Abstract
Objective
The aim of this study was to elaborate the link of thyroid hormones (THs) and metabolic syndrome (MetS) in a Chinese euthyroid employee population with MetS component(s).
Methods
An annual health checkup was performed on employees in 2019. Anthropometric parameters, metabolic parameters, and thyroid function were measured. A questionnaire was used in conjunction with Zhenhai Lianhua Hospital database to receive employees' medication records and thyroid surgical history records.
Results
A total of 5486 eligible employees were included; the prevalence of MetS was generally higher in males than in females (38.9 vs. 30.4%, P < 0.001). Among employees with central obesity, hypertriglyceridemia, hyperglycemia, hypertension, and low high-density lipoprotein cholesterol (HDL-C), the prevalence of MetS was 68.8, 63.6, 68.2, 48.8, and 60.0% in males and 72.6, 63.3, 61.3, 42.3, and 42.3% in females, respectively. Logistic regression analysis showed that thyroid-stimulating hormone and free thyroxine (FT4) quartiles had no significant impact on MetS. Free triiodothyronine/free thyroxine (FT3/FT4) and free triiodothyronine (FT3)) quartiles were positively associated with the increased odds ratio (OR) for MetS and dyslipidemia (hypertriglyceridemia and low HDL-C), regardless of gender. In males, FT3 and FT3/FT4 quartiles were positively associated with the OR for central obesity, whereas FT4 quartiles were negatively associated; both FT3 and FT4 quartiles were positively associated with increased OR of hyperglycemia, while similar results were not observed in females. Interaction analysis indicated no significant effect of gender and TH interactions on risk of MetS.
Conclusion
High FT3 and FT3/FT4 were strongly linked with MetS and dyslipidemia in our study, even in the euthyroid individuals. Tighter control of thyroid function was necessary for those with preexisting MetS component(s).
Key Words: euthyroid, thyroid stimulating hormone, free triiodothyronine, free thyroxine, metabolic syndrome
Introduction
Metabolic syndrome (MetS) is defined as a cluster of cardiovascular risk factors that occur together in individuals (1). It is diagnosed when the individual has three or more of its components (central obesity, hypertension, hypertriglyceridemia, hyperglycemia, and low high-density lipoprotein cholesterol (HDL-C)). Although not a specific disease, MetS is a complex pathophysiologic state in which multiple risk factors converge to add the risk of many familiar diseases (2). With the growing number of individuals with obesity, dyslipidemia, type 2 diabetes, and hypertension all over the world, MetS has gradually become a serious concern for global public health (3). Alarmingly, one-third of the general population was reported to have MetS (4).
Thyroid hormones (THs) are vital hormones that regulate the metabolism of substances (5) and therefore affect MetS and its components by regulating energy homeostasis, lipid metabolism, and gluconeogenesis. However, the association between THs and MetS in the euthyroid population is still controversial. A survey of 20,053 euthyroid Korean adults found that neither serum thyroid stimulating hormone (TSH) nor free thyroxine (FT4) was significantly associated with MetS (6). Mehran et al. argued that FT4, but not TSH, was associated with the development of MetS and its components (7), while Ruhla et al. found that high normal TSH was associated with an increased risk of MetS (8). More importantly, there are limited relevant findings in the euthyroid population with MetS component(s) (9), as most of the above literature reports are from the general population. We choose these specific individuals because MetS was more prevalent than general population. In a study of 15,048 adults in Korea, among those with body mass index (BMI) < 27.5, 22.6% had MetS, but the percentage rose to 65.4% among those with BMI ≥ 27.5 (10). The percentage of MetS among type 2 diabetic patients in sub-Saharan African countries was up to 59.62% (11). Consequently, it is appropriate to be more concerned with the prevention of MetS in these individuals.
The Zhejiang Zhenhai study has been well documented in the previous literature (12, 13, 14, 15). These studies covered a variety of health checkup items, such as carotid, thyroid, liver, blood pressure, and uric acid, and provide valuable advice to workers on the early management of many diseases. The association of MetS and its components and THs is controversial as described above, and its evaluation in different regions and ethnicities is of importance. This study is part of the 2019 Zhejiang Zhenhai study, and the aim was to discuss the relationship between TSH, free triiodothyronine (FT3), FT4, and FT3/FT4 levels within the normal range with MetS and its components. In light of the findings, we further propose how to better manage thyroid function in specific populations such as hypertension, dyslipidemia, hyperglycemia, and central obesity to provide a basis for early prevention of MetS.
Materials and methods
Participants
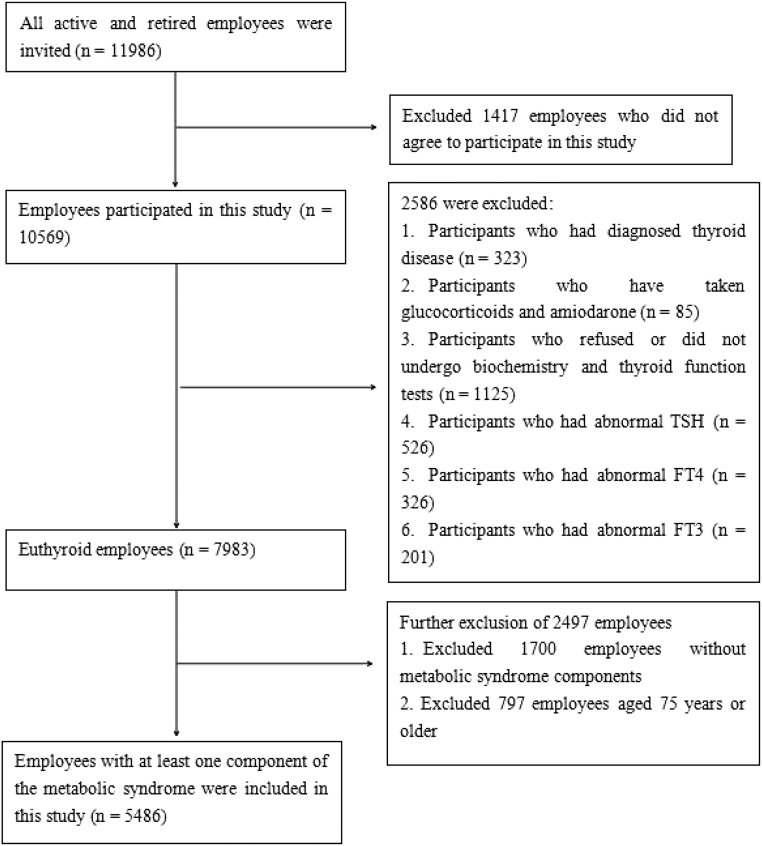
As in the previous Zhejiang Zhenhai study (15), all active and retired employees (a total of 11,986, age range 20–99 years old) of Zhenhai Refining & Chemical Company (Ningbo, China) were invited to participate. Employees who volunteered to participate in the 2019 Zhejiang Zhenhai study (n = 10,569) were included in this study, and the exclusion criteria were: (i) employees who did not agree to participate in this study; (ii) employees who were diagnosed with thyroid disease; (iii) employees who have taken glucocorticoids and amiodarone within the recent month; (iv) employees who refused or did not undergo biochemistry and thyroid function tests; (v) employees who had abnormal TSH, FT3, and FT4; (vi) employees without MetS components; and (vii) employees aged 75 years or older. The specific details of the screening procedures are shown in Fig. 1. This study was complied with the Declaration of Helsinki and authorized by the Ethics Committee of the Affiliated Hospital of Ningbo University School of Medicine (approval number: KY20181209). After full explanation of the purpose of the study, all participants were given written informed consent.
