Abstract
Spontaneous mutants of susceptible clinical and laboratory isolates of Streptococcus pneumoniae exhibiting reduced susceptibility to evernimicin (SCH27899; MIC, 0.5 to 4.0 mg/liter) were selected on plates containing evernimicin. Four isolates that did not harbor mutations in rplP (which encodes ribosomal protein L16) were further analyzed. Whole chromosomal DNA or PCR products of the 23S ribosomal DNA (rDNA) operons from these mutants could be used to transform the susceptible S. pneumoniae strain R6 to resistance at frequencies of 10−5 and 10−4, respectively, rates 10- to 100-fold lower than that for a single-allele chromosomal marker. The transformants appeared slowly (48 to 72 h) on selective medium, and primary transformants passaged on nonselective medium produced single colonies that displayed heterogeneous susceptibilities to evernimicin. A single passage on selective medium of colonies derived from a single primary transformant homogenized the resistance phenotype. Sequence analysis of the 23S rDNA and rRNA from the resistant mutants revealed single, unique mutations in each isolate at the equivalent Escherichia coli positions 2469 (A → C), 2480 (C → T), 2535 (G → A), and 2536 (G → C). The mutations map within two different stems of the peptidyltransferase region of domain V. Because multiple copies of rDNA are present in the chromosome, gene conversion between mutant and wild-type 23S rDNA alleles may be necessary for stable resistance. Additionally, none of the characterized mutants showed cross-resistance to any of a spectrum of protein synthesis inhibitors, suggesting that the target site of evernimicin may be unique.
Everninomicins are a class of oligosaccharide antibiotics isolated from Micromonospora carbonaceae (25). One such compound, evernimicin (SCH27899), has been evaluated as a therapeutic agent (Ziracin) (6). Evernimicin has been shown to have potent activity against many gram-positive bacteria, including vancomycin-resistant enterococci, methicillin-resistant staphylococci, and penicillin-resistant pneumococci (9). In fact, there were no staphylococcal, enterococcal, or pneumococcal isolates that displayed resistance to evernimicin in either the investigation by Jones and Barrett (9) or a more recent worldwide survey of clinical isolates, including isolates known to be resistant to other antibiotics (R. S. Hare, F. J. Sabatelli, and The Ziracin Susceptibility Testing Group, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., Abstr. E-119, p. 204, 1998). The lack of cross-resistance to evernimicin suggests that the mechanism of action is novel, and prior selection for resistance to antimicrobial agents currently in clinical use will not impact the efficacy of evernimicin. Recently, in Streptococcus pneumoniae, we have shown that mutations in ribosomal protein L16 (encoded by rplP) cause a reduced susceptibility to evernimicin and that evernimicin has an inhibitory effect on in vivo protein synthesis (1). Another study demonstrated evernimicin-mediated inhibition of protein synthesis in cell-free assays derived from Escherichia coli and Staphylococcus aureus (13). Furthermore, radiolabeled evernimicin was found to bind the large ribosomal subunit at a single, unique high-affinity site (13).
The rrn operon is unique because unlike most chromosomal genes, which are present in a single copy, rrn operons are typically present as multiple loci in the genome. Therefore, rrn operon mutations that confer resistance to protein synthesis inhibitors are typically recessive or weakly codominant since the majority of the ribosomes must harbor the mutant allele for normal growth to occur in the presence of the inhibitor (19). Consequently, selectable spontaneous mutations in rrn loci in organisms with a high copy number of rrn operons are rare and have not had a clinical impact. Alternative resistance mechanisms, such as alterations to the ribosomal proteins (5), ribosome modification or protection (e.g., rRNA methylation [26]), and drug metabolism or efflux, are more clinically relevant. In contrast, ribosomal DNA (rDNA) mutations resulting in resistance to antimicrobial agents occur more frequently in bacteria that harbor a single rrn operon (e.g., Mycobacterium abscessus, Mycobacterium chelonae [24], Mycobacterium avium [14], and Halobacterium halobium [12]) or two rrn operons (e.g., Helicobacter pylori [23] and Mycobacterium smegmatis [17]). However, even in organisms with two rrn operons, the mode of action of the drug can influence whether a heterogeneous rRNA population confers resistance. Heterogeneous rrn alleles of M. smegmatis conferring erythromycin resistance appear to be a dominant phenotype (18), whereas heterogeneous rrn alleles conferring aminoglycoside resistance appear as a recessive phenotype (17). In a strain of Staphylococcus aureus that harbored an additional rplP allele on a plasmid, mutations to L16 which conferred evernimicin resistance were recessive (unpublished data).
