Abstract
A rabbit model of coccidioidal meningitis was used to compare the therapeutic efficacies of terbinafine (TBF) and fluconazole (FCZ). Hydrocortisone acetate-treated New Zealand White male rabbits were infected intracisternally with either 2.2 × 104 or 6.4 × 104 Coccidioides immitis arthroconidia. Oral treatment with polyethylene glycol 200 (PEG) twice daily (n = 8), TBF twice daily (n = 9; 200 mg/kg of body weight/day), or FCZ once daily (n = 8; 80 mg/kg/day) began on day 5 and continued for 21 days. Mean survival times were 20, 24, and 32 days for rabbits treated with PEG, TBF, and FCZ, respectively. All of the FCZ-treated animals (100%; P = 0.003), 56% of the TBF-treated animals (P = 0.4), and 25% of the PEG-treated animals survived the length of the study. Both FCZ and TBF were effective at reducing the incidence of paresis. Only FCZ was effective at reducing most neurological and systemic signs. FCZ treatments resulted in lower cerebrospinal fluid (CSF) protein concentrations and leukocyte counts and faster clearing of CSF fungal cultures compared with those for PEG-treated controls, but TBF treatments had no significant effect on these parameters. Neither drug affected CSF glucose levels. Mean serum TBF levels by bioassay were within the range of 3.5 to 6.2 μg/ml at 1, 2, and 4 h postdosing and 0.35 to 7.0 μg/ml at 14 h postdosing. No TBF was detected in CSF. Mean FCZ levels (24 to 25.5 h postdosing) by bioassay were 16.4 to 19.2 and 13.5 to 19.2 μg/ml in serum and CSF, respectively. The reduction in the numbers of CFU in the spinal cord and brain was over 100-fold (P = 0.0005) in FCZ-treated animals and 2-fold (P ≤ 0.2) in TBF-treated animals compared with those in PEG-treated animals. Histopathologic severity (semiquantitative scoring system) was significantly attenuated by FCZ treatment (P = 0.05) and was slightly attenuated by TBF treatment compared with that for the controls. In conclusion, TBF appeared to have a slight effect on survival, histology, and reduction of the numbers of CFU in tissue; however, these effects were not significant. FCZ was effective at controlling coccidioidal meningitis.
Coccidioidal meningitis is one of the most severe and devastating fungal diseases. Currently, therapy with an azole will control this meningitis in 80% of patients. This therapy is usually considered lifelong, and a cure is unusual (2). Patients receiving azole therapy are still at risk for other complications associated with coccidioidal meningitis, such as stroke, vasculitis, hydrocephalus, and encephalitis (17).
Terbinafine (TBF) is a naftifine analog with a mechanism of action distinct among antimycotics. TBF inhibits fungal growth by inhibiting the fungal enzyme squalene epoxidase, a key enzyme in the synthesis of the fungal sterol ergosterol (1). TBF is fungicidal in vitro against a broad range of fungi (9). The drug is highly lipophilic and distributes widely into adipose- and keratin-rich tissues (1, 6). Tissue TBF concentrations are much higher than plasma TBF concentrations, and it is highly bound to plasma components (11). This protein binding along with lipoprotein binding and rapid drug metabolism by rodents has been suggested as the reason for the lack of efficacy of TBF in experimental models of systemic fungal infection. However, a few reports have suggested that TBF may be effective for the treatment of systemic fungal infections (12, 16).
This study evaluated the efficacy of TBF in a rabbit model of coccidioidal meningitis in comparison with that of a conventional treatment, fluconazole (FCZ).
MATERIALS AND METHODS
Animals and study design.
Male New Zealand White rabbits (weight, 3 to 4 kg) obtained from Myrtle's Rabbitry (Thompson Station, Tenn.) were used in the study. To avoid potential complications with opportunistic pathogens in the rabbit, these rabbits were specific pathogen free and were free of Bordetella. The study was done in two parts. Each part used 12 to 13 rabbits, with 4 or 5 rabbits per treatment group.
