ABSTRACT.
Bacterial resistance in community-acquired urinary tract infections (UTIs) is increasing worldwide. Our study aimed to assess the microbiological epidemiology and antimicrobial susceptibility patterns of community-acquired urine bacterial isolates in French Amazonia. Our study is retrospective. It was conducted from January 2015 to December 2019 in the microbiology laboratory of the Cayenne General Hospital (French Guiana). It includes all positive urine samples from adult (> 18 years) outpatients (N = 2,533). Isolated microorganisms were Gram-negative rods in 83.9%, mainly Enterobacterales (98.4%). The main isolated bacteria were Escherichia coli (58.7%) and Klebsiella pneumoniae (13.3%). Among the isolated E. coli, 37.2% were susceptible to amoxicillin, 77.9% to amoxicillin/clavulanic acid, 94.9% to cefotaxime, 78.9% to ofloxacin, and 98.9% to nitrofurantoin. In 106 cases (5.1%), isolated Enterobacterales were extended-spectrum β-lactamase producers (5% of E. coli and 8.9% of K. pneumoniae). Overall, high levels of cross- and co-resistance were registered. The main isolated Gram-positive bacteria was Staphylococcus saprophyticus (28.9%). It was resistant to oxacillin in 52.5% of cases and susceptible to nitrofurantoin in 99.1% of cases. Patients with S. saprophyticus were young women in almost all cases. In conclusion, the most isolated microorganisms from outpatient urinalyses were E. coli and K. pneumoniae. They showed a high resistance rate to amoxicillin, but they were susceptible to the most remaining antibiotics. S. saprophyticus was isolated mainly in young women and was resistant to oxacillin in half of the cases. Interestingly, nitrofurantoin was active against most isolated organisms and can be considered as empirical treatment in uncomplicated UTIs.
INTRODUCTION
Antibiotic resistance is increasing worldwide, resulting in a serious threat problem in the community.1 In Latin America, resistance in community-acquired urinary tract infections (UTIs) is also increasing.2 In French Guiana, Baizet et al.,3 reported in a retrospective study of adult patients diagnosed with community-acquired UTI that Escherichia coli was predominant (74.1%) and had decreased susceptibility to ampicillin, amoxicillin/clavulanic acid, fluoroquinolones, cotrimoxazole, and furans compared with the susceptibility profile observed in mainland France. In that study, 3.1% of E. coli and 31.6% of Klebsiella pneumoniae were extended-spectrum β-lactamase (ESBL) producers.3
Urinary tract infection is a common infectious presentation in community practice worldwide. It is often treated with broad-spectrum antibiotics because of concerns about infection with resistant organisms. Consequently, the extensive use of antimicrobial agents has invariably resulted in the development of antibiotic resistance. Indeed, antibiotic resistance in uropathogens has changed in recent years, in both the community and hospitals.4,5 In addition, there is little available information on the resistance pattern of microorganisms causing community-acquired UTIs in French Amazonia.
Initial antibiotic treatment of UTIs is typically empirical, and the appropriate treatment is initiated after urine culture and susceptibility tests. The empiric treatment should include an antimicrobial to which all probable uropathogens are susceptible.6 For this, it is essential to know the most common etiological agents for UTIs, and antibiotic resistance rates in the related geographic area because resistance rates in different geographic regions can vary.7 Additionally, most antibiotics are eliminated by glomerular filtration and high antibiotics concentrations were documented in the urines of treated patients.8 This raises the question of whether antibiotic resistance is a major concern in treating UTIs. Indeed, resistance or susceptible profile are defined in vitro according to the minimal inhibitory concentration of the antibiotic on the causal bacteria. High levels of antibiotics in urines and the kidney parenchyma suggest that some antibiotics can effectively treat UTIs despite their in vitro resistance profile. For this, some authors encourage rethinking urinary antibiotic breakpoints.8 In addition to the resistance levels,9 monitoring co- and cross-resistance to the available antibiotics is important to select appropriate alternatives.
We conducted this retrospective study to search for the microbiological epidemiology and antimicrobial susceptibility patterns of community-acquired urine bacterial isolates in French Amazonia.
MATERIALS AND METHODS
We conducted this retrospective study over 5 years (January 2015 to December 2019) in the microbiology laboratory of the Cayenne General Hospital (French Guiana). We included all community-acquired isolates in urine samples from adult patients (> 18 years) attending the emergency department (ED), outpatient clinics (OC), or remote health care centers (RHCC) with a significant density of growth independently of the clinical diagnosis of UTI and the prior antibiotics exposure. The Cayenne general hospital is a 742-bed general center that provides first-line medical care for an urban population of 150,000. It manages 18 RHCC, providing care for additional 50,000 inhabitants. It is also a referral center for a larger population coming from all over French Guiana and the border countries.10
We reviewed all positive urine cultures and antibiotic susceptibility testing. Fungal cultures, anaerobic bacterial cultures, polymicrobial cultures, and negative cultures were excluded from this study.
Data collection.
We collected data from the computerized database of the microbiology laboratory. A community-acquired isolate is defined as a culture collection from an outpatient (i.e., consulting in the ED, OC, or RHCC). Significant density of growth refers to the breakpoints defined by the French society of microbiology.11 In women, they are defined as ≥ 103 colony-forming units (CFU)/mL for E. coli and Staphylococcus saprophiticus; ≥ 104 CFU/mL for enterobacterales and Pseudomonas aeruginosa; and ≥ 105 CFU/mL for Streptococcus agalactiae, nonsaprophiticus coagulase-negative staphylococci (CNS), and non–P. aeruginosa nonfermentative Gram-negative bacteria (GNB). In men, they are defined as ≥ 103 CFU/mL for E. coli, S. saprophiticus, Enterobacterales, and P. aeruginosa; and ≥ 105 CFU/mL for S. agalactiae, nonsaprophiticus CNS, and non–P. aeruginosa non fermentative GNB. Redundant cases are defined as positive culture urinalysis in the same patient growing to the same organism in an interval of < 6 months. Each patient with nonredundant results is considered as a new patient. Enterobacterales were divided into three groups according to their enzymatic resistance profile to β-lactams at the basal state (natural resistance).12 Group I are those without enzymatic resistance at the basal state (e.g., E. coli, Proteus mirabilis), group II are those producing low-level penicillinase (e.g., K. pneumoniae, Citrobacter koseri), and group III are those producing low-level AmpC (e.g., Enterobacter spp., Serratia marcessens, Citrobacter freundii). Cross-resistance refers to resistance to several antibiotics with a similar mechanism of action. Coresistance refers to resistance to more than one class of antibiotics. For each included urine culture, we collected the patient’s age and gender, the isolated bacteria, and its susceptibility to different classes of antibiotics.
