Abstract
The role of Clove (Cv) and Tulsi (Ts) supplementation on broiler growth performance and gut health as an alternative to antibiotic growth promoters has already been established. Therefore, the objectives of this study were to investigate the role of Cv and Ts on the serum biochemical profile and meat quality traits in broilers. A total of sixty (60) one-day-old commercial broiler chicks were randomly allotted into four homogenous groups (15 birds per group). They were then fed Cv powder and Ts extract from day (d) 8 to d 28 with drinking water i.e. zero level of Cv or Ts (T0), 0.5% Cv + 2% Ts (T1), 1.0% Cv + 3% Ts (T2), 1.5% Cv + 4% Ts (T3). Blood and meat samples were collected on d 14, 21, and 28 to analyze the serum-biochemical profile and meat quality. Supplementation of Cv and Ts improved serum-biochemical profile by reducing total cholesterol and low-density lipoprotein levels in broilers. However, they did not affect the serum protein levels while the 0.5–1% Cv and 2–3% Ts supplemented groups had higher glucose levels on d 21. Production of breast and thigh meat increased with increased size, and density of myofibers while fed 0.5–1% Cv and 2–3% Ts. On the contrary, 1.5% Cv powder and 4% Ts extract supplementation improved thigh meat color, breast and thigh meat pH as well as the water-holding capacity. The current study findings suggest that Cv and Ts can be used in combination to improve broiler health, production, and meat quality.
Keywords: Broiler, Clove, Tulsi, Serum biochemistry, Meat quality
1. Introduction
Global meat consumption is expected to grow by about 14% by 2030, having a 17.8% growth in poultry meat consumption, in comparison to the base period (2018-'20) which will be mostly driven by the increased income and population (OECD-FAO, 2021). However, there have been rising concerns regarding the emergence of antibiotic-resistant pathogens (Agyare et al., 2018). Even though, the use of growth promoters (GPs) has ensured production but also sacrificed the quality of production creating ubiquitous public health risks. Therefore, an organic production system needs to be established in no time to achieve sustainability in the growth of this sector.
Production scientists are evaluating different medicinal plants and their active components as feasible alternatives to antibiotics to ensure health and production for many years (Chowdhury et al., 2018, Arif, 2022). The beneficial impacts of these plants or plant products are mostly brought about by their antioxidant, antimicrobial, appetite-stimulating, and immune-boosting capacities (Eevuri and Putturu, 2013). Clove (Cv) bud is a phytochemical-rich spice that contains different bioactive components like hidroxibenzoic acids, quercetin, kaempferol, hydroxiphenyl propens, hydroxicinamic acids, ferulic, caeic, ellagic, salicylic acids, eugenol, eugenol acetate and β-cariofileno which have potent antioxidant, antimicrobial, anticancer, and antidiabetic properties (Batiha et al., 2020). Tulsi (Ts) is another aromatic plant that is rich in bioactive ingredients eugenol, oleanolic acid, β-elemene, ursolic acid, rosmarinic acid, carvacrol, β-caryophyllene, linalool, and germacrene D (Eevuri and Putturu, 2013). These active components exert antimicrobial, antioxidant, immunomodulatory, digestive stimulating, hepatoprotective, cardioprotective effects, and so on (Eevuri and Putturu, 2013, Diaz-Sanchez et al., 2015).
Both Cv and Ts reportedly promote the gut health and growth rate of broilers (Chowdhury et al., 2018, Naeem et al., 2021, Islam et al., 2023). So, they may be used as effective low-cost alternatives to synthetic GPs like antibiotics or steroids in producing safe organic broiler meat. However, meat production is not the only challenge to overcome by poultry farmers or the poultry industries. There are negative perceptions about broiler meat among consumers as it is usually pale, soft, and exudative in nature (Mir et al., 2017). The slow-growing indigenous breeds of chicken produce higher quality meat compared to the fast-growing broilers (Li et al., 2019). According to the earlier report, rapid growth and weight gain in broilers compromise their meat quality (Petracci et al., 2015). Color, pH, water-holding capacity (WHC), drip loss (DL), cook loss (CL), tenderness, juiciness, and characteristics of myofibers are the major physicochemical and histological indicators that are routinely used to evaluate the quality of meat (Mir et al., 2017). The pH is linked to meat quality properties i.e. color, WHC, juiciness, shelf-life, and processing ability of the meat (Mir et al., 2017, Islam et al., 2022a). High-quality meat is attributed to optimum pH and WHC, increased juiciness, and tenderness (Mir et al., 2017, Suliman et al., 2020, Islam et al., 2022a). The histomorphometric properties of myofibers like size or diameter and density of myofibers also affect meat production and quality attributes like shear value, tenderness, etc. (Ismail and Joo, 2017).
There are abundant resources on the use of Cv and Ts in terms of growth performance and gut health in broilers. However, data on serum biochemical profile and meat quality indices in broilers fed Cv and Ts are very limited. It is also noteworthy that there is no previous study depicting the combined roles of Cv and Ts on the aforementioned aspects in broilers. Supplementation of phytobiotic extracts in varying dosages (lower to higher) is easier while supplied with drinking water and it is also easier to prepare a homogenous mixture with water (Grashorn, 2010). Moreover, supplementation of aqueous extract eliminates the risks of lower feed intake and feed wastage. The treatment levels were set based on the earlier study reports. The previous study reports showed that supplementation of 2–5% tulsi in broilers improves their growth performances (Hasan et al., 2016). In another study, Mufarrej et al. (2019) fed 1–6% clove to the broilers and found that feeding clove at a concentration of 2% or more negatively affects the growth performance of broilers. It is mentionable that similar combinations of clove and tulsi were used to investigate their effects on broiler growth performance and gut health (Islam et al., 2023).Therefore, the present study was carried on to picture the roles of the combined inclusion of different levels (0.5%, 1%, and 1.5% Cv and 2%, 3%, and 4% Ts) of Cv and Ts on the dynamics of serum biochemical profile and meat quality in broilers.
2. Materials and methods
2.1. Ethic al statement
The “Animal welfare and Experimentation Ethics Committee” of Bangladesh Agricultural University (BAU) approved the experimental design followed for the present study. The ethical approval identification is - AWEEC/BAU/2021(5).
2.2. Broiler housing and management
Sixty (60) unsexed one-day-old Cobb-500 broiler chicks were collected from the Nourish Poultry & Hatchery Ltd., Bangladesh for conducting the present study. Following an acclimatization period of seven days, they were randomly parted into four groups (15 birds in each group) and housed in separate cages (Size: 20 square feet). The broilers were reared under standard conditions (75-95°F brooding temperature,70°F rearing temperature, and 50–60% relative humidity) (Islam et al., 2021). Standard biosecurity protocols were followed throughout the trial period. Vaccination of broilers was done against the Newcastle Disease (BCRDV vaccine, Bangladesh Livestock Research Institute (BLRI), Bangladesh) at five days of age and Infectious Bursal Disease (GumboMed Plus Vet, Incepta Vaccine Ltd., Bangladesh) at 11 days of age.
2.3. Clove and Tulsi supplement preparation
Very fine Cv (Syzygium aromaticum) powder was prepared from the sun-dried Cv buds and stored in airtight containers to prepare an aqueous extract. Ts (Ocimum sanctum) extract (aqueous) was prepared on a daily basis from fresh leaves by blending (Model: VIS-SBL-005, VISION Blender, Bangladesh) them in adequate amount of water (2% Ts extract- 20 g fresh leaves/liter drinking water, 3% Ts extract − 30 g fresh leaves/liter drinking water, and 4% Ts extract − 40 g fresh leaves/liter drinking water) (Islam et al., 2023).
2.4. Feeding management
The broilers were offered a balanced diet (Nourish Feeds Limited, Bangladesh) and fresh drinking water (Islam et al., 2022b; Supplementary table 1). From day (d) 8, the prepared Cv and Ts supplements were supplied with drinking water in three different concentrations.
Treatment (T) T0: Control group, received no additional supplement; T1: 0.5% Cv and 2% Ts; T2: 1% Cv and 3% Ts; T3: 1.5% Cv and 4% Ts (Islam et al., 2023). The broilers received the Cv and Ts supplement up to 28 days of age.
