Highlights
-
•
Voltage-gated channels (Na+, K+, Ca2+ channels), transient receptor potential channels (TRP), purinergic (P2X) channels and acid-sensing ion channels (ASICs) are heavily involved in both acute and chronic pain.
-
•
Natural products of plant origin have been identified as novel treatments for pain and many act by targeting these ion channels.
-
•
Here we review 79 natural compounds/extracts that are reported to interact with ion channels as part of their analgesic mechanism of action.
-
•
Further study is required to develop new plant-based compounds towards clinical trials to develop novel analgesics.
Keywords: Pain, Nociception, Ion channel, Natural product, Extract, Plant
Abstract
Chronic pain affects approximately one-fifth of people worldwide and reduces quality of life and in some cases, working ability. Ion channels expressed along nociceptive pathways affect neuronal excitability and as a result modulate pain experience. Several ion channels have been identified and investigated as potential targets for new medicines for the treatment of a variety of human diseases, including chronic pain. Voltage-gated channels Na+ and Ca2+ channels, K+ channels, transient receptor potential channels (TRP), purinergic (P2X) channels and acid-sensing ion channels (ASICs) are some examples of ion channels exhibiting altered function or expression in different chronic pain states. Pharmacological approaches are being developed to mitigate dysregulation of these channels as potential treatment options. Since natural compounds of plant origin exert promising biological and pharmacological properties and are believed to possess less adverse effects compared to synthetic drugs, they have been widely studied as treatments for chronic pain for their ability to alter the functional activity of ion channels. A literature review was conducted using Medline, Google Scholar and PubMed, resulted in listing 79 natural compounds/extracts that are reported to interact with ion channels as part of their analgesic mechanism of action. Most in vitro studies utilized electrophysiological techniques to study the effect of natural compounds on ion channels using primary cultures of dorsal root ganglia (DRG) neurons. In vivo studies concentrated on different pain models and were conducted mainly in mice and rats. Proceeding into clinical trials will require further study to develop new, potent and specific ion channel modulators of plant origin.
Introduction
Chronic pain affects approximately one-fifth of people worldwide and reduces quality of life and in some cases working ability. It is a global public health problem and a leading cause of disability all over the world (59 Global Prescription Medication Statistics: Strong Growth and CNS Well Represented, 2015, Gaskin and Richard, 2012, Stevens and Stephens, 2018). Nonsteroidal anti-inflammatory drugs (NSAIDs), tricyclic antidepressants (TCAs), anticonvulsants, muscle relaxants, and opioids are often prescribed as pharmacologic treatments of pain; however, their adverse effects, especially after long-term use, including gastrointestinal bleeding, the renal function destruction, and clinical tolerance and dependence strongly limit their application (Allegaert et al., 2010, Benyamin et al., 2008, Dowell et al., 2016, Duca et al., 2022, Hassett et al., 2014, Lapeyre-Mestre et al., 2013, Trang et al., 2015, Woolf and Hashmi, 2004). Considering the above, new therapeutic agents with increased efficacy, less side effects, and lower costs and leading to an improved quality of life should become one of the primary objectives in modern medical research (Daniyal and Wang, 2021, Johansen et al., 2012, Tasneem et al., 2019, van Hecke et al., 2014).
Plants contain a vast natural supply of compounds that may be a source of novel drugs. The medicinal use of plants as analgesic drugs in alternative medicine is far older than the current sciences of medicine in developed countries. Since the 1880′s, the most popular active ingredients to treat pain have been morphine (which comes from opium and poppies), salicylic acid (which comes from the bark of the white willow tree), and THC (which comes from cannabis) (Brune, 2002, Goyal, 2014, Goyal et al., 2013, Jones, 2011, Rivera et al., 2005, Zeb and Lee, 2021). In recent years, the exploration for new therapeutic agents capable of inhibiting, decreasing, or relieving pain with few or no adverse effects from the enormous arrays of medicinal plant resources is growing. Therefore, the present review summarizes the evidences of analgesic abilities of natural compounds/extracts from plant origin with activity towards the ion channels.
Ion channels located at the nociceptor sensory peripheral terminal, facilitate the initiation of the signaling cascade in response to any noxious stimuli, affecting neuron excitability by altered action potential generation and propagation, axonal conduction and neurotransmitter release and further neuronal processing produces the experience of pain (Skerratt and West, 2015, Zhang et al., 2022). The role of voltage gated Na+ and Ca2+ channels, K+ channels, transient receptor potential channels (TRP), purinergic channels (P2X) and acid-sensing ion channels (ASICs) have been identified and investigated as potential targets for new medicines for the treatment of a variety of human diseases as well as acute and chronic pain (Bear et al., 2009, Bennett et al., 2019, Bernier et al., 2018, Birch et al., 2004, Cardoso and Lewis, 2018, Du et al., 2018, Du and Gamper, 2013, Lee and Chen, 2018, Markman and Dworkin, 2006, Moore et al., 2018, Ocaña et al., 2004, Takeda et al., 2011, Tsantoulas and McMahon, 2014, Zamponi et al., 2009) [Fig. 1]. Voltage-gated Na+ and Ca2+ ion channels are associated with setting neuronal excitability. VGSCs play a major role in action potential generation while the VGCCs control release of neurotransmitters. K+ channels are crucial in shaping action potentials and controlling the membrane potential, in excitable tissues including nociceptive sensory neurons, as a result of nerve or tissue injury (Bear et al., 2009, Bennett et al., 2019, Birch et al., 2004, Cardoso and Lewis, 2018, Du et al., 2018, Du and Gamper, 2013, Markman and Dworkin, 2006, Takeda et al., 2011, Zamponi et al., 2009). TRP channels are thermosensitive ion channels and have activation thresholds within the noxious range of temperatures (below 15 °C or above 43 °C) indicating possible involvement in thermal nociception, whereas the P2X channels are activated by extracellular ATP released from damaged or inflamed cells to initiate nociceptive signals (Bernier et al., 2018, Moore et al., 2018). Tissue acidosis is associated with inflammation and decreased extracellular pH (below pH = 6) opens ASIC channels resulting in activation of nociceptors (Lee and Chen, 2018).
Fig. 1.
Natural products targeting Ion channels involved in pain.
Although a large number of small molecules have been reported to alter the functional activity of these ion channels, the effect and mechanism of action of natural products on these channels are still a matter of investigation. Here we focus on the effects of natural products on different ion channels involved in pain processing. We discuss sources, active constituents and chemical structures (where known) of natural products and their reported ion channel targets.
Ion channel and drug targeting
Voltage gated Na+ and Ca2+ channels, K+ channels, transient receptor potential channels (TRP), purinergic channels (P2X) and acid-sensing ion channels (ASICs) are some of the ion channels classically involved in the pathogenesis of pain (Bear et al., 2009, Bennett et al., 2019, Bernier et al., 2018, Birch et al., 2004, Cardoso and Lewis, 2018, Du et al., 2018, Du and Gamper, 2013, Lee and Chen, 2018, Markman and Dworkin, 2006, Moore et al., 2018, Takeda et al., 2011, Zamponi et al., 2009).
Voltage-gated sodium channels (VGSCs)
are important determinants of sensory neuron excitability: they are essential for the initial transduction of sensory stimuli, the electrogenesis of the action potential, and neurotransmitter release from sensory neuron terminals. Their activation depolarizes the resting membrane potential to generate an action potential upstroke. Na+ channels consist of a pore-forming α-subunit, as well as associated β-subunits. The family of related α-subunits consists of 10 members, 9 of which (Nav1.1–1.9) are voltage gated and one further non-voltage-gated member, Nax, which is involved in salt sensing. Nav1.1, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 are all expressed by sensory neurons. The biophysical characteristics of these channels, as well as their unique expression patterns within subtypes of sensory neurons, define their functional role in pain signaling. Changes in the expression of VGSCs, as well as posttranslational modifications, contribute to the sensitization of sensory neurons in chronic pain states (Alles et al., 2020, Bennett et al., 2019, Black et al., 2012, Cox et al., 2006, Dib-Hajj et al., 2009, He et al., 2010, Luiz and Wood, 2016, Wang et al., 2011). Furthermore, gene variants in Nav1.7 (Alles et al., 2020, Bennett et al., 2019, Black et al., 2012, Cox et al., 2006), Nav1.8 (Bennett et al., 2019, He et al., 2010), and Nav1.9 (Bennett et al., 2019) have now been linked to the common pain disorders.
Effect of natural products on Voltage-gated sodium channels (VGSCs) (See Table 1)
Table 1.
Anti-nociceptive potential of natural products from plant origin by modulating the ion channels activity.
| Plant Product/Chemical Nature | Structure | Source | Target | Study Method | Type of pain/Sex/Species/Genetic background | Reference |
|---|---|---|---|---|---|---|
| Crude Extract | N/A | Radix paeoniae | Suppress voltage dependent sodium current | Whole cell patch clamp 0.8 mg/ml |
Male-female/Wistar rat/Wild type | (Dong and Xu, 2002) |
| Bulleyaconitine A/ Diterpenoid alkaloid |
 |
Aconitum bulleyanum | Inhibit Nav1.3 and Nav1.7 | Pituitary GH3 cells tested with 10 μM. Sensory and motor block of rat sciatic nerve, tested with 0.375 to 0.75 mM Rat spared nerve injury model of neuropathic pain IC50: Nav1.3, Nav1.7, and Nav1.8 were 995.6 ± 139.1 nM, 125.7 ± 18.6 nM, and 151.2 ± 15.4 lM, respectively |
Male/SD rats/Wild types Chronic pain/Male/SD rats/Wild type |
(Wang et al., 2007, Xie et al., 2018a, Xie et al., 2018b) |
| Eugenol/Phenolic essential oil |  |
Syzygium aromaticum | Suppress voltage dependent sodium current | Dental primary afferent neurons. IC50 = 0.6 mM Chronic constriction injury (CCI) Dose: 10 & 50 μg, i.t. |
SD rats/Wild type Chronic pain /SD rats/ Wild type |
(Lionnet et al., 2010, Park et al., 2006) |
| (-)-Hardwickiic acid ((-)-HDA)/ Diterpenoid |  |
Croton californicus | Inhibit Nav1.7 | Rat DRGs tested with 20 μM. HEK cells tested with 20 μM. Alleviates HIV- and chemotherapy- induced neuropathy Dose: 2 μg/5 μl. |
Chronic pain /Male-female/SD rats/Wild type | (Cai et al., 2018) |
| Hautriwaic acid (HTA)/ Diterpenoid |  |
Eremocarpus setigerus | Suppress voltage dependent sodium current | Rat DRGs tested with 20 μM. Alleviates HIV- and Chemotherapy- induced neuropathy Dose: 2 μg/5 μl. |
Chronic pain /Male -female/SD rats/Wild type | (Cai et al., 2018) |
| Puerarin/ Isoflavonoid |  |
Pueraria montana | Inhibit Nav1.8 | Rat DRG neurons IC50 = 481.5 μM. Paclitaxel-induced neuropathic pain at 8 mg/kg Dose: 0.1,1.0,10 uM, i.t. |
Chronic pain /Male/SD rats/Wild type | (Zhang et al., 2018a) |
| Lappaconitine/ Diterpene alkaloid |  |
Aconitum sinimontanum | Inhibit Nav1.7 |
HEK293 cells (Acute 30, 60, 100 μM). IC50 = 27.67 μM. |
HEK293 cells | (Liao et al., 2019) |
| Brucine/ Second abundant alkaloid |  |
Strychnos nuxvomica | Suppress voltage dependent sodium current | Chronic constriction injury (CCI) mouse model Dose: 10, 30 mg/kg |
Chronic pain /Male/C57Bl/6 mice/Wild type | (Yu et al., 2019) |
| Gingerol and Shogaol/Beta-hydroxy ketone Phenol |
 
|
Zingiber officinale | Inhibit Nav1.8 | Rat oral ulcerative mucositis model HEK293 cells Human CHO cells 300 μM and 150 μM. Spinal nerve ligation (SNL) Dose: 100–400 mg/kg, oral |
HEK293 cells Human CHO cells Chronic pain /Male/SD rats/Wild type |
(Hitomi et al., 2017, Shen et al., 2022) |
| Neoline/ Alkaloid |  |
Goshajinkigan extract formulation | Inhibit Nav1.7 | HEK293 cells (20 mg/ml)Streptozotocin (STZ) -induced diabetic neuropathy Dose: 7.5 mg/kg |
Chronic pain /Male/ICR mice | (Nakatani et al., 2020) |
| Crude extract | N/A | Allium macrostemon | Inhibit Nav1.7 |
Human embryonic kidney 293 T (HEK293T) 50 mg/L. Formalin-induced, Acetic-acid-induced and Thermal pain. Dose: 50 and 100 mg/kg/i.p. |
Acute pain/Male/C57Bl/6 mice/Wild type | (Yang et al., 2021b) |
| L-Tetrahydropalmatine and protopine/ Alkaloids |
 
|
Cordyalis yanhusuo | Inhibit Nav1.7 | CHO cells IC50 = 7.05 μM. Formalin-induced pain model. Dose: 10,20 and 40 mg/kg,i.p. |
Acute pain/ mice/wild type | (Xu et al., 2021a) |
| Magnolol/ Hydroxylated biphenyl |  |
Magnolia officinalis | Suppress voltage dependent sodium current | NG108-15 cells. IC50 = 15 and 30 μM DRG neurons, TTX-S IC50 = 9.4 μM. TTX-R IC50 = 7 μM |
NG108-15 cells Male/ICR mice |
(Gong et al., 2012, Qiu et al., 2021) |
| Saikosaponins A/ Pentacyclic triterpenoid |  |
Bupleurum chinense | Inhibit Nav1.7 | Nav1.7 CHO cells IC50 = 28.6 nM Analgesic activity in thermal and formalin-induced pain in mice. Dose: 2.5,5.0,10.0 mg/kg, i.p. |
Acute pain/Male -Female/Kunming mice | (Xu et al., 2021b) |
| Imperatorin/ Pentacyclic triterpenoid |  |
Angelica biserrata | Inhibit Nav1.7 |
Nav1.7 CHO cells Thermal and formalin induced nociception Dose: 3.8,7.5,15.0 mg/kg, i.g. |
Acute pain/Male -Female/Kunming mice | (Xu et al., 2021b) |
| Rhodojaponin III/ Grayanane-type diterpenoid |  |
Rhododendron molle | Inhibit Nav1.7 and Nav1.8 |
hNaV1.5-CHL, hNaV1.7HEK293 and hNaV1.8HEK293 cell lines Thermal and acetic acid induced nociception Dose: 0.01–0.2 mg/kg Chronic constriction injury (CCI) Dose: 0.075,0.15,0.3 mg/kg |
Acute-chronic pain/Male -Female/Kunming mice Male -Female/SD rat/Wild type |
(Yang et al., 2022) |
| Ethanolic Extract | N/A | Aconiti Brachypodi | Suppressed TTX-sensitive sodium current | DRG culture Whole cell patch clamp 10 µg/ml-8.0 mg/ml Thermal and acetic acid induced nociception Dose: 1.0–20.0 mg/kg, i.g. |
Male -Female/Wistar rat/Wild type Acute pain/Female/Kunming mice |
(Ren et al., 2012) |
| Neochamaejasmin A (NCA)/ biflavonoid |  |
Stellera chamaejasme |
Modulate Kv1.4 channels | Human Kv1.4 CHO cell lines Whole cell patch clamp IC50 of 7.55 µM |
Human Kv1.4 CHO cell lines | (Ren et al., 2018) |
| Chlorogenic acid (CGA)/ flavonoid |  |
Eucommiae folium | Modulate IK,A and IK,V channels | TG neurons culture Whole cell patch clamp Dose: 0.2 and 1 mmolL-1 |
Male/SD rats/Wild type | (Zhang et al., 2014) |
| Gintonin/ ginseng saponins |  |
Panax ginseng | inhibited Kv1.2 channel | Xenopus oocytes IC50 0.58 ± 0.4 ug/mL |
Xenopus laevis frog oocytes | (Lee et al., 2013) |
| Oleanolic acid / Pentacyclic triterpene |  |
Eriope blanchetii | Activation of ATP-gated K+ channels | Capsaicin induced nociception Dose: 10, 30 and 100 mg/kg, oral |
Acute pain/Male/Swiss albino mice/Wild type | (Maia et al., 2006) |
| Methanolic extract | N/A | Dioscorea bulbifera | Activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel | CFA, LPS, PGE2 and Capsaicin induced nociception Dose: 500 mg/kg, oralPartial ligation of sciatic nerve (PLSN) Dose: 500 mg/kg, oral |
Acute-chronic pain/Male-female/Swiss mice/Wild type | (Nguelefack et al., 2010) |
| Kaurenoic acid/Diterpene |  |
Sphagneticola trilobata | Activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel | Phenyl-p-benzoquinone, acetic acid and formalin induced nociception Dose: 3–30 mg/kg, i.p. |
Acute pain/Male/Swiss mice/Wild type | (Mizokami et al., 2012) |
| Pimaradienoic acid/Pimarane diterpene |  |
Vigueira arenaria | Activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel | Acetic acid, formalin and CFA induced nociception Dose: 1, 3 and 10 mg/kg, i.p. |
Acute pain/Male/Swiss mice/Wild type | (Possebon et al., 2014) |
