ABSTRACT.
We conducted a case-control study to identify risk and protective factors during a cholera outbreak in Jijiga, Ethiopia, in June 2017. A case-patient was defined as anyone > 5 years old with at least three loose stools in 24 hours who was admitted to a cholera treatment center in Jijiga on or after June 16, 2017. Two controls were matched to each case by type of residency (rural or urban) and age group. We enrolled 55 case-patients and 102 controls from June 16 to June 23, 2017. Identified risk factors for cholera were male sex, eating cold food, and eating food outside the home. Eating hot food was protective, as was reported handwashing after defecation; no other reported water, sanitation, and hygiene factors were associated with cholera risk. Recommendations included continuing messaging about safe food handling practices at home, the dangers of consuming meals prepared away from home, and the importance of hand hygiene practices.
Cholera presents with acute watery diarrhea (AWD) and is caused by toxigenic Vibrio cholerae, serogroup O1 or O139, transmitted through the fecal-oral route.1 The WHO estimates that each year cholera causes 1.4–4.3 million illnesses and 30,000–140,000 deaths globally.2 From January 1 to July 10, 2017, 33,993 cases of AWD were reported in the Somali Region of Ethiopia, including 1,000 cases in Jijiga, the regional capital. Neighboring Somalia (75,414 cases; 1,007 deaths) and Kenya (5,866 cases; 80 deaths) reported large cholera outbreaks during 2017.3
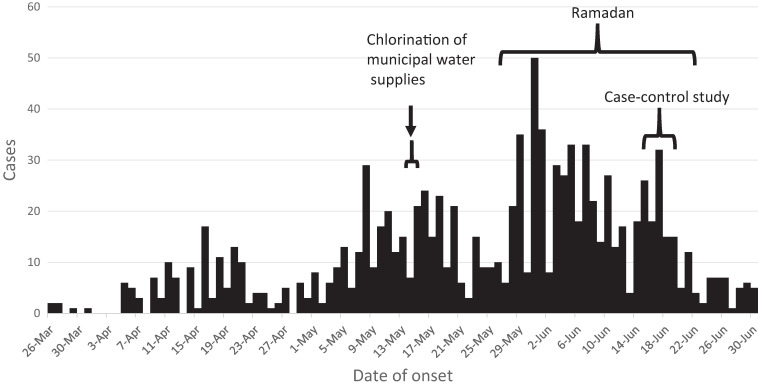
The Somali Regional Health Bureau (RHB) declared an outbreak in January. From March 26 to June 16, RHB reported 926 cases in Jijiga; the epidemic curve showed a gradual continued decrease after a city-wide intervention of bulk chlorination of city reservoirs and filling stations for water delivery carts and trucks,4 followed by an increase after Ramadan began in late May (Figure 1).
Figure 1.
Cholera cases in Jijiga, Ethiopia, March 26 through June 30, 2017.
Despite the intense water chlorination activities,4 cases continued to increase. In June, the RHB, WHO, and CDC implemented a case-control study to identify risks for cholera. A case was defined as AWD (at least three loose stools in 24 hours) in a patient > 5 years old admitted to a cholera treatment center (CTC) in Jijiga on or after June 16, 2017. Cases and controls were ineligible if anyone in their household had cholera since January 1, 2017. Cases must have lived in the same household in Jijiga for at least 5 days before becoming ill; controls must have lived in the same household for 7 days before the interview. Surveyors interviewed cases in the CTC and then visited the case’s home and observed water, sanitation, and hygiene (WASH) conditions.
Two controls were matched to each case by age group (6–14 years, 15–44 years, and 45 years or older) and urban/rural residency type (areas in which houses shared a common wall were considered urban). Surveyors identified controls by numbering the houses from 1 to 10 to the right and to the left along the street in front of the case’s house and selecting two houses using a random number list. If no eligible person was present, surveyors went to the next house.
Questionnaires covered demographics, water sources and storage, water treatment practices, home hygiene practices, and foods eaten in the previous 5 days. At the time of the outbreak, cholera was called “acute watery diarrhea”; all survey questions about onset, health communications, and contact with cases used this term. Surveyors tested the free chlorine residual (FCR) levels of stored drinking water in the homes of the participants using chlorine color disc test kits (Hach, Loveland, CO). Turbidity was measured subjectively.