2.5. Blood samples collection
Blood (5 ml) was collected from five broilers in each group from the wing vein on d 14, d 21, and d 28 of the experiment. After collecting the blood samples, they were transferred into sterile tubes and left undisturbed in a slanting position (at 4 °C temperature, overnight) for proper clotting. The serum samples were then collected and centrifuged at 1000 rpm for 15 min. The supernatant serum samples were collected, transferred into screw-capped vials, and preserved at −20 °C until further use. Following serum preparation, the glucose (GLU) profile, lipid profile, and protein profile of the serum were analyzed. Serum GLU was measured following the method followed by Sultana et al. (2022). The lipid profile, i.e. total cholesterol (TCHOL), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), was determined by using the protocols followed by Sultana et al., 2021, Islam et al., 2022c. The serum protein profile, i.e. total protein (TP), albumin (ALB), globulin (GLB), and albumin-globulin ratio (ALB:GLB), was measured following the methods specified by Grimsley and Pace, (2003). The biochemical analyses were performed by spectrophotometric technique using a Hyman type Humalyzer 2000 analyzer (Wiesbaden, Germany).
2.6. Collection of meat samples
Five broilers having homogenous weights were sacrificed on d 28 and immediately dissected to collect the meat (thigh and breast meat). The meat weights were then measured after trimming off the adipose tissues (Font-i-Furnols et al., 2015, Islam et al., 2022b).
2.7. Meat physicochemical properties analysis
As part of the meat physicochemical properties analysis, meat color [the color coordinates are lightness (L*), redness (a*), yellowness (b*), hue angle (HA), and saturation index (SI)], initial pH (pHi) at 1 h postmortem (PM), ultimate pH (pHu) at 24 h PM, drip loss,% (DL), cook loss, % (CL), and water holding capacity, % (WHC) were measured.
2.7.1. Meat color measurement
The meat samples were preserved (4 °C) for 24 h before the measurement of meat color. The instrumental meat color coordinates were determined by Chroma Meter (CR-400, Minolta Corporation, Japan). At first, the Chroma Meter was calibrated using a white tile. The standard values of the color coordinates were 55.31, 3.42, and 7.69 for the L*, a*, and b*, respectively. Chroma Meter reading for the color coordinates was taken from the external surfaces of the meat samples at 24 h PM (Font-i-Furnols et al., 2015; Islam et al., 2022b). The HA and SI were then calculated using the following formulas-
2.7.2. Measurement of meat pH
The pH values of the meat were determined (from three different spots for each meat sample) by pH meter (PH-25, Wincom, China) at 1 h (pHi) and 24 h (pHu) PM. The pH meter was calibrated using a buffered solution (pH: 7.0). The pH meter was recalibrated before taking each reading (Font-i-Furnols et al., 2015, Islam et al., 2022a).
2.7.3. Meat drip loss measurement
To measure the DL (%) of the meat samples, they were deboned and stored in separate zipper bags for 24 h at 4 °C after recording the initial weight of each sample. The weights of the meat samples were again measured at 24 h PM and the % of DL was calculated (Kerth, 2013, Font-i-Furnols et al., 2015).
2.7.4. Meat cook loss measurement
For the measurement of CL (%), deboned breast and thigh meat samples (size: 4 cm × 3 cm × 1 cm) were excised at 24 h PM and weighed. Then the samples were placed on aluminum foil paper and cooked at 163 °C in a convection oven (FH20-1, Prestige, India) until the internal temperature reached 80 °C. After cooling at room temperature, the cooked meat samples were reweighed (Kerth, 2013, Font-i-Furnols et al., 2015). The CL (%) was calculated using the formula-
2.7.5. Meat water holding capacity measurement
For the measurement of WHC (%) of the meat samples, 1 g (initial weight) of each meat sample was chopped properly with a chopper and loaded into a PCR tube. The PCR tubes were then set inside a high-performance centrifuge machine (ScanSpeed 1730R MicroCentrifuge, LaboGene, UK) and centrifuged at 10,000 RCF for 10 min (4 °C). After removing the supernatant fluid, the meat samples were reweighed (final weight) (Kerth, 2013, Font-i-Furnols et al., 2015). The WHC (%) was calculated using the following formula-
2.8. Muscle biology analysis
For the study of muscle biology, muscle samples were processed to prepare paraffin sections (5 μm)and stained with the Harris Hematoxylin and Eosin stain. Photomicrographs of the histoarchitecture of breast and thigh muscles were captured (400X actual magnification) using a photomicroscope (CX41U-LH50HG, Olympus Corp., Japan). The myofiber count (MFC) per microscopic field and the cross-sectional area (CSA, square micrometer) of the myofibers were analyzed using the “ImageJ freehand tool”.
2.9. Statistical analysis
All the data generated in the present study were analyzed (IBM SPSS Statistics, version 22) by using the one-way ANOVA approach and a totally randomized design. The unit of analysis for the present study was the individual bird (there was no pen replication). The normality of the data sets was checked by the Shapiro-Wilk test before ANOVA analysis. Duncan's Multiple Range Test (DMRT) was used to compare the mean values. When P < 0.05, differences were considered statistically significant.
3. Results
3.1. Serum-biochemical profile
3.1.1. Serum glucose profile
No noticeable variation (P < 0.05) in serum GLU levels was observed among the groups on d 14 and d 28. Interestingly, GLU level was substantially (P < 0.05) higher in the T2 group compared to the T0 and T3 on d 21. On the contrary, GLU level markedly dropped (P < 0.05) in the T3 group in comparison to the T1 and T2 on d 21 (data are given in Supplementary table 2).
3.1.2. Serum lipid profile
The role of Cv and Ts inclusion on the dynamics of the serum lipid profile are summarized in Table 1. On d 14, there was no mentionable difference (P > 0.05) in serum TCHOL and LDL levels among the experimental groups. However, serum TG levels substantially increased (P < 0.05) in the T2 and T3 groups. Likewise, serum HDL level was also markedly higher (P < 0.05) in the T3 group while no noticeable difference was observed among T0, T1, and T3 groups.
Table 1.
Effects of Clove powder and Tulsi extract supplementation on the dynamics of serum lipid profile in broilers.
| Parameter (mg/dl) |
Treatments1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||
| d 14 | ||||||
| TCHOL | 130.79 | 112.25 | 131.50 | 125.70 | 13.29 | 0.463 |
| TG | 103.74c | 99.66c | 128.65b | 149.84a | 8.56 | 0.001 |
| HDL | 35.31b | 37.68ab | 34.67b | 41.58a | 1.93 | 0.011 |
| LDL | 74.73 | 73.88 | 51.84 | 53.93 | 14.04 | 0.235 |
| d 21 | ||||||
| TCHOL | 183.67a | 175.44a | 150.42ab | 111.35b | 18.90 | 0.007 |
| TG | 79.54c | 91.48b | 98.29b | 117.34a | 5.41 | 0.001 |
| HDL | 45.28 | 42.75 | 44.33 | 42.27 | 2.61 | 0.641 |
| LDL | 85.81a | 86.03a | 70.22b | 71.84b | 3.90 | 0.001 |
| d 28 | ||||||
| TCHOL | 159.37a | 152.08ac | 140.22c | 122.38bc | 8.73 | 0.004 |
| TG | 79.23c | 82.58bc | 90.72ab | 96.94a | 3.90 | 0.001 |
| HDL | 40.17a | 38.00a | 34.04b | 37.52ab | 1.71 | 0.019 |
| LDL | 95.81a | 96.70a | 70.89b | 72.34b | 7.39 | 0.002 |
a,b,cWithin a row, values with different alphabetic superscripts differ significantly (P < 0.05).
1T0: represents the control group; T1, T2, and T3 groups represent supplementation of 0.5% Clove + 2% Tulsi, 1% Clove + 3% Tulsi, and 1.5% Clove and 4% Tulsi, respectively.
TCHOL - Total cholesterol, TG - Triglyceride, HDL - High-density lipoprotein, LDL - Low-density lipoprotein.
On d 21, serum TCHOL levels substantially decreased (P < 0.05) in the T3 group. On the contrary, the T3 group had the highest level of TG that was followed by T2, T1, and T0. Though there was no mentionable difference (P > 0.05) in serum HDL levels, serum LDL levels considerably (P < 0.05) decreased in the T2 and T3 groups.
On d 28, serum TCHOL levels substantially decreased (P < 0.05) in the Cv and Ts supplemented groups while the levels of TG markedly rose (P < 0.05). HDL levels substantially dropped(P < 0.05) in the T2 group while LDL levels also substantially dropped (P < 0.05) in both T2 and T3 groups.
3.1.3. Serum protein profile
There was no mentionable difference (P > 0.05) in serum protein levels among the groups on different days of the experiment. However, the serum TP, ALB, and GLB levels tended to be higher in the treatment groups, especially in the T2 in comparison to the T0 (data are given in Supplementary table 3).
