Abstract
Background & aims
The CFTR-modulating therapy Elexaftor – Tezacaftor - Ivacaftor (ETI) has been widely prescribed since its approval in 2020 in the European Union. The aim of this study was to methodically evaluate the effects of an ETI treatment on clinical, biochemical data and Pseudomonas colonization in order to demonstrate its efficacy.
Methods
This prospective monocentric study comprised 69 patients diagnosed with cystic fibrosis aged at least 12 years and treated with ETI between September 2020 and November 2021. Clinical and laboratory data of each patient and study visit were collected before and after 24 weeks of ETI treatment. Follow-up status of Pseudomonas aeruginosa (PsA) colonization was assessed after one year of therapy by regularly determined sputum or throat swab samples.
Results
Marked improvements biochemical markers of systemic inflammation as white blood cell count, levels of immunoglobulins A, G and M and albumin within 24 weeks of therapy were observed. ETI treatment proved to be effective as seen by amelioration of lung function and sweat chloride concentration. Assessment of PsA colonization status revealed a conversion from a positive to negative detection in 36% of the cases after one year of therapy.
Conclusions
ETI treatment effectively improves systemic inflammation parameters and shows promising results in PsA status conversion.
Keywords: Cystic fibrosis, CFTR modulator, Clinical data, Inflammation markers, Elexacaftor, Tezacaftor, Ivacaftor, ETI, Kaftrio
Highlights
-
•
24-week treatment with ETI improves biochemical markers of inflammation and as well as status of Pseudomonas colonization.
1. Introduction
Cystic fibrosis is a life-threatening disease due to mutations within Cystic Fibrosis Transmembrane Conductance Regulator gene (CFTR) that encodes for the equally named, cAMP-regulated chloride channel [1]. With one in 3.300–4800 neonates affected, cystic fibrosis is the most common autosomal-recessive hereditary disorder in Germany [2,3].
Cystic fibrosis may lead to organ manifestations like exocrine pancreas insufficiency and diabetes, liver cirrhosis as well as intestinal obstruction, but the most severe complication is pulmonary disease due to recurring infections (e.g., Pseudomonas aeruginosa) and subsequent deterioration of lung function. The subsequent chronic failure in pulmonary function still represents the most common cause for patients' death in almost 50% of the cases [4]. In the past decades, improvements in therapy of cystic fibrosis have clearly ameliorated as median age of survival increased from four to five years in the 1950’s to 50 years in Europe nowadays [[4], [5], [6]].
Until recently, the medical regimen was still limited to symptomatic treatment like inhalation of hypertonic saline for better secretolysis, substitution of pancreatic enzymes and antimicrobial therapy [7]. However, the development of a new class of drugs, the so-called CFTR modulators, has set a new milestone in the treatment of cystic fibrosis since Ivacaftor, a CFTR potentiator, proved to be very efficacious in patients carrying a G551D gating mutation and was subsequently introduced in 2012 [8]. Since then, research on this class of drugs has been further advanced and culminated with the approval of the triple combination therapy Elexacaftor – Tezacaftor – Ivacaftor (ETI) for patients with F508del homozygous mutations or F508del heterozygous and a minimal function mutation (Tricafta®: US 03/2020, Kaftrio®: EU 09/2020) in 2020.
Elexacaftor and Tezacaftor are two CFTR correctors that facilitate CFTR processing in the ER, whereas Ivacaftor as a potentiator prolongs CFTR channel opening and therefore (at least partially) restores CFTR function. In the phase III studies reported by Middleton and Heijerman [9,10], this drug combination led to an average improvement of lung function (FEV1) by 14% as well as a clear reduction of sweat chloride concentration. These impressive results are further underlined by our own clinical observations in the daily practice as quality of patients' life seemed to rise whereas exacerbation and hospitalization rate rather declined.
To demonstrate the drugs' efficacy in a real world setting and application in the daily clinical routine, we initiated this monocentric prospective cohort study that enables the objective collection and evaluation of clinical and biochemical data on the effectiveness of ETI therapy derived from a cohort comprising 69 participants. The aim of this study was to methodically evaluate the effects of an ETI treatment on clinical and laboratory data used as routine screening parameters in clinical care during an observational period of six months after treatment initiation.
2. Material and methods
2.1. Patients
The study cohort comprised 69 patients diagnosed with cystic fibrosis aged at least 12 years, between September 2020 and November 2021.
The study was approved by the local ethics' committee (vote number 20-485-B), registered (clinicaltrials.gov ID NCT05576324), and performed in accordance with the Declaration of Helsinki. Informed consent was obtained of all study participants or their legal custodians prior to study inclusion. Study visits up to four weeks prior and after six months of ETI therapy were arranged on the occasion of regular, usually quarterly appointments in the CF outpatient clinic. Here, actual body measures, body plethysmography, sweat chloride as well as biochemical parameters were assessed.