Figure 1.
Flowchart of the screening of research subjects.
Questionnaire
A questionnaire was administered by endocrinology specialists to the 10,569 employees who were identified for the health checkup to exclude those with diagnosed thyroid disease. The questionnaire covers four items about the thyroid: (i) ‘Have you ever had a history of thyroid surgery?’, (ii) ‘Have you taken or are you taking thyroxine medication?’, (iii) ‘Have you taken or are you taking thiamazole or propylthiouracil medication?’, and (iv) ‘Have you taken amiodarone or glucocorticoids (two common drugs affecting thyroid function) within the recent month?’.
Other relevant information collection
We screened out participants with records of glucose-lowering drugs, lipid-lowering drugs, blood-lowering drugs, or previous diagnosis of hypertension, hypertriglyceridemia, low HDL-C, and diabetes mellitus through a computerized database in Zhenhai Lianhua Hospital. These participants were assessed as having diagnosed hypertension, hypertriglyceridemia, low HDL-C, and hyperglycemia. According to the department to which the employee belongs, the work intensity of the employee was classified as light, moderate, and vigorous, and the retired employee was classified as light.
Measurement indicators
Anthropometric data of the employees, including height, weight, pulse, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP), were recorded in the medical examination report. Standing height and weight were measured in light clothing and without shoes. Height and weight measurements were made using the standard height- and weight-measuring instrument HGM-700 (Sheng Yuan, Zhengzhou, China). BMI = weight (kg)/height (m)2. WC was measured midway between the lowest rib and the iliac crest in the standing position during calm breathing. Blood pressure was measured on the right upper arm brachial artery in the quiet sitting position using the Omron HEM-7052 electronic sphygmomanometer (Omron Corp., Kyoto, Japan). Each employee was informed about fasting for at least 8 h or overnight before the physical examination, having their blood drawn intravenously by a professional nurse on the morning of the physical examination, and having their thyroid function tested through a blood sample. TSH, FT3, and FT4 were detected by professional testers with a DXI800 chemiluminescence immunoassay analyzer (BeckmanCoulter, Brea, CA, USA) and configured kits and reagents; the detection method was chemiluminescence immunoassay. The normal ranges of TSH, FT3, and FT4 were 0.3–4.7 mU/L, 4.0–8.3 pmol/L, and 9.0–28.0 pmol/L, respectively (14). The inter-assay coefficients of variation for the THs were as follows: TSH, 4.39% at a mean concentration of 1.14 mU/L; FT3, 7.78% at a mean concentration of 6.30 pmol/L; and FT4, 7.15% at a mean concentration of 16.37 pmol/L. Routine biochemical data including various metabolic parameters were tested using an Olympus AU640 automated analyzer (Olympus). Determination of serum triglycerides (TGs) was done by enzyme-coupled colorimetric method, HDL-C by direct method, and fasting blood glucose (FBG) by glucokinase method.
Diagnostic criteria
The diagnostic criteria of MetS were according to a Joint Interim Statement, in combination with the actual situation of WC in the Chinese population (1, 16). For each component, the diagnostic criteria were as follows: (i) central obesity (WC ≥ 90 cm in males and ≥ 85 cm in females), (ii) hypertriglyceridemia (TGs ≥ 1.7 mmol/L (150 mg/dL) or have received lipid-lowering medication or previously diagnosed hypertriglyceridemia), (iii) low HDL-C (HDL-C < 1.0 mmol/L in males and < 1.3 mmol/L in females or previously diagnosed low HDL-C), (iv) hypertension (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or have received anti-hypertensive medication or previously diagnosed hypertension), and (v) hyperglycemia (FBG ≥ 5.6 mmol/L or have received glucose-lowering medication or previously diagnosed diabetes mellitus). If any three or more of the above five items are met, the diagnosis of MetS was made. In the meantime, according to the questionnaire, employees with diagnosed thyroid disease included those who have taken or are taking thyroxine tablets alone (hypothyroidism), those who have taken or are taking anti-thyroid medication (hyperthyroidism), andthose with a history of thyroid surgery. Among those without thyroid disease, euthyroid was defined as TSH, FT3, and FT4 within the normal range.
Statistical analysis
The employees were grouped according to gender and THs quartile levels. The ranges of four TH parameters quartiles in males and females are presented in Supplementary Table 1 (see section on supplementary materials given at the end of this article). Continuous variables are expressed as means ± s.d., and categorical variables are expressed as percentages (%). Categorical variables were compared across quartiles by using chi-square tests. Continuous variables were compared between two groups by using independent samples t-test. After adjusting for age, work intensity, and/or BMI, a multiple linear regression model was established with metabolic parameters as independent variables and thyroid parameters as dependent variables to analyze whether there was a linear correlation. Multivariable-adjusted logistic regression was used to estimate the risk of MetS and its components according to the TSH, FT3, FT4, and FT3/FT4 quartiles (Tables 3, 4 and 5); the odds ratios (ORs) and 95% confidence intervals were calculated after adjusting for age, work intensity, and (or) BMI. Sex-interaction analyses were used to test for significant differences of the association estimated between males and females. The ORs in the model were the risk of MetS for males compared to females at the same TH quartile level. Gender in the role of THs in the effect of MetS was estimated by the addition of an interaction term to the relevant adjusted logistic regression model. All data analyses were two tailed, and P-value < 0.05 was considered statistically significant. All statistical analyses was performed with SPSS version 26.0 (IBM).
Table 3.