Immune suppression.
At 1 day prior to infection, on the day of infection, and for 3 consecutive days following infection, all rabbits received an intramuscular injection of hydrocortisone acetate (Steris Laboratory, Inc., Subsidiary of Schein Pharmaceutical, Inc., Florham Park, N.J.) at 2 mg/kg of body weight.
Test organism.
Coccidioides immitis strain Silveira (ATCC 28868) was used in the study with appropriate biohazard containment. A suspension of arthroconidia of C. immitis was prepared as described previously (18). The suspension was stored at 4°C.
Susceptibility testing.
Susceptibility testing in vitro was performed as described previously for arthroconidia (14) and endospores (5).
Infection.
Prior to infection, the stock suspension of arthroconidia was quantitated by serial plating on Mycosel agar plates (Becton Dickinson and Co., Cockeysville, Md.). The stock suspension was further diluted to make an inoculum containing 2.5 × 105 CFU/ml. Each rabbit was anesthetized and infected intracisternally as described previously (18). Rabbits were infected with either 2.2 × 104 or 6.4 × 104 arthroconidia in part 1 and part 2, respectively. Up to 0.6 ml of cerebrospinal fluid (CSF) was removed by gentle aspiration, and then 0.2 ml of the inoculum was injected and flushed with 0.6 ml of sterile saline. Blood was collected from either the marginal ear vein or the central ear artery. Each rabbit was given yohimbine at 0.2 mg/kg intravenously to aid recovery from anesthesia.
Postinoculation monitoring.
The rabbits were monitored twice daily for evidence of systemic, neurological, or discomfort sequelae. Evaluations consisted of food and water consumption, coat appearance, respiration, vocalization, head and body shake, posture, head and body cants, mobility, paresis or paralysis, awareness, reflex, pain sensation, convulsion, agitation, lethargy, other behavior changes, weight, and temperature. Animals exhibiting signs of discomfort were given buprenorphine subcutaneously at 0.008 mg/kg twice daily. A high-calorie diet supplement (Vitacal; Burns Veterinary Supply, Inc., Rockville Centre, N.Y.) and fluids (Lactated Ringer's Injection, USP; McGaw, Inc. Irvine, Calif.) were given as needed to stimulate appetite and prevent dehydration. Rabbits were euthanatized (as described in the euthanasia and tissue collection section) if they experienced undue discomfort, paralysis, convulsions, stupor, or prolonged anorexia or dehydration.
Treatment.
Half the rabbits were studied in each part of the study. Starting 5 days postinfection, the animals were divided into three groups and were given one of three oral treatments with a 3-ml syringe with an attached animal feeding needle (18 gauge by 3 in): (i) placebo (polyethylene glycol 200 [PEG]) twice daily, FCZ at 80 mg/kg once daily, or (iii) TBF at 100 mg/kg twice daily. The drugs were suspended in PEG at concentrations of 120 and 150 mg/ml for FCZ and TBF, respectively. The drug dosage was adjusted daily on the basis of the rabbit's weight. The animals were treated for 21 days. FCZ and TBF powders were provided by Pfizer (Groton, Conn.) and Novartis Pharma AG (Basel, Switzerland), respectively. No differences in any results were found between the two parts of the study, and the results were combined.
Collection of CSF and serum.
Every 7 to 12 days, rabbits were anesthetized to a surgical plane of anesthesia with isoflurane, and CSF and serum were collected as described previously (18).
Euthanasia and tissue collection.
Rabbits that required euthanasia or that survived 7 or 8 days after the last treatment (32 or 33 days postinfection) were anesthetized, CSF and serum were collected, and the rabbits were euthanatized by intravenous injection of a concentrated pentobarbital solution (Euthasol; Delmarvia Laboratories, Inc., Bristol, Tenn.). After euthanasia, the brains and proximal spinal cords were collected. The left half of the brain including the cerebellum and upper cervical cord was transected and placed into 10% buffered formalin for histopathologic study. The right half of the specimen was transected into the brain (cerebrum, cerebellum, pons, and medulla) and proximal spinal cord (approximately 1.5 cm) and was processed separately for quantitative fungal culture as described previously (18).
