ABSTRACT.
In endemic malaria areas, Plasmodium is currently diagnosed mainly through the use of rapid diagnostic tests (RDTs). However, in Senegal, many causes of fever remain unknown. Tick-borne relapsing fever, an often-neglected public health problem, is the main cause of consultation for acute febrile illness after malaria and flu in rural areas. Our objective was to test the feasibility of extracting and amplifying DNA fragments by quantitative polymerase chain reaction (qPCR) from malaria-negative RDTs for Plasmodium falciparum (malaria Neg RDTs P.f) to detect Borrelia spp. and other bacteria. Between January and December 2019, malaria Neg RDTs P.f were collected on a quarterly basis in 12 health facilities in four regions of Senegal. The DNA extracted from the malaria Neg RDTs P.f was tested using qPCR and the results were confirmed by standard PCR and sequencing. Only Borrelia crocidurae DNA was detected in 7.22% (159/2,202) of RDTs. The prevalence of B. crocidurae DNA was higher in July (16.47%, 43/261) and August (11.21%, 50/446). The annual prevalence was 9.2% (47/512) and 5.0% (12/241) in Ngayokhem and Nema-Nding, respectively, health facilities in the Fatick region. Our study confirms that B. crocidurae infection is a frequent cause of fever in Senegal, with a high prevalence of cases in health facilities in the regions of Fatick and Kaffrine. Malaria Neg RDTs P.f are potentially a good source of pathogen sampling for the molecular identification of other causes of fever of unknown origin, even in the most remote areas.
INTRODUCTION
The global reduction in the incidence of malaria in endemic countries of sub-Saharan Africa raises the challenge of identifying nonmalarial acute undifferentiated febrile illnesses (AUFIs).1–3 In Senegal, nonmalarial AUFIs, which include diseases vectored by arthropods, represent a very large proportion of the reasons for people presenting with febrile syndromes in rural areas.4–6 Plasmodium falciparum malaria is a major public health problem and an endemic disease in Senegal. It has long remained a major cause of morbidity and mortality.2,7,8 Malaria and tick-borne relapsing fever (TBRF), coendemic diseases in Senegal, have nearly identical clinical symptoms.8,9 In the absence of clinical signs of another disease, malaria is the first suspected cause of fever. Most patients are treated with antimalarials regardless of whether malaria parasites have been observed.1,8 As a result, this has resulted in ineffective treatment of many patients infected by TBRF with an unclear diagnosis and often considered as resistant to malaria drugs.10,11
TBRF, the second-most common identified cause of arthropod-borne febrile illness after malaria in Senegal, is very prominent among AUFIs, where P. falciparum trophozoite and Borrelia spirochete coinfections are rarely reported.4,6,8,12 The prevalence of malaria has drastically decreased in recent years in Senegal and has even disappeared in some areas such as the village of Dielmo, which was previously a malaria hyperendemic area.2,13 This has given way to TBRF, which has become the main reason patients consult a health professional with a febrile illness.2,4,7,14,15 This decrease in the incidence of malaria is the result of combined approaches including the intensification of vector control interventions, the use of insecticide-treated nets, the early detection of patients through the use of malaria rapid diagnostic tests (RDTs), and the implementation of effective antimalarial treatments.16,17 However, studies have shown that the prevalence of TBRF is higher than malaria in some places in Senegal.6,7,18
P. falciparum RDTs are the main form of medical diagnosis used for diagnosing malaria in underdeveloped countries. RDTs have become essential tools for rapidly detecting and managing malaria cases.19 Several studies have demonstrated that RDTs are a reliable source of parasite DNA for the molecular detection of Plasmodium, which has led to a better characterization of circulating Plasmodium species and reevaluation of malaria epidemiology.20–24 However, no studies have been conducted to determine whether it is practically feasible to use malaria RDTs as a reliable source of bacterial DNA for the investigation of AUFIs or neglected arthropod-borne tropical diseases.
In the Fatick region of Senegal, TBRF currently appears to be the leading cause of morbidity in the preelimination stage of malaria.2,5 As with malaria, TBRF registers high prevalence between July and September in the Niakhar and Dielmo health facilities. The peak of TBRF occurrence is observed in August in Senegal and Mauritania.2,6,25–27 Borrelia crocidurae has recently been reported as a probable cause of spontaneous miscarriage in Senegal.28 Studies on the causes of fever in rural Senegal have shown that B. crocidurae may be responsible for many cases of febrile illnesses.1,4,6,14,29 Results from the Dielmo point-of-care (POC) facility showed that, after malaria and flu, TBRF was the leading cause of consultation for febrile syndromes, with a prevalence of 9.5% in 2012.1,4
Our study aims to detect bacterial DNA from circulating arthropod-borne diseases in febrile patients attending health facilities in Senegal from RDTs that are negative for P. falciparum.
MATERIALS AND METHODS
Study design, timing, and location.
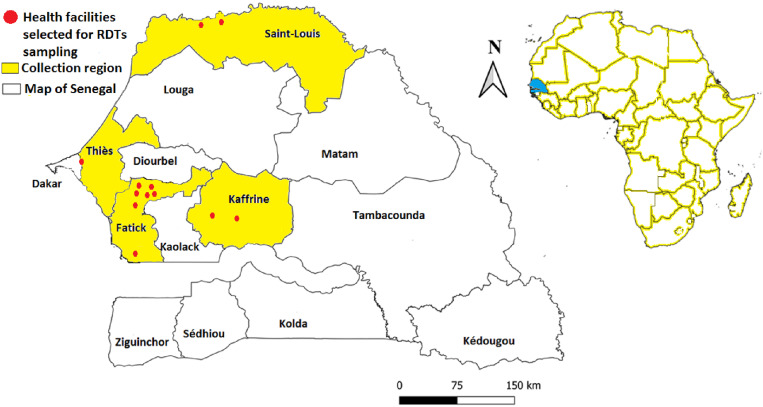
This retrospective study was conducted in 12 health facilities in four regions (between January and December 2019) from Senegal (Figure 1). The selected health facilities were seven in the Fatick region, two in the Kaffrine region, two in the Saint-Louis region, and one in the Thiès region (Table 1). RDTs negative for P. falciparum were obtained from patients seen in consultations in these health facilities. Climate is typically Sahelo-Saharian in the north, Sahelian in the center, and Sahelo-Sudanean and Sudano-Guinean in the south. It has two alternating seasons, a short rainy season variable according to region (June to mid-October) and a long dry season (mid-October to May), but is sometimes swept by the dry, dusty winds of the Harmattan (December to February).14,27 In the study areas, the incidence of fever rises during the winter season (rainy season), corresponding to the malaria peaks in Senegal. Authorization to use these RDTs negative for P. falciparum was granted by the authority of the National Malaria Control Program (PNLP) extended to the health facilities through the chief physicians of the health facilities under number 00001476.