Risk of having MetS in thyroid hormone quartiles by gender.
| Thyroid hormone quartiles | Males | Females | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | P-value for trend | Adjusted OR (95% CI) | P-value | P-value for trend | |
| TSH | ||||||
| Quartile 1 (reference) | 1.000 | 0.779 | 1.000 | 0.914 | ||
| Quartile 2 | 0.976 (0.801, 1.189) | 0.811 | 0.669 (0.453, 0.987) | 0.043 | ||
| Quartile 3 | 0.922 (0.755, 1.125) | 0.423 | 1.008 (0.690, 1.473) | 0.966 | ||
| Quartile 4 | 0.988 (0.811, 1.205) | 0.909 | 0.895 (0.614, 1.303) | 0.561 | ||
| FT3 | ||||||
| Quartile 1 (reference) | 1.000 | <0.001 | 1.000 | 0.001 | ||
| Quartile 2 | 0.951 (0.775, 1.165) | 0.626 | 1.412 (0.947, 2.105) | 0.090 | ||
| Quartile 3 | 1.489 (1.219, 1.818) | <0.001 | 1.697 (1.141, 2.524) | 0.009 | ||
| Quartile 4 | 1.416 (1.192, 1.791) | <0.001 | 1.852 (1.253, 2.736) | 0.002 | ||
| FT4 | ||||||
| Quartile 1 (reference) | 1.000 | 0.110 | 1.000 | 0.093 | ||
| Quartile 2 | 0.852 (0.701, 1.035) | 0.106 | 1.022 (0.703, 1.487) | 0.907 | ||
| Quartile 3 | 0.870 (0.714, 1.060) | 0.167 | 0.732 (0.500, 1.073) | 0.110 | ||
| Quartile 4 | 0.838 (0.687, 1.022) | 0.081 | 0.792 (0.539, 1.164) | 0.236 | ||
| FT3/FT4 | ||||||
| Quartile 1 (reference) | 1.000 | <0.001 | 1.000 | 0.003 | ||
| Quartile 2 | 1.097 (0.896, 1.343) | 0.370 | 1.369 (0.923, 2.030) | 0.118 | ||
| Quartile 3 | 1.349 (1.104, 1.649) | 0.003 | 1.405 (0.949, 2.082) | 0.090 | ||
| Quartile 4 | 1.435 (1.174, 1.754) | <0.001 | 1.815 (1.238, 2.660) | 0.002 | ||
Model adjusting for age, BMI, and work intensity.
Table 4.
Risk of having MetS components in males by thyroid hormone quartiles.
| Thyroid hormone quartiles | Central obesitya | Hypertriglyceridemia | Low HDL-C | Hypertension | Hyperglycemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | P for trend | Adjusted OR (95% CI) | P-value | P for trend | Adjusted OR (95% CI) | P-value | P for trend | Adjusted OR (95% CI) | P-value | P for trend | Adjusted OR (95% CI) | P-value | P for trend | |
| TSH | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.098 | 1.000 | 0.416 | 1.000 | 0.202 | 1.000 | 0.582 | 1.000 | 0.080 | |||||
| Quartile 2 | 1.016 (0.848, 1.217) | 0.862 | 0.992 (0.832, 1.182) | 0.926 | 0.988 (0.827, 1.180) | 0.892 | 1.024 (0.844, 1.243) | 0.810 | 0.936 (0.760, 1.151) | 0.529 | |||||
| Quartile 3 | 1.150 (0.961, 1.377) | 0.128 | 0.971 (0.813, 1.158) | 0.740 | 1.114 (0.932, 1.331) | 0.236 | 0.908 (0.749, 1.101) | 0.328 | 0.840 (0.679, 1.039) | 0.108 | |||||
| Quartile 4 | 1.126 (0.941, 1.347) | 0.194 | 1.087 (0.912, 1.296) | 0.353 | 1.086 (0.909, 1.296) | 0.364 | 1.103 (0.908, 1.341) | 0.323 | 0.850 (0.689, 1.048) | 0.128 | |||||
| FT3 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | <0.001 | 1.000 | <0.001 | 1.000 | 0.001 | 1.000 | 0.159 | 1.000 | 0.027 | |||||
| Quartile 2 | 1.299 (1.080, 1.564) | 0.006 | 1.100 (0.920, 1.315) | 0.297 | 1.100 (0.920, 1.315) | 0.584 | 0.855 (0.702, 1.040) | 0.117 | 1.030 (0.834, 1.272) | 0.784 | |||||
| Quartile 3 | 1.345 (1.119, 1.616) | 0.002 | 1.433 (1.199, 1.712) | <0.001 | 1.273 (1.064, 1.522) | 0.008 | 1.060 (0.869, 1.293) | 0.567 | 1.155 (0.937, 1.425) | 0.178 | |||||
| Quartile 4 | 1.739 (1.447, 2.090) | <0.001 | 1.431 (1.195, 1.714) | <0.001 | 1.251 (1.044, 1.500) | 0.015 | 1.076 (0.881, 1.315) | 0.472 | 1.245 (1.004, 1.543) | 0.046 | |||||
| FT4 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.011 | 1.000 | <0.001 | 1.000 | 0.792 | 1.000 | 0.026 | 1.000 | 0.001 | |||||
| Quartile 2 | 0.976 (0.818, 1.164) | 0.785 | 0.748 (0.626, 0.892) | 0.001 | 0.973 (0.815, 1.162) | 0.765 | 1.054 (0.870, 1.278) | 0.590 | 0.956 (0.773, 1.181) | 0.674 | |||||
| Quartile 3 | 0.764 (0.638, 0.915) | 0.003 | 0.582 (0.487, 0.696) | <0.001 | 1.115 (0.933, 1.332) | 0.230 | 1.167 (0.961, 1.418) | 0.119 | 1.065 (0.861, 1.317) | 0.562 | |||||
| Quartile 4 | 0.850 (0.710, 1.016) | 0.074 | 0.514 (0.430, 0.615) | <0.001 | 0.932 (0.779, 1.114) | <0.001 | 1.221 (1.004, 1.485) | 0.045 | 1.406 (1.140, 1.735) | 0.001 | |||||
| FT3/FT4 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | <0.001 | 1.000 | <0.001 | 1.000 | 0.046 | 1.000 | 0.318 | 1.000 | 0.527 | |||||
| Quartile 2 | 1.113 (0.927, 1.338) | 0.251 | 1.317 (1.102, 1.573) | 0.002 | 1.226 (1.026, 1.465) | 0.025 | 0.823 (0.677, 1.001) | 0.051 | 0.943 (0.766, 1.161) | 0.580 | |||||
| Quartile 3 | 1.377 (1.148, 1.651) | 0.001 | 1.701 (1.423, 2.034) | <0.001 | 1.348 (1.127, 1.611) | 0.001 | 0.897 (0.736, 1.093) | 0.282 | 0.915 (0.742, 1.130) | 0.411 | |||||
| Quartile 4 | 1.600 (1.336, 1.917) | <0.001 | 2.047 (1.710, 2.451) | <0.001 | 1.178 (0.984, 1.410) | 0.074 | 0.871 (0.715, 1.061) | 0.170 | 0.941 (0.761, 1.163) | 0.571 | |||||
Model adjusting for age, BMI, and work intensity.
aAdjusting for age and work intensity.
