Abstract
The world recently witnessed the emergence of new epidemic outbreaks like COVID-19 and mpox. The 2022 outbreak of mpox amid COVID-19 presents an intricate situation and requires strategies to combat the status quo. Some of the challenges to controlling an epidemic include present knowledge of the disease, available treatment options, appropriate health infrastructures facilities, current scientific methods, operations concepts, availability of technical staff, financial funds, and lastly international policies to control an epidemic state. These insufficiencies often hinder the control of disease spread and jeopardize the health of countless people. Also, disease outbreaks often put a huge burden on the developing economies. These countries are the worst affected and are immensely dependent on assistance provided from the larger economies to control such outbreaks. The first case of mpox was reported in the 1970s and several outbreaks were detected thereafter in the endemic areas eventually leading to the recent outbreak. Approximately, more than 80,000 individuals were infected, and 110 countries were affected by this outbreak. Yet, no definite vaccines and drugs are available to date. The lack of human clinical trials affected thousands of individuals in availing definite disease management. This paper focuses on the epidemiology of mpox, scientific concepts, and treatment options including future treatment modalities for mpox.
Keywords: Mpox, DNA virus infections, Orthopoxvirus, Public health emergency
Introduction
Amidst the COVID-19 pandemic, another public health concern emerged as a potential threat to afflict people globally, i.e. an abrupt increase in the incidence of mpox (monkeypox) cases. Indeed, starting from mid-May 2022, cases of human mpox have significantly risen in several non-endemic countries worldwide, leading to the declaration of the ongoing outbreak of mpox as a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO) in July 2022 [1, 2]. Mpox disease is caused by the mpox virus (MPXV), a double-stranded DNA virus from the Orthopoxvirus genus, belonging to the Poxviridae family [2, 3]. The same genus includes the variola virus, a known causative agent of smallpox [2]. Genetically, MPXV is identified with two types of clads. Clad I, also known as Congo Basin clad, is mostly clustered in the Central-South Cameroon region till DRC. Infections from this clad are more severe with case fatality rates (CRF) > 10%. Clad II also referred to as West African clad, commonly distributed in western Cameroon to the Sierra Leon area, is further divided into sub-clad groups as IIa and IIb (also now referred to as clad III) having a CRF < 1% [4, 5]. Overall reported human case fatality rates (CFRs) range between 3.6 and 10.6% in the endemic regions [2]. In the current 2022 outbreak, clad IIb was predominant [6, 7]. To date, the exact animal reservoir for the mpox virus (MPXV) has remained unknown. However, few native African rodents (Gambian giant rats) and squirrels are suspected to be natural reservoirs of the virus. Common species which were frequently infected with MPXV are squirrels, Gambian giant rats, strip mice, dormice, and primates [8].
Emergence of Mpox
The first outbreak of mpox was reported in 1958 in a group of 10 captive monkeys at the Statens Seruminstitut, Copehengan, Denmark, and Centre d’Enseignement et de Recherches de Medecine aeronautique, Paris. No human infection was reported in individuals who were in close contact with infected monkeys. Subsequently, the mpox outbreak occurred for the first time in humans between 1970 to 1971 [9]. The first case was reported in a 9-month-old boy residing in a remote village of the Democratic Republic of Congo (DRC), admitted to a local hospital suspected of smallpox infection. Samples from infected individuals were sent to the WHO Smallpox Reference Center, Moscow, revealing mpox infections in virus isolates [10]. When inspected from family, monkeys were part of the diet, and their skins were also processed in this area. However, no other cases including secondary infections were reported in the community. Nonetheless, seven more cases were reported during this time period [9]. The World Health Organization in 1967 took the initiative to collaborate with laboratories to conduct cooperative studies. This was to conduct serological surveys, identify mpox outbreaks, and determine the natural foci of the virus. However, these surveys failed to state any major findings and concluded mpox is not a widespread disease and can exist only in the local environment [9]. Ever since, there has been a subsequent upsurge of mpox cases, mostly recorded in the DRC province. Approximately, 80% of the cases were reported in this region from the years 1970–1997 [11]. For the past five decades, DRC is the most affected country with mpox; no other country had reported an mpox outbreak to such an extent [12].
