ABSTRACT.
Mosquito-borne diseases are a global burden; however, current methods of evaluating human–mosquito contact rates are expensive and time consuming. Validated surveys of self-reported mosquito bites may be an inexpensive way to determine mosquito presence and bite exposure level in an area, but this remains untested. In this study, a survey of self-reported mosquito bites was validated against household mosquito abundance from six communities in Esmeraldas, Ecuador. From February 2021 to July 2022, households were interviewed monthly, and five questions were used to ask participants how often they were bitten by mosquitoes at different times during the day. At the same time, adult mosquitoes were collected using a Prokopack aspirator. Species were identified and counted. Survey responses were compared with the total number of mosquitoes found in the home using negative binomial regression. More frequent self-reported mosquito bites were significantly associated with higher numbers of collected adult mosquitoes. These associations were driven by the prevalence of the dominant genera, Culex. These results suggest that surveys of perceived mosquito bites relate to actual mosquito presence, making them a potentially useful tool for determining the impact of vector–control interventions on community perceptions of risk but less useful for assessing the risk of nondominant species such as Aedes aegypti. Further work is needed to examine the robustness of these results in other contexts.
INTRODUCTION
Mosquito-borne diseases comprise a significant global burden that results in an estimated 700,000 deaths each year.1 Mosquito species from genera including Anopheles, Culex, and Aedes transmit diseases of significant public health impact including malaria, filariasis, and dengue, respectively, in addition to transmission of other assorted arboviruses. Vector surveillance is therefore a key component of mosquito-borne disease control strategy, and provides important data to help understand how mosquito abundance and presumably human–mosquito contact rates vary across changing climates and social structure, such as increases in urbanization.2,3
Current strategies to monitor vector populations and the intensity of mosquito–human interaction (i.e., human–mosquito contact rates4), however, present several challenges.5 The gold standard for assessing the human biting rate (number of mosquito bites per human per unit time) is human landing collection (HLC).6 Although it is the most accurate method currently available to evaluate mosquito bite exposure, it is time consuming, expensive, and potentially risky to those participating in the collection.6 As a result of these constraints, researchers have focused on establishing the efficacy of alternative methods of determining biting rate based on relative abundances measured by a variety of mosquito trapping methods.6 Unlike HLC, these alternative methods reflect the abundance of mosquitoes instead of direct host-seeking mosquitoes; however, they are often implemented because of simpler logistics and lower cost while still providing useful surrogates for the human biting rate.6 A method that has been widely used in other areas of public health research but has not been fully explored in the study of mosquito-borne diseases is that of self-report and recall surveys. Although self-report can be limited by recall bias, response bias, or social desirability bias, it is nevertheless useful in characterizing a wide range of environmental exposures and health outcomes.7–11 In theory, surveying individuals on how often they think they have been bitten by mosquitoes could be a feasible and low-cost method to estimate human biting rates, especially if combined with other information.12 Relatively few studies, however, have explored the potential of self-reported human biting rate of mosquitoes (or other arthropods).13 One such study was carried out in New Orleans using a self-reported human biting rate survey to parameterize a vector-borne transmission model; the survey, however, was not validated against human landing or adult mosquito density measures.12 Interestingly, the study found a positive correlation between participants who went outdoors during high-risk times and the reported number of bites. It also found that different locations were associated with different levels of reported bites, suggestive of differences in mosquito densities by geography.13 Self-report has also been used to capture information about mosquito nuisance,14–17 typically by asking how mosquitoes affected the respondents’ daily activities. When comparing nuisance survey responses against mosquito eggs collected from ovitraps, results have been mixed.15,17 However, associations were more consistent when surveys were compared with entomological surveillance methods that are more reflective of adult mosquito populations, such as CO2 traps. The results from these studies add to the argument that self-reported mosquito interactions are reflective of actual mosquito presence.
The aim of this study is to validate a five-question survey of self-reported mosquito bites by comparison to adult mosquito collections from participants’ homes. This research was nested within an ongoing longitudinal study of dengue virus transmission in rural coastal Ecuador.