At study initiation, patients who were already under ETI treatment, so the study visit prior to ETI treatment was analyzed retrospectively. Therefore, some of the parameters are missing for these patients prior to ETI therapy. For the study visit after 6 months of therapy, all patients received the standardized assessment of the above-mentioned parameters. Patients that did not have started ETI therapy prior to study inclusion received standardized assessment for both study visits.
Exclusion parameters were defined as pregnancy at initiation and during ETI treatment as well as systemic intake of corticosteroids. Patients that did not take ETI until the follow-up time point after six months were also excluded from the study. A sample size calculation was not performed prior to study initiation due to the observational character of the study.
2.2. Clinical data
Clinical and laboratory data of each patient and study visit were collected from the hospital’s electronic health files and recorded pseudonymized. The patients' demographic data are summarized in Table 1.
Table 1.
Baseline characteristics of the study cohort prior to ETI.
| Age | 27.56 yrs (SD ± 12.29 yrs; range: 12–56 yrs) | |
| <18 yrs | 17 (Mean 14.8 yrs, SD ± 1.5 yrs) | |
| >18 yrs | 52 (Mean 31.4 yrs, SD ± 11.1 yrs) | |
| Sex | f | 27 (39%) |
| m | 42 (61%) | |
| Mutation | F508del homozygous | 39 (57%) |
| F508del heterozygous | 29 (42%) | |
| other | 1 (1%) | |
| CF-related diabetes | yes | 24 (35%) |
| no | 45 (65%) | |
| Pretreatment with CFTR modulator other than ETI | Iva | 8 (11%) |
| Teza + Iva | 17 (25%) | |
| Luma + Iva | 3 (4%) | |
| FEV1 | 69.3% (SD ± 21.3%; range 27–108%) | |
| >80% | 25 (Mean 92.1%, SD ± 8.2%) | |
| <80% | 44 (Mean 56.3%, SD ± 14.0%) | |
| Pseudomonas aeruginosa colonization | yes | 33 (48%) |
| no | 36 (52%) | |
Clinical data included sex, gender, age, mutation and body mass index (BMI, kg/m2) for adults as well as and BMI Z-score for juveniles. For lung function tests, forced vital capacity (FVC, %), forced expiratory volume in 1 s (FEV1, % of nominal value defined by Global Lung Initiative Reference Values [11]), maximal expiratory flow at 25% of FVC (MEF25, %), effective airway resistance (sReff, %), residual volume (RV, %), total lung capacity (TLC, %) ratio of RV and TLC (RV/TLC, %) were analyzed.
Laboratory data contained white blood cell count (WBC, x103/μl), HbA1c (%), plasma creatinine (mg/dl), total bilirubin (mg/dl), direct bilirubin (mg/dl), gamma-glutamyltransferase (γ-GT, U/l), alanin-aminotransferase (ALT, U/l), aspartate-aminotransferase (AST, U/l), cholinesterase (CHE, kU/l), glutamate-dehydrogenase (GLDH, U/l), C-reactive protein (CRP, mg/l), albumin (g/l), immunoglobulin G, M, A (g/l), E (kU/l).
For the evaluation of PsA colonization status, microbiological testing (sputum or deep throat swabs) was provided by patients with CF routinely four to six times per year in accordance with the current CF guidelines. A singular positive result in either of both diagnostic modalities was sufficient to label a patient PsA positive. Chronic PsA colonization was defined by three or more positive results out of at least six tests within one year.
For the PsA status conversion, at least 3 negative results within a period of at least a year after initiation of ETI treatment was necessary in accordance with the german CF care guidelines [12].
2.3. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (Version 9, Graph Pad, San Diego, USA). Differences of clinical and laboratory data between baseline and after six months of ETI therapy were analyzed using multiple paired t-testing as the longitudinal data are compared from the same individual. Subcategory analyses for age (pediatric versus adult), LFT (FEV1, >80% versus <80%), Pseudomonas aeruginosa colonization (baseline positive versus baseline negative), previous medication with CFTR modulators other than ETI (CFTR modulator pretreatment versus CFTR modulator naïve) and genotype (F508del homozygous versus F508del heterozygous) were performed by multiple unpaired t-testing regarding differences between both time points.
Analysis of the association of the Pseudomonas aeruginosa colonization status and its alteration over time during the treatment with Kaftrio was assessed according to the guidelines after one year of therapy and was tested using Fisher’s exact test.
