Abstract
Short-coupled idiopathic ventricular fibrillation (IVF) is a subtype of IVF in which episodes of polymorphic ventricular tachycardia or ventricular fibrillation are initiated by short-coupled premature ventricular contractions (PVCs). Our understanding of the pathophysiology is evolving, with evidence suggesting that these malignant PVCs originate from the Purkinje system. In most cases, the genetic underpinning has not been identified. Whereas the implantation of an implantable cardioverter-defibrillator is uncontroversial, the choice of pharmacological treatment is the subject of discussion. In this review, we summarize the available knowledge on pharmacological therapy in short-coupled IVF and provide our recommendations for management of patients with this syndrome.
Keywords: Short-coupled idiopathic ventricular fibrillation, Ventricular arrhythmia, Ventricular fibrillation, Quinidine
1. Introduction
Sudden cardiac arrest (SCA) survivors in whom no underlying cause is identified after extensive diagnostic testing are diagnosed with idiopathic ventricular fibrillation (IVF) [1] In an unknown proportion of IVF patients, episodes of polymorphic ventricular tachycardia (pVT) or ventricular fibrillation (VF) are found to be initiated by premature ventricular contractions (PVCs) with an unusually short coupling interval. Nomenclature used for this phenotypic entity varies, with ‘short-coupled torsades de pointes (SCTdP)’ and ‘short-coupled (idiopathic) ventricular fibrillation (SCIVF)’ being the most common. We believe SCIVF to be the most appropriate term for this condition, because torsades de pointes is classically associated with prolongation of the QT-interval, which is absent in SCIVF. Case reports describing what could, in retrospect, be called SCIVF were first published more than 70 years ago [2], and Leenhardt et al. were the first to describe SCIVF as a distinct entity in a group of patients, naming it ‘short-coupled variant of torsade de pointes’ [3].
Steinberg et al. reported that 6.6% of IVF patients could be diagnosed with SCIVF, based on having recurrent pVT or VF initiated by a short-coupled PVC [4]. However, this is presumably an underestimation of the true incidence, given that recurrence of the ventricular arrhythmia is required to assess its initiation and diagnose SCIVF. A recent analysis by Groeneveld et al. of the Dutch IVF registry revealed that of the patients with recurrent pVT or VF in whom the initiation could be assessed, 91% fulfilled their definition of SCIVF [5]. These authors speculate that SCIVF may in fact be the common phenotype, rather than a distinct subtype of true IVF. Although arbitrary, a coupling interval <350 ms is most commonly used to define a short-coupled PVC [4,5].
In 2009, a founder risk haplotype on chromosome 7q36, involving the arrhythmia gene dipeptidyl-aminopeptidase-like protein 6 (DPP6), encoding a regulatory β-subunit of the rapidly recovering cardiac transient outward potassium current (Ito), was found to be associated with familial IVF in the Netherlands [6]. It is assumed that the resulting DPP6 overexpression increases the activity of Purkinje fiber Ito channels. Documented VF in these patients was invariably initiated by monomorphic short-coupled PVCs originating from the right ventricular apex/lower free wall and is considered to be a familial form of SCIVF. However, the genetic substrate of SCIVF remains unknown in the majority of patients, particularly in those from outside the Netherlands.
In patients diagnosed with SCIVF, implantation of an implantable cardioverter-defibrillator (ICD) is strongly recommended to reduce the risk of sudden cardiac death (SCD) [7]. However, the incidence rate of recurrent VF was 2.2 per 100 patient-months in the cohort described by Steinberg et al., with 21% of patients experiencing an electrical storm soon after the sentinel SCA [4]. Considering the substantial rate of ventricular arrhythmia recurrence and given that an ICD does not prevent their recurrence, drug therapy, or radiofrequency ablation if the required resources, including expertise in these kinds of complex procedures, is available, is indicated. The optimal choice of medication for patients with SCIVF is uncertain. In this review, we discuss the pathophysiology, the evidence for chronic and acute pharmacological treatment options, and provide our recommendations for management in SCIVF.
2. Genetic background and pathophysiology
The pathophysiological mechanism of SCIVF has not been resolved entirely. In particular, there is uncertainty regarding the origin of short-coupled trigger PVCs. The prevailing hypothesis is that the malignant PVCs originate from the Purkinje system, as was demonstrated by Haïssaguerre et al. using electroanatomical mapping in the first years of this century [8,9]. There is evidence of right ventricular outflow tract (RVOT) origin in a smaller subset of patients, which seems to be associated with longer coupling intervals and seems to be less malignant than Purkinje triggered PVCs [10]. Insights regarding the molecular mechanism of Purkinje trigger PVCs are provided by studies in DPP6 risk haplotype carriers, currently the only known monogenetic cause of SCIVF. Apparent overexpression of DPP6 appears to cause increased activity of Purkinje-fiber Ito which in turn increases the chance of short-coupled PVCs occurring, which then may result in IVF [11]. The apparent efficacy of quinidine, a potent Ito inhibitor, supports the Purkinje-fiber hypothesis. In addition, ablation of culprit trigger PVCs mapped to the Purkinje system with long-term absence of arrhythmia recurrence has been reported [12,13].
In most of the described patients, no causal variants are found with genetic analysis. However, there may be a hitherto undiscovered genetic substrate in a subset of cases, given the family history of SCD in a proportion of SCIVF patients [14]. Marsman et al. linked the pathogenic calmodulin 1 (CALM1) variant c.268T > C (p.F90L) to SCIVF in a family of Moroccan descent [15]. Apart from IVF, the phenotype associated with this mutation was characterized by mild QT prolongation in the recovery phase after exercise.