In this paper, we report the isolation and characterization of rDNA mutations in S. pneumoniae that confer reduced susceptibility to evernimicin. The locations of the mutations within the 23S rRNA have not been found to confer resistance to other protein synthesis inhibitors. If the positions of the mutations in the rRNA are considered to be potential points of contact with evernimicin or specific modifiers of the ribosomal structure required to interact with evernimicin, then the sites of interaction between evernimicin and the 50S ribosomal subunit appear to be unique. Identification of the unique sites of interaction may help to clarify the detailed mechanism of action of evernimicin.
MATERIALS AND METHODS
Bacterial strains.
S. pneumoniae ATCC 49619 and clinical isolate SP#3 (1) were used in the spontaneous-mutant selection experiments. A nonencapsulated laboratory strain of S. pneumoniae R6 was used for transformation experiments.
Selection of spontaneous mutants exhibiting resistance to evernimicin.
S. pneumoniae ATCC 49619 and clinical isolate SP#3 were grown to the mid-exponential phase of growth in Todd-Hewitt broth, pelleted, and resuspended at a concentration of approximately 1012 CFU/ml. Aliquots (0.1 ml) were plated on Mueller-Hinton agar (MHA) supplemented with 5% sheep blood and 0.125, 0.25, or 0.5 μg of evernimicin/ml. Spontaneous mutants appeared after incubating the plates at 37°C for 72 h in an atmosphere of 5% CO2. MICs for evernimicin were determined by E-test (AB Biodisk, Solna, Sweden) on MHA supplemented with 5% sheep blood as recommended by the manufacturer. Plates were incubated for 24 h, as described above and MICs were determined according to the manufacturer's guidelines.
DNA extraction.
Whole chromosomal DNA from S. pneumoniae strains was prepared by detergent lysis followed by phenol-chloroform extraction as described previously (3). The DNA was further purified by treatment with RNase followed by precipitation with 0.6 volumes of 20% polyethylene glycol 6000–2.5 M NaCl.
PCR amplification.
rDNA was amplified with an Expand Long Template PCR system (Boehringer Mannheim, Mannheim, Germany) in 50-μl reaction volumes containing 3 U of DNA polymerase mix, 1× polymerase buffer no. 1, 350 μM each deoxynucleoside triphosphate, 300 nM each primer, and 50 ng of template DNA. PCR conditions were 93°C for 60 s followed by 25 cycles of 92°C for 2 s, 55°C for 30 s, and 68°C for 195 s. For each of the last 15 cycles, the 68°C extension time was extended by 12 s. The primer sequences for the universal amplification of DNA operons (Table 1) are based on the preliminary sequence of S. pneumoniae obtained from The Institute for Genomic Research website at http://www.tigr.org.
TABLE 1.
Primers used for the amplification of rDNA operons and rDNA operon fragments
| Primer | Position | Strand | Nucleotide sequence |
|---|---|---|---|
| F1 | 5′ 16S rDNA | + | 5′ ACAACTCAGGTCCGTTGGTC 3′ |
| R1 | 3′ 5S rDNA | − | 5′ AGCTAAGCGACTTCCCTATC 3′ |
| F2 | 5′ 16S-23S spacer | + | 5′ GAGTTTATGACTGAAAGGTC 3′ |
| R2 | 5′ 23S rDNA | − | 5′ CTTCTGCCGTTTCGCTCG 3′ |
| E1 | A2058G 23S rDNA | + | 5′ ACCCGCGACAGGACGGGAAGACCCCATG 3′a |
The A2058G substitution conferring erythromycin resistance is underlined.
Transformation.