Data were collected on smartphones using Epi Info 7 (CDC, Atlanta, GA) and analyzed with SAS v9.4 (SAS, Cary, NC). To explore factors associated with cholera, multivariable logistic regression models were fit by treating case status as a dependent variable and a factor as an independent variable, controlling for age group and residency type. Adjusted odds ratios (ORs) of cholera and 95% CIs were calculated. The institutional review boards at CDC, RHB, and the Ethiopian Federal Ministry of Health determined that this research was public health practice and exempt from review. Oral consents were obtained from adult participants; for children 5–13 years old, a parent or a guardian was interviewed, with oral assent from the child.
Surveyors enrolled 55 cases and 102 controls from June 16 to June 23, 2017. Of these, 15 cases (27%) and 15 controls (15%) who reported living with someone who took oral rehydration therapy (ORS) for AWD in 2017 were excluded from the analysis; if a family member recently had cholera, it is possible that the case/control had a previous asymptomatic infection and some immunity to cholera and would bias the results. Finally, 40 cases and 87 controls were included in the analysis.
Most cases (57%) presented at the CTC on the day that symptoms began; 35% presented the following day. All 40 cases were assessed at the CTC and assigned one of three treatment plans based on WHO definitions for the severity of dehydration5,6: No case lacked signs of dehydration (treatment plan A), 27 (68%) cases had signs of some dehydration (plan B), and 13 (33%) had signs of severe dehydration (plan C). Three cases were given rapid diagnostic tests for cholera (Crystal VC, Span Diagnostics, Surat, India), and all tested positive.
Of all cases and controls, 27 (21%) were 6–14 years old, 86 (68%) were 15–44 years old, and 14 (11%) were ≥ 45 years old; 65% lived in rural areas. A majority of respondents were female (78%) and had not completed primary school (70%). One-third (33%) of cases and 17% of controls were male; male sex was associated with having been a cholera case (OR = 3.6, 95% CI = 1.3–9.5).
Cases and controls had similar WASH conditions in their homes (Table 1). Overall, 31 (27%) participants reported their primary drinking water source as unimproved (biyole [donkey cart with a small water tank], birka [pond], truck, surface, dam, bought on the street, other), and 63% lived in kebeles (wards) served by city reservoirs (Table 2). Thirty-two (26%) respondents reported treating their drinking water, and surveyors measured FCR > 0.2 mg/L in household stored water of 52 (43%) participants. Surveyors observed water for handwashing within 3 m of a latrine or a special container for carrying water to the latrine in 22 (18%) respondents’ homes, observed soap within 3 m of a latrine in 12 (10%) respondents’ homes, and observed handwashing soap anywhere in the house in 44 (36%) of respondents’ homes. Participants reporting handwashing after defecation (OR = 0.4, 95% CI = 0.2–0.8) were more likely to be controls than cases. No other WASH variable, including type of water source and storage, reporting treating water, soap observed in the house, and type of latrine, was associated with being a case (all P > 0.05).
Table 1.