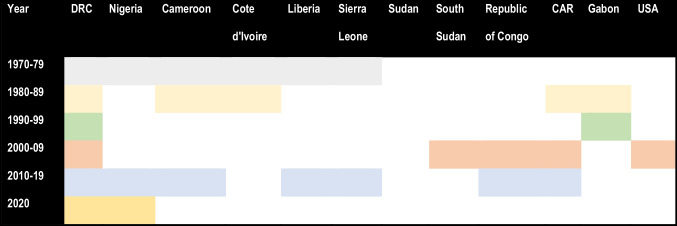
The initial mpox outbreak that was reported in DRC mostly affected children below 10 years of age. A slight male predominance was observed in the systemic review conducted by Beer and Rao [11]. Most of the initial outbreaks occurred among individuals living in small rural areas or residing close to humid evergreen tropical forests or individuals commonly involved with bushmeat hunting [11]. Geographically, the spread of infection from 1970 to 2003 concentrated in the Central and Western parts of Africa (Table 1). Countries which frequently reported infections were Cameroon, the Central African Republic, Gabon, Sierra Leone, Liberia, Nigeria, and Cote d’Ivoire, yet greater outbreaks were mostly detected in DRC [13]. An active surveillance programme was carried out by WHO between the years 1981 to 1986 reporting total confirmed cases of 338 and 33 deaths, an almost 20 times rise in the reported case after the surveillance [10, 11]. A slight drop in the incidence of disease was observed between the period of 1993–1995. But soon after, DRC witnessed a major outbreak from 1996–1997 [13]. A total of 511 cases were recorded with a surge in secondary transmission rates of up to 78% and a fatality rate between 1 and 5% [10, 13].
In 2003, the mpox outbreak occurred for the first time in the USA, outside the African continent. The index case was a 3-year-old girl, bitten by an infected prairie dog, imported from Ghana along with other African rodents to the USA [14]. A total of 71 cases were reported, including both suspected and laboratory-confirmed cases, as per the CDC report [15]. During the period of 2005, mpox was registered for the first time in the dry savannah region of Sudan. Overall, 40 cases both suspected and confirmed were recorded. In this outbreak, a change in the genomic structure of MPXV was observed as compared to the MPXV traditionally reported in DRC suggesting the adaptability of MPVX in dry regions from humid evergreen tropical forests [10]. In the year 2018, mpox travelled for the first time to the UK and was reported in the European continent. Only two cases were registered, in individuals, which had a travel history to Nigeria [16]. Nevertheless, with the advent of 2022, the world saw a major outbreak of mpox (Table 1).
Table 1.
Decade-wise spread of mpox across different countries between 1970 and 20209,10
Mpox Outbreak 2022
Mpox is endemic in Central and West Africa, where hundreds of cases were detected annually for many years, acquired mostly from wild animals and most rarely from infected humans [1, 3], which results in a sporadic spillover of cases in humans as observed in the MPVX endemic regions [5]. However, in the 2022 outbreak of mpox, most of the cases were reported in non-endemic countries like N. America, S. America, and Europe (Fig. 1) [17]. Although the origin of the 2022 outbreak is still unknown, it is highly likely that the initial infection has been imported from an endemic country, allowing the circulation of the virus through close physical contacts among humans [1, 18]. For the first time, mpox was documented with transmission chains in countries which had no immediate contact with Central or Western Africa [19, 20]. This suggests a probability of undetected MPXV circulating in the local population in the outbreak-hit regions causing disease transmission in humans [17]. Being a DNA virus, mpox is more stable in nature and may have possibly evolved as a potent virus causing infections in humans in the due course of time [17]. Daniel et al. reported 6–12 times higher mutation rates in mpox as previously estimated [6]. Human to human transmissibility of mpox has also evolved in these decades [21, 22]. Vertical infection of mpox has been also reported. Pregnant mothers infected with mpox had miscarriages during the first trimester of pregnancy [6]. Perinatally acquired mpox infection was registered in a 9-day year old neonate as well [23]. Transmissibility of infection within the family especially from parents to children have also been stated to increase [20, 22]. The degree of transmissibility of the diseases, popularly known as R0, reported in the 1980 for mpox was 0.83. However, in the 2022 outbreak, the R0 reported was 1.1–2.4 [21, 24]. Pan et al. suggested the increase in the R0 is due to decreased immunity of individuals due to the absence of smallpox immunization and high contact rates of infection in the MSM community [6].
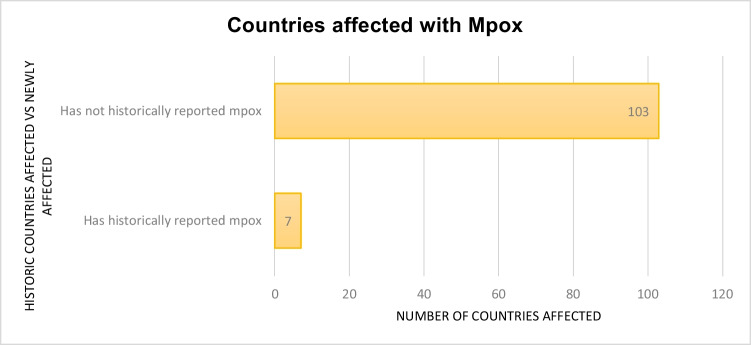
Fig. 1.