Histopathology scoring.
The central nervous system was sectioned in a paraffin block containing the cerebrum, brain stem, midbrain, cerebellum, and upper cervical cord. Sections were stained with hematoxylin-eosin and periodic acid-Schiff. The meningeal inflammation was scored on a scale of 0 to 6 (18). A score of 0 was given to normal tissue, and a score of 6 was given if severe inflammation was present. Scoring was done without knowledge of the treatment group or the clinical status of the animal.
CSF protein and glucose concentrations and fungal culture.
CSF protein and glucose concentrations were determined with a Syncron CX system with the microprotein (M-TP) and glucose (GLU3) analysis kits (Beckman Instruments, Inc., Brea, Calif.), respectively. Total leukocyte (WBC) counts in the CSF were determined by counting the cells in the freshly obtained CSF with a hemacytometer. The fungi in CSF cultures were quantitated by pelleting the freshly obtained CSF by centrifugation. The pellet was resuspended in 100 to 200 μl of phosphate-buffered saline and plated on Mycosel agar. The detectable level was less than 4 CFU/ml of CSF.
CSF and serum drug levels.
Data are presented as means ± standard deviations. The drug concentrations in the CSF and serum of FCZ- and TBF-treated animals were determined by bioassay as described previously (3, 4, 10, 15). Trichophyton mentagrophytes was used as the indicator organism for TBF. Lower detection limits in both the serum and CSF were 2.0 and 0.31 μg/ml for FCZ and TBF, respectively.
CSF and serum antibody.
Titers against coccidioidal antigen were determined by quantitative immunodiffusion (8).
Statistical analyses.
Data are presented as means ± standard deviations. GB-STAT for Microsoft Windows (version 6.0; Dynamic Microsystems, Inc., Silver Spring, Md.) was used in all statistical analyses with the exception of the survival analysis. Survival data were analyzed with Statview for Macintosh (version 5; SAS Institute, Inc., Cary, N.C.). Confidence (95%) intervals of the log10 geometric means for the culture data were compared, and a Kruskal-Wallis one-way analysis of variance was used to detect differences among the groups. In addition, a Mann-Whitney U test was used to compare each group.
The Student t test was used to compare means of WBC counts in the CSF, body fluid drug levels, and protein and glucose concentrations.
Histological scores were analyzed by the Student t test or analysis of variance by the Tukey-Kramer procedure or by Dunn's method with the Bonferroni correction for multiple comparisons.
A Fisher exact test was used to compare survival between treatment groups at day 32. A Kaplan-Meier survival analysis followed by a treatment group comparison by the Breslow-Gehan-Wilcoxon test was used to compare prolongation of survival. A P value of ≤0.05 was considered significant for all tests.
RESULTS
In vitro susceptibility.
The activities of TBF and FCZ against C. immitis strain Silveira were tested in vitro by the broth macrodilution method. TBF and FCZ MICs for the mycelial form were 0.3 and 6.25 μg/ml, respectively. The TBF MIC for the parasitic form (endospores) of the fungus was 0.6 μg/ml.
Survival.
Overall, two of eight PEG-treated rabbits survived to the end of the study, whereas five of nine (P = 0.2 by the Fisher exact test) and eight of eight (P = 0.004) TBF- and FCZ-treated rabbits survived, respectively. One designated additional TBF-treated animal was used for drug and tissue studies only and was not included in the survival study. Survival was not significantly affected by TBF treatment, and there was only a suggestion of efficacy. FCZ treatment was borderline superior compared to TBF treatment (P = 0.053).