Figure 1.
Geographic location of the 12 health facilities selected for the collection of malaria-negative RDTs P. falciparum in the communes of the Senegalese regions.
Table 1.
Sampling of malaria-negative RDTs P. falciparum in health facilities from four regions of Senegal between January and December 2019
| Region | Health facilities | Geographic coordinates | Quarterly collection 2019 | Collected P. falciparum RDTs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January–March | April–June | July–September | October–December | ||||||||||||
| Fatick | Nema-Nding | 13°42′N–16°24′W | 27 | 16 | 25 | 8 | 24 | 15 | 16 | 18 | 40 | 34 | 14 | 15 | 252 |
| Ngayokhem | 14°32′N–16°26′W | 33 | 39 | 40 | 28 | 24 | 59 | 45 | 15 | 135 | 66 | 37 | 36 | 557 | |
| Ndiambour | 14°33′N–16°20′W | 37 | 20 | 11 | 3 | – | 21 | 31 | 53 | 157 | 132 | 83 | 30 | 578 | |
| Patar Sine | 14°33′N–16°22′W | 15 | 10 | 10 | 15 | 16 | 6 | 16 | 63 | 46 | 55 | 55 | – | 307 | |
| Mbadatte | 14°32′N–16°21′W | – | – | – | – | – | – | – | – | 30 | 11 | 7 | 3 | 51 | |
| Sagne | 14°24′N–16°24′W | – | – | – | – | 9 | 15 | 30 | 23 | 62 | 85 | 33 | – | 257 | |
| Ndoss Diarraff | 14°27′N–16°21′W | 106 | 90 | 65 | 59 | – | 46 | 51 | 94 | 211 | 114 | 46 | 40 | 922 | |
| Thiès | Keur Moussa | 14°46′N–17°6′W | – | – | – | – | – | – | 17 | – | 253 | 220 | 105 | 36 | 631 |
| Kaffrine | Nguérane Fass | 14°14′N–14°50′W | – | – | – | – | – | – | – | 96 | 155 | 322 | 112 | – | 685 |
| Kathiotte | 14°6′N–14°33′W | – | – | – | – | – | – | – | 38 | 32 | 105 | 25 | 13 | 213 | |
| Saint-Louis | Taouey | 16°27′N–15°41′W | – | – | – | – | – | 3 | 25 | 40 | 242 | – | – | – | 310 |
| Gaaya | 16°15′N–15°53′W | – | – | – | – | – | – | – | 19 | 61 | 2 | – | – | 82 | |
| Total | 218 | 175 | 151 | 113 | 73 | 165 | 231 | 459 | 1,424 | 1,146 | 517 | 173 | 4,845 | ||
The eligibility criteria to collect these malaria-negative RDTs for P. falciparum (malaria Neg RDTs P.f) were as follows: all malaria RDTs for P. falciparum only performed on patients presenting at the above-mentioned health facilities with fever as the reason for consultation (axillary body temperature greater than 37.5°C) who had malaria Neg RDTs P.f results, with the initials of the collection site, date, name of patient, and/or allocation number of the health registry written on the results.
Testing ability to identify Borrelia DNA from malaria-negative RTDs P. falciparum.
For the first test, we used 10 malaria-positive RDTs P. falciparum with both antigenic bands visible (control and tests) and 10 malaria Neg RDTs P.f with only the control band being visible (SD Bioline Malaria Ag Pf 05FK50, Gyeonggi-do, Republic of Korea) to validate our protocol for the extraction and amplification of Plasmodium DNA fragments by quantitative polymerase chain reaction (qPCR) (Table 1, Supplemental Figure 1A). We used a mixture of 6 mL of human blood (group A+ containing anticoagulant from healthy donors obtained from the Etablissement Français du Sang under customer code 7831 VITROME) with 180 μL of the B. crocidurae strain cultivated in our laboratory. The bacterial load was estimated from a thick drop made with 5 μL of blood mixed with B. crocidurae by microscopic observation at 1,000× magnification in 200 visual fields (Supplemental Figure 1A). A total of 25 blank malaria RDTs P. falciparum (SD Bioline Malaria Ag Pf 05FK50, product 43813) were tested with the Borrelia blood mixture. As a second test control, 10 blank malaria RDTs P. falciparum were tested at the same time with unmixed insectarium blood. A 5-μL volume of blood was used for each malaria RDT P. falciparum and the antigenic test results were read after the recommended reading time, according to the manufacturer’s instructions. After at least 1 month of storage at room temperature, the three components of the malaria RDT strips (sample pad [S], nitrocellulose strip [N], and absorbent paper [F]) that come in contact with the blood sample during the detection process were evaluated separately as a specimen21,30 (Table 1, Supplemental Figure 1B).
