Highlights
-
•
SCA17 should be included in the differential diagnoses of PMEs.
-
•
SCA17 is characterized by cerebellar features, myoclonic epilepsy, cognitive decline, psychiatric features, and chorea.
-
•
Subtle clinical signs like chorea can provide additional diagnostic clues to SCA17(HDL4), a Huntington disease phenocopy.
Dear Editor,
Progressive myoclonic epilepsies (PMEs) are a group of uncommon clinically and genetically heterogenous disorders characterized by myoclonic epilepsies in combination with a relentlessly progressive neurologic decline. We reported a spinocerebellar ataxia type 17 (SCA17) patient presenting with PME.
A 39-year-old female with no significant past medical history presented to our clinic for evaluation of progressive cerebellar features including ataxia and slurred scanning speech, along with cognitive decline, delusional psychosis, and myoclonic epilepsy. Her initial presentation was at the age of 31, when she gradually developed scanning speech, difficulty walking and imbalance. At the age of 33, she had recurrent episodes of generalized myoclonic jerks with preserved consciousness, and also unprovoked generalized tonic-clonic seizures. Her seizures were controlled with valproic acid. At the age of 36, she developed psychosis including delusion, visual and auditory hallucinations requiring antipsychotic medications. Her psychiatric symptoms were refractory to multiple antipsychotics, and she developed drug-induced parkinsonism. Electroconvulsive therapy (ECT) was then introduced, and in combination with antipsychotic medications, resulted in good control of psychiatric symptoms. Family history on both paternal and maternal sides, as well as her brother and his child were negative for epilepsy, psychiatric diseases, movement disorders, and neurodegenerative diseases. She had no children. Examination at age 39 demonstrated cerebellar features including slurred scanning speech, head and truncal titubation, prominent dysmetria of both arms and legs, and markedly wide-based gait. Oculomotor examination showed macrosaccadic oscillations, saccadic pursuits, oculomotor apraxia in both horizontal and vertical gazes, as well as hypometric saccades. Mild intermittent chorea of the left foot was noted (Supplementary Material). Ten years after the clinical onset, she had progressive cognitive impairment, gait deterioration, and became wheelchair-dependent.
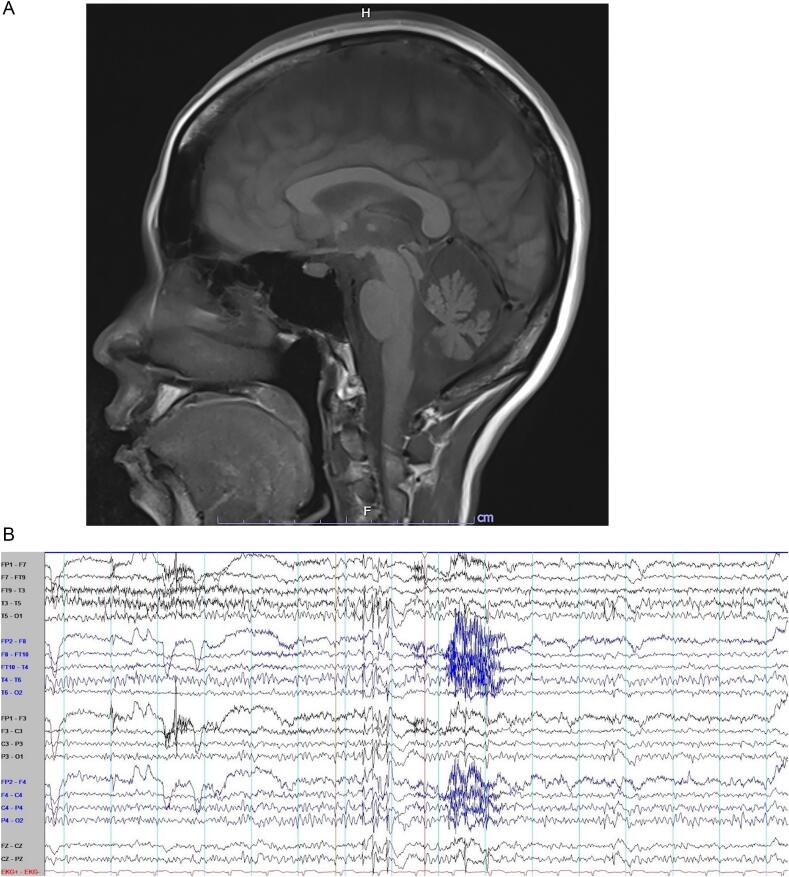
Brain MRI demonstrated cerebellar atrophy (Fig. 1A). Electroencephalography (EEG) showed normal awake EEG background. Interictal EEG showed generalized 2–2.5 Hz polyspike-and-wave complexes with maximum negativity at bifrontal electrodes (Fig. 1B). Indirect ophthalmoscopic examination to search for cherry red spot, serum ceruloplasmin, 24-h urine copper and alpha-fetoprotein were all unremarkable. Genetic testing revealed CAG/CAA repeat expansion (53 repeats in the pathological allele) in the TATA-box binding protein (TBP) gene, confirming the diagnosis of SCA17. Pathogenic mitochondrial DNA mutation associated with myoclonic epilepsy with ragged red fibers (MERRF) was not found.