Microbiological technique.
Bacterial inoculation was performed on Uriselect® media (Bio-Rad, Marnes-la-Coquette, France) incubated at 35–37°C for 18 to 24 hours under an aerobic atmosphere. Indole positive pink colonies were identified as E. coli; other bacteria were identified by mass spectrometry (MALDI biotyper—Bruker). The antimicrobial susceptibility tests were performed by using VITEK 2 AST-N372 card (BioMérieux, Marcy l’Étoile, France) or by disk diffusion method in Mueller–Hinton medium (BioMérieux). The susceptibility to antibiotics was estimated according to the Antibiogram Committee of the French Society for Microbiology.13 The identification of ESBL-producing enterobacterales (ESBL-PE) was confirmed by the disc diffusion method to detect synergy.14
Statistical analysis.
Results are reported as median and interquartiles ranges (IQR: 1st–3rd quartiles) or numbers with percentages. Qualitative variables were compared using Fisher’s exact test, and continuous variables the Mann–Whitney U test. We used linear regression, and calculated the correlation coefficient (R2) to determine the trend of the event’s prevalence (susceptibility to the studied antibiotic) according to the quarter of the study. Statistical significance was defined as a P value ≤ 0.05. Statistical analyses were carried out with Excel (2010 Microsoft Corporation, Redmond, WA) and IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY).
Ethical considerations.
This study was approved by the ethics committee of our hospital. Written information was distributed to all patients or their relatives, stating that their data could be used for research purposes and that they can oppose that. Our database has been registered at the Commission National de l’Informatique et des Libertés (registration no. 2217629), complying with French law on electronic data sources.
RESULTS
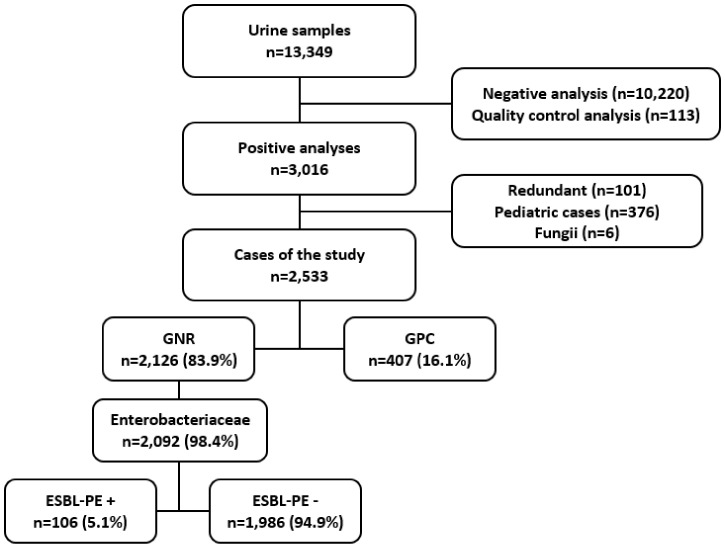
During the study period, 13,349 urinalyses were performed in our bacteriology laboratory. After exclusions, 2,533 positive urinalyses were studied (Figure 1). The distribution of the exams according to the year of the study showed an average of 635 positive urinalyses per year. The urinalyses were sampled in the emergency department in 64% of cases, in the remote healthcare centers in 29% of cases, and in the outpatient clinics in 7% of cases. Patients were women in 74.1% of cases. The median age of patients was 44 years (IQR: 30–66). It was 38 (IQR: 28–57) in women and 63 (IQR: 46–76) in men (P < 0.001).
Figure 1.
Flow chart of the study. ESBL-PE = extended-spectrum β-lactamase producer Enterobacterales; GNR = Gram-negative rods; GPC = Gram-positive cocci.
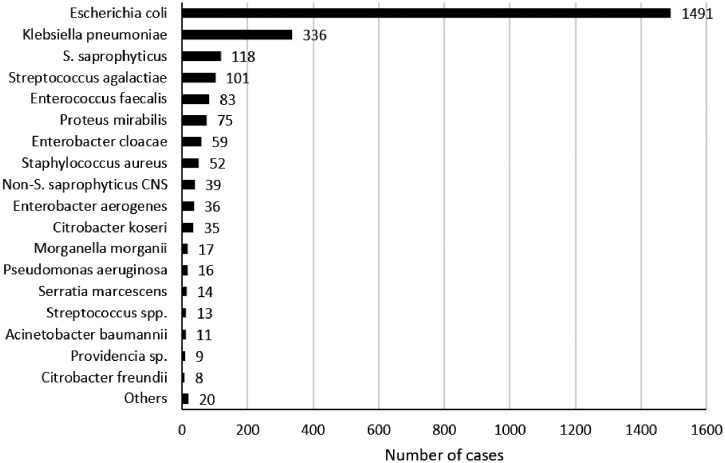
The distribution of the isolated microorganisms is reported in Figure 2. Isolated microorganisms were Gram-negative rods (GNR) in 2,126 cases (83.9%) and Gram-positive cocci (GPC) in 407 cases (16.1%). Among the isolated GNR, 2,092 (98.4%) were Enterobacterales. They were group I Enterobacterales in 1,572 cases (75%), group II in 374 cases (17.9%), and group III in 146 cases (7.1%) (Table 1). The main isolated bacteria were E. coli (1,491 cases; 58.7%) and K. pneumoniae (336 cases; 13.3%). Patients with E. coli were 42 years old (IQR: 30–63), and 79.1% were women. Patients with K. pneumoniae were 52 years old (IQR: 31–72), and 68.8% of them were women (P < 0.001 for both values compared with E. coli group).
Figure 2.
The microorganisms isolated in urine cultures. CNS = coagulase-negative staphylococci.
Table 1.
