Summary
Background
The pandemic of COVID-19 raised the urgent need for safe and efficacious vaccines against SARS-CoV-2. We evaluated the efficacy and safety of a new SARS-CoV-2 virus receptor-binding domain (RBD) vaccine.
Methods
A phase 3, multicentre, randomised, double-blind, placebo-controlled trial was carried out at 18 clinical sites in three provinces of the south-eastern region of Cuba. Subjects (healthy or those with controlled chronic diseases) aged between 19 and 80 years, who gave written informed consent were eligible. Subjects were randomly assigned (1:1, in blocks) to two groups: placebo, and 50 μg RBD vaccine (Abdala). The product was administered intramuscularly, 0.5 mL in the deltoid region, in a three-dose immunization schedule at 0-14-28 days. The organoleptic characteristics and presentations of the vaccine and placebo were identical. All participants (subjects, clinical researchers, statisticians, laboratory technicians, and monitors) remained blinded during the study period. The main endpoint was to evaluate the efficacy of the Abdala vaccine in the prevention of symptomatic COVID-19. The trial is registered with the Cuban Public Registry of Clinical Trials, RPCEC00000359.
Findings
Between March 22 to April 03, 2021, 48,290 subjects were included (24,144 and 24,146 in the placebo and Abdala groups, respectively) in the context of predominant D614G variant circulation. The evaluation of the main efficacy outcomes occurred during May–June 2021, starting at May 3rd, in the context of high circulation of mutant viruses, predominantly VOC Beta. The incidence of adverse reactions for individuals in the placebo and Abdala vaccine groups were 1227/24,144 (5.1%) and 1621/24,146 (6.7%), respectively. Adverse reactions were mostly mild, and from the injection site, which resolved in the first 24–48 h. No severe adverse events with demonstrated cause–effect relationship attributable to the vaccine were reported. Symptomatic COVID-19 disease was confirmed in 142 participants in the placebo group (78.44 incidence per 1000 person-years, 95% confidence interval [CI], 66.07–92.46) and in 11 participants in Abdala vaccine group (6.05 incidence per 1000 person years; 95% CI 3.02–10.82). The Abdala vaccine efficacy against symptomatic COVID-19 was 92.28% (95% CI 85.74–95.82). Moderate/serious forms of COVID-19 occurred in 30 participants (28 in the placebo group and only 2 in the Abdala vaccine group) for a vaccine efficacy of 92.88% (95% CI 70.12–98.31). There were five critical patients (of which four died), all in the placebo group.
Interpretation
The Abdala vaccine was safe, well tolerated, and highly effective, fulfilling the WHO target product profile for COVID-19 vaccines. Those results, along with its immunization schedule and the advantage of easy storage and handling conditions at 2–8 °C, make this vaccine an option for the use in immunization strategies as a key tool for the control of the pandemic.
Funding
Centre for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba.
Keywords: COVID-19, Vaccine, SARS-CoV-2, Controlled clinical trial, Efficacy, Randomised, Double-blind, Placebo-controlled clinical trial, Phase 3, Spike RBD protein
Research in context.
Evidence before this study
We searched PubMed website on February 27, 2023, for published research articles, with no language restrictions, using the search terms “COVID-19”, “SARS-CoV-2”, “vaccine” and “efficacy”. We found 3490 results that were restricted to 69 by the addition of “phase 3 clinical trial”. In general, the articles included results of different efficacy trials of vaccines already authorized and widely used, and different technologies mostly mRNA, inactivated vaccines and non-replicating viral vectors, among others. Although several subunit vaccines based on RBD protein are currently in advanced phases of clinical development, we found few phase 3 publications regarding vaccine efficacy: Novavax/NVXCoV2373 (full length recombinant S protein-micelle nanoparticle/matrix M adjuvant) 89.7% (95% CI 80.2–94.6), Soberana-02/Soberana-Plus (heterologous three doses combination of recombinant RBD conjugated to tetanus toxoid/RBD-dimer in Alum) 92.0% (95% CI 80.4–96.7), and ZF2001 vaccine, based in a tandem-repeat dimeric RBD of the SARS-CoV-2 spike protein that is produced in Chinese hamster ovary cells and then adjuvanted with aluminium hydroxide, 81.4% (95% CI 73.3–87.3).
Even after the distribution of millions of doses of different approved COVID-19 vaccines all over the world, there is still a global need for new vaccines to control the pandemic, by increasing availability as well as better storage and distribution conditions.
Added value of this study
We previously reported the Phase 1–2 clinical trial of Abdala vaccine in adults from 19 to 80 years of age, using a three-dose immunization schedule at 0-14-28 days. The vaccine was well tolerated and no safety concerns were raised. High anti-RBD IgG immune responses were elicited as well as neutralizing antibodies against SARS-CoV-2. In this phase 3, randomised, double-blind, placebo-controlled clinical trial in 48,290 participants using the same immunization schedule, we confirmed the satisfactory safety profile previously found, but now in a larger population of individuals where around 74% had some previous coexisting illness conditions. Abdala vaccine efficacy against symptomatic COVID-19 was 92.28% (95% CI 85.74–95.82) and 92.88% (95% CI 70.12–98.31) against moderate/serious forms of the disease. There were five critical patients (of which four died), all in the placebo group. The efficacy was evaluated in the context of high circulation of mutant viruses, predominantly VOC Beta.
In this phase 3 trial, we demonstrated for the first time the safety and efficacy of a COVID-19 vaccine based on a SARS-CoV-2 recombinant spike RBD protein produced in the yeast Pichia pastoris, with a very well-known technology platform that we have been used for the large-scale production of hepatitis B vaccine in the last 30 years.
Implications of all the available evidence
Abdala vaccine is safe and highly protective against symptomatic COVID-19, including the most severe forms of the disease and death, fulfilling the WHO target product profile for COVID-19 vaccines. Those results, along with its compact three doses immunisation schedule, the advantages that offer the use of a very well-established technology platform, the demonstrated vaccine thermo stability and the easy storage and handling conditions at 2–8 °C, make this affordable vaccine an option for the use in massive immunisation strategies, that can contribute to worldwide efforts in the control of the pandemic.
Introduction
The causal agent of the COVID-19 pandemic is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of 21 August 2022, 593 million confirmed cases and 6.4 million deaths had been reported globally, according to data recorded by the World Health Organization (WHO).1
There is consensus that, as long as no safe and effective preventive vaccines are available for SARS-CoV-2, in quantities sufficient to implement comprehensive immunization programmes, the world will not return to normality. Vaccines are urgently needed to mitigate the consequences of this pandemic and protect humanity from future epidemics caused by this virus.2
The Cuban COVID-19 Abdala vaccine (from now onwards Abdala) was designed at the Genetic Engineering and Biotechnology Centre (CIGB) in Havana, Cuba, considering the state-of-the-art research on COVID-19 vaccine, especially the immunological aspects necessary for the development of vaccines against this disease. The vaccine antigen is based on the recombinant receptor-binding domain (RBD) subunit of the SARS-CoV-2 spike protein, produced in P. pastoris yeast, adjuvanted to alumina.3,4 This is a very well-known technology platform previously used by this institution.
An exploratory phase 1-2 study, carried out in adults 19–80 years of age, evaluated Abdala's safety and immunogenicity. It was safe, well-tolerated and induced strong humoral immune responses against SARS-CoV-2.5
The aim of this study was to evaluate Abdala's efficacy and safety in preventing symptomatic disease due to SARS-CoV-2 infection in adults, 19–80 years of age.
Methods
Study design
A phase 3, multicentre, randomised, double-blind, placebo-controlled clinical trial was carried out at 18 clinical sites in three provinces of the south-eastern region of Cuba.