Countries reporting mpox historically vs countries reporting an mpox outbreak as recorded in an early March 2023 report by CDC19
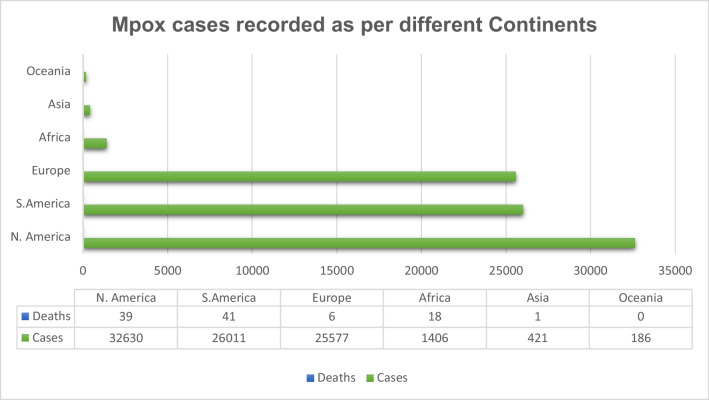
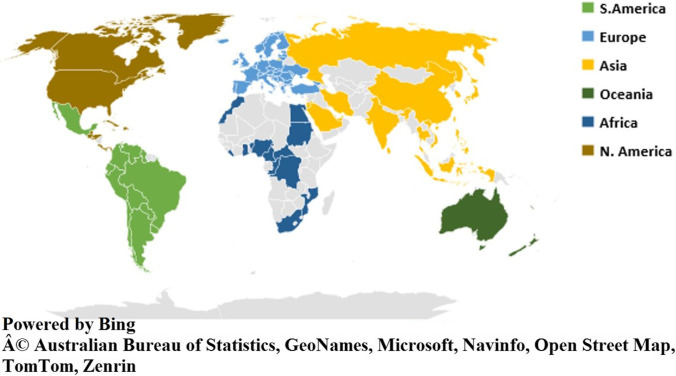
As per the WHO, till 17 March 2023, the total confirmed cases for mpox were 86,601, with 1265 probable cases reported with 112 deaths. Globally, 110 countries were affected by mpox so far (Fig. 2) [20]. Approximately 34.7% of cases were reported in America, the worst affected country [20]. Majority of the infection occurred through household contacts (43%) and by sexual encounters (43%) [20, 22]. Commonly affected individuals were young males who were not vaccinated against smallpox and have had sex with men. There was a slight male predisposition, with the median age reported as 34 years (IQR: 29–41). Around, 98% of individuals who were infected were either gay or bisexual, among which 41% of the people were HIV infected [4].
Fig. 2.
Mpox number of cases and deaths recorded in early March 2023 across the continent as per the report by CDC19
On 23 July 2022, mpox was declared a public health emergency by the Public Health Emergency of International Concern (PHEIC), depicting a risk of international spread, along with significant international coordination to control the disease [25].