Water, sanitation, and hygiene (WASH) characteristics of cholera cases and controls, and odds ratio (OR) of cholera, Ethiopia, 2017
| WASH questions | All (N = 127) | Cases (N = 40) | Controls (N = 87) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| n* (%) | n* (%) | n* (%) | |||
| Reported primary drinking water source at home† | |||||
| Unimproved | 31 (27) | 9 (26) | 22 (27) | 0.9 (0.3–2.2) | 0.75 |
| Improved | 85 (73) | 26 (74) | 59 (73) | Ref | |
| Respondent lives in a section of Jijiga supplied by a municipal reservoir | |||||
| Yes | 80 (63) | 24 (60) | 56 (64) | 0.9 (0.4–2.0) | 0.76 |
| No | 47 (37) | 16 (40) | 31 (36) | Ref | |
| Reported drinking water source† at work/school | |||||
| Unimproved | 77 (77) | 26 (87) | 51 (73) | 2.4 (0.7–7.9) | 0.15 |
| Improved | 23 (23) | 4 (13) | 19 (27) | Ref | |
| Reported drinking untreated water in the past 5 days | |||||
| Yes | 44 (35) | 13 (33) | 31 (36) | 0.9 (0.4–1.9) | 0.73 |
| No | 82 (65) | 26 (67) | 56 (64) | Ref | |
| Have a water tap at home | |||||
| Yes | 27 (25) | 10 (30) | 17 (22) | 1.6 (0.6–4.3) | 0.32 |
| No | 83 (75) | 23 (70) | 60 (78) | Ref | |
| Reported treating drinking water in past 5 days | |||||
| Yes | 32 (26) | 10 (25) | 22 (26) | 0.9 (0.4–2.1) | 0.78 |
| No | 93 (74) | 30 (75) | 63 (74) | Ref | |
| Free chlorine > 0.2 mg/L in stored water | |||||
| Yes | 52 (43) | 18 (49) | 34 (41) | Ref | |
| No | 68 (57) | 19 (51) | 49 (59) | 1.1 (0.5–2.5) | 0.73 |
| Water storage container at home (observed)‡ | |||||
| Unimproved | 13 (11) | 2 (6) | 11 (13) | 0.4 (0.1–2.0) | 0.26 |
| Improved | 102 (89) | 30 (94) | 72 (87) | Ref | |
| A child's hand can fit inside the opening of the water storage container (observed) | |||||
| Yes | 51 (41) | 16 (43) | 35 (40) | 1.2 (0.5–2.7) | 0.64 |
| No | 73 (59) | 21 (57) | 52 (60) | Ref | |
| Turbidity of stored water (observed by surveyor) | |||||
| Cloudy | 4 (3) | 3 (9) | 1 (1) | 9.8 (0.9–106.1) | 0.06 |
| Clear | 117 (97) | 32 (91) | 85 (99) | Ref | |
| Handwashing water or a special jerrycan for carrying water to latrine observed < 3 m of latrine | 22 (18) | 4 (11) | 18 (21) | 0.4 (0.1–1.8) | 0.17 |
| Soap observed < 3 m of latrine | 12 (10) | 1 (3) | 11 (13) | 0.2 (0.02–1.4) | 0.10 |
| Handwashing soap observed anywhere in the house | 44 (36) | 13 (36) | 31 (36) | 1.0 (0.4–2.2) | 0.91 |
| Reported handwashing practices | |||||
| Before eating | 103 (81) | 30 (75) | 73 (84) | 0.5 (0.2–1.4) | 0.19 |
| After eating | 69 (54) | 19 (47) | 50 (57) | 0.6 (0.3–1.3) | 0.24 |
| After defecating | 77 (61) | 18 (45) | 59 (68) | 0.4 (0.2–0.8) | 0.01 |
| After cleaning up baby’s defecation | 39 (31) | 11 (28) | 28 (32) | 0.7 (0.3–1.6) | 0.42 |
| Before religious gathering | 6 (5) | 2 (5) | 4 (5) | 1.1 (0.2–6.6) | 0.90 |
| Reported any handwashing (any one of the above) | 122 (96) | 37 (93) | 85 (98) | 0.2 (0.03–1.3) | 0.08 |
| Where do you defecate? | |||||
| Flush toilet | 2 (2) | 1 (3) | 1 (1) | 2.0 (0.1–33.6) | 0.65 |
| Pit latrine | 117 (92) | 35 (88) | 82 (94) | 0.4 (0.1–1.4) | 0.15 |
| Open defecation | 5 (4) | 1 (3) | 4 (5) | 0.6 (0.1–5.5) | 0.63 |
| Other | 2 (2) | 1 (3) | 1 (1) | 2.1 (0.1–34.3) | 0.61 |
| At work/school | |||||
| Have water close to latrine | 66 (52) | 20 (57) | 46 (58) | 1.0 (0.5–2.3) | 0.97 |
| Have handwashing soap | 34 (30) | 13 (37) | 21 (27) | 1.5 (0.6–3.2) | 0.36 |
| Neighbor | |||||
| Have any of neighbors with AWD in the past week | 43 (35) | 9 (24) | 34 (39) | 0.5 (0.2–1.3) | 0.14 |
| Have anyone sharing a latrine have AWD in the last 3 months | 3 (2) | 2 (6) | 1 (1) | 5.0 (0.4–60.5) | 0.20 |
AWD = acute watery diarrhea; OR = odds ratio; WASH = water, sanitation, and hygiene.