Susceptibility profile of Gram-negative rods recovered from urine samples
| Tested antibiotics | GNR | Enterobacterales | Group I EB | Group II EB | Group III EB | NF-GNR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | Nb | Result | Nb | Result | Nb | Result | |
| ESBL-PE | 2,092 | 106 (5.1%) | 2,092 | 106 (5.1%) | 1,572 | 75 (4.8%) | 374 | 30 (8%) | 146 | 1 (0.7%) | 29 | 0 (0%) |
| Penicillins | ||||||||||||
| Ampicillin | 2,100 | 608 (29%) | 2,092 | 608 (29.1%) | 1,572 | 608 (38.7%) | 374 | 0 (0%) | 146 | 0 (0%) | – | – |
| Amoxicillin/clavulanic acid | 2,099 | 1,566 (74.6%) | 2,092 | 1,565 (74.8%) | 1,572 | 1,240 (78.9%) | 374 | 325 (86.9%) | 146 | 0 (0%) | – | – |
| Piperacillin | 2,124 | 732 (34.5%) | 2,092 | 706 (33.7%) | 1,572 | 610 (38.8%) | 374 | 0 (0%) | 146 | 96 (65.8%) | 29 | 26 (89.7%) |
| Ticarcillin | 2,125 | 752 (35.4%) | 2,092 | 725 (34.7%) | 1,572 | 629 (40%) | 374 | 0 (0%) | 146 | 96 (65.8%) | 29 | 27 (93.1%) |
| Piperacillin/tazobactam | 2,124 | 2,012 (94.7%) | 2,091 | 1,982 (94.8%) | 1,571 | 1,502 (95.6%) | 374 | 344 (92%) | 146 | 136 (93.2%) | 29 | 28 (96.6%) |
| Temocillin | 1,318 | 1,286 (97.6%) | 1,317 | 1,286 (97.6%) | 1,044 | 1,030 (98.7%) | 193 | 186 (96.4%) | 80 | 70 (87.5%) | – | – |
| Mécillinam | 1,055 | 970 (91.9%) | 1,055 | 970 (91.9%) | 1,004 | 933 (92.9%) | 44 | 34 (77.3%) | 7 | 3 (42.9%) | – | – |
| Aztreonam | 687 | 622 (90.5%) | 669 | 611 (91.3%) | 625 | 585 (93.6%) | 37 | 21 (56.8%) | 7 | 5 (71.4%) | 16 | 11 (68.8%) |
| Cephalosporins | ||||||||||||
| Cefotaxime | 2,096 | 1,971 (94%) | 2,092 | 1,968 (94.1%) | 1,572 | 1,495 (95.1%) | 374 | 344 (92%) | 146 | 129 (88.4%) | – | – |
| Ceftazidime | 2,126 | 2,011 (94.6%) | 2,092 | 1,982 (94.7%) | 1,572 | 1,507 (95.9%) | 374 | 344 (92%) | 146 | 131 (89.7%) | 29 | 26 (89.7%) |
| Cefepime | 2,126 | 2,017 (94.9%) | 2,092 | 1,984 (94.8%) | 1,572 | 1,507 (95.9%) | 374 | 346 (92.5%) | 146 | 131 (89.7%) | 29 | 29 (100%) |
| Carbapenems | ||||||||||||
| Imipenem | 2,124 | 2,118 (99.7%) | 2,091 | 2,086 (99.8%) | 1,572 | 1,571 (99.9%) | 374 | 374 (100%) | 145 | 141 (97.2%) | 29 | 29 (100%) |
| Meropenem | 121 | 120 (99.2%) | 90 | 90 (100%) | 48 | 48 (100%) | 35 | 35 (100%) | 7 | 7 (100%) | 29 | 29 (100%) |
| Ertapenem | 2,080 | 2,076 (99.8%) | 2,078 | 2,074 (99.8%) | 1,567 | 1,567 (100%) | 368 | 367 (99.7%) | 143 | 140 (97.9%) | – | – |
| Aminoglycosides | ||||||||||||
| Amikacin | 2,120 | 2,084 (98.3%) | 2,089 | 2,054 (98.3%) | 1,570 | 1,546 (98.5%) | 373 | 364 (97.6%) | 146 | 144 (98.6%) | 29 | 28 (96.6%) |
| Tobramycin | 1,185 | 1,080 (91.1%) | 1,154 | 1,049 (90.9%) | 879 | 805 (91.6%) | 203 | 183 (90.1%) | 72 | 61 (84.7%) | 29 | 29 (100%) |
| Gentamycin | 2,111 | 1,960 (92.8%) | 2,080 | 1,929 (92.7%) | 1,564 | 1,453 (92.9%) | 373 | 351 (94.1%) | 143 | 125 (87.4%) | 29 | 29 (100%) |
| Fluoroquinolones | ||||||||||||
| Ofloxacin | 2,097 | 1,710 (81.5%) | 2,092 | 1,707 (81.6%) | 1,572 | 1,256 (79.9%) | 374 | 328 (87.7%) | 146 | 123 (84.2%) | – | – |
| Ciprofloxacin | 1,977 | 1,797 (90.9%) | 1,943 | 1,766 (90.9%) | 1,449 | 1,311 (90.5%) | 362 | 331 (91.4%) | 132 | 124 (93.9%) | 29 | 28 (96.6%) |
| Others | ||||||||||||
| TMP/SMX | 2,097 | 1,402 (66.9%) | 2,082 | 1,387 (66.6%) | 1,567 | 955 (60.9%) | 372 | 317 (85.2%) | 143 | 115 (80.4%) | 13 | 13 (100%) |
| Nitrofurantoin | 2,084 | 1,786 (85.7%) | 2,083 | 1,785 (85.7%) | 1,565 | 1,475 (94.2%) | 373 | 238 (63.8%) | 145 | 72 (49.7%) | – | – |
| Fosfomycin | 765 | 750 (98%) | 764 | 749 (98%) | 714 | 708 (99.2%) | 43 | 35 (81.4%) | 7 | 6 (85.7%) | – | – |
EB = Enterobacteriaceae; ESBL = extended-spectrum β-lactamases; GNR = Gram-negative rods; Nb = number of tested isolates; NF-GNR = nonfermentative GNR; PE = producing enterobacterales; TMP/SMX = trimethoprim/sulfamethoxazole.
The susceptibility of GNR to antimicrobials is reported in Table 1. Antibiotic susceptibilities of E. coli and K. pneumoniae are reported in Table 2. Among the 1,491 tested E. coli, 37.2% were susceptible to amoxicillin, 77.9% to amoxicillin/clavulanic acid, 94.9% to cefotaxime, 100% to imipenem, 98.5% to amikacin, 78.9% to ofloxacin, and 98.9% to nitrofurantoin.
Table 2.