The malaria RDTs were opened using dissecting forceps. The terminal absorbent paper strips and the sample pad were easily lifted from their underlying supports with the help of a pair of forceps and an individual sterile slide for each malaria RDT. In contrast, the nitrocellulose membrane, which was firmly adhered to the holder, was scraped off using a separate sterile scalpel for each malaria RDT (Table 1, Supplemental Figure 1B). The three parts of each malaria RDT constituting a sample were transferred to a 1.5-mL tube containing 180 μL of G2 lysis buffer and 20 μL of proteinase K (Qiagen, Hilden, Germany) and incubated at 37°C overnight. DNA extraction was performed using an EZ1 DNA tissue kit (Qiagen) according to the manufacturer’s recommendations. DNA from each sample was eluted with 100 μL of Tris-ethylenediaminetetraacetate buffer (Qiagen), monitored with a NanoDrop 1000, and stored at −20°C until further analysis. The DNA obtained from each of the three components of the malaria RDT strip was subjected to qPCR for the detection of actin to verify our extraction and also to qPCR for the detection of Borrelia DNA.6,31
Choice of fragments to be used to detect pathogen DNA in malaria-negative RDTs.
The malaria Neg RDTs P.f from the health facilities of each region were sorted in the VITROME-Dakar laboratory according to the criteria described below, grouped per collection month in individual plastic zipper bags for each month, and stored in polystyrene boxes. The polystyrene boxes were sent to the VITROME unit of Institut Hospitalo-Universitaire Méditerranée Infection (Marseille, France) to be stored at +4°C until further analysis. Each malaria Neg RDT P.f was processed individually by recording the information marked on it (health facility code, date, and/or number). The blood deposit pad (sample pad) and the nitrocellulose membrane of each malaria Neg RDT P.f were collected as described above as samples for molecular screening of microorganisms.
Subsequently, qPCR was performed on DNA extracted from the malaria Neg RDTs P.f targeting a wide range of fastidious bacteria (Coxiella burnetii, Rickettsia spp., Bartonella spp., Borrelia spp., and Anaplasma spp.). A control quantitative real-time PCR (qPCR) targeting β-actin was performed on some of our DNA samples obtained from the malaria Neg RDTs P.f.
Samples that were positive during the first qPCR targeting Borrelia spp. genera using the ITS4 gene were subjected to a second qPCR specific to B. crocidurae using the glpQ gene.32,33 Samples were considered positive only if the qPCR had a cycle threshold (Ct) below 36.31 To ensure the reliability of our results, we included DNA from cultured strains of Rickettsia montanensis, Bartonella elizabethae, Anaplasma phagocytophilum, C. burnetii, Borrelia burgdorferi, and B. crocidurae from our laboratory as positive controls and DNA from Rhipicephalus sanguineus ticks negative for the tested microorganisms as negative controls in each qPCR. The sequences of primers and probes used for the detection of microorganisms are listed in Supplemental Table 1.6,34,35 The qPCRs for each sample contained 5 µL of DNA template, 10 µL of Roche Master Mix (Eurogentec), 0.5 µL of UDG, 0.5 µL of each primer (forward and reverse), 0.5 µL of probe, and 3 µL of DNase/RNase-free distilled water for a total reaction volume of 20 µL. TaqMan cycling conditions included two hold steps at 50°C for 2 minutes, followed by 95°C for 15 minutes and 40 cycles of two steps each (95°C for 30 seconds and 60°C for 30 seconds).36
Standard PCR and sequencing.
Standard PCR was performed on qPCR samples that were positive for B. crocidurae using glpQ genes randomly selected for comparison with circulating B. crocidurae species in different hosts by sequencing the flaB (flagellin) gene from a 750-bp fragment.34,37,38 The PCR contained 5 μL of DNA extract, 12.5 μL of AmpliTaq Gold (ATG) DNA polymerases, 6 μL of DNase/RNase-free distilled water, and 0.75 μL of each primer. The reaction mixture was amplified with initial denaturation at 95°C for 15 min, followed by 39 cycles of 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute, and final heating at 72°C for 5 minutes. Successful amplification was confirmed by electrophoresis on a 1.5% agarose gel. Purification of PCR products was performed using NucleoFast 96 PCR plates (Macherey-Nagel, Hoerdt, France) according to the manufacturer’s instructions. Amplicons were sequenced using the BigDye Terminator v. 3.1 Cycle sequencing kit (Applied Biosystems, Foster City, CA).39 The obtained sequences were assembled and processed using ChromasPro software (ChromasPro v. 1.7, Technelysium, Tewantin, Australia).33,35 The corrected sequences were compared with the existing sequences in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). MEGA software v. 7.0.21 was used to perform our sequence alignments and to construct phylogenetic trees by neighbor-joining algorithms with 1,000 bootstrap replications.
Statistical analysis.
The results obtained were statistically tested using R v. 3.2.1 software to assess whether time period and/or locality influenced the detection of B. crocidurae by qPCR from RDTs negative for P. falciparum. The significance of the explanatory variables and their interactions was determined by log-likelihood ratio tests. The Pearson χ2 test was used to compare the proportions of Borrelia infection. Tests with 95% confidence intervals and a P value of < 0.05 were considered statistically significant.
RESULTS
Sampling results of malaria-negative RDTs P. falciparum.
A total of 4,845 malaria RDTs were collected, including 2,924 (60%) in Fatick, 898 (19%) in Kaffrine, 631 (13%) in Thiès, and 392 (8%) in Saint-Louis. Only in the health facilities in Nema-Nding and Ngayokhem were malaria Neg RDTs P.f collected throughout the year, namely from January to December. The numbers of malaria Neg RDTs P.f collected by month and by health facility are presented in Table 1. The largest numbers of malaria Neg RDTs P.f were collected in the four regions in September and October, accounting for 29.4% (1,424/4,845) and 23.7% (1,146/4,845) of malaria RDTs, respectively (Table 1).
Fragments chosen for pathogen DNA detection in malaria-negative RDTs.
A total of 20 field malaria RDTs P. falciparum (10 positive and 10 negative malaria RDT P. falciparum strips) were analyzed to confirm the feasibility of amplifying DNA fragments by Plasmodium qPCR. A total of 19/20 malaria RDT DNA samples were actin positive, with Ct values between 23 and 27 (mean 25.50). Plasmodium qPCR was positive only for malaria-positive RDT Plasmodium strips, with 70% (7/10) having a Ct between 31.39 and 34.92 (mean 33.29) (Supplemental Table 2, Supplemental Figure 1A).