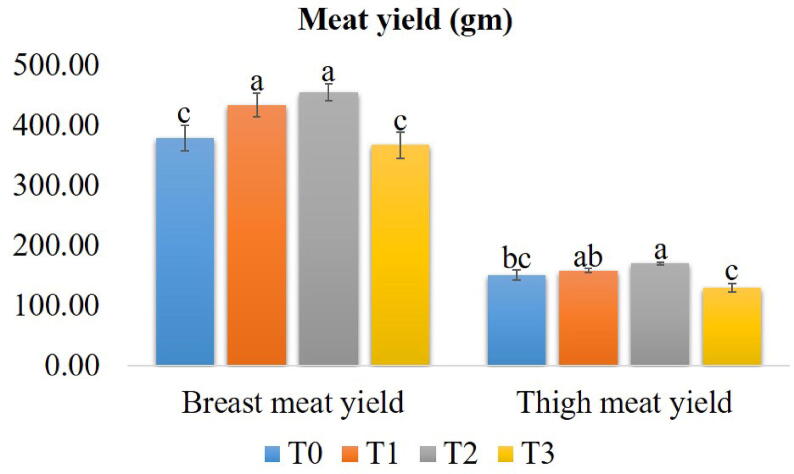
3.2. Breast and thigh meat yield
The yields of breast meat and thigh meat in different experimental groups are shown in Fig. 1. The T2 group had the highest meat yield while the T3 had the lowest. However, no mentionable variation (P > 0.05) was found between T2 and T1. Though the meat yield tended to decrease in T3 in comparison to T0, the difference was negligible (P > 0.05).
Fig. 1.
Yields of breast and thigh meat in broilers supplemented with different concentrations of Cv and Ts. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts. Data are presented as mean ± SEM. Differences among the groups of birds were compared using one-way ANOVA followed by DMRT. Mean values with different alphabetic superscripts significantly differ from each other (P < 0.05).
3.3. Physicochemical properties of meat
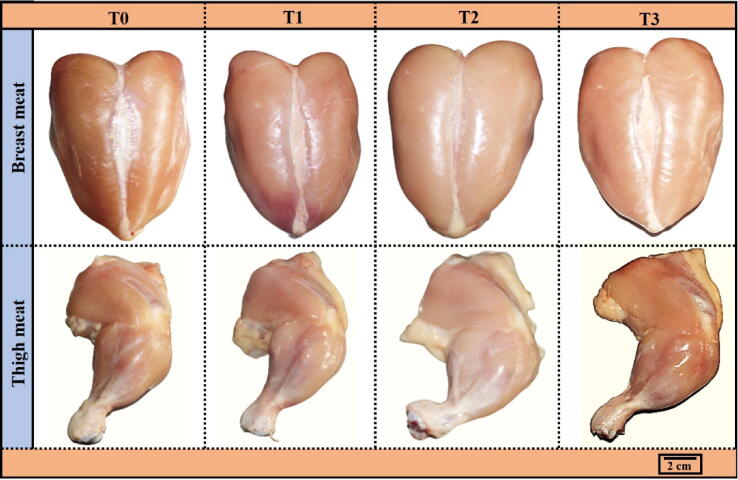
3.3.1. Meat color
In naked eye observation, no noticeable change in breast meat color was observed. On the other hand, the thigh meat was found comparatively darker and more reddish in the T3 group (Fig. 2).
Fig. 2.
Representative images showing the breast and thigh meat color of broilers supplemented with different concentrations of Cv and Ts. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts.
The color coordinates of the breast meat tended to improve in the Cv and Ts supplemented groups (P > 0.05). However, no noticeable variation (P > 0.05) was observed among the groups. Nevertheless, the T1 and T2 had slightly higher b* values (P > 0.05) indicating increased yellowness of breast meat in these groups. The groups had no mentionable difference (P > 0.05) in the L*, b*, and SI values of the thigh meat. However, a* value increased markedly (P < 0.05) in the T3 indicating increased thigh meat redness. The T3 group also revealed a substantial drop (P < 0.05) in HA compared to the rest of the experimental groups (Table 2).
Table 2.
Effects of Clove powder and Tulsi extract supplementation on the physicochemical properties of breast and thigh meat in the broiler.
| Attributes |
Treatments1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||
| Breast meat | ||||||
| L* | 61.22 | 58.82 | 56.42 | 56.82 | 2.21 | 0.195 |
| a* | 3.21 | 3.36 | 3.45 | 3.87 | 1.09 | 0.937 |
| b* | 8.10 | 9.01 | 8.87 | 7.79 | 1.47 | 0.809 |
| HA | 67.07 | 69.54 | 69.84 | 63.88 | 5.91 | 0.733 |
| SI | 24.91 | 30.72 | 32.93 | 31.18 | 13.15 | 0.933 |
| pHi (1 h) | 6.80b | 6.75c | 6.76c | 6.87a | 0.02 | 0.001 |
| pHu (24 h) | 6.15b | 6.11b | 6.13b | 6.34a | 0.04 | 0.003 |
| DL (%) | 1.62 | 1.01 | 0.81 | 1.28 | 0.36 | 0.209 |
| CL (%) | 24.68b | 23.89b | 27.97a | 23.58b | 1.07 | 0.012 |
| WHC (%) | 83.20b | 80.59c | 80.13c | 86.57a | 1.12 | 0.001 |
| Thigh meat | ||||||
| L* | 57.40 | 55.59 | 55.00 | 53.42 | 2.60 | 0.529 |
| a* | 5.97ab | 4.23c | 4.84bc | 6.78a | 0.71 | 0.027 |
| b* | 7.81 | 6.76 | 8.70 | 6.96 | 1.17 | 0.060 |
| HA | 52.90a | 57.61a | 60.71a | 35.27b | 6.24 | 0.015 |
| SI | 46.78 | 29.10 | 42.23 | 33.42 | 9.15 | 0.275 |
| pHi (1 h) | 6.97b | 6.90c | 6.89c | 7.03a | 0.02 | 0.002 |
| pHu (24 h) | 6.42 | 6.37 | 6.29 | 6.44 | 0.08 | 0.251 |
| DL (%) | 1.89 | 1.67 | 2.03 | 1.16 | 0.28 | 0.064 |
| CL (%) | 25.84 | 26.59 | 27.53 | 24.04 | 1.13 | 0.073 |
| WHC (%) | 82.35b | 82.55b | 81.01b | 85.51a | 0.10 | 0.011 |
a,b,cWithin a row, values with different alphabetic superscripts differ significantly (P < 0.05).
1T0: represents the control group; T1, T2, and T3 groups represent supplementation of 0.5% Clove + 2% Tulsi, 1% Clove + 3% Tulsi, and 1.5% Clove and 4% Tulsi, respectively.
L* - Lightness, a* - Redness, b* - Yellowness, HA - Hue angle, SI - Saturation index, pHi - Initial pH (1 h postmortem), pHu - Ultimate pH (24 h postmortem), CL - Cook loss, DL - Drip loss, WHC - Water holding capacity.
3.3.2. Meat pH
The pH values of the meat samples are shown in Table 2. The pHi of the breast meat substantially dropped (P < 0.05) in the T1 and T2 groups while it was considerably higher (P < 0.05) in the T3 in comparison to the T0. However, no mentionable difference (P > 0.05) in pHu was found among the T0, T1, and T2 groups. Notwithstanding, the T3 group had substantially higher (P < 0.05) pHu in comparison to the rest.
The pHi of the thigh meat also dropped markedly (P < 0.05) in the T1 and T2 groups while the T3 group had significantly higher (P < 0.05) pHi compared to the rest of the groups. There was no mentionable change (P > 0.05) in pHu among the experimental groups.
3.3.3. Drip loss, cook loss and water holding capacity of meat
The impacts of Cv and Ts on the DL, CL, and WHC of meat are presented in Table 2. The experimental groups had no mentionable variation (P > 0.05) in terms of DL in both the breast meat and thigh meat. The CL of the breast meat was markedly higher (P < 0.05) in the T2 while no mentionable difference (P > 0.05) was observed in the case of thigh meat. The breast meat of the T1 and T2 had considerably lower (P < 0.05) WHC. On the other hand, the T3 had markedly higher (P < 0.05) WHC in comparison to the rest. The WHC of thigh meat was substantially higher (P < 0.05) in the T3 but no difference was observed among the T0, T1, and T2 groups.