| Ellagic acid/ Polyphenolic secondary metabolite |  |
Punica granatum | Activation of L-arginine/NO/cGMP/KATP channel pathway | Formalin induced nociception Dose: 30–300 μg/paw/i.pl. |
Acute pain/Male/Wistar rats/Wild type | (Ghorbanzadeh et al., 2014) |
| Patuletin/ Trimethoxyflavone flavonoid | 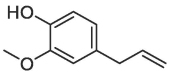 |
Inula britannica | Activation of L-arginine/NO/cGMP/KATP channel pathway | Acetic acid, glutamate and formalin induced nociception Dose: 30 mg/kg, i.p. |
Acute pain/Male/Swiss albino mice/Wild type | (Zarei et al., 2018) |
| Methanolic extract | N/A | Cnicus benedictus | Modulation of L-arginine/ nitric oxide/cGMP/ATP-sensitive potassium channel pathway | Acetic acid and formalin induced nociception Dose: 150, 150 and 300 mg/kg, i.p. |
Acute pain/Male/Wistar rats/Wild type | (Ahmadimoghaddam et al., 2020) |
| Methanolic extract | N/A | Bougainvillea spectabilis | Modulation of ATP-sensitive K+ channel | Acetic acid, glutamate and formalin induced nociception Dose: 50, 100 and 200 mg/kg, oral |
Acute pain/Male/Swiss albino mice/Wild type | (Ferdous et al., 2020) |
| Essential oil | N/A | Artemisia biennis | Activation of L-arginine/NO/cGMP/KATP channel pathway | Acetic acid, glutamate and formalin induced nociception Dose: 30, 60 and 120 mg/kg, oral |
Acute pain/Male/Swiss mice/Wild type | (Zarei et al., 2021) |
| Essential oil | N/A | Bupleurum falcatum | Activation of L-arginine/NO/cGMP/KATP channel pathway | Formalin induced nociceptionCervical spinal cord hemicontusion (CSC) Dose: 25, 50 and 100 mg/kg, oral |
Acute-chronic pain/Male/Swiss mice/Wild type | (Ahmadimoghaddam et al., 2021) |
| Cannabidiol/Phytocannabinoid Camphene and alpha‑bisabolol/ Terpenes |
 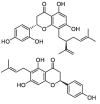 
|
Cannabis sativa | Inhibit Cav3.1 and Cav3.2 | HEK293 cells & TG neurons culture HEK tsA-201 cells DRG neurons culture Whole-cell patch clamp Complete Freund’s Adjuvant (CFA) and formalin induced nociception Partial sciatic nerve ligation (PSNL) |
Male/C57Bl/6 mice/Wild type Acute and Chronic pain /Male-female/C57Bl/6J miceMale Cacna1h mice (Cav3.2 null mice) |
(Gadotti et al., 2021, Harding et al., 2023, Ross et al., 2008) |
| Eugenol/Phenolic essential oil |  |
Syzygium aromaticum | Inhibit Cav3.1 and Cav3.2 | HEK293 cells TG neurons culture Whole-cell patch clamp |
SD rats/Wild type | (Seo et al., 2013) |
| Linalool/ Essential oil |  |
Lavandula stoechas and Rosmarinus officinalis | Inhibit Cav3.2 | HEK-293 T Whole-cell patch clamp Ca2+ imagingOlfactory receptor cells (ORCs) Newt retinal neurons Cerebellar Purkinje cells |
Wister rats/Wild type | (El Alaoui et al., 2017, Narusuye et al., 2005) |
| Sophoraflavanone G/6-prenylnaringenin |
 
|
Sophorae radix | Inhibit Cav3.1 and Cav3.2 | Cav3.1HEK293 cells and Cav3.2HEK293 cells Partial sciatic nerve ligation Oxaliplatin induced neuropathy |
Chronic pain /Male/Wistar rat ddy mice/C57Bl/6j miceCacna1h mice (Cav3.2 null mice) |
(Sekiguchi et al., 2018) |
| Betulinic acid/ Pentacyclic triterpenoid |  |
Hyptis emoryi | Suppress voltage dependent calcium current | DRG neurons culture Whole cell patch clamp Ca2+ imaging HEKtsA-201 cells Voltage clamp recording Chemotherapy induced peripheral neuropathy (CIPN) HIV associated peripheral neuropathy Partial sciatic nerve ligation (PSNL) |
Chronic pain /Male-female/SD rats/Wild type | (Bellampalli et al., 2019) |
| Astilbin/Flavonoid |  |
Paeonia lactiflora | Suppress voltage dependent calcium current | Acetic acid, hot plate and formalin induced nociception Dose: 10 and 30 mg/kg |
Acute pain/Female/BALB/c mice | (Bi et al., 2019) |
| Physalin F/Secosteroid |  |
Physalis acutifolia | Inhibit Cav2.2 | DRG neurons culture Paclitaxel-induced peripheral neuropathy Spinal nerve ligation Dose: 2ug/5ul, i.t. |
Chronic pain /Male/SD rats/Wild type | (Shan et al., 2019) |
| Ethanolic extract | N/A | Solanum virginianum | Inhibit Cav2.2 | Chronic construction injury (CCI) Dose: 100 and 200 mg/kg, oral |
Chronic pain /Male-female/Wistar rats/Wild type | (Verma et al., 2020) |
| Argentatin-C/Triterpene |  |
Parthenium incanum | Inhibit Cav3.1 Cav3.2 Cav3.3 Nav1.7 Nav1.8 Nav1.9 |
HEK293 cells Rat DRG neurons culture Molecular docking Paw incision mouse model of postoperative pain |
Acute pain/Female/SD rats/Wild type Male CD1 mice |
(Duran et al., 2022) |
| Heantos-4 | N/A | Mixture of organic herbs | Inhibit Cav3.1 and Cav3.3 | hCav3.1Flp-In293 cells, hCav3.2Flp-In293 cells and hCav3.3Flp-In293 cells Whole-cell patch clamp Acute brain slice electrophysiology 1 mg/ml |
Male-female/Wistar rats/Wild type | (Cain et al., 2016) |
| Baicalin/ Glycosyloxyflavone |  |
Scutellaria baicalensis | Modulation of TRPV1 channels | DRG neuron culture Ca2+ imaging Chronic constriction injury (CCI) Dose: 15 & 30 µg/kg, i.p. |
New-born SD rats Chronic pain/Male/SD rats/Wild type |
(Sui et al., 2010, Wang et al., 2020) |
| Vitexin/Flavonoid |  |
Vitex agnus | Modulation of TRPV1 channels | Acetic acid, formalin, Complete Freund’s Adjuvant (CFA), capsaicin and thermal induced nociception Dose: 1.0,3.0,10.0 mg/kg/i.p. |
Acute pain/Male/Swiss mice/Wild type | (Borghi et al., 2013) |
| Ethanolic extract | N/A | Pterodon pubescens | Modulation of TRPV1 and TRPA1 channels | Partial sciatic nerve ligation Dose: 30.0, 100 and 300 mg/kg, i.g. Capsaicin 5 µl, i.t. Cinnamaldehyde 5 µl, i.t. |
Acute and chronic pain /Female/Swiss mice/Wild type | (Nucci-Martins et al., 2015) |
| Methanol/methylene chloride extract | N/A | Croton macrostachyus | Modulation of TRPV1 channels | Complete Freund adjuvant (CFA), PGE2 and Capsaicin induced nociception Partial sciatic nerve ligation (PSNL) Dose: 250 and 500 mg/kg, oral |
Acute and chronic pain/Male-female/Swiss mice/Wild type | (Nguelefack et al., 2015) |
| Coumarins/Aromatic organic compound |  |
Angelicae pubescentis | Modulation of TRPV1 channels | Spared nerve injury (SNI) Dose: 5,10 and 20 mg/kg, i.g. |
Chronic pain/Male/SD rats/Wild type | (Li et al., 2017) |
| Crude extract | N/A | Ephedra sinica | Modulation of TRPV1 channels | Flp-In293 cells, mTRPV1/Flp-In293 cells Capsaicin induced nociception, 0.03–3.1 ug/paw/i.d. Dose: 0.3–10 mg/paw, intra-planter 3 mg/paw,i.d. Dose: 87.5–700 mg/kg, oral |
Acute pain/Male/ddY mice | (Nakamori et al., 2017) |
| Crude extract and dichloromethane fraction | N/A | Amphilophium crucigerum | Modulation of TRPV1 channels | Hot water tail-flick, Capsaicin and Complete Freund adjuvant (CFA) induced nociception Partial sciatic nerve ligation (PSNL) Dose: Crd 30, 100 and 300 mg/kg, i.g. Dcm 3,10 and 30 mg/kg, i.g. |
Acute and chronic pain/Male/ albino swiss mice | (De Prá et al., 2017) |
| Water extract of frankincense and myrrh | N/A | Boswellia carterii and Commiphora myrrha | Modulation of TRPV1 channels | DRG neurons culture Ca2+ imaging Hot water tail-flick and Capsaicin induced nociception Chronic constriction injury (CCI) Dose: 1.5 and 7.5 mg/kg, i.g. |
Acute and chronic pain/Male/ C57Bl/6 mice/Wild type | (Hu et al., 2017) |
| Polyacetylene fraction | N/A | Echinophora platyloba | Modulation of TRPA1 channels | HEK293 cells Thermo-TRPs receptor assays |
HEK293 cells | (Chianese et al., 2018) |
| Ethanolic Extract | N/A | Nypa fruticans | Modulation of TRPV1 channels | Sciatic nerve crush injury Dose: 500 mg/kg, oral |
Chronic pain/Male/SD rats/Wild type | (Kang and Hyun, 2020) |
| Ginger Extract and 6-shogaol/Phenols Zingiber officinale |
 |
Zingiber officinale | Modulation of TRPV1 channels | STZ induced diabetic peripheral neuropathy Dose: Ginger extract 100, 200 and 400 mg/kg, oral 6-shogaol 5, 10 and 15 mg/kg, oral |
Chronic pain/Male/Balb/C mice | (Fajrin et al., 2020) |
| Crude extract | N/A | Corydalis Saxicola | Modulation of TRPV1 channels | Cisplatin induced neuropathic pain Dose: 30, 60 and 120 mg/kg, oral |
Chronic pain/Male/SD rats/Wild type | (Kuai et al., 2020) |
| Berberine/Alkaloid |  |
Coptis chinensis | Modulation of TRPV1 channels | Cisplatin induced peripheral neuropathy (CIPN) Dose: 60, 90 and 120 mg/kg, orally Partial sciatic nerve ligation (PSNL) |
Chronic pain/Male/ C57Bl/6 mice (Wild type)/ TRPV1 Knockout mice |
(Meng et al., 2021, Yang et al., 2020b) |
| Methanolic extract | N/A | Ononis spinosa | Modulation of TRPV1 channels | Capsaicin induced nociception, Dose: 40 µg/paw, ipl Dose: 100 µg/paw, ipl |
Acute pain/Male/Wistar Rats/Wild type | (Jaffal et al., 2021) |
| Lectin/ Heterogeneous group of proteins | N/A | Parkia platycephala | Modulation of TRPV1 channels | Formalin induced-temporomandibular joint pain Infraorbital nerve transection- induced neuropathic pain Capsaicin 40.93 µM; 5.0 µl Lectin 0.025 mg/mL, 5.0 µl (Zebrafish), 0.25 mg/kg, ip (Rat) |
Acute and chronic pain/Male/Wild Zebrafish Swiss mice/Wistar rats/Wild type |
(de Oliveira Leite et al., 2022) |
| Viphyllin (standardized extract) | N/A | Piper nigrum | Modulation of TRPV1 channels | Acetic acid-induced writhing test, Formalin-induced paw licking test, hot plate test, Tail flick test capsazepine 0.1 mg/kg, i.p. Viphyllin 10–50 mg/kg, ip |
Acute pain/Male/Balb/C mice/Wild type | (Venkatakrishna et al., 2022) |
| Citral/Terpene |  |
Cymbopogon citratus | Modulation of TRPV1, TRPM3 and TRPM8 channels | Formalin, Cinnamaldehyd, Menthol and Capsaicin induced orofacial nociception Dose: 0.1, 0.3, 1.0, 3.0, 10.0, 30.0, 100 and 300 mg/Kg, oral Formalin temporomandibular joint (TMJ) nociception, Mustard oil-induced craniofacial nociception and Infraorbital nerve transection- induced neuropathic pain (IONX) Dose: 0.1 mg/kg, oral |
Acute and chronic pain/Swiss mice/Wistar rats/Wild type | (Alves Rodrigues Santos et al., 2022) |
| Sodium ferulate (SF)/Sodium salt of ferulic acid |  |
Angelica sinensis, Cimicifuga heracleifolia, Lignsticum chuangxiong | Modulation of P2X3 receptor channels | Rat DRG neurons culture Whole cell patch clamp Chronic constriction injury (CCI) Dose: 50 and 100 mg/kg, i.p. |
Chronic pain/Male/SD rats/Wild type | (Zhang et al., 2010, Zhang et al., 2008) |
| Tetramethylpyrazine/Alkaloid |  |
Ligusticum wallichii | Modulation of P2X3 receptor channels | Controlled cortical impact (CCI) Dose: 4 mM/scHot-water immersion (Burn-injury pain) DRG neurons culture Whole cell patch clamp Dose: 100 mg/kg, ip |
Chronic pain/Male/C57Bl/6 mice/SD rats/Wild type | (Gao et al., 2010, Gao et al., 2008, Wang et al., 2017) |
| Lappaconitine (LA)/ Aconitum alkaloid |  |
Extracted from the plants of Aconitum species | Modulation of P2X3 receptor channels | DRG neurons culture Whole cell patch clamp Chronic constriction injury (CCI) Dose: 4 mg/kg, ip |
Chronic pain/Male/SD rats/Wild type | (Ou et al., 2011) |
| Methanolic Extract and some fractions | N/A | Rheedia longifolia | Modulation of P2X7 receptor channels | Dye uptake assay Whole-cell patch clamp IC50 = 2 μg/mL |
Acute pain/Mice/Wild type | (Santos et al., 2011) |
| Emodin/ Natural anthraquinone |
 |
Rheum rhabarbarum | Modulation of P2X2 and P2X3 receptor channels | Chronic constriction injury (CCI) Dose: 50 mg/kg, ip |
Chronic pain/Male/SD rats/Wild type | (Gao et al., 2011) |
| Puerarin/Isoflavonoid | 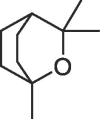 |
Radix puerariae | Modulation of P2X3 and P2X7 receptor channels | Chronic constriction injury (CCI) Dose: 100 mg/kg, ip Clinical studies |
Chronic pain/Male/SD rats/Wild type | (Li et al., 2011, Xu et al., 2012, Zhang et al., 2013) |
| Sinomenine/Alkaloid |  |
Sinomenium acutum | Modulation of P2X3 receptor channels | Carrageenan induced inflammation Photochemically induced sciatic nerve injury Photochemically induced spinal cord injury Dose: 20,40 and 80 mg/kg, ip HEK293 cells Whole cell patch clamp STZ induced diabetic neuropathy Dose: 40 mg/kg, ip |
Acute-chronic pain/Male-female/SD rats/male C57Bl/6 mice//Wild type Chronic pain/Male/SD rats/Wild type |
(Gao et al., 2013, Rao et al., 2017) |
| Curcumin/Beta-diketone |  |
Curcuma longa | Modulation of P2X3 receptor channels | DRG neurons culture Whole cell patch clamp HIV-gp120-induced neuropathic pain Dose: 4 mg/ml,sl |
Chronic pain/Male/SD rats/Wild type | (Zhao et al., 2017) |
| Artemisinin/ Sesquiterpene lactone |  |
Artemisia annua | Modulation of P2X4 receptor channels | HEK293 cells Whole cell patch clamp Chronic constriction injury (CCI) Dose: 5 mg/kg, ip |
Chronic pain/Male/SD rats/Wild type | (Ying et al., 2017) |
| Osthole/Derivative of coumarin |  |
Cnidium monnieri | Modulation of P2X4 receptor channels | STZ induced diabetic neuropathy Dose: 20 mg/kg, ip |
Chronic pain/Male/SD rats/Wild type | (Yuan et al., 2018) |
| 1,8-cineole/ Monoterpene cyclic ether |  |
Eucalyptus | Modulation of P2X3 receptor channels | Chronic constriction injury (CCI) Dose: 50 and 100 mg/kg, ig |
Chronic pain/Male-female/SD rats/Wild type | (Zhang et al., 2018b) |
| Gardenoside/Natural reactive aglycone | 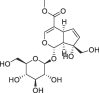 |
Gardenia jasminoides | Modulation of P2X3 and P2X7 receptor channels | Chronic constriction injury (CCI) Dose: 300 umol/l, iv |
Chronic pain/Male/SD rats/Wild type | (Yu et al., 2018a, Yu et al., 2018b) |
| Hesperidin/ Bioflavonoid |  |
Citrus fruits (family Rutaceae) | Modulation of P2X3 receptor channels | HEK293 cells Whole cell patch clamp Chronic constriction injury (CCI) Dose: 50 mg/kg, ip |
Chronic pain/Male/SD rats/Wild type | (Tao et al., 2019) |
| Crude extract | N/A | Hericium erinaceus | Modulation of P2X4 and P2X7 receptor channels | Human neuroblastoma SH-SY5Y cellsL5-spinal nerve ligation (SNL) Dose: 100 mg/kg, ig |
Chronic pain/Male/C57BL/6 NARL mice | (Yang et al., 2020a) |
| Crude ethanolic extract | N/A | Physalis angulata | Modulation of P2X7 receptor channels | HEK-293 cells Mouse peritoneal macrophages culture Dye uptake assay Whole cell patch clamp ATP-induced paw edema Dose: 0.001–100 mg/kg, ip |
Acute pain/Male/Swiss webster mice | (Arruda et al., 2021) |
| Gallic acid/Phenolic acid | 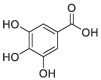 |
Found in gallnuts, sumac, witch hazel, tea leaves, oak bark | Modulation of P2X7 receptor channels | Neonatal colorectal dilation (CRD) Dose: 20 mg/kg, ig HEK293 cells Whole cell patch clamp Chronic constriction injury (CCI) Dose: 100 mg/kg,ip |
Chronic pain/Male/SD rats/Wild type | (Wen et al., 2022, Yang et al., 2021a) |
| Resveratrol/ polyphenol |  |
Plant species, including aliments, such as grapes, peanuts, and wines | Modulation of P2X3 and P2X7 receptor channels | STZ-induced diabetic neuropathy Dose: 25,100 and 400 mg/kg, ig Partial sciatic nerve ligation (PSNL) HEK 293 cells Whole cell patch clamp HIV-gp120 induced neuropathy Dose: 30 mg/kg, ip DRG neurons culture Whole cell patch clamp Chronic constriction injury (CCI) Dose: 25 mg/kg, oral |
Chronic pain/Male/SD rats/Wild type | (Cui et al., 2020, Guo et al., 2021, Wu et al., 2017, Xie et al., 2017) |
| Astragalin/Flavonoid | 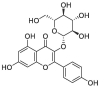 |
White stamen of flowers | Modulation of P2X4 receptor channels | HEK 293 cells Whole cell patch clamp Chronic constriction injury (CCI) Dose: 50 mg/kg, ig |
Chronic pain/Male/SD rats/Wild type | (Wang et al., 2021) |
| Crude water extract | N/A | Aconitum jaluense | Modulation of P2X7 receptor channels | L5-Spinal nerve ligation (SNL) Dose: 10,30,100 and 300 μg/10 μl, it |
Chronic pain/Male/SD rats/Wild type | (Yang et al., 2016) |
| Ethanolic extract | N/A | Azadirachta indica | Modulation of ASIC hannels | Glutamate, formalin, cinnamaldehyde, capsaicin, menthol and acidic saline induced nociception Dose: 0.5,1.0,2.5,5.0 and 10.0 mg/ml, 20 μl, ip |
Acute pain/Male-female/Wild zebra fish | (Batista et al., 2018) |
Radix paeoniae
The effect of whole extract of Radix paeoniae (RP) on sodium currents (INa) was examined in freshly isolated rat hippocampal CA1 neurons using whole-cell patch-clamp. The results suggested that the whole extract of RP suppressed hippocampal CA1 INa. This mechanism was driven by a shift in the inactivation curve towards hyperpolarization. The effect was decreased recovery time from inactivation which attenuated the number of activity-dependent activatable channels in a dose dependent manner. Thus, RP whole extract may be used to reduce neuronal hyperexcitability (Dong and Xu, 2002).
