, specific species could not be identified within the pericardial culture and antibacterial susceptibility testing was not performed either.
A multi-disciplinary team approach that included cardiology, infectious disease, and nephrology helped guide pharmacotherapy management. The patient was started on intravenous meropenem for a total of five days and as he clinically improves his regimen was de-escalated to oral ciprofloxacin 500 mg twice daily for fourteen days. A regimen of low-dose colchicine 0.3 mg twice daily was started for an expected course of four weeks along with a tapered dose of ibuprofen 800 mg three times daily for a total of fourteen days as well. As for the COVID-19 pneumonia, the patient was started on intravenous dexamethasone, though he was not discharged on an oral regimen. Before discharge, repeat laboratory data were within normal limits, and a repeat TTE showed complete resolution of the pericardial effusion and recovered left ventricular function with an improved 55% ejection fraction.
Discussion
Arcobacter spp. is an emerging food and water-borne zoonotic pathogen with increasing reports of documented cases of infection in humans. While generally associated with diarrheal illness and peritonitis, bacteremia, and pericarditis are rare presentations with an overall prevalence of 0.2–3.6% in humans [6]. To our knowledge, there have been no documented cases of bacterial pericarditis associated with Arcobacter spp. in either immunocompetent or immunocompromised individuals. We presented a young male with well-controlled HIV found to have COVID-19 with diarrhea with progression to Arcobacter spp. pericarditis that was complicated by purulent pericardial effusion with tamponade requiring urgent pericardiocentesis.
Indeed, pericarditis is typically associated with viral etiology, however, other causes such as neoplastic, autoimmune, and post-myocardial infractions are common etiologies as well. As for bacterial pericarditis, it is a relatively rare etiology contributing to less than 1% of all cases of pericarditis, and of bacterial causes, Staphylococcus spp., Streptococcus spp., and Mycobacterium tuberculosis are most implicated [7]. If not identified early in the disease process and treated accordingly, bacterial pericarditis can lead to purulent pericarditis with the presence of gross pus – as was the case for our patient – which is associated with mortality as high as 50% [7]. Further, bacterial pericarditis can lead to prolonged hospital courses and increased healthcare costs and burdens. Thus, with bacterial pericarditis itself an uncommon entity, an instance of Arcobacter spp. associated pericarditis reported in the setting of bacteremia has never been reported.
Despite initially being identified in the late 1970 s, human cases of Arcobacter spp. remain uncommon. Historically, case reports of Arcobacter spp. reported in the literature revolved around gastrointestinal-related illnesses. A 2014 case report describes a 26-year-old Spanish man male who presented with bloody diarrhea that was initially misattributed to Campylobacter but was caused by A. cryaerophilus after gene sequencing [5]. In another case, a patient with gastroenteritis caused by A. butzleri had consumed undercooked chicken and subsequently developed diarrhea, abdominal pain, and fever [8]. Laboratory tests confirmed the presence of A. butzleri in stool samples, and the infection was self-limited and resolved without complications.
The novelty of Arcobacter spp. remains so, with as recent as 2017 of cases depicting first-time reporting of Arcobacter, such as one diarrheic patient in Costa Rica that was identified as A. cryaerophilus [4]. In one retrospective study, several infections of Arcobacter spp were reported with all cases including some gastrointestinal symptomatology, but none with any cardiac manifestations [9]. With sparse case reports and likely underestimation of Arcobacter spp., the prevalence of this emerging pathogen is not well documented. In a 2020 prospective prevalence study of Arcobacter spp. in German patients, the species was isolated among only 0.85% of study participants [10]. The authors further noted that the isolation and identification of Arcobacter spp. may not be possible with routine diagnostic settings. As it relates to our patient, our institution lacked the advanced biochemical tests and molecular techniques to help differentiate between the closely related species of Arcobacter. There is the ongoing development of rapid diagnostic assays to help aid in early diagnosis so that further transmission of Arcobacter spp. into the food chain can be prevented [10].
