Abstract
Objective
A novel transdermal arterial gasotransmitter sensor (TAGS) has been tested as a diagnostic tool for lower limb microvascular disease in individuals with and without diabetes mellitus (DM).
Methods
The TAGS system noninvasively measures hydrogen sulfide (H2S) emitted from the skin. Measurements were made on the forearm and lower limbs of individuals from three cohorts, including subjects with DM and chronic limb-threatening ischemia, to evaluate skin microvascular integrity. These measurements were compared with diagnosis of peripheral artery disease (PAD) using the standard approach of the toe brachial index. Other measures of vascular health were made in some subjects including fasting blood glucose, hemoglobin A1c, plasma lipids, blood pressure, estimated glomerular filtration, and body mass index.
Results
The leg:arm ratio of H2S emissions correlated with risk factors for microvascular disease (ie, high-density lipoprotein levels, estimated glomerular filtration rate, systolic blood pressure, and hemoglobin A1c). The ratios were significantly lower in symptomatic DM subjects being treated for chronic limb-threatening ischemia (n = 8, 0.48 ± 0.21) compared with healthy controls (n = 5, 1.08 ± 0.30; P = .0001) and with asymptomatic DM subjects (n = 4, 0.79 ± 0.08; P = .0086). The asymptomatic DM group ratios were also significantly lower than the healthy controls (P = .0194). Using ratios of leg:arm transdermal H2S measurement (17 subjects, 34 ratios), the overall accuracy to identify limbs with severe PAD had an area under the curve of the receiver operating curve of 0.93.
Conclusions
Ratios of transdermal H2S measurements are lower in legs with impaired microvascular function, and the decrease in ratio precedes clinically apparent severe microvascular disease and diabetic ulcers. The TAGS instrument is a novel, sensitive tool that may aid in the early detection and monitoring of PAD complications and efforts for limb salvage.
Keywords: Peripheral vascular disease, Hydrogen sulfide, Diabetic wounds
Diabetes mellitus (DM) currently affects more than 34 million Americans1 and is strongly associated with peripheral arterial disease (PAD)2,3 and other cardiovascular disorders. PAD, asymptomatic in many diabetic patients due to peripheral neuropathy,4,5 is frequently difficult to evaluate using the ankle-brachial index (ABI) or toe brachial index (TBI) due to characteristic tibial vessel incompressibility in chronic diabetes.4, 5, 6, 7 Given the significant role of cutaneous microcirculation in wound healing,8 there is a critical need for accurate, easy-to-use diagnostic techniques to detect and monitor PAD severity, especially in diabetic patients. The most severe form of PAD, chronic limb-threatening ischemia (CLTI), is present in nearly 11% of patients with PAD and projected to impact more than 4 million Americans by 2030,9 presenting a significant risk of nonhealing wounds and major amputation. Chronic diabetic-related wounds are a significant global cause of morbidity and public health care burden10 generating more than 60% of nontraumatic limb amputations and 27% of the $116 billion diabetic health care costs in the United States.1,11,12 Despite improved treatments with new diabetic wound therapies,13,14 healing rates are variable with frequent recurrence15 exacerbated by loss of epidermal and dermal architecture, which contributes to the risk of lower-limb amputations.16,17 A critical gap is how to determine which patients will benefit from revascularization procedures and to delineate the optimal timing of such interventions.
Currently, the ABI/TBI18,19 represents the best, albeit indirect, measure of microcirculatory flow in a lower limb. Unfortunately, tibial vessel calcification limits the usefulness of this approach in many individuals with chronic diabetes.19 Transcutaneous oximetry is an alternative noninvasive approach that is sensitive to microvascular impairment, but is costly and cumbersome and therefore not widely used. The ideal clinical tool to determine microvascular status would be a small, easy-to-use point-of-care device that could be used outside of specialized centers.20
Hydrogen sulfide (H2S), a gasotransmitter critical in cardiovascular homeostasis, is produced in vascular endothelial cells by cystathionine γ-lyase21,22 where its ability to stimulate angiogenesis and vasodilation enhances recovery from tissue ischemia,23,24 limb ischemia,25 myocardial ischemia,26 and cerebral artery occlusion.27 H2S stimulates angiogenesis, acts as an anticoagulant and antiadhesive agent,28 inhibits inflammation and apoptosis,29,30 and upregulates antioxidant pathways.31 Endothelial cells exposed to hyperglycemia have decreased H2S production and bioavailability,32 and both animal models of diabetes and human subjects with diabetes33,34 have a demonstrable loss of H2S production that may contribute to vascular dysfunction35 and poor wound healing. Indeed, low plasma levels of H2S in hemodialysis patients correlate with accelerated atherosclerosis,36 as well as vascular inflammation.37 H2S can pass through cellular membranes and freely permeates through tissues38,39 enabling the noninvasive measurement of transdermal H2S as an indicator of the H2S generation within the skin microcirculation. Thus, H2S transpiration from the skin is a potential direct biomarker of skin microvascular function.40
The transdermal arterial gasotransmitter sensor (TAGS) is a device that noninvasively measures transdermal H2S levels via a sample chamber sealed to the skin to collect and concentrate surface-emitted H2S. The collected sample is routed through a uniquely developed sensor to measure trace levels of H2S as described previously.41 The present study is the first to evaluate transdermal H2S measurements as a biomarker of microvascular disease using the TAGS device.
Methods
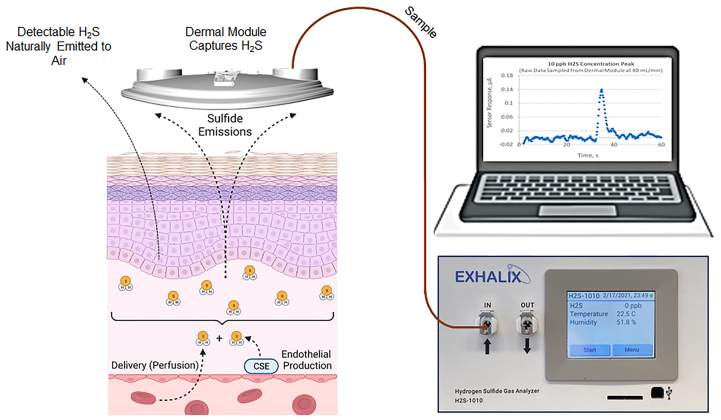
The study protocols were approved by the UNM Health Sciences Center institutional review board (HRRC: 19-153). As depicted in Fig 1 and described in detail elsewhere, gas samples are collected from the skin to measure emitted H2S using the TAGS system.41 In addition, blood and urine samples were collected to measure hemoglobin A1c (HgbA1c), serum lipid profile, basic metabolic indices, estimated glomerular filtration rate, and microalbuminuria to evaluate the severity of microvascular disease.