3.3.4. Muscle biology
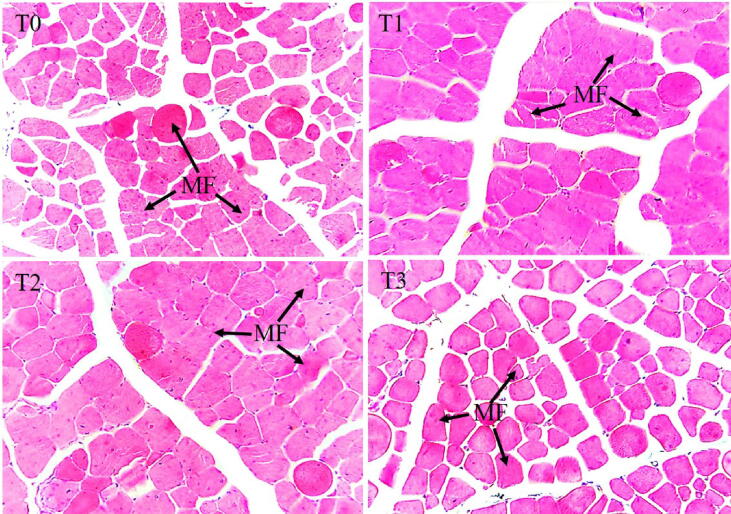
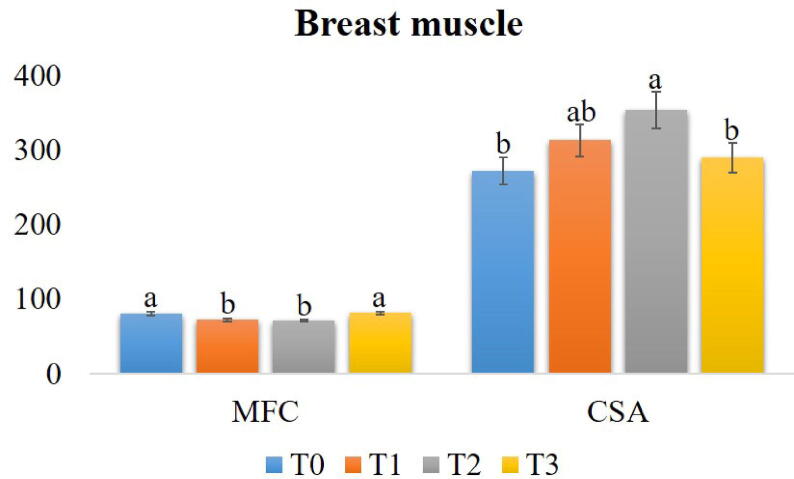
3.3.4.1. Breast muscle
The breast muscle histoarchitecture is shown in Fig. 3. The T1 and T2 had significantly lower (P < 0.05) MFC compared to the T0 and T3. The CSA of myofibers was markedly higher (P < 0.05) in the T2 compared to the T0 and T3. However, there was no mentionable variation (P > 0.05) between the T1 and T2 groups (Fig. 4).
Fig. 3.
Representative photomicrographs showing the histoarchitecture of breast muscle of the broiler supplemented with different concentrations of Cv and Ts. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts.
Fig. 4.
Effects of Cv powder and Ts extract on the myofiber count (MFC) and cross-sectional area (CSA) of myofibers in breast muscle of broiler chicken. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts. Data are presented as mean ± SEM. Differences among the groups of birds were compared using one-way ANOVA followed by DMRT. Mean values with different alphabetic superscripts significantly differ from each other (P < 0.05).
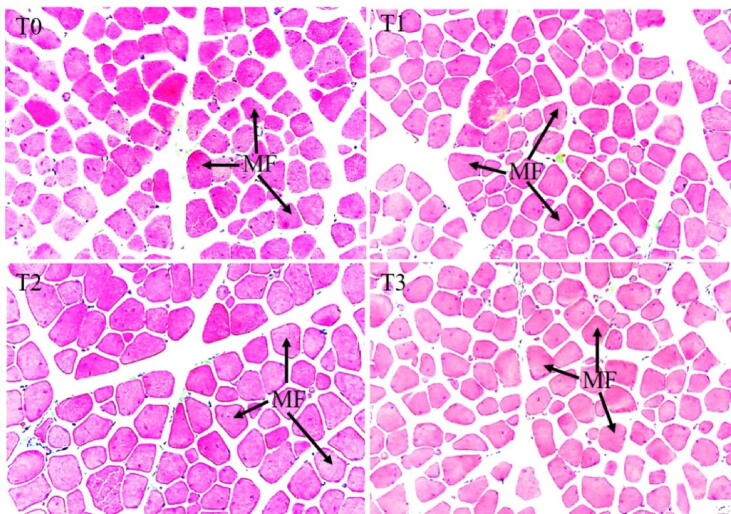
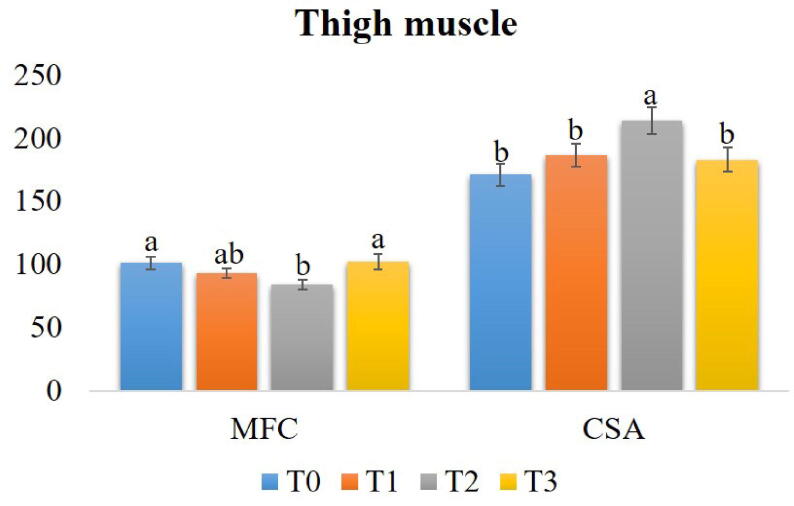
3.3.4.2. Thigh muscle
The thigh muscle histoarchitecture is shown in Fig. 5. The MFC was substantially lower (P < 0.05) in the T2. The CSA of myofibers was substantially higher (P < 0.05) in the T2 in comparison to the rest of the experimental groups. However, there was no noticeable variation (P > 0.05) among the T1, T2, and T3 groups (Fig. 6).
Fig. 5.
Representative photomicrographs showing the histoarchitecture of thigh muscle of the broiler supplemented with different concentrations of Cv and Ts. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts.
Fig. 6.
Effect of Cv powder and Ts extract on the myofiber count (MFC) and cross-sectional area (CSA) of myofibers in thigh muscle of broiler chicken. Treatment (T) T0: Control group, did not receive any plant extract; T1: 0.5% Cv and 2% Ts; T2: 1% Cv, and 3% Ts; T3: 1.5% Cv and 4% Ts. Data are presented as mean ± SEM. Differences among the groups of birds were compared using one-way ANOVA followed by DMRT. Mean values with different alphabetic superscripts significantly differ from each other (P < 0.05).
3.3.5. Benefit-cost analysis
The benefit-cost analyses of the broilers fed Cv and Ts are shown in Table 3. The results showed that the feed cost was significantly (P < 0.05) higher in the T2 group indicating higher feed intake. The total cost of production per broiler was also higher (P < 0.05) in the Cv and Ts-fed groups. It is mentionable that the cost of production per kg live broiler was lower (P < 0.05) in the T2 group generating more profit (P < 0.05) than the other groups. In line with this, the T2 group also had a higher (P < 0.05) benefit-cost ratio.
Table 3.
Benefit-cost analysis of broilers fed different combinations of Clove and Tulsi.
| Parameters |
Treatments1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||
| Chick cost (Tk./bird) | 25 | 25 | 25 | 25 | – | – |
| Feed cost (Tk./bird) | 108.12b | 113.26ab | 116.9a | 107.7b | 2.87 | 0.015 |
| Cost of clove and tulsi (Tk./bird) | 0.00 | 2.67 | 5.33 | 8.00 | – | – |
| Bedding, disinfectant, vaccine (Tk./bird) | 21 | 21 | 21 | 21 | – | – |
| Mortality cost (Tk./bird) | 0.00 | 0.00 | 0.00 | 0.00 | – | – |
| Labor cost (Tk./bird) | 16 | 16 | 16 | 16 | – | – |
| Electricity and others (Tk./bird) | 09 | 09 | 09 | 09 | – | – |
| Total cost of production (Tk./bird) | 179.12c | 186.93b | 193.23a | 186.7b | 2.87 | 0.002 |
| Total cost of production (Tk/kg. body weight) | 113.58b | 112.61bc | 109.29c | 121.95a | 1.74 | 0.000 |
| Total Income (Tk./bird) (Sale price @ 160 Tk./kg) | 252.32c | 265.60b | 282.88a | 244.96c | 4.46 | 0.000 |
| Profit (Tk./ bird) | 73.2b | 78.67ab | 89.65a | 58.26c | 5.30 | 0.000 |
| Profit (Tk./kg live bird) | 46.42b | 47.39ab | 50.71a | 38.05c | 1.74 | 0.000 |
| Benefit-cost ratio | 1.41a | 1.42a | 1.46a | 1.31b | 0.03 | 0.002 |
a,b,cWithin a row, values with different alphabetic superscripts differ significantly (P < 0.05).