Susceptibility profile of isolated Escherichia coli and Klebsiella pneumoniae
| Tested antibiotics | E. coli and K. pneumoniae | E. coli | K. pneumoniae | |||
|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | |
| ESBL | 1,827 | 104 (5.7%) | 1,491 | 74 (5%) | 336 | 30 (8.9%) |
| Penicillins | ||||||
| Amoxicillin | 1,827 | 554 (30.3%) | 1,491 | 554 (37.2%) | 336 | 0 (0%) |
| Amoxicillin/clavulanic acid | 1,827 | 1,450 (79.4%) | 1,491 | 1,162 (77.9%) | 336 | 288 (85.7%) |
| Piperacillin | 1,827 | 555 (30.4%) | 1,491 | 555 (37.2%) | 336 | 0 (0%) |
| Piperacillin/Tazobactam | 1,826 | 1,727 (94.6%) | 1,490 | 1,421 (95.4%) | 336 | 306 (91.1%) |
| Temocillin | 1,138 | 1,117 (98.2%) | 964 | 950 (98.5%) | 174 | 167 (96%) |
| Mecillinam | 1,042 | 961 (92.2%) | 1,001 | 930 (92.9%) | 41 | 31 (75.6%) |
| Cephalosporins | ||||||
| Cefotaxime | 1,827 | 1,721 (94.2%) | 1,491 | 1,415 (94.9%) | 336 | 306 (91.1%) |
| Ceftazidime | 1,827 | 1,733 (94.9%) | 1,491 | 1,427 (95.7%) | 336 | 306 (91.1%) |
| Cefepime | 1,827 | 1,735 (95%) | 1,491 | 1,427 (95.7%) | 336 | 308 (91.7%) |
| Carbapenems | ||||||
| Imipenem | 1,827 | 1,827 (100%) | 1,491 | 1,491 (100%) | 336 | 336 (100%) |
| Ertapenem | 1,817 | 1,816 (99.9%) | 1,487 | 1,487 (100%) | 330 | 329 (99.7%) |
| Aminoglycosides | ||||||
| Amikacin | 1,824 | 1,792 (98.2%) | 1,489 | 1,466 (98.5%) | 335 | 326 (97.3%) |
| Tobramycin | 1,023 | 930 (90.9%) | 839 | 766 (91.3%) | 184 | 164 (89.1%) |
| Gentamycin | 1,819 | 1,687 (92.7%) | 1,483 | 1,373 (92.6%) | 336 | 314 (93.5%) |
| Fluoroquinolones | ||||||
| Ofloxacin | 1,827 | 1,467 (80.3%) | 1,491 | 1,177 (78.9%) | 336 | 290 (86.3%) |
| Ciprofloxacin | 1,692 | 1,525 (90.1%) | 1,368 | 1,232 (90.1%) | 324 | 293 (90.4%) |
| Others | ||||||
| TMP/SMX | 1,820 | 1,166 (64.1%) | 1,486 | 886 (59.6%) | 334 | 280 (83.8%) |
| Nitrofurantoin | 1,819 | 1,671 (91.9%) | 1,484 | 1,468 (98.9%) | 335 | 203 (60.6%) |
| Fosfomycin | 751 | 737 (98.1%) | 711 | 705 (99.2%) | 40 | 32 (80%) |
Nb = number of tested isolates; TMP/SMX = trimethoprim/sulfamethoxazole.
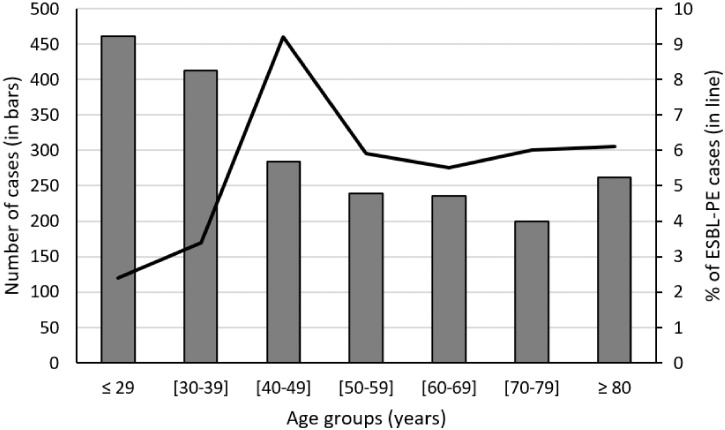
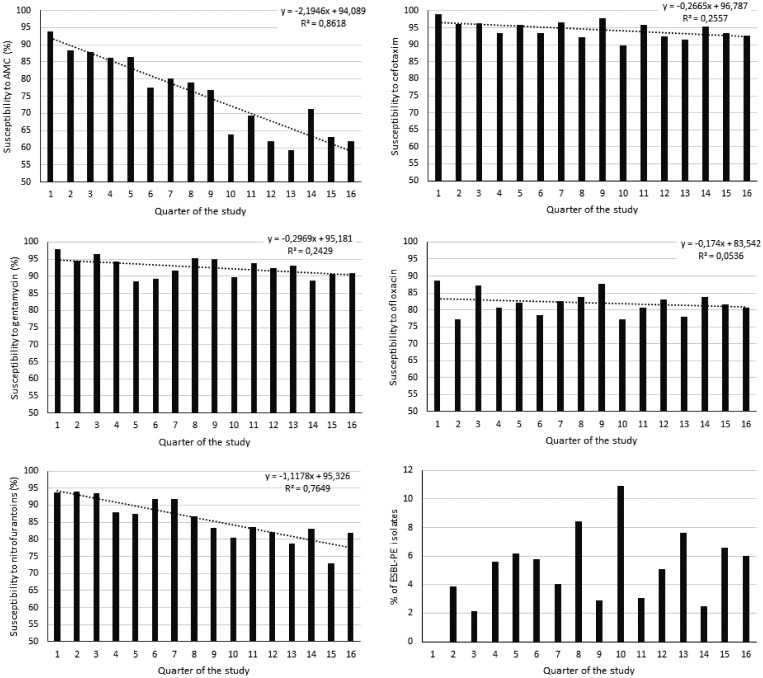
In 106 cases (5.1%), isolated Enterobacterales were ESBL producers (5% of E. coli and 8.9% of K. pneumoniae; P = 0.008). The patient’s age was 52 years (IQR: 40–70) in patients with ESBL-PE versus 45 (31–67) in those without (P = 0.002). The female gender was 60% in patients with ESBL-PE versus 74.9% in those without (P = 0.001). The percentages of ESBL-PE according to the age groups are reported in Figure 3. ESBL-PE rate was 4.8% in group I, 8% in group II, and 0.7% in group III Enterobacterales. Most of the isolated ESBL-PE in urinalyses were sampled in the ED and the outpatient clinics. The susceptibility trend of Enterobacterales and ESBL-PE profile across the 16 quarters of the study showed a decreasing susceptibility profile for amoxicillin/clavulanic acid (P < 0.001), cefotaxime (P = 0.045), and nitrofurantoin (P < 0.001). Although the susceptibility trends for gentamycin (P = 0.052) and ofloxacin (P = 0.388) were stable (Figure 4). Cross- and co-resistance among Enterobacterales are reported in Table 3.
Figure 3.
Percentage of extended spectrum β-lactamase producing Enterobacteriaceae (ESBL-PE) (in line) according to the age group (in bars).
Figure 4.
The susceptibility trend of Enterobacterales and extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-PE) profile across the 16 quarters of the study. P value was < 0.001 for amoxicillin/clavulanate, 0.045 for cefotaxime, 0.052 for gentamycin, 0.388 for ofloxacin, and < 0.001 for nitrofurantoin. AMC = amoxicillin/clavulanic acid.