3. Results
3.1. Demographic characteristics of the study population
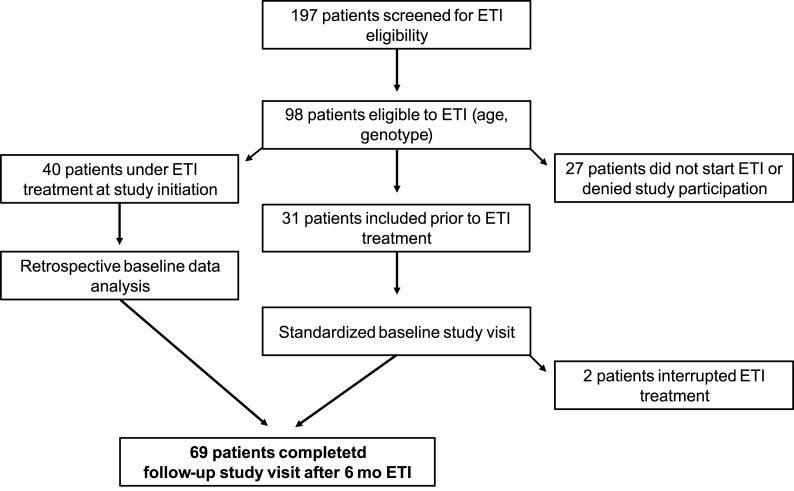
For this study, 98 patients were screened. Baseline characteristics were analyzed retrospectively for 40 patients, whereas 31 patients could be included prospectively. 2/31 did not reach study end point due to non-compliance. In total, 69 patients completed the follow-up study visit after six months of ETI therapy. The study flow is summarized in Fig. 1. The average follow-up interval was 6.37 months. The demographic data on our study cohort are summarized in Table 1. Subcategory analyses are also indicated in Table 1. Notably, one patient (m, 15 years) could be included taking ETI in off-label use though not having a F508del mutation because ETI treatment had been shown to be efficacious for his specific mutation combination (R347P/M1101K) [13,14].
Fig. 1.
Study flow.
3.2. ETI treatment effectively improves lung function parameters
Analysis of the most routinely used lung function parameters as determined by body plethysmography demonstrated improvements of almost all relevant markers except for TLC under ETI therapy (Table 2). As expected, there was a clear augmentation of FEV1 for the entire study cohort with an overall increase of 10 %-points, but also MEF25 as a parameter for obstruction of smaller airways and FVC proved to be ameliorated by 15.5 and 5.7 %-points, respectively. In contrast to that, we could observe very distinct decreases in the values for airway resistance (sReff, −36.8 %-points), RV (−17.7 %-points) and ratio of RV/TLC (−0.2) over the course of ETI therapy. Moreover, patients' BMI and BMI z-Score showed an evident increase, too.
Table 2.
Lung function parameters and BMI at baseline and after six months ETI treatment. BMI values are indicated for all adult patients whereas BMI z-Score is provided for juveniles.
| Baseline |
Follow-up |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean [t0] | SD [t0] | 95% CI [t0] lower limit | 95% CI [t0] upper limit | n | Mean [t6] | SD [t6] | 95% CI [t6] lower limit | 95% CI [t6] upper limit | n | Difference | SE | P-value | |
| FVC (%) | 86.1 | 15.2 | 82.5 | 89.8 | 69 | 91.6 | 17.6 | 87.3 | 95.9 | 67 | 5.7 | 1.57 | <0.001 |
| FEV 1 (%) | 69.3 | 21.3 | 64.2 | 74.4 | 69 | 79.4 | 21.0 | 74.3 | 84.5 | 67 | 10.0 | 1.31 | <0.001 |
| FEV1/FVC | 0.8 | 0.2 | 0.8 | 0.8 | 69 | 0.9 | 0.4 | 0.8 | 1.0 | 67 | 0.1 | 0.05 | 0.015 |
| MEF 25 (%) | 44.2 | 33.3 | 36.2 | 52.2 | 69 | 60.0 | 42.1 | 49.7 | 70.3 | 67 | 15.5 | 2.45 | <0.001 |
| sReff (%) | 184.6 | 93.2 | 161.5 | 207.7 | 65 | 144.7 | 92.5 | 121.8 | 167.6 | 65 | −36.8 | 7.92 | <0.001 |
| RV (%) | 142.9 | 51.9 | 130.1 | 155.6 | 66 | 123.3 | 34.5 | 114.7 | 131.8 | 65 | −17.7 | 4.66 | <0.001 |
| TLC (%) | 105.2 | 13.1 | 102.0 | 108.4 | 66 | 105.5 | 12.1 | 102.5 | 108.5 | 65 | 0.6 | 0.95 | 0.125 |
| RV/TLC | 1.4 | 0.5 | 1.3 | 1.5 | 66 | 1.2 | 0.3 | 1.1 | 1.2 | 65 | −0.2 | 0.05 | <0.001 |
| BMI | 21.7 | 2.8 | 20.9 | 22.5 | 52 | 22.4 | 2.8 | 21.6 | 23.2 | 51 | 0.7 | 0.39 | <0.001 |