The trial was conducted in medical wards and certified areas for the vaccination process. The clinical researchers selected the participants in the trial. They were specialists in family medicine, internal medicine and intensive care, and were also in charge of clinical and safety evaluations, as well as the proper collection of the primary information generated in the study. Trial on-site monitoring (by the promoter centre monitors) verified this process as well as the accuracy of all case report forms, and all Good Clinical Practices procedures. Data management was carried out by direct supervision of specialized and independent engineers. All statistical analyses were performed by independent statisticians at the Cybernetics, Mathematics and Physics Institute, in Havana. An independent data monitoring committee (constituted with one of the following independent specialists: statistician, pathologist, clinician, epidemiologist and pharmacist) was established before starting the study to assesses safety and primary trial data, to make timely recommendations to the sponsor. The protocol followed the Helsinki Declaration Guidelines and was evaluated by an ad hoc Centralized Ethics Committee, integrated by members of the Ethics and Review Committee of the “Saturnino Lora” Provincial Hospital in Santiago de Cuba (main clinical site), extended with Research Ethics Committees members from the Universities of Medical Sciences of Santiago de Cuba, Guantánamo and Granma provinces, who granted ethical approval of the study (approval No 1/2021). This Review Board was constituted with highly qualified medical specialists not linked to the study, as well as a member of the community. This committee made the research following-up, ensuring the protection of the rights, safety and well-being of the participants in the study. Additionally, the Cuban Centre for State Control of Medicines, Equipment and Medical Devices (CECMED) approved the beginning of the clinical trial after considering the scientific, methodological and ethical aspects. The manuscript adheres to CONSORT reporting guidelines.
Participants
The study universe included adults of any sex, with permanent residence in the capital cities of Santiago de Cuba, Guantánamo and Granma provinces, who responded to the call for the study. Once the selection criteria were verified, the subjects were included in the trial and identified with a code including a consecutive number. Subjects aged from 19 to 80 years (healthy adults or with controlled comorbidities), who signed their written informed consent to participate, were enrolled. Exclusion criteria were: subjects with previous SARS-CoV-2 infection confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR) or at the moment of inclusion; individuals suspected to have the infection or contact with a COVID-19 case, or previous acute infections in the last 15 days; those with uncontrolled chronic diseases at the time of inclusion; subjects who had received a vaccine candidate against COVID-19 or with any medical condition that required an immunomodulator, systemic steroid or cytostatic during the study; Body Mass Index ≤18 or ≥35 kg/m2; subjects with tattoos on both deltoid regions that interfered with the local safety assessment; those who received blood, blood products, or any investigational product in the last three months; subjects with known hypersensitivity to thiomersal and to any component of the formulation under study; history or suspicion of alcoholism or drug dependence; pregnancy or lactation; mental disability to issue consent.
Randomisation and masking
The study subjects were randomly distributed (1:1) into 2 groups: I) placebo and II) 50 μg RBD (Abdala vaccine). Randomisation was carried out at the CIGB's Clinical Research Direction, in blocks of 4 individuals, for each clinical site, by means of a computerised random number generator. The clinical sites received the product (Abdala and placebo) in such blocks, in masked vials, to avoid their identification. Their organoleptic characteristics and presentations were identical. As a result, the decision to accept or reject a participant was made without knowing the assignment in the sequence. The trial participants, researchers, and monitors, were unaware of the trial-group assignments during the whole trial. Statistical analyses were done without the knowledge of the group's identity. This was known after the analyses were concluded.
Procedures
Abdala is a slightly opaque greyish-white suspension. After a settling time it separates into two phases: the upper one is a transparent liquid and the lower is a precipitated gel, easily resuspended after shaking and is easily resuspended and is essentially free of foreign particles. Each mL contains 100 μg RBD (active ingredient), 0.60 mg aluminium hydroxide gel, 0.56 mg disodium hydrogen phosphate, 0.62 mg sodium dihydrogen phosphate dihydrate, 8.5 mg sodium chloride and 0.05 mg thiomersal (antimicrobial). Placebo vial had the same excipients, except the RBD. Their organoleptic characteristics and presentations were identical.
Preparation and administration of both formulations was done by personnel trained for these purposes, under the researcher indication and supervision. Each clinical site had a stock with the masked products (Abdala and placebo), stored at 2–8 °C, in quantity sufficient to ensure the progress of the study protocol without interruptions due to this fact. The sponsor was responsible for the continuous supply of the products.
The products (Abdala or placebo) were administered intramuscularly, 0.5 mL in the deltoid region, in a three-dose immunization schedule at 0-14-28 days.
Symptomatic COVID-19 cases were defined as participants with a nasopharyngeal swab positive for SARS-CoV-2 by RT-PCR, who presented at least one major symptom or sign (new onset dyspnoea or worsening, oxygen saturation [SpO2] ≤92% by pulse oximetry without oxygen supplement, persistence of chest pain, change of behaviour or alteration in the state of consciousness, local or generalized cyanosis or pneumonia by clinical or imaging diagnosis) or at least two minor symptoms or signs (fever ≥38 °C, headache, chills, odynophagia, myalgia, fatigue that interferes with daily activity, vomiting and/or diarrhoea, anosmia and/or ageusia).
RT-PCR SARS-CoV-2 positive cases, detected in nasopharyngeal swabs at the Hygiene, Epidemiology and Microbiology Provincial Centres, were notified to the research team at clinical sites participating in the trial. SARS-CoV-2 infection was diagnosed using RIDA®GENE SARS-CoV-2 (R-Biopharm AG, Germany). Cases were confirmed using the LightMix Modular SARS-CoV-2 (COVID19) RdRP-gene (TIB Molbiol/Roche Diagnostics, Germany).
Cases were also captured by the spontaneous notification of the volunteers through an emergency 24-h telephone number to contact the main researcher. Additionally, as part of the epidemiological strategies of the Cuba's Ministry of Public Health for the identification of new COVID-19 cases and their contacts, an active daily search by family doctors was also in place that allowed the identification of new cases among the volunteers through their identity card as participants in the trial. All positive cases were immediately sent to the “Joaquín Castillo Duany” Hospital in Santiago de Cuba, as the centralized care unit for all the COVID-19 patients in the trial, who remained hospitalized for a better follow-up.
Clinical data were judged by an independent adjudication committee of medical doctors at the hospital, which was unaware to which study group the patient was assigned. Clinical forms of symptomatic COVID-19 disease were classified into four categories: mild, moderate, serious and critical disease, following the same international criteria used when addressing the clinical spectrum of SARS-CoV-2 infection.6
Efficacy analyses were carried out in individuals without evidence of SARS-CoV-2 exposure before the first dose of the immunization schedule (“modified intention to treat” [mITT] and “per protocol” [PP] populations). For that purpose, serum samples (taken at basal time from all the participants in the trial) were tested using the validated UMELISA ANTI SARS-CoV-2, a qualitative assay for the detection of total antibodies against SARS-CoV-2 in human serum or plasma (manufactured by Immunoassay Centre, Havana. Registration number D2010-42, CECMED).
Adverse events (AE) were carefully registered according to the type, duration, severity, outcome and causality relationship. AE's severity was classified into five levels: (1) mild, if no therapy was necessary; (2) moderate, if a specific treatment was needed; (3) severe or medically significant but not immediately life-threatening; cause hospitalization or prolongation of hospitalization; disabling; (4) life-threatening, requires urgent intervention and (5) AE-related death.7 A qualitative assessment was used to classify the causal relationship following WHO recommendation.8 Adverse reactions (AR) expected with vaccination were especially sought (pain at the injection site, erythema, induration, headache, fever, among others).