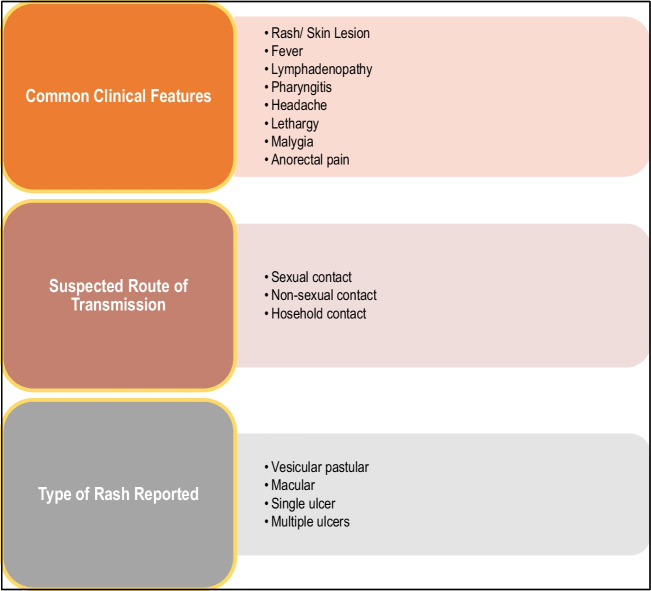
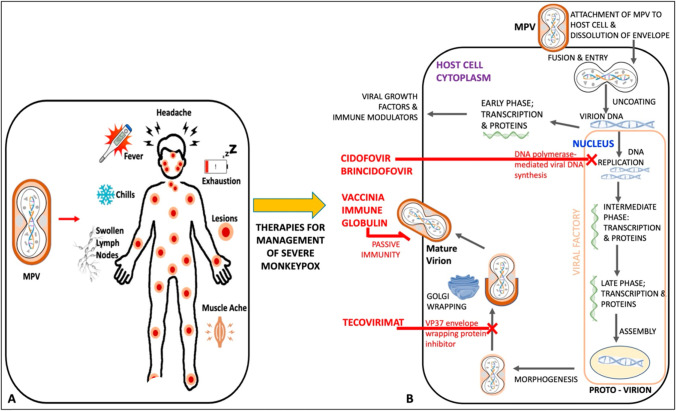
Clinical presentation of this disease includes three distinct phases, i.e. incubation, prodrome, and rash [2]. The incubation period can last for 3 to 20 days with the median being 7 days followed by the prodrome phase that is characterized by lethargy, myalgia, headache, fever, and lymphadenopathy which may last up to 5 days (Fig. 3) [2, 4, 18]. Lymphadenopathy is one of the critical features of the progression of the disease and often reported before the development of skin lesions [18]. Fever is usually followed by multiple papular, ulcerative, and vesiculopustular skin lesions [4], which progress from macules to papules, vesicles, pustules, crusts, and lastly scab, presenting for up to 4 weeks [2, 18]. In 95% of the cases, skin lesions appears [4]. Common anatomical sites for skin lesions were anogenital with approximately 73% of cases followed by trunk, arms or legs, face, and eventually palms and soles, only accounting for 10% of the cases. Lesions developed contain infectious virus particles, through which the infection can be transmitted directly with human contacts [2]. Secondary complications include pneumonia, encephalitis, keratitis, gastroenteritis, sepsis, and secondary bacterial infections, affecting mostly patients with a previous diagnosis of HIV infection [2, 4, 5].
Fig. 3.
Common features reported in mpox infection4
Current Treatment Modalities and Prevention
The strategy for the prevention and treatment of mpox is very similar to the treatment of Orthomyxovirus infection [26]. The 2022 outbreak revealed the urgency to control the spread of mpox as it has caused a potential threat in many countries [27]. Presently, there is no definitive cure for mpox infection, mild symptoms are manageable, and further complications can be avoided in patients with mpox with the help of supportive care [28, 29]. Studies have depicted that patients with mild symptoms recover without any treatment [30–32]. Treatment options available for smallpox are also effective in the treatment of mpox, as the clinical presentation of mpox and smallpox is very similar. These include the vaccinia vaccine, vaccinia immune globulin (IVG), and antiviral agents such as cidofovir, tecovirimat, and brincidofovir [32]. Furthermore, CDC recommends the use of the potential treatment options should be done depending upon the severity of the cases and for serious emergency cases, as the current drugs pose severe adverse effects, and their therapeutic efficacy is still uncertain [33]. Antiviral drugs are a choice of treatment in immunocompromised patients, in patients with complicated lesions, in pregnant women infected with mpox, in breast-feeding women, and in the paediatric population [34]. Tecovirimat is the first line of action antiviral recommended for the treatment of smallpox; it works by inhibiting the viral envelope protein, thereby blocking the final steps of virus maturation and release from infected cells, inhibiting the spread. As per the CDC guidelines, emergency access use of tecovirimat is allowed for compassionate use, for the treatment of Orthopoxvirus infections, such as mpox [35, 36]. Cidofovir and its oral analogue brincidofovir are commonly approved drugs for the treatment of smallpox; both act by inhibiting viral DNA polymerase. Different studies have evaluated the effect of brincidofovir against Orthopoxvirus infections [37]. Studies done by Lanier et al. and others on the effect of cidofovir and brincidofovir have been evaluated for mpox with some success [34, 37]. As per the recommended guidelines by CDC, preexposure smallpox vaccination has been advised for veterinarians, monkeypox contacts, healthcare workers caring for mpox patients, researchers, and field investigators [38]. Prior immunization with the smallpox vaccine has demonstrated some proven protective effects against mpox due to the cross-protective immunity provided by the smallpox vaccine. Furthermore, the severity of clinical manifestations is also reduced [39]. Currently, three available smallpox vaccines with the US national stockpile, i.e. JYNNEOSTM, ACAM2000, have been licenced (2007) for smallpox, the most recent being Aventis Pasteur Smallpox Vaccine (APSV) which could be potentially used for mpox on a case-to-case basis, under an investigational new drug (IND) protocol. JYNNEOSTM, a third-generation and live viral vaccine, is produced from the modified vaccinia Ankara-Bavarian Nordic [40–42]. Licenced in 2019, JYNNEOSTM is an attenuated non-replicating orthopoxvirus. It is now indicated for