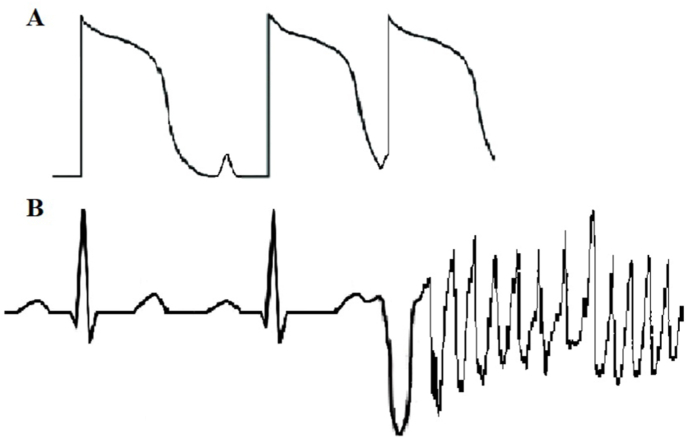
A potential link to mutations in the gene encoding the cardiac ryanodine receptor (RYR2) was suggested by Cheung et al., who described a mother and daughter with a SCIVF phenotype carrying a RYR2 mutation, causing diastolic sarcoplasmatic reticulum (SR) leak of calcium under non-stress conditions [16]. This mechanism is similar to catecholaminergic polymorphic ventricular tachycardia (CPVT), although the occurrence under non-stress conditions is very atypical. Cheung et al. reported multiple novel RYR2 variants found in apparent SCIVF patients [17]. However, one of these variants causes loss-of-function of the cardiac ryanodine receptor and may be related to calcium release deficiency syndrome [18]. These reports indicate a potential role of abnormal intracellular calcium handling in the pathophysiology of SCIVF. Spontaneous diastolic SR calcium release may cause delayed afterdepolarizations in Purkinje fibers which have been shown to be especially sensitive to afterdepolarizations, leading to malignant PVCs (Fig. 1) [19]. However, the precise molecular mechanism behind the short-coupled PVCs remains speculative.
Fig. 1.
Two delayed afterdepolarizations (A), the latter initiating a premature ventricular contraction during ventricular repolarization causing polymorphic ventricular tachycardia.
3. β-blockers
Reports of β-blocker usage in SCIVF are limited and results have been poor. The initial case series by Leenhardt et al. included two patients treated with β-blockers, both of whom died suddenly after 24 and 46 months of follow-up [3]. One case report described a patient treated with β-blockers who experienced VF storms on five occasions across five years, until he underwent radiofrequency ablation [20]. Another patient had recurrent ICD shocks for VF on bisoprolol during the six weeks following the index event, after which ablation was performed [21]. An increase in the frequency of non-sustained ventricular tachycardia episodes after the initiation of atenolol was reported in one case [22]. Other case reports asserted that β-blockers were ineffective at suppressing ventricular arrhythmia without further qualitative or quantitative specification [12,23,24]. In a recent review on SCIVF, including a literature review of different chronic pharmacological treatment options, Belhassen and Tovia-Brodie concluded that β-blockers were effective in only 13% of published cases [25]. Importantly, the reported success rates in this review were (simplistically) defined as the proportion of patients who experienced a recurrent event using the total number of patients treated with that particular medication as the denominator, but without considering differences in follow-up durations. In conclusion, we believe that β-blockers should not be primarily used in patients with SCIVF.
4. Verapamil
In their aforementioned seminal paper, Leenhardt et al. reported that verapamil was the only drug able to consistently suppress ventricular arrhythmia by suppressing short-coupled PVCs and prolonging their coupling interval [3]. However, they also noted that verapamil did not prevent SCD. Three of the twelve patients who were treated with verapamil experienced ventricular arrhythmia recurrence, of whom one died suddenly. The remaining nine patients remained asymptomatic during the mean follow-up duration of 7 years.
A number of case reports showed positive results with verapamil. One report described a patient treated with verapamil for 6 months without recurrences after an index SCA [26]. Rabah et al. reported an event-free 8-month follow-up on verapamil in a patient who presented with a VF storm [27]. Another patient was discharged with a prescription of verapamil after a VF storm and remained asymptomatic for one year [28]. However, the erratic nature of arrhythmia recurrences in SCIVF was demonstrated by a report describing a patient who was asymptomatic for 18 years using amiodarone until a VF storm occurred, prompting a switch to verapamil [29]. Although there were no recurrences during the 6 months that followed, it seems more reasonable to conclude that ventricular arrhythmia did not reappear than surmise that verapamil prevented recurrence. This unpredictable nature, matching our clinical experience, perfectly illustrates why it is unwise to draw conclusions based on case reports with a short duration of follow-up.
Numerous articles presented unfavorable effects of verapamil in patients with SCIVF. Two patients experienced VF recurrence only 2 months after verapamil was started [30,31]. Conte et al. reported the history of a patient who was free of recurrence on verapamil for 12 months until an electrical storm occurred. The verapamil dose was increased, and no arrhythmic events occurred during the 3-year follow-up [32]. Another patient suddenly died at home secondary to VF six years after his initial presentation, despite being treated with a daily dose of 480 mg verapamil [33]. In conclusion, verapamil may be effective in some patients with SCIVF, but breakthrough events can occur. The advantages of verapamil, as compared with quinidine, are its worldwide availability and low incidence of side-effects and adverse events.