S. pneumoniae R6 was grown to an optical density at 650 nm of 0.08 in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 5% horse serum. Aliquots were stored at −70°C with 10% glycerol for use in transformation experiments. Transformations were performed by incubating thawed cells (1 ml) with competence-stimulating peptide (1 μg/ml) (8) and donor DNA (1 μg/ml) at 30°C for 30 min. The cells were allowed to recover for 60 min at 37°C in the transformation medium before being plated on MHA supplemented with 5% sheep blood and the appropriate antibiotic. Transformation frequencies are expressed as ratios of the number of transformants obtained on selective medium to the number of colonies obtained without selection. For positive controls with chromosomal DNA, strains harboring mutations in rpsL and rplP, which confer resistance to streptomycin and evernimicin, respectively, were used. For transformation experiments using isolated rDNA operons, an erythromycin resistance-conferring allele (A2058G at the equivalent E. coli 23S rDNA position) was constructed by the megaprimer method of site-directed mutagenesis (20) with primers F1, R1, and E1 (Table 1). The presence of the mutation in erythromycin-resistant transformants was verified by DNA sequencing.
DNA sequencing and analysis.
Sequencing reactions were performed by cycle sequencing with a Big Dye termination kit (Perkin-Elmer Applied Biosystems, Branchburg, N.J.) according to the manufacturer's recommendations. The reaction products were separated on an ABI PRISM 310 (Perkin-Elmer Applied Biosystems) automated sequencer. Double-stranded sequencing was performed on the DNA from resistant mutants and their isogenic parent strains. Sequencher software (Gene Codes Corp., Ann Arbor, Mich.) was used for sequence comparisons and alignments.
rRNA sequencing.
Cells were grown in 200 ml of brain heart infusion broth (Difco) to an optical density at 600 nm of 0.1. Cells were harvested, resuspended in 1 ml H2O with 0.1% Triton X-100, and incubated for 15 min at room temperature, and then their RNA was extracted sequentially with H2O-saturated phenol (acidic), phenol-chloroform, and chloroform. The RNA was precipitated and sequenced by a primer extension method, as described previously (21), with a primer (5′ GGTCCTCTCGTACTAGGAGCAG 3′) which is complementary to the E. coli 23S rRNA α-sarcine loop (E. coli position 2660).
RESULTS
Selection for evernimicin resistance.
At evernimicin concentrations of 0.125, 0.25, and 0.5 μg/ml, colonies became visible on plates only after 72 h of incubation. The frequency at which colonies appeared was ∼5.3 × 10−9 for both S. pneumoniae ATCC 49619 and clinical isolate SP#3. The MIC of evernimicin for susceptible strains ATCC 49619, SP#3, and R6 was 0.03 μg/ml.
Transformation with rplP.
Eight spontaneously resistant isolates obtained from each parental strain were propagated with selection for evernimicin resistance (0.125 μg/ml) for further analysis. As a means of discriminating between mutations in rplP and other loci, PCR products encompassing rplP and 1 kb of flanking sequence from each strain were amplified and used to transform the evernimicin-sensitive recipient strain R6. None of the PCR products from the ATCC 49619-derived mutants conferred resistance to evernimicin. PCR products from two of the SP#3-derived mutants transformed R6 to evernimicin resistance at a frequency of 10−3. The transformants were selected on medium containing 0.125 μg of evernimicin/ml, and colonies were visible after 24 h. DNA sequence analysis of the two transforming PCR products revealed two new mutations in rplP: a GCT-to-CAT change resulted in an Ala49-to-His substitution, and an ATC-to-ATG change resulted in an Ile49-to-Met substitution. The evernimicin MICs for the transformants were 0.75 and 0.19 μg/ml, respectively.
Non-rplP alleles.