Aconitum bulleyanum
Bulleyaconitine A, a diterpenoid alkaloid isolated from Aconitum bulleyanum plants. Experimental studies have revealed that bulleyaconitine A at therapeutic doses potently inhibits peripheral and central sensitization driven by upregulation of protein kinase C and VGSCs in DRG neurons (M.-X. Xie et al., 2018a; M. X. Xie et al., 2018b). Bulleyaconitine A effect is enhanced dose dependently via blocking voltage dependent Nav1.3 and Nav1.7 channels in DRG neurons and therefore, inhibits the ectopic discharges (Wang et al., 2007). Together, bulleyaconitine A is able to suppress nociception by targeting the voltage dependent sodium channels.
Syzygium aromaticum
Eugenol, an essential oil from Syzygium aromaticum plant, inhibited action potentials and voltage dependent sodium current (INa) in neurons contributes to its analgesic effect (Park et al., 2006). The studies demonstrated that eugenol may alleviate neuropathic pain, both allodynia and hyperalgesia in CCI rats, by acting on central sensitization. The most probable site of action is at the level of the dorsal horn of the spinal cord, a location implicated heavily in nociception (Lionnet et al., 2010).
Croton californicus
Hardwickiic acid a diterpenoid isolated from plant Croton californicus inhibited voltage dependent Nav1.7 channels in DRG neurons. Therefore, this compound may be used as an antagonist alleviating Nav1.7 activation in the presence of non-noxious stimuli (Cai et al., 2018).
Eremocarpus setigerus
Hautriwaic acid a diterpenoid isolated from plants Eremocarpus setigerus inhibited voltage dependent sodium channels in the DRG neurons may novel specific sodium channel antagonists for pain relief (Cai et al., 2018).
Pueraria montana
Puerarin is a major isoflavonoid isolated from the root of Pueraria montana (Kudzu root) which has been used traditionally for treatment of cardiovascular disorders and brain injury. Additionally, puerarin acts on the β1 subunit of Nav1.8 channels in DRG neurons to attenuate hyperexcitability in neuropathic rats. The suppression of voltage dependent sodium currents contributed to its anti-paclitaxel induced neuropathic pain effect (Zhang et al., 2018a).
Aconitum sinimontanum
Lappaconitine is a diterpene alkaloid isolated from Aconitum sinimontanum and widely employed in Chinese and Japanese medicine mainly for analgesic indications. Studies reported that lappaconitine irreversibly inhibited Nav1.7 channels in a voltage dependent manner. Nav1.7 was stably expressed in human embryonic kidney (HEK293) cells which supports its application as a potent analgesic (Liao et al., 2019).
Strychnos nuxvomica
Strychnos nuxvomica is grown extensively in South Asia. Brucine, the second abundant alkaloid constituent of Strychnos nuxvomica, alleviated thermal hypersensitivity and mechanical allodynia in CCI induced neuropathic pain. This kind of inhibition is due to brucine which inhibits voltage dependent sodium channels. The result is reduced excitability of DRG neurons through a reduction of action potential firing frequency (Yu et al., 2019).
Zingiber officinale
Root extract of Zingiber officinale rich in gingerols and shogaols, exhibit antagonistic effects on voltage dependent Nav1.8 channels in oral ulcerative mucositis (Hitomi et al., 2017) and SNL induced neuropathic pain (Shen et al., 2022). Therefore, both ingredients demonstrate inhibitory effects on the generation of action potentials in DRG neurons, which contributes to the analgesic effects of Zingiber officinale in neuropathic pain.
Goshajinkigan extract formulation
Goshajinkigan extract formulation (GJG), an aqueous extract of a combination of 10 herbal medicines, a traditional Japanese Kampo formula, has been demonstrated to have an ameliorative effect on diabetic and chemotherapy associated peripheral neuropathic pain. Kampo formulae are composed of two or more kinds of natural crude drugs, and the decoctions of their mixtures are generally administered. Neoline as the active ingredient of GJG demonstrated antinociceptive effect via the inhibition of Nav1.7 current in streptozotocin as well as oxaliplatin-induced neuropathic pain in mice (Nakatani et al., 2020).
Allium macrostemon
Allium macrostemon is an edible herb traditionally used for the treatment of thoracic pain, stenocardia, asthma and diarrhea. Crude extract of Allium macrostemon significantly reduced pain behaviors in rodent pain models. Moreover, Allium macrostemon significantly reduced the excitability of sensory neurons by inhibition of the voltage dependent Nav1.7 channel contributing to a reduction in the firing frequency of action potentials thus reducing peripheral neuronal excitability (Yang et al., 2021b).
Corydalis yanhusuo
L-Tetrahydropalmatine and protopine monomers derived from Corydalis yanhusuo were tested in vivo and in vitro, to determine their analgesic properties. The results demonstrated that both monomers showed strong analgesic activity and inhibited the peak currents, which promoted the activation and inactivation phases of Nav1.7 channels (Xu et al., 2021a).
Magnolia officinalis
Magnolol, a hydroxylated biphenyl compound isolated from the bark of Magnolia officinalis, showed inhibitory effect on voltage dependent sodium currents at sensory neurons in a concentration-dependent manner (Gong et al., 2012). In addition, Magnolol significantly postponed recovery of voltage dependent Na+ currents from inactivation and produced frequency dependent blocks of both subtypes of Na+ currents (Qiu et al., 2021). These results suggest that the inhibitory effects of magnolol on Na+ channels may contribute to its analgesic effect.
Bupleurum chinense
Saikosaponin A, monomer derived from Bupleurum chinense was tested in vivo and in vitro, to determine its analgesic properties. The results showed that Saikosaponin A in Bupleurum chinense inhibited the peak currents of Nav1.7 in a concentration-dependent manner, suggesting they may be potential inhibitors of Nav1.7, thus indicates analgesic potential. Further, the study demonstrated that Saikosaponin A made Nav1.7 more easily activated and made it more difficult for the cell to return to its resting membrane potential thus delaying the regulation process of Nav1.7 channel as a whole. However, this did not affect the inactivation state of the channel. In vivo study of Saikosaponin A showed analgesic potential in thermal pain test and formalin-induced pain test in mice (Xu et al., 2021b).
Angelica biserrata
Imperatorin, monomer derived from Angelica biserrate, was tested in vivo and in vitro to determine its analgesic properties. The results showed that Imperatorin in Angelica biserrata inhibited the peak currents of Nav1.7 in a concentration-dependent manner, suggesting analgesic potential. Further study demonstrated that Imperatorin modulated Nav1.7 activation and inactivation thresholds. In vivo studies showed analgesic potential of Imperatorin in thermal pain and formalin-induced pain tests in mice (Xu et al., 2021b).
Rhododendron mole
Rhodojaponin III, an active constituent of Rhododendron mole, significantly inhibited the latency of the nociceptive response in the hot plate, tail-immersion, acetic acid and formalin-induced pain tests. Furthermore, Rhodojaponin III improved hyperalgesia in CCI rats. Electrophysiological experiments demonstrated that Rhodojaponin III mildly blocks Nav1.7 and Nav1.8 sodium channels to different degrees in a significant dose-dependent manner to ameliorate nociceptive and peripheral neuralgia-associated pain. Hepatotoxicity and leukopenia are associated as chronic side effects with Rhodojaponin III, hence, further investigation of sub-acute toxicity is necessary (Yang et al., 2022).
Aconiti Brachypodi
Ethanolic extract of Aconiti Brachypodi produced dose-dependent analgesic effects on hot plate tests, acetic acid induced writhing test, and formalin test in mice. In vitro studies of Aconiti Brachypodi extract indicated that temperate concentrations of extract reduced TTX-sensitive peak sodium current amplitudes in a dose-dependent way in rat’s dorsal root ganglion neurons, suggesting the modulation of Ethanolic extract of Aconiti Brachypodi on the TTX-sensitive sodium currents involved in its intervention in the input of nociceptive information (Ren et al., 2012).
K+ channels superfamily is a very
large group of ion channels. Voltage-gated potassium channels (VGKKs) are important physiological regulators of membrane potentials, action potential shape, and firing adaptation in excitable tissues including nociceptive sensory neurons. (Jain et al., 2003, Johnston et al., 2010, Lázaro-Ibáñez et al., 2001, MacKinnon, 2003, Ocaña et al., 2004, Ortiz et al., 2003, Tsantoulas and McMahon, 2014, Yamazumi et al., 2001). Recent studies in various pain models identified the voltage gated potassium channels and non-VGKK channels including calcium-activated (KCa) or ATP-sensitive potassium (KATP) channels as potential therapeutic targets for pain (Abd-Elsayed et al., 2019, Du and Gamper, 2013, Wickenden and McNaughton-Smith, 2009).
Effect of natural products on K+ channels (Table 1)
Stellera chamaejasme
Neochamaejasmin A (NCA), a biflavonoid, one of the main active ingredients in the plant roots of Stellera chamaejasme, inhibited Kv1.4 channels in whole cell patch clamp of transfected human Kv1.4 CHO cell lines with IC50 of 7.55 µM via direct binding to the pore domain. Three mutations, V549A, A553V and V560A, occurred inside the pore, were found to significantly alleviate the NCA blocking effects, suggesting that they are the important binding sites of NCA (Ren et al., 2018).
Eucommiae folium
Chlorogenic acid (CGA), a flavonoid, obtained from dried leaves of Eucommiae folium, decreased the peak current density of IK,A channels in whole cell patch clamp of rat trigeminal ganglion (TG) neurons. It caused significant reduction in the activation and inactivation thresholds of IK,A and IK,V channels and exhibited a strong effect on the activation and inactivation velocities of IK,A and IK,V channels. These findings provided novel evidence, explaining the biological effects of CGA, especially regarding its anti-nociceptive action (Zhang et al., 2014).
Panax ginseng
Gintonin, devoid of ginseng saponins, prepared from leaves of Panax ginseng, inhibited Kv1.2 channel activity in two electrode voltage-clamp experiment, in reversible and concentration-dependent manners in Xenopus oocytes after injection of RNA encoding the human Kv1.2α subunit. Gintonin mediated regulation of Kv1.2 channel activity might explain one of the modulations of gintonin mediated neuronal activities in nervous system (Lee et al., 2013).
Eriope blanchetii
Oleanolic acid, pentacyclic triterpene, isolated from aerial part of Eriope blanchetii inhibited capsaicin evoked acute nociception in mice. The study suggested that its antinociceptive action is at least, in part, related to the activation of ATP-gated K+ channels (Maia et al., 2006).
Dioscorea bulbifera
The methanolic extract of Dioscorea bulbifera indicated significant antinociceptive effects in persistent pain induced by intraplantar injection of complete Freund’s adjuvant and on neuropathic pain induced by partial ligation of sciatic nerve. This study demonstrated the antinociceptive activities of Dioscorea bulbifera on both inflammatory and neuropathic pain and these effects may result, at least partially, from its ability to activate the NO–cGMP–ATP-sensitive potassium channels pathway (Nguelefack et al., 2010).
Sphagneticola trilobata
Kaurenoic acid is a diterpene isolated from Sphagneticola trilobata, which dose-dependently inhibited inflammatory nociception induced by acetic acid, phenyl-p-benzoquinone, complete Freund's adjuvant, or formalin. Results indicate that kaurenoic acid exhibits a consistent analgesic effect and that its mechanism involves the activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel signaling pathway (Mizokami et al., 2012).
Vigueira arenaria
Pimaradienoic acid is a pimarane diterpene extracted at high concentration from Vigueira arenaria. Pimaradienoic acid dose dependently inhibited inflammatory nociception induced by carrageenan-induced paw edema, acetic acid, complete Freund's adjuvant, and formalin. The study data show that pimaradienoic acid exhibits an analgesic effect and that its mechanisms involve the activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel signaling pathway (Possebon et al., 2014).