All participants were evaluated in the first hour after the administration of each dose by anamnesis, vital signs (temperature, blood pressure, respiratory and cardiac frequencies), an inspection of the injection site (to detection local symptoms) as well as general physical examination. Subsequently, an active/passive surveillance of AE was in place for the 14 days-period between each dose, combining home visits of family physicians at the community level (within 72 h after each dose) with the self-reporting of any AE that may occur throughout the trial.
Outcomes
The primary endpoint of the trial was to evaluate Abdala's efficacy in preventing the first occurrence of symptomatic COVID-19, in individuals without evidence of prior exposure to SARS-CoV-2 infection with onset 14 days after the third dose of the immunization schedule (PP population). The consistency of vaccine efficacy (VE) was evaluated in various subgroups, including sex, age groups, ethnicity and comorbidities. Secondary endpoints were the prevention of mild and moderate/serious forms of COVID-19 (in PP population) as well as the prevention of symptomatic COVID-19 cases that occurred in the subset of individuals analysed by “intention-to-treat” (ITT) without evidence of prior exposure to SARS-CoV-2 infection at the time of inclusion in the study, before the first dose of the investigational product (mITT population). Safety outcome included the collection of AE in all enrolled volunteers that received at least one dose of the vaccine or placebo (ITT population).
Statistical analysis
The sample size was calculated considering the total number of cases needed to demonstrate Abdala's VE to prevent symptomatic COVID-19. Assuming proportional risks over time with 1:1 randomization of vaccine and placebo groups, a total of 151 cases of COVID-19 would be needed to detect a 60% reduction in the hazard rate (i.e., 60% VE) with 30% as the lower limit of 95% confidence interval (95% CI) to reject the null hypothesis H0: VE ≤ 30% with a statistical power of 0.90. Based on the estimated incidence rate of 0.286% in two months for the placebo group in the study sites, the enrolment of 48,000 participants was required, anticipating a 2% dropout rate. Details of the sample size calculation are shown in the study protocol (Appendix).
Two interim analyses were planned. Supplemental Material (S3, Table S3).
Participants’ data were collected at the time of symptom onset and censored at the end of their follow-up. In each analysis, non-parametric estimates of the survival functions of the control and experimental groups were calculated using the Kaplan–Meier method for censored data. Graphs of the cumulative hazard functions were obtained. Log-rank hypothesis tests were performed to check for differences between the survival curves of the study groups. VE, defined as the percentage reduction in the hazard ratio, was calculated by the expression VE = 1-HR, where HR represents the ratio between the hazard rate functions of the experimental and control groups. This ratio was estimated by Cox regression models for proportional hazards. For the primary endpoint, the Cox regression was stratified by age groups, from 19 to 50 years and from 51 to 80, as planned in the protocol. To model mild and moderate/serious cases, multi-state Cox regression was used. The validation of the Cox models was done by analysing their respective residual graphs and testing their Schoenfeld residuals.
To assess safety, AEs were tabulated and plotted by study group. AEs’ incidence rates in each group were described in the ITT population, defined as the total cohort of participants who received at least one injection, and considered in the group where they were randomized.
The mITT population was defined as the subset of individuals in the ITT definition that showed no previous immunologic or virological evidence of COVID-19 infection at the time of study inclusion, prior to the first dose of the investigational product. This population was used for the secondary outcome of VE.
The primary efficacy end point in the interim and primary analyses was assessed in the PP population, defined as the subset of volunteers included, who met all the eligibility criteria, with no previous immunologic or virological evidence of COVID-19 infection at the time of study inclusion, prior to the first dose of the investigational product, who received the complete three-dose immunization schedule, in whom the primary endpoint assessment was available and who did not have any major protocol deviations. Participants were considered in the group where they were randomized. Subgroup analyses were performed by baseline characteristics, including age, sex, ethnicity and presence or absence of coexisting conditions, also carried out in the PP population. Subgroups analyses were based on any symptomatic infection. Incidence rates per 1000 person-years were also calculated.
Statistical analyses were conducted using R version 3.6.2. The input of the primary information in databases built with the OpenClinica software (www.openclinica.com), was performed remotely (from the clinical site) and in duplicate (independently by two operators) for subsequent automatic comparison and correction of the databases, necessary for statistical analysis with accurate trial information. Access to these databases at the CIGB (with a user name and password) was made through the national health system network (INFOMED). For debugging errors, data not matching the one recorded in the original forms were corroborated, to avoid confusion.
This study was registered in the Cuban Public Clinical Trial Registry, RPCEC00000359.
Role of the funding source
The funder of the study had a role in study design, data interpretation, and writing of the report, but had no role in data collection or data analysis.
Results
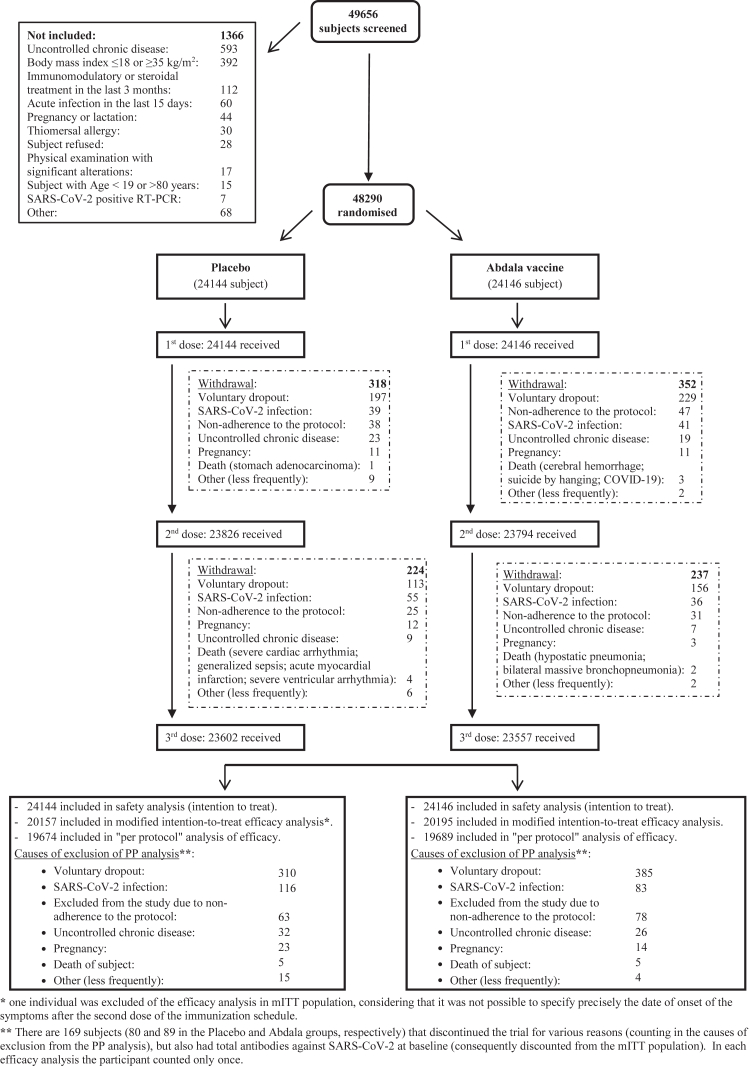
From 22 March to 03 April, 2021, a total of 48,290 subjects were included out of 49,656 that were screened in the context of predominantly SARS-CoV-2's D614G variant circulation. Their disposition is shown in Fig. 1. Subjects were randomly distributed into two study groups of the vaccination schedule (0-14-28 days): placebo and Abdala (RBD 50 μg). All volunteers completed the vaccination schedule (three doses), except for 1131 subjects that discontinued vaccination (542 in the placebo group, and 589 in Abdala group). Fig. 1 also summarizes individuals, by cause, who discontinued the 2nd and 3rd administration of the investigational product. The main cause of withdrawal was voluntary dropout which occurred in 61.5% (695/1131) of the participants. Other causes were: SARS-CoV-2 infection, non-adherence to the protocol by subjects (optional decision provided in the research protocol) and uncontrolled chronic diseases, among others. Considering all subjects included, high compliance with the planned vaccination was observed (97.7%).