Table 5.
Risk of having MetS components in females by thyroid hormone quartiles.
| Thyroid hormone quartiles | Central obesitya | Hypertriglyceridemia | Low HDL-C | Hypertension | Hyperglycemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95%CI) | P-value | P for trend | Adjusted OR (95%CI) | P-value | P for trend | Adjusted OR (95%CI) | P-value | P for trend | Adjusted OR (95%CI) | P-value | P for trend | Adjusted OR (95%CI) | P-value | P for trend | |
| TSH | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.170 | 1.000 | 0.380 | 1.000 | 0.068 | 1.000 | 0.831 | 1.000 | 0.035 | |||||
| Quartile 2 | 1.131 (0.768, 1.665) | 0.533 | 0.687 (0.494, 0.955) | 0.025 | 0.712 (0.521, 0.974) | 0.034 | 1.154 (0.826, 1.613) | 0.401 | 1.033 (0.722, 1.476) | 0.860 | |||||
| Quartile 3 | 1.222 (0.833, 1.794) | 0.306 | 0.876 (0.633, 1.214) | 0.428 | 1.120 (0.818, 1.533) | 0.481 | 1.116 (0.798, 1.560) | 0.521 | 0.894 (0.622, 1.285) | 0.544 | |||||
| Quartile 4 | 1.290 (0.883, 1.886) | 0.188 | 1.072 (0.777, 1.479) | 0.674 | 1.171 (0.854, 1.606) | 0.328 | 1.051 (0.753, 1.466) | 0.772 | 0.688 (0.474, 0.998) | 0.049 | |||||
| FT3 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.371 | 1.000 | <0.001 | 1.000 | 0.013 | 1.000 | 0.480 | 1.000 | 0.395 | |||||
| Quartile 2 | 0.946 (0.644, 1.388) | 0.775 | 1.466 (1.047, 2.053) | 0.026 | 1.226 (0.896, 1.678) | 0.204 | 1.105 (0.785, 1.556) | 0.566 | 0.702 (0.482, 1.022) | 0.065 | |||||
| Quartile 3 | 1.018 (0.695, 1.491) | 0.927 | 1.639 (1.173, 2.290) | 0.004 | 1.367 (0.998, 1.871) | 0.051 | 1.045 (0.746, 1.465) | 0.796 | 0.957 (0.665, 1.376) | 0.812 | |||||
| Quartile 4 | 1.165 (0.801, 1.694) | 0.424 | 1.858 (1.333, 2.590) | <0.001 | 1.465 (1.071, 2.005) | 0.017 | 0.902 (0.646, 1.258) | 0.543 | 1.064 (0.743, 1.523) | 0.737 | |||||
| FT4 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.839 | 1.000 | <0.001 | 1.000 | 0.029 | 1.000 | 0.316 | 1.000 | 0.103 | |||||
| Quartile 2 | 1.220 (0.834, 1.783) | 0.305 | 0.778 (0.564, 1.073) | 0.126 | 0.961 (0.700, 1.318) | 0.803 | 0.651 (0.467, 0.909) | 0.012 | 1.508 (1.038, 2.193) | 0.031 | |||||
| Quartile 3 | 1.170 (0.799, 1.711) | 0.420 | 0.650 (0.470, 0.899) | 0.009 | 0.769 (0.562, 1.053) | 0.101 | 0.885 (0.632, 1.239) | 0.475 | 1.373 (0.942, 2.000) | 0.099 | |||||
| Quartile 4 | 1.061 (0.722, 1.558) | 0.764 | 0.489 (0.351, 0.681) | <0.001 | 0.746 (0.545, 1.020) | 0.066 | 1.087 (0.772, 1.531) | 0.631 | 1.448 (0.996, 2.106) | 0.053 | |||||
| FT3/FT4 | |||||||||||||||
| Quartile 1 (reference) | 1.000 | 0.458 | 1.000 | <0.001 | 1.000 | 0.005 | 1.000 | 0.216 | 1.000 | 0.310 | |||||
| Quartile 2 | 1.322 (0.907, 1.927) | 0.147 | 1.743 (1.241, 2.449) | 0.001 | 1.057 (0.867, 1.288) | 0.582 | 0.687 (0.489, 0.966) | 0.031 | 0.877 (0.613, 1.256) | 0.475 | |||||
| Quartile 3 | 1.091 (0.739, 1.611) | 0.661 | 1.854 (1.319, 2.605) | <0.001 | 1.128 (1.085, 1.173) | <0.001 | 0.833 (0.591, 1.173) | 0.296 | 0.780 (0.540, 1.127) | 0.185 | |||||
| Quartile 4 | 1.241 (0.850, 1.812) | 0.263 | 2.678 (1.915, 3.745) | <0.001 | 1.152 (1.043, 1.273) | 0.005 | 0.742 (0.528, 1.043) | 0.085 | 0.854 (0.597, 1.222) | 0.387 | |||||
Model adjusting for age, BMI, and work intensity.
aAdjusting for age and work intensity.
Results
Baseline characteristics of the study population
A total of 5486 employees (age range, males: 25~74 years old, females: 26~74 years old) were enrolled in our study, of which 4139 were males and 1347 were females. The characteristics of males and females are presented separately in Table 1. Even though males were younger than females, they appeared to have more unfavorable metabolic profiles, such as higher BMI, SBP, DBP, FBG, TG, WC, and lower HDL-C (all P < 0.05). As a consequence, males had a significantly higher prevalence of MetS (38.9%), central obesity (37.4%), hypertriglyceridemia (49.0%), and hypertension (68.9%) but a significantly lower prevalence of low HDL-C (45.8%); all differences were statistically significant (P < 0.001). Conversely, males have a significantly lower percentage of medication treatment than females. Moreover, the prevalence of MetS in employees with central obesity, hypertriglyceridemia, low HDL-C, hypertension, or hyperglycemia was 68.8, 63.6, 60.0, 48.9, and 68.2% in males and 72.6, 63.3, 42.3, 42.3, and 61.3% in females, respectively. Considering these differences, in the following analysis, we explore men and women separately.
Table 1.