| BMI z-Score | −0.4 | 1.2 | −1.0 | 0.3 | 17 | 0.1 | 1.0 | −0.4 | 0.6 | 17 | 0.5 | 0.001 | 0,027 |
With respect to subcategory analyses, the group with a FEV1 restriction <80% displayed a much more pronounced change of FEV1 (13.4 vs 7.4 %-points, q ≤ 0.01), RV (28.5 vs −7.6 %-points; q ≤ 0.01) and RV/TLC (−0.34 vs −0.08, q ≤ 0.01) in comparison to patients with FEV1 > 80%, respectively. Furthermore, patients receiving a CFTR modulator other than ETI prior to the initiated Kaftrio® treatment displayed a tendency to a rather minor increase of FEV1 than patients naïve to a CFTR modulator. However, this effect did not reach the set threshold of statistical significance (7.1 vs 12.2 %-points, q = 0.15). Yet, the change of sReff proved to be a significantly for this subanalysis: Whereas CFTR-modulator pretreated patients showed almost no difference of sReff values (+8.52 %-points) before and under treatment, CFTR-modulator naïve patients had a marked decrease of sReff values on average (−52.0 %-points; q = 0.02). Subcategory analysis regarding age, Pseudomonas aeruginosa colonization status and genotype yielded no relevant results.
3.3. ETI therapy ameliorates biochemical parameters of patients with CF
Regarding biochemical parameters (Table 3), we also observed clear signs of a restored CFTR channel function under ETI treatment as assessed by sweat chloride as a direct surrogate parameter for CFTR function. In our patient cohort, we detected a highly significant reduction of sweat chloride. Moreover, the average levels of serum sodium showed a trend for an increase in the therapy course, whereas serum chloride did not change.
Table 3.
Laboratory parameters at baseline and after six months ETI treatment.
| Baseline |
Follow-up |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean [t0] | SD [t0] | 95% CI [t0] lower limit | 95% CI [t0] upper limit | n | Mean [t6] | SD [t6] | 95% CI [t6] lower limit | 95% CI [t6] upper limit | n | Difference | SE | P-value | |
| WBC (x10^3/μl) | 8.9 | 3.2 | 8.1 | 9.6 | 69 | 6.4 | 2.1 | 5.9 | 6.9 | 68 | −2.5 | 0.36 | <0.001 |
| HbA1c (%) | 6.0 | 1.0 | 5.7 | 6.2 | 61 | 5.6 | 0.9 | 5.4 | 5.9 | 66 | −0.3 | 0.08 | 0.0006 |
| Kreatinin (mg/dl) | 0.7 | 0.2 | 0.7 | 0.8 | 68 | 0.9 | 0.9 | 0.7 | 1.1 | 69 | 0.2 | 0.05 | 0.009 |
| Bilirubin (total, mg/dl) | 0.6 | 0.5 | 0.5 | 0.7 | 58 | 0.9 | 0.6 | 0.8 | 1.1 | 69 | 0.3 | 0.02 | <0.001 |
| Bilirubin (direct, mg/dl) | 0.3 | 0.4 | 0.2 | 0.4 | 52 | 0.4 | 0.3 | 0.3 | 0.5 | 69 | 0.1 | 1.58 | <0.001 |
| AST (U/l) | 25.8 | 9.9 | 23.4 | 28.2 | 69 | 28.2 | 16.1 | 24.3 | 32.1 | 69 | 2.4 | 2.37 | 0.13 |
| ALT (U/l) | 27.0 | 15.3 | 23.3 | 30.6 | 69 | 31.7 | 21.7 | 26.5 | 36.9 | 69 | 4.7 | 0.04 | 0.047 |
| γGT (U/l) | 31.9 | 40.9 | 22.0 | 41.8 | 68 | 35.6 | 57.0 | 21.9 | 49.3 | 69 | 3.6 | 2.6 | 0.177 |
| CHE (U/l) | 7.7 | 2.6 | 7.1 | 8.4 | 62 | 7.4 | 1.8 | 7.0 | 7.8 | 69 | −0.3 | 0.2 | 0.131 |
| GLDH (U/l) | 6.8 | 12.0 | 3.2 | 10.5 | 43 | 5.7 | 6.0 | 4.3 | 7.2 | 67 | −1.4 | 1.85 | 0.459 |
| CRP (mg/l) | 7.9 | 10.1 | 5.3 | 10.5 | 59 | 8.6 | 13.4 | 4.1 | 13.2 | 35 | 0.0 | 2.86 | 0.987 |