Punica granatum
Ellagic acid, a polyphenolic secondary metabolite isolated from Punica granatum, produced a dose related peripheral antinociception during late phases of the formalin test which is comparable with morphine. The purposed mechanism involves activation of the l-arginine/NO/cGMP/KATP channels pathway followed by hyperpolarization of primary afferent neurons (Ghorbanzadeh et al., 2014).
Inula britannica
Patuletin is a trimethoxyflavone flavonoid isolated from Inula britannica, demonstrated significant antinociception potential in pain assessment tests including acetic acid induced writhing, formalin and glutamate induced paw licking. The results indicated that patuletin exhibits an analgesic effect. Its mechanisms involve the activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel signaling pathway (Zarei et al., 2018).
Cnicus benedictus
Methanolic extract of Cnicus benedictus exhibited an antinociceptive effect on acetic acid-induced writhing and tail-flick tests. The mechanism of Cnicus benedictus antinociception involved activation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel signaling pathway (Ahmadimoghaddam et al., 2020).
Bougainvillea spectabilis
The methanolic extract of Bougainvillea spectabilis indicated significant antinociception potential in pain assessment tests including acetic acid-induced writhing and formalin induced paw licking. The study data showed that Bougainvillea spectabilis exhibits potent peripheral antinociceptive effects and that its mechanisms involve the modulation of the NO-cyclic GMP-protein kinase GATP-sensitive potassium channel signaling pathway (Ferdous et al., 2020).
Artemisia biennis
Essential oil derived from Artemisia biennis had significant anti-nociceptive activity in the acetic acid induced writhing, tail-flick and formalin and glutamate induced paw licking assays and mechanical allodynia induced by cervical spinal cord contusion. Study data output indicated activation of the L-arginine-NO-cGMP-KATP system as a result of the anti-nociceptive abilities of Artemisia biennis extract (Zarei et al., 2021).
Bupleurum falcatum
Essential oil obtained from Bupleurum falcatum showed significant anti nociceptive activity in the formalin induced paw licking and mechanical allodynia induced by cervical spinal cord contusion. The study data indicated that Bupleurum falcatum exhibits potent anti-nociceptive effect and that its mechanisms involve the modulation of the L-arginine-NO-cGMP-KATP system pathway (Ahmadimoghaddam et al., 2021).
Voltage-gated Ca2+ channels (VGCCs)
are expressed in excitable cells including DRG neurons where they control the release of neurotransmitters and neuronal excitability. VGCCs are responsible for depolarization-induced influx of Ca2+, triggers consequent release of neurotransmitter from synaptic vesicles and increase excitability thus blocking or genetically deleting these channels in hyperexcitable nociceptive neurons may reduce net excitability. These channels are well established mediators of pain signals in primary afferent neurons. The Ca2+ channel is composed of pore forming α1 subunit and the auxiliary subunits; β, γ and α2δ. On the basis of α1 subunit, they fall into three categories as Cav1-3. (Bourinet et al., 2014, Dolphin, 2018a, Dolphin, 2018b, Field et al., 2006, Rettig et al., 1996, Sheng et al., 1996, Zamponi et al., 2015). N-type Ca2+ channels are localized to synaptic nerve terminals in laminae 1 and 2 of the dorsal horn where their opening results in the release of neurotransmitters while T-type VGCCs are likely localized to nerve endings where they regulate cellular excitability. Consequently, inhibition of N-type and T-type VGCCs has the propensity to mediate analgesia. (Alles and Smith, 2018, Montera et al., 2021, Todorovic and Jevtovic-Todorovic, 2013).
Effect of natural products on VGCCs (Table 1)
Cannabis sativa
Cannabidiol obtained from Cannabis sativa, at moderately hyperpolarized potentials, Cannabidiol inhibited peak Cav3.1 and Cav3.2 currents by about 45%, but were less potent on Cav3.3 channels. Cannabidiol produced a significant hyperpolarizing shift in the steady state inactivation potentials for each of the Cav3 channels, which accounts for inhibition of channel currents and analgesic potential (Harding et al., 2023, Ross et al., 2008). Camphene and alpha-bisabolol, terpenes, isolated from Cannabis sativa, significantly inhibited Cav3.2 channels expressed in HEK tsA-201 cells, as well as native T-type channels in mouse DRG neurons by inhibiting peak current in the low micromolar range, and mediated an additional small hyperpolarizing shift in half-maximal inactivation threshold. Both terpenes inhibited nocifensive responses in mice that had received an intraplantar injection of formalin, reduced thermal hyperalgesia in mice injected with CFA and also inhibited mechanical hypersensitivity induced by partial sciatic nerve ligation. These effects were absent in Cav3.2 null mice, indicated that these compounds mediated their analgesic properties by acting on Cav3.2 channels (Gadotti et al., 2021).
Syzygium aromaticum
Eugenol, an essential oil from Syzygium aromaticum plant inhibited Cav3.1, Cav3.2, and Cav3.3 channels in a concentration-dependent manner by negatively shifting the steady-state inactivation curves of the T-type channel isoforms. Eugenol showed little effect on the current kinetics of Cav3.1 and Cav3.2, but it accelerated the inactivation kinetics of Cav3.3 currents and reduction of channel availability enhanced eugenol inhibition sensitivity for Cav3.1 and Cav3.2, but not for Cav3.3. T-type currents recorded from rat TG neurons were inhibited by eugenol with a similar potency to Cav3.1 and Cav3.2 isoforms. These findings suggested that T-type Ca2+ channels are additional molecular targets for the pain relieving effects of eugenol (Seo et al., 2013).
Lavandula stoechas and Rosmarinus officinalis
Methanolic extract of Lavandula stoechas and Rosmarinus officinalis containing active constituent essential oils, linalool and rosmarinic acid, respectively, inhibited Cav3.2 channels in a concentration dependent manner by negative shift of the steady-state inactivation of Cav3.2 channels with no change in the activation properties. These results demonstrated that the Cav3.2 calcium channels are molecular target of the linalool and rosmarinic acid for their antinociception activity (El Alaoui et al., 2017, Narusuye et al., 2005).
Sophorae radix
Sophoraflavanone G isolated from Sophorae radix, reported to blocked Cav3.1 and Cav3.2 channels. In mice, sophoraflavanone G, abolished the mechanical allodynia following intraplantar administration of a hydrogen sulfide donor, strongly suppressed visceral pain and spinal ERK phosphorylation, and alleviated the neuropathic allodynia induced by partial sciatic nerve ligation or oxaliplatin. The data demonstrated that sophoraflavanone G blocks T-type calcium channels and alleviates neuropathic and visceral pain (Sekiguchi et al., 2018).
Hyptis emoryi
Betulinic acid (BA) found in Hyptis emoryi inhibited depolarization evoked calcium influx in DRG neurons predominantly through targeting low-voltage gated Cav3.2, Cav3.3 and high-voltage gated Cav2.2 calcium channels resulting in reduced spontaneous excitatory post synaptic currents and depolarization-evoked release of calcitonin gene-related peptide (CGRP) from lumbar spinal cord slices. Voltage clamp electrophysiology experiments revealed a reduction of Ca2+, but not Na+, currents in sensory neurons following BA exposure. BA showed reversed mechanical allodynia in chemotherapy and partial sciatic nerve ligation, induced peripheral neuropathy. All these results highlighted BA as a potential non-opioid therapy for management of chronic pain (Bellampalli et al., 2019).
Paeonia lactiflora
Astilbin (AB), mainly obtained from Paeonia lactiflora, showed analgesic activities via regulation of the Ca2+ channels. AB strongly reduced the expression levels of c-Fos and phosphorylated calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) in the mice brain. These effects correlated with changes of the Ca2+ channel and intracellular Ca2+ influx which indicated evidence that astilbin-mediated analgesia is related to Ca2+ channels (Bi et al., 2019).
Physalis acutifolia
The natural product physalin F, isolated from the Physalis acutifolia, demonstrated antinociceptive effects in models of inflammatory pain. Physalin F reported to blocks Cav2.2 (N-type) voltage-gated calcium channels in DRG neurons without any effect on Cav3 calcium channels, voltage-gated sodium and potassium channels. It inhibited the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in spinal cord slices and reversed tactile hypersensitivity in models of paclitaxel-induced peripheral neuropathy and spinal nerve ligation (Shan et al., 2019).
Solanum virginianum
Ethanolic extract of Solanum virginianum significantly debilitated hyperalgesia and allodynia in CCI rats. Further docking simulation studies of solasodine (active constituent in Solanum virginianum extract) revealed that solasodine properly positioned at Cav2.2 may inactivate calcium channels (Verma et al., 2020).
Parthenium incanum
Argentatin C obtained from Parthenium incanum blocked the activity of both voltage-gated sodium and T-type calcium channels in calcium imaging assays. Docking analysis predicted that argentatin C may bind to Nav1.7–1.9 and Cav3.1–3.3 channels. Furthermore, argentatin C reversed mechanical allodynia in a paw incision mouse model of postsurgical pain by reducing the Na+ and T-type Ca2+ currents as well as excitability in rat and macaque DRG neurons (Duran et al., 2022).
Heantos-4
Heantos-4, is a mixture of organic herbs developed in Vietnam, significantly inhibited Cav3.1 and Cav3.3 currents in whole-cell voltage clamp study on exogenously expressed T-type calcium channels. These findings indicated that Heantos-4 has selective effects on specific T-type calcium channel isoforms makes it possible candidate with antinociceptive potential (Cain et al., 2016).
Transient receptor potential channels (TRP channels)
are wide collection of a gene family involved in pain and itch sensory function. This family is made up of ion channel proteins that function as non-selective cation-permeable channels, virtually all of which conduct Ca2+ (Nilius, 2007). They function as molecular sensors of multiple physical and chemical stimuli, including changes in pH, chemical irritants including pungent peppers, wasabi, mustard, and menthol, as well as thermal, mechanical, osmotic, and actinic (radiation) cues. The TRP superfamily is composed of 28 members divided into six subfamilies, classified as canonical (TRPC), vanilloid (TRPV), ankyrin (TRPA), melastatin (TRPM), polycystin (TRPP), and mucolipin (TRPML) (Vennekens et al., 2008). The involvement of the TRPV1 (Chen et al., 2009), TRPV2 (Cheng et al., 2007), TRPA1 (Cho et al., 2012) and TRPM8 (McKemy et al., 2002, Peier et al., 2002, Weyer and Lehto, 2017) channels in thermal nociception has been well documented. Temperatures below 15 °C or above 43 °C evoke thermal sensation accompanied by the sensation of pain and these channels exhibits distinct thermal activation thresholds (Caterina et al., 1997, Caterina and Julius, 2001, Moore et al., 2018, Vennekens et al., 2008). Moreover, it is clear that spinal as well brain synaptic plasticity is an important procedure for the pain transition from acute to chronic in which TRP channels play critical roles presynaptically and postsynaptically (Choi et al., 2016, Duitama et al., 2020, Kim et al., 2008).
Effect of natural products on transient receptor potential channels (TRP) (Table 1)
Scutellaria baicalensis
Intraperitoneal (i.p.) 16-day administration of baicalin, a glycosyloxyflavone isolated from Scutellaria baicalensis, significantly reduced the mechanical and thermal nociceptive responses induced by CCI surgery in rats in a dose-dependent manner. The mRNA expression levels of TRPV1 and TRPA1 were significantly increased in the DRG of CCI rats. Moreover, baicalin administration, reversed mRNA expression level of TRPV1 (Sui et al., 2010) and suppressed TRPV1 upregulation and phosphorylation of extracellular signal-regulated kinases (MAPK/ERK pathway) (Wang et al., 2020) in DRG neurons after peripheral nerve injury might account for the anti-nociceptive mechanism of baicalin.
Vitex agnus
Vitexin, a flavonoid extracted from Vitex agnus, dose-dependently inhibited pain-like behavior i.e. mechanical and thermal hyperalgesia induced by capsaicin (an agonist of TRPV1), demonstrated that Vitexin exhibits an analgesic effect by targeting TRPV1 channels (Borghi et al., 2013).
Pterodon pubescens
The antinociceptive effects of ethanolic extract of Pterodon pubescens were reported on mechanical and thermal hyperalgesia in neuropathic pain induced by partial sciatic nerve ligation in mice along with nociceptive response induced by TRPV1 and TRVA1 agonists (capsaicin and cinnamaldehyde, respectively). Results indicated that oral administration of ethanolic extract of Pterodon pubescens, attenuate neuropathic pain associated thermal and mechanical hyperalgesia, without inducing tolerance, along with significant inhibition of TRPV1 and TRPA1 channels activators. This study added evidence for the therapeutic potential of Pterodon pubescens in the management of neuropathic pain (Nucci-Martins et al., 2015).
Croton macrostachyus
Methanol/methylene chloride extract of Croton macrostachyus was tested on CFA-induced persistent thermal and mechanical pain, neuropathic pain induced by partial sciatic nerve ligation (PSNL), prostaglandin E2 induced acute mechanical hyperalgesia, as well as on nociception induced by capsaicin in mice. Croton macrostachyus induced long lasting and significant antihyperalgesic effects on CFA-inflammatory and PSNL-induced neuropathic pain, reduced the mechanical hyperalgesia induced by PGE2 and time dependently inhibited the capsaicin-induced nociception. The results indicated that Croton macrostachyus exerted anti-nociception potential through the modulation of TRPV1 channels (Nguelefack et al., 2015).
Angelicae pubescentis
Coumarins, isolated from dried roots of Angelicae pubescentis, significantly prevented neuropathic pain and attenuated the development of mechanical hypersensitivity induced by spared nerve injury in rat. Molecular profiling revealed that coumarins reduced the levels of proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) and significantly attenuated the expression of TRPV1 and pERK in damaged DRG neurons (Li et al., 2017).
Ephedra sinica
Ephedra herb extract (EHE) of Ephedra sinica significantly increased the intracellular Ca2+ concentration in stable mouse TRPV1-expressing mTRPV1/Flp-In293 cells, which was inhibited by the TRPV1 antagonist BCTC, indicated that EHE activated TRPV1 channels. In vivo study demonstrated that EHE induced paw licking behavior in a dose-dependent manner which was also inhibited by TRPV1 antagonist BCTC. Administration of EHE before administration of capsaicin suppressed capsaicin-induced paw licking by regulating TRPV1 activity on sensory neurons, without affecting the physical performance of the mice (Nakamori et al., 2017).
Amphilophium crucigerum
Crude extract and dichloromethane fraction of Amphilophium crucigerum reported antinociceptive effect in the hot water tail-flick and capsaicin intraplantar tests. Furthermore, these preparations exhibited anti-nociceptive and anti-inflammatory effects in a chronic inflammatory pain model, CFA, and anti-nociceptive effects in neuropathic pain model in mice. Moreover, crude extract and dichloromethane fraction reduced capsaicin-induced Ca2+ influx and diminished the [3H]-resiniferatoxin specific binding to spinal cord membranes. Results supported the analgesic effect of Amphilophium crucigerum and suggested the presence of compounds that may act as TRPV1 antagonists (De Prá et al., 2017).
Water extract of frankincense and myrrh (WFM)
Frankincense and myrrh are widely used in clinics as a pair of herbs obtained from Boswellia carterii and Commiphora myrrha, respectively, for their synergistic effects that relieve pain. In vivo study showed that the nociceptive response in mouse by heat and capsaicin induced were relieved by WFM treatment. Calcium response to capsaicin was also decreased in DRG neurons of CCI mouse after WFM treatment Furthermore, thermal hypersensitivity and mechanical allodynia were also alleviated by WFM treatment in a CCI neuropathic pain model by reverting the TRPV1 expression at both the mRNA and protein levels in predominantly small-to-medium neurons. In conclusion, WFM alleviated CCI-induced mechanical allodynia and thermal hypersensitivity via modulating TRPV1 expression (Hu et al., 2017).
Echinophora platyloba
Polyacetylene fraction isolated from Echinophora platyloba were evaluated for their modulation of six thermo-TRP channels (TRPA1, TRPM8, TRPV2-4, TRPM8) and they revealed a selective activity on TRPA1, an ion channel involved in the mediation of neuropathic and inflammatory pain (Chianese et al., 2018).
Nypa fruticans
In a sciatic crush injury rat model, a significant level of antinociceptive effect was reported in the thermal hyperalgesia test in which ethanolic extract of Nypa fruticans was orally administered. Protein quantification of the sciatic nerve and L4–L6 spinal cord showed a decreased TRPV1 expression, the inflammatory expression factor, COX2, and proinflammatory factors in the test groups. These results indicated that Nypa fruticans affects anti-nociceptive and anti-inflammatory by controlling TRPV1 in sciatic neuropathic pain models (Kang and Hyun, 2020).