For each parental strain, two spontaneously resistant isolates that did not contain selectable mutations in rplP were analyzed further. Chromosomal DNA from the four non-rplP isolates was used to transform R6 and select for evernimicin resistance; the rate of transformation to evernimicin resistance was approximately 10−5 (Table 2). This rate is markedly different from those obtained in previous transformation experiments using rplP and rpsL controls (in which transformation efficiencies were approximately 10−3). In addition, unlike the rpsL and rplP resistance markers, for which transformants arose within 24 h, the time periods required for the appearance of colonies when using chromosomal DNA from the four non-rplP isolates were 48 and 72 h on media containing 0.06 and 0.125 μg of evernimicin/ml, respectively (Table 2). Thus, the primary transformants mimicked the slow growth rate of the spontaneously derived isolates. However, when either the spontaneous isolates or the primary transformants were replated (either with or without 0.125 μg of evernimicin/ml), they grew within 24 h; despite the initial slow growth rate on the primary selection medium, the growth rate of these strains on selective or nonselective medium was now normal.
TABLE 2.
Frequency of transformation of S. pneumoniae R6 to evernimicin resistance with whole chromosomal DNA from four selected spontaneous mutants and their parent strains at different drug concentrations
| Donor DNA | Time (h) | Transformation frequency at evernimicin concn (μg/ml)a:
|
|||
|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | ||
| ATCC 49619 | 24 | NG | NG | NG | NG |
| 48 | tntc | 1 × 10−6 | NG | NG | |
| 72 | tntc | tntc | NG | NG | |
| ATCC 49619-1 | 24 | 1.3 × 10−5 pp | NG | NG | NG |
| 48 | tntc | 6.1 × 10−5 | 2.0 × 10−6 | NG | |
| 72 | tntc | tntc | 2.2 × 10−5 | 2.0 × 10−6 | |
| ATCC 49619-2 | 24 | 1.8 × 10−5 pp | NG | NG | NG |
| 48 | tntc | 4.5 × 10−5 | 2.0 × 10−6 | NG | |
| 72 | tntc | tntc | 4.0 × 10−5 | 1.0 × 10−6 | |
| SP#3 | 24 | NG | NG | NG | NG |
| 48 | tntc | NG | NG | NG | |
| 72 | tntc | tntc | NG | NG | |
| SP#3-1 | 24 | 6.0 × 10−6 pp | NG | NG | NG |
| 48 | tntc | 3.8 × 10−5 | NG | NG | |
| 72 | tntc | tntc | 2.5 × 10−5 | 3.0 × 10−6 | |
| SP#3-2 | 24 | NG | NG | NG | NG |
| 48 | tntc | 1.6 × 10−5 | NG | NG | |
| 72 | tntc | tntc | 4.0 × 10−6 | NG | |
Abbreviations: NG, no growth; tntc, too numerous to count; pp, pinprick colonies.
rDNA transformation.
We next determined if the uncharacterized isolates had alterations in the rrn operon sequence. In S. pneumoniae there are four rrn operons (4). The rrn operons from the four resistant strains were amplified with universal primers F1 and R1 (Table 1), and the 5,221-bp amplicons were used to transform S. pneumoniae R6. In all four cases, after 48 to 72 h, evernimicin-resistant colonies arose at a frequency of 10−4 to 10−5 (Table 3). In a control experiment, S. pneumoniae R6 was transformed with an rrn operon that carried a mutation conferring erythromycin resistance (nucleotide 2058 was changed from A to G) (Table 4). The rate of transformation of S. pneumoniae R6 to erythromycin resistance mimicked that for evernimicin resistance in that transformation rates of 10−4 to 10−5 occurred with prolonged incubation (48 to 72 h) at drug concentrations lying between the MIC and the minimum bactericidal concentration. Similar to the transformation experiments with chromosomal DNA, both the evernimicin-resistant and the erythromycin-resistant primary transformants exhibited a slow initial growth phase on selective medium followed by a normal growth rate after a second passage on medium (with or without the selective drug). To further localize the resistance allele, two smaller overlapping PCR products of 2,388 and 3,123 bp were generated; one (obtained using primer pair F1-R2 [Table 1]) consisted of the 16S rDNA and part of the 5′ end of the 23S rDNA, pair, and the other (obtained using primer pair F2-R1) consisted of the 23S and 5S rDNA. Evernimicin-resistant transformants were obtained only with the F2-R1 PCR products, implying that the mutations are located in either the 5S or 23S rDNA.
TABLE 3.