In our experimental design, we used a total of 25 blank malaria RDTs tested with Borrelia-positive blood and 10 malaria RDTs with Borrelia-negative blood as controls (Supplemental Table 2, Supplemental Figure 1B). The bacterial density of the blood mixture with the B. crocidurae strain checked under a thick-film microscope had loads of 200–500 spirochetes per microscopic field (Supplemental Figure 1B). Extraction of the different parts of the malaria Neg RDTs P.f samples (blood deposit, nitrocellulose strip, and final absorbent paper) was successful after storage at room temperature for 1 month. DNA was detected in the three selected parts of the malaria RDTs by actin qPCR with Ct values ranging from 27.16 to 35.50 (mean 29.72). The Ct values for identification were low for all samples in the S- and N-positive parts, ranging from 28.42 to 32.20 Ct (mean 30.07) and 27.16 to 34.05 Ct (mean 29.61), respectively, for each part. The F part had 7/25 positive samples, with high Ct values ranging from 34.74 to 35.50 Ct (mean 35.10) (Supplemental Table 2, Supplemental Figure 1B). Laboratory tests for Borrelia identification in malaria Neg RDTs P.f showed that on all samples, the first two parts, S and N, had low Ct values ranging from 22.60 and 27.36 Ct (mean 25.35) to 22.34 and 26.08 Ct (mean 24.01), respectively. Considering the final part (F), we obtained 24/25 positive samples with high Ct values between 29.33 and 33.96 Ct (mean 32.20) (Supplemental Table 2, Supplemental Figure 1B). However, all 10 malaria RDTs that were tested with Borrelia-negative blood and used as a control remained negative. Thus, for the rest of our study on collected field samples, we used only the first two parts, namely the blood deposit part and the nitrocellulose strip, to search C. burnetii, Rickettsia spp., Bartonella spp., Borrelia spp., and bacteria of the Anaplasmataceae family.
Molecular detection of bacteria in malaria-negative RDTs P. falciparum.
The DNA extracted from 2,202 malaria Neg RDTs P.f representing 45.4% of malaria RDTs collected was submitted to qPCR for the detection of bacteria. Only Borrelia spp. DNA was detected in 7.2% (159) of malaria RDT samples using the ITS4 gene (Tables 2 and 3). The qPCR tests targeting C. burnetii, Rickettsia spp., Bartonella spp., and Anaplasma spp. were all negative. All samples that were positive for Borrelia spp. were identified as B. crocidurae by the specific qPCR using the GlpQ gene with Ct values between 27.66 and 35.85 (average 33.09, SD ±2.08).
Table 2.
Prevalence of TBRF Borrelia-positive cases identified in malaria-negative RDTs P. falciparum in health facilities from four regions of Senegal during the third quarter in 2019
| Region | Health facility | July | August | September | Positives/tested malaria RDTs | Total % region (positives/tested) | |||
|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive | Tested | Positive | Tested | Positive | ||||
| Fatick | Nema-Nding | 16 | 3 | 18 | 3 | 42 | 0 | 6/76 | 10.25 (99/966) |
| Ngayokhem | 45 | 8 | 15 | 2 | 124 | 8 | 18/184 | ||
| Patar Sine | 16 | 0 | 63 | 6 | 46 | 7 | 13/125 | ||
| Ndiambour | 31 | 6 | 49 | 5 | 80 | 3 | 14/160 | ||
| Mbadatte | – | – | – | – | 30 | 1 | 1/30 | ||
| Ndoss Diarraff | 81 | 11 | 85 | 11 | 110 | 3 | 25/276 | ||
| Sagne | 30 | 13 | 23 | 8 | 62 | 1 | 22/115 | ||
| Thiès | Keur Moussa | 17 | 1 | – | – | 162 | 2 | 3/179 | 1.7 (3/179) |
| Kaffrine | Nguérane Fass | – | – | 96 | 3 | 149 | 3 | 6/245 | 5.4 (17/316) |
| Kathiotte | – | – | 38 | 11 | 33 | 0 | 11/71 | ||
| Saint-Louis | Taouey | 25 | 1 | 40 | 0 | 101 | 0 | 1/166 | 2 (5/246) |
| Gaaya | – | – | 19 | 2 | 61 | 2 | 4/80 | ||
| Total % period (positives/tested) | 16.5 (43/261) | 11.43 (51/446) | 3 (30/1,000) | 7.26 (124/1,707) | |||||
Table 3.