General characteristics of study population.
| Variables | All (n = 5486) | Males (n = 4139) | Females (n = 1347) | t/χ2 | P-value |
|---|---|---|---|---|---|
| Age (years) | 53.24 ± 12.50 | 51.89 ± 12.68 | 57.41 ± 10.93 | 14.35 | <0.001 |
| BMI (kg/m2) | 23.84 ± 2.97 | 24.20 ± 2.86 | 22.73 ± 3.01 | 16.22 | <0.001 |
| SBP (mmHg) | 132.71 ± 15.57 | 133.21 ± 15.06 | 131.18 ± 16.95 | 4.18 | <0.001 |
| DBP (mmHg) | 80.94 ± 10.59 | 82.19 ± 10.32 | 77.10 ± 10.52 | 15.67 | <0.001 |
| WC (cm) | 84.64 ± 8.88 | 86.76 ± 7.99 | 78.14 ± 8.27 | 34.10 | <0.001 |
| FBG (mmol/L) | 5.39 ± 1.22 | 5.41 ± 1.28 | 5.33 ± 0.98 | 2.10 | 0.036 |
| TG (mmol/L) | 1.77 ± 1.15 | 1.84 ± 1.24 | 1.54 ± 0.81 | 8.33 | <0.001 |
| HDL-C (mmol/L) | 1.15 ± 0.33 | 1.10 ± 0.31 | 1.33 ± 0.35 | 23.17 | <0.001 |
| TSH (mU/L) | 1.79 ± 0.81 | 1.73 ± 0.79 | 1.96 ± 0.85 | 8.91 | <0.001 |
| FT3 (pmol/L) | 5.22 ± 0.55 | 5.29 ± 0.54 | 5.03 ± 0.52 | 15.69 | <0.001 |
| FT4 (pmol/L) | 11.41 ± 1.33 | 11.46 ± 1.35 | 11.27 ± 1.25 | 4.43 | <0.001 |
| FT3/FT4 ratio | 0.46 ± 0.067 | 0.47 ± 0.067 | 0.45 ± 0.064 | 7.93 | <0.001 |
| Glucose-lowering drug therapy, n (%) | 255 (4.6%) | 179 (4.3%) | 76 (5.6%) | 3.98 | 0.046 |
| Lipid-lowering drug treatment, n (%) | 477 (8.7%) | 311 (7.5%) | 166 (12.3%) | 29.61 | <0.001 |
| Blood-lowering drug treatment, n (%) | 728 (13.3%) | 517 (12.5%) | 211 (15.7%) | 8.89 | 0.003 |
| Work intensity | |||||
| Light, n (%) | 3376 (61.5%) | 2224 (53.7%) | 1152 (85.5%) | 433.9 | <0.001 |
| Moderate, n (%) | 854 (15.6%) | 773 (18.7%) | 81 (6.0%) | 124.0 | <0.001 |
| Vigorous, n (%) | 1256 (22.9%) | 1142 (27.6%) | 114 (8.5%) | 210.6 | <0.001 |
| MetS, n (%) | 2021 (36.8%) | 1612 (38.9%) | 409 (30.4%) | 32.17 | <0.001 |
| Central obesity, n (%) | 1835 (33.4%) | 1550 (37.4%) | 285 (21.2%) | 121.16 | <0.001 |
| Hypertriglyceridemia, n (%) | 2567 (46.8%) | 2027 (49.0%) | 540 (40.1%) | 32.22 | <0.001 |
| Low HDL-C, n (%) | 2657 (48.4%) | 1896 (45.8%) | 761 (56.5%) | 46.48 | <0.001 |
| Hypertension, n (%) | 3704 (67.5%) | 2853 (68.9%) | 851 (63.2%) | 15.33 | <0.001 |
| Hyperglycemia, n (%) | 1430 (26.1%) | 1094 (26.4%) | 336 (24.9%) | 1.17 | 0.280 |
BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; FT3, free triiodothyronine; FT4, free thyroxine; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride; TSH, thyroid-stimulating hormone; WC, waist circumference.
The relationship between TSH and MetS and its components
After adjustment of age, work intensity, and/or BMI, TSH was positively correlated with TG (β = 0.041, P = 0.010) and negatively correlated with HDL-C (β = 0.039, P = 0.018) in males and not correlated with any metabolic parameters in females (Table 2). Furthermore, TSH has no significant effect on the risk of MetS and its components regardless of gender (Tables 3, 4, and 5). Multivariable-adjusted logistic regression in Tables 4 and 5 showed that after adjustment of age, work intensity, and (or) BMI, only the OR of hyperglycemia in the fourth quartile was significantly lower than that in the first quartile (P = 0.049), and the OR tended to decrease as the quartile increased in females (P for trend = 0.035), but there was no statistically significant difference in males (P for trend = 0.080). However, TSH quartiles were not significantly associated with the risk of MetS (males: P for trend = 0.779, females: P for trend = 0.914, Table 3).
Table 2.
The relationship between thyroid hormones and metabolic paraments.
| TSH | FT3 | FT4 | FT3/FT4 | |||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| Males | ||||||||
| SBP (mmHg) | –0.014 | 0.409 | 0.037 | 0.020 | 0.020 | 0.228 | 0.014 | 0.391 |
| DBP (mmHg) | –0.002 | 0.925 | 0.068 | <0.001 | –0.015 | 0.351 | 0.061 | <0.001 |
| WCa (cm) | 0.020 | 0.200 | 0.100 | <0.001 | –0.035 | 0.024 | 0.101 | <0.001 |
| FBG (mmol/L) | –0.019 | 0.228 | 0.023 | 0.136 | 0.091 | <0.001 | –0.049 | 0.002 |
| TG (mmol/L) | 0.041 | 0.010 | 0.070 | <0.001 | –0.106 | <0.001 | 0.134 | <0.001 |
| HDL-C (mmol/L) | –0.039 | 0.018 | –0.064 | <0.001 | 0.017 | 0.297 | –0.058 | <0.001 |
| Females | ||||||||
| SBP (mmHg) | –0.008 | 0.791 | 0.037 | 0.219 | 0.025 | 0.410 | 0.006 | 0.839 |
| DBP (mmHg) | 0.050 | 0.069 | 0.055 | 0.053 | 0.029 | 0.305 | 0.018 | 0.526 |
| WCa (cm) | 0.053 | 0.061 | 0.078 | 0.006 | –0.027 | 0.338 | 0.072 | 0.010 |
| FBG (mmol/L) | –0.041 | 0.145 | 0.046 | 0.010 | 0.088 | 0.002 | –0.028 | 0.325 |
| TG (mmol/L) | 0.016 | 0.577 | 0.126 | <0.001 | –0.147 | <0.001 | 0.204 | <0.001 |
| HDL-C (mmol/L) | –0.029 | 0.307 | –0.079 | 0.005 | 0.025 | 0.369 | –0.077 | 0.006 |
Model adjusting for age, BMI, and work intensity.
aAdjusting for age and work intensity.
β, standardized coefficients.