Fig 1.
Transdermal arterial gasotransmitter sensor (TAGS) approach for determining endothelial dysfunction. Hydrogen sulfide (H2S) is a reactive thiol with reducing activities that is synthesized within the vasculature primarily by cystathionine γ-lyase (CSE). H2S can react with cysteine residues on multiple proteins through sulfhydration, shown to modulate the function of multiple ion channels, structural proteins, and enzymes to cause vasorelaxation within seconds, and its effects persist to angiogenesis within days of exposure to H2S, illustrating how this molecule is critical to angiogenic pathways. Therefore, from the TAGS measurement point of view, the two mechanisms giving rise to bioavailability of H2S within the dermal layer, as shown on the left, are local production of H2S by the endothelial layer and delivery of systemic H2S to the measurement location. The TAGS transdermal sampler placed on the surface of the skin collects and measures the portion reaching the surface, proportional to the skin bioavailability (concentration) of H2S.
Study subjects
Subjects were recruited in from three groups: healthy control subjects without either DM or PAD (healthy group), those with DM but without overt clinical PAD (DM group), and those with both DM and clinical severe PAD with CLTI (DM+PAD group). Subjects were classified as diabetic by HgbA1c ≥6.5%.42 PAD and CLTI were diagnosed as having the following: weak or absent pulse at the ankle with tissue loss or foot wound and/or nonhealing prior foot amputation site and ipsilateral TBI less than 0.6. ABI and TBI were performed clinically in an Intersocietal Accreditation Commision Vascular Laboratory-accredited noninvasive clinical vascular laboratory and abstracted from the medical record for subjects with CLTI. Subjects recruited in the control group were free from major comorbid conditions including heart failure, cancer, renal failure, and pulmonary hypertension (Table I). Written informed consent was obtained from all participants.
Table I.
Clinical characteristics of individuals in each of the three cohorts
| Characteristic | Non-DM | DM (–PAD) | DM (+PAD) |
|---|---|---|---|
| Male/female, n | 6/9, 15 | 9/8, 17 | 7/5, 12 |
| Age, years | 40.3 ± 45.5 | 44.1 ± 8.1 | 64.6 ± 13.2a,b |
| BMI, kg/m2 | 24.9 ± 2.4 | 28.8 ± 6.7a | 26.7 ± 6.0a,b |
| SBP, mm Hg | 122.9 ± 13.6 2 | 130.9 ± 6.7a | 138.8 ± 13.4a,b |
| ASCVD risk score, %c | 1.4 ± 1.8 | 3.5 ± 3.3 | 25.1 ± 13.4a |
| ΔTAGS ratio (healthy – disease present) | 0.20 ± 0.37 | 0.37 ± 0.34 | 0.24 ± 0.18 |
| HgbA1c, % | 5.2 ± 0.3 | 8.0 ± 2.0a | 9.5 ± 2.1a |
| Total cholesterol, mg/dL | 193.6 ± 33.9 | 180.9 ± 35.3 | 153.8 ± 28.4 |
| HDL, mg/dL | 69.5 ± 17.4 | 55.1 ± 18.8 | 43.5 ± 5.8a |
| LDL, mg/dL | 106.7 ± 29.0 | 100.7 ± 32.1 | 75.8 ± 24.4 |
| Triglycerides, mg/dL | 87.6 ± 34.9 | 125.9 ± 86.7 | 173.2 ± 49.6a |
| Blood urea nitrogen, mg/dL | 13.6 ± 3.8 | 16.9 ± 13.1 | 27.5 ± 15.1a |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.4 | 1.8 ± 1.5a,b |
| Fasting glucose, mg/dL | 81.7± 4.3 | 175.1 ± 46.8a | 169.5 ± 61.0a |
| Urine creatinine, mg/dL | 109.1 ± 80.3 | 105.8 ± 62.3 | 118.2 ± 96.1 |
| Urine ratio microalbumin/creatinine | 3.8 ± 3.7 | 54.3 ± 121.8a | – |
ASCVD, Atherosclerotic cardiovascular disease; BMI, body mass index; DM, diabetes mellitus; HDL, high-density lipoprotein; HgbA1c, hemoglobin A1c; LDL, low-density lipoprotein; PAD, peripheral artery disease; SBP, systolic blood pressure; TAGS, transdermal arterial gasotransmitter sensor.
Values are shown as mean ± standard deviation.
Indicates different from non-DM for P < .05, one-way analysis of variance with the post hoc Tukey test.
Indicates different from DM (–PAD) for P < .05, one-way analysis of variance with the post hoc Tukey test.
Estimate of the 10-year risk of developing coronary heart disease taking into account age, sex, total cholesterol, HDL cholesterol, blood pressure, treatment for hypertension, and smoking. ASCVD values <7.5% are considered low risk.
Study design
Before H2S measurements and blood draws for lipid panel and fasting glucose, participants fasted overnight and arrived in the morning. Blood pressure (BP) was measured following standard guidelines, and the same BP device was used for all study participants.43 Briefly, BP was measured in both arms and then repeated in the arm with the highest systolic BP. Height, weight, and heart rate were also recorded. Blood samples were collected to measure HgbA1c, lipids, and for a basic blood metabolic panel. Urine was collected to measure microalbuminuria and creatinine. All samples were analyzed at a core clinical reference laboratory. These values were used to determine an atherosclerotic cardiovascular disease (ASCVD) risk score.43 The ASCVD risk is an estimate of the 10-year risk of ASCVD, defined as coronary death or nonfatal myocardial infarction and fatal or nonfatal stroke.44 The variables used include systolic BP, HDL cholesterol, total cholesterol, age, race, sex, diabetes, hypertension treatment, and smoking status. An ASCVD risk of ≥7.5% is considered high risk (Table II).43
Table II.