Annual prevalence of TBRF Borrelia-positive cases identified in malaria-negative RDTs P. falciparum in two health facilities in the Fatick region, Senegal
| Health facility | RDTs P. falciparum | January/February/March | April/May/June | July/August/September | October/November/December | Total by health facility (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ngayokhem | Positives/tested by month | 3/26 | 0/19 | 2/36 | 3/28 | 4/24 | 4/59 | 8/45 | 2/15 | 8/124 | 6/63 | 4/37 | 3/36 | 47/512 (9.2) |
| Positives/tested (%) by quarter | 5/81 (6.2) | 11/111 (9.9) | 18/184 (9.8) | 13/136 (9.5) | ||||||||||
| Nema-Nding | Positives/tested by month | 0/24 | 0/16 | 2/25 | 1/8 | 2/24 | 0/15 | 3/16 | 3/18 | 0/42 | 0/27 | 1/13 | 0/15 | 12/243 (4.9) |
| Positives/tested (%) by quarter | 2/65 (3.1) | 3/47 (6.4) | 6/76 (7.9) | 1/55 (1.8) | ||||||||||
| Total by quarter | 7/146 (4.8) | 14/158 (8.9) | 24/260 (9.2) | 14/191 (7.3) | 59/755 (7.8) | |||||||||
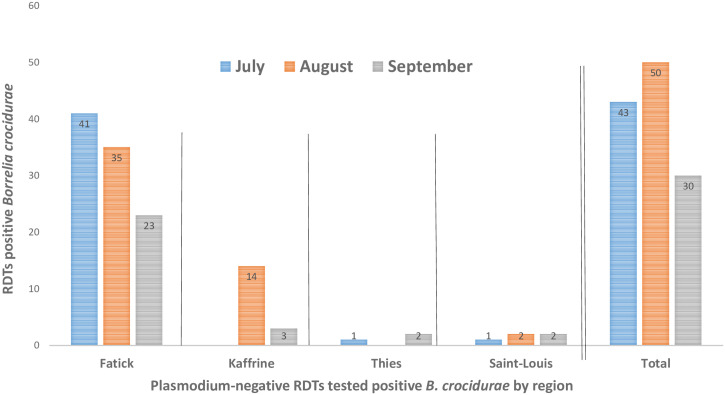
A total of 261, 446, and 1,000 malaria Neg RDTs P.f were tested during July, August, and September, respectively (Table 2). An overall B. crocidurae prevalence of 7.3% (124/1,707) was detected on RDTs, namely 16.5% in July, 11.4% in August, and 3% in September. The prevalence of B. crocidurae was significantly higher in July and August than September (Pearson χ2 test = 6.391; P = 0.041 < 0.05 [significance threshold]) (Table 2, Figure 2). The highest number of TBRF cases was obtained in August, when the DNA of 51 malaria Neg RDTs P.f was positive for B. crocidurae. Between July and August, the prevalence of DNA of Borrelia varied from region to region, with 16%, 10%, 6%, and 4% in Fatick, Kaffrine, Thiès, and Saint-Louis, respectively (Supplemental Figure 2). The most cases of TBRF were found in the Fatick region, with a prevalence of 10.3% (99/966) from July to September (Table 2, Figure 2). In the Fatick region, we recorded 18.7% (41/219), 13.8% (35/253), and 4.7% (23/494) of prevalence in July, August, and September, respectively (Table 2, Figure 2). In the Kaffrine region, a prevalence of 5.4% (17/316) was obtained over the 2 months of collection, 10.4% (14/134) in August and 1.7% (3/182) in September. In the Thiès region, a prevalence of 1.6% (3/179) was obtained over the 2 months of collection, 5.9% (1/17) in July and 1.2% (2/162) in September. In the Saint-Louis region, a prevalence of 2% (5/246) was obtained, 4% (1/25) in July only in the Taouey health facility and 3.4% (2/59) in August and 1.2% (2/162) in September (Table 2, Supplemental Figure 3).
Figure 2.
Distribution of Borrelia in malaria-negative RDTs P. falciparum from July to September in four Senegalese regions among febrile patients.
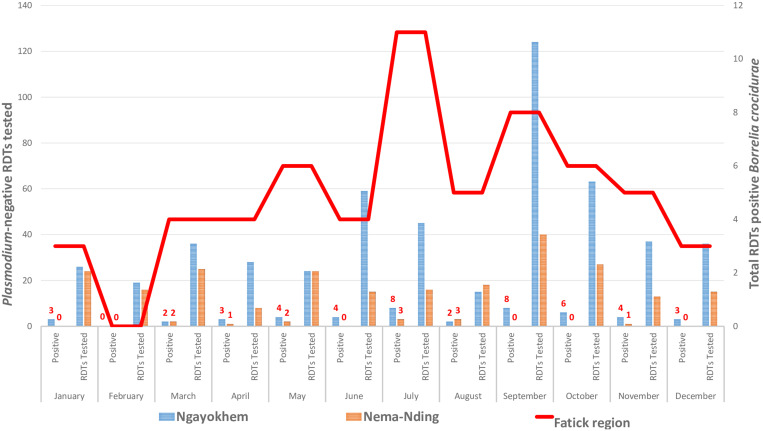
For the two health facilities in the Fatick region where annual collection was available, a total of 753 malaria Neg RDTs P.f were obtained from the health facilities in Ngayokhem (512 malaria Neg RDTs P.f) and Nema-Nding (241 RDTs) (Table 3). In the Fatick region, 7.8% (59/755) of annual prevalence of B. crocidurae was detected in malaria Neg RDTs P.f (Table 3, Figure 3). The prevalence of TBRF cases was 9.2% (47/512) in the health facility in Ngayokhem and 4.9% (12/243) in the health facility in Nema-Nding. The prevalence of B. crocidurae was significantly different between the two health facilities (Pearson χ2 test = 4.0037; P = 0.0454 < 0.05 [significance threshold]). In the Fatick region, analysis of the four quarters of the year shows a prevalence of 4.7% (7/146) between January and March, 8.9% (14/158) between April and June, 9.2% (24/260) between July and September, and 7.3% (14/191) between October and December. The prevalence of B. crocidurae was significantly different between the four quarters of the year (Pearson χ2 test = 0.48154; P = 0.041 < 0.05 [significance threshold]) (Table 3, Figure 3). The highest TBRF case numbers were obtained in the third quarter of the year, with 18 and 6 malaria Neg RDTs P.f being positive for B. crocidurae in the Ngayokhem and Nema-Nding health facilities, respectively (Table 3, Figure 3).
Figure 3.
Monthly distribution of Borrelia in malaria-negative RDTs P. falciparum in two health facilities in the Fatick region among febrile patients.
Borrelia standard PCR and phylogenetic analysis.