The relationship between FT3, FT4, FT3/FT4 and MetS and its components
Interestingly, the data in Tables 3, 4 and 5 showed that elevated FT3 and FT3/FT4 increased the OR of MetS and dyslipidemia (low HDL-C and hypertriglyceridemia) in both males and females (all P for trend < 0.05). Conversely, we found that higher FT4 quartiles within the normal range were related with lower OR of low HDL-C and hypertriglyceridemia in females and that the risk tended to decrease as FT4 quartile increased (all P for trend < 0.05). In males, FT4 was also negatively associated with the risk of hypertriglyceridemia (P for trend < 0.001), and only in the fourth FT4 quartile a significant lower OR of low HDL-C was observed (P < 0.001). However, trend analysis could not produce a significant downward trend between the FT4 quartiles and the risk of low HDL-C (P for trend = 0.792). In line with this, Table 2 also confirmed the correlation between FT3, FT4, FT3/FT4 and lipid parameters (TG and HDL-C). In detail, FT3 and FT3/FT4 were positively associated with TG and negatively associated with HDL-C, while FT4 was negatively associated with TG and not significantly associated with HDL-C. In males, FT3 and FT3/FT4 quartiles were also positively associated with the OR of central obesity and FT4 quartiles were negatively associated (all P for trend < 0.01), while in females they were not. Moreover, FT3 and FT4 were also positively associated with an increased OR of hyperglycemia in males (both P for trend < 0.05).
Sex-interaction analysis
Table 6 revealed that after adjusting for age, BMI, and work intensity, the risk of MetS was generally higher in males than in females, with no significant interaction between gender and THs effects on MetS (all P value for interaction > 0.05).
Table 6.
Associations of MetS with gender in subgroups of subjects stratified by thyroid hormone quartiles.
| Thyroid hormone quartiles | MetS % | Males | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | P-value for interaction | ||
| TSH | ||||
| Quartile 1 | 36.5% (498/1364) | 1.267 (0.930, 1.728) | 0.134 | 0.896 |
| Quartile 2 | 35.3% (486/1376) | 1.686 (1.224, 2.322) | 0.001 | |
| Quartile 3 | 37.2% (506/1362) | 1.031 (0.749, 1.420) | 0.851 | |
| Quartile 4 | 38.4% (531/1384) | 1.298 (0.956, 1.763) | 0.094 | |
| FT3 | ||||
| Quartile 1 | 32.3% (437/1352) | 1.485 (1.069, 2.063) | 0.018 | 0.854 |
| Quartile 2 | 33.9% (455/1342) | 0.944 (0.683, 1.306) | 0.729 | |
| Quartile 3 | 39.8% (553/1390) | 1.515 (1.117, 2.055) | 0.008 | |
| Quartile 4 | 41.1% (576/1402) | 1.358 (0.999, 1.846) | 0.051 | |
| FT4 | ||||
| Quartile 1 | 41.1% (558/1359) | 1.319 (0.969, 1.796) | 0.078 | 0.586 |
| Quartile 2 | 37.8% (521/1378) | 1.012 (0.745, 1.375) | 0.937 | |
| Quartile 3 | 34.5% (475/1376) | 1.513 (1.106, 2.069) | 0.010 | |
| Quartile 4 | 34.0% (467/1373) | 1.430 (1.031, 1.982) | 0.032 | |
| FT3/FT4 | ||||
| Quartile 1 | 31.8% (436/1369) | 1.392 (1.007, 1.924) | 0.045 | 0.932 |
| Quartile 2 | 34.3% (472/1375) | 1.143 (0.834, 1.567) | 0.405 | |
| Quartile 3 | 39.2% (537/1369) | 1.481 (1.084, 2.025) | 0.014 | |
| Quartile 4 | 42.0% (576/1373) | 1.227 (0.906, 1.663) | 0.187 | |
Model adjusting for age, BMI, and work intensity.
Discussion
This study was part of the Zhejiang Zhenhai study, initiated in 2019, and aimed to explore the relationship between TH parameters and MetS and its components in a euthyroid employee population with unfavorable metabolic profiles. The main results were as follows: (i) in both genders, TSH and FT4 levels had no significant effect on MetS but elevated FT3 and FT3/FT4 significantly increased the risk of MetS as well as dyslipidemia; (ii) only in males, elevated FT3, FT3/FT4, and reduced FT4 increased the risk of central obesity and elevated FT3, and FT4 increased the risk of hyperglycemia.
As previous studies have confirmed certain disparities in the prevalence of MetS and its components by gender, we discuss each gender separately in this study. Physical activity level (work intensity was used instead in this study), age, and BMI in particular are known to significantly influence thyroid parameters and metabolic characteristics; so we adjusted for these three factors in our regression model. In addition, the literature recommended establishing age-sex specific TH reference ranges for people over 75 years of age because of their wide individual differences (17); for this, we excluded elderly people aged over 75 years. Of these 5486 eligible employees, 36.8% had MetS, which was actually higher than several occupational population surveys (18, 19). Apart from the fact that the study population itself had unfavorable metabolic characteristics, this was also related to the high percentage of retired employees in the structure of the study population (20). Furthermore, employees with central obesity were more susceptible to MetS. It was undeniable that obesity played an instrumental role in MetS. IDF's diagnostic criteria for MetS published in 2005 even emphasized that central obesity was essential (21).
THs have an essential effect on hepatic fatty acid and cholesterol anabolism and therefore have a close relationship with dyslipidemia (22). Dyslipidemia was proved to be common in people with thyroid dysfunction, especially hypothyroidism and subclinical hypothyroidism (23, 24). In the present survey, we observed a strong correlation between normal range THs and dyslipidemia. First, FT3, FT4, and FT3/FT4 were associated with lipid parameters in both genders. Second, elevated FT3 and FT3/FT4 and decreased FT4 were positively associated with increased ORs of hypertriglyceridemia and low HDL-C. These findings implied that the impact of THs on lipid metabolism extends into the range of normal thyroid function. Although high TSH was associated with unfavorable lipid metabolism (25), this relationship was not observed within the normal range in this study. Some researches supported our view. Mehran et al. concluded that a normal range of TSH was not significantly associated with dyslipidemia after adjustment of relevant confounders (7). Shin et al. also found no correlation between TSH and dyslipidemia in a Korean female in their survey (6).
A lower basal metabolic rate (BMR) rate can lead to obesity and overweight. It has been identified that THs are a crucial regulator of BMR and that THs increase BMR via increased production of ATP during metabolism and maintenance of ionic gradients (5). Nevertheless, the effect of a normal range of THs on weight was not well understood. In this study, higher FT3 and FT3/FT4 and lower FT4 increased the risk of central obesity; however, this was not found in females, which may be related to the protective effect of estrogen in females (26, 27).