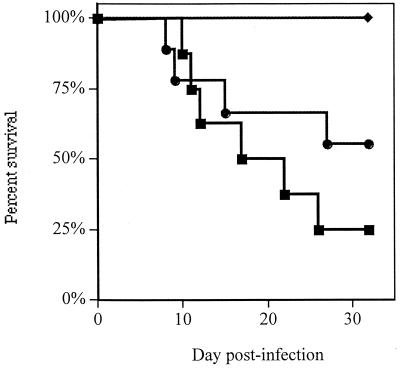
When time to death was analyzed by the Kaplan-Meier test (Fig. 1), treatment with FCZ prolonged survival over the survival times after both PEG and TBF treatments (P ≤ 0.04). TBF treatment did not prolong survival compared to the survival time after the control PEG treatment (P = 0.4). Mean survival times were 20, 32, and 24 days for PEG-, FCZ-, and TBF-treated animals, respectively.
FIG. 1.
Survival of rabbits infected intracisternally with C. immitis arthroconidia. Animals were treated orally with either PEG twice daily (n = 8) (■), FCZ at 80 mg/kg once daily (n = 8) (⧫), or TBF at 100 mg/kg twice daily (n = 9) (●) starting 5 days after infection.
Fungal tissue burden.
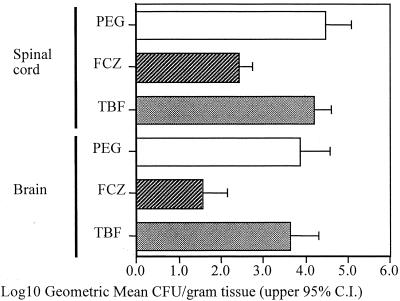
The same nonsignificant suggestion of efficacy seen from the survival of TBF-treated animals was also seen in the reduction of C. immitis counts in the spinal cords and brains (Fig. 2). A twofold reduction in the numbers of CFU of C. immitis in both the spinal cords and brains of TBF-treated animals was seen compared to the numbers in the PEG-treated animals (P ≤ 0.19). FCZ-treated animals showed over a 100-fold reduction in the numbers of CFU in both tissues compared to the numbers in both tissues of PEG-treated animals (P ≤ 0.0005). FCZ treatment was better than TBF treatment (P ≤ 0.0004). None of the tissues from any treatment group were free of detectable infection after 3 weeks of treatment.
FIG. 2.
Log10 geometric mean numbers of C. immitis CFU recovered from spinal cord and brains. C.I., confidence interval.
Histopathology.
Histologic disease scores were 5.75 ± 0.70, 4.24 ± 1.58, and 5.56 ± 1.01 for PEG-, FCZ-, and TBF-treated animals, respectively. Nearly all PEG-treated animals had severe meningeal inflammation. FCZ-treated animals had less central nervous system inflammation than PEG-treated animals (P < 0.05) but not TBF-treated animals. Endarteritis was noted in four of eight PEG-treated animals and in four of eight and seven of nine animals treated with FCZ and TBF, respectively; ischemia and/or infarcts were noted in seven, six, and five animals treated with PEG, TBF, and FCZ, respectively.
CSF WBC concentrations.