Fig. 1.
Study flow chart.
Table 1 shows the demographic and baseline characteristics of participants. Most of them were women (25,286, 52.4%); mean (±SD) age was 48.9 ± 16.2 years. Overall, 52.2% (25,186) were mestizo, 26.1% (12,605) white and 21.7% (10,499) black, coinciding with the ethnic distribution of the Cuban population in the south-eastern region of the country where the study was conducted. No relevant imbalances between the study groups were observed. Supplemental Material (S1, Table S1) shows the demographic characterization of these individuals by age ranges.
Table 1.
Demographic and baseline characteristics of the participants in the ABDALA-3 study at enrolment.
| Variable | Placebo | Abdala vaccine | Total |
|---|---|---|---|
| N (%) | 24,144 (50.0) | 24,146 (50.0) | 48,290 (100) |
| Sex – no. (%) | |||
| Female | 12,689 (52.6) | 12,597 (52.2) | 25,286 (52.4) |
| Male | 11,455 (47.4) | 11,549 (47.8) | 23,004 (47.6) |
| Age (years) | |||
| Means ± SD | 48.9 ± 16.2 | 48.9 ± 16.1 | 48.9 ± 16.2 |
| Median (IQR) | 50 (15) | 50 (15) | 50 (15) |
| Ethnicity– no. (%) | |||
| White | 6248 (25.9) | 6357 (26.3) | 12,605 (26.1) |
| Black | 5233 (21.7) | 5266 (21.8) | 10,499 (21.7) |
| Mestizo | 12,633 (52.4) | 12,523 (51.9) | 25,186 (52.2) |
| BMI (kg/m2) | |||
| Means ± SD | 26.5 ± 4.4 | 26.5 ± 4.3 | 26.5 ± 4.3 |
| Median (IQR) | 26.4 (6.2) | 26.4 (6.2) | 26.4 (6.2) |
| Subjects with some comorbidity – no. (%) | 17,928 (74.3) | 17,931 (74.3) | 35,859 (74.3) |
| Main comorbidities/risk factors – no. (%) | |||
| Overweight/type I obesity | 11,806 (48.9) | 11,756 (48.7) | 23,562 (48.8) |
| High blood pressure | 8223 (34.1) | 8261 (34.2) | 16,484 (34.1) |
| Smoking | 6339 (26.3) | 6197 (25.7) | 12,536 (26.0) |
| Diabetes mellitus | 1959 (8.1) | 1945 (8.1) | 3904 (8.1) |
| Bronchial asthma | 1881 (7.8) | 1928 (8.0) | 3809 (7.9) |
| Heart disease | 897 (3.7) | 897 (3.7) | 1794 (3.7) |
| Other CNS and peripheral disorders | 878 (3.6) | 899 (3.7) | 1777 (3.7) |
| Allergy | 852 (3.5) | 853 (3.5) | 1705 (3.5) |
| Gastrointestinal disorders | 789 (3.3) | 799 (3.3) | 1588 (3.3) |
| COPD | 259 (1.1) | 243 (1.0) | 502 (1.0) |
| Cancer | 250 (1.0) | 227 (0.9) | 477 (1.0) |
| Ischemic/hemorrhagic cerebral infarction | 151 (0.6) | 163 (0.7) | 314 (0.7) |
| Genitourinary disorders | 149 (0.6) | 132 (0.5) | 281 (0.6) |
| Disorders of red blood cells and platelets | 85 (0.4) | 82 (0.3) | 167 (0.3) |
| Deep and superficial vein thrombosis | 28 (0.1) | 36 (0.1) | 64 (0.1) |
| Subjects with total antibodies against SARS-CoV-2 at baseline | 3986 (16.5) | 3951 (16.4) | 7937 (16.4) |
BMI: Body-mass index (the weight in kilograms divided by the square of the height in meters. The calculation was based on the weight and height measured at the time of screening); CNS: Central Nervous System; COPD: Chronic Obstructive Pulmonary Disease; IQR: Interquartile range; SD: standard deviation.
Seventy-four per cent of participants had some comorbidity or risk factor, with a similar distribution in both study groups. The most frequent were overweight/type I obesity, hypertension, smoking, diabetes mellitus and bronchial asthma, being of interest due to their clinical relevance in relation to COVID-19 (Table 1). The population involved in this Abdala's efficacy clinical trial was very close to real-world conditions.9
Only 19% of subjects reported some AE; 15,937 AEs were reported of 150 different types. AEs’ frequency by affected organ systems, according to dose and study group is presented in Supplemental Material (S2, Table S2). With consistent causality to vaccination, 21.8% (1933 AEs) were reported in the 1st dose, 22.9% (1214) in the 2nd dose and 23.5% (417) in the 3rd dose. The remaining AEs were not related to the formulations under study or their cause–effect relationship was indeterminate. Table 2 characterizes the AEs at each dose, by study group.
Table 2.
Overall distribution and characterization of adverse events, according to dose and study group (reported from the beginning of the study until 14 days after completing the vaccination schedule).