5. Quinidine
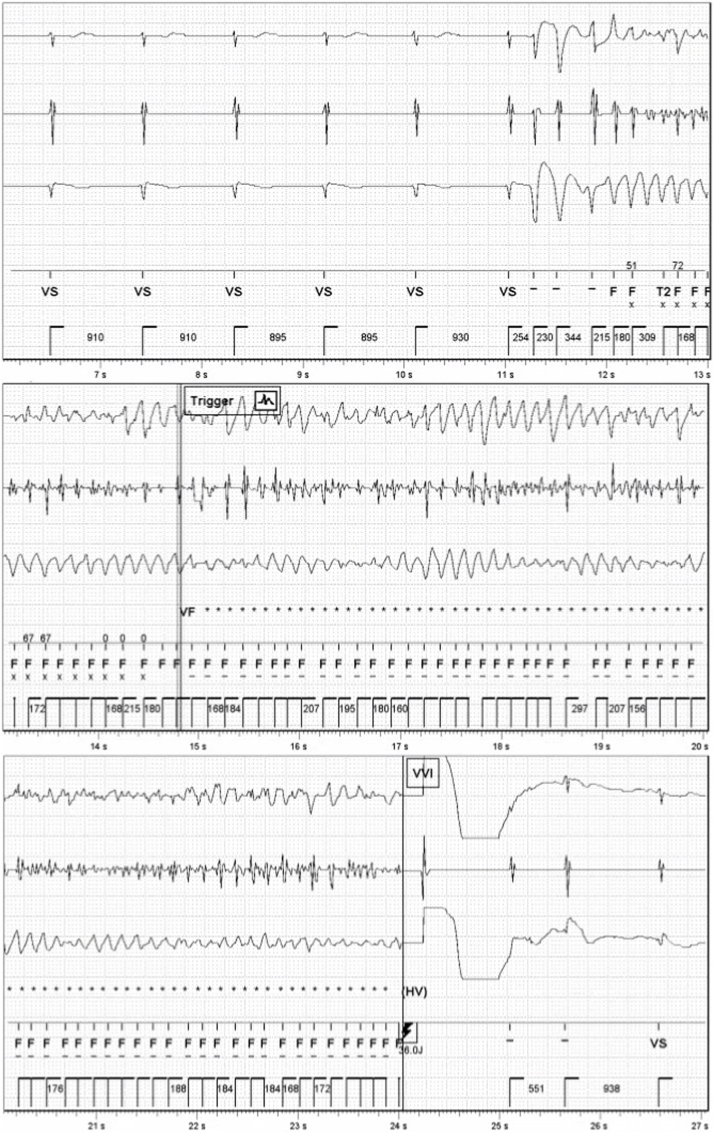
Quinidine is an antiarrhythmic drug with multi-channel blocking properties that has slowly gained popularity in the past decades for use in SCIVF. Interestingly, the first case report of what can retrospectively be called SCIVF specifically mentions that oral quinidine effectively prevented VF attacks [2]. Among the largest published cohort of patients with SCIVF, 12 patients were treated with quinidine, of whom 2 had a recurrence of VF during the follow-up of 65.5 months [4]. Both patients used quinidine doses of <300 mg daily. An early case report describes a 38-year-old man presenting with multiple episodes of syncope caused by self-limiting VF episodes, who was treated with quinidine and had no recurrence for a remarkable period of 37 years [34]. Pinnelas et al. reported on a young woman suffering SCA who was treated with quinidine after hospital discharge and remained asymptomatic at last follow-up of 18 months [35]. The brief case history of one of the several patients who were successfully treated with quinidine at our center is illustrated in Fig. 2.
Fig. 2.
Implantable cardioverter-defibrillator electrogram showing polymorphic ventricular tachycardia initiated by a short-coupled premature ventricular contraction degenerating into ventricular fibrillation before a successful shock was applied in a 47-year old man treated with bisoprolol 2.5 mg daily who initially presented with out-of-hospital cardiac arrest one year earlier. Following this recurrence, bisoprolol was replaced with quinidine 600 mg daily, and he remained asymptomatic until last follow-up (3.5 years).
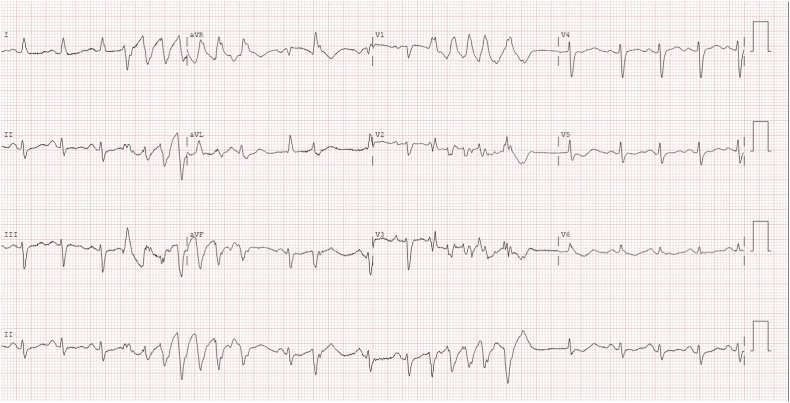
Quinidine does not appear to be universally effective. One patient experienced arrhythmic event recurrence only three months after quinidine was started despite adherence with an adequate dose [36]. Kataoka et al. described a patient in whom quinidine alone was insufficient to suppress short-coupled ventricular arrhythmia. However, she remained asymptomatic during the 32 months following the addition of verapamil [37]. We have also observed patients who experience breakthrough arrhythmic events despite the use of adequate dosages of quinidine (see below and Fig. 3A, Fig. 3B).