Bold indicates statistically significant at 0.05
Values for N do not sum to total due to data missing for some variables.
Improved: borehole/well/any pipe; Unimproved: any biyole/birka/truck/surface/dam/street/other.
Improved: tap stand, bucket with a tap, jerrican, barrel, roto; Unimproved: bucket with no lid, other.
Table 2.
Foods eaten in the past 5 days among cholera cases and controls, and odds ratio (OR) of cholera, Ethiopia, 2017
| Food eaten at home | All (N = 127) | Cases (N = 40) | Controls (N = 87) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Hot rice | 107 (84) | 30 (75) | 77 (89) | 0.4 (0.2–1.1) | 0.07 |
| Cold or leftover rice | 47 (37) | 18 (45) | 29 (33) | 1.5 (0.7–3.3) | 0.29 |
| Hot macaroni | 87 (69) | 21 (54) | 66 (76) | 0.4 (0.2–0.9) | 0.02 |
| Cold or leftover macaroni | 29 (23) | 11 (28) | 18 (21) | 1.6 (0.7–3.8) | 0.30 |
| Hot pasta | 109 (86) | 30 (77) | 79 (91) | 0.3 (0.1–0.9) | 0.04 |
| Cold or leftover pasta | 36 (29) | 11 (28) | 25 (29) | 0.9 (0.4–2.2) | 0.88 |
| Hot injera | 99 (78) | 27 (68) | 72 (83) | 0.4 (0.2–1.0) | 0.06 |
| Cold or leftover injera | 45 (36) | 13 (33) | 32 (37) | 0.8 (0.4–1.9) | 0.67 |
| Hot shuro* | 16 (13) | 5 (13) | 11 (13) | 1.2 (0.4–3.7) | 0.79 |
| Cold or leftover shuro | 12 (10) | 6 (16) | 6 (7) | 2.9 (0.8–9.9) | 0.09 |
| Hot sorghum | 47 (38) | 16 (42) | 31 (36) | 1.3 (0.6–3.0) | 0.48 |
| Cold or leftover sorghum | 24 (19) | 10 (26) | 14 (16) | 1.8 (0.7–4.9) | 0.24 |
| Hot sambusa | 64 (51) | 17 (44) | 47 (54) | 0.7 (0.3–1.5) | 0.34 |
| Cold or leftover sambusa | 46 (36) | 17 (43) | 29 (33) | 1.6 (0.7–3.5) | 0.26 |
| Hot meat | 49 (39) | 11 (28) | 38 (44) | 0.5 (0.2–1.2) | 0.13 |
| Cold or leftover meat | 18 (14) | 4 (11) | 14 (16) | 0.6 (0.2–2.1) | 0.44 |
| Hot cooked vegetables | 28 (23) | 10 (27) | 18 (21) | 1.6 (0.6–3.9) | 0.34 |
| Cold or leftover vegetables | 21 (17) | 10 (26) | 11 (13) | 2.8 (1.1–7.6) | 0.04 |
| Raw vegetables | 34 (27) | 11 (29) | 23 (27) | 1.1 (0.5–2.7) | 0.79 |
| Watermelon | 32 (26) | 7 (18) | 25 (29) | 0.6 (0.2–1.6) | 0.32 |
| Mango | 35 (28) | 9 (24) | 26 (30) | 0.8 (0.3–2.0) | 0.65 |
| Orange | 24 (19) | 7 (18) | 17 (20) | 1.1 (0.4–2.8) | 0.94 |
| Timir† | 91 (73) | 22 (56) | 69 (80) | 0.3 (0.1–0.8) | 0.01 |
| Other fruit | 15 (12) | 4 (11) | 11 (13) | 0.9 (0.3–-3.1) | 0.86 |
| Fruit juice | 20 (16) | 8 (21) | 12 (14) | 1.6 (0.6–4.5) | 0.33 |
| Sour milk | 27 (22) | 7 (18) | 20 (23) | 0.8 (0.3–2.1) | 0.63 |
| Milk | 84 (67) | 22 (56) | 62 (72) | 0.5 (0.2–1.1) | 0.10 |
| Hulbat‡ | 58 (46) | 19 (47) | 39 (45) | 1.2 (0.6–2.7) | 0.60 |
| Cake | 18 (15) | 3 (8) | 15 (17) | 0.5 (0.1–1.7) | 0.25 |
| Bread | 36 (29) | 14 (38) | 22 (26) | 1.8 (0.8–4.2) | 0.15 |
| Any hot food | 124 (98) | 38 (95) | 86 (99) | 0.2 (0.01–2.07) | 0.17 |
| Any cold food | 84 (66) | 30 (75) | 54 (62) | 1.8 (0.8–4.2) | 0.17 |
| Ate food prepared outside the home | 25 (20) | 17 (43) | 8 (9) | 7.8 (2.9–20.8) | <0.01 |
| Attended any gatherings§ where food was served | 9 (7) | 6 (15) | 3 (3) | 4.7 (1.1–20.4) | 0.04 |
OR = odds ratio.