| Albumin (g/l) | 41.6 | 3.8 | 40.6 | 42.6 | 61 | 44.5 | 3.2 | 43.7 | 45.3 | 69 | 3.1 | 0.53 | <0.001 |
| IgG (g/l) | 13.9 | 4.0 | 12.9 | 14.9 | 67 | 11.4 | 2.9 | 10.8 | 12.1 | 69 | −2.5 | 0.27 | <0.001 |
| IgM (g/l) | 1.1 | 0.7 | 0.9 | 1.4 | 26 | 1.2 | 0.7 | 1.0 | 1.3 | 69 | −0.1 | 0.04 | 0.0589 |
| IgE (kU/l) | 187.3 | 340.1 | 99.5 | 275.2 | 60 | 186.9 | 495.9 | 67.7 | 306.0 | 69 | 18.0 | 50.17 | 0.717 |
| IgA (g/l) | 3.0 | 1.8 | 2.5 | 3.4 | 55 | 2.3 | 1.3 | 2.0 | 2.6 | 68 | −0.6 | 0.09 | <0.001 |
| Albumin/Globulin ratio | 3.3 | 1.1 | 3.0 | 3.5 | 59 | 4.2 | 1.2 | 3.9 | 4.5 | 68 | 0.9 | 0.09 | <0.001 |
| Sweat chloride (mmol/l) | 95.3 | 21.6 | 88.7 | 102.0 | 44 | 48.6 | 20.1 | 39.7 | 52.6 | 41 | −46.7 | 3.17 | <0.001 |
| Sodium (mmol/l) | 138.1 | 2.1 | 137.7 | 138.7 | 69 | 139.8 | 2.0 | 139.3 | 140.3 | 67 | 1.6 | 0.32 | <0.001 |
| Chloride (mmol/l) | 102.5 | 12.0 | 99.5 | 105.6 | 63 | 106.8 | 18.9 | 103.1 | 112.3 | 68 | 4.3 | 2.6 | 0.104 |
In the context of ETI treatment, special attention needs to be paid on a potential elevation of liver enzymes as indicated in the Summary of Product Characteristics. In our study cohort, persistently elevated liver enzymes were present in 15/69 patients (about 22%), especially with respect to ALT. Out of these 15 patients, two patients displayed more than a threefold elevation in comparison to baseline. In both patients, therapy could be resumed after normalization of serum transaminases during a short ETI-free interval with a reduced dosage. Moreover, a slight but highly significant increase in the levels of total and direct bilirubin could also be observed. In addition to that, γ-GT as another cholestatic parameter tendentially increased under ETI, however this finding did not reach the level of statistical significance. With respect to hepatic protein synthesis capacity, a pronounced increase of serum albumin levels could be observed in the longitudinal study course. Other hepatic enzymes like CHE or GLDH did not show any difference. Also, renal function parameter creatinine was not significantly altered during ETI therapy.
ETI treatment did also evidently affect “inflammation markers”, as there was a clear decrease of peripheral leukocytes (WBC) as well as the levels of the immunoglobulins A, G and M. Contrarily, C-reactive protein does not show any relevant differences between study points as there were not any relevant elevations prior to treatment. Also, immunoglobulin E is not significantly altered during ETI therapy.
We also found evidence that the levels of HbA1c decreased slightly during ETI treatment. The change did not differ between patients with CF-related diabetes or without. Unfortunately, in this study, the assessment of parameters specific for evaluating a pancreatic insufficiency was not included.
Subcategory analysis for age, CFTR modulator pretreatment, Pseudomonas aeruginosa status as well as genotype (homozygous F508del vs. heterozygous F508del) did not show any differences for neither of the mentioned parameters.