1T0: represents the control group; T1, T2, and T3 groups represent supplementation of 0.5% Clove + 2% Tulsi, 1% Clove + 3% Tulsi, and 1.5% Clove and 4% Tulsi, respectively.
4. Discussion
The careless use of subtherapeutic levels of antibiotics has led to the emergence of antibiotic-resistant pathogenic bacteria (Hussein et al., 2020). Moreover, there is rising concern among consumers about the contamination of edible tissues by these antibiotic residues (Agyare et al., 2018). In contrast to conventional farming, the use of antibiotics is prohibited in organic production systems (Wierup, 2001). Therefore, alternative solutions to augment the growth and production are thus in dire need for both traditional and organic poultry farming (Diaz-Sanchez et al., 2015). Earlier studies have shown the beneficial impacts of Cv and Ts on broilers' growth rate and gut health (Agostini et al., 2012, Eevuri and Putturu, 2013, Al-Mufarrej et al., 2019, Batiha et al., 2020, Arif, 2022). Hence, the present study investigated the potential effects of the combinations of Cv powder and Ts extract supplementation on the serum biochemistry and meat quality in broilers for the first time.
4.1. Serum-biochemical profile
Serum-biochemical attributes are very good indicators of the health status of any living animal. The current study revealed no substantial difference in GLU levels among the experimental groups except on d 21 where a marked rise in GLU levels was found in the treated groups supplemented with 0.5–1% Cv powder + 2–3% Ts extract and 1% Cv powder + 3% Ts extract. Even so, none of the serum GLU levels indicated hyperglycemia as all the values were very close to the normal values (Sultana et al., 2022). However, Naeem et al. (2021) reported significantly reduced blood GLU levels in broilers in response to Ts supplementation. Chowdhury et al. (2018) reported that Cv supplementation doesn't alter the serum GLU. Mohammadi et al. (2014) stated that serum GLU drops with the increase in Cv oil concentration. The level of blood GLU remains under strict regulation due to its crucial role in maintaining body homeostasis, given that excessively high or low levels of GLU can cause detrimental impacts on the body (Ji et al., 2020).
A complete lipid profile including TCHOL, TG, HDL, and LDL is routinely used for predicting cardiovascular risks. Myocardial infarction and heart disorders are linked to elevated blood TCHOL and TG levels (Langsted et al., 2011). Elevated TCHOL level is also strongly associated with increased ischaemic heart disease related mortality (Lewington et al., 2007). In the current study, serum TCHOL remained unaffected by the treatment until d 14 but substantially decreased afterward. This finding coincides with Mahrous et al., 2017, Hussein et al., 2019, and Sehitoglu and Kaya, (2021) who also reported an increase in serum TCHOL in response to Cv supplementation. On the contrary, Naeem et al. (2021) showed that serum TCHOL substantially increases following the dietary inclusion of Ts. The hypolipidemic activity of the Cv might be linked to its capacity to mitigate free radicals produced from the body of the broilers and thus improves their health status (Hussein et al., 2019).
Dietary inclusion of Ts (Naeem et al., 2021) or Cv (Hussein et al., 2019) reduces the TG level in serum. However, these findings contradict our findings as the serum TG levels were found to be increased substantially in the treatment groups in the current study. Circulatory TG allows the liver to transport adipose tissue and blood sugar in both directions (Ji et al., 2020, Xu et al., 2022). So, the higher GLU and TG levels found in the current study might be linked to each other. The serum HDL levels tended to increase initially but decreased onward. According to earlier study reports, Cv oil or Ts supplementation leads to a significant rise in serum HDL (Hussein et al., 2019, Naeem et al., 2021). Prior research has also shown that HDL levels are inversely related to the risk of cardiovascular disease and may be used to predict risk (Rader and Hovingh, 2014). Serum LDL levels showed an inverse relationship with the concentration of Cv and Ts supplementation. LDL levels considerably decreased in the broilers supplemented with 1–1.5% Cv powder and 2–3% Ts extract. This finding coincides with the previous report where a significant reduction in LDL level was reported in response to Cv oil (0.75–1.5 ml/kg diet) supplementation (Hussein et al., 2019). On the other hand, Naeem et al. (2021) reported an increase in serum LDL while 1% Ts powder is supplemented with diet. Higher blood LDL levels are linked to a lower chance of survival since they pile up in the heart and arteries, causing severe hypertension (Kalantar-Zadeh et al., 2003). Therefore, the findings indicate an overall improvement in the lipid profile of broilers treated with Cv powder and Ts extract. Serum lipoproteins not only affect the health status of broiler but also affects the meat quality. However, there is very limited data depicting the possible linkage between serum biochemical attributes and meat quality in broiler. Higher serum lipoprotein concentration causes excessive deposition of lipid particles in the meat of broilers resulting in undesirable appearance of the meat (Alvarenga et al., 2011). Serum TCHOL has strong positive correlation with meat TG and LDL and a strong negative correlation with meat HDL whereas serum TG has strong positive correlation with meat LDL (Ozlem et al., 2020). TG is also positively correlated with intramuscular fat accumulation in broilers (Hailemariam et al., 2022). Therefore, lipoproteins in serum could be directly related with lipoproteins of chicken products and thus can be transferred into human creating human health risks (Alvarenga et al., 2011, Ozlem et al., 2020, Hailemariam et al., 2022).
Serum TP, ALB, and GLB concentrations are often used to evaluate the nutritional status of animals. In the current study, no discernible change in protein levels was observed even though their levels tended to increase in the treatment groups. However, Mahrous et al., 2017, Hussein et al., 2019 indicated an increase in serum TP levels in response to Cv treatment while Naeem et al. (2021) showed increased levels of TP in broilers fed Ts powder. Serum TP level is an indicator of the capacity of protein synthesis by the liver. During the growing period of broiler, the liver increases protein synthesis to maintain the extensive demand of amino acids for building muscle mass within a short period (Tóthová et al., 2019). So, this might be the reason behind having no significant difference in the protein levels in the broilers supplemented with different concentrations of Cv and Ts. However, the tendency to increase protein levels in the supplement groups is also an indicatorof boosting the capacity of protein synthesis by the liver (Tóthová et al., 2019). Nevertheless, a detailed investigation is necessary to picture the underlying mechanism of regulation of serum protein in broiler.
It is noted that we assessed the overall health status of broilers by investigating the glucose, lipid, and protein profile. Serum biochemical profiling is frequently used to assess the health status of broilers (Tóthová et al., 2019, Ayman et al., 2022). However, “Health” is a very broad idea and therefore, needs to be investigated further from multiple aspects like enzyme and hormone levels, antioxidant status, immunity, etc.
4.2. Breast and thigh meat yield
The ultimate goal of using any kind of growth promoter is to increase meat production. Dietary inclusion of Cv oil promotes weight gain in poultry (Hussein et al., 2019, Arif, 2022). 0.5–1.5% Ts inclusion in diet enhances feed consumption, weight gain, and meat production in poultry (Vasanthakumar et al., 2013, Naeem et al., 2021). In the present study, the meat yield increased at 0.5–1% Cv and 2–3%Ts inclusion levels. Tariq et al., 2015, Azadegan et al., 2014 also reported improved weight gain and breast meat yield while fed Cv oil with diet. Increased meat yield was reported in quails while Cv oil was supplied at 0.75 ml/kg with feed (Hussein et al., 2019). Dietary inclusion of Cv decreases amino acid breakdown and increases their absorption, thus promoting meat production (Lee and Shibamoto, 2002, Mansoub, 2011). Ts leaf extract contains several active components such as essential oils, organic acids, etc. which have anti-oxidative and antimicrobial properties which also boost the production (Kelm et al., 2000). However, meat yield substantially dropped while Cv and Ts supplemented at a higher concentration (1.5% and 4%, respectively). This indicates that the positive impacts of Cv and Ts are dependent on their inclusion level. This finding concurs with the previous study where decreased growth rate and weight gain were reported while Cv was supplemented with the broiler diet at a higher concentration (Mohammadi et al., 2014).