Fig. 3A.
12-lead ECG showing two salvo's of nonsustained polymorphic ventricular tachycardia in a patient with short-coupled idiopathic ventricular fibrillation during electrical storm despite treatment with quinidine 800 mg daily and an adequate quinidine trough level of 3.4 mg/L.
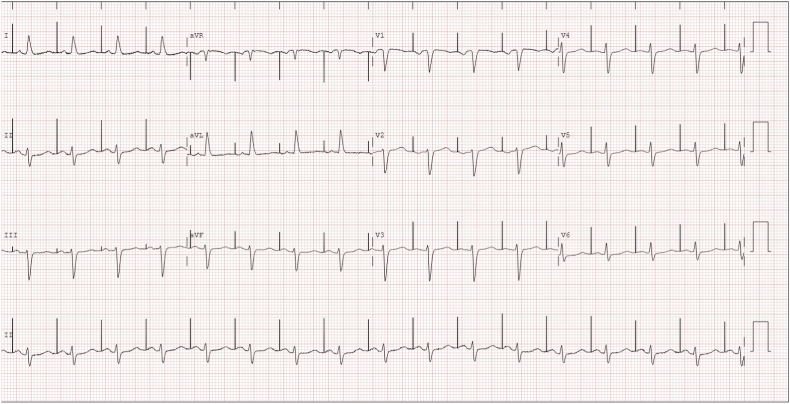
Fig. 3B.
Resolution of electrical storm after quinidine was replaced with cilostazol 300 mg daily.
Threatening patients with certain inherited arrhythmia syndromes, including SCIVF, is the problem of global availability of quinidine, which has been lamented by numerous experts [35,38]. Quinidine is available in the Netherlands, but the long-acting type is not, despite the latter being preferable for adherence. Most commonly, we initially prescribe a dose of 200 mg thrice daily. If ventricular arrhythmia recurs, dosage should be adjusted based on blood levels to reach the therapeutic range of 2.5–5.0 mg/l (trough level). Side effects, most often gastrointestinal in nature, are common, and can quite often be effectively treated by the addition of cholestyramine. Adverse effects, particularly QTc prolongation, and rarely thrombocytopenia and hepatotoxicity, should be monitored in patients treated with quinidine. In addition, a pronounced increase in ICD defibrillation threshold related to quinidine was observed in one patient at our center, requiring the discontinuation of quinidine. Therefore, testing of the defibrillation threshold after the initiation of quinidine may be prudent.
The lack of worldwide accessibility of quinidine has generated outcry among the medical community. A survey conducted in 2013 revealed that quinidine was not available in 76% of the surveyed countries, and only accessible through regulatory procedures that may take up to 90 days in 10% of countries [38]. In 2017, Eli Lilly, the sole manufacturer of quinidine in the United States, discontinued their production of parenteral quinidine. Despite its drawbacks, quinidine is uniquely indicated in multiple inherited arrhythmia diseases, including Brugada syndrome, early repolarization syndrome and IVF [39,40]. Though there are no data describing the evolution of quinidine availability since 2013, there is little reason to assume the situation has improved.
6. Cilostazol
Cilostazol, a selective phosphodiesterase III inhibitor, is primarily used to treat intermittent claudication [41], and also has effects in cardiac tissue. These effects may include a positive chronotropic effect, potentially related to inhibition of adenosine reuptake, and a positive inotropic effect caused by an increased intracellular cyclic AMP concentration [42]. It has been found that cilostazol may suppress Ito, as a result of its positive chronotropic effect, attenuation the electrical heterogeneity of action potentials and prolonging the action potential duration [43].
This hypothesis has spurred interest in the drug for application in certain inherited arrhythmia syndromes. Cilostazol showed mixed results in Brugada syndrome [[43], [44], [45]]. To the best of our knowledge, only one case report describing the use of cilostazol in SCIVF has been published, detailing the history of a young woman who was unsuccessfully treated with cilostazol 100 mg twice daily during an arrhythmic storm while pregnant [46]. Cilostazol is contra-indicated in patients with chronic heart failure and should be used cautiously in combination with verapamil, as the CYP3A4-inhibiting effect of verapamil may result in elevated cilostazol blood levels (although this effect could actually be utilized).
We have prescribed cilostazol 200 mg in a severely affected IVF patient carrying the DPP6 risk haplotype who, following his sentinel SCA at age 34, suffered numerous VF recurrences including electrical storms, after radiofrequency ablation proved insufficiently effective. The cilostazol dose was later increased to 300 mg daily, but despite this, VF recurred at least once per year. In another patient at our center who experienced an electrical storm under quinidine, replacing quinidine by cilostazol 300 mg daily successfully suppressed the ventricular arrhythmias (Fig. 3A, Fig. 3B). Nonetheless, currently available data is insufficient to recommend the routine use of cilostazol in SCIVF.