3.4. ETI treatment improves Pseudomonas aeruginosa (PsA) colonization status and protects from PsA colonization
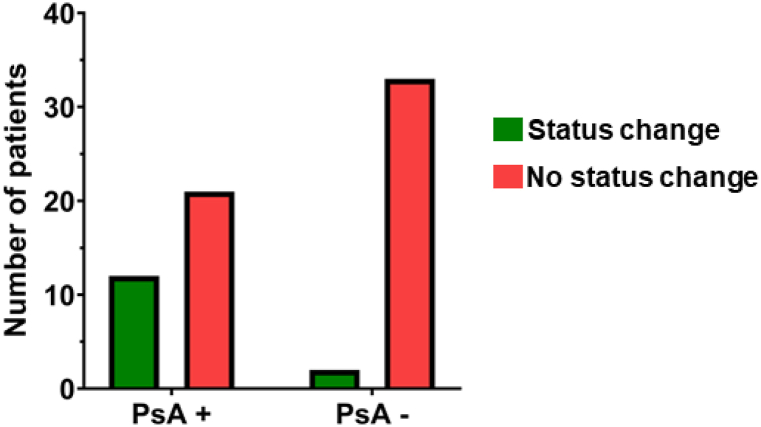
Furthermore, we sought to determine whether we could detect any changes regarding the Pseudomonas aeruginosa (PsA) colonization status during the ETI therapy. At least one positive result for PsA (sputum or throat swab) in the microbiological testing was deemed to label a patient PsA positive. In our cohort, 15/69 patients received deep throat swabs prior to therapy. Out of these 15 patients, 13 patients were followed-up by throat swabs and two patients by sputum after six months of therapy. 52/69 patients were able to produce sputum prior to therapy. Under ETI treatment, 31 out of these 52 patients continued to produce sputum, whereas 21 patients had to be tested by throat swab. Most patients were already PsA positive at initiation of ETI treatment (57%) and thus displayed a rather chronic PsA colonization. Of note, we found a substantial number of PsA positive patients prior to ETI that sustainably displayed three or more negative results in the further course of the ETI treatment. In numbers, out of 33 PsA+ patients, 12 patients (36%) were considered PsA− (Fig. 2, PsA−, green bar) after one year of ETI therapy. Among those 12 patients with PsA status conversion, PsA status was assessed by sputum culture in seven patients, whereas four patients received deep throat swabs. Only one patient was not able to expectorate sputum under ETI and thus received deep throat swabs in the therapy course.
Fig. 2.
Proportion of Pseudomonas aeruginosa (PsA) status change after one year of ETI therapy.
In the other 22 PsA+ patients (64%), colonization status did not change (Fig. 2, PsA+, red bar). In contrast to that, only two patients out of 35 PsA− patients (6%) at baseline were PsA colonized as confirmed by positive culture during the treatment (Fig. 2, PsA−, green bar). The remaining 33 patients (94%) stayed PsA− throughout the therapy.
Statistical analysis using Fisher’s exact test of these numbers in a 2 × 2 table revealed an odds ratio of 8.8 (95% CI 1.9 to 40.9, p = 0.0038) for a favorable outcome, i.e., a change in PsA status from PsA positive to negative or remaining PsA negative under ETI treatment.
4. Discussion
The introduction of the CFTR-modulating triple therapy Elexacaftor – Tezacaftor – Ivacaftor represents a hallmark in the treatment of most patients with CF. Here, we now provide real-world data on the effectiveness of ETI therapy two years after market admission in Germany. The aim of our study was to systematically analyze therapy effects on clinical and laboratory parameters assessed in daily practice.
In the overall patient cohort, we can report major improvements regarding lung function: First and foremost a large increase in FEV1 by roughly 10%-points and also other lung function parameters such as MEF25, RV or sReff improve significantly. In patients with CF naïve to any kind of CFTR modulators other than ETI, the improvement in FEV1 even accounted for almost 14%-points. Therefore, our findings are nearly coherent with the phase 3 study of Middleton et al. [9] where 14.3%-points FEV1 increase following a 24 week ETI treatment interval has been reported in patients with CF with a F508del/minimal function phenotype. Our findings are also in line with the results of the PROMISE study comprising a rather large US-American CF patient cohort that have reported similar results from daily practice [15].
Moreover, levels of sweat chloride also decreased markedly underscoring the gain of function effect that the small molecules Elexacaftor, Tezacaftor and Ivacaftor exert on CFTR channel function [9,15].
Also, we can confirm the results of Middleton and Heijerman’s findings regarding an increase in BMI [9,10] as we could also show a relevant improvement by 0.71 points and 0.3 points for BMI Z-score respectively. The obvious assumption that ETI treatment may positively influence the metabolic status is further underlined by the improvements of patients' HbA1c levels by −0,31 points. This result has also been confirmed by the reports of Petersen et al. [16].
We only detected mild to moderate elevations of liver enzymes, however, except for two patients these did not cross the threshold of a 5-fold increase of the upper limit, where ETI treatment is recommended to pause. However, after 24 weeks of therapy, there still was a significant elevation of ALT in the overall patient cohort compared to therapy initiation. Moreover, 15 out of 69 patients (21%) displayed elevated transaminases, a percentage twice as high as the findings reported in the approval study [9]. We also found a significant increase of both, direct and total bilirubin, whereas γ-GT as another cholestatic parameter did not show any relevant changes. Here, our findings stand contrarily to the reported results derived from a smaller, retrospective monocentric study where significant changes in AST and γ-GT have been described [17].