Pearson correlation coefficient (r value) and level of significant relationship (P value) of toe brachial index (TBI) values (left panel) vs transdermal arterial gasotransmitter sensor (TAGS) ratios (right panel) between six vascular health biomarkers: age, hemoglobin A1c (HgbA1c), high-density lipoprotein (HDL), estimated glomerular filtration rate, systolic blood pressure, and atherosclerotic cardiovascular disease (ASCVD) risk score
| Toe brachial index | TAGS ratio | |
|---|---|---|
| Age, years |
P = .6662 r = −0.1028 |
P = .0244 r = −0.3554 |
| HgbA1c, % |
P = .5562 r = −0.1399 |
P < .0001 r = −0.5792 |
| HDL, mg/dL |
P = .5864 r = −0.1965 |
P ≤ .0001 r = 0.7033 |
| Estimated glomerular filtration rate |
P = .6747 r = −0.1000 |
P = .0069 r = 0.4307 |
| Systolic blood pressure, mm Hg |
P = .8899 r = 0.0589 |
P = .0040 r = −0.5649 |
| ASCVD risk score, % |
P = .8700 r = 0.0869 |
P = .0026 r = −0.6093 |
Data analyzed via linear regression analysis. There is a significant correlation between TAGS ratios and the five measured vascular health biomarkers (P < .05, boldface).
Participants were seated on an examination table and allowed to reach a resting condition. Subsequently, skin surface was cleaned with 70% isopropyl alcohol followed by purified water and wiped dry with a clean cloth before placement of the transdermal sampler. The samplers were sealed to the arm with the highest systolic BP reading and on the distal calf of each leg (3 sample sites per person). A gas-tight seal was achieved using Eakin cohesive as the interface to the skin. Each sampling chamber remained in place for 10 minutes before gas retrieval and measurement. Transdermal H2S is reported as concentration within the sample chamber in parts-per-billion (ppb) and converted to skin emission rate in fmol/cm2/min. The TAGS system was calibrated using a linear standard curve created with multiple-point calibration from 0 to 100 ppb at 10-ppb intervals. Calibration gas mixtures were generated by diluting 10-ppm stock H2S (balance N2 with a purity of ±2%; CalGas Direct Inc) with N2 gas.
Data analysis
Each leg was counted as a single data point because disease can be bilateral or unilateral and the test is to discriminate individual limbs with adequate microcirculation from those with impaired microcirculation. Data are presented as mean ± standard deviation (SD). Pearson correlation coefficients were estimated to evaluate the strength of the linear relationships between measured biomarkers and the ASCVD score with TBI or with transdermal H2S readings. Differences in transdermal H2S measurements among the three groups (non-DM, DM-PAD, and DM+PAD) were analyzed using a one-way analysis of variance with the Tukey multiple comparison test.
Results
Transdermal H2S measurements differentiate between groups
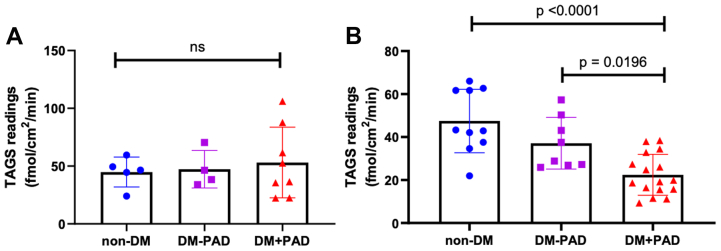
Transdermal H2S readings from the arm were not significantly different between groups (Fig 2, A), whereas transdermal leg H2S readings in DM + PAD (8 subjects, 16 legs) were significantly lower than readings from control (5 subjects, 10 legs; P < .0001; Fig 2, B). Expressing transdermal H2S emissions as a ratio of leg to arm (much like ABI) increased discrimination between groups such that all groups were statistically distinct from one another (Fig 3).
Fig 2.
Transdermal hydrogen sulfide (H2S) levels (transdermal arterial gasotransmitter sensor [TAGS] readings) in parts-per-billion (ppb) measured in the (A) arms and in the (B) leg in each group. Data analyzed using one-way analysis of variance. Significant differences between the control group (non-diabetes mellitus [DM]) and DM + peripheral artery disease (PAD) for the leg measurements.
Fig 3.
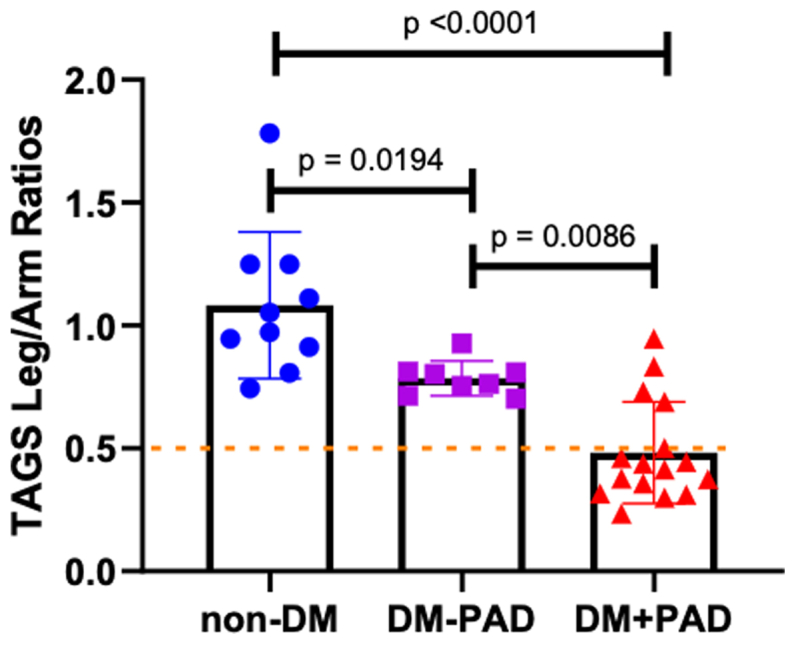
Transdermal arterial gasotransmitter sensor (TAGS) ratios of the legs and arms across different vascular health groups. Data analyzed via ordinary one-way analysis of variance. Significant difference in ratios measured between the control group (non-diabetes mellitus [DM]) and both DM groups (P < .0001 and P < .05) and between the DM – peripheral artery disease (PAD) group and the DM + PAD group (P < .05).