Fig. 1.
Brain MRI and electroencephalogram of our patient. A. The mid-sagittal view of brain MRI demonstrated cerebellar atrophy. B. Electroencephalography (EEG) showed normal awake EEG background. Interictal EEG demonstrated generalized 2–2.5 Hz polyspike-and-wave complexes with maximum negativity at bifrontal electrodes.
The constellation of her clinical features including progressive cognitive decline, myoclonic epilepsies and cerebellar features are compatible with progressive myoclonic epilepsies (PME) or progressive myoclonic ataxia (PMA, aka. Ramsay Hunt syndrome). It has been well-recognized that PME and PMA have the same list of differential diagnoses, and cerebellar involvement is common in PME. The differential diagnoses then include myoclonic epilepsy with ragged red fibers (MERRF), dentatorubral-pallidoluysian atrophy (DRPLA), sialidosis, and neuronal ceroid lipofuscinosis, among others. The onset at age 31 is unusual for Unverricht-Lundborg disease and Lafora body disease which typically have earlier ages at onset. DRPLA is uncommon in Thai population. There were no cherry red spot to suggest sialidosis, and no mitochondrial mutation supporting the diagnosis of MERRF.
Approaching from another perspective of her clinical features which is cerebellar features, SCAs are also included in the differential diagnosis. Common SCAs in Thai population include SCA1, SCA2, SCA3, SCA6 and SCA17 [[1], [2], [3]]. Given associated cognitive decline and psychiatric features, SCA17 was one of our top differential diagnoses, which was then confirmed by the genetic testing. In retrospect, the patient also had subtle chorea involving her left foot on physical examination. SCA17 is known to be one of the Huntington disease phenocopies, hence named Huntington disease-like 4 (HDL4). Oculomotor apraxia is a feature that can be seen in ataxia-telangiectasia (AT) and ataxia with oculomotor apraxia, the most common of which are ataxia with oculomotor apraxia types 1 (AOA1) and 2 (AOA2). Normal alpha-fetoprotein argues against AT and AOA2. Genetic testing for the APTX mutations responsible for AOA1 was not performed in our case. To our knowledge, oculomotor apraxia has not been reported in SCA17 as well.
SCA17 is a rare neurodegenerative disease causing by CAG/CAA repeat expansions in the TATA box-binding protein (TBP) gene. The phenotypes of SCA17 are complex and variable, and in some cases overlap with that of Huntington disease (HD) or Parkinson's disease (PD) [4]. Phenotypic expressions of SCA17 often correlate with the length of the CAG/CAA expansion, with HD and PD phenotypes associated with less than 50 repeats. The known clinical features of SCA17 include progressive cerebellar ataxia, psychiatric abnormalities, and dementia. Seizures have been reported in SCA17. To our knowledge, PMEs have never been reported. Given its rarity, EEG data in SCA17 patients in the literature have been limited [5]. Nocturnal frontal lobe epilepsy was reported in a patient with SCA17 [6].
With regard to SCAs in general, epilepsy is common in some types such as SCA10 [7]. However, in common SCAs found in Thailand including SCA1, SCA2, SCA3, SCA6, and SCA17, epilepsy has not been reported [[1], [2], [3]]. The descriptions of seizure semiology among SCAs are mainly restricted to generalized tonic-clonic seizures, but data on further specification on seizure semiology and EEG findings are limited.5 7 We present a case with unique clinical presentation compatible with the definition of PME in the SCA17 patient, broadening the phenotypic spectrum of SCA17. The lack of family history of neurological disorders may be explained by various reasons including de novo mutation, anticipation associated with triplet repeat expansions, or unrecognized diagnosis in other family members. This issue cannot be certain since DNA of the family members is not available for analysis. Diffuse alterations of cortical and subcortical networks initiated by pathogenetic insults may play an important role in epileptogenesis and neuropsychiatric symptoms. It has been well-recognized that cortical myoclonus can occur even when the primary pathology is in the cerebellum such as in celiac disease [[8], [9], [10]]. PME in our patient may support the connections between the cerebellum and cerebral cortices where myoclonic epilepsy is originated from. In conclusion, SCA17 should be included in the differential diagnosis of adult-onset PMEs. Important clinical clues leading to this diagnosis include progressive cerebellar ataxia, psychiatric features, dementia and chorea.
Consent statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images and video. A copy of the written consent is available for review.