Regarding inflammation parameters, we can state that ETI treatment effectively normalizes systemic inflammation markers such as WBC or immunoglobulins A and G. These findings have also been shown for a CFTR-modulating therapy with Lumacaftor and Ivacaftor [18]. For ETI, this had not been reported yet. Also the slight increase of serum albumin of ∼3 g/dl can be seen in that context, as albumin has been shown to have a reciprocal relationship to the aforementioned inflammation markers and is also able to predict the severity of lung deterioration and systemic inflammation in the longitudinal course [19,20]. In cystic fibrosis, hyperinflammation has been shown to not only occur due to extrinsic factors like colonization with pathogenic germs but also as intrinsically caused by CFTR deficiency or dysfunction in immune cells itself [21,22]. Consequently, we postulate that the observed normalization of the inflammation parameters WBC, IgG and IgA as well as the Albumin/Globulin-ratio is at least partially due to a direct therapy effect on restoration or normalization of the immune system activity. This notion is further supported by the study of Favia et al. where the colleagues could show that Ivacaftor monotherapy is able to restore CFTR expression and function in mononuclear cells [23]. Also, treatment with Ivacaftor has been shown to restore proper immune cell responses like the formation of extracellular traps (NETs) in CF neutrophils [24] or phagocytic function against Pseudomonas aeruginosa in CF macrophages [25].
The beneficial effect of ETI treatment on systemic inflammation parameters is also reflected by our finding of a significant PsA status conversion in initially PsA+ patients within a year after therapy initiation. There are several studies that have described a relative reduction of PsA abundancy under a CFTR modulator treatment [[26], [27], [28]]. Moreover, Sosinski et al. also reported a decrease of PsA RNA quantity in sputum samples of patients with CF under ETI treatment [29].
Regarding our observed proportions of PsA status conversion under ETI treatment, our findings are in line with current literature as Heltshe et al. reported similar findings with a shift towards PsA− cultures in ∼30% of initially PsA+ patients with G551D mutation and treated with Ivacaftor [30]. This effect may be ascribed to an increased bacterial clearance due to improved rheologic behavior of the airway surface liquid (ASL) [31], but also restored immune cellular functions like phagocytosis or a differentiation shift within the T helper cell compartment under CFTR modulator treatment [32,33] might to contribute to this effect. However, further functional studies are necessary to validate our findings.
Clearly, the presented study also displays some limitations: On the one hand, due to the monocentric study design and partially retrospective data analysis, only a limited number of patients could be enrolled with some data missing for some patients. Moreover, the heterogeneity of our study cohort must be considered which is why we have performed subcategory analyses for each potential confounder. Regarding PsA colonization status, results have to be interpreted with caution as results derived from sputum and to a smaller amount also from deep throat swab samples.
Taken together, we can summarize that a 24-week treatment interval with Elexacaftor, Tezacaftor and Ivacaftor effectively attenuates inflammatory parameters like WBC counts, immunoglobulins and albumin and improves lung function, BMI as well as sweat chloride. Another important finding is a promising decline of Pseudomonas aeruginosa colonization in our patient cohort. ETI treatment proved also relatively safe, however special attention and monitoring should be given to liver associated transaminases, bilirubin and γ-GT.
Grant support
AH received funding support from the Frieda-Marohn-Stiftung.
Author contributions
AS, JW and AH conceived and designed the study. RR, AG, CT, JS and EP contributed reagents, materials, analysis tools or data. HH, NK and AS performed the experiments, analyzed and interpreted the data. AS and AH wrote the manuscript.
Declaration of competing interest
AS is stock owner of Vertex Pharmaceuticals. AH has received research grants for clinical studies, speaker’s fees, honoraria or travel expenses from Abbvie, Astellas, MSD, Novartis, Nutricia and Shire/Takeda.
Acknowledgements
The authors would like to thank the patients and their parents for participating in this study. This study was supported by the Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg (Laboratory Rotation). We acknowledge financial support by Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding programme “Open Access Publication Funding”.