4.3. Physicochemical properties of meat
Nowadays, the quality of meat has become a major concern for the producers as well as meat scientists as the desired rate of meat production has already been achieved (Mir et al., 2017). Due to rising concerns about residual contamination of meat with antibiotics, steroids, or other synthetic growth promoters, organic poultry farming is gaining popularity (Islam et al., 2022a; Wireup, 2001). There is no previous study on the impacts of Ts on meat quality to the best of our knowledge while very few study reports are available regarding the dietary inclusion of Cv. Paleness, softness, and higher exudative characteristics of broiler meat are the major problems for poultry farmers (Baeza et al., 2002). Therefore, color is considered one of the most significant meat quality parameters as it directly affects the acceptance of meat by consumers (Mir et al., 2017). The data obtained in the present study showed that Cv and Ts inclusion had no impact on breast meat color which coincides with Sanwo et al. (2019). However, the addition of 1.5% Cv and 4% Ts resulted in increased L* and decreased HA of the thigh meat. Kyakma et al. (2022) showed that the inclusion of 4 g Cv/kg feed markedly increases the L* and a* values of meat without affecting the b* value. On the contrary, Suliman et al. (2020) reported that meat color coordinates remain unaffected while 1–2% Cv is supplied with the diet.
Meat pH is linked to the color and WHC of meat (Husak et al., 2008). The pHu of meat is related to the muscle glycogen content and the postmortem conversion of this glycogen into lactic acid is responsible for pH drop (Nissen and young, 2006). The current study data implies that Cv and Ts supplementation had significant effects on the meat's pH. The pHi of the breast meat substantially dropped in the groups where 0.5–1% Cv powder and 2–3% Ts were supplied. Nevertheless, an increased pHi was observed while Cv and Ts were added at a combination of 1.5% and 4%, respectively. The previous study reports on the effect of Cv on meat pH are a bit inconsistent. According to Suliman et al. (2020), the pHi decreases while 1% Cv is supplemented but it remains unaffected while supplied at a higher dose (>1%). In another study, Sanwo et al. (2019) stated that the meat pH remains unaffected in response to 4 g/kg Cv supplementation. In the present study, the breast meat pHu was substantially higher in the broilers supplemented with 1.5% Cv powder and 4% Ts extract which matches the findings of Kyakma et al. (2022). However, this finding contradicts the previous study report where a marked drop in the final pH of meat was reported (Suliman et al., 2020). Lower meat pH causes the muscle proteins to separate resulting in uneven reflection of light from the surface leading to the pale appearance of meat (Swatland, 2008, Mir et al., 2017). Therefore, the present study results imply that the meat pH is improved while Cv and Ts are supplemented at a higher concentration (1.5% Cv + 4% Ts).
The DL, CL, and WHC of meat are some major indicators of meat quality that exhibit the functional properties of meat. They have a linear correlation with the color, pH, and tenderness of meat (Mir et al., 2017). Results from the current study showed no mentionable difference in DL of the meat samples at 24 h PM which implies that Cv and Ts have no impact on DL. However, there was an increased amount of CL in the case of breast meat of the broilers supplied 1% Cv powder and 3% Ts extract. This indicates the poor ability of the breast meat to hold water during processing (Mir et al., 2017, Kyakma et al., 2022). Suliman et al. (2020) reported a lower amount of CL in the meat of broilers fed Cv seeds. The WHC of the breast meat was considerably less in the groups supplemented with 0.5–1% Cv powder and 2–3% Ts extract but a higher WHC was noticed while fed 1.5% Cv powder and 4% Ts extract. Sanwo et al. (2019) reported a higher WHC of meat of broilers fed 3 g/kg Cv which is in line with the current study finding. However, a previous report showed that dietary inclusion of Cv seeds at different levels does not affect the WHC of meat which contradicts our findings (Suliman et al., 2020). Higher WHC is an indicator of improved tenderness as well as juiciness of meat (Mir et al., 2017). Lower WHC of meat causes increased water outflow and thus resulting in the nutrient loss (Otto et al., 2004).
4.4. Muscle biology
The characteristics of myofibers are strongly linked to meat quality (Tůmová and Teimouri, 2009). The density of myofiber influences the sensory perception of meat while the CSA of the myofibers also affects the shear force value as well as the tenderness of meat (Verdiglione and Cassandro, 2013). Myofiber characteristics also have a significant role in determining meat color. The diameter and density of myofibers are negatively correlated with meat redness and lightness, respectively (Li et al., 2019).However, there is no conclusive evidence to establish a relationship between myofiber characteristics and meat quality. To the best of our knowledge, there is no previous study reporting the effect of Cv and Ts on myofiber characteristics. The results of the current study showed a substantial reduction in MFC with increased CSA of myofibers in the broilers fed 0.5–1% Cv powder and 2–3% Ts extract. The MFC has an inverse linear relationship with the CSA of myofibers whereas MFC and CSA both have a linear relationship with the muscle mass (Rehfeldt et al., 2004). In the present study, the high meat-yielding broilers had the larger CSA of myofibers. The increased yield of meat is connected to the larger diameter and density of myofibers which also justifies our findings (Li et al., 2019).
It is noted that phytobiotics contain many pharmacologically active components and are “Generally Recognized as Safe” (Grashorn, 2010). Phytobiotics contain different irritating ingredients that may be harmful to the body. The higher concentration of clove and tulsi reduces palatability, decreases feed intake, and gut motility in broilers as it contains a large amount of eugenol (Daniel et al., 2009). According to Kreydiyyeh et al. (2000), a high concentration of clove may inhibit the intestinal absorption of some nutrients. On the other hand, some studies on humans reported that eugenol,a major constituent of both clove and tulsi, has an allergic efficacy, spermicidal effect, disseminated intravascular coagulopathy, generalized seizures, and hepatotoxicity (Batiha et al., 2020).
4.5. Benefit-cost analysis
Generating a handsome amount of profit is the primary goal of every business enterprise that can be achieved by either lowering the production cost or raising the selling price or both. If the supplementation of phytobiotics to the broiler's diet doesn't make it more profitable, the producers won't use them even if they ensure healthier and organic production. However, the profit margin depends on so many factors like the availability and cost of different inputs, diseases and mortality, quantity and quality of the output, market demand, etc. (Ahammed and Rahman, 2022, Janjić et al., 2022). The current study findings showed that the profit margin (50.71 Tk./kg liver bird) notably higher in the groups supplemented with 1% Cv and 3% Ts despite having a higher production cost determined by the higher feeding cost and the costs of the phytobiotics. This is in line with the earlier studies where the supplementation of synbiotic, probiotic, and phytobiotic in broilers generated Tk. 42.87, 38.89, and 37.30, respectively (Ahammed and Rahman, 2022). The benefit-cost ratio (1.46) was the maximum for the broilers fed 1% Cv and 3% Ts. This finding also correspondence with an earlier report where a benefit-cost ratio of 1.47 was found in broilers (Jahan and Kamruzzaman, 2022). Therefore, phytobiotic supplementations like Cv and Ts had positive impacts on enhancing the economic viability of broiler production. There are not many published articles that have investigated the role of different types of phytobiotics on economic viability of broiler production. Therefore, the current study will open up numerous opportunities for further research.
5. Conclusion
The results from the current study suggest that Cv and Ts have the potential to bring about positive impacts on the broiler which is largely dependent on the concentration supplied. They have the potential to improve serum biochemistry by reducing serum TCHOL and LDL levelswhich indicates an improvement in the health status of the broilers. Supplementation of 0.5–1% Cv powder and 2–3% Ts extract increased breast and thigh meat yield which also resulted in more profit generation. On the other hand, 1.5% Cv powder and 4% Ts extract were found to be more effective in maintaining a healthy serum-biochemical profile as well as the meat quality attributes (i.e. color, pH, WHC, and myofiber characteristics). In these contexts, it may be concluded that Cv and Ts can be used in combination to improve broiler health, production, and meat quality. Nevertheless, further study is suggested to evaluate the quality of meat from the panel taste, microbiological and nutritional aspects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We are grateful to the department of “Anatomy and Histology”, BAU for providing the laboratory facilities. We are also expressing our heartfelt gratitude to the department of “Animal Science”, BAU for the research support.
Funding
This work was supported by the Ministry of Science and Technology (MoST), Bangladesh. Dr. Nasrin Sultana received the research grant (Grant No: BS-56/2021-22).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103654.
Contributor Information
Nasrin Sultana, Email: nasrin.sultana@bau.edu.bd.