| Variable | DOSE 1 |
DOSE 2 |
DOSE 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Abdala | Total | Placebo | Abdala | Total | Placebo | Abdala | Total | |
| N | 24,144 | 24,146 | 48,290 | 23,826 | 23,794 | 47,620 | 23,602 | 23,557 | 47,159 |
| Subjects with adverse events | 3000 (12.4%) | 3177 (13.2%) | 6177 (12.8%) | 1749 (7.3%) | 1983 (8.3%) | 3732 (7.8%) | 579 (2.5%) | 639 (2.7%) | 1218 (2.6%) |
| Total of adverse events (15,937) | 4318 (48.7%) | 4545 (51.3%) | 8863 (55.6%) | 2442 (46.0%) | 2861 (54.0%) | 5303 (33.3%) | 838 (47.3%) | 933 (52.7%) | 1771 (11.1%) |
| Intensity | |||||||||
| Mild | 3942 (91.3%) | 4166 (91.7%) | 8108 (91.5%) | 2208 (90.4%) | 2598 (90.8%) | 4806 (90.6%) | 719 (85.8%) | 781 (83.7%) | 1500 (84.7%) |
| Moderate | 371 (8.6%) | 366 (8.1%) | 737 (8.3%) | 230 (9.4%) | 260 (9.1%) | 490 (9.2%) | 114 (13.6%) | 150 (16.1%) | 264 (14.9%) |
| Severe (Grade 3) | 2 (0.04%) | 1 (0.02%) | 3 (0.03%) | 0 | 0 | 0 | 5 (0.6%) | 2 (0.2%) | 7 (0.4%) |
| Serious (Grade 4) | 2 (0.04%) | 1 (0.02%) | 3 (0.03%) | 0 | 1 (0.03%) | 1 (0.01%) | 0 | 0 | 0 |
| Serious (Grade 5) | 1 (0.02%) | 3 (0.07%) | 4 (0.05%) | 4 (0.2%) | 2 (0.07%) | 6 (0.1%) | 0 | 0 | 0 |
| Causality (WHO) | |||||||||
| Inconsistent with vaccination | 2871 (66.5%) | 2849 (62.7%) | 5720 (64.5%) | 1632 (66.8%) | 1746 (61.0%) | 3378 (63.7%) | 588 (70.2%) | 561 (60.1%) | 1149 (64.9%) |
| Indeterminate | 596 (13.8%) | 614 (13.5%) | 1210 (13.7%) | 334 (13.7%) | 377 (13.2%) | 711 (13.4%) | 97 (11.6%) | 108 (11.6%) | 205 (11.6%) |
| Consistent with vaccination | 851 (19.7%) | 1082 (23.8%) | 1933 (21.8%) | 476 (19.5%) | 738 (25.8%) | 1214 (22.9%) | 153 (18.3%) | 264 (28.3%) | 417 (23.5%) |
| Action taken | |||||||||
| None | 2817 (65.2%) | 3025 (66.6%) | 5842 (65.9%) | 1629 (66.7%) | 1962 (68.6%) | 3591 (67.7%) | 584 (69.7%) | 628 (67.3%) | 1212 (68.4%) |
| Non-drug therapy | 132 (3.1%) | 133 (2.9%) | 265 (3.0%) | 44 (1.8%) | 52 (1.8%) | 96 (1.8%) | 9 (1.1%) | 16 (1.7%) | 25 (1.4%) |
| Drug therapy | 1372 (31.8%) | 1385 (30.5%) | 2757 (31.1%) | 762 (31.2%) | 842 (29.4%) | 1604 (30.2%) | 241 (28.8%) | 289 (31.0%) | 530 (29.9%) |
| Hospitalization | 1 (0.02%) | 0 | 1 (0.01%) | 3 (0.1%) | 0 | 3 (0.05%) | 4 (0.5%) | 0 | 4 (0.2%) |
| Withdrawal from study | 6 (0.1%) | 11 (0.2%) | 17 (0.2%) | 4 (0.2%) | 5 (0.2%) | 9 (0.2%) | 0 | 0 | 0 |
| Outcome | |||||||||
| Completely resolved | 4277 (99.1%) | 4499 (99.0%) | 8776 (99.0%) | 2421 (99.1%) | 2845 (99.4%) | 5266 (99.3%) | 822 (98.1%) | 919 (98.5%) | 1741 (98.3%) |
| Resolved with sequelae | 4 (0.09%) | 4 (0.09%) | 8 (0.09%) | 2 (0.08%) | 2 (0.07%) | 4 (0.07%) | 0 | 0 | 0 |
| Getting better | 28 (0.6%) | 27 (0.6%) | 55 (0.6%) | 11 (0.5%) | 9 (0.3%) | 20 (0.4%) | 12 (1.4%) | 10 (1.1%) | 22 (1.2%) |
| Present and unchanged | 8 (0.2%) | 11 (0.2%) | 19 (0.3%) | 4 (0.2%) | 3 (0.1%) | 7 (0.1%) | 4 (0.5%) | 4 (0.4%) | 8 (0.5%) |
| Worsening | 0 | 1 (0.02%) | 1 (0.01%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Death from adverse event | 1 (0.02%) | 3 (0.07%) | 4 (0.05%) | 4 (0.2%) | 2 (0.07%) | 6 (0.1%) | 0 | 0 | 0 |
The variable Action taken was tabulated following a hierarchical structure, to ensure that its categories are exclusive. Levels of this variable are ordered from least to most “drastic” measures: None, Non-pharmacological therapy, Pharmacological therapy, Hospitalization and Withdrawal from the study.
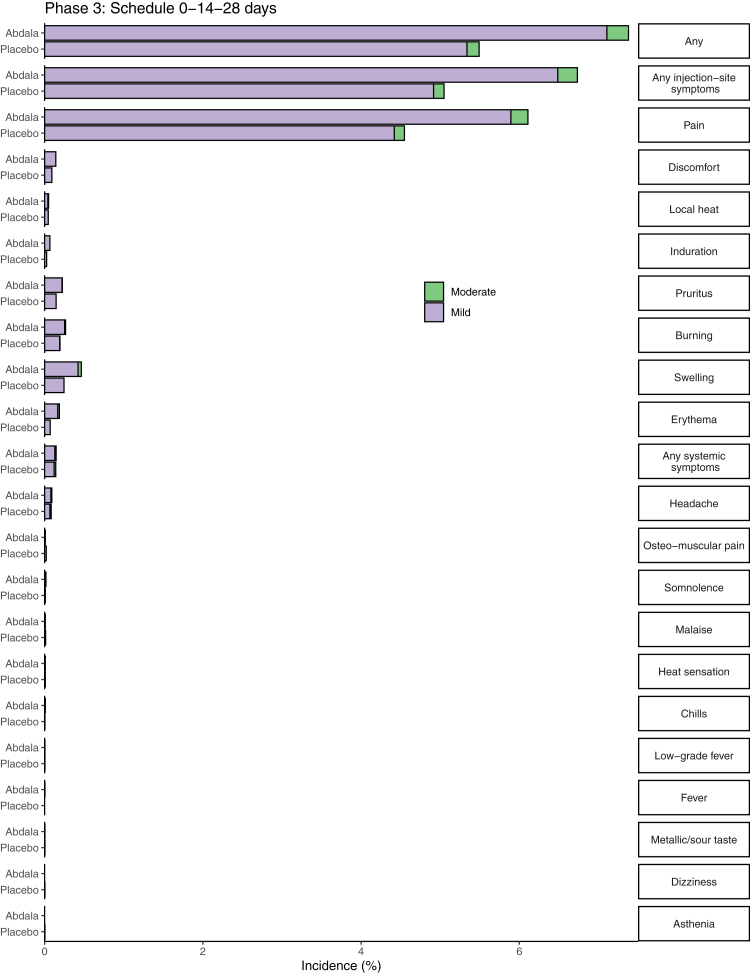
Abdala was well tolerated. The overall incidence of AR was 1227/24,144 (5.1%) for individuals in the placebo group and 1621/24,146 (6.7%) for Abdala group. Reactogenicity was largely absent or mild in most reports, and next doses were neither withheld nor delayed for this cause (Fig. 2).
Fig. 2.
Percentage of participants according to the occurrence of adverse reactions, by study group. The percentage of participants in each study group (Abdala vaccine, Placebo) with adverse reactions according to the maximum toxicity grade (mild or se) from first dose up to 14 days after the third dose is plotted by signs or symptoms. Participants who reported 0 events make up the remainder of the 100%.
Depending on the number of doses administered, reactogenicity was very low: 1480 events in 71,572 doses administered (2.1%) in the placebo group, and 2084/71,497 (2.9%) in the Abdala group, with a huge predominance (>97%) of local AR, mainly pain at the injection site, which represented 84.3% of the total reported. These reports were similar between study groups, slightly higher in the Abdala group. More than half of these AR were reported during the observation period corresponding to the first dose and were significantly reduced in the following doses. Most of the AR resolved spontaneously in the first 24–48 h without medication.
No severe AEs with demonstrated cause–effect relationship attributable to the investigational product were reported and there were no withdrawals for this cause. No episodes of anaphylaxis, immune-mediated medical conditions, myocarditis as well as other events of special interest relevant to COVID-19 were reported in this research.
During the vaccination period, a total of 10 serious AEs leading to death occurred in five participants in the placebo group, and five subjects in the Abdala group, described in Fig. 1. No deaths were considered by researchers to be related to the vaccine or placebo. Subsequently, during the evaluation of the VE (from 14 days after completion of the vaccination schedule), there were four deaths related to COVID-19, all in the placebo group.