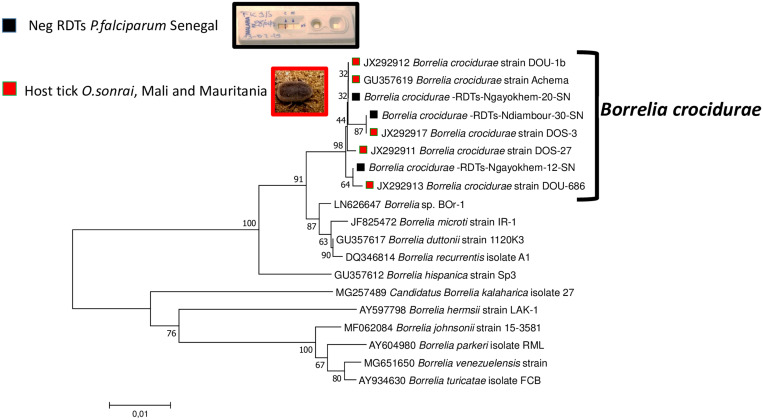
To compare the B. crocidurae DNA sequences amplified by qPCR from malaria RDTs in this study with some of the B. crocidurae sequences available at GenBank, 16 qPCR-positive samples with Ct values < 30 were subjected to standard PCR, amplifying fragments of the Borrelia flaB genes. We obtained 7/16 usable sequences with the flagellin flaB genes. The obtained sequences were 99.86–100% identical to the sequence of B. crocidurae (accession number JX292917) isolated from the blood of a laboratory mouse inoculated from an Ornithodoros sonrai tick from Mali. The two sequences with identity scores less than 100% have been deposited in the GenBank database with the accession numbers OP547439 and OP547440. The phylogenetic tree constructed with the B. crocidurae sequences of the flaB gene detected in this work was clustered with those obtained in ticks from Mali (JX292911–JX292913, JX292917) and Mauritania (GU357619) (Figure 4).
Figure 4.
Phylogenetic tree (neighbor-joining, bootstrap 1,000) of partial sequence data of the flagellin gene, FlaB (663 bp), of B. crocidurae identified from malaria-negative RDTs P. falciparum (black cube). Sequences of the B. crocidurae FlaB gene found in O. sonrai ticks (red cube) and sequences from other Borrelia species were processed for species comparison.
DISCUSSION
In this study of the causes of nonmalarial AUFIs, in the third quarter of the year, we recorded a relative high prevalence of 7% of B. crocidurae in malaria Neg RDTs P.f in Senegal, and showed a high prevalence of 16.5% in July and 11.4% in August of malaria Neg RDTs P.f that were positive for Borrelia with a low number of malaria Neg RDTs P.f tested. In contrast, in September, when a large number of malaria Neg RDTs P.f were tested, a low prevalence (3%) of Borrelia was recorded. The annual prevalence of 7.8% of DNA of Borrelia recorded from malaria Neg RDTs P.f tested in the Fatick region is close to that obtained by the homemade syndromic surveillance system called EPIMIC 2016 alarms.18 Analysis of malaria Neg RDTs P.f between January and December in the two health facilities in the Fatick region showed cases of TBRF occurring almost every month, with cases peaking between July and September, comparable to the results obtained by the Dielmo POC facility between 2011 and 2013.1 Our results perfectly follow the periodic fluctuations of human cases of borreliosis, which are highest between July and September, corresponding to the winter period. The month of September marks the beginning of the high prevalence of malaria in Senegal, which lasts until November.6,26,27 In Senegal, the months of July and August have been reported as periods of high prevalence of TBRF cases.6,40 These peak periods with a higher number of TBRF cases obtained in this study have been previously reported in Morocco and Senegal.6,26 We recorded a prevalence of 16% in the Fatick region, 10% in the Kaffrine, 6% in the Thiès. and 4% in that of the Saint-Louis (Supplemental Figure 2). This prevalence is comparable to that obtained by studies conducted in some of these different localities, namely in Thiès with a prevalence of 9% in children under the age of 14 years, in the Fatick region with a prevalence of 13% in 2008 and 10% in 2011, 19% in Sine-Saloum in 2011, 12% in 2017 in Niakhar, and 5% in the Louga region.4,6,14,41
In rural areas of underdeveloped countries, malaria Neg RDTs P.f are most often the only medical diagnostic tool for vector-borne diseases.42 We validated our methodologies by taking solid references allowing us to confirm the possibility of detecting arthropod vectorized diseases from bacterial DNA contained in malaria Neg RDTs P.f from the field.21,23,24,30 This study is the first to demonstrate that it is possible to search for neglected tropical diseases or circulating pathologies in malaria RDTs independent of the specific search for Plasmodium parasites. Our study allowed us to assess, for the first time, the prevalence of DNA of B. crocidurae agent of TBRF, a disease coendemic with malaria in Senegal, using malaria Neg RDTs P.f.
In Senegal, most studies have shown a high prevalence and continuous annual transmission of B. crocidurae.5,6,40 Malaria Neg RDTs P.f from the field, which we consider a robust source of clinical samples for the causes of nonmalarial fever, could allow us to provide answers as to the distribution, circulation, and prevalence of TBRF B. crocidurae among febrile patients attending health facilities. Limitations of our study include lack of knowledge of patient demographics (age and sex) and clinical data (in addition to fever). In this study, we were not able to evaluate cases of coinfection with TBRF and malaria because of the small number of malaria-positive RDTs P. falciparum obtained in the field and in some localities. The Senegalese Ministry of Health uses these malaria-positive RDTs P. falciparum for confirmation and monitoring Plasmodium parasites circulating in the context of control strategies for malaria elimination.
B. crocidurae bacteria detected from our malaria Neg RDTs P.f DNA samples, confirmed by standard PCR, have already been identified in O. sonrai ticks in Senegal, Mauritania, and Mali.43,44 The phylogenetic analysis of the FlaB gene allowed us to show that our Borrelia obtained was close to the Borrelia already found in Senegal.44,45 The results presented in this study confirm the single circulation of the B. crocidurae pathogen causing TBRF in febrile patients in Senegal. Given the interesting results obtained in these Borrelia endemic areas, it is important to note the potential limitations of our approach. Not all pathogens causing febrile illnesses will be present in the blood in sufficiently high and thus detectable numbers. Considering that a large proportion of the samples tested were negative for the microorganisms we were looking for, there are many other causes of fever to be found in Senegal.