Rafiqul Islam, Email: mrislam.ah@bau.edu.bd.
Sonali Bhakta, Email: sonali.dvm@bau.edu.bd.
Akash Saha John, Email: john1701131@bau.edu.bd.
Sadia Islam Sinza, Email: sadia45818@bau.edu.bd.
Md. Abul Hashem, Email: hashem.as@bau.edu.bd.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Agostini P.S., Sola-Oriol D., Nofrarías M., Barroeta A.C., Gasa J., Manzanilla E.G. Role of in-feed clove supplementation on growth performance, intestinal microbiology, and morphology in broiler chicken. Livest. Sci. 2012;147:113–118. doi: 10.1016/j.livsci.2012.04.010. [DOI] [Google Scholar]
- Agyare, C., Boamah, V.E., Zumbi, C.N., Osei, F.B., 2018. Antibiotic use in poultry production and its effects on bacterial resistance, in: Kumar, Y., (Ed.), Antimicrobial resistance—A global threat. IntechOpen, pp. 33-51. https://dx.doi.org/10.5772/intechopen.73725.
- Ahammed M., Rahman M.N. Synbiotic, probiotic and neem leaf as alternatives to antibiotic growth promoter in broiler diet. Bangladesh J. Anim. Sci. 2022;51:122–132. doi: 10.3329/bjas.v51i3.61788. [DOI] [Google Scholar]
- Al-Mufarrej S.I., Al-Baadani H.H., Fazea E.H. Effect of level of inclusion of clove (Syzygium aromaticum) powder in the diet on growth and histological changes in the intestines and livers of broiler chickens. S. Afr. J. Anim. 2019;49:166–175. doi: 10.4314/sajas.v49i1.19. [DOI] [Google Scholar]
- Alvarenga R.R., Zangeronimo M.G., Pereira L.J., Rodrigues P.B., Gomide E.M. Lipoprotein metabolism in poultry. World's Poult. Sci. J. 2011;67:431–440. doi: 10.1017/S0043933911000481. [DOI] [Google Scholar]
- Arif, M., urRehman, A., Naseer, K., Abdel-Hafez, S.H., Alminderej, F.M., El-Saadony, M.T., Abd. El-Hack, M.E., Taha, A.E., Elnesr, S.S., Salem, H.M., Alagawany, M., 2022. Effect of Aloe vera and clove powder supplementation on growth performance, carcass and blood chemistry of Japanese quails. Poult. Sci. 101, 101702. https://doi.org/10.1016/j.psj.2022.101702. [DOI] [PMC free article] [PubMed]
- Ayman U., Akter L., Islam R., Bhakta S., Rahman M.A., Islam M.R., Sultana N., Sharif A., Jahan M.R., Rahman M.S., Haque Z. Dietary chitosan oligosaccharides improves health status in broilers for safe poultry meat production. Ann. Agric. Sci. 2022;67:90–98. doi: 10.1016/j.aoas.2022.05.003. [DOI] [Google Scholar]
- Azadegan M.M., Hassanabadi A., Nasiri M.H., Kermanshahi H. Supplementation of clove essential oils and probiotic to the broiler’s diet on performance, carcass traits and blood components. Iran. J. Appl. Anim. Sci. 2014;4:117–122. [Google Scholar]
- Baéza E., Dessay C., Wacrenier N., Marche G., Listrat A. Effect of selection for improved body weight and composition on muscle and meat characteristics in Muscovy duck. Br. Poult. Sci. 2002;43:560–568. doi: 10.1080/0007166022000004471. [DOI] [PubMed] [Google Scholar]
- Batiha G.E.S., Alkazmi L.M., Wasef L.G., Beshbishy A.M., Nadwa E.H., Rashwan E.K. Syzygiumaromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10:202. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K., Kumar P., Samanta I., Pradhan S., Samanta A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018;236:39–47. doi: 10.1016/j.anifeedsci.2017.12.003. [DOI] [Google Scholar]
- Daniel A.N., Sartoretto S.M., Schmidt G., Caparroz-Assef S.M., Bersani-Amado C.A., Cuman R.K.N. Anti-inflammatory and antinociceptive activities A of eugenol essential oil in experimental animal models. Rev. Bras. Farmacogn. 2009;19:212–217. doi: 10.1590/S0102-695X2009000200006. [DOI] [Google Scholar]
- Diaz-Sanchez S., D'Souza D., Biswas D., Hanning I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015;94:1419–1430. doi: 10.3382/ps/pev014. [DOI] [PubMed] [Google Scholar]
- Eevuri T.R., Putturu R. Use of certain herbal preparations in broiler feeds-a review. Vet. World. 2013;6:172–179. doi: 10.5455/vetworld.2013.172-179. [DOI] [Google Scholar]
- Font-i-Furnols, M., Čandek-Potokar, M., Maltin, C., PrevolnikPovše, M., 2015. A handbook of reference methods for meat quality assessment. European Cooperation in Science and Technology (COST): Brussels, Belgium.
- Grashorn M.A. Use of phytobiotics in broiler nutrition–an alternative to infeed antibiotics. J. Anim. Feed Sci. 2010;19:338–347. doi: 10.22358/jafs/66297/2010. [DOI] [Google Scholar]
- Grimsley G.R., Pace C.N. Spectrophotometric determination of protein concentration. Curr. Protoc. Protein Sci. 2003;33:3–11. doi: 10.1002/0471140864.ps0301s33. [DOI] [PubMed] [Google Scholar]
- Hailemariam A., Esatu W., Abegaz S., Urge M., Assefa G., Dessie T. Serum biochemical and meat fatty acid profiles of different chicken genotypes. Open J. Anim. Sci. 2022;12:287–302. doi: 10.4236/ojas.2022.122022. [DOI] [Google Scholar]
- Husak R.L., Sebranek J.G., Bregendahl K. A survey of commercially available broilers marketed as organic, free-range, and conventional broilers for cooked meat yields, meat composition, and relative value. Poult. Sci. 2008;87:2367–2376. doi: 10.3382/ps.2007-00294. [DOI] [PubMed] [Google Scholar]
- Hasan M.N., Mostofa M., Sorwar M.G., Hasan M.T., Das K., Hossain M.N. Effects of Tulsi leaf extract on body weight gain in broiler production. Bangl. J. Vet. Med. 2016;14(1):21–25. [Google Scholar]
- Hussein M.M., Abd El-Hack M.E., Mahgoub S.A., Saadeldin I.M., Swelum A.A. Effects of clove (Syzygium aromaticum) oil on quail growth, carcass traits, blood components, meat quality, and intestinal microbiota. Poult. Sci. 2019;98:319–329. doi: 10.3382/ps/pey348. [DOI] [PubMed] [Google Scholar]
- Hussein E.O., Ahmed S.H., Abudabos A.M., Aljumaah M.R., Alkhlulaifi M.M., Nassan M.A., Suliman G.M., Naiel M.A., Swelum A.A. Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals. 2020;10:507. doi: 10.3390/ani10030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, R., Sultana, N., Alam, R., Akter, A., Anisuzzaman., Haque, Z., Khan, M.A.H.N.A., 2022b. Dose and time-dependent effects of glucocorticoid: A morphologic and morphometric study in the broiler lung. Turkish J. Vet. Anim. Sci. 46, 617-628. https://doi.org/10.55730/1300-0128.4233.