The relationship between various THs and MetS in euthyroid subjects has been inconsistent in recent years. Two studies reported no association between FT4 levels and MetS in euthyroid Korean population (6, 28), whereas Mehran et al. showed that higher FT4 was a protective factor for the development of MetS in euthyroid adults (7). A study identified that those with TSH levels between 2.5 and 4.5 mU/L had higher BMI and TGs than those below 2.5 mU/L and increased the risk of MetS by 1.7 times (8), while another study considered that normal range TSH was not significantly associated with MetS (29). Conflicting conclusions also exist about FT3 (30, 31). In contrast, normal high values of FT3/FT4 were consistently associated with increased prevalence of MetS in recent studies (32, 33). Reasons for the differences and uncertainties in the results of these studies were related to differences in the study populations, adjustment for different confounders, and various diagnostic criteria for MetS. In our study, higher FT3 and FT3/FT4 significantly increased the risk of MetS in both genders. Males showed higher FT3 and FT3/FT4 at baseline characteristics and had more severe metabolic profiles. In the above, it is clear that the overconversion of FT4 to FT3 observed in our study was closely bound to unfavorable metabolic characteristics, which was in accordance with some findings (32, 33, 34). Recently, a large data survey suggested increased conversion of FT4 to FT3 with age (35); this change may contribute to account for the high prevalence of MetS in the elderly. Previous studies have shown that higher FT3/FT4 and FT3 and lower FT4 were positively associated with increased risk of insulin resistance (IR) (30, 36, 37), and IR made a crucial contribution to the development of the MetS (38). FT3 had been proved in many studies to be positively correlated with unfavorable metabolic parameters, while FT4 was negatively correlated (31, 34, 39). In addition, metabolic disorders itself may also have a negative impact on thyroid function, with increased deiodinase activity in poor metabolizers and obese people, thus promoting the conversion of FT4 to FT3 (34). Hyperleptinemia in obese patients may have an adverse effect on lipid metabolism through the action of the hypothalamic–pituitary–thyroid axis (40); however, further research is needed to confirm these mechanisms. Interestingly, we found that normal range FT4, despite affecting components of MetS, did not significantly reduce the risk of MetS after adjusting for confounding factors. This may be due to the fact that the activity of FT4 in circulation was much lower than that of FT3, suggesting that slight changes in FT4 within the normal range have a lower predictive value for MetS than FT3 and FT3/FT4.
This study inevitably has the following deficiencies. First, due to the cross-sectional study, although we found that THs were associated with MetS and its components, we could not confirm the causal relationship. As this study population is relatively stable, prospective longitudinal studies can be carried out in the future to further explore; secondly, information on relevant confounders such as smoking, alcohol consumption, diet, and menopause was not available. Furthermore, the health check at Zhenhai Lianhua Hospital did not include thyroid autoantibody test, and so we were unable to discuss the effect of thyroid autoantibodies (thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibody (TgAb)) on the prevalence of MetS and its components in this population.
In summary, we confirmed that THs, even in the normal range, were strongly associated with the MetS and its components in this employee population. The increased conversion of FT4 to FT3 appeared to be an adverse metabolic profile. At the same time of reducing blood pressure, lipid, glucose, and weight for these individuals, stricter control of the upper limit of FT3 may be beneficial in reducing the risk of MetS, and more multicenter, large studies should be conducted to establish reasonable FT3 values for individuals with MetS component(s).
Supplementary Material
Declaration of interest
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by Ningbo Natural Science Foundation (grant number 2018A610248), NINGBO Medical & Health Leading Academic Discipline Project (grant number 2022-F24), the medical and health research project of Zhejiang province (grant numbers 2018ZH029 and 2020KY871), the Major Project for Science & Technology Innovation 2025 in Ningbo, China (grant number 2019B10035), Ningbo Social Development, China (grant number 2019C50080), and Ningbo Social Welfare Research (grant number 2022S047).
Acknowledgements
The authors extend their thanks to all employees who participated in our study. Thr authors sincerely thank Dr Zhongwei Zhu of Ningbo Zhenhai Lianhua Hospital for his permanent assistance of this study.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 20091201640–1645. ( 10.1161/CIRCULATIONAHA.109.192644) [DOI] [PubMed] [Google Scholar]
- 2.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, et al. Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology (Elmsford, NY) 2017683–33. ( 10.1016/j.reprotox.2016.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saklayen MG. The global epidemic of the metabolic syndrome. Current Hypertension Reports 201820 12. ( 10.1007/s11906-018-0812-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engin A. The definition and prevalence of obesity and metabolic syndrome. Advances in Experimental Medicine and Biology 20179601–17. ( 10.1007/978-3-319-48382-5_1) [DOI] [PubMed] [Google Scholar]
- 5.Mullur R Liu YY & Brent GA. Thyroid hormone regulation of metabolism. Physiological Reviews 201494355–382. ( 10.1152/physrev.00030.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin KA & Kim EJ. Association between thyroid hormone and components of metabolic syndrome in euthyroid Korean adults: A population-based study. Medicine 2021100 e28409. ( 10.1097/MD.0000000000028409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehran L Amouzegar A Tohidi M Moayedi M & Azizi F. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid 2014241566–1574. ( 10.1089/thy.2014.0103) [DOI] [PubMed] [Google Scholar]
- 8.Ruhla S Weickert MO Arafat AM Osterhoff M Isken F Spranger J Schöfl C Pfeiffer AF & Möhlig M. A high normal TSH is associated with the metabolic syndrome. Clinical Endocrinology 201072696–701. ( 10.1111/j.1365-2265.2009.03698.x) [DOI] [PubMed] [Google Scholar]
- 9.Tarcin O, Abanonu GB, Yazici D & Tarcin O. Association of metabolic syndrome parameters with TT3 and FT3/FT4 ratio in obese Turkish population. Metabolic Syndrome and Related Disorders 201210137–142. ( 10.1089/met.2011.0098) [DOI] [PubMed] [Google Scholar]
- 10.Kim YM Kim S Kim SH & Won YJ. Clinical and body compositional factors associated with metabolic syndrome in obese koreans: a cross-sectional study. Metabolic Syndrome and Related Disorders 201816290–298. ( 10.1089/met.2017.0174) [DOI] [PubMed] [Google Scholar]
- 11.Shiferaw WS Akalu TY Gedefaw M Anthony D Kassie AM Misganaw Kebede W Mulugeta H Dessie G & Aynalem YA. Metabolic syndrome among type 2 diabetic patients in Sub-Saharan African countries: a systematic review and meta-analysis. Diabetes and Metabolic Syndrome 2020141403–1411. ( 10.1016/j.dsx.2020.07.013) [DOI] [PubMed] [Google Scholar]