MATERIALS AND METHODS
Data were collected between February 2021 and July 2022 from six communities in the Esmeraldas province of Ecuador.18–20 These communities were selected to provide variation in population size and relative accessibility, with one larger town (Borbón), three smaller, road-accessible communities (Maldonado, Colon Eloy, and Timbiré), and two rural communities accessible only by river (Santo Domingo and Santa Maria). Around 30% of households in each town were randomly selected for inclusion in the study based on a previously conducted community census (Supplemental Table 1).
Each selected household was visited once per month by trained community entomologists. Visits typically took place around noon (median, 12:17 pm; interquartile range [IQR], 10:08 am to 3:10 pm). During these visits, adult mosquitoes were collected using Prokopack 1419 aspirators (John W. Hock, Gainesville, FL) for a duration of 15 minutes. Adults were aspirated from areas where occupants spent most of their time, such as bathrooms, bedrooms, the living room, and the dining room. Within each area, aspirations occurred where Aedes aegypti were most likely to rest.21 Collected mosquitoes were chilled for 2 hours before being identified as Ae. aegypti, Culex spp., or other species. Species Ae. aegypti and Culex spp. were further identified as male or female, and blood-fed female Ae. aegypti, versus unfed females, were further enumerated. Other species were not enumerated by sex.
During each entomological data collection, we used a household survey to assess knowledge of dengue transmission, and practices related to mosquito control accompanied the entomological activities. The survey was programmed in Open Data Kit22 and administered using Android tablets. The survey was administered to any adult member of the household who consented to participate in the study and was available at the time of the mosquito collection. The survey took a median of 7.0 minutes to complete (IQR, 4.4–12.4 minutes).
The self-report mosquito bite survey was included as a pilot module of this survey and was based on questionnaires from Thongsripong et al.13 The self-report survey included five questions, three of which asked the participants to recall the frequency of bites they received in their home on the previous day at three different time periods: during the day, at night, and at dawn and dusk. Each of these three questions had four possible answers to how often they were bitten: none, rarely, sometimes, and often. Participants were asked two additional questions on the overall numbers of bites in their house during the past day for themselves and their child (see Supplemental Materials for survey questions). To assess interrater reliability, we asked a second adult member of the household the same biting rate questions in a small subset (2.5%) of interviews.
Ethics statement.
The study protocol was reviewed and approved as part of an arboviral surveillance study. The study was approved by the Universidad San Francisco de Quito (2017-159M) and the University of Michigan (HUM00140967) ethics review boards. It was also reviewed and approved by the Ecuadorian Ministry of Health (Ministerio de Salud Publica) at the local and national levels (MSPCURI000237-2). Written informed consent was obtained from all adult participants.
Statistical analysis.
We descriptively summarized the total number of Culex spp., Ae. aegypti, and “other” species resting mosquito counts using histograms and by calculating median values and IQRs. We also tabulated the responses to each survey question. The correlation between resting Culex spp. and Ae. aegypti counts was examined via Spearman correlation coefficients. Interrater reliability was examined using weighted kappa statistics to compare biting rate responses in the subset of interviews where two adult household members were surveyed separately.
To validate self-reported human biting rate questions, we examined correlation between survey questions using Spearman correlation coefficients. We also compared responses to resting adult mosquito counts. We considered our primary outcome of interest to be total counts of adult mosquitoes (of all species). As secondary outcomes, we considered 1) the counts of Culex spp., the species with the largest presence in the area, and 2) the counts of Ae. aegypti, which are unlikely to contribute substantially to the bites received by participants but are critical to dengue virus transmission. We also considered 3) the counts of female Culex spp. and Ae. aegypti and 4) the counts of male Culex spp. and Ae. aegypti. To further test whether resting mosquito counts were associated with self-reported biting rates, we used negative binomial regression models with a random effect to account for repeated observations per household. A negative binomial model was selected because variances within each level of perceived biting rate were higher than the means within each level, suggesting overdispersion, and confirmed by graphing observed and average estimated probabilities for each count. The outcome of these models was the total mosquito count, and the exposure was the perceived biting rate. Each question was included in a separate model. In adjusted models, we adjusted for community of residence as a categorical (fixed) covariate and seasonality by including the terms sine(2*m*pi/t) and cosine(2*m*pi/t), where m is the calendar month of collection and t is 12.23
RESULTS
Between February 2021 and July 2022, 10,295 household visits were completed, which resulted in 9,326 paired household adult mosquito collections and surveys collected from 681 unique households. This included 5,001 mosquito collections (426 unique households) from the larger community of Borbón, and 538–1,210 mosquito collections (39–142 unique households) from five increasingly rural communities with and without road access. The number of completed surveys and mosquito collections varied slightly from month to month because of participant availability at the time surveys were being collected (Supplemental Table 1).