Zingiber officinale
Ethanolic extract of Zingiber officinale and its active constitute 6-shogaol, alleviated hyperalgesia and allodynia in the STZ induced diabetic peripheral neuropathy mice model. Both ginger extract (400 mg/kg) and 6-shogaol (15 mg/kg) significantly reduced TRPV1 and NMDAR2B expressions in the spinal cord with very limited effect on pancreatic islets, compared to the diabetic control group. TRPV1 functionally interacts with N-methyl-D-aspartate receptors (NMDAR) and contributes to the development of pain behavior. Research found that the expression of NMDAR subunit 2B (NMDAR2B) in the spinal cord's dorsal horn is higher in mice models of diabetic neuropathy (Fajrin et al., 2020).
Corydalis Saxicola
Crude extract of Corydalis Saxicola reported anti-nociception potential in cisplatin-induced mechanical, heat, and cold hyperalgesia. Corydalis Saxicola exerted its therapeutic effects by ameliorating neuronal damages, improving intraepidermal nerve fiber (IENF) loss, and inhibiting inflammation-induced p38 phosphorylation to block TRPV1 activation (Kuai et al., 2020).
Coptis chinensis
Berberine obtained from Coptis chinensis demonstrated to increase both mechanical and thermal pain thresholds in a dose-dependent manner in partial sciatic nerve ligation (PSNL) (Yang et al., 2020b) and cisplatin-induced (CIPN) peripheral neuropathy (Meng et al., 2021). The pain reducing potential of berberine exerted by reversed the mRNA and protein expression of TRPV1 in dorsal root ganglion neurons after peripheral nerve injury (Yang et al., 2020b). Moreover, berberine mediated the neuroinflammatory reaction induced by cisplatin by inhibiting the overexpression of TRPV1 and NF-κB and activating the JNK/p38 MAPK pathways in early injury, which inhibited the expression of p-JNK and mediated the expression of p38 MAPK/ERK in late injury in vivo at dorsal root ganglion neurons (Meng et al., 2021).
Ononis spinosa
Methanolic extract of Ononis spinosa alleviated capsaicin induced mechanical allodynia through the direct modulation of TRPV1 and the involvement of β2 adrenoreceptor signaling (Jaffal et al., 2021).
Parkia platycephala
Lectin isolated from Parkia platycephala reduced nociceptive behavior in adult zebrafish, and this is related to the activation of the TRPV1 channels since antinociception was effectively inhibited by capsazepine and by capsaicin-induced desensitization. Lectin reduced allodynic nociceptive behavior associated with formalin induced temporomandibular joint pain and infraorbital nerve transection induced neuropathic pain in rats. The results confirmed the potential pharmacological relevance of Parkia platycephala as an inhibitor of orofacial nociception in acute and chronic pain through the modulation of TRPV1 (de Oliveira Leite et al., 2022).
Piper nigrum
Viphyllin, a standardized extract of Piper nigrum seeds, was reported to significantly inhibit the acetic acid induced writhing and formalin induced paw licking. It increased the withdrawal latency in hot plate and tail flick test. Capsazepine abolished the analgesic effect of Viphyllin, clearly suggested the involvement of TRPV1 ion channel in Viphyllin mediated antinociceptive effect (Venkatakrishna et al., 2022).
Cymbopogon citratus
Citral, a naturally occurring terpene, extracted from Cymbopogon citratus, produced significant antinociception on acute nociceptive behaviors, and these effects were attenuated by TRPV1 antagonist capsazepine, TRPM3 antagonist mefenamic acid and by TRPM8 desensitization. The infraorbital nerve transection (IONX) animals developed facial mechanical hypersensitivity that was significantly reduced by citral. The docking experiments revealed that citral may interact with TRPV1 and TRPM8 channels. These results indicated the potential use of citral as an inhibitor of orofacial nociception in both acute and chronic pain states through TRPV1, TRPM3 and TRPM8 channels (Alves Rodrigues Santos et al., 2022).
Purinergic receptor cation channels (P2X)
also known as the ATP-gated P2X receptor cation channel family that consists of seven receptor subtypes named P2X1-P2X7, is made up of cation-permeable ligand-gated ion channels that open in response to extracellular adenosine 5′-triphosphate binding (ATP). ATP released from damaged or inflamed cells activates the excitatory and calcium-permeable P2X receptor channels to initiate and maintain the nociceptive signals, therefore, their selective targeting represents a therapeutic opportunity for pain management. The P2X channels are reported to play crucial role in central nervous system pain transmission and persistent modulation upon and following the occurrence of neuropathic pain. Recent advances in the structural, functional and pharmacological characterization of rodent and human ATP-gated P2X receptor channels have shed brighter light on the role of P2X2, P2X3, P2X4 (Westlund et al., 2021) and P2X7 receptor channels in the pathogenesis of central pain including the mediation of fast transmission in the peripheral nervous system and modulation of neuronal activity in the central nervous system (Bernier et al., 2018, Gever et al., 2006, Khakh and Alan North, 2006, Kuan and Shyu, 2016, North, 2002).
Effect of natural products on purinergic receptor cation channels (P2X) (Table 1)
Sodium ferulate
Sodium ferulate (SF) is an active principle of Angelica sinensis, Cimicifuga heracleifolia, Lignsticum chuangxiong and expressed antioxidant and anti-inflammatory activities. SF indicated reduced thermal and mechanical hyperalgesia in CCI rat model by decreasing the pain transmitted by primary afferent neurons mediated by P2X3 receptor. In CCI rats treated with SF, the Mechanical withdrawal threshold, and thermal withdrawal latency were increased while the upregulated expression of P2X3 receptors in DRG neurons was reduced followed decreased the increment of P2X3 agonist-activated currents and P2X3 mRNA expression, compared to the normal saline group (Zhang et al., 2010, Zhang et al., 2008).
Ligusticum wallichii
Tetramethylpyrazine (TMP), an alkaloid, is an important compound in Ligusticum wallichii, reported to inhibit the primary afferent transmission of neuropathic pain induced by P2X3 receptor in CCI rats. TMP reduced the mechanical withdrawal threshold and thermal withdrawal latency by downregulation of the P2X3 receptor expression in L4/L5 DRG neurons and spinal cord (Wang et al., 2017). TMP reported to alleviates nociceptive transmission of burn-injury pain mediated by the P2X3 receptor (Gao et al., 2008, Gao et al., 2010).
Lappaconitine
Lappaconitine (LA) is an aconitum alkaloid extracted from the plants of genus Aconitum, showed increased pain thresholds, the down-regulated P2X3 receptor expression and the reduced P2X3 receptor agonists ATP- and α, β-meATP-induced inward currents (IATP and Iα, β-meATP) in the acutely dissociated rat DRG neurons of CCI rats. These results indicated that the analgesic effect of LA involves decreased expression and sensitization of the P2X3 receptors of the rat DRG neurons following CCI (Ou et al., 2011).
Rheedia longifolia
The Rheedia longifolia extract and some fractions showed an analgesic and anti-inflammatory activity by inhibitory effect on the P2X7 purinergic receptor in a dose-dependent manner. The ethyl acetate fraction exhibited the most potent inhibitory effects than others like methanol extract and the butanol fraction. Further investigation is needed to determine the pattern of inhibition and selectivity (Santos et al., 2011).
Rheum rhabarbarum
Emodin, an anthraquinone obtained from Rheum rhabarbarum extract, demonstrated anti-hyperalgesic potential associated with significant reduction of P2X2/3 expression of L4/L5 DRG neurons in CCI rats. The data of immunohistochemistry, in situ hybridization (ISH) and RT-PCR in P2X2 and P2X3 mRNA expression suggested that the antinociceptive mechanism of emodin is involved in the nucleic acid level (Gao et al., 2011).
Radix puerariae
Puerarin, an isoflavonoid, obtained from Radix puerariae, decreased the thermal and mechanical hyperalgesia by inhibiting the up-regulated expression of P2X3 receptors from DRG neurons of CCI rats (Xu et al., 2012). The inflammation and associated pain involved in dressing changes of burn patients were relieved by puerarin treatment and this effect were correlated with the decreased expression level of P2X3/7 receptors mRNA and protein in peripheral blood mononuclear cells (PBMCs) of burn patients (Li et al., 2011, Zhang et al., 2013).
Sinomenium acutum
Sinomenine, an alkaloid originally isolated from the root of the plant Sinomenium acutum, significantly inhibited P2X3 agonist ATP-activated currents in HEK293 cells transfected with the P2X3 receptor. Sinomenine was reported to relieve the hyperalgesia in rats by suppressed the up-regulated expression and activation of the P2X3 receptor followed by decreased the phosphorylation and activation of P38MAPK in Type-2 diabetes mellitus (T2DM) inflicted DRG (Rao et al., 2017). In conclusion, sinomenine demonstrated potential to effectively alleviate mechanical and cold allodynia in rats and mice after photochemically induced sciatic nerve and spinal cord injury (Gao et al., 2013).
Curcuma longa
Study showed that peripheral nerve exposure to HIV gp120 increased neuropathy associated mechanical and thermal hyperalgesia accompanied by upregulated expression of the P2X3 receptor in the DRG of the gp120-treated model rats. Nano curcumin (Curcuma longa) treatment decreased the upregulated expression of the P2X3 receptor in DRG of gp120-treated model rats, followed by suppressed phosphorylation of ERK1/2 thus reduced the sensitization of DRG primary afferents and relieved mechanical and thermal hyperalgesia in gp120-treated rats (Zhao et al., 2017).
Artemisia annua
Artemisinin, extracted from Artemisia annua leaves, is a type of sesquiterpene lactone, relieved pain behaviors in the CCI rats, inhibited the expression of P2X4 receptor in the DRG, and decreased the ATP-activated currents in HEK293 cells transfected with P2X4 plasmid. Dual-labeling immunofluorescence study showed that the artemisinin significantly decreased the co-expression of P2X4 receptor and glial fibrillary acidic protein (GFAP) in DRG neurons of CCI rats (Ying et al., 2017).
Cnidium monnieri
Osthole is a component extracted from Cnidium monnieri plant seeds and has anti-inflammatory and anti-oxidative properties. Osthole treatment data showed decreased the P2X4 receptor upregulation and SGC activation in DRG neurons, followed by the down-regulation of IL1β, TNF-α, BDNF and p-p38MAPK and the up-regulation of IL-10 in diabetic mellitus (DM) rats. Osthole treatment may act on the P2X4 receptor to alleviate the mechanical and thermal hyperalgesia in DM rats (Yuan et al., 2018).
Eucalyptus
1,8-cineole is a natural monoterpene cyclic ether present in eucalyptus and has been reported to exhibit anti-inflammatory and antioxidant effects. 1,8-cineole treatment indicated decreased the mechanical withdrawal threshold and thermal withdrawal latency by down-regulation of P2X3 receptor mRNA expression and P2X3 receptor protein expression in the L4-L5 DRG neurons of CCI rats. These results demonstrated that 1,8-cineole can alleviate pathological pain caused by P2X3 receptor stimulation (Zhang et al., 2018b).
Gardenia jasminoides
Gardenoside, also known as genipin, is a natural reactive aglycone isolated from the fruit of Gardenia jasminoides, significantly improved the sciatica by partially restored the decreased of mechanical withdrawal threshold and thermal withdrawal latency in CCI rats. Further, results indicated that the levels of iNOS, IL-1β, TNF-α, p-ERK/ERK and p-p38/p38, and expressions of P2X3 and P2X7 receptors in the L4-L5 DRG neurons were significantly decreased in the CCI rats after gardenoside treatment. It was also reported that gardenoside combined with ozone could alleviated chronic neuropathic pain. The effects of gardenoside and ozone may be mediated by the inhibition of P2X3 and P2X7 receptor expression in the rat DRG (Yu et al., 2018a, Yu et al., 2018b).
Hesperidin
Hesperidin is a bioflavonoid, found in citrus fruits (family Rutaceae) with cardioprotective, neuroprotective, antioxidative and anti-inflammatory activities. Hesperidin reported to relieved the abnormal mechanical and thermal hyperalgesia in CCI rats by suppressed the upregulated expression of P2X3 protein and mRNA in DRG neurons which was accompanied by activation of ERK1/2 (Tao et al., 2019).
Hericium erinaceus
Crude extract of Hericium erinaceus reported to suppressed, the increased level of IL-6, activation of astrocytes and microglia and upregulated expression of P2X4 and P2X7 receptors at DRG neurons in L5-spinal nerve ligation mice model, thus relieved the neuropathic pain (Yang et al., 2020a).
Physalis angulata
Crude ethanolic extract of Physalis angulata enriched with physalin B, D, F, and G forms, showed dose-dependent inhibition of P2X7 receptor function and ATP-induced paw edema was potently inhibited in mice (Arruda et al., 2021).
Gallic acid
The results showed that CCI rats treated with gallic acid, the mechanical withdrawal threshold and thermal withdrawal latency were increased, accompanied by inhibition of the upregulated expression of P2X7 and TNF-α at both mRNA and protein levels, and reduced NF-κB and phosphorylated-STAT3 in the dorsal root ganglia. Gallic acid significantly decreased the co-expression of P2X7 and glial fibrillary acidic protein (GFAP) in the DRG. In addition, gallic acid could suppress ATP activated current in human embryonic kidney 293 (HEK293) cells transfected with the plasmid expressing P2X7 (Wen et al., 2022, Yang et al., 2021a).
Resveratrol
Resveratrol (RES) is a natural polyphenol obtained by a wide variety of plant species, including aliments, such as grapes, peanuts, and wines. The results suggested that RES ameliorated neuropathic pain in a dose-dependent manner induced by partial sciatic nerve ligation (PSNL) and STZ (DNP) in rats, by suppressing P2X3 up-regulation and ERK phosphorylation in DRG neurons and spinal dorsal horn terminals (SDH) (Cui et al., 2020, Guo et al., 2021). RES was also reported to decrease the sensitization of the P2X7 receptors in the satellite glial cells of DRG neurons after CCI and HIV envelope glycoprotein 120 (gp120) treated rats and increase the threshold of thermal and mechanical hypersensitivity in rats with chronic neuropathic pain (Wu et al., 2017, Xie et al., 2017).
Astragalin
Astragalin (AST), is a flavonoid extracted from the white stamen of some flowers, demonstrated partly abrogated the upregulation of P2X4, inhibited SGC activation, and alleviated pain behavior in CCI rats. It also suppressed ATP-activated currents in HEK293 cells overexpressing P2X4 (Wang et al., 2021).
Aconitum jaluense
Crude water extract of Aconitum jaluense showed anti-allodynic effects in neuropathic pain by suppression of P2X7 receptor expression as well as reduced microglial activation in the spinal cord of SNL rats (Yang et al., 2016).
Acid-sensing ion channels (ASICs)
are voltage-independent depolarizing sodium channels, expressed in somatosensory neurons, belonging to the degenerin/ENaC superfamily and activated by extracellular protons. Six isoforms have been identified encoded by four different genes: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4. During inflammation, tissue damage and ischemia, the extracellular pH values decreases, which activates nociceptors by activating a particular pH-specific ASIC. All ASICs except ASIC4 are expressed in DRG neurons. ASIC subunits are differentially expressed in different DRG neuronal subtypes after nerve injury suggesting a role in different sensory modalities (Deval et al., 2010, Lee and Chen, 2018, Papalampropoulou-Tsiridou et al., 2020).
Effect of natural products on acid-sensing ion channels (ASICs) (Table 1)
Azadiractha indica
Ethanolic extract of Azadiractha indica, significantly inhibited the acute nociception induced by acidic saline (0.1%) in an adult zebra fish experimental model. There was no difference between these groups treated with extract or morphine or naïve ones. The antinociceptive effect of extract was abolished by amiloride suggested that the antinociceptive effect of this extract on acute pain seems to be modulated by the acid-sensing ion channels (ASIC channels) (Batista et al., 2018).