both smallpox and mpox prevention for adults. Further, ACAM2000, a second-generation vaccine constituted of live vaccinia virus, under the emergency access ACAM2000 is allowed for mpox during the outbreak. Researchers have demonstrated that these vaccines can be used as pre- and post-treatment options, i.e. either in preventing the infection and the disease or in ameliorating the infection and disease [34, 43, 44]. Studies have demonstrated that pregnant women, children less than 8 years of age, and immunocompromised patients should be given antiviral treatment than vaccination. These vaccines, although approved, have shown some local and systematic side effects such as fever, muscle pain, vaccinia, abdominal and back pain, fatigue, headache, lymphadenopathy, etc. [42–44]. Researchers have also highlighted the need for maintaining appropriate social barriers such as avoiding close contact with affected individuals, avoiding contact with skin lesions of individuals infected with MPXV, etc. [44–46]. Vaccinia immune globulin intravenous (VIGIV) is a choice of treatment in case of severe infection with mpox, though there is a paucity of data about its effectiveness in treating mpox. VIGIV is also under SNS and can be administered under investigational new drugs held by CDC [29, 30, 47–49]. Therefore, the treatment options and the repurposing of vaccines need to be considered on a case-to-case basis depending on the severity of cases and the immune state of patients [50] (Fig. 4).
Fig. 4.
A Symptoms and B mechanism of action of mpox antiviral therapy: cidofovir, brincidofovir, vaccinia immune globulin, and tecovirimat [50]
Key Fundamental Findings of the Narrative Review
Some major key findings related to mpox are as follows: mpox was solely endemic to the region of DRC [11]. There has been a slow and steady increase in mpox cases which has adapted itself to develop into the current outbreak. Secondly, the 1996–1997 DRC outbreak highlighted the increase in secondary transmission rates of mpox, potentially getting adapted to spread in the human population [13]. Thirdly, the MPXV had adapted to thrive itself from the humid evergreen regions to the dry savannah region of Sudan, as observed in the 2005 outbreak, thus further demonstrating its environmental adaptability to flourish [10]. Lastly, international travel and commerce have given a wider chance for the disease to spread as reported in the 2003 and 2018 outbreaks of mpox in the USA and the UK [14, 16]. All these above factors have led to the 2022 outbreak of mpox, affecting every continent across the globe (Fig. 5).
Fig. 5.
Global spread of mpox in 2023 outbreak19
Most of the consistent outbreak guidelines available from WHO or CDC only account for people with a high risk of exposure; these guidelines are based on the best available evidence which is based upon risk–benefit analysis and other factors [51]. Available drugs for the treatment of choice are also limited and lack evidence-based studies in humans [29, 30, 48]. This depicts an extensive need to increase the sustainable funding option to enhance our understanding of the development of new drugs and vaccines to curtail the spread of mpox.
Implication in Future Research
The environmental, behavioural, and social reasons behind the 2022 mpox outbreak remain unknown to date [1]. A deeper understanding of mpox genetics and biochemistry is essential to control its outbreak. It is currently unclear how mpox is closely related and linked to the viral strain that is primarily found in western Africa, as well as the potential routes of rapid transmission. To further understand the immune defence mechanisms against MPXV, more research is needed on the human systemic and mucosal immune responses. As DNA viruses are more adept to correct mutations; therefore, it is unlikely that the mpox virus will suddenly change during human transmission [24, 52]. It is yet unknown, whether vaccinations and earlier infections have given the population immunity. Additionally, exploratory studies are required to pinpoint the precise mpox virus reservoir, understand how the virus spreads naturally, and determine the causes of the present increase in cases across several nations. Currently, no potent drugs are available and limited evidence-based studies are being conducted for the treatment of mpox [29]. Most of the available choices of treatment are discussed in this paper (Fig. 6). Therefore, it becomes essential to investigate the domain of natural products with antiviral properties. This provides alternative treatment options, to prevent human to human spread