When asked about the frequency of perceived bites during the day, the most common response was “rarely” in Borbón (40.4%). The most frequent response in all other communities was split as “sometimes” (53.6%).
All five self-reported biting rate questions had moderate, but not high, positive correlations, indicating that no two questions were redundant (Table 1). A total of 258 households provided data from two separate adults instead of just one. For these households, the weighted kappa ranged from 0.56 (bites per day) to 0.75 (bites to the child).
Table 1.
Spearman correlation coefficients between the five survey questions
| Survey question | Bites during day | Bites at night | Bites at dawn/dusk | Bites, last 24 hours | Children’s bites, last 24 hours |
|---|---|---|---|---|---|
| Bites during day | – | – | – | – | – |
| Bites at night | 0.46 | – | – | – | – |
| Bites at dawn/dusk | 0.49 | 0.65 | – | – | – |
| Bites, last 24 hours | 0.59 | 0.63 | 0.68 | – | – |
| Children’s bites, last 24 hours | 0.43 | 0.49 | 0.52 | 0.62 | – |
All P values are < 0.0001.
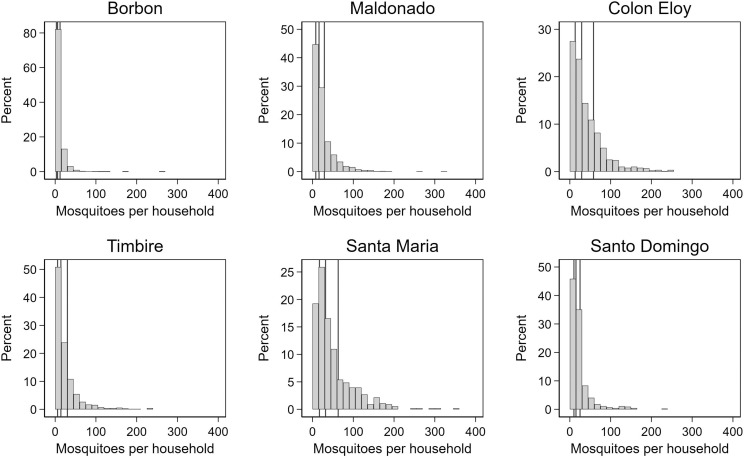
Households in the most urbanized community, Borbón, had a median of 3 resting mosquitoes per household (IQR, 9) of all species collected. All other communities had a median of 20 resting mosquitoes per household (IQR, 32) (Figure 1). Borbón had a higher proportion of households that had 0 collected resting mosquitoes per household compared with the more rural communities (6.7 versus 1.1%); 95.8% of captured mosquitoes were Culex spp., 4.1% were Ae. aegypti, and 0.1% were other species. The proportion of captured mosquitoes that were Ae. aegypti varied by community (11.1% in Borbón versus 2.8% in Maldonado, 1.1% in Colon Eloy, 1.0% in Timbiré, 4.0% in Santo Domingo, and 1.0% Santa Maria). The median number of resting female Culex spp. or Ae. aegypti mosquitoes per household was 5 (IQR, 8), and the median number of resting male Culex spp. or Ae. aegypti mosquitoes per household was 5 (IQR, 11). The mean percentage of mosquitoes that were female was 49.6% in Borbón, and 49.6, 43.3, 41.5, 41.3, and 41.0% in Maldonado, Colon Eloy, Timbiré, Santo Domingo, and Santa Maria, respectively.
Figure 1.
Histograms of resting mosquitoes collected from households in each community. The 25th, 50th, and 75th percentiles for each community are indicated by black vertical lines.