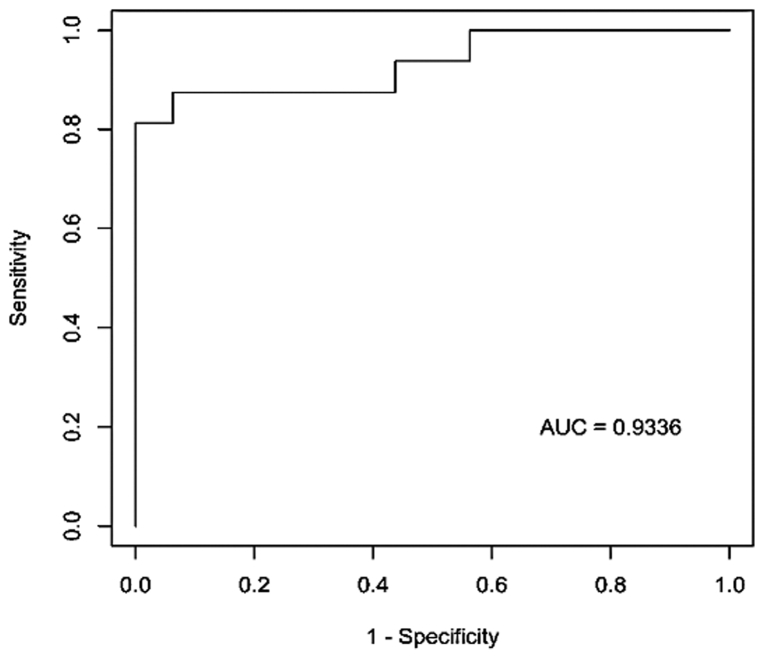
The receiver operating curve (ROC) analyses of the leg:arm transdermal H2S ratio gave an area under the curve (AUC) of 0.9336 (Fig 4). The Youden index was maximized at 0.8125 for two separate cut-points. Defining leg:arm transdermal H2S ratios of less than 0.695 as testing positive for PAD resulted in a sensitivity of 81.25% (95% confidence interval [CI]: 54.3%-95.6%) and a specificity of 100% (95% CI: 79.4%-100.0%). Defining leg:arm transdermal H2S ratios of less than 0.735 as indicative of PAD gave a sensitivity of 87.5% (95% CI: 61.6%-98.4%) and a specificity of 93.75% (95% CI: 79.4%-100.0%).
Fig 4.
Receiver operator characteristic curve illustrating the ability of transdermal arterial gasotransmitter sensor (TAGS) leg:arm ratios to discriminate between those with and without detection of chronic limb-threatening ischemia (CLTI) in the entire population of participants tested. AUC, Area under the curve.
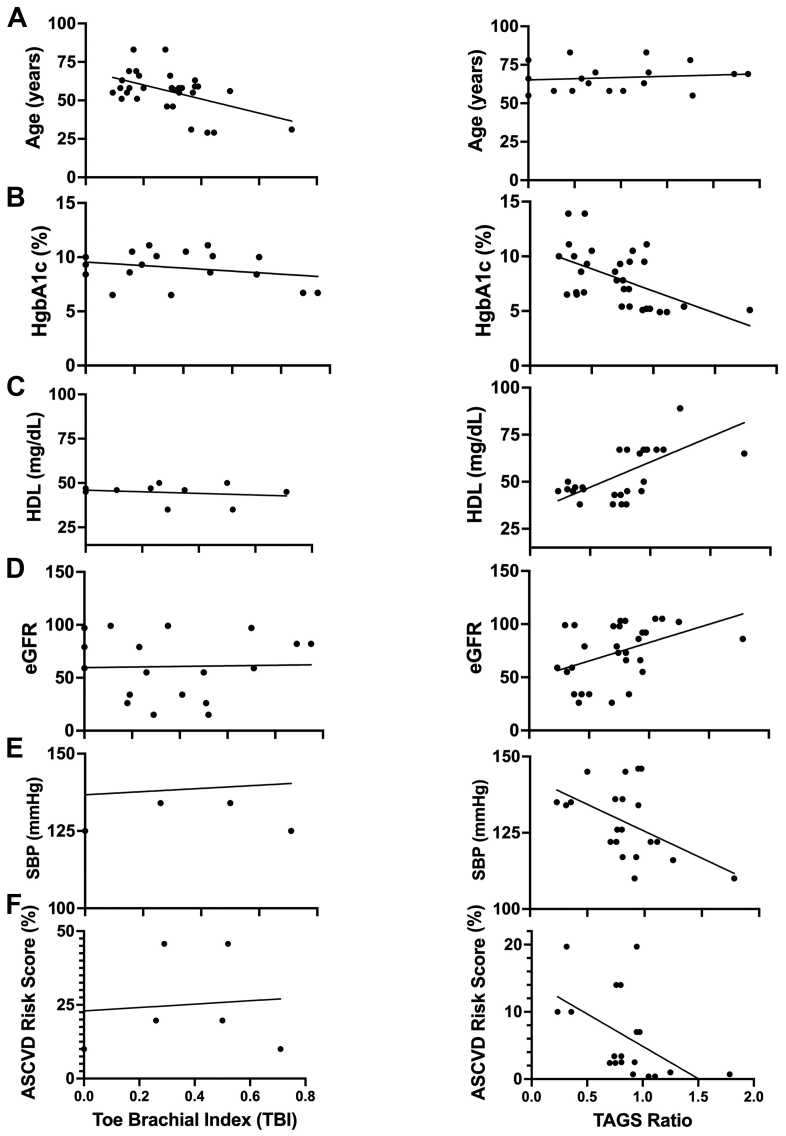
Transdermal H2S measurements correlate with cardiovascular risk whereas TBI does not
Pearson correlation coefficients in Fig 5 show the plots of measured blood biomarkers and ASCVD correlated with either TBI (left panels) or H2S emission rate (right panels). TBI was not significantly correlated with any clinical biomarker including age, HgbA1c, HDL, estimated glomerular filtration rate and systolic BP, and ASCVD risk score percentage. Transdermal H2S was significantly correlated with all listed biomarkers with decreasing H2S predicting increasing cardiovascular risk (P < .05).