7. Bepridil
Bepridil is an antiarrhythmic drug classified primarily as a calcium antagonist that also has class Ia and class III antiarrhythmic properties [47]. The drug is currently only marketed in Japan. A voltage clamp study in sheep Purkinje fibers demonstrated that bepridil, among other ion currents, strongly reduces Ito current, meaning that this drug could be of interest for future research efforts in SCIVF [48]. Bepridil has been studied in the context of early repolarization syndrome. Katsuumi et al. described three patients with early repolarization syndrome and repeated episodes of ventricular arrhythmia, of whom two remained free of recurrences after starting bepridil [49]. Some reports describing the use of bepridil in Brugada syndrome are available, with seemingly positive results [50,51]. Bepridil 200 mg daily was added to cilostazol in the severely affected DPP6 carrier we described earlier. However, the patient continued to experience appropriate ICD shocks secondary to VF. We are not aware of any case reports describing the clinical use of bepridil in these patients with SCIVF.
8. Acute management
The clinical course of SCIVF tends to be erratic, exhibiting long periods without arrhythmic events, punctuated by severe recurrences, and it is not uncommon for patients to initially present with electrical storm [3], necessitating the use of intravenous medication. What little is known about the efficacy of various drugs in the setting of electrical storm derives from case reports in which a wide range of medications have been described.
To our knowledge, only one report described the administration of i.v. quinidine in emergency settings in SCIVF. Spectacular results were obtained in a young woman who was placed on extracorporeal membrane oxygenation after developing refractory (I)VF lasting for 8 h shortly following admission for out-of-hospital cardiac arrest and was restored to sinus rhythm by one defibrillation following the administration of two intravenous boluses of quinidine 300 mg, and had no recurrence of any ventricular ectopy [35].
In their case series, Ruan and Wang outlined the results obtained with the use of i.v. verapamil in three patients experiencing recurrent syncope, found to be caused by short-coupled polymorphic ventricular tachycardia. After administration of verapamil, ventricular tachycardia did not recur and the PVC burden decreased [52]. In a sedated and intubated patient with repeated episodes of pVT, verapamil was administered via nasogastric tube after other medications were found to be ineffective, terminating all arrhythmia [27]. Another case report described a 24-week pregnant woman with electrical storm unresponsive to multiple other antiarrhythmics, in whom a 2.5 mg bolus of i.v. verapamil eliminated all ventricular ectopy and PVT [46]. Contrarily, Rabah et al. reported a patient in whom verapamil boluses failed to control repeating short-coupled PVC-induced PVT/VF [26].
Isoproterenol, a β-adrenoceptor agonist, is another commonly used drug for the management of electrical storms in SCIVF as well as certain inherited arrhythmia syndromes. Isoproterenol increases the L-type calcium current, thereby counteracting the (increased) Ito current and lengthening an abbreviated Purkinje fiber action potential. At our center, where i.v. quinidine is not available, isoproterenol is the drug of choice for the management of electrical storms in SCIVF. In this setting, we increase the dosage of isoproterenol until the short-coupled PVCs disappear. However, published clinical results have been mostly negative. In multiple reports, isoproterenol failed to control arrhythmic storm [27,46], or appeared to cause an increase in the frequency of PVCs and ventricular tachycardia [53]. Successful use of isoproterenol, similar to our clinical experience, has also been described [28].
A few other medications were described by single case reports. Phenytoin, a class Ib antiarrhythmic drug, successfully eliminated almost all ventricular ectopy in an arrhythmic storm in one patient [54]. Intravenous dopamine titrated to a heart rate of 110 beats per minute was successful in suppressing pVT/VF storm [26]. Lastly, another patient with incessant VF storm was treated with bretylium with dramatic positive effects [55].
9. Our recommendations
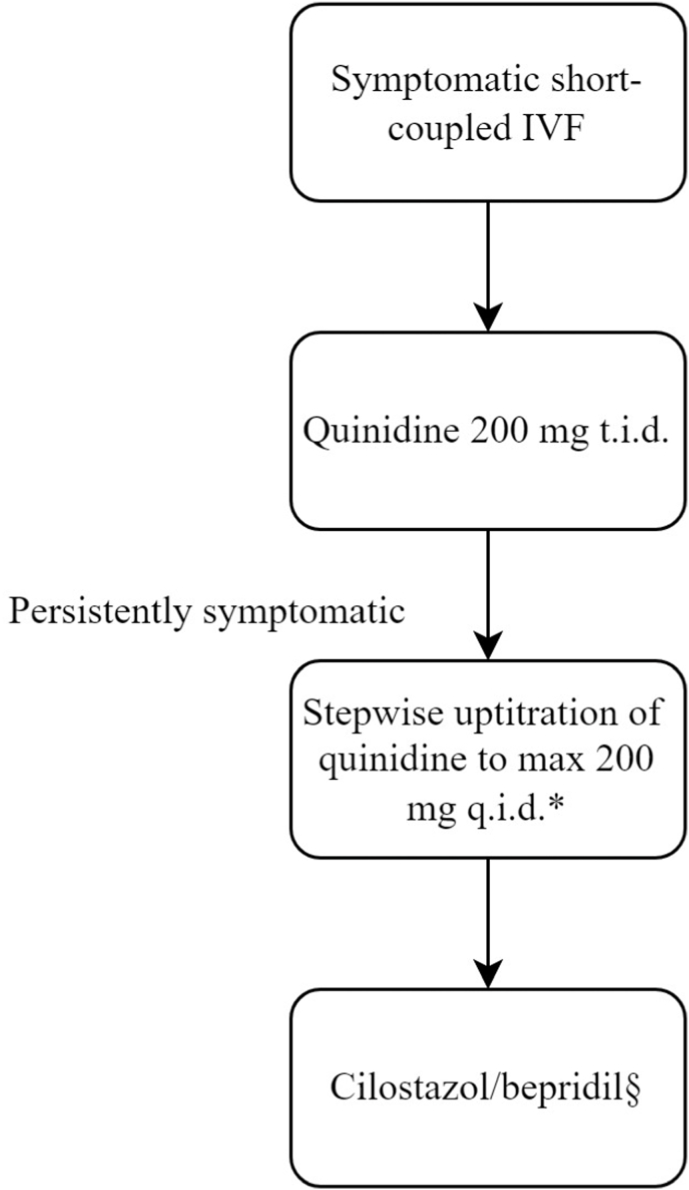
Given the lack of solid evidence, the pharmacological management of SCIVF is challenging and mostly empirical. Although verapamil appears to have been the preferred choice in the years following the first description of SCIVF, quinidine has become more popular in recent times and, in our experience, seems to be quite effective in our experience. However, QT prolongation and gastrointestinal side-effects tend to limit the dose that can be reached and we usually prescribe a dose of 200 mg three times daily. In most cases, further uptitration is not needed. The availability in certain parts of the world an important limitation for quinidine. In some parts of the world, long-acting quinidine tablets are available, which are preferable for adherence. Verapamil does not have these drawbacks, but it is our experience that verapamil does not always provide equal protection as quinidine. In the rare situation where quinidine and verapamil are insufficient to control the arrhythmia or are not tolerated, cilostazol and/or bepridil may be considered. Our recommendations are summarized in Fig. 4.