Frequency of transformation of S. pneumoniae R6 to evernimicin resistance with rDNA operons from four selected spontaneous mutants and their parent strains at different drug concentrations
| Donor DNA | Time (h) | Transformation frequency at evernimicin concn (μg/ml)a:
|
|||
|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | ||
| ATCC 49619 | 24 | NG | NG | NG | NG |
| 48 | tntc | NG | NG | NG | |
| 72 | tntc | tntc | 1.0 × 10−6 | NG | |
| ATCC 49619-1 | 24 | 6.8 × 10−5 | 1.9 × 10−5 | 2.0 × 10−6 | NG |
| 48 | tntc | 8.6 × 10−5 | 1.7 × 10−5 | 3.0 × 10−6 | |
| 72 | tntc | tntc | 3.7 × 10−5 | 8.0 × 10−6 | |
| ATCC 49619-2 | 24 | 6.7 × 10−5 | 1.9 × 10−5 | 1.0 × 10−6 | NG |
| 48 | tntc | 9.0 × 10−5 | 2.2 × 10−5 | 1.0 × 10−6 | |
| 72 | tntc | tntc | 3.4 × 10−5 | 3.0 × 10−6 | |
| SP#3 | 24 | NG | NG | NG | NG |
| 48 | tntc | 1.0 × 10−6 | NG | NG | |
| 72 | tntc | tntc | NG | NG | |
| SP#3-1 | 24 | 2.0 × 10−4 | 7.6 × 10−5 | 1.3 × 10−5 | NG |
| 48 | tntc | 1.3 × 10−4 | 4.8 × 10−5 | 1.2 × 10−5 | |
| 72 | tntc | tntc | 1.1 × 10−4 | 2.1 × 10−5 | |
| SP#3-2 | 24 | 7.0 × 10−5 | 1.3 × 10−5 | 1.0 × 10−6 | NG |
| 48 | tntc | 8.4 × 10−5 | 1.7 × 10−5 | 2.0 × 10−6 | |
| 72 | tntc | tntc | 2.4 × 10−5 | 4.0 × 10−6 | |
Abbreviations: NG, no growth; tntc, too numerous to count.
TABLE 4.
Frequency of transformation of S. pneumoniae R6 to erythromycin resistance with a 23S rDNA allele containing an A2058G substitution at the equivalent E. coli position
| Time (h) | Transformation frequency at erythromycin concn (μg/ml)a:
|
|||||
|---|---|---|---|---|---|---|
| 0.06 | 0.12 | 0.25 | 0.5 | 1.0 | 2.0 | |
| 24 | tntc | 5.6 × 10−6 | 1.2 × 10−6 | NG | NG | NG |
| 48 | tntc | tntc | 2.5 × 10−5 | 1.1 × 10−5 | 8.0 × 10−6 | NG |
| 72 | tntc | tntc | 4.5 × 10−5 | 1.4 × 10−5 | 1.1 × 10−5 | NG |
Abbreviations: NG, no growth; tntc, too numerous to count.
Characterization of rDNA mutations by DNA and RNA sequencing.
The universal 23S rDNA PCR products from the four spontaneous mutants detailed above and the ATCC 49619 and SP#3 parent strains were sequenced. Compared to the parental strains, the four spontaneous mutants each exhibited a single nucleotide change at the following equivalent E. coli positions: 2469 (A → C), 2480 (C → T), 2535 (G → A), and 2536 (G → C) (Table 5; Fig. 1). These mutations are located on two different stems in domain V of the 23S rRNA that are proximal to the peptidyltransferase loop. Distinct single-nucleotide peaks in the sequencing chromatograms of the mutant and parent strains suggested that the intragenomic populations of 23S rDNA alleles were homogeneous. Sequence analysis of the 23S rDNA in the R6 isolates that were transformed with the 23S rDNA PCR products confirmed the presence of identical mutations in the evernimicin-resistant transformants. rRNAs from these evernimicin-resistant transformants were extracted and sequenced to confirm that the mutant rrn alleles were homogeneous. The mutant 23S rRNA represented >90% of the sequence band for all four mutants strains.
TABLE 5.