Fig 5.
Comparison of the relationship of toe brachial index (TBI) values (left panels) vs transdermal arterial gasotransmitter sensor (TAGS) ratios (right panels) between five vascular health biomarkers: (A) age, (B) hemoglobin A1c (HgbA1c), (C) high-density lipoprotein (HDL), (D) estimated glomerular filtration rate (eGFR), and (E) systolic blood pressure (SBP), and (F) atherosclerotic cardiovascular disease (ASCVD) risk score percentage. Data analyzed via linear regression analysis. There is a significant correlation between TAGS ratios and the six measured vascular health biomarkers (P < .05).
Discussion
Before these studies, experiments on healthy human subjects were carried out to validate the TAGS reading specificity for H2S measurement.45 In those experiments, TAGS readings were compared with measurements made with a commercial device that measures H2S at low concentrations comparable to those measured with TAGS. The Serinus 57 TRS device (ACOEM Ecotech) thermocatalytically converts H2S to SO2 before chemiluminescent detection of SO2 and has a limit of detection of less than 1 ppb. Although not suitable for clinical use, our earlier studies demonstrated that the TAGS device measurements have excellent correlation to those made by the Serinus device (r = 0.9359 and P < .0001).45
Emissions of volatile organic compounds (VOCs) have been used as disease biomarkers since the time of Hippocrates, who taught that breath odor identifies liver dysfunction.46 Indeed, approximately 1849 VOCs have been identified in humans,47,48 500 of which are released through the skin. Recent studies49 have mapped the VOC profile of healthy human skin using whole body gas samples (breath gas excluded) analyzed with a gas chromatograph-mass spectrometery system. This study identified 33 skin-emitted molecules including terpenes, aldehydes, alcohols, heterocycles, ketones, and dimethyl sulfide. However, no study to date has evaluated transdermal H2S emission rates.
H2S is difficult to measure in living systems owing to its volatile nature and the low concentrations of free H2S in tissues due to protein binding, oxidation, metabolism, and conversion to stable storage as polysulfides, so-called sulfane sulfur.50 Current results describe, for the first time, noninvasive measurement of transdermal H2S emission rates in human subjects and differences in emission rates between diabetic patients with and without PAD. This corroborates prior studies in subjects with diabetes showing positive correlations between plasma levels of H2S measured using liquid chromatography or methylene blue assays and endothelial function. Importantly, these measurement techniques are difficult to conduct and interpret51 due to nuances in published methods and the invasive nature of sample collection. The real-time transdermal measurement approach addresses many of the problems associated with measuring H2S levels in blood samples, which need to be stabilized for later analysis. Real-time measurement prevents sample oxidation and loss to off-gassing, which frequently gives falsely low results.
The H2S measurements made with the TAGS device address these prior issues by making readings transdermally and noninvasively. The current studies demonstrate significant variability between subjects in transdermal H2S transmission. We also observed that there are no differences between the control and DM groups in forearm measurements. In contrast, even with the high variability, transdermal H2S emissions are significantly lower in the legs of PAD patients with DM compared with healthy controls. When the data are expressed as a ratio of the lower limb to the upper limb (leg:arm) to control for inherent variability between subjects, differences are even more apparent. This corroborates previous observations that vascular production52 and circulating levels53,54 of H2S are decreased in diabetic subjects. In addition, this supports prior evidence that small arteries generate more H2S compared with large arteries.54, 55, 56 Indeed, it is tempting, based on these results, to speculate that differences in H2S production in the lower limb skin circulation may contribute to lower limb sequelae in patients with PAD and DM. Indeed, the loss of microvascular production of H2S may explain some of the poor correlations between the duration of diabetes and the development of nonhealing ulcers and PAD.57
The ROC analysis of the leg:arm transdermal H2S ratio resulted in an AUC estimate of 0.9336, suggesting that this ratio differentiates a leg with limb-threatening PAD from a leg with non-limb-threatening PAD with a probability greater than 90%. The candidate cut-points provide estimates of sensitivity and specificity that suggest that this ratio has excellent potential to correctly classify limb risk according to PAD status. These results were derived from a study with a relatively small sample size; a larger study would improve the estimate of AUC and provide refined cut-points that increase assessments of sensitivity and specificity. Because early diagnosis of CLTI increases the opportunity to mitigate disease and wound progression, transdermal H2S readings have the potential to impact the significant socioeconomic burdens of CLTI and downstream complications. Furthermore, lower limb ulceration complications, which include chronic immobility and amputation, are more effectively prevented by early detection, thereby allowing earlier intervention.58, 59, 60 Therefore, efficient early diagnosis of impaired skin microcirculation is a major gap in effective medical management of diabetes that may be bridged by the use of the TAGS diagnostic technique.
Although chronic diabetic foot ulcers are known to be associated with recurrent vascular endothelial damage,8,61,62 detecting this disease at an early stage has proved challenging. Potential PAD biomarkers including plasma sortilin and omentin-1 in subjects with diabetes have long been investigated.63 However, discordant observations raise questions on the biomarkers to diagnose PAD. In addition, all blood-borne markers require phlebotomy and laboratory analysis and cannot determine that changes are unique to individual extremities.
An alternative noninvasive method for evaluating microvascular function in the forearm is reactive hyperemia index (RHI) measured by pulse amplitude tonometry. A recent study measuring RHI in groups similar to the current study observed that patients with diabetes have a lower RHI compared with both healthy subjects and patients with diabetes and no microvascular complications.64,65 Similar to our study, endothelial dysfunction evaluated by RHI indicates that skin endothelial dysfunction correlates with the severity of PAD. This supports the current results that suggest that skin microvascular damage correlates with the severity of diabetic foot disease. However, we observed that the TAGS ratios were much more sensitive, with an impressive ROC for differentiating severe disease from both healthy controls and subjects with diabetes without severe disease. Thus, evaluating skin microcirculatory function appears to be an effective measure of skin healing capacity. In addition, TAGS measurements are more effective than current clinical standards and other novel approaches such as laser speckle contrast imaging.66 TAGS leg:arm ratios in this study were able to identify high-risk presymptomatic diabetic PAD patients those with more severe disease (Fig 3). In addition, TAGS ratios strongly correlate with numerous risk factors for microvascular disease, supporting the contention that lower leg readings are caused by diminished local vascular endothelial health.