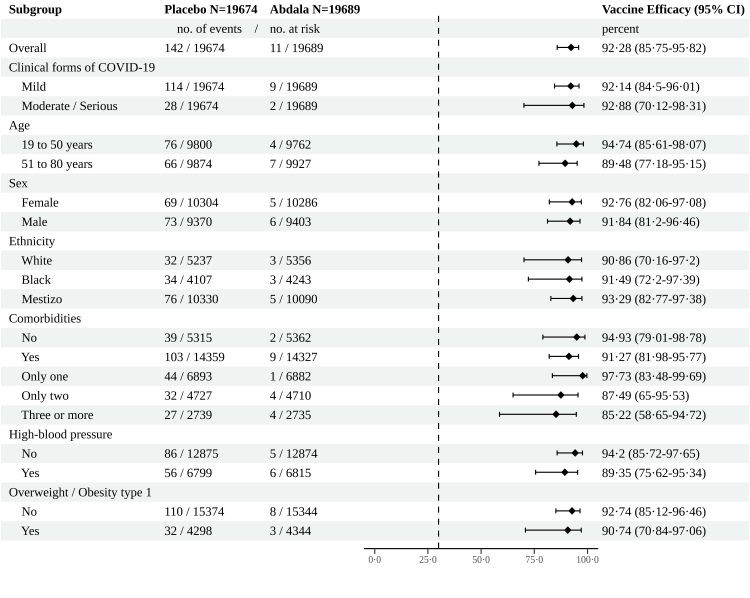
The evaluation of the main efficacy outcomes occurred during May–June 2021, starting at May 3rd, in the context of high circulation of mutant viruses, predominantly variant of concern (VOC) Beta. In the PP population of 39,363 participants, 153 cases of virologically confirmed symptomatic COVID-19 with an onset at least 14 days after the third dose occurred: 142 in placebo recipients and 11 in Abdala recipients. This corresponded to a VE of 92.28% (95% CI 85.74–95.82) which was the primary end-point of the trial (Fig. 3).
Fig. 3.
Abdala vaccine efficacy to prevent symptomatic COVID-19. Secondary end-points and Subgroups analyses. Analysis of Abdala's efficacy in the prevention of symptomatic COVID-19 by clinical forms of the disease, death and various subgroups in the per-protocol population, was based on adjudicated assessments starting 14 days after the third injection. Vaccine efficacy, defined as 1 minus the hazard ratio (Abdala vs. placebo), and 95% confidence intervals were estimated with the use of a stratified Cox proportional-hazards model. The dashed vertical line represents a vaccine efficacy of 30%, based on the null hypothesis that the primary efficacy of Abdala vaccine is 30% or less.
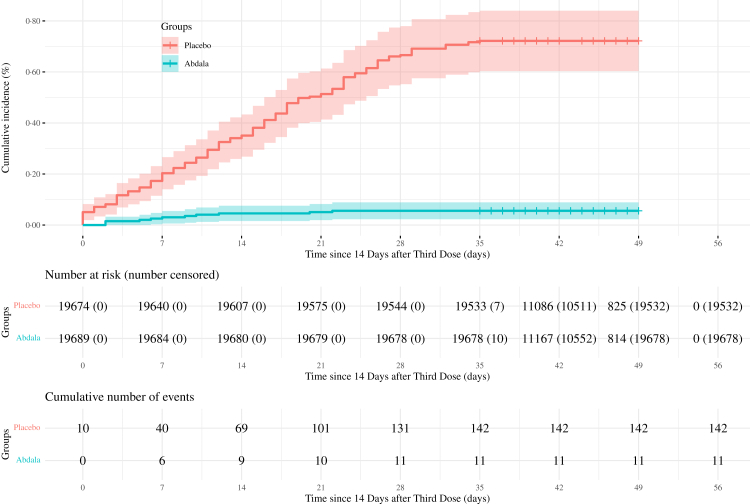
Cumulative incidences of COVID-19 related events in Abdala and placebo groups are shown in Fig. 4. The case incidence per 1000 person-years (95% CI) was 78.44 (95% CI 66.07–92.46) for the placebo group and 6.05 (95% CI 3.02–10.82) for the Abdala group.
Fig. 4.
Kaplan–Meier plots of efficacy of abdala vaccine against symptomatic COVID-19. Cumulative incidence of symptomatic COVID-19 in the per-protocol population is shown. The timing of surveillance for symptomatic COVID-19 participants began at least 14 days after the administration of the third dose (i.e., on day 42) through approximately the first 2 months of follow-up.
A secondary endpoint was efficacy evaluation according to COVID-19 clinical forms. In mild forms (114 cases in the placebo group and 9 in the Abdala group), VE was 92.14% (95% CI 84.50–96.01). Moderate/serious forms of COVID-19 (including critical disease) occurred in 30 participants (28 in the placebo group and only two in the Abdala group) for a VE of 92.88% (95% CI 70.12–98.31). In the placebo group, five critically ill patients as well as four deaths occurred, but none of those outcomes were found in Abdala recipients.
VE in preventing symptomatic COVID-19 was calculated in age subgroups of 19–50 and 51–80 years, as planned in the protocol (Fig. 3). In the age group of 19–50 years, VE was 94.74% (95% CI 85.61–98.07) and 89.48% (95% CI 77.18–95.15) for the group of 51–80 years. In the older group, 66 cases of COVID-19 occurred in the placebo group vs. only 7 in those who received Abdala. Interestingly, in the subset of the oldest individuals (66–80 years), 19 out of 3735 from the placebo group had symptomatic COVID-19 but it did not occur in the 3670 individuals who received Abdala.
Additional analyses of VE in preventing COVID-19 were performed in subgroups of defined sex, and ethnicity, as well as the presence or absence of coexisting conditions (Fig. 3). VE according to sex and ethnicity was above 90%. In participants with/without comorbidities, the efficacy was 91.27% (95% CI 81.98–95.77) and 94.93% (95% CI 79.01–98.72), respectively. The point estimate of efficacy decreased as the number of comorbidities increased. Finally, the two most frequent comorbidities found in the trial (hypertension and overweight/obesity type 1) were analysed separately, showing VE estimates also above 90% (Fig. 3). Most of symptomatic cases in the efficacy analyses had some comorbidity (103/142 cases in the placebo group and 9/11 for the Abdala group). Incidence rate per 1000 person-years (95% CI) for all secondary variables is shown in Supplemental Material (S4, Table S4).
VE in preventing symptomatic COVID-19 in the mITT population was 83.29% (95% CI 76.55–88.1). Details showed in Supplemental Material (S5, Table S5, Fig. S1).
Discussion
A three-dose vaccination schedule of Abdala administered at 0-14-28 days showed high efficacy against symptomatic COVID-19 (92.28%), fulfilling the WHO target product profile for COVID-19 vaccines that suggests a “clear demonstration of efficacy (on a population basis) ideally with a point estimate around 50%” should be a minimum acceptable criterion for any COVID-19 vaccine, and as a preferred condition, at least 70% with consistent results in older individuals.10 Both were achieved in this phase 3 trial. WHO recommends that successful vaccines should show an estimated hazard reduction of at least half, accurately enough to conclude that the true efficacy of the vaccine is greater than 30%.10 It is estimated that a 50% effective vaccine could significantly reduce the incidence of COVID-19 among those vaccinated and could provide useful herd immunity. As a result, even if a much higher efficiency of 50% would be preferable, that would represent substantial progress.11
Worldwide, more than 360 vaccines against SARS-CoV-2 based on a broad range of technological platforms are currently under development, 170 of them already in clinical trials, including 43 in phase 3 and 11 vaccines have been included in the WHO's emergency authorisation list.12 Results of the efficacy of different vaccines are already published13, 14, 15, 16, 17, 18, 19, 20 but the comparison between them has been difficult considering the differences in study protocols, dose regimen, the evaluation in non-equivalent conditions in different countries and economic conditions, various stages of COVID-19 pandemic and SARS-CoV-2 variants, among other factors.21, 22, 23
Currently, according to the results of clinical trials, highly efficacious vaccines against symptomatic COVID-19 have been obtained19: mRNA-BNT162b2 vaccine 95% (95% CI 90.3–97.6), ChAdOx1 nCoV-19 (adenovirus vector vaccine) 70.4% (95% CI 95% 54.8–80.6), rAd26 and rAd5 vector-based (Gam-COVIDVac) 91.6% (95% CI 85.6–95.2), Ad26.COV2.S (adenovirus vector vaccine) 66.1% (95% CI 55.0–74.8) and mRNA-1273 94.1% (95% CI 89.3–96.8). According to these results, Abdala's VE, shown in the phase 3 trial, compares favourably to all of this vaccines, produced using very different technology platforms.