Bold indicates statistically significant at 0.05.
Ground chickpea stew.
Dates.
Meat and potato stew
Funeral, wedding, religious ceremony, or other gathering.
Participants who reported having eaten cold food, including vegetables (OR = 2.8, 95% CI = 1.1–7.6), or food prepared outside the home at a restaurant or food vendor (OR = 7.8, 95% CI = 2.9–20.8) had increased odds for cholera, and those who reported having eaten hot food (macaroni: OR = 0.4, 95% CI = 0.2–0.9; pasta: OR = 0.3, 95% CI = 0.1–0.9) or dates (OR = 0.3, 95% CI = 0.1–0.8) had lower odds for cholera (Table 2). Reporting attending a gathering away from home where food was served was associated with being a case (OR = 4.7, 95% CI = 1.1–20.4).
During a time of intense water chlorination interventions, contaminated food likely prolonged cholera transmission in Jijiga. This study found that persons with cholera were more likely than those who remained well to report having eaten food from outside the home and cold food and less likely to report safe handwashing practices and consumption of hot foods. Cholera has been grown in the laboratory on cold rice and other foods, and experiments have shown that properly heating food inactivates V. cholerae.7,8 Investigations of previous cholera outbreaks identified eating seafood, uncooked vegetables, and cold leftover rice as risks.9–11 In Jijiga, an increase in cases coincided with Ramadan. RHB reported an increase in street food vendors that sold foods for the evening Iftar meal; anecdotally, many of these vendors were unlicensed and may not have been as accustomed to safe food preparation as regular vendors.
If eating out and eating cold foods introduced cholera into homes, then poor hygiene in homes (few households had soap anywhere in the house or water at latrines) may have contributed to spreading cholera to other household members. We found poor hygiene in participants’ homes, consistent with a survey conducted across Somali Region at the same time.12 Our results indicated that 15 (27%) of the initial 55 cases were excluded from the analysis because they reported a household member treated with ORS, suggesting that co-primary transmission was ongoing. This can occur through local contamination of food or water in the household by symptomatic or asymptomatically infected household members, facilitated by poor hygiene.13,14 A systematic review and meta-analysis for risks of typhoid, which is also transmitted via the fecal-oral route, concluded that consumption of food and beverages outside the home was associated with infection, as was poor household hygiene.15 These are consistent with our findings.
Although the statistical link between household water practices and cholera was not found in the study, the importance for cholera prevention cannot be overlooked.1,16,17 The proportion of respondents who had FCR > 0.2 mg/L in stored water was greater than the proportion of people who reported treating their water (43% versus 26%), suggesting that chlorinated water in municipal distribution systems was reaching households. A systematic review and meta-analysis of risks for cholera found that factors thought to be protective against cholera are not always protective, possibly due to multiple routes of cholera transmission and variation in the quality of implementations of WASH interventions.18 This may explain our finding that only one WASH intervention was protective.
The study was subject to several limitations. The population was limited to Jijiga and may not be generalizable to the rest of the Somali region or Ethiopia. Cases were excluded if they had a household member who took ORS for diarrhea anytime in 2017, so we were not able to directly assess risks associated with transmission within the home. Only three cases were confirmed with a rapid test, and controls were not tested for cholera. Approximately half of cholera infections are asymptomatic19; if this were true for the controls, results would be biased toward the null hypotheses. We did not ask about household income or other socio-economic indicators.