References
- 1.Ratjen F., Doring G. Cystic fibrosis. Lancet. 2003;361(9358):681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Farrell P.M. The prevalence of cystic fibrosis in the European Union. J. Cyst. Fibros. 2008;7(5):450–453. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Naehrig S., Chao C.M., Naehrlich L. Cystic fibrosis. Dtsch. Arztebl. Int. 2017;114(33–34):564–574. doi: 10.3238/arztebl.2017.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Registry C.F.F.P. Cystic Fibrosis Foundation; 2021. Patient Registry Annual Data Report. [Google Scholar]
- 5.McKone E.F., et al. Survival estimates in European cystic fibrosis patients and the impact of socioeconomic factors: a retrospective registry cohort study. Eur. Respir. J. 2021;58(3) doi: 10.1183/13993003.02288-2020. [DOI] [PubMed] [Google Scholar]
- 6.McBennett K.A., Davis P.B., Konstan M.W. Increasing life expectancy in cystic fibrosis: advances and challenges. Pediatr. Pulmonol. 2022;57(Suppl 1):S5–s12. doi: 10.1002/ppul.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafeeq M.M., Murad H.A.S. Cystic fibrosis: current therapeutic targets and future approaches. J. Transl. Med. 2017;15(1) doi: 10.1186/s12967-017-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey B.W., et al. A CFTR potentiator in patients with cystic fibrosis and theG551DMutation. N. Engl. J. Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton P.G., et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heijerman H.G.M., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall G.L., et al. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur. Respir. J. 2021;57(3) doi: 10.1183/13993003.00289-2020. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz C., et al. [CF lung disease - a German S3 guideline: module 2: diagnostics and treatment in chronic infection with Pseudomonas aeruginosa] Pneumologie. 2018;72(5):347–392. doi: 10.1055/s-0044-100191. [DOI] [PubMed] [Google Scholar]
- 13.Laselva O., et al. Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator. Eur. Respir. J. 2021;57(6) doi: 10.1183/13993003.02774-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veit G., et al. A precision medicine approach to optimize modulator therapy for rare CFTR folding mutants. J. Personalized Med. 2021;11(7):643. doi: 10.3390/jpm11070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols D.P., et al. Clinical effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: a clinical trial. Am. J. Respir. Crit. Care Med. 2022;205(5):529–539. doi: 10.1164/rccm.202108-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen M.C., et al. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J. Cyst. Fibros. 2022;21(2):265–271. doi: 10.1016/j.jcf.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomassen J.C., et al. Monatsschrift Kinderheilkunde; 2022. 6 Monate Zulassung von Kaftrio® in Deutschland – erste Erfahrungen aus dem „wahren Leben“ von Menschen mit CF. [Google Scholar]
- 18.Sommerburg O., et al. CFTR modulator therapy with lumacaftor/ivacaftor alters plasma concentrations of lipid-soluble vitamins A and E in patients with cystic fibrosis. Antioxidants (Basel) 2021;10(3) doi: 10.3390/antiox10030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J., et al. Application of albumin/globulin ratio in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. J. Thorac. Dis. 2018;10(8):4923–4930. doi: 10.21037/jtd.2018.07.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., et al. Albumin/Globulin Ratio as Yin–Yang in rheumatoid arthritis and its correlation to inflamm-aging cytokines. J. Inflamm. Res. 2021;14:5501–5511. doi: 10.2147/JIR.S335671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratner D., Mueller C. Immune responses in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;46(6):715–722. doi: 10.1165/rcmb.2011-0399RT. [DOI] [PubMed] [Google Scholar]
- 22.Polverino F., et al. CFTR regulates B cell activation and lymphoid follicle development. Respir. Res. 2019;20(1):133. doi: 10.1186/s12931-019-1103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favia M., et al. Treatment of Cystic Fibrosis Patients Homozygous for F508del with Lumacaftor-Ivacaftor (Orkambi®) Restores Defective CFTR Channel Function in Circulating Mononuclear Cells. Int. J. Mol. Sci. 2020;21(7):2398. doi: 10.3390/ijms21072398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robledo-Avila F.H., et al. Dysregulated calcium homeostasis in cystic fibrosis neutrophils leads to deficient antimicrobial responses. J. Immunol. 2018;201(7):2016–2027. doi: 10.4049/jimmunol.1800076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratcher P.E., et al. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J. Cyst. Fibros. 2016;15(1):67–73. doi: 10.1016/j.jcf.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe S.M., et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014;190(2):175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graeber S.Y., et al. Effects of Lumacaftor–Ivacaftor on Lung Clearance Index, Magnetic Resonance Imaging, and Airway Microbiome in Phe508del Homozygous Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2021;18(6):971–980. doi: 10.1513/AnnalsATS.202008-1054OC. [DOI] [PubMed] [Google Scholar]
- 28.Ronan N.J., et al. CORK study in cystic fibrosis. Chest. 2018;153(2):395–403. doi: 10.1016/j.chest.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Sosinski L.M., et al. A restructuring of microbiome niche space is associated with Elexacaftor-Tezacaftor-Ivacaftor therapy in the cystic fibrosis lung. J. Cyst. Fibros. 2022;21(6):996–1005. doi: 10.1016/j.jcf.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heltshe S.L., et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin. Infect. Dis. 2015;60(5):703–712. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison C.B., et al. Treatment of cystic fibrosis airway cells with CFTR modulators reverses aberrant mucus properties via hydration. Eur. Respir. J. 2022;59(2) doi: 10.1183/13993003.00185-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hector A., et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am. J. Respir. Crit. Care Med. 2015;191(8):914–923. doi: 10.1164/rccm.201407-1381OC. [DOI] [PubMed] [Google Scholar]
- 33.Tiringer K., et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]