In addition to providing a strong correlation between transdermal H2S emissions and nonhealing diabetic foot ulcers, the results of this study support the hypothesis that decreased H2S levels contribute to decreased wound healing in diabetic patients. This relationship is suggested by the known proangiogenic properties of H2S,27,67, 68, 69, 70 described antioxidant properties,33, 71, 72, 73 H2S inhibition of platelet aggregation, and H2S-induced vasodilation.33,54,56 Thus, the loss of H2S in diabetes53,74,75 may contribute to and serve as a marker for diminished microvascular function and wound healing.
Studies in cultured endothelial cells show that hyperglycemia directly decreases both cystathionine γ-lyase expression and H2S production,52 suggesting that the control of diabetes has an impact on microcirculatory function. In addition, H2S appears to increase insulin production76,77 and may also increase sensitivity to insulin,77 although other studies have suggested that this relationship is more complex.78 Additional studies are needed to delineate the molecular signaling pathways that regulate the role of H2S in the pathophysiology of diabetic wound healing and PAD and determine if the microcirculation of the skin in the lower limb is more susceptible to damage than the skin in the arm. It is clear that H2S plays a critical role in vascular health, but many questions also remain.
Conclusions
The data presented in this paper provide evidence of the association between microvascular health and transdermal H2S emission rate. Although further clinical studies are needed to characterize larger populations, our results indicate that limbs affected by CLTI can be differentiated from asymptomatic nondiabetic or diabetic limbs without severe PAD with an accuracy of approximately 88%. Compared with TBI, TAGS demonstrated better detection accuracy in identifying tissue loss-afflicted limbs from asymptomatic limbs.
These findings demonstrate that the TAGS ratio is a novel and sensitive indicator of impaired healing capacity in diabetic patients. We anticipate that TAGS addresses an urgent clinical need inherent in using ABI as a diagnostic modality due to the severe limitation of incompressible tibial vessels often present in advanced diabetes. Although TBI and Doppler waveform analysis can be used as surrogates to quantify distal limb perfusion, these are unfortunately imperfect measures as many patients with advanced diabetic-related CLTI frequently have some degree of digital vessel calcification, leading to inaccuracies in toe pressure. In addition, the prognostic value of toe pressure alone in predicting limb outcomes and need for (or response to) revascularization is not well established. In many other cases, TBI is rendered impossible by the presence of either great toe amputation or transmetatarsal amputation. TAGS provides a direct assessment of microvascular status and is not affected by the presence of amputation or macrovascular vessel incompressibility. Ultimately, TAGS may be used as a point-of-care diagnostic that provides a qualitative measure that can be used in every subject to screen for impaired microvascular function and as a follow-up tool to evaluate the efficacy of revascularization procedures.
Limitations
One limitation of this study is that fewer TBI measurements were available to calculate correlations as only subjects with severe PAD with CLTI underwent vascular laboratory testing. Although it can be inferred that subjects recruited into the healthy control group presumably had normal macrovascular flow to the lower limbs, the lack of available noninvasive testing prevents complete assurance of this.
Footnotes
This work was funded by National Heart, Lung, and Blood Institute (NHLBI) grants R44 HL121871 (N.L.K. and R.S.) and HL123301 (N.L.K.).
Author conflict of interest: B.J.B., D.M.F., M.K., and R.S. have financial ties with Exhalix, manufacturer of the TAGS instrument. The other authors have no competing interests.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. 2020. p. 2. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed April 17, 2023.
- 2.Selvin E., Erlinger T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Olin J.W., Sealove B.A. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–692. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey S.L., Lanting S.M., Chuter V.H. The ankle brachial index in people with and without diabetes: intra-tester reliability. J Foot Ankle Res. 2020;13:1–6. doi: 10.1186/s13047-020-00389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamil S., Sehested T.S.G., Carlson N., Houlind K., Lassen J.F., Bang C N., et al. Diabetes and risk of peripheral artery disease in patients undergoing first-time coronary angiography between 2000 and 2012—a nationwide study. BMC Cardiovasc Disord. 2019;19:234. doi: 10.1186/s12872-019-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben A.J.H.M., Martens R.J.H., Stehouwer C.D.A. Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol. 2017;28:3461–3472. doi: 10.1681/ASN.2017020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronow W.S. Peripheral arterial disease of the lower extremities. Arch Med Sci. 2012;8:375–388. doi: 10.5114/aoms.2012.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasubramanian G.V., Chockalingam N., Naemi R. The role of cutaneous microcirculatory responses in tissue injury, inflammation and repair at the foot in diabetes. Front Bioeng Biotechnol. 2021;9:732753. doi: 10.3389/fbioe.2021.732753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes J.A., Eid M.A., Creager M.A., Goodney P.P. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1808–1817. doi: 10.1161/ATVBAHA.120.314595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen C.K., Gordillo G.M., Roy S., Kirsner R., Lambert L., Hunt T.K., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordois A., Scuffham P., Shearer A., Oglesby A., Tobian J.A. The health care costs of diabetic peripheral neuropathy in the U.S. Diabetes Care. 2003;26:1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 12.Driver V.R., Fabbi M., Lavery L.A., Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S–22S. doi: 10.1016/j.jvs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Shen J.T., Falanga V. Innovative therapies in wound healing. J Cutan Med Surg. 2003;7:217–224. doi: 10.1007/s10227-002-0106-5. [DOI] [PubMed] [Google Scholar]