Conclusion
Traditional medicine is used by different cultural groups all over the world and remedies have been passed down from generation to generation to maintain health. Ion channels like voltage-gated channels (Na+, Ca2+ channels), K+ channels, transient receptor potential channels (TRP), purinergic (P2X) channels and acid-sensing ion channels (ASICs) are critical for establishing acute and chronic pain and modulating the function of these channels can significantly alleviate pain. In recent years, natural medicinal agents of plant origin possess ion channel-modulating potential and have been recognized as a valuable source of new therapeutics for pain management. This review summarizes 79 natural products (53 isolated compounds and 26 crude extracts or formula) based on 97 research articles that show potent analgesic potential with activity at ion channels. Some of these natural products have undergone clinical trials, while others warrant further investigation for their mechanisms on pain signaling pathways. Most of the compounds/extracts identified did not present any toxicity or known side effects and were at least as efficient as currently used synthetic drugs. Out of 97 research articles, 30 articles used both male and female rodents while 52 used males, 4 used female rodents, and the remaining 11 articles are based on cell lines. Few studies mentioned in this review have used more than one species of rodents for different experiments to assess the anti-nociceptive activity of natural products. A total 55 studies used rats (wild type) of which 43 used Sprague Dawley rats and 12 are Wistar rats. Forty studies used mice of which 11 used C57Bl/6 mice, 2 used ICR mice, 4 used Kunning mice, 14 used Swiss albino mice, 2 used ddy mice, 3 used Balb/c mice, 1 used CD1 mice and 3 used knockout-mice. Three studies used non-mammalian models and five studies used human ion channel-expressing CHO cells. This comprehensive review, addressed both acute and chronic pain studies. Twenty-two studies are on acute pain models, 34 studies are on various chronic pain models (HIV-related, chemotherapy-induced and diabetic neuropathies, nerve injury and spinal cord injury), and 12 studies address both acute and chronic pain models. Future studies should focus on investigating mechanisms of action, dose ranges, clinical efficacy, safety of the extracts, sex as a biological variable, and active constituents to find more specific and safer molecules to target ion channels. In addition, more studies should be carried out on human neuronal cells to address translational potential (Renthal et al., 2021). However, the findings of this review are promising regarding the development of new potential therapeutic agents from natural products for treating acute and chronic pain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the Research Endowment Fund of the Department of Anesthesiology and Critical Care Medicine, University of New Mexico School of Medicine. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abd-Elsayed, A., Jackson, M., Gu, S.L., Fiala, K., Gu, J., 2019. Neuropathic pain and Kv7 voltage-gated potassium channels: The potential role of Kv7 activators in the treatment of neuropathic pain. Mol. Pain 15, 1744806919864256. 10.1177/1744806919864256. [DOI] [PMC free article] [PubMed]
- Ahmadimoghaddam D., Sadeghian R., Ranjbar A., Izadidastenaei Z., Mohammadi S. Antinociceptive activity of Cnicus benedictus L. leaf extract: a mechanistic evaluation. Res. Pharm. Sci. 2020;15:463–472. doi: 10.4103/1735-5362.297849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadimoghaddam D., Zarei M., Mohammadi S., Izadidastenaei Z., Salehi I. Bupleurum falcatum L. alleviates nociceptive and neuropathic pain: Potential mechanisms of action. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.113990. [DOI] [PubMed] [Google Scholar]
- Allegaert K., De Hoon J., Debeer A., Gewillig M. Renal Side Effects of Non-Steroidal Anti-Inflammatory Drugs in Neonates. Pharmaceuticals (Basel) 2010;3:393–405. doi: 10.3390/ph3020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles S.R.A., Nascimento F., Luján R., Luiz A.P., Millet Q., Bangash M.A., Santana-Varela S., Zhou X., Cox J.J., Okorokov A.L., Beato M., Zhao J., Wood J.N. Sensory neuron– derived NaV1.7 contributes to dorsal horn neuron excitability. Sci. Adv. 2020;6:eaax4568. doi: 10.1126/sciadv.aax4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles S.R.A., Smith P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018;70:315–347. doi: 10.1124/pr.117.014399. [DOI] [PubMed] [Google Scholar]
- Alves Rodrigues Santos, S.A., de Barros Mamede Vidal Damasceno, M., Alves Magalhães, F.E., Sessle, B.J., Amaro de Oliveira, B., Alves Batista, F.L., Vieira-Neto, A.E., Rolim Campos, A., 2022. Transient receptor potential channel involvement in antinociceptive effect of citral in orofacial acute and chronic pain models. EXCLI J. 21, 869–887. 10.17179/excli2022-5042. [DOI] [PMC free article] [PubMed]
- Arruda, J.C.C., Rocha, N.C., Santos, E.G., Ferreira, L.G.B., Bello, M.L., Penido, C., Costa, T.E.M.M., Santos, J.A.A., Ribeiro, I.M., Tomassini, T.C.B., Faria, R.X., 2021. Physalin pool from Physalis angulata L. leaves and physalin D inhibit P2X7 receptor function in vitro and acute lung injury in vivo. Biomed. Pharmacother. 142, 112006. 10.1016/j.biopha.2021.112006. [DOI] [PubMed]
- Batista, Francisco Lucas A, Lima, L.M.G., Abrante, Izamar A, de Araújo, J.I.F., Batista, Francisca Leidivania A, Abrante, Izabel A, Magalhães, E.A., de Lima, D.R., Lima, M. da C.L., do Prado, B.S., Moura, L.F.W.G., Guedes, M.I.F., Ferreira, M.K.A., de Menezes, J.E.S.A., Santos, S.A.A.R., Mendes, F.R.S., Moreira, R.A., Monteiro-Moreira, A.C.O., Campos, A.R., Magalhães, F.E.A., 2018. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 108, 408–416. 10.1016/j.biopha.2018.08.160. [DOI] [PubMed]
- Bear B., Asgian J., Termin A., Zimmermann N. Small molecules targeting sodium and calcium channels for neuropathic pain. Curr. Opin. Drug Discov. Devel. 2009;12:543–561. [PubMed] [Google Scholar]
- Bellampalli S.S., Ji Y., Moutal A., Cai S., Wijeratne E.M.K., Gandini M.A., Yu J., Chefdeville A., Dorame A., Chew L.A., Madura C.L., Luo S., Molnar G., Khanna M., Streicher J.M., Zamponi G.W., Gunatilaka A.A.L., Khanna R. Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels. Pain. 2019;160:117–135. doi: 10.1097/j.pain.0000000000001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.L., Clark A.J., Huang J., Waxman S.G., Dib-Hajj S.D. The role of voltage-gated sodium channels in pain signaling. Physiol. Rev. 2019;99:1079–1151. doi: 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- Benyamin R., Trescot A.M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Glaser S.E., Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Bernier L.-P., Ase A.R., Séguéla P. P2X receptor channels in chronic pain pathways. Br. J. Pharmacol. 2018;175:2219–2230. doi: 10.1111/bph.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H., Sun Z., Chu Q., Li L., Guan X., Zhou Y., Li Z. Analgesic effects of astilbin partially via calcium channels through regulation on CaMKII. Food Agric. Immunol. 2019;30:309–319. doi: 10.1080/09540105.2019.1580677. [DOI] [Google Scholar]
- Birch P.J., Dekker L.V., James I.F., Southan A., Cronk D. Strategies to identify ion channel modulators: Current and novel approaches to target neuropathic pain. Drug Discov. Today. 2004;9:410–418. doi: 10.1016/S1359-6446(04)03043-0. [DOI] [PubMed] [Google Scholar]
- Black J.A., Frézel N., Dib-Hajj S.D., Waxman S.G. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol. Pain. 2012;8:82. doi: 10.1186/1744-8069-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi S.M., Carvalho T.T., Staurengo-Ferrari L., Hohmann M.S.N., Pinge-Filho P., Casagrande R., Verri W.A.J. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013;76:1141–1149. doi: 10.1021/np400222v. [DOI] [PubMed] [Google Scholar]
- Bourinet E., Altier C., Hildebrand M.E., Trang T., Salter M.W., Zamponi G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014;94:81–140. doi: 10.1152/physrev.00023.2013. [DOI] [PubMed] [Google Scholar]
- Brune K. Next generation of everyday analgesics. Am. J. Ther. 2002;9:215–223. doi: 10.1097/00045391-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Cai S., Bellampalli S.S., Yu J., Li W., Ji Y., Wijeratne E.M.K., Dorame A., Luo S., Shan Z., Khanna M. (−)-Hardwickiic acid and Hautriwaic acid induce Antinociception via blockade of Tetrodotoxin-sensitive voltage-dependent sodium channels. ACS Chem. Nerosci. 2018;10:1716–1728. doi: 10.1021/acschemneuro.8b00617. [DOI] [PubMed] [Google Scholar]
- Cain S.M., Ahn S., Garcia E., Zhang Y., Waheed Z., Tyson J.R., Yang Y., Van Sung T., Phillips A.G., Snutch T.P. Heantos-4, a natural plant extract used in the treatment of drug addiction, modulates T-type calcium channels and thalamocortical burst-firing. Mol. Brain. 2016;9:94. doi: 10.1186/s13041-016-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F.C., Lewis R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018;175:2138–2157. doi: 10.1111/bph.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen Y., Willcockson H.H., Valtschanoff J.G. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp. Neurol. 2009;220:383–390. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Yang F., Takanishi C.L., Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chianese G., Sirignano C., Shokoohinia Y., Mohammadi Z., Bazvandi L., Jafari F., Jalilian F., Moriello A.S., De Petrocellis L., Taglialatela-Scafati O., Rigano D. TRPA1 modulating C14 polyacetylenes from the Iranian endemic plant Echinophora platyloba. Molecules. 2018;23:1–8. doi: 10.3390/molecules23071750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.-H., Jeong M.-Y., Choi I.-S., Lee H.-J., Jang I.-S. TRPA1-like channels enhance glycinergic transmission in medullary dorsal horn neurons. J. Neurochem. 2012;122:691–701. doi: 10.1111/j.1471-4159.2012.07817.x. [DOI] [PubMed] [Google Scholar]
- Choi S.-I., Lim J.Y., Yoo S., Kim H., Hwang S.W. Emerging Role of Spinal Cord TRPV1 in Pain Exacerbation. Neural Plast. 2016;2016:5954890. doi: 10.1155/2016/5954890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.J., Reimann F., Nicholas A.K., Thornton G., Roberts E., Springell K., Karbani G., Jafri H., Mannan J., Raashid Y., Al-Gazali L., Hamamy H., Valente E.M., Gorman S., Williams R., McHale D.P., Wood J.N., Gribble F.M., Woods C.G. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Li Y., Ning J., Mi Y., Wang X., Qiu Z., Li L., Gou X. Resveratrol alleviates diabetic mechanical allodynia in rats by downregulating P2X3R. Mol. Med. Rep. 2020;22:957–963. doi: 10.3892/mmr.2020.11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniyal M., Wang W. Molecular pharmacology of inflammation: Medicinal plants as antiinflammatory agents. Inflammation and Natural Products. Elsevier. 2021:21–63. [Google Scholar]
- de Oliveira Leite G., Santos S.A.A.R., dos Santos Silva R.R., Teixeira C.S., Campos A.R. Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation. Molecules. 2022;27:7506. doi: 10.3390/molecules27217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Prá S.D.T., Ferro P.R., Milioli A.M., Rigo F.K., Chipindo O.J., Trevisan G., Camponogara C., de Oliveira S.M., Casoti R., Manfron M.P., Ferreira J. Antinociceptive activity and mechanism of action of hydroalcoholic extract and dichloromethane fraction of Amphilophium crucigerum seeds in mice. J. Ethnopharmacol. 2017;195:283–297. doi: 10.1016/j.jep.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Deval E., Gasull X., Noël J., Salinas M., Baron A., Diochot S., Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Black J.A., Waxman S.G. Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 2009;10:1260–1269. doi: 10.1111/j.1526-4637.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. Voltage-gated calcium channels: their discovery, function and importance as drug targets. Brain Neurosci. Adv. 2018;2 doi: 10.1177/2398212818794805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin, A.C., 2018b. Voltage-gated calcium channel α (2)δ subunits: an assessment of proposed novel roles. F1000Research 7. 10.12688/f1000research.16104.1. [DOI] [PMC free article] [PubMed]
- Dong X.-P., Xu T.-L. Radix paeoniae rubra suppression of sodium current in acutely dissociated rat hippocampal CA1 neurons. Brain Res. 2002;940:1–9. doi: 10.1016/s0006-8993(02)02555-6. [DOI] [PubMed] [Google Scholar]
- Dowell D., Haegerich T.M., Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. J. Am. Med. Assoc. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Gamper N. Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr. Neuropharmacol. 2013;11:621–640. doi: 10.2174/1570159X113119990042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Gao H., Jaffe D., Zhang H., Gamper N. M-type K+ channels in peripheral nociceptive pathways. Br. J. Pharmacol. 2018;175:2158–2172. doi: 10.1111/bph.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca L.M., Helmick C.G., Barbour K.E., Nahin R.L., Von Korff M., Murphy L.B., Theis K., Guglielmo D., Dahlhamer J., Porter L., Falasinnu T., Mackey S. A Review of Potential National Chronic Pain Surveillance Systems in the United States. J. Pain. 2022;23:1492–1509. doi: 10.1016/j.jpain.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duitama M., Vargas-López V., Casas Z., Albarracin S.L., Sutachan J.-J., Torres Y.P. TRP Channels Role in Pain Associated With Neurodegenerative Diseases. Front. Neurosci. 2020;14:782. doi: 10.3389/fnins.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran P., Loya-López S., Ran D., Tang C., Calderon-Rivera A., Gomez K., Stratton H.J., Huang S., Xu Y., Wijeratne E.M.K., Perez-Miller S., Shan Z., Cai S., Gabrielsen A.T., Dorame A., Masterson K.A., Alsbiei O., Madura C.L., Luo G., Moutal A., Streicher J., Zamponi G.W., Gunatilaka A.A.L., Khanna R. The natural product Argentatin C attenuates postoperative pain via inhibition of voltage-gated sodium and T-type voltage-gated calcium channels. Br. J. Pharmacol. 2022;n/a doi: 10.1111/bph.15974. [DOI] [PubMed] [Google Scholar]
- El Alaoui C., Chemin J., Fechtali T., Lory P. Modulation of T-type Ca2+ channels by Lavender and Rosemary extracts. PLoS One. 2017;12:e0186864. doi: 10.1371/journal.pone.0186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajrin F.A., Nugroho A.E., Nurrochmad A., Susilowati R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J. Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112396. [DOI] [PubMed] [Google Scholar]
- Ferdous A., Janta R.A., Arpa R.N., Afroze M., Khan M., Moniruzzaman M. The leaves of Bougainvillea spectabilis suppressed inflammation and nociception in vivo through the modulation of glutamatergic, cGMP, and ATP-sensitive K(+) channel pathways. J. Ethnopharmacol. 2020;261 doi: 10.1016/j.jep.2020.113148. [DOI] [PubMed] [Google Scholar]
- Field M.J., Cox P.J., Stott E., Melrose H., Offord J., Su T.-Z., Bramwell S., Corradini L., England S., Winks J., Kinloch R.A., Hendrich J., Dolphin A.C., Webb T., Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. PNAS. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti V.M., Huang S., Zamponi G.W. The terpenes camphene and alpha-bisabolol inhibit inflammatory and neuropathic pain via Cav3.2 T-type calcium channels. Mol. Brain. 2021 doi: 10.1186/s13041-021-00876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Hao J., Wiesenfeld-Hallin Z., Wang D.-Q., Xu X.-J. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur. J. Pharmacol. 2013;721:5–11. doi: 10.1016/j.ejphar.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Gao Y., Xu C., Liang S., Zhang A., Mu S., Wang Y., Wan F. Effect of tetramethylpyrazine on primary afferent transmission mediated by P2X3 receptor in neuropathic pain states. Brain Res. Bull. 2008;77:27–32. doi: 10.1016/j.brainresbull.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Gao Y., Xu C., Yu K., Li G., Wan F., Liu S., Lin J., Liu H., Zhang J., Li X., Liang S. Effect of tetramethylpyrazine on DRG neuron P2X3 receptor involved in transmitting pain after burn. Burns. 2010;36:127–134. doi: 10.1016/j.burns.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Gao Y., Liu H., Deng L., Zhu G., Xu C., Li G., Liu S., Xie J., Liu J., Kong F., Wu R., Li G., Liang S. Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res. Bull. 2011;84:406–413. doi: 10.1016/j.brainresbull.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Gaskin D.J., Richard P. The economic costs of pain in the United States. J. Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Gever J.R., Cockayne D.A., Dillon M.P., Burnstock G., Ford A.P.D.W. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Ghorbanzadeh B., Mansouri M.T., Hemmati A.A., Naghizadeh B., Mard S.A., Rezaie A. Involvement of L-arginine/NO/cGMP/K(ATP) channel pathway in the peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacol. Biochem. Behav. 2014;126:116–121. doi: 10.1016/j.pbb.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Global Prescription Medication Statistics: Strong Growth and CNS Well Represented, 2015. ACS Chem. Neurosci. 6, 505–506. 10.1021/acschemneuro.5b00098. [DOI] [PubMed]
- Gong C.-L., Wong K.-L., Cheng K.-S., Kuo C.-S., Chao C.-C., Tsai M.-F., Leung Y.-M. Inhibitory effects of magnolol on voltage-gated Na+ and K+ channels of NG108-15 cells. Eur. J. Pharmacol. 2012;682:73–78. doi: 10.1016/j.ejphar.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Goyal S., Khilnani G., Singhvi I., Singla S., Khilnani A.K. Guggulipid of Commiphora mukul, with antiallodynic and antihyperalgesic activities in both sciatic nerve and spinal nerve ligation models of neuropathic pain. Pharm. Biol. 2013;51 doi: 10.3109/13880209.2013.796392. [DOI] [PubMed] [Google Scholar]
- Goyal, S., 2014. Medicinal plants of the genus sapindus (sapindaceae) – a review of their botany, phytochemistry, biological activity and traditional uses. J. Drug Deliv. Ther. 4. 10.22270/jddt.v4i5.949.