Evernimicin-resistant 23S rDNA mutants and their evernimicin MICs
| Strain | 23S rDNA mutation | Evernimicin MIC (μg/ml) for:
|
|
|---|---|---|---|
| Spontaneous mutant | R6 transformant | ||
| ATCC 49619 | None | 0.047 | NA |
| ATCC 49619-1 | C2480T | 0.75 | 3.0 |
| ATCC 49619-2 | G2535A | 0.38 | 0.75 |
| SP#3 | None | 0.047 | NA |
| SP#3-1 | A2469C | 0.75 | 1.0 |
| SP#3-2 | G2536C | 0.38 | 0.75 |
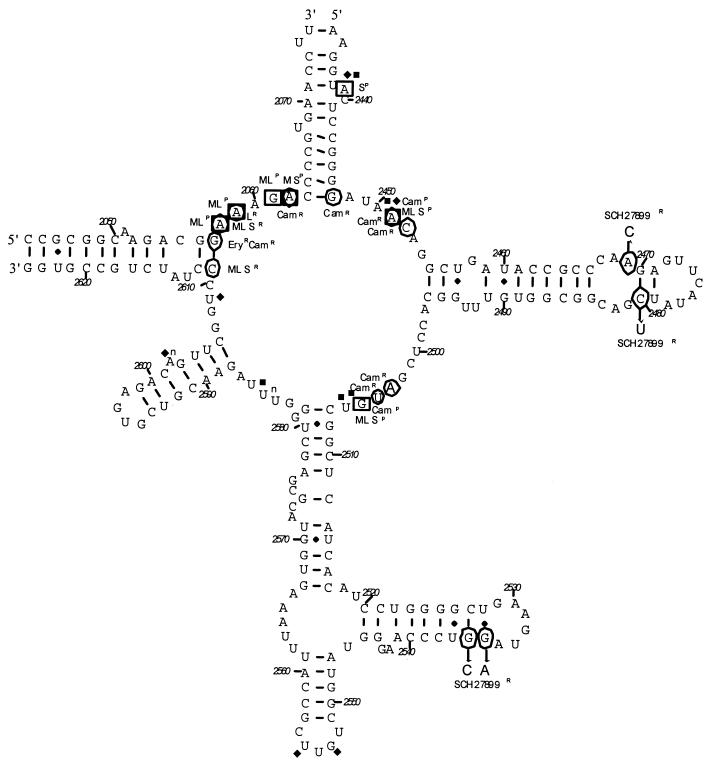
FIG. 1.
Peptidyltransferase region of domain V of the 23S rRNA (E. coli numbering). The residues that when mutated confer resistance (R) to protein synthesis inhibitors are circled. Mutations that confer resistance to evernimicin are indicated (SCH27899R). Residues which are protected (P) from modification by bound drug are boxed (26). Residues protected by tRNA bound in the A and P sites are labeled with the symbols ⧫ and ■, respectively (15). M, macrolides; L, lincosamides; S, streptogramin B; Cam, chloramphenicol; Ery, erythromycin.
MICs of evernimicin for 23S rDNA mutants.
The primary transformants, after initial selection on medium containing evernimicin, yielded two different phenotypes with respect to evernimicin resistance, depending on whether they were next passaged on selective or nonselective medium. Those passaged on selective medium produced homogeneous populations of evernimicin-resistant organisms, such that the MICs for the collection of transformants obtained for each specific mutation were consistent. After this second round of growth, the MIC remained consistent irrespective of further exposure to the drug. The second phenotypic class arose from primary transformants passaged on nonselective medium. These transformants produced a heterogeneous phenotype with respect to the evernimicin MIC. This was evident on the evernimicin E-tests, which showed uniform growth in the zone of a predicted sensitive strain but showed a concentration gradient of satellite colonies extending from evernimicin sensitive to the maximum MIC obtained for transformants that been maintained on selective medium. The evernimicin MICs for the original spontaneous mutant and the corresponding 23S rDNA transformants are shown in Table 5. The 23S rDNA mutants were tested alongside the original parental strains for any changes in sensitivity to other antimicrobial agents. No change in MIC was observed with any of the following antimicrobial agents: ampicillin, benzylpenicillin, chloramphenicol, clarithromycin, ciprofloxacin, clindamycin, erythromycin, fusidic acid, gentamicin, lincomycin, rifampin, spectinomycin, streptomycin, Synercid, tetracycline, or vancomycin.