- 14.Brem H., Sheehan P., Boulton A.J.M. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187:S1–S10. doi: 10.1016/S0002-9610(03)00299-X. [DOI] [PubMed] [Google Scholar]
- 15.Sweitzer S.M., Fann S.A., Borg T.K., Baynes J.W., Yost M.J. What is the future of diabetic wound care? Diabetes Educ. 2006;32:197–210. doi: 10.1177/0145721706286897. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. New Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13:16. doi: 10.1186/s13047-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D., Li J., Zou L., Xu Y., Hu D., Pagoto S.L., et al. Sensitivity and specificity of the ankle—brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. 2010;15:361–369. doi: 10.1177/1358863X10378376. [DOI] [PubMed] [Google Scholar]
- 19.Ix J.H., Miller R.G., Criqui M.H., Orchard T.J. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg. 2012;56:721–727. doi: 10.1016/j.jvs.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakol M., Ashraf S., Brener S.J. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. 2012;4:65–93. doi: 10.5539/gjhs.v4n1p65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G., Wu L., Bryan S., Khaper N., Mani S., Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res. 2010;86:487–495. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 22.Patel S., Fedinec A.L., Liu J., Weiss M.A., Pourcyrous M., Harsono M., et al. H2S mediates the vasodilator effect of endothelin-1 in the cerebral circulation. Am J Physiol Heart Circ Physiol. 2018;315:H1759–H1764. doi: 10.1152/ajpheart.00451.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coletta C., Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr Vasc Pharmacol. 2013;11:208–211. [PubMed] [Google Scholar]
- 24.Kolluru G.K., Bir S.C., Yuan S., Shen X., Pardue S., Wang R., et al. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res. 2015;107:590–600. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bir S.C., Kolluru G.K., McCarthy P., Shen X., Pardue S., Pattillo C.B., et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor–dependent angiogenesis. J Am Heart Assoc. 2022;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan J, Guo W, Huang C, Bao G., Zhu Y., Zhu Y.Z. S-Propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal. 2014;20:2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang H., Oh M.Y., Kim Y.J., Choi I.Y., Yang H.S., Ryu W.S., et al. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92:1520–1528. doi: 10.1002/jnr.23427. [DOI] [PubMed] [Google Scholar]
- 28.Zanardo R.C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G.W.J. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 29.Muzaffar S., Jeremy J.Y., Sparatore A., Del Soldato P., Angelini G.D., Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong L., Lv L., Yang J., Liao X., Yu J., Wang R., et al. Inhibitory effect of hydrogen sulfide on platelet aggregation and the underlying mechanisms. J Cardiovasc Pharmacol. 2014;64:481–487. doi: 10.1097/FJC.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 31.Bos E.M., Wang R., Snijder P.M., Boersema M., Damman J., Fu M., et al. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki K., Olah G., Modis K., Coletta C., Kulp G., Gerö D., et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S., et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta M., Biswas U.K., Chakraborty R., Banerjee P., Raychaudhuri U., Kumar A. Evaluation of plasma H2S levels and H2S synthesis in streptozotocin induced type-2 diabetes-an experimental study based on Swietenia macrophylla seeds. Asian Pac J Trop Biomed. 2014;4:S483–S487. doi: 10.12980/APJTB.4.201414B58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altaany Z., Moccia F., Munaron L., Mancardi D., Wang R. Hydrogen sulfide and endothelial dysfunction: relationship with nitric oxide. Curr Med Chem. 2014;21:3646–3661. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 36.Wang W., Feng S.-J., Li H., Zhang X.-D., Wang S.-X. Correlation of lower concentrations of hydrogen sulfide with activation of protein kinase CβII in uremic accelerated atherosclerosis patients. Chin Med J (Engl) 2015;128:1465–1470. doi: 10.4103/0366-6999.157653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Feng S.-J., Zhang G.-Z., Wang S.-X. Correlation of lower concentrations of hydrogen sulfide with atherosclerosis in chronic hemodialysis patients with diabetic nephropathy. Blood Purif. 2014;38:188–194. doi: 10.1159/000368883. [DOI] [PubMed] [Google Scholar]
- 38.Cuevasanta E., Denicola A., Alvarez B., Möller M.N. Solubility and permeation of hydrogen sulfide in lipid membranes. PLoS One. 2012;7:3–8. doi: 10.1371/journal.pone.0034562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi H., Mano Y., Terasaka S., Sakurai T., Furuya A., Urano H., et al. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp Anim. 2011;60:374–384. doi: 10.1538/expanim.60.373. [DOI] [PubMed] [Google Scholar]
- 41.Shekarriz R., Friedrichsen D.M., Brooks B., Silaski G., Rios L., Wiest E., et al. Sensor of transdermal biomarkers for blood perfusion monitoring. Sens Biosensing Res. 2020;28:100328. [Google Scholar]
- 42.Understanding A1c: diagnosis. American Diabetes Association. https://diabetes.org/diabetes/a1c#:∼:text=The%20goal%20for%20most%20adults,that%20is%20less%20than%207%25.&text=If%20your%20A1C%20level%20is,were%20in%20the%20diabetes%20range. Accessed April 17, 2023.