Table 3.
Cross-resistance and coresistance among Enterobacterales isolated from urine samples
| Tested antibiotics | AMX | AMC | PTZ | TEM | CTX | CAZ | IPM | AMK | GEN | OFL | CIP | SXT | FT | FS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESBL-PE | 100 | 78.3 | 38.7 | 22.8 | 100 | 100 | 0.0 | 24.5 | 44.3 | 88.7 | 83.8 | 77.9 | 16.8 | 12.2 |
| AMX | 100 | 35.5 | 7.3 | 4.4 | 8.4 | 7.4 | 0.3 | 2.2 | 10.1 | 23.0 | 12.0 | 44.8 | 16.7 | 2.8 |
| AMC | 100 | 100 | 20.5 | 6.7 | 19.2 | 16.5 | 0.8 | 5.1 | 17.5 | 30.6 | 16.0 | 51.1 | 20.2 | 3.7 |
| PTZ | 100 | 99.1 | 100 | 22.9 | 41.3 | 38.5 | 0.9 | 12.0 | 29.9 | 51.4 | 40.2 | 61.5 | 19.8 | 9.0 |
| TEM | 100 | 77.4 | 51.6 | 100 | 51.6 | 48.4 | 3.2 | 9.7 | 53.3 | 54.8 | 44.0 | 48.1 | 36.7 | 10.0 |
| CTX | 100 | 81.5 | 36.3 | 23.5 | 100 | 88.7 | 1.6 | 21.8 | 46.0 | 84.7 | 78.3 | 75.2 | 23.5 | 12.0 |
| CAZ | 100 | 79.1 | 38.2 | 24.6 | 100 | 100 | 1.8 | 21.8 | 50.0 | 83.6 | 78.0 | 73.1 | 23.8 | 13.8 |
| IPM | 100 | 80.0 | 20.0 | 20.0 | 40.0 | 40.0 | 100 | 0.0 | 0.0 | 20.0 | 20.0 | 20.0 | 100 | 0.0 |
| AMK | 94.3 | 77.1 | 37.1 | 15.0 | 77.1 | 68.6 | 0.0 | 100 | 57.1 | 91.4 | 86.4 | 77.1 | 29.4 | 12.0 |
| GEN | 98.7 | 60.3 | 21.2 | 18.6 | 37.7 | 36.4 | 0.0 | 13.2 | 100 | 75.5 | 57.3 | 75.5 | 18.4 | 9.5 |
| OFL | 88.8 | 41.8 | 14.5 | 8.2 | 27.3 | 23.9 | 0.3 | 8.3 | 29.6 | 100 | 75.0 | 65.5 | 12.9 | 4.7 |
| CIP | 91.5 | 40.1 | 20.9 | 22.9 | 40.7 | 36.2 | 0.6 | 10.7 | 33.3 | 100 | 100 | 67.4 | 13.4 | 10.3 |
| TMP/SMX | 95.1 | 38.1 | 9.2 | 3.7 | 13.1 | 11.4 | 0.1 | 3.9 | 16.0 | 36.1 | 19.7 | 100 | 8.4 | 3.4 |
| FT | 82.6 | 35.2 | 7.0 | 4.8 | 9.4 | 8.4 | 1.7 | 3.4 | 9.1 | 16.4 | 8.4 | 19.5 | 100 | 11.5 |
| FS | 93.3 | 73.3 | 40.0 | 20.0 | 73.3 | 73.3 | 0.0 | 20.0 | 57.1 | 60.0 | 53.8 | 78.6 | 21.4 | 100 |
AMC = amoxicillin/clavulanic acid; AMK = amikacin; AMX = amoxicillin; CAZ = ceftazidime; CIP = ciprofloxacin; CTX = cefotaxime; ESBL-PE = extended-spectrum β-lactamase producing Enterobacteriaceae; FS = fosfomycin; FT = nitrofurantoin; GEN = gentamycin; IPM = imipenem; PTZ = piperacillin/tazobactam; OFL = ofloxacin, TMP/SMX = trimethoprim/sulfamethoxazole; TEM = temocillin. Values indicate the resistance rate to the antibiotic in the column when the strain is resistant to the antibiotic in the row.
GPCs were isolated in 407 cases (16.1%). They were CNS in 157 cases (38.6%), S. agalactiae in 101 cases (24.8%), Enterococcus faecalis in 83 cases (20.4%), and Staphylococcus aureus in 52 cases (12.8%). CNS was S. saprophyticus in 118 cases (75.2% of CNS). It was resistant to oxacillin in 52.5% of cases and susceptible to nitrofurantoin and tetracycline in 99 and 90% of cases respectively. Patients with S. saprophyticus were women in 94.5% of cases and were 29 years old (IQR: 21–36). S. aureus was resistant to oxacillin in 13.5% of cases. Susceptibility profiles of GPC are reported in Table 4.
Table 4.