DISCUSSION
The identification of rDNA mutations that confer resistance to evernimicin allows further speculation about the precise mechanism of action of the drug. Experiments with cross-linkable oligonucleotide probes complimentary to 23S rRNA nucleotides 2475 to 2483 identified interactions between this region of domain V and ribosomal protein L16 (16). The identification of mutations conferring resistance to evernimicin that occur in L16 (1) and positions around the 2465-to-2485 stem of the 23S rRNA (Fig. 1) strengthen the postulate that these two ribosomal components are structurally and functionally linked. Anticodon stem-loop analogues bound to the A site of the ribosome have been shown to interact with the 2465-to-2485 stem of domain V (10), suggesting that this loop forms part of the A site of the ribosome. This finding is congruent with the proposed function of L16, which is not essential for peptidyltransferase activity (11) or GTP-mediated tRNA hydrolysis (22) but appears to be involved in the fixation of the aminoacyl stem of the tRNA to the ribosome at its A site (11). Therefore, it is possible that evernimicin inhibits protein synthesis by interfering with the binding or positioning of the 3′ end of the tRNA in the A site of the ribosome. This mechanism is not refuted by the presence of point mutations on the 23S rRNA stem at positions 2535 and 2536 that also confer resistance to evernimicin, since the function of this stem and its point of interaction with other domains in the ribosome are not clearly defined. It is possible that this rRNA region also forms part of the A site and thus results in resistance to evernimicin in a manner similar to the position 2469 and 2480 mutations. Alternatively, the residues of this stem may not interact at all with evernimicin but rather cause distortions elsewhere in the ribosome which prevent the binding of evernimicin in the A site of the ribosome.
A summary of rRNA footprints and mutations which confer resistance to protein synthesis inhibitors that act at the peptidyltransferase center is shown in Fig. 1. The consistent equivalence of nucleotides protected by drug binding and implicated in drug resistance suggests that the evernimicin resistance-conferring mutations are likely to pinpoint the site of drug interaction with the 23S rRNA. The unique locations of mutations which confer evernimicin resistance, compared to those for other peptidyltransferase inhibitors, imply that evernimicin has unique mechanistic properties. This observation appears to be confirmed by the lack of cross-resistance associated with evernimicin-resistant mutants and other inhibitors of protein synthesis.
Isolation of selectable spontaneous rrn mutations is primarily limited to organisms with one or two rrn alleles. However, experimental models using either plasmid-borne rrn alleles overexpressing a mutant rrn that dominates the sensitive phenotype (12, 19) or engineered strains with reduced numbers of chromosomal rrn operons (2, 17) have been used to examine the effect of rrn mutations on translation and antibiotic resistance. The emergence of S. pneumoniae strains that maintain homogeneous rrn alleles conferring resistance to evernimicin may be a result of high-frequency intragenomic allelic exchange or gene conversion. Genetic exchange between rRNA alleles has been observed to occur at a frequency of 6 × 10−5 in E. coli (7). In M. smegmatis, recA-mediated gene conversion between aminoglycoside-resistant and -sensitive rRNA alleles has been shown to occur at a frequency of 10−4 (17). S. pneumoniae is known to achieve very high rates of homologous recombination following transformation, and therefore intragenomic gene conversion frequencies may also be relatively high in this bacterium.