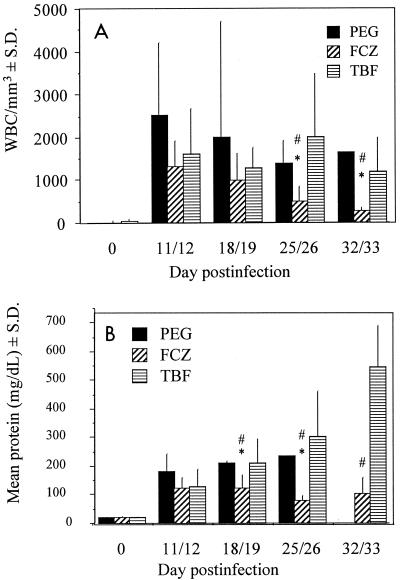
Before infection, rabbits had few WBCs in their CSF (0, 16 ± 44, and 28 ± 55 WBCs/mm3 in the PEG-, FCZ-, and TBF-treated groups, respectively). Only three animals in all the groups had any WBCs in their CSF. This was most likely related to the trauma of initial puncture. After infection, mean WBC counts increased dramatically (range, 1,297 to 2,522 WBCs/mm3) and did not return to baseline (day 0) levels for any of the treatment groups (P ≤ 0.05) throughout the study (Fig. 3A). WBC counts for TBF-treated animals were not different from those for PEG-treated animals throughout the study. FCZ caused a reduction in WBC counts on days 25 to 26 and 32 to 33 compared to those for PEG-treated (P ≤ 0.01) and TBF-treated (P ≤ 0.02) animals sampled on the same days. FCZ treatment reduced WBC counts on days 25 to 26 and 32 to 33 compared to those on days 11 to 12 (P ≤ 0.005) and 32 to 33 compared to those on days 18 to 19 (P = 0.008).
FIG. 3.
(A) WBC counts in CSF. The number of animals sampled at each of the time points shown were eight, eight, four, three, and one, respectively, for PEG-treated animals; nine, seven, six, six, and five, respectively, for TBF-treated animals; and eight at all times for FCZ-treated animals. ∗, P ≤ 0.01 for difference from PEG-treated animals within same sampling period; #, P ≤ 0.02 for difference from TBF-treated animals within same sampling period. (B) CSF protein concentrations. The number of animals sampled at each of the time points shown were seven, seven, eight, six, and eight, respectively, for FCZ-treated animals; seven, seven, six, five, and four, respectively, for TBF-treated animals; and eight, five, three, one, and zero, respectively, for PEG-treated animals. ∗, P ≤ 0.04 for difference from PEG-treated animals within same sampling period; #, P ≤ 0.03 for difference from TBF-treated animals within same sampling period.
CSF glucose concentrations.
CSF glucose concentrations were similar for all treatment groups throughout the study. Glucose concentrations decreased from 104.1 ± 12.7, 96.6 ± 9.9, and 103.6 ± 9.2 mg/dl before infection to 58.5 ± 15.9, 51.0 ± 6.7, and 50.3 ± 4.8 mg/dl for PEG-, FCZ-, and TBF-treated animals, respectively, 11 to 12 days after infection with C. immitis. This decline was followed by a slow rebound for all groups and was not dependent upon the animal's response or lack of response to therapy. At the end of the study, mean glucose levels in the various groups were between 76.0 and 80.6 mg/dl, which was still less than the baseline levels before the infection (P ≤ 0.01).
CSF protein concentrations.
CSF protein concentrations before infection were 21.5 ± 2.7, 20.8 ± 4.8, and 21.7 ± 3.0 mg/dl for PEG-, FCZ-, and TBF-treated animals, respectively (Fig. 3B). By 11 to 12 days postinfection, protein levels increased at least fivefold for all treatment groups. While CSF protein concentrations in PEG- and TBF-treated animals continued to increase, FCZ-treated animals showed a decline in CSF protein concentration 18 to 19 days postinfection, and the concentration continued to decrease on day 25 to 26 postinfection compared to those for both PEG- and TBF-treated animals (P ≤ 0.04). Although the protein concentrations for FCZ-treated animals decreased with time, they did not return to the levels found before infection (P = 0.02). Not enough CSF from the remaining PEG-treated rabbits was available on days 32 to 33 postinfection to obtain protein concentrations.
CFU in CSF.
More than 50% of the CSF specimens collected from rabbits treated with PEG or TBF were positive for C. immitis on culture on days 11 to 12 postinfection (Table 1). Although not statistically significant, the CSF of fewer FCZ-treated rabbits (13%) tested positive for C. immitis for this early sampling period. The number of positive CSF cultures decreased for PEG- and TBF-treated animals on days 18 to 19. None of the rabbits had culture-positive CSF after days 25 to 26 postinfection.