of infection and restrict virus amplification in the host organisms. There is a recent increased interest among the scientific community to look into the numerous bioactivities of structurally unrelated natural compounds [53, 54]. Plant-derived polyphenol resveratrol has beeb shown to significantly suppress replication of MPXV affecting probably the viral DNA synthesis and inducing a comparable effect to the well-characterized Orthopoxvirus inhibitor, i.e. cytosine-1-β-d-arabinofuranoside (AraC) [55]. Due to the pleiotropic action of natural compounds and lack of systemic toxicity, plant-derived agents may represent target compounds to be explored in future clinical trials to enrich the drug arsenal against Orthopoxvirus infections. Parallel to this, early detection of infected patients who are potentially capable of transmitting the infection is also crucial, pointing to the need for improved diagnosis (particularly in atypical clinical presentations and asymptomatic cases), and better availability of molecular tests. Besides, such continual efforts of preclinical scientists and pharmaceutical companies, availability of health infrastructures, and medical staff are of critical importance—a situation still aggravated by the ongoing COVID-19 pandemic. A high-risk patient population is possibly in danger of mpox nosocomial transmission and deserves more attention. Therefore, it is crucial to administer the proper supportive care [24]. Consequently, it is necessary to improve genomic sequencing capabilities to identify the mpox viral clade(s). The primary necessities are to combat the spread of mpox while dealing with the ongoing COVID-19 pandemic and to include suitable and timely information campaigns for people at risk. It is challenging to create an evidence-based classification of drug safety and effectiveness having a brief history of mpox. Further studies on various animal models, which may affect medication exposure, are also encouraged. The focus of larger research should be on identifying the patients who are most at risk for consequences from mpox infection as well as the best timing for initiating and completing antiviral therapy.
Fig. 6.
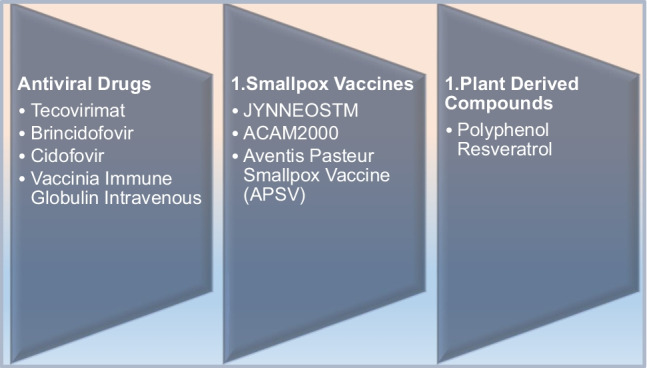
Different treatment modalities in mpox
Conclusion
The emergence of new diseases is one of the incessant threats which mankind can face. Persistent interference between the environment and humans creates an opportunity for new infections to evolve. Over 75% of the pathogens, which are newly emerging, are zoonotic in nature [56]. Several diseases like HIV/AIDS, Nipah, SARS, and Ebola including mpox have recently appeared. International travel and commerce and human behaviour often help disease to spread [56]. With the first emergence of mpox in 1958, little is still known about its reservoir host and vector of the disease. Despite repeated outbreaks of mpox over the past years, it has failed to gather scientific attention. There is a lack of understanding of mpox transmission dynamics and disease evolution. In the areas endemic to mpox, regular disease surveillance is lacking. This also includes the need to promote funding for capacity building required for surveillance of the disease, research activities, and testing facilities [17, 57]. The role of central bodies like the World Health Organization plays a major role in controlling such outbreaks. However, non-compliance to guidelines and regulations by health agencies like WHO severely impacts the control measures [25]. Boosting vaccine development and effective drug development is essential to prevent future outbreaks. In addition, new plant-derived products could be further developed and can be promoted as they potentially have lesser side effects for mpox treatment.
Abbreviations
- Mpox
Monkeypox
- MPXV
Mpox virus
- PHEIC
Public Health Emergency of International Concern
- DRC
Democratic Republic of Congo
- SNS
Strategic National Stockpile
- VIGIV
Vaccinia immune globulin intravenous
Author Contribution
SR, KV, and KS: conceptualization and writing; HST: editing and proofreading. All authors reviewed the manuscript.
Data Availability
This document includes citations for all the data that were analysed throughout the literature review.
Compliance with Ethical Standards
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have their consent to publish.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samriddhi Ranjan, Email: sranjan@masonlive.gmu.edu.
Kanupriya Vashishth, Email: dr.kanupriyavashishth@gmail.com.
Katrin Sak, Email: katrin.sak.001@mail.ee.
Hardeep Singh Tuli, Email: hardeep.biotech@gmail.com.