Subunits vaccines also showed high efficacy levels against symptomatic COVID-19 in clinical trials24: Novavax/NVXCoV2373 (full length recombinant S protein-micelle nanoparticle/matrix M) 89.7% (95% CI 80.2–94.6), Soberana-02/Soberana-Plus (recombinant RBD conjugated to tetanus toxoid/RBD-dimer in Alum) 92.0% (95% CI 80.4–96.7),25 SCB-2019 (recombinant trimeric spike protein/AS03 or CpG/Alum) 67.2% (95% CI.72% CI 54.3–76.8).26 Our results show that Abdala's VE is very high, even though it is a simple vaccine formulation based on recombinant RBD in alum.
Consequently, regarding VE, Abdala behaves satisfactorily, as other vaccines widely use in worldwide mass immunization programs. Abdala has an excellent safety profile, previously observed in phase 1–2 clinical trial5 and confirmed in the current phase 3 study. It was well tolerated, without any serious toxicity, and no deaths were attributed to its administration. In general, subunit vaccines have been very safe, in accordance with this technological platform.24 One strength of Abdala is that the technology of gene expression of proteins in the yeast P. pastoris has more than thirty years-experience at CIGB.27 Other key strength of Abdala is its high thermostability.4 This offers a great advantage for the vaccination programs considering that no freezing conditions are required for storage and transportation, that makes this vaccine more affordable for countries or regions of difficult access, without conditions to maintain those freezing requirements.
Abdala's VE showed other encouraging results, such as those regarding with the efficacy against moderate/serious forms of COVID-19 (92.88%) as well as the fact that, the five critical patients found in the trial (of which four died) were all in the placebo group. VE according to age was also high, above 90%, consistent with the primary efficacy end-point. Considering that mortality from SARS-CoV-2 disproportionately affects older adults, it is very important to develop vaccination strategies to protect this highly vulnerable subgroup. In the study, no symptomatic COVID-19 cases occurred in the oldest participants (66–80 years). An elevated proportion of participants had pre-existing conditions, such as hypertension and overweight type I obesity in accordance with the occurrence in real world settings in Cuba. When the absence or presence of any comorbidity was analysed, the efficacy values were again higher than 90%. The higher the number of comorbidities, the lower the point estimates of efficacy. Efficacy results regarding comorbidities are relevant, considering the influence of these control variables on the clinical outcome of COVID-19 patients, where severe forms of the disease and mortality in adults have been associated with the presence of pre-existing medical conditions.28,29
To our knowledge, Abdala's study is one of the largest phase 3 trials published on COVID-19 vaccines, enrolling more than 48,000 people in less than 14 days. The trial performed well, without deviations and a minimum of dropouts (1.4%). This was also facilitated by the compact immunization schedule of three doses administered at 0-14-28 days, previously selected in the phase 2 trial, very useful to face the urgency of the pandemic situation.5 Randomisation assured a homogenous demographic and baseline characteristics of the participants in both placebo and Abdala groups. Blinding was maintained throughout the trial up to the estimation of clinical efficacy. Therefore, the internal validity of the study was adequate. Several factors such as the inclusion of participants from both sexes and different ethnic groups, the wide range of ages (19–80 years), the fact that 74% of the study population reported previous comorbidities/risk factors, as well as the high sample size of the trial, contribute to the generalisability (external validity, applicability) of the trial findings, narrowing the gap between the clinical trial and real-world populations, in which the same immunization schedule was used.
In Cuba, three epidemic periods have been defined as associated with the circulation of three major variants. Our phase 3 clinical trial was conducted from March to June 2021, corresponding exactly with the second period/second wave of the pandemic in the country,30 characterized by a continuous increase in the incidence of cases associated to the expansion of the circulation of the Beta variant. Variant distribution changed over time, according to the emergence and expansion of those with the greatest evolutionary advantage. Abdala's vaccine was evaluated in the context of high circulation of mutant viruses in the country. According to the epidemiological data from the eastern province of Santiago de Cuba, that included most of the phase 3 volunteers, the huge increase in the number of COVID-19 cases in that period was explained by the predominant circulation of VOC Beta strain. Detailed information in Supplemental Material (S6).
An observational retrospective cohort study, carried out in Havana, analysed Cuba's Ministry of Public Health databases to estimate Abdala's real-world vaccine effectiveness.31 The study, that included 1,355,638 persons age ≥19, demonstrated that Abdala was highly effective in preventing severe illness and death from COVID-19 (primary outcomes) under real-life conditions. In fully-vaccinated individuals, estimated Abdala's effectiveness was 98.2% (95% CI 97.9–98.5) against severe illness and 98.7% (95% CI 98.3–99.0) against death. Effectiveness exceeded 92.0% in all age groups. Those results, obtained in real-world conditions, complemented our findings obtained in the setting of the phase 3 placebo controlled, randomised, double blind clinical trial. The evaluation of Abdala's VE and effectiveness occurred in two separated geographically regions (eastern provinces and the western city of Havana, respectively) and two different epidemic waves.30 As mentioned previously, Abdala's VE was evaluated during the second wave/second period of the pandemic characterized by the expansion of Beta variant. The evaluation of effectiveness occurred in the third transmission period, coinciding with the dissemination of the Delta variant. In Havana, the epidemic peak occurred in August, 2021, corresponding with the almost exclusive circulation of the Delta strain, considered the most transmissible variant globally at that time.
This study has several limitations. First, the short duration of the efficacy follow-up, as has previously occurred for many other vaccine candidates in Phase 3 trials. It is important to take into consideration that we carried out a unique inclusion strategy, where the 48,290 participants were included in less than 14 days, with a uniform immunization schedule of three doses in a 28 days period and a median time of follow-up since randomization up to efficacy database closure of 75 days (IQR 73, 79 days). On the other hand, as the incidence of SARS-CoV-2 increased in the country with the circulation of VOC, the time required to meet the efficacy end-point decreased. Second, this study did not assess the efficacy in other populations such as children, adolescents and pregnant and lactating women, but further studies in those populations were also conducted after EUA was granted. Third, in the current report, data regarding neutralizing antibody titters as well as the duration of the immune response were not included nor the demonstration of vaccine effectiveness in the current epidemic context, where different sub lineages of Omicron VOC are prevalent. The evaluation of all these issues is ongoing and we will be included in other publications.
To our knowledge, Abdala is the first COVID-19 vaccine based on a subunit RBD-protein obtained by recombinant-DNA technology in the yeast P. pastoris, which demonstrated clinical efficacy in phase 3 clinical trials. Abdala vaccine is safe and highly protective against symptomatic COVID-19, including the most severe forms of the disease and death. Those results, along with its immunization schedule and the advantage of easy storage and handling conditions at 2–8 °C, make this vaccine an option for the use in immunization strategies as a key tool for the control of the pandemic.