TABLE 1.
Fungal CFU in cultures of CSF
| Treatment | No. of cultures positive/total no. of cultures on the following days postinfection:
|
|||
|---|---|---|---|---|
| 11–12 | 18–19 | 25–26 | 32–33 | |
| PEG | 5/8 | 1/5 | 0/4 | 0/1 |
| FCZ | 1/8 | 1/8 | 0/8 | 0/8 |
| TBF | 5/7 | 2/7 | 0/7 | 0/5 |
Antibody titers.
Antibody was present in the serum of only one animal (in the PEG-treated group) at days 8 to 11. On days 15 to 18, about half the animals in each group were seropositive (five of eight, two of four, and four of seven in the FCZ, PEG, and TBF groups, respectively), with all titers being <1:4 except in one PEG-treated animal, which had an immunoglobulin G (IgG) titer of 1:64 and an IgM titer of 1:4. Nine animals were IgG seropositive and four animals were IgM seropositive. On day 25, only two animals were then seronegative (one in the FCZ treatment group and one in the TBF treatment group); however, only three animals (one in each group) had titers of ≥1:16 for IgG, and only three animals were IgM seropositive. By days 27 to 32 only three animals, all in the FCZ group, were seronegative. In the latter group, of the five seropositive animals, four had titers of <1:16 and only one was IgM seropositive. In contrast, all surviving PEG- and TBF-treated animals were seropositive, with all but one animal having titers of ≥1:16, and IgM seropositivity persisted in more than half of them. Thus, serum antibody titers suggest a responsiveness to FCZ.
No animals had antibody in their CSF on days 8 to 11. On days 15 to 18, only two animals (in the FCZ group) were positive (IgG). However, by day 25, all PEG-treated animals and half the FCZ- and TBF-treated animals had antibody titers (IgG), which were low (<1:8); one TBF-treated animal, however, had a titer of 1:8 to 1:16. On days 27 to 32, all PEG- and TBF-treated animals were positive, with titers of 1:4 to 1:32 (IgG), whereas four of nine FCZ-animals remained seronegative. The only IgM titer (positive undiluted only) in any animal on any day was detected in the TBF-treated group on day 32. Thus, the CSF IgG antibody titers also reflected a more favorable overall response to FCZ.
Drug levels.
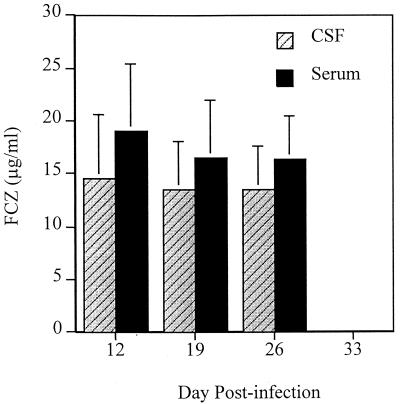
FCZ levels in the serum and CSF at 24 to 25.5 h postdosing were well above the MIC for the C. immitis strain used in the study (Fig. 4). FCZ levels were generally 15 to 20% higher in serum than in CSF.
FIG. 4.
FCZ concentrations measured in the CSF and serum between 24 and 25.5 h postdosing. For assays with serum, eight animals were sampled at each time, and for assays with CSF, seven animals were sampled at day 26 and eight animals were sampled at the other times.
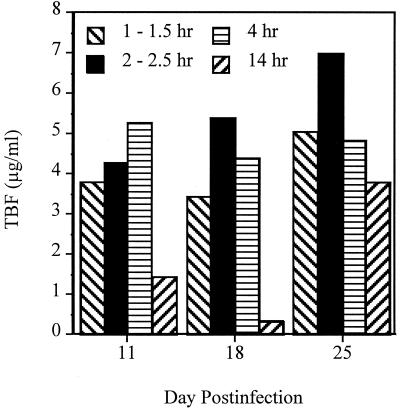
TBF levels in serum were measured during the study at 1 to 1.5, 2 to 2.5, 4, or 14 h postdosing (Fig. 5). Serum TBF levels were well above the MIC for C. immitis for over 4 h and were above or close to the MIC at 14 h. Peak serum drug levels were reached sooner as treatments continued. On day 25 postinfection, a sample obtained at 14 h postdosing showed high levels of TBF. No TBF was detected in the CSF at any of the time points.