References
- 1.Capobianchi MR, Di Caro A, Piubelli C, Mori A, Bisoffi Z, Castilletti C. Monkeypox 2022 outbreak in non-endemic countries: open questions relevant for public health, nonpharmacological intervention and literature review. Front Cell Infect Microbiol. 2022;12:1005955. doi: 10.3389/fcimb.2022.1005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forni D, Cagliani R, Molteni C, Clerici M, Sironi M. Monkeypox virus: the changing facets of a zoonotic pathogen. Infect Genet Evol. 2022;105:105372. doi: 10.1016/j.meegid.2022.105372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee AG, Wanjari UR, Kannampuzha S, et al. The pathophysiological and immunological background of the monkeypox virus infection: an update. J Med Virol. 2023;95(1):e28206. doi: 10.1002/jmv.28206. [DOI] [PubMed] [Google Scholar]
- 4.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 5.Risk assessment: Monkeypox multi-country Outbreak. European Centre for Disease Prevention and Control. 2022. https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak. Accessed 11 Oct 2023.
- 6.Pan D, Nazareth J, Sze S, et al. Transmission of monkeypox/mpox virus: a narrative review of environmental, viral, host, and population factors in relation to the 2022 international outbreak. J Med Virol. 2023;95(2):e28534. doi: 10.1002/jmv.28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monkeypox: experts give virus variants new names. World Health Organization. 2022. https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names. Accessed 16 Mar 2023.
- 8.Factsheet for health professionals on mpox (monkeypox). European Centre for Disease Prevention and Control. 2022. https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals. Accessed 16 Mar 2023.
- 9.Arita I, Gispen R, Kalter SS, et al. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull World Health Organ. 1972;46(5):625–631. [PMC free article] [PubMed] [Google Scholar]
- 10.Sklenovská N, Van Ranst M. Emergence of monkeypox as the most important Orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. Holbrook MR, ed. PLoS Negl Trop Dis. 2019;13(10):e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat?. A systematic review Gromowski G, ed. PLoS Negl Trop Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15(4):280–287. doi: 10.1053/j.spid.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(27):642–6. [PubMed]
- 16.Rapid risk assessment: Monkeypox cases in the UK imported by travellers returning from Nigeria, 2018. European Centre for Disease Prevention and Control. 2018. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-monkeypox-cases-uk-imported-travellers-returning-nigeria. Accessed 11 Oct 2022.
- 17.Zumla A, Valdoleiros SR, Haider N, et al. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis. 2022;22(7):929–931. doi: 10.1016/S1473-3099(22)00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafaati M, Zandi M. State-of-the-art on monkeypox virus: an emerging zoonotic disease. Infection. 2022 doi: 10.1007/s15010-022-01935-3. [DOI] [PubMed] [Google Scholar]
- 19.2022 Mpox Outbreak Global Map. Mpox. 2023. https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html. Accessed 16 Mar 2023.
- 20.2022-23 Mpox (Monkeypox) Outbreak: Global Trends. World Health Organization. 2023. https://worldhealthorg.shinyapps.io/mpx_global/. Accessed 16 Mar 2023.
- 21.Tiecco G, Degli Antoni M, Storti S, Tomasoni LR, Castelli F, Quiros-Roldan E. Monkeypox, a literature review: what is new and where does this concerning virus come from? Viruses. 2022;14(9):1894. doi: 10.3390/v14091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramnarayan P, Mitting R, Whittaker E, et al. Neonatal Monkeypox Virus Infection. N Engl J Med. 2022;387(17):1618–1620. doi: 10.1056/NEJMc2210828. [DOI] [PubMed] [Google Scholar]
- 24.Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022;14(7):e26531. doi: 10.7759/cureus.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenham C, Eccleston-Turner M. Monkeypox as a PHEIC: implications for global health governance. Lancet. 2022;400(10369):2169–2171. doi: 10.1016/S0140-6736(22)01437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9(7):ofac310. 10.1093/ofid/ofac310. [DOI] [PMC free article] [PubMed]
- 27.Harapan H, Ophinni Y, Megawati D, et al. Monkeypox: a comprehensive review. Viruses. 2022;14(10):2155. doi: 10.3390/v14102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel M, Adnan M, Aldarhami A, et al. Current insights into diagnosis, prevention strategies, treatment, therapeutic targets, and challenges of monkeypox (mpox) infections in human populations. Life (Basel). 2023;13(1):249. doi: 10.3390/life13010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treatment information for healthcare professionals. Mpox Health Professionals Clinical Guidance. 2023. https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html. Accessed 18 Mar 2023.