In Senegal, reported data on TBRF cases are low and most of the available data come from the two POC laboratories installed in Niakhar and Dielmo in the Fatick region.1,4,6,18,46 This study is the first to use an innovative approach to assess the prevalence of TBRF in febrile patients attending health facilities based on malaria Neg RDTs P.f from different regions. Our results confirm that TBRF transmission occurs all year long. However, peak periods for human cases are noted from July to September in Senegal and Mauritania.6,26 This study demonstrates, once more, the need to develop TBRF RDTs that are specific to B. crocidurae, and then to make them available to health facilities in rural areas for better management of this endemic zoonotic infection. The development of this promising tool could shed light on unclear diagnoses, improve patient management, and help health care workers choose the right treatment of the best management of TBRF cases.
Financial Disclosure
This study was supported by the Institut Hospitalo-Universitaire Méditerranée Infection, the French National Research Agency under the “Investissements d’avenir” program, reference ANR-10-IAHU-03, the Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMMI (Fonds Européen de Développement Régional-Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection).
Supplemental Materials
ACKNOWLEDGMENTS
We thank the coordinator, Dr. Doudou Sene, and his assistant, Dr. Ibrahima Diallo, from the National Malaria Control Program (PNLP) for allowing us to collect malaria-negative RDTs P.f from health facilities, and the chief physicians of the health facilities of Niakhar and Sokone (in Fatick), Kaffrine and Koungheul (in Kaffrine), Pout (in Thiès), and Richard-Toll and Dagana (in Saint-Louis) for their involvement. We are grateful to the head nurses of each health facility targeted in this study for their participation in the collection and conservation of malaria-negative RDTs P. falciparum in the field.
Note: Supplemental materials appear at www.ajtmh.org.
References
- 1. Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape J-F, Drancourt M, Raoult D, 2013. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis 7: e1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wotodjo AN, Doucoure S, Gaudart J, Diagne N, Diene Sarr F, Faye N, Tall A, Raoult D, Sokhna C, 2017. Malaria in Dielmo, a Senegal village: is its elimination possible after seven years of implementation of long-lasting insecticide-treated nets? PLoS One 12: e0179528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhargava A, Ralph R, Chatterjee B, Bottieau E, 2018. Assessment and initial management of acute undifferentiated fever in tropical and subtropical regions. BMJ 363: k4766. [DOI] [PubMed] [Google Scholar]
- 4. Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Trape JF, Raoult D, 2011. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis 17: 883–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diatta G. et al. , 2016. An alternative strategy of preventive control of tick-borne relapsing fever in rural areas of Sine-Saloum, Senegal. Am J Trop Med Hyg 95: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ndiaye EHI, Diouf FS, Ndiaye M, Bassene H, Raoult D, Sokhna C, Parola P, Diatta G, 2021. Tick-borne relapsing fever borreliosis, a major public health problem overlooked in Senegal. PLoS Negl Trop Dis 15: e0009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trape J-F. et al. , 2014. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis 14: 476–488. [DOI] [PubMed] [Google Scholar]
- 8. Diallo MA, Kane BS, Ndiaye M, Dieng M, Diongue K, Badiane AS, Seck MC, Ndiaye D, 2017. Plasmodium falciparum malaria co-infection with tick-borne relapsing fever in Dakar. Malar J 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hovette P, Aubron C, Perrier-Gros-Claude JD, Schieman R, N’Dir MC, Camara P, 2001. Value of quantitative buffy coat (QBC) in borreliasis-malaria co-infection. Med Trop (Mars) 61: 196–197. [PubMed] [Google Scholar]
- 10. Flatau E, Reichman N, Elias M, Raz R, 2000. Malaria and Borrelia co-infection. J Travel Med 7: 98–99. [DOI] [PubMed] [Google Scholar]
- 11. Nordstrand A, Bunikis I, Larsson C, Tsogbe K, Schwan TG, Nilsson M, Bergström S, 2007. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg Infect Dis 13: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trape J-F. et al. , 2013. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS One 8: e78473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wotodjo AN, Doucoure S, Diagne N, Sarr FD, Parola P, Gaudart J, Sokhna C, 2021. The impact of renewing long-lasting insecticide-treated nets in the event of malaria resurgence: lessons from 10 years of net use in Dielmo, Senegal. Am J Trop Med Hyg 104: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mediannikov O, Socolovschi C, Bassene H, Diatta G, Ratmanov P, Fenollar F, Sokhna C, Raoult D, 2014. Borrelia crocidurae infection in acutely febrile patients, Senegal. Emerg Infect Dis 20: 1335–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Malaria Control Program (PNLP) , 2021. PLAN STRATEGIQUE NATIONAL DE LUTTE CONTRE LE PALUDISME AU SENEGAL 2021–2025. Available at: https://senegal-cocreation.com/wp-content/uploads/2021/02/PSN_PNLP_Senegal_Version-finale_-Fevrier-2021.pdf. Accessed January 25, 2023.
- 16.National Malaria Control Program (PNLP) , 2015. PLAN STRATEGIQUE NATIONAL 2011–2015. Available at: https://pspdb.dev.gouv.bj/server/storage/app/PolitiqueFichiers/57_Plan_strategique_2011_2015.pdf. Accessed January 25, 2023.