FIG. 5.
TBF levels measured in the serum at 1 to 1.5, 2 to 2.5, 4, and 14 h postdosing. Bars represent mean concentrations for two rabbits for all four time points on day 11, for the first three time points on day 18, and for the second and fourth time points on day 25. The means for three animals are shown for the first and third time points on day 25. One animal provided the concentration for last time point on day 18. On day 32 (data not shown), five animals had mean concentrations of 0.08 ± 0.18 μg/ml. No TBF was detectable in the CSF of any animal (days 7 to 32 postinfection for 31 samples, 1 to 14.5 h postdosing for 25 samples, and 44 to 161 h postdosing for six samples).
DISCUSSION
The general decline in the numbers of CFU in CSF contrasts with the culture positivity of the brain and spinal cord, suggesting that the organism is embedded in the meningeal and parenchymal inflammatory reaction and is no longer liberated into the CSF. This evolution is the same as that in the human disease (2).
In this study, despite the in vitro susceptibility of the organism to TBF, TBF appeared to have only a modest effect on survival, histologic findings, and tissue CFU reduction; however, the effects were not significant. This leaves open the question of whether TBF may be effective when used in combination with other antifungals.
In a study with rats, TBF was shown to cross the blood-brain barrier at levels higher than expected from the free fraction available (7). Thus, it was conceivable that measurable amounts of TBF could cross the blood-brain barrier and decrease the fungal burden in a meningeal disease. In the study with rats, TBF was injected directly into the carotid artery, which may not stimulate the conditions encountered when the drug is given orally.
One could argue that the lack of measurable TBF in the CSF was responsible for the inferior performance of TBF compared to that of FCZ. However, in a previous study (13) we showed that penetration or accumulation of measurable itraconazole in the CSF was not needed for itraconazole to have efficacy equal to that of FCZ in this model. Presumably, the accumulation of itraconazole in the meninges is responsible for the efficacy. Like itraconazole, TBF is known to accumulate in tissues (6). The accumulation of TBF in adipose- and keratin-rich tissues at concentrations significantly higher than those in plasma are most likely responsible for a good response against superficial infections (1).
FCZ was effective at controlling coccidioidal meningitis and reducing C. immitis levels in the CSF and tissue. The antibody titers in serum and CSF were consistent with this. As was shown in a previous study with this model (13), FCZ penetrates the CSF at levels slightly below the levels obtained in serum. However, it did not eliminate C. immitis from the tissues. Although FCZ is efficacious in the treatment of human coccidioidal meningitis, it likewise appears not to be curative (2).
ACKNOWLEDGMENTS
We thank Novartis Pharma AG, Kaweah Delta District Hospital, and the Bank of Stockton, Stockton, Calif., for financial support; Pfizer, Inc. for donating FCZ; and M. Martinez, R. Ramirez, C. Reed, D. Hewitt, and C. Zimmermann for assistance.