Ethical statement
The patient that appears on the video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
Funding
The work has not been externally funded.
Contributors
The acquisition, analysis and interpretation of data by AB, PT, TP. Writing the first draft by AB. Revision of the manuscript for intellectual content by AB, PT, TP. Approval of the final manuscript: AB, PT, TP.
The following are the supplementary data related to this article.
Segment 1 displays examination in the emergency room. There are generalized low-amplitude myoclonic jerks involving bilateral arms, legs and trunk.
Segment 2 displays examination in the office. Mild intermittent chorea of the left foot is noted. There is mild-to-moderate head and truncal titubation (which makes identification of co-existing myoclonic jerks difficult), as well as prominent slurred and scanning speech. Ocular motor examination demonstrates macrosaccadic oscillations, saccadic pursuits, oculomotor apraxia (i.e., marked delay in saccade initiation and the patient has to thrust her head or blink her eyes in order to initiate saccades) in both horizontal and vertical gazes, as well as hypometric saccades. There is marked overshoot dysmetria of both arms on finger-to-nose-to-finger and finger following tests. She has markedly wide-based gait.
Declaration of Competing Interest
The authors declare no relevant conflict of interest.
Acknowledgment
We are grateful to Thananan Thammongkolchai, MD for assistance with video editing.
Contributor Information
Apisit Boongird, Email: apisit.bon@mahidol.ac.th.
Pichet Termsarasab, Email: pichet.ter@mahidol.ac.th.
Teeratorn Pulkes, Email: teeratorn.pul@mahidol.ac.th.
References
- 1.Choubtum L., Witoonpanich P., Hanchaiphiboolkul S., et al. Analysis of SCA8, SCA10, SCA12, SCA17 and SCA19 in patients with unknown spinocerebellar ataxia: a Thai multicentre study. BMC Neurol. 2015;15:166. doi: 10.1186/s12883-015-0425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choubtum L., Witoonpanich P., Kulkantrakorn K., et al. Trinucleotide repeat expansion of TATA-binding protein gene associated with Parkinson’s disease: a Thai multicenter study. Parkinsonism Relat Disord. 2016;28:146–149. doi: 10.1016/j.parkreldis.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Boonkongchuen P., Pongpakdee S., Jindahra P., et al. Clinical analysis of adult-onset spinocerebellar ataxias in Thailand. BMC Neurol. 2014;14:75. doi: 10.1186/1471-2377-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoshima Y., Takahashi H. Spinocerebellar Ataxia type 17 (SCA17) Adv Exp Med Biol. 2018;1049:219–231. doi: 10.1007/978-3-319-71779-1_10. [DOI] [PubMed] [Google Scholar]
- 5.Liang L., Chen T., Wu Y. The electrophysiology of spinocerebellar ataxias. Neurophysiol Clin. 2016;46(1):27–34. doi: 10.1016/j.neucli.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Belluzzo M., Musho-Ilbeh S., Monti F., et al. A case of nocturnal frontal lobe epilepsy in a patient with spinocerebellar ataxia type 17. Seizure. 2012;21(10):805–806. doi: 10.1016/j.seizure.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan R., Yau W.Y., O’Connor E., et al. Spinocerebellar ataxia: an update. J Neurol. 2019;266(2):533–544. doi: 10.1007/s00415-018-9076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia K.P., Brown P., Gregory R., et al. Progressive myoclonic ataxia associated with coeliac disease. The myoclonus is of cortical origin, but the pathology is in the cerebellum. Brain. 1995;118(Pt 5):1087–1093. doi: 10.1093/brain/118.5.1087. (published Online First: 1995/10/01) [DOI] [PubMed] [Google Scholar]
- 9.Ganos C., Kassavetis P., Erro R., et al. The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord. 2014;29(4):437–443. doi: 10.1002/mds.25867. [DOI] [PubMed] [Google Scholar]
- 10.Tijssen M.A., Thom M., Ellison D.W., et al. Cortical myoclonus and cerebellar pathology. Neurology. 2000;54(6):1350–1356. doi: 10.1212/wnl.54.6.1350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Segment 1 displays examination in the emergency room. There are generalized low-amplitude myoclonic jerks involving bilateral arms, legs and trunk.
Segment 2 displays examination in the office. Mild intermittent chorea of the left foot is noted. There is mild-to-moderate head and truncal titubation (which makes identification of co-existing myoclonic jerks difficult), as well as prominent slurred and scanning speech. Ocular motor examination demonstrates macrosaccadic oscillations, saccadic pursuits, oculomotor apraxia (i.e., marked delay in saccade initiation and the patient has to thrust her head or blink her eyes in order to initiate saccades) in both horizontal and vertical gazes, as well as hypometric saccades. There is marked overshoot dysmetria of both arms on finger-to-nose-to-finger and finger following tests. She has markedly wide-based gait.