- Islam R., Sultana N., Ayman U., Islam M.R., Hashem M.A. Role of steroid growth promoter on growth performance and meat quality traits in broiler. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam R., Sultana N., Ayman U., Akter A., Afrose M., Haque Z. Morphological and morphometric adaptations of testes in broilers induced by glucocorticoid. Vet. Med. 2021;66:520–529. doi: 10.17221/38/2021-VETMED. [DOI] [Google Scholar]
- Islam R., Sultana N., Khan M.A.H.N.A. Sero-biochemical and cardiac morphological alterations in broiler chicken triggered by dietary dexamethasone. Adv. Anim. Vet. Sci. 2022;10:829–837. doi: 10.17582/journal.aavs/2022/10.4.829.837. [DOI] [Google Scholar]
- Islam R., Sultana N., Bhakta S., Haque Z., Hasan A., Siddique M.P., Islam M.R. Modulation of growth performance, gut morphometry, and cecal microbiota in broilers by clove (Syzygium aromaticum) and tulsi (Ocimum sanctum) supplementation. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan M., Kamruzzaman M. Economic assessment of commercial Turkey and broiler farming in Bangladesh: an application of binary probit model. Can. J. Bus. Inf. Stud. 2022;4:65–71. doi: 10.34104/cjbis.022.065071. [DOI] [Google Scholar]
- Janjić J., Šević-Savić K., Marković R., Šefer D., Nedić D., Đurić S., Vejnović B., Mirilović M. Influence of phytobiotics in feed on the cost-effectiveness of broiler production during fattening. Meat Tech. 2022;63:51–58. doi: 10.18485/meattech.2022.63.1.5. [DOI] [Google Scholar]
- Ji J., Tao Y., Zhang X., Pan J., Zhu X., Wang H., Du P., Zhu Y., Huang Y., Chen W. Dynamic changes of blood glucose, serum biochemical parameters and gene expression in response to exogenous insulin in Arbor Acres broilers and Silky fowls. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-63549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K., Block G., Humphreys M.H., Kopple J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- Kerth C.R. Wiley-Blackwell Publishing; USA: 2013. The science of meat quality. [Google Scholar]
- Kreydiyyeh S.I., Usta J., Copti R. Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum. Food Chem. Toxicol. 2000;38:755–762. doi: 10.1016/S0278-6915(00)00073-9. [DOI] [PubMed] [Google Scholar]
- Kyakma S.S., Tella T.K., Sanwo K.A. Some meat quality parameters of broiler chickens fed diets containing different additives. Niger. J. Anim. Prod. 2022;49:33–45. doi: 10.51791/njap.v49i2.3459. [DOI] [Google Scholar]
- Langsted A., Freiberg J.J., Tybjaerg-Hansen A., Schnohr P., Jensen G.B., Nordestgaard B.G. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J. Int. Med. 2011;270:65–75. doi: 10.1111/j.1365-2796.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- Lee K.G., Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J. Agric. Food Chem. 2002;50:4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto R., Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- Li J., Yang C., Peng H., Yin H., Wang Y., Hu Y., Yu C., Jiang X., Du H., Li Q., Liu Y. Effects of slaughter age on muscle characteristics and meat quality traits of Da-Heng meat type birds. Animals. 2019;10:69. doi: 10.3390/ani10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrous H.S., El-Far A.H., Sadek K.M., Abdel-Latif M.A. Effects of different levels of Clove Bud (Syzygium Aromaticum) dietary supplementation on immunity, antioxidant status, and performance in broiler chickens. Alex. J. Vet. Sci. 2017;54:29–39. doi: 10.5455/ajvs.272231. [DOI] [Google Scholar]
- Mansoub N.H. Comparison of effects of using nettle (Urtica dioica) and probiotic on performance and serum composition of broiler chickens. Glob. Vet. 2011;6:247–250. [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Tech. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Z., Ghazanfari S., Moradi M.A. Effect of supplementing clove essential oil to the diet on microflora population, intestinal morphology, blood parameters and performance of broilers. Eur. Poult. Sci. 2014;78:51. [Google Scholar]
- Naeem H., Naqvi S.Z.H., Hussain J., Abbas N., Hayat S., Arshad L., Ghayas A., Rehman A. Efficacy of Tulsi (Ocimum Sanctum) plant powder on health, growth and Carcass Traits of Japanese Quail (Coturnix Japonica) Braz. J. Poult. Sci. 2021;24:1–8. doi: 10.1590/1806-9061-2021-1453. [DOI] [Google Scholar]
- Nissen P.M., Young J.F. Creatine monohydrate and glucose supplementation to slow-and fast-growing chickens changes the postmortem pH in pectoralis major. Poult. Sci. 2006;85:1038–1044. doi: 10.1093/ps/85.6.1038. [DOI] [PubMed] [Google Scholar]
- OECD-FAO., 2021. OECD-FAO Agricultural Outlook 2021-2030 (Edition 2021). OECD Agriculture Statistics (database), https://doi.org/10.1787/4bde2d83-en (accessed on 16 August 2022)
- Otto G., Roehe R., Looft H., Thoelking L., Kalm E. Comparison of different methods for determination of drip loss and their relationships to meat quality and carcass characteristics in pigs. Meat Sci. 2004;68:401–409. doi: 10.1016/j.meatsci.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ozlem K., Tarkan S., Mukremin O., Akın Y., Bulent O. Changes in Serum biochemical and lipid profile, and fatty acid composition of breast meat of broiler chickens fed supplemental grape seed extract. Turk. J. Vet. Anim. Sci. 2020;44:182–190. doi: 10.3906/vet-1906-37. [DOI] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World's Poult. Sci. J. 2015;71:363–374. doi: 10.1017/S0043933915000367. [DOI] [Google Scholar]
- Rader D.J., Hovingh G.K. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- Rehfeldt, C.H.A.R.L.O.T.T.E., Fiedler, I., Stickland, N.C., 2004. Number and size of muscle fibres in relation to meat production, in: te Pas, M.F.W., Everts, M.E., Haagsman, H.P. (Eds.), Muscle development of livestock animals: Physiology, genetics and meat quality. CABI Books, CABI International, pp. 1-38. https://doi.org/10.1079/9780851998114.0001.
- Sanwo K.A., Adegoke A.V., Akinola O.S., Njoku C.P., Okolo S.O., Oladipo N.A., Oladejo A.S. Meat quality characteristics of improved indigenous chickens (FUNAAB-ALPHA) fed turmeric (Curcuma longa) or clove (Syzygium aromaticum) as feed additives. J. Agric. Sci. Environ. 2019;19:102–112. doi: 10.51406/jagse.v19i1.2018. [DOI] [Google Scholar]
- Şehitoğlu M., Kaya H. The effect of Clove oil supplementation in laying hen diets on performance, egg quality, some blood parameters, and yolk TBARS. Turk. J. Agric. Food. Sci. Tech. 2021;9:2213–2218. doi: 10.24925/turjaf.v9i12.2213-2218.4482. [DOI] [Google Scholar]
- Suliman G.M., Alowaimer A.N., Al-Mufarrej S.I., Hussein E.O., Fazea E.H., Naiel M.A., Alhotan R.A., Swelum A.A. The effects of clove seed (Syzygium aromaticum) dietary administration on carcass characteristics, meat quality, and sensory attributes of broiler chickens. Poult. Sci. 2020;100 doi: 10.1016/j.psj.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N., Islam R., Akter A., Ayman U., Bhakta S., Rony S.A., Nahar A., Alam R. Biochemical and morphological attributes of broiler kidney in response to dietary glucocorticoid, dexamethasone. Saudi J. Biol. Sci. 2021;28:6721–6729. doi: 10.1016/j.sjbs.2021.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N., Islam R., Das R.R., Khan M.A.H.N.A., Rafiq K., Haque Z. Steroid growth promoter modified glucose profile and liver morphology in broiler by altering the localization and expression pattern of hepatic glucocorticoid receptors. Res. Vet. Sci. 2022;152:277–288. doi: 10.1016/j.rvsc.2022.08.024. [DOI] [PubMed] [Google Scholar]
- Swatland H.J. How pH causes paleness or darkness in chicken breast meat. Meat Sci. 2008;80:396–400. doi: 10.1016/j.meatsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Tariq H., Rao P.R., Raghuvanshi R.S., Mondal B.C., Singh S. Effect of Aloe vera and clove powder supplementation on carcass characteristics, composition and serum enzymes of Japanese quails. Vet. World. 2015;8:664–668. doi: 10.14202/vetworld.2015.664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóthová C., Sesztáková E., Bielik B., Nagy O. Changes of total protein and protein fractions in broiler chickens during the fattening period. Vet. World. 2019;12:598–604. doi: 10.14202/vetworld.2019.598-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tůmová E., Teimouri A. Chicken muscle fibres characteristics and meat quality: a review. Sci. Agric. Bohem. 2009;40:253–258. [Google Scholar]
- Vasanthakumar P., Sasikumar P., Pangayarselvi B., Chandrasekaran D., Doraisamy K.A., Senthilkumar S., Purushothaman M.R. Performance of broiler chicken fed Tulsi leaf powder and leaf extract supplemented diets during summer to alleviate heat stress. Indian J. Anim. Sci. 2013;83:930–931. [Google Scholar]
- Verdiglione R., Cassandro M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013;92:2433–2437. doi: 10.3382/ps.2013-03013. [DOI] [PubMed] [Google Scholar]
- Wierup M. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb. Drug Resist. 2001;7:183–190. doi: 10.1089/10766290152045066. [DOI] [PubMed] [Google Scholar]
- Xu W., Song Z., Wang W., Li X., Yan P., Shi T., Fu C., Liu X. Effects of in ovo feeding of t10, c12-conjugated linoleic acid on hepatic lipid metabolism and subcutaneous adipose tissue deposition in newly hatched broiler chicks. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.