- Guo J., Wang C., Niu X., Zhou F., Li H., Gao W. Effects of resveratrol in the signaling of neuropathic pain involving P2X3 in the dorsal root ganglion of rats. Acta Neurol. Belg. 2021;121:365–372. doi: 10.1007/s13760-019-01126-2. [DOI] [PubMed] [Google Scholar]
- Harding E.K., Souza I.A., Gandini M.A., Gadotti V.M., Ali M.Y., Huang S., Antunes F.T.T., Trang T., Zamponi G.W. Differential regulation of Cav3.2 and Cav2.2 calcium channels by CB1 receptors and cannabidiol. Br. J. Pharmacol. 2023 doi: 10.1111/bph.16035. [DOI] [PubMed] [Google Scholar]
- Hassett A.L., Aquino J.K., Ilgen M.A. The risk of suicide mortality in chronic pain patients. Curr. Pain Headache Rep. 2014;18:436. doi: 10.1007/s11916-014-0436-1. [DOI] [PubMed] [Google Scholar]
- He X.-H., Zang Y., Chen X., Pang R.-P., Xu J.-T., Zhou X., Wei X.-H., Li Y.-Y., Xin W.-J., Qin Z.-H., Liu X.-G. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151:266–279. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Hitomi S., Ono K., Terawaki K., Matsumoto C., Mizuno K., Yamaguchi K., Imai R., Omiya Y., Hattori T., Kase Y., Inenaga K. [6]-gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na(+) channels. Pharmacol. Res. 2017;117:288–302. doi: 10.1016/j.phrs.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Hu D., Wang C., Li F., Su S., Yang N., Yang Y., Zhu C., Shi H., Yu L., Geng X., Gu L., Yuan X., Wang Z., Yu G., Tang Z. A Combined Water Extract of Frankincense and Myrrh Alleviates Neuropathic Pain in Mice via Modulation of TRPV1. Neural Plast. 2017;2017 doi: 10.1155/2017/3710821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffal S.M., Al-Najjar B.O., Abbas M.A. <i>Ononis spinosa</i> alleviated capsaicin-induced mechanical allodynia in a rat model through transient receptor potential vanilloid 1 modulation. Korean J. Pain. 2021;34:262–270. doi: 10.3344/kjp.2021.34.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.K., Patil C.S., Singh A., Kulkarni S.K. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003;67:150–156. doi: 10.1159/000067802. [DOI] [PubMed] [Google Scholar]
- Johansen A., Romundstad L., Nielsen C.S., Schirmer H., Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromsø study. Pain. 2012;153:1390–1396. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Johnston J., Forsythe I.D., Kopp-Scheinpflug C. SYMPOSIUM REVIEW: Going native: voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 2010;588:3187–3200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.W. Early drug discovery and the rise of pharmaceutical chemistry. Drug Test. Anal. 2011;3:337–344. doi: 10.1002/dta.301. [DOI] [PubMed] [Google Scholar]
- Kang M.S., Hyun K.Y. Antinociceptive and anti-inflammatory effects of Nypa fruticans wurmb by suppressing TRPV1 in the sciatic neuropathies. Nutrients. 2020;12:1–11. doi: 10.3390/nu12010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B.S., Alan North R. P2X receptors as cell-surface ATP sensors in health and disease. at. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., Park C.-K., Cho I.-H., Jung S.J., Kim J.S., Oh S.B. Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J. Pain. 2008;9:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Kuai C.-P., Ju L.-J., Hu P.-P., Huang F. Corydalis saxicola Alkaloids Attenuate Cisplatin-Induced Neuropathic Pain by Reducing Loss of IENF and Blocking TRPV1 Activation. Am. J. Chin. Med. 2020;48:407–428. doi: 10.1142/S0192415X20500214. [DOI] [PubMed] [Google Scholar]
- Kuan Y.-H., Shyu B.-C. Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Mol. Brain. 2016;9:58. doi: 10.1186/s13041-016-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre-Mestre M., Grolleau S., Montastruc J.-L. Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002–2006. Fundam. Clin. Pharmacol. 2013;27:223–230. doi: 10.1111/j.1472-8206.2011.00991.x. [DOI] [PubMed] [Google Scholar]
- Lázaro-Ibáñez G.G., Torres-López J.E., Granados-Soto V. Participation of the nitric oxide-cyclic GMP-ATP-sensitive K(+) channel pathway in the antinociceptive action of ketorolac. Eur. J. Pharmacol. 2001;426:39–44. doi: 10.1016/s0014-2999(01)01206-7. [DOI] [PubMed] [Google Scholar]
- Lee C.-H., Chen C.-C. Roles of ASICs in Nociception and Proprioception. Adv. Exp. Med. Biol. 2018;1099:37–47. doi: 10.1007/978-981-13-1756-9_4. [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Choi S.-H., Lee B.-H., Hwang S.-H., Kim H.-J., Rhee J., Chung C., Nah S.-Y. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α. Neurosci. Lett. 2013;548:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang J., Gao Y., Yang Y., Xu C., Li G., Guo G., Liu S., Xie J., Liang S. Puerarin alleviates burn-related procedural pain mediated by P2X(3) receptors. Purinergic Signal. 2011;7:489–497. doi: 10.1007/s11302-011-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhao C., Yao M., Song Y., Wu Y., Wen A. Analgesic effect of coumarins from Radix angelicae pubescentis is mediated by inflammatory factors and TRPV1 in a spared nerve injury model of neuropathic pain. J. Ethnopharmacol. 2017;195:81–88. doi: 10.1016/j.jep.2016.11.046. [DOI] [PubMed] [Google Scholar]
- Liao C., Li Y., Tjong S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionnet L., Beaudry F., Vachon P. Intrathecal eugenol administration alleviates neuropathic pain in male Sprague-Dawley rats. Phyther. Res. 2010;24:1645–1653. doi: 10.1002/ptr.3174. [DOI] [PubMed] [Google Scholar]
- Luiz A.P., Wood J.N. Sodium Channels in Pain and Cancer: New Therapeutic Opportunities. Adv. Pharmacol. 2016;75:153–178. doi: 10.1016/bs.apha.2015.12.006. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/S0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- Maia J.L., Lima-Júnior R.C.P., Melo C.M., David J.P., David J.M., Campos A.R., Santos F.A., Rao V.S.N. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: possible mechanisms. Pharmacol. Res. 2006;54:282–286. doi: 10.1016/j.phrs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Markman J.D., Dworkin R.H. Ion channel targets and treatment efficacy in neuropathic pain. J. Pain. 2006;7:38–47. doi: 10.1016/j.jpain.2005.09.008. [DOI] [PubMed] [Google Scholar]
- McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Meng J., Qiu S., Zhang L., You M., Xing H., Zhu J. Berberine Alleviate Cisplatin-Induced Peripheral Neuropathy by Modulating Inflammation Signal via TRPV1. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.774795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami S.S., Arakawa N.S., Ambrosio S.R., Zarpelon A.C., Casagrande R., Cunha T.M., Ferreira S.H., Cunha F.Q., Verri W.A.J. Kaurenoic acid from Sphagneticola trilobata Inhibits Inflammatory Pain: effect on cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. J. Nat. Prod. 2012;75:896–904. doi: 10.1021/np200989t. [DOI] [PubMed] [Google Scholar]
- Montera M., Goins A., Cmarko L., Weiss N., Westlund K.N., Alles S.R.A. Trigeminal neuropathic pain is alleviated by inhibition of Ca(v)3.3 T-type calcium channels in mice. Channels (Austin) 2021;15:31–37. doi: 10.1080/19336950.2020.1859248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C., Gupta R., Jordt S.-E., Chen Y., Liedtke W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamori S., Takahashi J., Hyuga S., Tanaka-Kagawa T., Jinno H., Hyuga M., Hakamatsuka T., Odaguchi H., Goda Y., Hanawa T., Kobayashi Y. Ephedra Herb extract activates/desensitizes transient receptor potential vanilloid 1 and reduces capsaicin-induced pain. J. Nat. Med. 2017;71:105–113. doi: 10.1007/s11418-016-1034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani, Y., Negoro, K., Yamauchi, M., Katasho, M., Ishikura, K. ichiro, Iwaki, A., Tsukada, K., Yamaguchi, M., Uehara, A., Yoshida, M., Ishiuchi, K., Makino, T., Kitajima, M., Ohsawa, M., Amano, T., 2020. Neoline, an active ingredient of the processed aconite root in Goshajinkigan formulation, targets Nav1.7 to ameliorate mechanical hyperalgesia in diabetic mice. J. Ethnopharmacol. 259, 112963. 10.1016/j.jep.2020.112963. [DOI] [PubMed]
- Narusuye K., Kawai F., Matsuzaki K., Miyachi E. Linalool suppresses voltage-gated currents in sensory neurons and cerebellar Purkinje cells. J. Neural Transm. 2005;112:193–203. doi: 10.1007/s00702-004-0187-y. [DOI] [PubMed] [Google Scholar]
- Nguelefack T.B., Dutra R.C., Paszcuk A.F., Andrade E.L., Tapondjou L.A., Calixto J.B. Antinociceptive activities of the methanol extract of the bulbs of Dioscorea bulbifera L. var sativa in mice is dependent of NO-cGMP-ATP-sensitive-K(+) channel activation. J. Ethnopharmacol. 2010;128:567–574. doi: 10.1016/j.jep.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Nguelefack T.B., Dutra R.C., Paszcuk A.F., de Andrade E.L., Calixto J.B. TRPV1 channel inhibition contributes to the antinociceptive effects of Croton macrostachyus extract in mice. BMC Complement. Altern. Med. 2015;15:1–9. doi: 10.1186/s12906-015-0816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Transient receptor potential (TRP) cation channels: rewarding unique proteins. Bull. Mem. Acad. R. Med. Belg. 2007;162:244–253. [PubMed] [Google Scholar]
- North R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nucci-Martins C., Martins D.F., Nascimento L.F., Venzke D., Oliveira A.S., Frederico M.J.S., Silva F.R.M.B., Brighente I.M.C., Pizzolatti M.G., Santos A.R.S. Ameliorative potential of standardized fruit extract of Pterodon pubescens Benth on neuropathic pain in mice: Evidence for the mechanisms of action. J. Ethnopharmacol. 2015;175:273–286. doi: 10.1016/j.jep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Ocaña M., Cendán C.M., Cobos E.J., Entrena J.M., Baeyens J.M. Potassium channels and pain: present realities and future opportunities. Eur. J. Pharmacol. 2004;500:203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Ortiz M.I., Granados-Soto V., Castañeda-Hernández G. The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol. Biochem. Behav. 2003;76:187–195. doi: 10.1016/s0091-3057(03)00214-4. [DOI] [PubMed] [Google Scholar]
- Ou S., Zhao Y.D., Xiao Z., Wen H.Z., Cui J., Ruan H.Z. Effect of lappaconitine on neuropathic pain mediated by P2X 3 receptor in rat dorsal root ganglion. Neurochem. Int. 2011;58:564–573. doi: 10.1016/j.neuint.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Papalampropoulou-Tsiridou M., Labrecque S., Godin A.G., De Koninck Y., Wang F. Differential Expression of Acid - Sensing Ion Channels in Mouse Primary Afferents in Naïve and Injured Conditions. Front. Cell. Neurosci. 2020;14:103. doi: 10.3389/fncel.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.-K., Li H.Y., Yeon K.-Y., Jung S.J., Choi S.-Y., Lee S.J., Lee S., Park K., Kim J.S., Oh S.B. Eugenol inhibits sodium currents in dental afferent neurons. J. Dent. Res. 2006;85:900–904. doi: 10.1177/154405910608501005. [DOI] [PubMed] [Google Scholar]
- Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M., Earley T.J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Possebon M.I., Mizokami S.S., Carvalho T.T., Zarpelon A.C., Hohmann M.S.N., Staurengo-Ferrari L., Ferraz C.R., Hayashida T.H., de Souza A.R., Ambrosio S.R., Arakawa N.S., Casagrande R., Verri W.A.J. Pimaradienoic acid inhibits inflammatory pain: inhibition of NF-κB activation and cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. J. Nat. Prod. 2014;77:2488–2496. doi: 10.1021/np500563b. [DOI] [PubMed] [Google Scholar]
- Qiu J., Zhang L., Hong J., Ni X., Li J., Li G., Zhang G. Magnolol inhibits sodium currents in freshly isolated mouse dorsal root ganglion neurons. Clin. Exp. Pharmacol. Physiol. 2021;48:347–354. doi: 10.1111/1440-1681.13422. [DOI] [PubMed] [Google Scholar]
- Rao S., Liu S., Zou L., Jia T., Zhao S., Wu B., Yi Z., Wang S., Xue Y., Gao Y., Xu C., Li G., Xu H., Zhang C., Liang S. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X(3) receptor in dorsal root ganglia. Purinergic Signal. 2017;13:227–235. doi: 10.1007/s11302-016-9554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Pan L., Zhao W., Xu L., Xu J., Lu H., Chen Y. Neochamaejasmin A inhibits K(V)1.4 channel activity via direct binding to the pore. Brain Res. 2018;1683:17–26. doi: 10.1016/j.brainres.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Ren W., Yuan L., Li J., Huang X.-J., Chen S., Zou D.-J., Liu X., Yang X.-Z. Ethanolic extract of Aconiti Brachypodi Radix attenuates nociceptive pain probably via inhibition of voltage-dependent Na+ channel. African. J. Tradit. Complement. Altern. Med. AJTCAM. 2012;9:574–583. doi: 10.4314/ajtcam.v9i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W., Chamessian A., Curatolo M., Davidson S., Burton M., Dib-Hajj S., Dougherty P.M., Ebert A.D., Gereau R.W., 4th, Ghetti A., Gold M.S., Hoben G., Menichella D.M., Mercier P., Ray W.Z., Salvemini D., Seal R.P., Waxman S., Woolf C.J., Stucky C.L., Price T.J. Human cells and networks of pain: Transforming pain target identification and therapeutic development. Neuron. 2021;109:1426–1429. doi: 10.1016/j.neuron.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J., Sheng Z.H., Kim D.K., Hodson C.D., Snutch T.P., Catterall W.A. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. PNAS. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera D., Obon C., Inocencio C., Heinrich M., Verde A., Fajardo J., Llorach R. The ethnobotanical study of local Mediterranean food plants as medicinal resources in Southern Spain. J. Physiol. Pharmacol. an Off. J. Polish Physiol. Soc. 2005;56 Suppl 1:97–114. [PubMed] [Google Scholar]
- Ross H.R., Napier I., Connor M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008;283:16124–16134. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.A.A., Fidalgo-Neto A.A., Faria R.X., Simões A., Calheiros A.S., Bérenger A.L., Faria-Neto H.C.C., Figueiredo M.R., Frutuoso V.S.L., Alves L.A. Effect of Rheedia longifolia Leaf Extract and Fractions on the P2X7 Receptor In Vitro: Novel Antagonists? J. Med. Food. 2011;14:920–929. doi: 10.1089/jmf.2010.0184. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F., Fujita T., Deguchi T., Yamaoka S., Tomochika K., Tsubota M., Ono S., Horaguchi Y., Ichii M., Ichikawa M., Ueno Y., Koike N., Tanino T., Nguyen H.D., Okada T., Nishikawa H., Yoshida S., Ohkubo T., Toyooka N., Murata K., Matsuda H., Kawabata A. Blockade of T-type calcium channels by 6-prenylnaringenin, a hop component, alleviates neuropathic and visceral pain in mice. Neuropharmacology. 2018;138:232–244. doi: 10.1016/j.neuropharm.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Seo H., Li H.Y., Perez-Reyes E., Lee J.-H. Effects of eugenol on T-type Ca2+ channel isoforms. J. Pharmacol. Exp. Ther. 2013;347:310–317. doi: 10.1124/jpet.113.207936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z., Cai S., Yu J., Zhang Z., Vallecillo T.G.M., Serafini M.J., Thomas A.M., Pham N.Y.N., Bellampalli S.S., Moutal A., Zhou Y., Xu G.-B., Xu Y.-M., Luo S., Patek M., Streicher J.M., Gunatilaka A.A.L., Khanna R. Reversal of Peripheral Neuropathic Pain by the Small-Molecule Natural Product Physalin F via Block of CaV2.3 (R-Type) and CaV2.2 (N-Type) Voltage-Gated Calcium Channels. ACS Chem. Nerosci. 2019;10:2939–2955. doi: 10.1021/acschemneuro.9b00166. [DOI] [PubMed] [Google Scholar]