Fig. 4.
Proposed chronic pharmacological treatment algorithm for patients with SCIVF. SCIVF, short-coupled idiopathic ventricular fibrillation.
∗Radiofrequency ablation of the initiating malignant PVC may be considered depending on local resources and expertise and patient characteristics (e.g. the presence or absence of frequent isolated premature ventricular contractions similar to those initiating the ventricular arrhythmias to allow for activation mapping).§Recommended starting dose of cilostazol is 100 mg twice daily. Recommended dose of bepridil is 200–400 mg once daily.
10. Conclusion
Despite the condition being recognized for decades, there is uncertainty surrounding SCIVF with regard to its pathophysiology, the status as a separate disease entity and the optimal treatment options. Available reports indicate that quinidine is the best chronic pharmacological treatment option, and verapamil may be considered where quinidine is unavailable or not tolerated. In selected cases other drugs (e.g. cilostazol or bepridil) and ablation of VF initiating triggers may be critical. During electrical storms, i.v. quinidine and isoproterenol, depending on availability, appear to be the best therapies. More studies are needed to establish the efficacy of the various therapeutic strategies.
Funding
This work was supported by eRare (E-rare 3 - Joint Call 2015 to Prof. Wilde), the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences (PREDICT2 to Prof. Wilde), and the Dutch Heart Foundation (grant 03-003-2021-T061 to Dr. Postema).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Haïssaguerre M., Duchateau J., Dubois R., et al. Idiopathic ventricular fibrillation: role of purkinje system and microstructural myocardial abnormalities. JACC Clin Electrophysiol. 2020;6(6):591–608. doi: 10.1016/j.jacep.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moe T. A case of Morgagni-Adams-Stokes attacks caused by transient recurrent ventricular fibrillation without apparent organic heart disease. Acta Med Scand. 1948;130(5):416–435. doi: 10.1111/j.0954-6820.1948.tb10076.x. [DOI] [PubMed] [Google Scholar]
- 3.Leenhardt A., Glaser E., Burguera M., Nürnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89(1):206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg C., Davies B., Mellor G., et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: a report from the CASPER registry. Eur Heart J. 2021;42(29):2827–2838. doi: 10.1093/eurheartj/ehab275. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld S.A., van der Ree M.H., Mulder B.A., et al. Prevalence of short-coupled ventricular fibrillation in a large cohort of Dutch patients with idiopathic ventricular fibrillation. Circulation. 2022;145(18):1437–1439. doi: 10.1161/CIRCULATIONAHA.121.057878. [DOI] [PubMed] [Google Scholar]
- 6.Alders M., Koopmann T.T., Christiaans I., et al. Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet. 2009;84(4):468–476. doi: 10.1016/j.ajhg.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeppenfeld K., Tfelt-Hansen J., de Riva M., et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43(40):3997–4126. doi: 10.1093/eurheartj/ehac262. 2022. [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M., Hocini M., Cheniti G., et al. Localized structural alterations underlying a subset of unexplained sudden cardiac death. Circ Arrhythm Electrophysiol. 2018;11(7) doi: 10.1161/CIRCEP.117.006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haïssaguerre M., Shah D.C., Jaïs P., et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359(9307):677–678. doi: 10.1016/S0140-6736(02)07807-8. [DOI] [PubMed] [Google Scholar]
- 10.Wang G., Zhong L., Chu H., Wang C., Zhu X. Short-coupled variant of torsade de pointes: a systematic review of case reports and case series. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.922525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao L., Koopmann T.T., Ördög B., et al. Unique cardiac Purkinje fiber transient outward current β-subunit composition: a potential molecular link to idiopathic ventricular fibrillation. Circ Res. 2013;112(10):1310–1322. doi: 10.1161/CIRCRESAHA.112.300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinfurt J., Nazer B., Aguilar M., et al. Catheter ablation of short-coupled variant of torsade de pointes. Clin Res Cardiol. 2022;111(5):502–510. doi: 10.1007/s00392-021-01840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knecht S., Sacher F., Wright M., et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54(6):522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Haïssaguerre M., Shoda M., Jaïs P., et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106(8):962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 15.Marsman R.F., Barc J., Beekman L., et al. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J Am Coll Cardiol. 2014;63(3):259–266. doi: 10.1016/j.jacc.2013.07.091. [DOI] [PubMed] [Google Scholar]