Contributors
FHB conceived and designed this study, was its main coordinator, and took part in the analyses and interpretation of the results and paper writing; MCRC, ZNR, MPM, JBA, MCMR, LLL, VMVP, JSD, SLV, TMH, NCC and ERM were clinical researchers of the trial, participated in volunteers recruitment, data acquisition and interpretation of the results; YMB, KlUP, KaUP, JQG, IBC, ABI, APM, RMB and KMCA took part in trial coordination and data monitoring; ERM, COCC, MAV and RHR participated in the data management, statistical analyses and results interpretation; JLRR and GMS ensured masking of the investigational product and the cold chain throughout the process, as well as compliance with GCPs during vaccination; MVCN contributed to the diagnosis and virological follow-up by PCR-RT of patients with COVID-19; MAA, EPV and MLF took part in the trial design and coordination; VLMG participated in the trial design, advice, analyses and interpretation of the results and paper writing. All authors had full access to and verify all data in the study, and took the decision to submit the paper for publication. FHB, MCRC, YMB, ERM, KlUP, KaUP, JQG, COCC, MAV, KMCA and VLMG, verified the study data.
Data sharing statement
The study protocol is provided in the Appendix. Anonymised participant data will be made available when the trial is complete, upon requests directed to the corresponding author (hernandez.bernal@cigb.edu.cu). Proposals will be reviewed and approved by the sponsor, researcher, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Declaration of interests
FHB, YMB, KlUP, KaUP, JQG, JLRR, IBC, GMS, MLF, MAV, RHR, KMCA, MAA, and VLMG, are employees at the Genetic Engineering and Biotechnology Centre, Havana Network, where Abdala vaccine active ingredient is produced and the formulation was developed. The remaining authors have no conflict of interest. No honoraria, consulting fees or payments for seminar presentations, speeches or appearances have been received by any of the authors.
Acknowledgements
A special thanks to the thousands of volunteers in this trial, who maintained the highest adherence to the research protocol. Likewise, the authors wish to thank the government authorities of the provinces involved in the study and the Cuban Ministry of Public Health for their support.
Funding: Genetic Engineering and Biotechnology Centre (CIGB), Havana, Cuba.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100497.
Contributor Information
Francisco Hernández-Bernal, Email: hernandez.bernal@cigb.edu.cu.
ABDALA Research Group:
Francisco Hernández-Bernal, Maria C. Ricardo-Cobas, Yenima Martín-Bauta, Ernesto Rodríguez-Martínez, Klaudia Urrutia-Pérez, Karen Urrutia-Pérez, Joel Quintana-Guerra, Zadis Navarro-Rodríguez, Marjoris Piñera-Martínez, José L. Rodríguez-Reinoso, Cristina O. Chávez-Chong, Idania Baladrón-Castrillo, Grettel Melo-Suárez, Alejandro Batista-Izquierdo, Alexis Pupo-Micó, Ricardo Mora-Betancourt, Jacqueline Bizet-Almeida, Maria C. Martínez-Rodríguez, Leonardo Lobaina-Lambert, Vivian M. Velázquez-Pérez, Jalimy Soler-Díaz, Sandra Laurencio-Vallina, Tamara Meriño-Hechavarría, Norberto Carmenaty-Campos, Enri Rodríguez-Montero, Miladys Limonta-Fernández, Marel Alonso-Valdés, Reinier Hernández-Rodríguez, Eulogio Pimentel-Vázquez, Karem M. Catasús-Álvarez, Maria V. Cabrera-Núñez, Marta Ayala-Ávila, and Verena L. Muzio-González
Appendix A. Supplementary data
References
- 1.World Health Organization Weekly epidemiological update on COVID-19-24 August 2022. 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-24-august-2022 Available at:
- 2.Centers for Disease Control and Prevention COVID-19 risks and vaccine information for older adults. https://espanol.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html Available at:
- 3.Limonta-Fernández M., Chinea-Santiago G., Martín-Dunn A.M., et al. An engineered SARS-CoV-2 receptor-binding domain produced in Pichia pastoris as a candidate vaccine antigen. New Biotechnol. 2021 doi: 10.1016/j.nbt.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izquierdo M., Ramos Y., Costa L., et al. Demonstrating “Abdala” subunit vaccine thermostability. BioProcess J. 2022;21 doi: 10.12665/J21OA.Izquierdo. [DOI] [Google Scholar]
- 5.Hernández-Bernal F., Ricardo-Cobas M.C., Martin-Bauta Y., et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: a randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study) eClinicalMedicine. 2022;101383:46. doi: 10.1016/j.eclinm.2022.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health (NIH) Coronavirus disease 2019 (COVID-19) https://www.covid19treatmentguidelines.nih.gov/ Treatment Guidelines Panel 2021. Available in: [PubMed]
- 7.US Department of Health and Human Services . 2017. National institutes of health, common terminology criteria for adverse events. version 5.0. [Google Scholar]
- 8.World Health Organization . 2nd ed. WHO; Geneva: 2018. Causality assessment of an adverse event following immunisation (AEFI) [Google Scholar]
- 9.Nie P., Ding L., Sousa-Poza A., et al. Socioeconomic position and the health gradient in Cuba: dimensions and mechanisms. BMC Publ Health. 2020;866:20. doi: 10.1186/s12889-020-08980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Target product profiles for COVID-19 vaccines. 2020. https://www.who.int/who-documents-detail/who-target-product-profiles-for-covid-19-vaccines Available at:
- 11.Krause P., Fleming T.R., Longini I., et al. COVID-19 vaccine trials should seek worthwhile efficacy. Lancet. 2020;396(10253):741–743. doi: 10.1016/S0140-6736(20)31821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 13.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voysey M., Costa S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath P.T., Galiza E.P., Baxter D.N., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Kaabi N., Zhang Y., Xia S., et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng H., Peng Z., Luo W., et al. Efficacy and safety of COVID-19 vaccines in phase III trials: a meta-analysis. Vaccines. 2021;582(6):9. doi: 10.3390/vaccines9060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharif N., Alzahrani K.J., Ahmed S.N., Dey S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;714170:12. doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotshild V., Hirsh-Raccah B., Miskin A., et al. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;22777:11. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidary M., Kaviar V.H., Shirani M., et al. A comprehensive review of the protein subunit vaccines against COVID-19. Front Microbiol. 2022;927306:13. doi: 10.3389/fmicb.2022.927306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toledo-Romani M.E., Garcia-Carmenate M., Valenzuela-Silva C., et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of heterologous three-dose combination with SOBERANA-Plus: a double blind, randomised, placebo-controlled phase 3 clinical trial. Lancet Reg Health Am. 2023;100423:18. doi: 10.1016/j.lana.2022.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo L., Smolenov I., Htay H., et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy E., Martinez E., Diago D., et al. Large-scale production of recombinant hepatitis B surface antigen from Pichia pastoris. J Biotechnol. 2000;77(2–3):157–167. doi: 10.1016/S0168-1656(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 28.Mahamat-Saleh Y., Fiolet T., Rebeaud M.E., et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021;11(10):e052777. doi: 10.1136/bmjopen-2021-052777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y., Lv Y., Zha W., et al. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2021;154373:117. doi: 10.1016/j.metabol.2020.154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzmán M.G., Pérez L., Tejero Y., et al. Emergence and evolution of SARS-CoV-2 genetic variants during the Cuban epidemic. J Clin Virol. 2022;2(4):100104. doi: 10.1016/j.jcvp.2022.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Más-Bermejo P.I., Dickinson-Meneses F.O., Almenares-Rodríguez K., et al. Cuban Abdala vaccine: effectiveness in preventing severe disease and death from COVID-19 in Havana, Cuba; a cohort study. Lancet Reg Health Am. 2022;100366:16. doi: 10.1016/j.lana.2022.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.