- 30.Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rallapalli S, Razai MS, Majeed A, Drysdale SB. Diagnosis and management of monkeypox in primary care. J R Soc Med. 2022;115(10):384–9. 10.1177/01410768221131914. [DOI] [PMC free article] [PubMed]
- 32.Shamim MA, Padhi BK, Satapathy P, et al. The use of antivirals in the treatment of human monkeypox outbreaks: a systematic review. Int J Infect Dis. 2023;127:150–161. doi: 10.1016/j.ijid.2022.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johri N, Kumar D, Nagar P, Maurya A, Vengat M, Jain P. Clinical manifestations of human monkeypox infection and implications for outbreak strategy. Health Sci Rev (Oxf). 2022;5:100055. doi: 10.1016/j.hsr.2022.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82(9):957–963. doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Highlights of prescribing information dosage forms and strengths TPOXX. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208627s000lbl.pdf. Accessed 25 Mar 2023.
- 36.Desai AN, Thompson GR, 3rd, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA. 2022;328(13):1348–1350. doi: 10.1001/jama.2022.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the Treatment of Poxvirus Infections. Viruses. 2010;2(12):2740–2762. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox - West and Central Africa, 1970–2017. Centers for Disease Control and Prevention. 2018. https://www.cdc.gov/mmwr/volumes/67/wr/mm6710a5.htm. Accessed 30 Mar 2023.
- 39.Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7(1):373. doi: 10.1038/s41392-022-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang RM, Zheng YJ, Zhou L, et al. Diagnosis, treatment, and prevention of monkeypox in children: an experts' consensus statement. World J Pediatr. 2023;19(3):231–42. 10.1007/s12519-022-00624-3. [DOI] [PMC free article] [PubMed]
- 41.Petersen E, Kantele A, Koopmans M, et al. Human monkeypox. Infect Dis Clin North Am. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022;22(12):e349–e358. doi: 10.1016/S1473-3099(22)00574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saied AA, Dhawan M, Metwally AA, Fahrni ML, Choudhary P, Choudhary OP. Disease history, pathogenesis, diagnostics, and therapeutics for human monkeypox disease: a comprehensive review. Vaccines. 2022;10(12):2091. doi: 10.3390/vaccines10122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemati S, Farhadkhani M, Sanami S, Mohammadi-Moghadam F. A review on insights and lessons from COVID-19 to the prevent of monkeypox pandemic. Travel Med Infect Dis. 2022;50:102441. doi: 10.1016/j.tmaid.2022.102441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadar S, Khan T, Omri A. Reemergence of monkeypox: prevention and management. Expert Rev Anti Infect Ther. 2022;20(11):1425–1433. doi: 10.1080/14787210.2022.2128763. [DOI] [PubMed] [Google Scholar]
- 46.Di Gennaro F, Veronese N, Marotta C, et al. Human monkeypox: a comprehensive narrative review and analysis of the public health implications. Microorganisms. 2022;10(8):1633. doi: 10.3390/microorganisms10081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection — United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(32):1023–8. 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed]
- 48.McCarthy MW. Therapeutic strategies to address monkeypox. Expert Rev Anti Infect Ther. 2022;20(10):1249–1252. doi: 10.1080/14787210.2022.2113058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam MR, Hossain MJ, Roy A, et al. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: A rapid review on comparative benefits and risks. Health Sci Rep. 2022;5(5):e798. 10.1002/hsr2.798. [DOI] [PMC free article] [PubMed]
- 50.Rajsri KS, Rao M. A review of monkeypox: the new global health emergency. Venereology. 2022;1(2):199–211. 10.3390/venereology1020014.
- 51.Mpox Vaccination Basics. 2023. https://www.cdc.gov/poxvirus/monkeypox/vaccines/index.html.
- 52.Lum FM, Torres-Ruesta A, Tay MZ, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022;22(10):597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sak K. Anticancer action of plant products: changing stereotyped attitudes. Explor Drug Sci. 2022;15:423–427. doi: 10.37349/etat.2022.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behl T, Rocchetti G, Chadha S, et al. Phytochemicals from plant foods as potential source of antiviral agents: an overview. Pharmaceuticals. 2021;14(4):381. doi: 10.3390/ph14040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao S, Realegeno S, Pant A, Satheshkumar PS, Yang Z. Suppression of poxvirus replication by resveratrol. Front Microbiol. 2017;8:2196. doi: 10.3389/fmicb.2017.02196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fauci AS. Emerging and reemerging infectious diseases: the perpetual challenge. Acad Med. 2005;80(12):1079–1085. doi: 10.1097/00001888-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Rothenburg S, Yang Z, Beard P, et al. Monkeypox emergency: urgent questions and perspectives. Cell. 2022;185(18):3279–3281. doi: 10.1016/j.cell.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This document includes citations for all the data that were analysed throughout the literature review.