- 16.Cheung J.W., Meli A.C., Xie W., et al. Short-coupled polymorphic ventricular tachycardia at rest linked to a novel ryanodine receptor (RyR2) mutation: leaky RyR2 channels under non-stress conditions. Int J Cardiol. 2015;180:228–236. doi: 10.1016/j.ijcard.2014.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii Y., Itoh H., Ohno S., et al. A type 2 ryanodine receptor variant associated with reduced Ca2+ release and short-coupled torsades de pointes ventricular arrhythmia. Heart Rhythm. 2017;14(1):98–107. doi: 10.1016/j.hrthm.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Roston T.M., Wei J., Guo W., et al. Clinical and functional characterization of ryanodine receptor 2 variants implicated in calcium-release deficiency syndrome. JAMA Cardiol. 2022;7(1):84–92. doi: 10.1001/jamacardio.2021.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruyama M., Joung B., Tang L., et al. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of purkinje fibers and triggered activity. Circ Res. 2010;106(2):399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts T.R., Yue A., Roberts P.R., Morgan J.M. Radiofrequency ablation of idiopathic ventricular fibrillation guided by noncontact mapping. J Cardiovasc Electrophysiol. 2004;15(8):957–959. doi: 10.1046/j.1540-8167.2004.03655.x. [DOI] [PubMed] [Google Scholar]
- 21.Reithmann C., Beckmann B.M., Kääb S. Purkinje-related ventricular fibrillation associated with a homozygous H558R polymorphism in the sodium channel SCN5A gene. Europace. 2016;18(6):896. doi: 10.1093/europace/euv134. [DOI] [PubMed] [Google Scholar]
- 22.Lipton J., Klein G.J., Sy R.W. Challenges in the diagnosis and management of idiopathic ventricular fibrillation. HeartRhythm Case Rep. 2015;1(5):269–274. doi: 10.1016/j.hrcr.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin C.A., Nunn L., Lambiase P.D. Syncope in a young man: role of Purkinje fibres in idiopathic ventricular fibrillation. Indian Pacing Electrophysiol J. 2017;17(4):113–115. doi: 10.1016/j.ipej.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajiyama T., Miyazawa K., Kondo Y., Nakano M., Kobayashi Y. SCN5A mutation and a short coupled variant of Torsades de Pointes originating from the right ventricle: a case report. J Cardiol Cases. 2019;21(3):104–105. doi: 10.1016/j.jccase.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belhassen B., Tovia-Brodie O. Short-coupled idiopathic ventricular fibrillation: a literature review with extended follow-up. JACC Clin Electrophysiol. 2022;8(7):918–936. doi: 10.1016/j.jacep.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Bogaard K., van der Steen M.S., Tan H.L., Tukkie R. Short-coupled variant of torsade de pointes. Neth Heart J. 2008;16(7–8):246–249. doi: 10.1007/BF03086155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabah H., Khalaf Z., Rabah A. Dopamine in idiopathic polymorphic ventricular tachycardia/ventricular fibrillation. J Innov Card Rhythm Manag. 2021;12(9):4699–4703. doi: 10.19102/icrm.2021.120908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillen R.H., Chort C., Mantilla L., Sriram C.S., Gonzalez M.D. Short coupled torsade de pointes: critical timing of the ventricular premature beats. J Electrocardiol. 2021;65:69–72. doi: 10.1016/j.jelectrocard.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Godinho A.R., Frutuoso C., Vasconcelos M., et al. Arrhythmic storm: short-coupled variant torsade de pointes. Rev Port Cardiol. 2016;35(5):307.e3075. doi: 10.1016/j.repc.2016.01.007. 307.e1. [DOI] [PubMed] [Google Scholar]
- 30.Saliba W., Abul Karim A., Tchou P., Natale A. Ventricular fibrillation: ablation of a trigger? J Cardiovasc Electrophysiol. 2002;13(12):1296–1299. doi: 10.1046/j.1540-8167.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 31.Strohmer B., Schernthaner C., Pichler M. T-wave oversensing by an implantable cardioverter defibrillator after successful ablation of idiopathic ventricular fibrillation. Pacing Clin Electrophysiol. 2006;29(4):431–435. doi: 10.1111/j.1540-8159.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 32.Conte G., Coppini L., Demola M.A., Ardissino D. Un caso di tempesta aritmica in una paziente con variante di torsione di punta ad intervallo di accoppiamento breve [A case of electrical storm in a patient with short-coupled variant of torsade de pointes] G Ital Cardiol. 2013;14(1):76–78. doi: 10.1714/1207.13375. [DOI] [PubMed] [Google Scholar]