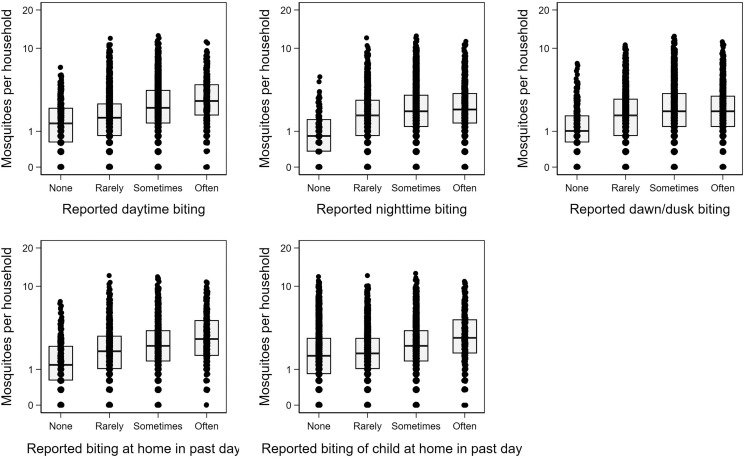
Aggregating the data across communities and time, we found a positive relationship between self-reported biting rates and the number of collected mosquitoes in the unadjusted model for all survey questions (Figure 2).
Figure 2.
Boxplots of total resting mosquito counts by self-report question.
Adjusting the model for season did not have an appreciable effect on the estimates (< 10% change across all estimates). Adjusting the model for community, however, had a clear effect on the estimates. Although associations between self-reported biting rate and resting mosquito counts remained statistically significant, the effect sizes were slightly attenuated.
Our results were suggestive of a dose–response relationship: for example, respondents who chose “rarely” when asked how often they were bitten by mosquitoes during the day had 0.96 times as many mosquitoes in their home compared with those who responded “none” (95% CI = 0.89, 1.03). Those who responded “sometimes” had 1.17 (95% CI = 1.09, 1.26) times as many mosquitoes, and those who responded “often” had 1.26 (95% CI = 1.15, 1.38) times as many (Table 2). However, because the CIs for each response option were overlapping, a true dose–response relationship cannot be confirmed. Nevertheless, this trend was present across self-report questions. For example, among those who reported being bitten at night rarely, sometimes, and often, households had resting mosquito counts that were 1.27, 1.45, and 1.50 times higher than those who reported no bites at night, respectively. Similarly, individuals who reported being bitten rarely, sometimes, or often at dawn and dusk had 1.11, 1.29, and 1.38 times more resting mosquitoes per household than those who reported no bites; those who reported being bitten rarely, sometimes, or often in the past overall 24 hours had 1.32, 1.47, and 1.63 times more resting mosquitoes than those who reported no bites; and those who reported that their children had been bitten rarely, sometimes, or often in the past 24 hours had 1.01, 117, and 1.27 times more resting mosquitoes than those who reported their children had not been bitten (Supplemental Table 2). To further investigate the effect of community on this relationship, we also stratified the analysis by community (Supplemental Table 3). In stratified analyses, only Borbón maintains a statistically significant dose–response similar to that seen in the aggregated model. This stratified result may be due to sample size because Borbón provided most surveys for the analysis. Secondary analyses stratified by species (Ae. aegypti only and Culex spp. only) suggested that these results were driven by Culex, which is the dominant genera in the region (Supplemental Table 4), and were similar for both males and females (Supplemental Table 5).
Table 2.
Unadjusted and adjusted IRR, estimated from negative binomial regression of the relationship between total mosquitoes resting indoors and self-reported bite frequency categories during the day
| Reported bite frequency | Unadjusted | Community adjusted | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| None | <ref> | <ref> | <ref> | <ref> |
| Rarely | 0.99 | 0.92, 1.07 | 0.96 | 0.89, 1.03 |
| Sometimes | 1.20 | 1.11, 1.29 | 1.17 | 1.09, 1.26 |
| Often | 1.32 | 1.20, 1.44 | 1.26 | 1.15, 1.38 |
IRR = incidence rate ratio; <ref> = reference category.