REFERENCES
- 1.Abdel-Rahman S M, Nahata M C. Oral terbinafine: a new antifungal agent. Ann Pharmacother. 1997;31:445–456. doi: 10.1177/106002809703100412. [DOI] [PubMed] [Google Scholar]
- 2.Dewsnup D H, Galgiani J N, Graybill J R, Diaz M, Rendon A, Cloud G A, Stevens D A. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med. 1996;124:305–310. doi: 10.7326/0003-4819-124-3-199602010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hostetler J S, Heykants J, Clemons K V, Woestenborghs R, Hanson L H, Stevens D A. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother. 1993;37:2224–2227. doi: 10.1128/aac.37.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kan V L, Henderson D K, Bennett J E. Bioassay for SF 86-327, a new antifungal agent. Antimicrob Agents Chemother. 1986;30:628–629. doi: 10.1128/aac.30.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine H, Stevens D, Cobb J, Gebhardt A. Miconazole in coccidioidomycosis. I. Assays of activity in mice and in vitro. J Infect Dis. 1975;132:407–414. doi: 10.1093/infdis/132.4.407. [DOI] [PubMed] [Google Scholar]
- 6.Leyden J. Pharmacokinetics and pharmacology of terbinafine and itraconazole. J Am Acad Dermatol. 1998;38:S42–S47. doi: 10.1016/s0190-9622(98)70483-9. [DOI] [PubMed] [Google Scholar]
- 7.Machard B, Misslin P, Lemaire M. Influence of plasma protein binding on the brain uptake of an antifungal agent, terbinafine, in rats. J Pharm Pharmacol. 1989;41:700–704. doi: 10.1111/j.2042-7158.1989.tb06344.x. [DOI] [PubMed] [Google Scholar]
- 8.Pappagianis D, Zimmer B L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3:247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petranyi G, Meingassner J G, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987;31:1365–1368. doi: 10.1128/aac.31.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex J H, Hanson L H, Amantea M A, Stevens D A, Bennett J E. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1991;35:846–850. doi: 10.1128/aac.35.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryder N S, Frank I. Interaction of terbinafine with human serum and serum proteins. J Med Vet Mycol. 1992;30:451–460. doi: 10.1080/02681219280000611. [DOI] [PubMed] [Google Scholar]
- 12.Schiraldi G F, Colombo M D, Harari S, Lo Cicero S, Ziglio G, Ferrarese M, Rossato D, Soresi E. Terbinafine in the treatment of nonimmunocompromised compassionate cases of bronchopulmonary aspergillosis. Mycoses. 1996;39:5–12. doi: 10.1111/j.1439-0507.1996.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen K N, Sobel R A, Clemons K V, Pappagianis D, Stevens D A, Williams P L. Comparison of fluconazole and itraconazole in a rabbit model of coccidioidal meningitis. Antimicrob Agents Chemother. 2000;44:1512–1517. doi: 10.1128/aac.44.6.1512-1517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens D A, Aristizabal B H. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 and UR-9751, and comparison with fluconazole. Diagn Microbiol Infect Dis. 1997;29:103–106. doi: 10.1016/s0732-8893(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 15.Tucker R M, Williams P L, Arathoon E G, Stevens D A. Treatment of mycoses with itraconazole. Ann N Y Acad Sci. 1988;544:451–470. doi: 10.1111/j.1749-6632.1988.tb40443.x. [DOI] [PubMed] [Google Scholar]
- 16.Villars V V, Jones T C. Special features of the clinical use of oral terbinafine in the treatment of fungal diseases. Br J Dermatol. 1992;126(Suppl. 39):61–69. doi: 10.1111/j.1365-2133.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams P L, Johnson R, Pappagianis D, Einstein H, Slager U, Koster F T, Eron J J, Morrison J, Aguet J, River M E. Vasculitic and encephalitic complications associated with Coccidioides immitis infection of the central nervous system in humans: report of 10 cases and review. Clin Infect Dis. 1992;14:673–682. doi: 10.1093/clinids/14.3.673. [DOI] [PubMed] [Google Scholar]
- 18.Williams P L, Sobel R A, Sorensen K N, Clemons K V, Shuer L M, Royaltey S S, Yao Y, Pappagianis D, Lutz J E, Reed C, River M E, Lee B C, Bhatti S U, Stevens D A. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J Infect Dis. 1998;178:1217–1221. doi: 10.1086/515689. [DOI] [PubMed] [Google Scholar]