- 33.Touat-Hamici Z., Blancard M., Ma R., et al. A SPRY1 domain cardiac ryanodine receptor variant associated with short-coupled torsade de pointes. Sci Rep. 2021;11(1):5243. doi: 10.1038/s41598-021-84373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kontny F., Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med. 1990;227(3):211–213. doi: 10.1111/j.1365-2796.1990.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 35.Pinnelas R., Friedman J., Gidea C., et al. The case for quinidine: management of electrical storm in refractory ventricular fibrillation. HeartRhythm Case Rep. 2020;6(7):375–377. doi: 10.1016/j.hrcr.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton J., Klein G.J., Sy R.W. Challenges in the diagnosis and management of idiopathic ventricular fibrillation. HeartRhythm Case Rep. 2015;1(5):269–274. doi: 10.1016/j.hrcr.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kataoka N., Nagase S., Okawa K., Aiba T., Kinugawa K., Kusano K. Multifocal Purkinje-related premature contractions and electrical storm suppressed by quinidine and verapamil in a case with short-coupled ventricular fibrillation. J Cardiol Cases. 2021;25(6):338–342. doi: 10.1016/j.jccase.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viskin S., Wilde A.A., Guevara-Valdivia M.E., et al. Quinidine, a life-saving medication for Brugada syndrome, is inaccessible in many countries. J Am Coll Cardiol. 2013;61(23):2383–2387. doi: 10.1016/j.jacc.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 39.Belhassen B., Rahkovich M., Michowitz Y., Glick A., Viskin S. Management of Brugada syndrome: thirty-three-year experience using electrophysiologically guided therapy with class 1A antiarrhythmic drugs. Circ Arrhythm Electrophysiol. 2015;8(6):1393–1402. doi: 10.1161/CIRCEP.115.003109. [DOI] [PubMed] [Google Scholar]
- 40.Haïssaguerre M., Sacher F., Nogami A., et al. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53(7):612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 41.Brown T., Forster R.B., Cleanthis M., Mikhailidis D.P., Stansby G., Stewart M. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2021;6(6):CD003748. doi: 10.1002/14651858.CD003748.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanlop N., Chattipakorn S., Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med. 2011;12(2):88–95. doi: 10.2459/JCM.0b013e3283439746. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya T., Ashikaga K., Honda T., Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13(7):698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 44.Ağaç M.T., Erkan H., Korkmaz L. Conversion of Brugada type I to type III and successful control of recurrent ventricular arrhythmia with cilostazol. Arch Cardiovasc Dis. 2014;107(8–9):476–478. doi: 10.1016/j.acvd.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Abud A., Bagattin D., Goyeneche R., Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17(2):210–212. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 46.Burrows K., Fox J., Biblo L.A., Roth J.A. Pregnancy and short-coupled torsades de pointes. Pacing Clin Electrophysiol. 2013;36(3):e77–e79. doi: 10.1111/j.1540-8159.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 47.Prystowsky E.N. Effects of bepridil on cardiac electrophysiologic properties. Am J Cardiol. 1992;69(11):63D–67D. doi: 10.1016/0002-9149(92)90961-w. [DOI] [PubMed] [Google Scholar]
- 48.Berger F., Borchard U., Hafner D. Effects of the calcium entry blocker bepridil on repolarizing and pacemaker currents in sheep cardiac Purkinje fibres. Naunyn-Schmiedeberg’s Arch Pharmacol. 1989;339(6):638–646. doi: 10.1007/BF00168656. [DOI] [PubMed] [Google Scholar]
- 49.Katsuumi G., Shimizu W., Watanabe H., et al. Efficacy of bepridil to prevent ventricular fibrillation in severe form of early repolarization syndrome. Int J Cardiol. 2014;172(2):519–522. doi: 10.1016/j.ijcard.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Sugao M., Fujiki A., Nishida K., et al. Repolarization dynamics in patients with idiopathic ventricular fibrillation: pharmacological therapy with bepridil and disopyramide. J Cardiovasc Pharmacol. 2005;45(6):545–549. doi: 10.1097/01.fjc.0000159660.16793.84. [DOI] [PubMed] [Google Scholar]
- 51.Ohgo T., Okamura H., Noda T., et al. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4(6):695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Ruan Y., Wang L. Short-coupled variant of torsade de pointes. J Tongji Med Univ. 2001;21(1):30–31. doi: 10.1007/BF02888030. [DOI] [PubMed] [Google Scholar]
- 53.Kabunga P., Medii C., Yeates L., Sy R.W. Malignant ventricular arrhythmic storm triggered by short-coupled premature ventricular contractions arising from the anterolateral papillary muscle. Euro J Arrhythmia Electrophysiol. 2016;2(1):33–36. [Google Scholar]
- 54.Golian M., Bhagirath K.M., Sapp J.L., Jassal D.S., Khadem A. Idiopathic ventricular fibrillation controlled successfully with phenytoin. J Cardiovasc Electrophysiol. 2011;22(4):472–474. doi: 10.1111/j.1540-8167.2010.01891.x. [DOI] [PubMed] [Google Scholar]
- 55.Nakstad A.R., Eek C., Aarhus D., Larsen A., Haugaa K.H. Survival after prolonged resuscitation with 99 defibrillations due to Torsade De Pointes cardiac electrical storm: a case report. Scand J Trauma Resuscitation Emerg Med. 2010;18:7. doi: 10.1186/1757-7241-18-7. [DOI] [PMC free article] [PubMed] [Google Scholar]