Susceptibility profile of Gram-positive cocci isolates from urine samples
| Tested antibiotics | GPC | CNS | S. saprophyticus | S. aureus | S. agalactia | E. faecalis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | Nb | Result | Nb | Result | Nb | Result | |
| Penicillins | ||||||||||||
| Penicillin G | 213 | 36 (16.9%) | 157 | 23 (14.6%) | 118 | 16 (13.6%) | 52 | 9 (17.3%) | 4 | 4 (100%) | 0 | – |
| Oxacillin | 209 | 113 (54.1%) | 157 | 68 (43.3%) | 118 | 56 (47.5%) | 52 | 45 (86.5%) | 0 | – | 0 | – |
| Amoxicillin | 185 | 184 (99.5%) | – | – | – | – | – | – | 101 | 101 (100%) | 83 | 83 (100%) |
| Aminoglycosides | ||||||||||||
| Kanamycin | 209 | 197 (94.3%) | 157 | 146 (93%) | 118 | 115 (97.5%) | 52 | 51 (98.1%) | – | – | – | – |
| Tobramycin | 209 | 197 (94.3%) | 157 | 146 (93%) | 118 | 115 (97.5%) | 52 | 51 (98.1%) | – | – | – | – |
| Gentamycin | 209 | 198 (94.7%) | 157 | 146 (93%) | 118 | 115 (97.5%) | 52 | 52 (100%) | – | – | – | – |
| Macrolids | ||||||||||||
| Tetracyclin | 218 | 166 (76.1%) | 157 | 122 (77.7%) | 118 | 106 (89.8%) | 52 | 43 (82.7%) | 9 | 1 (11.1%) | – | – |
| Erythromycin | 267 | 123 (46.1%) | 157 | 77 (49%) | 118 | 55 (46.6%) | 52 | 38 (73.1%) | 9 | 7 (77.8%) | 49 | 1 (2%) |
| Lincomycin | 203 | 189 (93.1%) | 153 | 139 (90.8%) | 114 | 104 (91.2%) | 50 | 50 (100%) | – | – | – | – |
| Fluoroquinolones | ||||||||||||
| Ofloxacin | 209 | 183 (87.6%) | 157 | 139 (88.5%) | 118 | 111 (94.1%) | 52 | 44 (84.6%) | – | – | – | – |
| Glycopeptides | ||||||||||||
| Vancomycin | 268 | 265 (98.9%) | 157 | 156 (99.4%) | 118 | 117 (99.2%) | 52 | 52 (100%) | 9 | 9 (100%) | 49 | 48 (98%) |
| Others | ||||||||||||
| TMP/SMX | 256 | 189 (73.8%) | 157 | 107 (68.2%) | 118 | 79 (66.9%) | 52 | 48 (92.3%) | – | – | 47 | 34 (72.3%) |
| Rifampicin | 210 | 201 (95.7%) | 157 | 149 (94.9%) | 118 | 115 (97.5%) | 52 | 52 (100%) | – | – | – | – |
| Nitrofurantoin | 253 | 251 (99.2%) | 146 | 145 (99.3%) | 109 | 108 (99.1%) | 49 | 49 (100%) | 9 | 8 (88.9%) | 49 | 49 (100%) |
| Fosfomycin | 209 | 57 (27.3%) | 157 | 6 (3.8%) | – | – | 52 | 51 (98.1%) | – | – | – | – |
| Fucidic acid | 209 | 68 (32.5%) | 157 | 22 (14%) | – | – | 52 | 46 (88.5%) | – | – | – | – |
| Linezolid | 259 | 254 (98.1%) | 149 | 144 (96.6%) | 112 | 107 (95.5%) | 51 | 51 (100%) | 9 | 9 (100%) | 49 | 49 (100%) |
CNS = coagulase-negative staphylococci; GPC = Gram-positive cocci; Nb = number of tested isolates; TMP/SMX = trimethoprim/sulfamethoxazole.
DISCUSSION
Our study shows that the most isolated microorganisms from outpatient urinalyses were Enterobacterales, mainly E. coli and K. pneumoniae. They were susceptible to most antibiotics (wild type) in most cases. We highlight an elevated resistance level of Enterobacterales to amoxicillin and a high susceptibility rate to nitrofurantoin and fosfomycin. Additionally, we found a decreasing susceptibility profile in Enterobacterales for amoxicillin/clavulanate, cefotaxime, and nitrofurantoin across the 16 quarters of the study.
In Amazonia, Baizet et al.3 conducted a retrospective study of adults attending the ED of Cayenne Hospital with a diagnosis of UTI. They found that E. coli was predominant (74.1%). We indeed found 5 years later that Enterobacterales mainly E. coli, as the first isolate from urine cultures (58.7% in absolute). Despite a high representation of GPCs (16.1%), the flora and the resistance profile found in our study are different from those reported in Latin America.2 This is probably explained by the flow of populations in French Guiana, mostly from the French islands and the European continent rather than from the surrounding countries.
Enterobacterales were resistant to amoxicillin in 70.9%, to amoxicillin/clavulanic acid in 25.2%, and to cefotaxime in 5.9% of cases. Interestingly, they were sensitive to fosfomycin and nitrofurantoin in 98% and 86% of cases, respectively. For this, fosfomycin and nitrofurantoin seem to be a reasonable option for empirical treatment of uncomplicated UTIs.15–18 Indeed, fosfomycin and nitrofurantoin are active against common causes of UTIs, mainly E. coli, whereas nonfermentative GNRs are naturally resistant. Overall, resistance to these two antibiotics is uncommon in Enterobacterales and many multidrug resistant organisms retain susceptibility.15 On the other hand, fluoroquinolones were active against 90% of Enterobacteriaceae isolates. They were reported as effective for clinical and microbiological cures in patients with uncomplicated UTIs.19 However, they should be spared in the first- and second-line UTI treatment because of their selection pressure and also because they should be saved for more severe infections.19,20 On the other hand, our study shows that the susceptibility trend of Enterobacterales showed a decreasing susceptibility profile over time for amoxicillin/clavulanic acid, cefotaxime, and nitrofurantoin. In contrast, the susceptibility trends for gentamycin and ofloxacin were stable. This can be explained by the antibiotic pressure in the community.
In our study, 407 specimens grew to GPC, 157 of them (38.6% of GPCs and 5.8% of all positive urinalyses) grew to CNS, and 118 to S. saprophyticus (75.2% of CNS). It is well known that CNS—namely, S. saprophyticus—can cause community-acquired UTI. Indeed, S. saprophyticus is part of the normal human flora that colonizes the perineum, urinary, and gastrointestinal tracts. It causes 5% to 20% of community-acquired UTIs21 and up to 42% of UTIs among 16- to 25-year-old women.22 In our study, microbiological results showed that S. saprophyticus was resistant to oxacillin in 52.5% of cases and was susceptible to nitrofurantoin and tetracycline in 99% and 90% of cases, respectively. Patients were young women in the majority as described in the literature.23 It is noteworthy that UTI symptoms caused by S. saprophyticus are similar but can be more severe than in patients with E. coli UTIs, and 40% of patients present with acute pyelonephritis.23
Community-acquired UTIs caused by multidrug-resistant bacteria has become a growing and challenging to treat concern24,25 with decreased susceptibility to ampicillin, amoxicillin/clavulanic acid, fluoroquinolones, cotrimoxazole, and furans.9,26 Indeed, the reported resistance rates were 21% to 63.4% for ampicillin, 1.2% to 9.6% for amoxicillin/clavulanic acid, 1% to 5.4% for cefuroxime, 0.5% to 12.9% for ciprofloxacin, 14% to 45.4% for trimethoprim-sulfamethoxazole, 0% to 2.9% for fosfomycin, and 6.3% to 32.6% for nalidixic acid.27 Our study shows similar results with a decreased susceptibility of E. coli to amoxicillin, quinolones, and to trimethoprim/sulfamethoxazole. However, the isolated E. coli had a higher susceptibility rate to amoxicillin/clavulanic acid and full susceptibility to furans. Regarding ESBL-PE rate, it requires close monitoring and a high-priority prevention strategy. Overall, local susceptibility rates are compatible with the antibiotics recommended by the French society of infectious diseases for the treatment of community UTI9,26 and therefore reinforce their relevance in our context.