In the last decade, genomic sequencing of one of the species of Arcobacter helped identify 10 genes involved in virulence and pathogenesis [6]. The exact incubation period for Arcobacter infections is not completely known, however, in an outbreak reported at a wedding reception, it was shown incubation ranged between 6 h and 3days post-ingestion with a mean duration of 31 h [11]. As previously stated, the clinical spectrum of symptoms can vary from enteritis to bacteremia depending largely on an individual’s immune status. In HIV-associated diarrhea and enteritis, Arcobacter spp. is uncommon with an incidence of only 2.67% with enteritis [12]. Usually, infection in immunocompetent individuals is self-limiting not requiring antimicrobial drugs whereas in the immunocompromised definitive therapy with antimicrobial agents is required. Specifically, fluoroquinolones, tetracyclines, macrolides, aminoglycosides, and β-lactams with β-lactamase inhibitors are effective against Arcobacter spp. with some concern towards increasing resistance and reduced susceptibility [12]. As for our patient, we did not have antimicrobial susceptibilities and relied upon prior literature for empiric treatment.
Finally, it is worth discussing the role of both the COVID-19 virus coinfection and the recent COVID-19 mRNA vaccine administered to the patient. As stated, the Arcobacter bacteria translocated from the gut to the bloodstream and then spread to other areas of the body, such as the pericardium. However, there is some evidence to suggest that COVID-19 infection can increase the risk and severity of secondary bacterial infections [13]. Indeed, COVID-19 can cause damage to the respiratory tract and compromise the immune system, which can create an environment that is more conducive to bacterial infections with secondary bacterial infections such as pneumonia, bloodstream infections, and urinary tract infections have been reported in COVID-19 patients, particularly in those who require hospitalization or have underlying health conditions. These bacterial infections can worsen the symptoms of COVID-19 and lead to more severe outcomes. As it relates to the role of the COVID-19 mRNA vaccine, it is possible that in an immunocompromised patient who receives the vaccine he or she may experience more severe side effects or a weaker immune response to the vaccine, which could potentially make them more susceptible to certain infections. There is sparse literature reporting uncommon severe side effects ranging from acute interstitial pneumonia exacerbations related to COVID-19 mRNA vaccination to acute respiratory failure [14], [15]. However, in such cases, like our patient, the pathomechanism is uncertain at best. The timing of the patient’s vaccination and soon thereafter presentation to the hospital may explain some immunologic mechanism at play though.
Conclusion
In summation, we reported an immunocompromised patient with COVID-19 coinfection, he developed enteritis, bacteremia, and a tamponade pericardial effusion with bacterial isolates of the first reported case of Arcobacter genus. The role of COVID-19 coinfection precipitating progression to bacterial pericarditis, and to a much lesser extent, COVID-19 vaccination also were considered. Given the emerging nature and ease of transmissibility of foodborne illnesses such as Arcobacter spp., this case highlights a unique and potentially fatal etiology of pericarditis. Increased awareness of this pathogen and improved diagnostic tools should be considered by clinicians and additional stakeholders.
Author contribution
Azeem Rathore, Falguni Patel, and Nidhi Gupta helped write the draft of the manuscript. Denis D. Asiimwe, Fabiana Rollini, and Malleswari Ravi helped with revisions and syntax.
Ethical approval
Not required.
Consent
Consent was obtained by the patient.
Disclosures
There are no financial conflicts of interest to disclose.
Funding
There are no disclosures of funding nor any other financial relationships to be stated.
Conflict of interest
None.