DISCUSSION
The continued global burden of mosquito-borne diseases stresses the need for low-cost, scalable approaches to quantify population risks. Self-report approaches have been previously used but not thoroughly investigated. We found that self-reported mosquito biting data have a positive relationship to the number of resting mosquitoes found in respondents’ households. This suggests that self-report surveys could be used to provide crude measures of mosquito abundance, potentially as a complement to more time-consuming and costly surveillance methods. However, there are clear limitations of self-report surveys that must be considered as well. For example, people cannot discern the biting species, and therefore self-reported biting estimates are only predictors of the most common genera present, in this case Culex. We also noted that combined male and female densities predicted self-reported biting frequencies as well as female densities alone. Self-reported biting frequencies based on the day before the survey are also an imperfect substitute for indoor resting mosquitoes. These limitations all suggest that self-reported biting rates may reflect annoyance with mosquitoes, or awareness of the presence of mosquitoes in one’s environment, rather than bites per se.
Another caveat of these data is that the community with the largest number of surveys, Borbón, also had the clearest association between self-reported bites and resting mosquito counts. Borbón also had the lowest resting mosquito counts on average. This could be a result of the difference in sample size, but it might also be an indicator of a fundamental difference between the communities. As the most urban of the communities, houses in Borbón are better equipped to prevent outdoor mosquitoes from entering, resulting in lower numbers of indoor adult mosquitoes captured. Therefore, bite habituation may have been in operation in the more rural/remote communities, namely a mosquito biting event may be more noticeable for those in Borbón than in communities that have more frequent, and thus less memorable, interactions with mosquitoes.24
Given these limitations, self-reported biting data are very unlikely to be a generalized predictor of dengue risk; however, they may still be useful in understanding changes in the overall mosquito burden over time (i.e., increased or decreased), as well as the community’s perception of the burden of mosquitoes in their home. For example, self-reported data could be used to understand whether the perceived and actual mosquito presence increased, decreased, or remained the same after intervention. In addition, surveys could be combined with a limited number of mosquito captures to provide a robust measure of intervention effectiveness, or to characterize how human knowledge, attitude, and behaviors influence levels of mosquito bite exposure.25
There are several opportunities to improve upon human biting rate surveys in the future. The biting rate questions in our survey were moderately but not highly (> 0.80) correlated with one another, which suggests that they provided complementary, rather than redundant, information. In the future, combining several questions to develop a “biting score,” similar to what is used to assess pain, headache, or other subjective symptoms,26–28 may increase the accuracy of the survey. Direct questions about perceived biting frequency may be complemented by questions related to mosquito nuisance, as others have shown that questions about the extent to which mosquitoes disrupt meals or chores14–17 may also correlate with vector abundance. Interestingly, we found that the survey question focused on bites received by their child was strongly associated with adult mosquito densities, likely a result of parents’ awareness of their children’s ailments, and was also reliably reported by different members of the household.
Self-report surveys are a promising method for mosquito-borne disease research. Although on the surface the use of a self-report survey to measure mosquito presence in an area may seem unreliable, we found a clear dose-dependent effect captured by the responses. Because of the low cost of implementation, self-reported data could work well as an initial assessment of relative mosquito density before implementing more rigorous methods. When used in combination with other methods and in the correct circumstances, self-report mosquito biting surveys can be a viable way to assess mosquito presence. Further research into self-report assessments should be conducted to ensure the validity of self-reported data in other contexts.
Supplemental Materials
Note: Supplemental tables and materials appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2022. Vector-Borne Diseases. Available at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed May 26, 2022.