Results of this study were shared with the Federal Ministry of Health, RHB, Jijiga municipal government, kebele leaders, international humanitarian actors, and WHO. Recommendations included 1) continue to support chlorination activities, 2) implement interventions with food vendors and restaurants, and 3) increase messaging about hygiene and food preparation in the home. WASH interventions continued to be implemented, but a lack of resources limited education and communication interventions. Cases declined slowly in Jijiga, and the outbreak was declared over in December 2017. This investigation highlights the importance of addressing food preparation and hand hygiene in the home as drivers of cholera.
REFERENCES
- 1. Davis W, Narra R, Mintz ED, 2018. Cholera. Curr Epidemiol Rep 5: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali M, Nelson AR, Lopez AL, Sack DA, 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9: e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO , 2018. Cholera, 2018. Weekly Epidemiological Record. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4. Rajasingham A, Harvey B, Taye Y, Kamwaga S, Martinsen A, Sirad M, Aden M, Gallagher K, Handzel T, 2020. Improved chlorination and rapid water quality assessment in response to an outbreak of acute watery diarrhea in Somali Region, Ethiopia. J Water Sanit Hyg Dev 10: 596–602. [Google Scholar]
- 5.WHO , 2005. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th rev. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.WHO , 2020. GTFCC Cholera App. Geneva, Switzerland: World Health Organization. Available at: https://play.google.com/store/apps/details?id=com.cholera&hl=en_US&gl=US. Accessed June 21, 2021.
- 7. Tang JY, Izenty BI, Nur’Izzati AJ, Masran SR, Yeo CC, Roslan A, Abu Bakar CA, 2013. Survivability of Vibrio cholerae O1 in cooked rice, coffee, and tea. Int J Food Sci 2013: 581648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makukutu CA, Guthrie RK, 1986. Behavior of Vibrio cholerae in hot foods. Appl Environ Microbiol 52: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DuBois AE, Sinkala M, Kalluri P, Makasa-Chikoya M, Quick RE, 2006. Epidemic cholera in urban Zambia: hand soap and dried fish as protective factors. Epidemiol Infect 134: 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St Louis ME, Porter JD, Helal A, Drame K, Hargrett-Bean N, Wells JG, Tauxe RV, 1990. Epidemic cholera in West Africa: the role of food handling and high-risk foods. Am J Epidemiol 131: 719–728. [DOI] [PubMed] [Google Scholar]
- 11. Dinede G, Abagero A, Tolosa T, 2020. Cholera outbreak in Addis Ababa, Ethiopia: a case-control study. PLoS One 15: e0235440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yikii G HA, Odindo R, Kakaire CN, Challa AB, 2019. Knowledge, attitudes, and practice (KAP) from baseline data of acute watery diarrhoea (AWD)/cholera among adults (15–49) in the Somali Region of Ethiopia. Texila Int J Pub Health 7.
- 13. Sugimoto JD. et al. , 2014. Household transmission of Vibrio cholerae in Bangladesh. PLoS Negl Trop Dis 8: e3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weil AA. et al. , 2014. Bacterial shedding in household contacts of cholera patients in Dhaka, Bangladesh. Am J Trop Med Hyg 91: 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brockett S, Wolfe MK, Hamot A, Appiah GD, Mintz ED, Lantagne D, 2020. Associations among water, sanitation, and hygiene, and food exposures and typhoid fever in case-control studies: a systematic review and meta-analysis. Am J Trop Med Hyg 103: 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lantagne D, Yates T, 2018. Household water treatment and cholera control. J Infect Dis 218: S147–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snow J, 1856. Cholera and the water supply in the south districts of London in 1854. J Public Health Sanit Rev 2: 239–257. [PMC free article] [PubMed] [Google Scholar]
- 18. Wolfe M, Kaur M, Yates T, Woodin M, Lantagne D, 2018. A systematic review and meta-analysis of the association between water, sanitation, and hygiene exposures and cholera in case-control studies. Am J Trop Med Hyg 99: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A, 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 7: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]