- 43.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D’Agostino R.B., Gibbons R., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:49–73. [Google Scholar]
- 44.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Matheson B.T., Osofsky R.B., Friedrichsen D.M., Brooks B.J., Clark R.M., Shekarriz R., et al. Validation of the novel transdermal arterial gasotransmitter sensor (TAGSTM) system in measuring transdermal hydrogen sulfide in human subjects. Sens Biosensing Res. 2022;38:100523. doi: 10.1016/j.sbsr.2022.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace M.A.G., Pleil J.D. Evolution of clinical and environmental health applications of exhaled breath research: review of methods and instrumentation for gas-phase, condensate, and aerosols. Anal Chim Acta. 2018;1024:18–38. doi: 10.1016/j.aca.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amann A., Costello Bde L., Miekisch W., Schubert J., Buszewski B., Pleil J., et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014;8:34001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 48.Mazzatenta A., Pokorski M., Di Giulio C. Real time analysis of volatile organic compounds (VOCs) in centenarians. Respir Physiol Neurobiol. 2015;209:47–51. doi: 10.1016/j.resp.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Shen X., Pattillo C.B., Pardue S., Bir S.C., Wang R., Kevil C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson K.R. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan Q., Liu W., Liu Y., Fan Y., Wang X., Yu C., et al. High glucose induces the release of endothelin-1 through the inhibition of hydrogen sulfide production in HUVECS. Int J Mol Med. 2015;35:810–814. doi: 10.3892/ijmm.2014.2059. [DOI] [PubMed] [Google Scholar]
- 52.Candela J., Velmurugan G.V., White C. Hydrogen sulfide depletion contributes to microvascular remodeling in obesity. Am J Physiology-Heart Circulatory Physiol. 2016;310:H1071–H1080. doi: 10.1152/ajpheart.00062.2016. [DOI] [PubMed] [Google Scholar]
- 53.Whiteman M., Gooding K., Whatmore J., Ball C., Mawson D., Skinner K., et al. Plasma hydrogen sulfide (H2S) and correlations with physical, clinical and vascular indices of obesity, metabolic syndrome and type II diabetes. Free Radic Biol Med. 2009;47:S89. [Google Scholar]
- 54.Jackson-Weaver O., Osmond J.M., Riddle M.A., Naik J.S., Gonzalez Bosc L.V., Walker B.R., et al. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am J Physiol Heart Circ Physiol. 2013;304:H1446–H1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naik J.S., Osmond J.M., Walker B.R., Kanagy N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol. 2016;311:H1437–H1444. doi: 10.1152/ajpheart.00465.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrofsky J.S. The effect of type-2-diabetes-related vascular endothelial dysfunction on skin physiology and activities of daily living. J Diabetes Sci Technol. 2011;5:657–667. doi: 10.1177/193229681100500319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drovandi A., Seng L., Crowley B., Fernando M.E., Evans R., Golledge J. Health professionals’ opinions about secondary prevention of diabetes-related foot disease. Sci Diabetes Self Manag Care. 2022;48:349–361. doi: 10.1177/26350106221112115. [DOI] [PubMed] [Google Scholar]
- 58.Pastore D., Deja-Simoni A., De Stefano A., Pacifici F., Cela E., Infante M., et al. Risk factors for diabetic foot ulcers: an Albanian retrospective study of inpatients with type 2 diabetes. Eur Rev Med Pharmacol Sci. 2022;26:558–572. doi: 10.26355/eurrev_202201_27883. [DOI] [PubMed] [Google Scholar]
- 59.Dinoto E., Ferlito F., La Marca M.A., Tortomasi G., Urso F., Evola S., et al. The role of early revascularization and biomarkers in the management of diabetic foot ulcers: a single center experience. Diagnostics (Basel) 2022;12:538. doi: 10.3390/diagnostics12020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stougaard E.B., Winther S.A., Amadid H., Frimodt-Møller M., Persson F., Hansen T.W., et al. Endothelial glycocalyx and cardio-renal risk factors in type 1 diabetes. PLoS One. 2021;16:1–15. doi: 10.1371/journal.pone.0254859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jørgensen M.E., Bjerg L., Witte D.R., Carstensen B., Charles M., Hulman A. Effect of duration and burden of microvascular complications on mortality rate in type 1 diabetes: an observational clinical cohort study. Diabetologia. 2019;41:2297–2305. doi: 10.1007/s00125-019-4812-6. [DOI] [PubMed] [Google Scholar]
- 62.Giovannini S., Biscetti F., Brau F., Biscotti L., Santoliquido A., Pitocco D., et al. Sortilin/Omentin-1 ratio in peripheral artery disease: a cross-sectional study on 295 unselected elderly patients. Mech Ageing Dev. 2022;205:111677. doi: 10.1016/j.mad.2022.111677. [DOI] [PubMed] [Google Scholar]
- 63.Lanting S.M., Barwick A.L., Twigg S.M., Johnson N.A., Baker M.K., Chiu S.K., et al. Post-occlusive reactive hyperaemia of skin microvasculature and foot complications in type 2 diabetes. J Diabetes Complications. 2017;31:1305–1310. doi: 10.1016/j.jdiacomp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Barwick A., Lanting S., Chuter V. Intra-tester and inter-tester reliability of post-occlusive reactive hyperaemia measurement at the hallux. Microvasc Res. 2015;99:67–71. doi: 10.1016/j.mvr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Mennes O.A., van Netten J.J., van Baal J.G., Slart R.H.J.A., Steenbergen W. The association between foot and ulcer microcirculation measured with laser speckle contrast imaging and healing of diabetic foot ulcers. J Clin Med. 2021;10:3844. doi: 10.3390/jcm10173844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polhemus D.J., Kondo K., Bhushan S., Bir S.C., Kevil C.G., Murohara T., et al. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6:1077–1086. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majumder A., Singh M., George A.K., Behera J., Tyagi N., Tyagi S.C. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-β-synthase mutant mice via PPAR-γ/VEGF axis. Physiol Rep. 2018;6:e13858. doi: 10.14814/phy2.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang G.-G., Li W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran J Basic Med Sci. 2019;22:1192–1197. doi: 10.22038/ijbms.2019.36551.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Pan L., Zhuo Y., Gong Q., Rose P.Z.Y. Hypoxia-inducible factor-1α is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull. 2010;33:1550–1554. doi: 10.1248/bpb.33.1550. [DOI] [PubMed] [Google Scholar]
- 70.Calabrese V., Scuto M., Salinaro A.T., Dionisio G., Modafferi S., Ontario M.L., et al. Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis. Antioxidants (Basel) 2020;9:1303. doi: 10.3390/antiox9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen Y.-D., Wang H., Kho S.-H., Rinkiko S., Sheng X., Shen H.-M., et al. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corsello T., Komaravelli N., Casola A. Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants (Basel) 2018;7:129. doi: 10.3390/antiox7100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng Y., Ndisang J.F., Tang G., Cao K., Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 74.Choi S.-J., Jang B.-H., Lee S.-J., Min B.K., Rothschild A., Kim I.-D. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl Mater Inter. 2014;6:2588–2597. doi: 10.1021/am405088q. [DOI] [PubMed] [Google Scholar]
- 75.Kalisz M., Baranowska B.B.W. Do novel adipokines play a causative or only modulating role in the pathogenesis of obesity and metabolic disorders? Neuro Endocrinol Lett. 2012;33:11–15. [PubMed] [Google Scholar]
- 76.Kimura H., Shibuya N., Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bełtowski J., Wójcicka G., Jamroz-Wiśniewska A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: implications for the pathogenesis and treatment of diabetes mellitus. Biochem Pharmacol. 2018;149:60–76. doi: 10.1016/j.bcp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Geng B., Cai B., Liao F., Zheng Y., Zeng Q., Fan X., et al. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PLoS One. 2013;8:e73892. doi: 10.1371/journal.pone.0073892. [DOI] [PMC free article] [PubMed] [Google Scholar]