- 17. Thwing J. et al. , 2017. Declines in malaria burden and all-cause child mortality following increases in control interventions in Senegal, 2005–2010. Am J Trop Med Hyg 97: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abat C, Colson P, Chaudet H, Rolain J-M, Bassene H, Diallo A, Mediannikov O, Fenollar F, Raoult D, Sokhna C, 2016. Implementation of syndromic surveillance systems in two rural villages in Senegal. PLoS Negl Trop Dis 10: e0005212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis KM, Gibson LE, Haselton FR, Wright DW, 2014. Simple sample processing enhances malaria rapid diagnostic test performance. Analyst (Lond) 139: 3026–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veron V, Carme B, 2006. Recovery and use of Plasmodium DNA from malaria rapid diagnostic tests. Am J Trop Med Hyg 74: 941–943. [PubMed] [Google Scholar]
- 21. Cnops L, Boderie M, Gillet P, Van Esbroeck M, Jacobs J, 2011. Rapid diagnostic tests as a source of DNA for Plasmodium species-specific real-time PCR. Malar J 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishengoma DS, Lwitiho S, Madebe RA, Nyagonde N, Persson O, Vestergaard LS, Bygbjerg IC, Lemnge MM, Alifrangis M, 2011. Using rapid diagnostic tests as source of malaria parasite DNA for molecular analyses in the era of declining malaria prevalence. Malar J 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris U. et al. , 2013. Rapid diagnostic tests for molecular surveillance of Plasmodium falciparum malaria—assessment of DNA extraction methods and field applicability. Malar J 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srisutham S, Suwannasin K, Mathema VB, Sriprawat K, Smithuis FM, Nosten F, White NJ, Dondorp AM, Imwong M, 2020. Utility of Plasmodium falciparum DNA from rapid diagnostic test kits for molecular analysis and whole genome amplification. Malar J 19: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diatta G, Vial L, Niang O, Bouganali C, Trape J-F, 2005. Enquête sur la borréliose à tiques à Borrelia crocidurae au Sénégal. Available at: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/num-dakar-02/010039439.pdf. Accessed January 27, 2023.
- 26. Diatta G. et al. , 2012. Epidemiology of tick-borne borreliosis in Morocco. PLoS Negl Trop Dis 6: e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diouf I, Rodriguez Fonseca B, Caminade C, Thiaw WM, Deme A, Morse AP, Ndione J-A, Gaye AT, Diaw A, Ndiaye MKN, 2020. Climate variability and malaria over West Africa. Am J Trop Med Hyg 102: 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fall NS, Diagne N, Mediannikov O, Fenollar F, Parola P, Sokhna C, Raoult D, Lagier J-C, 2020. Detection of Borrelia crocidurae in a vaginal swab after miscarriage, rural Senegal, western Africa. Int J Infect Dis 91: 261–263. [DOI] [PubMed] [Google Scholar]
- 29. Parola P, Raoult D, 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 32: 897–928. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland C, Ropper C, Raman J, Naidoo I, Ishengoma D, Alifrangis M, Cnops L, Jacobs J , 2011. Preparation of rapid diagnostic tests (RDTs) for DNA extraction v1.1. Molecular Module, WorldWide Antimalarial Resistance Network, Procedure MOL06. Available at: https://www.wwarn.org/sites/default/files/MOL06_RDTsForDNAExtraction.pdf. Accessed January 25, 2023.
- 31. Elbir H. et al. , 2013. Multiplex real-time PCR diagnostic of relapsing fevers in Africa. PLoS Negl Trop Dis 7: e2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Konkel ME, 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol 34: 2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ndiaye EHI, Diatta G, Diarra AZ, Berenger JM, Bassene H, Mediannikov O, Bouganali C, Sokhna C, Parola P, 2022. Morphological, molecular and MALDI-TOF MS identification of bedbugs and associated Wolbachia species in rural Senegal. J Med Entomol 59: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 34. Assous MV, Wilamowski A, Bercovier H, Marva E, 2006. Molecular characterization of tickborne relapsing fever Borrelia, Israel. Emerg Infect Dis 12: 1740–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diarra AZ, Almeras L, Laroche M, Berenger J-M, Koné AK, Bocoum Z, Dabo A, Doumbo O, Raoult D, Parola P, 2017. Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl Trop Dis 11: e0005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Medkour H. et al. , 2021. Potential zoonotic pathogens hosted by endangered bonobos. Sci Rep 11: 6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toledo A, Anda P, Escudero R, Larsson C, Bergstrom S, Benach JL, 2010. Phylogenetic analysis of a virulent Borrelia species isolated from patients with relapsing fever. J Clin Microbiol 48: 2484–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diarra AZ. et al. , 2020. Molecular detection of microorganisms associated with small mammals and their ectoparasites in Mali. Am J Trop Med Hyg 103: 2542–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahal M, Medkour H, Diarra AZ, Bitam I, Parola P, Mediannikov O, 2020. Molecular identification and evaluation of Coxiella-like endosymbionts genetic diversity carried by cattle ticks in Algeria. Ticks Tick Borne Dis 11: 101493. [DOI] [PubMed] [Google Scholar]
- 40. Vial L, Diatta G, Tall A, Hadj Ba E, Bouganali H, Durand P, Sokhna C, Rogier C, Renaud F, Trape J-F, 2006. Incidence of tick-borne relapsing fever in West Africa: longitudinal study. Lancet 368: 37–43. [DOI] [PubMed] [Google Scholar]
- 41. Lecompte Y, Trape JF, 2003. West African tick-borne relapsing fever. Ann Biol Clin (Paris) 61: 541–548. [PubMed] [Google Scholar]
- 42. Bouzid D, Zanella M-C, Kerneis S, Visseaux B, May L, Schrenzel J, Cattoir V, 2021. Rapid diagnostic tests for infectious diseases in the emergency department. Clin Microbiol Infect 27: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elbir H, FotsoFotso A, Diatta G, Trape JF, Arnathau C, Renaud F, Durand P, 2015. Ubiquitous bacteria Borrelia crocidurae in western African ticks Ornithodoros sonrai . Parasit Vectors 8: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fotso Fotso A, Mediannikov O, Nappez C, Azza S, Raoult D, Drancourt M, 2016. Monoclonal antibodies for the diagnosis of Borrelia crocidurae . Am J Trop Med Hyg 94: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, McCoy BN, Safronetz D, Sogoba N, Maïga O, Traoré SF, 2012. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Negl Trop Dis 6: e1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mediannikov O, Socolovschi C, Bassene H, Diatta G, Ratmanov P, Fenollar F, Sokhna C, Raoult D, 2014. High incidence of Borrelia crocidurae in acute febrile patients in Senegal. Int J Infect Dis 21: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.