- 2. Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, Caminade C, Lowe R, 2021. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health 5: e404–e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolimenakis A, Heinz S, Wilson ML, Winkler V, Yakob L, Michaelakis A, Papachristos D, Richardson C, Horstick O, 2021. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit—a systematic review. PLoS Negl Trop Dis 15: e0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thongsripong P, Hyman JM, Kapan DD, Bennett SN, 2021. Human–mosquito contact: a missing link in our understanding of mosquito-borne disease transmission dynamics. Ann Entomol Soc Am 114: 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monroe A, Moore S, Okumu F, Kiware S, Lobo NF, Koenker H, Sherrard-Smith E, Gimnig J, Killeen GF, 2020. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J 19: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong J, Bayoh N, Olang G, Killeen GF, Hamel MJ, Vulule JM, Gimnig JE, 2013. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J 12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Althubaiti A, 2016. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc 9: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aerts C, Revilla M, Duval L, Paaijmans K, Chandrabose J, Cox H, Sicuri E, 2020. Understanding the role of disease knowledge and risk perception in shaping preventive behavior for selected vector-borne diseases in Guyana. PLoS Negl Trop Dis 14: e0008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cordioli M. et al. , 2014. A comparison between self-reported and GIS-based proxies of residential exposure to environmental pollution in a case-control study on lung cancer. Spat Spatio-Temporal Epidemiol 9: 37–45. [DOI] [PubMed] [Google Scholar]
- 10. Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ, 2013. The Motivation and Pleasure Scale–Self-Report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Compr Psychiatry 54: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harder H, Shilling VM, May SF, Cella D, Schmid P, Fallowfield LJ, 2020. The development and initial evaluation of the Diarrhoea Management Diary (DMD) in patients with metastatic breast cancer. Breast Cancer Res Treat 183: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones AV. et al. , 2017. GWAS of self-reported mosquito bite size, itch intensity and attractiveness to mosquitoes implicates immune-related predisposition loci. Hum Mol Genet 26: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thongsripong P, Qu Z, Yukich JO, Hyman JM, Wesson DM, 2020. An investigation of human-mosquito contact using surveys and its application in assessing dengue viral transmission risk. J Med Entomol 57: 1942–1954. [DOI] [PubMed] [Google Scholar]
- 14. Brown JA, Larson KL, Lerman SB, Cocroft A, Hall SJ, 2021. Resident perceptions of mosquito problems are more influenced by landscape factors than mosquito abundance. Sustainability 13: 11533. [Google Scholar]
- 15. Gaillard B, Simard F, Dormont L, Jay-Robert P, D’Abadie de Lurbe D, Etienne M, Baudin A, Raude J, 2019. Is perceived exposure to mosquitoes associated with actual exposure? Results from studies in high-risk and low-risk geographic areas. Am J Trop Med Hyg 101: 976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulla MS, Thavara U, Tawatsin A, Kong-Ngamsuk W, Chompoosri J, 2001. Mosquito burden and impact on the poor: measures and costs for personal protection in some communities in Thailand. J Am Mosq Control Assoc 17: 153–159. [PubMed] [Google Scholar]
- 17. Carrieri M, Bellini R, MacCaferri S, Gallo L, Maini S, Celli G, 2008. Tolerance thresholds for Aedes albopictus and Aedes caspius in Italian urban areas. J Am Mosq Control Assoc 24: 377–386. [DOI] [PubMed] [Google Scholar]
- 18. Lee GO. et al. , 2021. A dengue outbreak in a rural community in northern coastal Ecuador: an analysis using unmanned aerial vehicle mapping. PLoS Negl Trop Dis 15: e0009679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Echegaray F. et al. , 2021. Adapting rapid diagnostic tests to detect historical dengue virus infections. Front Immunol 12: 703887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Márquez S, Lee GO, Andrade P, Zuniga J, Trueba G, Eisenberg JN, Coloma J, 2022. A chikungunya outbreak in a dengue-endemic region in rural northern coastal Ecuador. Am J Trop Med Hyg 107: 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talbot B. et al. , 2021. Determinants of Aedes mosquito density as an indicator of arbovirus transmission risk in three sites affected by co-circulation of globally spreading arboviruses in Colombia, Ecuador and Argentina. Parasit Vectors 14: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G, 2010. Open Data Kit: tools to build information services for developing regions. Proceedings of the 4th ACM/IEEE International Conference on Information and Communication Technologies and Development. December 13–16, 2010, London, United Kingdom, 1–12.
- 23. Stolwijk AM, Straatman H, Zielhuis GA, 1999. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health 53: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng Z, Simons FER, 1998. A prospective study of naturally acquired sensitization and subsequent desensitization to mosquito bites and concurrent antibody responses. J Allergy Clin Immunol 101: 284–286. [DOI] [PubMed] [Google Scholar]
- 25. Paz-Soldán VA. et al. , 2015. Dengue knowledge and preventive practices in Iquitos, Peru. Am J Trop Med Hyg 93: 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly AM, 2001. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 18: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart WF, Lipton RB, Kolodner K, 2003. Migraine disability assessment (MIDAS) score: relation to headache frequency, pain intensity, and headache symptoms. Headache 43: 258–265. [DOI] [PubMed] [Google Scholar]
- 28. Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord I, 2003. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 58: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.