- 12.Xu C Xu L Yu C Miao M & Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clinical Endocrinology 201175240–246. ( 10.1111/j.1365-2265.2011.04016.x) [DOI] [PubMed] [Google Scholar]
- 13.Xu L Xie J Chen S Chen Y Yang H Miao M Zhu Z Li Y Yu C & Xu C. Light-to-moderate alcohol consumption is associated with increased risk of type 2 diabetes in individuals with nonalcoholic fatty liver disease: a nine-year cohort study. American Journal of Gastroenterology 2020115876–884. ( 10.14309/ajg.0000000000000607) [DOI] [PubMed] [Google Scholar]
- 14.Mao YS Liu ZM Chen CX Zhu ZW & Hong ZL. Ningbo thyroid dysfunction prevalence study: a cross-sectional survey in an employees-cohort. Chinese Medical Journal 20101231673–1678. [PubMed] [Google Scholar]
- 15.Xu C Yu C Ma H Xu L Miao M & Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai study. American Journal of Gastroenterology 20131081299–1304. ( 10.1038/ajg.2013.104) [DOI] [PubMed] [Google Scholar]
- 16.He J Lai Y Yang J Yao Y Li Y Teng W & Shan Z. The relationship between thyroid function and metabolic syndrome and its components: a cross-sectional study in a Chinese population. Frontiers in Endocrinology 202112 661160. ( 10.3389/fendo.2021.661160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann J Heinen E Kröll HJ Rudorff KH & Krüskemper HL. Thyroid function and thyroid hormone metabolism in elderly people. Low T3-syndrome in old age? Klinische Wochenschrift 198159315–323. ( 10.1007/BF01525000) [DOI] [PubMed] [Google Scholar]
- 18.Mehrdad R Pouryaghoub G & Moradi M. Association between metabolic syndrome and job rank. International Journal of Occupational and Environmental Medicine 2018945–51. ( 10.15171/ijoem.2018.1197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J Ouyang F Qiu D Duan Y Luo D & Xiao S. Association of nap duration after lunch with prevalence of metabolic syndrome in a Chinese government employee population. International Journal of Environmental Research and Public Health 2020174268. ( 10.3390/ijerph17124268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzouk L & Muntner P. Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Current Hypertension Reports 200911127–132. ( 10.1007/s11906-009-0023-8) [DOI] [PubMed] [Google Scholar]
- 21.Huang PL. A comprehensive definition for metabolic syndrome. Disease Models and Mechanisms 20092231–237. ( 10.1242/dmm.001180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha RA Singh BK & Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nature Reviews. Endocrinology 201814259–269. ( 10.1038/nrendo.2018.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delitala AP Fanciulli G Maioli M & Delitala G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. European Journal of Internal Medicine 20173817–24. ( 10.1016/j.ejim.2016.12.015) [DOI] [PubMed] [Google Scholar]
- 24.Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Current Cardiology Reports 20046451–456. ( 10.1007/s11886-004-0054-3) [DOI] [PubMed] [Google Scholar]
- 25.Duntas LH & Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Medical Clinics of North America 201296269–281. ( 10.1016/j.mcna.2012.01.012) [DOI] [PubMed] [Google Scholar]
- 26.Palmer BF & Clegg DJ. The sexual dimorphism of obesity. Molecular and Cellular Endocrinology 2015402113–119. ( 10.1016/j.mce.2014.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepard BD. Sex differences in diabetes and kidney disease: mechanisms and consequences. American Journal of Physiology. Renal Physiology 2019317 F456–F462. ( 10.1152/ajprenal.00249.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim BJ Kim TY Koh JM Kim HK Park JY Lee KU Shong YK & Kim WB. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clinical Endocrinology 200970152–160. ( 10.1111/j.1365-2265.2008.03304.x) [DOI] [PubMed] [Google Scholar]
- 29.Huang CY & Hwang LC. The association of thyroid hormones and TSH with the metabolic syndrome in euthyroid Taiwanese individuals. Endocrine Practice 2016221303–1309. ( 10.4158/EP161260.OR) [DOI] [PubMed] [Google Scholar]
- 30.Ding X, Zhu CY, Li R, Wu LP, Wang Y, Hu SQ, Liu YM, Zhao FY, Zhao Y, Zhang M, et al. Lower normal free thyroxine is associated with a higher risk of metabolic syndrome: a retrospective cohort on Chinese population. BMC Endocrine Disorders 202121 39. ( 10.1186/s12902-021-00703-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolffenbuttel BHR Wouters HJCM Slagter SN Van Waateringe RP Muller Kobold AC Van Vliet-Ostaptchouk JV Links TP & Van Der Klauw MM. Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocrine Disorders 201717 65. ( 10.1186/s12902-017-0215-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urrunaga-Pastor D Guarnizo-Poma M Moncada-Mapelli E Aguirre LG Lazaro-Alcantara H Paico-Palacios S Pantoja-Torres B & Benites-Zapata VA. High free triiodothyronine and free-triiodothyronine-to-free-thyroxine ratio levels are associated with metabolic syndrome in a euthyroid population. Diabetes & Metabolic Syndrome 2018155–161. ( 10.1016/j.dsx.2017.12.003) [DOI] [PubMed] [Google Scholar]
- 33.Park SY Park SE Jung SW Jin HS Park IB Ahn SV & Lee S. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clinical Endocrinology 20178787–96. ( 10.1111/cen.13345) [DOI] [PubMed] [Google Scholar]
- 34.Roef GL Rietzschel ER Van Daele CM Taes YE De Buyzere ML Gillebert TC & Kaufman JM. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 201424223–231. ( 10.1089/thy.2013.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strich D Karavani G Edri S & Gillis D. TSH enhancement of FT4 to FT3 conversion is age dependent. European Journal of Endocrinology 201617549–54. ( 10.1530/EJE-16-0007) [DOI] [PubMed] [Google Scholar]
- 36.Štěpánek L Horáková D Štěpánek L Janout V Janoutová J Bouchalová K & Martiník K. Free triiodothyronine/free thyroxine (FT3/FT4) ratio is strongly associated with insulin resistance in euthyroid and hypothyroid adults: a cross-sectional study. Endokrynologia Polska 2021728–13. ( 10.5603/EP.a2020.0066) [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Zeng J, Huang B, Yan F, Ye J, Chen Y, Zeng X, Zheng X, Xiao F, Lin M, et al. Independent associations of thyroid-related hormones with hepatic steatosis and insulin resistance in euthyroid overweight/obese Chinese adults. BMC Gastroenterology 202121 431. ( 10.1186/s12876-021-02011-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gluvic Z Zaric B Resanovic I Obradovic M Mitrovic A Radak D & Isenovic ER. Link between metabolic syndrome and insulin resistance. Current Vascular Pharmacology 20171530–39. ( 10.2174/1570161114666161007164510) [DOI] [PubMed] [Google Scholar]
- 39.Xu R Huang F Zhang S Lv Y & Liu Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: a cohort study. BMC Endocrine Disorders 201919 58. ( 10.1186/s12902-019-0383-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y Yin Q Xu M Ni Q Wang W & Wang Q. BMI modulates the effect of thyroid hormone on lipid profile in euthyroid adults. International Journal of Endocrinology 20172017 8591986. ( 10.1155/2017/8591986) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a