ESBL-PE in the community is a significant concern worldwide. Surveillance networks revealed a predominance of ESBL-P K. pneumoniae in Latin America (44%) and Asia Pacific regions (22%), with a lower incidence in Europe (13.3%) and North America (7.5%).28,29 Moreover, E. coli producing ESBL type CTX-M in the community are endemic in Asia, South America, and Europe.30 In Latin America, the incidence rate of ESBL-PE is among the highest in the world, varying from 45% to 51% for K. pneumoniae and 8.5% to 18% for E. coli.31,32 In addition, ESBL production can be worsened by developing combined resistance mainly to fluoroquinolones and aminoglycosides in E. coli and K. pneumoniae. In our study, combined resistance was 88.7% to ofloxacin and 24.5% to amikacin in case of ESBL production in Enterobacterales. ESBL-PE among UTI agents has widely been reported and should be suspected mainly in case of prior exposure to antibiotics.33–35 MacVane et al.36 showed that the main involved ESBL-PE among UTI isolates are E. coli and K. pneumoniae. They result in an ineffective empiric antibiotic treatment (62% versus 6%, P < 0.001) and a delayed effective antibiotic therapy (51 versus 2.5 hours, P < 0.001) compared with non-ESBL organisms. In addition, they are responsible for prolonged hospital stays (6 days versus 4 days, P = 0.02) and higher hospital costs. They were also responsible for higher infection-related mortality (7.2% versus 1.8%) and readmission rates in 30 days (7.2% versus 3.6%) without reaching the significance level.36 In French Guiana, Baizet et al.3 reported in a retrospective study of adults consulting at the ED of Cayenne Hospital with a diagnosis of UTI, that ESBL production was detected in 3.1% of E. coli and 31.6% of K. pneumoniae. Our study diagnosed ESBL-PE in 106 cases (5.1% of isolated Enterobacterales). It was 5% among E. coli and 8.9% among K. pneumoniae isolates. These results are concordant with those reported by former surveys3,37 and approximate the figures of mainland France. Unlike its neighbors in Latin America, the French Amazonia seems to be spared from the South America continental dissemination of ESBL-PE in the community.2 Nevertheless, a robust prevention strategy is required to stop the spread of antibiotic resistance. Further, cross-resistance to β-lactams and coresistance to other classes of antimicrobials in ESBL-PE are frequent.38 Indeed, coresistance to fluoroquinolones prevails in E. coli and K. pneumoniae strains.39,40 For this reason, fluoroquinolones should be considered only in documented infections caused by quinolone-susceptible ESBL-PE.41 In addition, ESBL-PE can develop coresistance to aminoglycosides mainly through aminoglycoside-modifying enzymes coproduced by CTX-M ESBL on the same plasmid.42 In our study, coresistance to amikacin and gentamycin was diagnosed in (24.5% and 44.3% of cases) and to ofloxacin in 88.7%. However, cross-resistance to piperacillin/tazobactam was found in 38.7% of cases. This suggests the possibility of piperacillin/tazobactam use in case of complicated UTI caused by ESBL-PE. Indeed, Seo et al.43 found a clinical and microbiological response to piperacillin/tazobactam treatment in 94% of cases with UTI caused by ESBL producing E. coli, similar to the response to ertapenem treatment. Chastain et al.8 found that renally eliminated antibiotics can achieve sufficient urinary concentrations for effective eradication of organisms determined to be resistant per in vitro susceptibility testing. This led some authors to rethink antibiotic treatment strategies for UTIs in the era of antimicrobial resistance.44,45
Our study has two significant limitations. First, it deals with the resistance profile of the isolated microorganisms independently of the clinical diagnosis of the UTI and the prior exposure to antibiotics. Second, it is monocentric and was focused on the capital city of French Guiana and its surroundings (the center and the east of the department) and deserves to be widen toward the other population basins, notably the west of French Guiana, which is more subject to cross-border exchanges. However, it shows the local microbial ecology, which should be considered in daily practice when selecting empirical treatment in case of community-acquired UTI.
CONCLUSION
Our study shows that the most isolated microorganisms from outpatient urinalyses were Enterobacterales, mainly E. coli and K. pneumoniae. They showed a high resistance rate to amoxicillin but susceptibility to most remaining antibiotics. Additionally, our study shows a decreasing susceptibility profile in Enterobacterales for amoxicillin/clavulanic acid, cefotaxime, and nitrofurantoin across the 16 quarters of the study. Interestingly, nitrofurantoin, and fosfomycin were active against most GNRs and can be considered as empirical treatment of uncomplicated UTIs. Fluoroquinolones are active against most GNRs and Enterobacterales isolates, reflecting a low antibiotic pressure in the community in French Guiana. However, they should be spared in the first- and second-line UTI treatment. This study is helpful for clinicians to guide the empiric treatment of outpatients with UTI symptoms in our region. Moreover, it would help local authorities in developing antibiotic policies for treating UTIs. Further clinical investigations are needed to identify predictive factors and outcomes of patients with community-acquired UTI in the Amazonian region.
References
- 1.Centers for Disease Control and Prevention , 2018. The Biggest Antibiotic-Resistant Threats in the U.S. Available at: https://www.cdc.gov/drugresistance/biggest_threats.html. Accessed November 26, 2018.
- 2. Bours PHA, Polak R, Hoepelman AIM, Delgado E, Jarquin A, Matute AJ, 2010. Increasing resistance in community-acquired urinary tract infections in Latin America, five years after the implementation of national therapeutic guidelines. Int J Infect Dis 14: e770–e774. [DOI] [PubMed] [Google Scholar]
- 3. Baizet C, Ouar-Epelboin S, Walter G, Mosnier E, Moreau B, Djossou F, Epelboin L, 2019. Decreased antibiotic susceptibility of Enterobacteriaceae causing community-acquired urinary tract infections in French Amazonia. Med Mal Infect 49: 63–68. [DOI] [PubMed] [Google Scholar]
- 4. Manges AR, Natarajan P, Solberg OD, Dietrich PS, Riley LW, 2006. The changing prevalence of drug-resistant Escherichia coli clonal groups in a community: evidence for community outbreaks of urinary tract infections. Epidemiol Infect 134: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahan NR, Chinitz DP, Waitman D-A, Dushnitzky D, Kahan E, Shapiro M, 2006. Empiric treatment of uncomplicated urinary tract infection with fluoroquinolones in older women in Israel: another lost treatment option? Ann Pharmacother 40: 2223–2227. [DOI] [PubMed] [Google Scholar]
- 6. Dellinger RP. et al. , 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shepherd AK, Pottinger PS, 2013. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin North Am 97: 737–757, xii. [DOI] [PubMed] [Google Scholar]
- 8. Chastain DB, King ST, Stover KR, 2018. Rethinking urinary antibiotic breakpoints: analysis of urinary antibiotic concentrations to treat multidrug resistant organisms. BMC Res Notes 11: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caron F. et al. , 2018. Practice guidelines for the management of adult community-acquired urinary tract infections. Med Mal Infect 48: 327–358. [DOI] [PubMed] [Google Scholar]
- 10. Kallel H, Resiere D, Houcke S, Hommel D, Pujo JM, Martino F, Carles M, Mehdaoui H, Antilles-Guyane Association of Critical Care Medicine , 2021. Critical care medicine in the French Territories in the Americas: current situation and prospects. Rev Panam Salud Publica 45: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French Society of Microbiology, 2018. Urinary infections. Référentiel en Microbiologie Médicale (REMIC). Paris, France: French Society of Microbiology, 181–97.