References
- 1.Lehmann D., Alter T., Lehmann L., Uherkova S., Seidler T., Gölz G. Prevalence, virulence gene distribution and genetic diversity of Arcobacter in food samples in Germany. Berl Munch Tierarzt Woche. 2015;128(3–4):163–168. doi: 10.2376/0005-9366-128-163. [DOI] [PubMed] [Google Scholar]
- 2.Collado L., Figueras M.J. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev. 2011;24(1):174–192. doi: 10.1128/CMR.00034-10. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arguello E., Otto C.C., Mead P., Babady N.E. Bacteremia caused by Arcobacter butzleri in an immunocompromised host. J Clin Microbiol. 2015;53(4):1448–1451. doi: 10.1128/JCM.03450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barboza K., Cubillo Z., Castro E., Redondo-Solano M., Fernández-Jaramillo H., Echandi M.L.A. First isolation report of Arcobacter cryaerophilus from a human diarrhea sample in Costa Rica. Rev Inst Med Trop Sao Paulo. 2017;59 doi: 10.1590/S1678-9946201759072. Published 2017 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueras M.J., Levican A., Pujol I., Ballester F., Rabada Quilez M.J., Gomez-Bertomeu F. A severe case of persistent diarrhoea associated with Arcobacter cryaerophilus but attributed to Campylobacter sp. and a review of the clinical incidence of Arcobacter spp. New Microbes New Infect. 2014;2(2):31–37. doi: 10.1002/2052-2975.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brückner V., Fiebiger U., Ignatius R., et al. Prevalence and antimicrobial susceptibility of Arcobacter species in human stool samples derived from out- and inpatients: the prospective German Arcobacter prevalence study Arcopath. Gut Pathog. 2020;12:21. doi: 10.1186/s13099-020-00360-x. Published 2020 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankuweit S., Ristić A.D., Seferović P.M., Maisch B. Bacterial pericarditis: diagnosis and management. Am J Cardiovasc Drugs. 2005;5(2):103–112. doi: 10.2165/00129784-200505020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Çelik E., Otlu S. Isolation of Arcobacter spp. and identification of isolates by multiplex PCR from various domestic poultry and wild avian species. Ann Microbiol. 2020;70:60. doi: 10.1186/s13213-020-01603-7. [DOI] [Google Scholar]
- 9.Ferreira S., Queiroz J.A., Oleastro M., Domingues F.C. Insights in the pathogenesis and resistance of Arcobacter: A review. Crit Rev Microbiol. 2016;42(3):364–383. doi: 10.3109/1040841X.2014.954523. (May) [DOI] [PubMed] [Google Scholar]
- 10.Puram Ramees Thadiyam, Dhama Kuldeep, Karthik Kumaragurubaran, Singh Rathore Ramswaroop, Kumar Ashok, Saminathan Mani, et al. Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control – a comprehensive review. Vet Q. 2017;37(1):136–161. doi: 10.1080/01652176.2017.1323355. doi: 10.1080/01652176.2017.1323355. (To link to this article) [DOI] [PubMed] [Google Scholar]
- 11.Lappi V., Archer J.R., Cebelinski E., et al. An outbreak of foodborne illness among attendees of a wedding reception in Wisconsin likely caused by Arcobacter butzleri. Foodborne Pathog Dis. 2013;10(3):250–255. doi: 10.1089/fpd.2012.1307. [DOI] [PubMed] [Google Scholar]
- 12.Patyal A., Rathore R.S., Mohan H.V., Dhama K., Kumar A. Prevalence of Arcobacter spp. in humans, animals and foods of animal origin including sea food from India. Transbound Emerg Dis. 2011;58(5):402–410. doi: 10.1111/j.1865-1682.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 13.Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13:5. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiya S., Fujimoto J., Matsumoto K., et al. Case report: Acute exacerbation of interstitial pneumonia related to messenger RNA COVID-19 vaccination. Int J Infect Dis. 2022;116:255–257. doi: 10.1016/j.ijid.2022.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bando T., Takei R., Mutoh Y., et al. Two cases of acute respiratory failure following SARS-CoV-2 vaccination in post-COVID-19 pneumonia. Respirol Case Rep. 2022;10(8) doi: 10.1002/rcr2.995. Published 2022 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]