- Shen C.-L., Wang R., Ji G., Elmassry M.M., Zabet-Moghaddam M., Vellers H., Hamood A.N., Gong X., Mirzaei P., Sang S. Dietary supplementation of gingerols-and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022;100 doi: 10.1016/j.jnutbio.2021.108904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z.-H., Rettig J., Cook T., Catterall W.A. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- Skerratt S.E., West C.W. Ion channel therapeutics for pain. Channels (Austin) 2015;9:344–351. doi: 10.1080/19336950.2015.1075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E.B., Stephens G.J. Recent advances in targeting ion channels to treat chronic pain. Br. J. Pharmacol. 2018;175:2133–2137. doi: 10.1111/bph.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui F., Zhang C.-B., Yang N., Li L.-F., Guo S.-Y., Huo H.-R., Jiang T.-L. Anti-nociceptive mechanism of baicalin involved in intervention of TRPV1 in DRG neurons in vitro. J. Ethnopharmacol. 2010;129:361–366. doi: 10.1016/j.jep.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Takeda M., Tsuboi Y., Kitagawa J., Nakagawa K., Iwata K., Matsumoto S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain. 2011;7:5. doi: 10.1186/1744-8069-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu L., Fan Y., Wang M., Li L., Zou L., Yuan H., Shi L., Yang R., Liang S., Liu S. Role of hesperidin in P2X3 receptor-mediated neuropathic pain in the dorsal root ganglia. Int. J. Neurosci. 2019;129:784–793. doi: 10.1080/00207454.2019.1567512. [DOI] [PubMed] [Google Scholar]
- Tasneem S., Liu B., Li B., Choudhary M.I., Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019;139:126–140. doi: 10.1016/j.phrs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Todorovic S.M., Jevtovic-Todorovic V. Neuropathic pain: role for presynaptic T-type channels in nociceptive signaling. Pflugers Arch. 2013;465:921–927. doi: 10.1007/s00424-012-1211-y. [DOI] [PubMed] [Google Scholar]
- Trang T., Al-Hasani R., Salvemini D., Salter M.W., Gutstein H., Cahill C.M. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J. Neurosci. 2015;35:13879–13888. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantoulas C., McMahon S.B. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci. 2014;37:146–158. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hecke O., Austin S.K., Khan R.A., Smith B.H., Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. PAIN®. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Venkatakrishna K., Sundeep K., Sudeep H.V., Gouthamchandra K., Shyamprasad K. ViphyllinTM, a Standardized Black Pepper Seed Extract Exerts Antinociceptive Effects in Murine Pain Models via Activation of Cannabinoid Receptor CB2, Peroxisome Proliferator-Activated Receptor-Alpha and TRPV1 Ion Channels. J. Pain Res. 2022;15:355. doi: 10.2147/JPR.S351513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R., Owsianik G., Nilius B. Vanilloid transient receptor potential cation channels: An overview. Curr. Pharm. Des. 2008;14 doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- Verma S., Kuhad A., Bhandari R., Prasad S.K., Shakya A., Prasad R.S., Sinha S.K. Effect of ethanolic extract of Solanum virginianum Linn. on neuropathic pain using chronic constriction injury rat model and molecular docking studies. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393:1715–1728. doi: 10.1007/s00210-020-01872-8. [DOI] [PubMed] [Google Scholar]
- Wang M., Cai X., Wang Y., Li S., Wang N., Sun R., Xing J., Liang S., Liu S. Astragalin Alleviates Neuropathic Pain by Suppressing P2X4-Mediated Signaling in the Dorsal Root Ganglia of Rats. Front. Neurosci. 2021 doi: 10.3389/fnins.2020.570831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-F., Gerner P., Wang S.-Y., Wang G.K. Bulleyaconitine A isolated from aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. Anesthesiology. 2007;107:82–90. doi: 10.1097/01.anes.0000267502.18605.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Gu J., Li Y.-Q., Tao Y.-X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang Q., Wang C., Xu X., Yu H. Tetramethylpyrazine attenuates periorbital allodynia and neuroinflammation in a model of traumatic brain injury. J. Inflamm. 2017;14:13. doi: 10.1186/s12950-017-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ling D., Wu C., Han J., Zhao Y. Baicalin prevents the up-regulation of TRPV1 in dorsal root ganglion and attenuates chronic neuropathic pain. Vet. Med. Sci. 2020;6:1034–1040. doi: 10.1002/vms3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Tang L., Zhang M., Wang C., Li S., Wen Y., Tu H., Tian H., Wei J., Liang P., Yang C., Li G., Gao Y. Gallic Acid Alleviates Visceral Pain and Depression via Inhibition of P2X7 Receptor. Int. J. Mol. Sci. 2022 doi: 10.3390/ijms23116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund K.N., Montera M.A., Goins A.E., Alles S.R.A., Suri N., McIlwrath S.L., Bartel R., Durvasula R.V., Kunamneni A. Single-Dose P2 X4R Single-Chain Fragment Variable Antibody Permanently Reverses Chronic Pain in Male Mice. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222413612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer A.D., Lehto S.G. Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals (Basel) 2017;10 doi: 10.3390/ph10020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden A.D., McNaughton-Smith G. Kv7 channels as targets for the treatment of pain. Curr. Pharm. Des. 2009;15:1773–1798. doi: 10.2174/138161209788186326. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Hashmi M. Use and abuse of opioid analgesics: potential methods to prevent and deter non-medical consumption of prescription opioids. Curr. Opin. Invest. Drugs. 2004;5:61–66. [PubMed] [Google Scholar]
- Wu, B., Ma, Y., Yi, Z., Liu, S., Rao, S., Zou, L., Wang, S., Xue, Y., Jia, T., Zhao, S., Shi, L., Li, L., Yuan, H., Liang, S., 2017. Resveratrol-decreased hyperalgesia mediated by the P2X(7) receptor in gp120-treated rats. Mol. Pain 13, 1744806917707667. 10.1177/1744806917707667. [DOI] [PMC free article] [PubMed]
- Xie, M.-X., Zhu, H.-Q., Pang, R.-P., Wen, B.-T., Liu, X.-G., 2018a. Mechanisms for therapeutic effect of bulleyaconitine A on chronic pain. Mol. Pain 14, 1744806918797243. 10.1177/1744806918797243. [DOI] [PMC free article] [PubMed]
- Xie J., Liu S., Wu B., Li G., Rao S., Zou L., Yi Z., Zhang C., Jia T., Zhao S., Schmalzing G., Hausmann R., Nie H., Li G., Liang S. The protective effect of resveratrol in the transmission of neuropathic pain mediated by the P2X(7) receptor in the dorsal root ganglia. Neurochem. Int. 2017;103:24–35. doi: 10.1016/j.neuint.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Xie M.X., Yang J., Pang R.P., Zeng W.A., Ouyang H.D., Liu Y.Q., Liu X.G. Bulleyaconitine A attenuates hyperexcitability of dorsal root ganglion neurons induced by spared nerve injury: The role of preferably blocking Nav1.7 and Nav1.3 channels. Mol. Pain. 2018;14 doi: 10.1177/1744806918778491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sun J., Li W., Zhang S., Yang L., Teng Y., Lv K., Liu Y., Su Y., Zhang J., Zhao M. Analgesic effect of the main components of Corydalis yanhusuo (yanhusuo in Chinese) is caused by inhibition of voltage gated sodium channels. J. Ethnopharmacol. 2021;280 doi: 10.1016/j.jep.2021.114457. [DOI] [PubMed] [Google Scholar]
- Xu C., Xu W., Xu H., Xiong W., Gao Y., Li G., Liu S., Xie J., Tu G., Peng H., Qiu S., Liang S. Role of puerarin in the signalling of neuropathic pain mediated by P2X3 receptor of dorsal root ganglion neurons. Brain Res. Bull. 2012;87:37–43. doi: 10.1016/j.brainresbull.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yu Y., Wang Q., Li W., Zhang S., Liao X., Liu Y., Su Y., Zhao M., Zhang J. Active components of Bupleurum chinense and Angelica biserrata showed analgesic effects in formalin induced pain by acting on Nav1. 7. J. Ethnopharmacol. 2021;269 doi: 10.1016/j.jep.2020.113736. [DOI] [PubMed] [Google Scholar]
- Yamazumi I., Okuda T., Koga Y. Involvement of potassium channels in spinal antinociceptions induced by fentanyl, clonidine and bethanechol in rats. Jpn. J. Pharmacol. 2001;87:268–276. doi: 10.1254/jjp.87.268. [DOI] [PubMed] [Google Scholar]
- Yang P.-P., Chueh S.-H., Shie H.-L., Chen C.-C., Lee L.-Y., Chen W.-P., Chen Y.-W., Shiu L., Liu P.-S. Effects of Hericium erinaceus Mycelium Extracts on the Functional Activity of Purinoceptors and Neuropathic Pain in Mice with L5 Spinal Nerve Ligation. Evidence-Based Complement. Altern. Med. 2020;2020:2890194. doi: 10.1155/2020/2890194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Dai Y., Ji Z., Zhang X., Fu W., Han C., Xu Y. Allium macrostemon Bunge. exerts analgesic activity by inhibiting NaV1. 7 channel. J. Ethnopharmacol. 2021;281 doi: 10.1016/j.jep.2021.114495. [DOI] [PubMed] [Google Scholar]
- Yang R., Li Z., Zou Y., Yang J., Li L., Xu X., Schmalzing G., Nie H., Li G., Liu S., Liang S., Xu C. Gallic Acid Alleviates Neuropathic Pain Behaviors in Rats by Inhibiting P2X7 Receptor-Mediated NF-κB/STAT3 Signaling Pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.680139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Park K.S., Yoon J.J., Bae H.-B., Yoon M.H., Choi J.I. Anti-allodynic effect of intrathecal processed Aconitum jaluense is associated with the inhibition of microglial activation and P2X7 receptor expression in spinal cord. BMC Complement. Altern. Med. 2016;16:214. doi: 10.1186/s12906-016-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yang Q., Zhao J., Sun S., Liu M., Wang Y., Feng Y., Zhang J. Evaluation of Rhodojaponin III from Rhododendron molle G. Don on oral antinociceptive activity, mechanism of action, and subacute toxicity in rodents. J. Ethnopharmacol. 2022;294 doi: 10.1016/j.jep.2022.115347. [DOI] [PubMed] [Google Scholar]
- Yang S., Yu Z., Sun W., Jiang C., Ba X., Zhou Q., Xiong D., Xiao L., Deng Q., Hao Y. The antiviral alkaloid berberine ameliorates neuropathic pain in rats with peripheral nerve injury. Acta Neurol. Belg. 2020;120:557–564. doi: 10.1007/s13760-018-1006-9. [DOI] [PubMed] [Google Scholar]
- Ying M., Liu H., Zhang T., Jiang C., Gong Y., Wu B., Zou L., Yi Z., Rao S., Li G., Zhang C., Jia T., Zhao S., Yuan H., Shi L., Li L., Liang S., Liu S. Effect of artemisinin on neuropathic pain mediated by P2X(4) receptor in dorsal root ganglia. Neurochem. Int. 2017;108:27–33. doi: 10.1016/j.neuint.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Yu G., Qian L., Yu J., Tang M., Wang C., Zhou Y., Geng X., Zhu C., Yang Y., Pan Y., Shen X., Tang Z. Brucine alleviates neuropathic pain in mice via reducing the current of the sodium channel. J. Ethnopharmacol. 2019;233:56–63. doi: 10.1016/j.jep.2018.12.045. [DOI] [PubMed] [Google Scholar]
- Yu M., Su B., Zhang X. Gardenoside suppresses the pain in rats model of chronic constriction injury by regulating the P2X3 and P2X7 receptors. J. Recept. Signal Transduct. Res. 2018;38:198–203. doi: 10.1080/10799893.2018.1468782. [DOI] [PubMed] [Google Scholar]
- Yu M., Zhao Y., Zhang X. Gardenoside combined with ozone inhibits the expression of P2X3 and P2X7 purine receptors in rats with sciatic nerve injury. Mol. Med. Rep. 2018;17:7980–7986. doi: 10.3892/mmr.2018.8803. [DOI] [PubMed] [Google Scholar]
- Yuan H., Ouyang S., Yang R., Li S., Gong Y., Zou L., Jia T., Zhao S., Wu B., Yi Z., Liu H., Shi L., Li L., Gao Y., Li G., Xu H., Liu S., Zhang C., Liang S. Osthole alleviated diabetic neuropathic pain mediated by the P2X(4) receptor in dorsal root ganglia. Brain Res. Bull. 2018;142:289–296. doi: 10.1016/j.brainresbull.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Zamponi G.W., Lewis R.J., Todorovic S.M., Arneric S.P., Snutch T.P. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res. Rev. 2009;60:84–89. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Mohammadi S., Komaki A. Antinociceptive activity of Inula britannica L. and patuletin: In vivo and possible mechanisms studies. J. Ethnopharmacol. 2018;219:351–358. doi: 10.1016/j.jep.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Zarei M., Ahmadimoghaddam D., Mohammadi S. Artemisia biennis Willd.: Anti-Nociceptive effects and possible mechanisms of action. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113604. [DOI] [PubMed] [Google Scholar]
- Zeb M., Lee C.H. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules. 2021;26 doi: 10.3390/molecules26020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.-L., Cao, X.-Y., Lai, R.-C., Xie, M.-X., Zeng, W.-A., 2018. Puerarin Relieves Paclitaxel-Induced Neuropathic Pain: The Role of Na(v)1.8 β1 Subunit of Sensory Neurons. Front. Pharmacol. 9, 1510. 10.3389/fphar.2018.01510. [DOI] [PMC free article] [PubMed]
- Zhang A., Gao Y., Zhong X., Xu C., Li G., Liu S., Lin J., Li X., Zhang Y., Liu H., Linag S. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. 2010;1313:215–221. doi: 10.1016/j.brainres.2009.11.067. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li X., Gao Y., Guo G., Xu C., Li G., Liu S., Huang A., Tu G., Peng H., Qiu S., Fan B., Zhu Q., Yu S., Zheng C., Liang S. Effects of puerarin on the inflammatory role of burn-related procedural pain mediated by P2X(7) receptors. Burns. 2013;39:610–618. doi: 10.1016/j.burns.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-L., Liu Y.-G., Li Q., Wang X.-D., Zheng X.-B., Yang B.-L., Wan B., Ma J.-M., Liu Z.-X. 1,8-cineole decreases neuropathic pain probably via a mechanism mediating P2X3 receptor in the dorsal root ganglion. Neurochem. Int. 2018;121:69–74. doi: 10.1016/j.neuint.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-J., Lu X.-W., Song N., Kou L., Wu M.-K., Liu F., Wang H., Shen J.-F. Chlorogenic acid alters the voltage-gated potassium channel currents of trigeminal ganglion neurons. Int. J. Oral Sci. 2014;6:233–240. doi: 10.1038/ijos.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Xu C., Liang S., Gao Y., Li G., Wei J., Wan F., Liu S., Lin J. Role of sodium ferulate in the nociceptive sensory facilitation of neuropathic pain injury mediated by P2X(3) receptor. Neurochem. Int. 2008;53:278–282. doi: 10.1016/j.neuint.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yao J., Rong M. Editorial: Role of Ion Channels in Pain. Front. Pharmacol. 2022 doi: 10.3389/fphar.2022.884665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Yang J., Han X., Gong Y., Rao S., Wu B., Yi Z., Zou L., Jia T., Li L., Yuan H., Shi L., Zhang C., Gao Y., Li G., Liu S., Xu H., Liu H., Liang S. Effects of nanoparticle-encapsulated curcumin on HIV-gp120-associated neuropathic pain induced by the P2X(3) receptor in dorsal root ganglia. Brain Res. Bull. 2017;135:53–61. doi: 10.1016/j.brainresbull.2017.09.011. [DOI] [PubMed] [Google Scholar]