- 12. Durand C, Boudet A, Lavigne J-P, Pantel A, 2020. Evaluation of two methods for the detection of third generation cephalosporins resistant enterobacterales directly from positive blood cultures. Front Cell Infect Microbiol 10: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soussy CJ. et al. , 2000. [Antibiogram Committee of the French Microbiology Society. Report 2000–2001]. Pathol Biol (Paris) 48: 832–871. [PubMed] [Google Scholar]
- 14. Jarlier V, Nicolas MH, Fournier G, Philippon A, 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis 10: 867–878. [DOI] [PubMed] [Google Scholar]
- 15. Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY, 2019. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr 42: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huttner A. et al. , 2018. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women. JAMA 319: 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKinnell JA, Stollenwerk NS, Jung CW, Miller LG, 2011. Nitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysis. Mayo Clin Proc 86: 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montelin H, Forsman K-J, Tängdén T, 2019. Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PLoS One 14: e0211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grigoryan L, Trautner BW, Gupta K, 2014. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 312: 1677–1684. [DOI] [PubMed] [Google Scholar]
- 20. Stewardson AJ, Gaïa N, François P, Malhotra-Kumar S, Delémont C, Martinez de Tejada B, Schrenzel J, Harbarth S, Lazarevic V, SATURN WP1 and WP3 Study Groups , 2015. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 21: 344.e1–344.e11. [DOI] [PubMed] [Google Scholar]
- 21. Hooton TM, Stamm WE, 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 11: 551–581. [DOI] [PubMed] [Google Scholar]
- 22. Wallmark G, Arremark I, Telander B, 1978. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis 138: 791–797. [DOI] [PubMed] [Google Scholar]
- 23. Kline KA, Lewis AL, 2016. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr 4. doi: 10.1128/microbiolspec.UTI-0012-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta K, Bhadelia N, 2014. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin North Am 28: 49–59. [DOI] [PubMed] [Google Scholar]
- 25. Foxman B, 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28: 1–13. [DOI] [PubMed] [Google Scholar]
- 26.Société de Pathologie Infectieuse de Langue Française (SPILF) , 2015. Diagnostic et antibiothérapie des infections urinaires bactériennes communautairesde l’adulte. Available at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.infectiologie.com/UserFiles/File/spilf/recos/infections-urinaires-spilf-argumentaire.pdf. Accessed April 10, 2023.
- 27. Tandogdu Z, Wagenlehner FME, 2016. Global epidemiology of urinary tract infections. Curr Opin Infect Dis 29: 73–79. [DOI] [PubMed] [Google Scholar]
- 28. Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Fishman NO, Bilker WB, Mao X, Lautenbach E, 2005. Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis 40: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 29. Zavascki AP, 2004. Assessing risk factors for acquiring antimicrobial-resistant pathogens: a time for a comparative approach. Clin Infect Dis 39: 871–872, author reply 872–873. [DOI] [PubMed] [Google Scholar]
- 30. Munoz-Price LS. et al. , 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaye KS, Engemann JJ, Mozaffari E, Carmeli Y, 2004. Reference group choice and antibiotic resistance outcomes. Emerg Infect Dis 10: 1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris AD, Karchmer TB, Carmeli Y, Samore MH, 2001. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis 32: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 33. Esteve-Palau E, Solande G, Sánchez F, Sorlí L, Montero M, Güerri R, Villar J, Grau S, Horcajada JP, 2015. Clinical and economic impact of urinary tract infections caused by ESBL-producing Escherichia coli requiring hospitalization: a matched cohort study. J Infect 71: 667–674. [DOI] [PubMed] [Google Scholar]
- 34. Hanna-Wakim RH. et al. , 2015. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kizilca O, Siraneci R, Yilmaz A, Hatipoglu N, Ozturk E, Kiyak A, Ozkok D, 2012. Risk factors for community-acquired urinary tract infection caused by ESBL-producing bacteria in children. Pediatr Int 54: 858–862. [DOI] [PubMed] [Google Scholar]
- 36. MacVane SH, Tuttle LO, Nicolau DP, 2014. Impact of extended-spectrum β-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med 9: 232–238. [DOI] [PubMed] [Google Scholar]
- 37. Woerther P-L. et al. , 2013. Characterization of fecal extended-spectrum-β-lactamase-producing Escherichia coli in a remote community during a long time period. Antimicrob Agents Chemother 57: 5060–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pitout JDD, 2010. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 70: 313–333. [DOI] [PubMed] [Google Scholar]
- 39. Merino I, Shaw E, Horcajada JP, Cercenado E, Mirelis B, Pallarés MA, Gómez J, Xercavins M, Martínez-Martínez L, De Cueto M, Cantón R, Ruiz-Garbajosa P, ITUBRAS-GEIH-SEIMC Group , 2016. CTX-M-15-H30Rx-ST131 subclone is one of the main causes of healthcare-associated ESBL-producing Escherichia coli bacteraemia of urinary origin in Spain. J Antimicrob Chemother 71: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 40. Mathers AJ, Peirano G, Pitout JDD, 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28: 565–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aslan AT, Akova M, 2019. Extended spectrum β-lactamase producing enterobacteriaceae: carbapenem sparing options. Expert Rev Anti Infect Ther 17: 969–981. [DOI] [PubMed] [Google Scholar]
- 42. Ramirez MS, Tolmasky ME, 2010. Aminoglycoside modifying enzymes. Drug Resist Updat 13: 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seo YB, Lee J, Kim YK, Lee SS, Lee J-A, Kim HY, Uh Y, Kim H-S, Song W, 2017. Randomized controlled trial of piperacillin-tazobactam, cefepime and ertapenem for the treatment of urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli. BMC Infect Dis 17: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zahar JR, Lecuit M, Carbonnelle E, Ribadeau-Dumas F, Nassif X, Lortholary O, 2007. Is it time to reconsider initial antibiotic treatment strategies for severe urinary tract infections in Europe? Clin Microbiol Infect 13: 219–221. [DOI] [PubMed] [Google Scholar]
- 45. Bader MS, Loeb M, Brooks AA, 2017. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med 129: 242–258. [DOI] [PubMed] [Google Scholar]