The spontaneous mutants were selected after a long incubation period (72 h) in medium with drug concentrations of two to eight times the MIC. It is conceivable that under these conditions sufficient growth and genetic recombination occur to allow recombinant conversion of susceptible rrn alleles to alleles conferring resistance. Once two copies of the resistance-conferring rrn allele are established, cell growth is likely to increase in parallel with the rate of conversion of other rrn alleles. Under continued selective pressure, a homogeneous condition is likely to develop, and rrn-mediated resistance becomes stable. The removal of selective pressure before allele conversion is complete is likely to explain the heterogeneous evernimicin resistance phenotype found in each transformed colony after the initial selective pressure. An extended period of selective pressure at drug concentrations lying between the MIC and the minimum bactericidal concentration appears to be crucial for the isolation of stable evernimicin-resistant S. pneumoniae mutants that are homogeneous at the rrn alleles. Similarly, the erythromycin-resistant transformation controls also required a prolonged recovery time and could not be selected on medium with >2.0 μg of erythromycin/ml, despite the fact that strains homogeneous for the resistant rrn allele exhibited erythromycin MICs of >256 μg/ml.
Although higher transformation frequencies might be expected for a multicopy target site (in this case, rrn alleles), the lower rate of transformation to evernimicin resistance may result from a higher rate of reversion of the single modified rrn allele mediated by recombination with one of the three susceptible alleles. The slow emergence of resistant rrn mutants is most likely due to a combination of both the time taken to convert a sufficient proportion of the rrn alleles to confer a resistance phenotype and the time it takes for the cell to replace drug-susceptible ribosomes with resistant variants. Cells that were transformed with PCR products of rrn alleles arose more quickly than those transformed with whole chromosomal DNA, which may reflect a higher initial rate of direct conversion of susceptible alleles by the transforming DNA.
ACKNOWLEDGMENTS
We thank Liqun Xiong and Alexander Mankin for performing the 23S rRNA sequencing.
REFERENCES
- 1.Adrian P V, Black T A, Zhao W, Shaw K J, Hare R S, Klugman K P. Mutations in ribosomal protein L16 conferring reduced susceptibility to evernimicin ( SCH27899): implications for mechanism of action. Antimicrob Agents Chemother. 2000;44:732–738. doi: 10.1128/aac.44.3.732-738.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai T, Condon C, Voulgaris J, Zaporojets D, Shen B, Al-Omar M, Squires C, Squires C L. Construction and initial characterization of Escherichia coli strains with few or no intact chromosomal rRNA operons. J Bacteriol. 1999;181:3803–3809. doi: 10.1128/jb.181.12.3803-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1995. [Google Scholar]
- 4.Bacot C M, Reeves R H. Novel tRNA gene organization in the 16S-23S intergenic spacer of the Streptococcus pneumoniae rRNA gene cluster. J Bacteriol. 1991;173:4234–4236. doi: 10.1128/jb.173.13.4234-4236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chittum H S, Champney W S. Ribosomal protein gene sequence changes in erythromycin-resistant mutants of Escherichia coli. J Bacteriol. 1994;176:6192–6198. doi: 10.1128/jb.176.20.6192-6198.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganguly A K, Girijavallabhan V M, Miller G H, Sarre O Z. Chemical modification of everninomicin. J Antibiot. 1982;35:561–570. doi: 10.7164/antibiotics.35.561. [DOI] [PubMed] [Google Scholar]
- 7.Harvey S, Hill C W. Exchange of spacer regions between rRNA operons in Escherichia coli. Genetics. 1990;125:683–690. doi: 10.1093/genetics/125.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones R N, Barrett M S. Antimicrobial activity of everninomicin (evernimicin), an oligosaccharide antimicrobial with a potent Gram-positive spectrum. Clin Microb Infect. 1995;1:35–43. doi: 10.1111/j.1469-0691.1995.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 10.Joseph S, Weiser B, Noller H F. Mapping the inside of the ribosome with an RNA helical ruler. Science. 1997;278:1093–1098. doi: 10.1126/science.278.5340.1093. [DOI] [PubMed] [Google Scholar]
- 11.Maimets T, Remme J, Villems R. Ribosomal protein L16 binds to the 3′-end of transfer RNA. FEBS Lett. 1984;166:53–56. doi: 10.1016/0014-5793(84)80043-5. [DOI] [PubMed] [Google Scholar]
- 12.Mankin A S, Zyrianova I M, Kagramanova V K, Garrett R A. Introducing mutations into the single-copy chromosomal 23S rRNA gene of the archaeon Halobacterium halobium by using an rRNA operon-based transformation system. Proc Natl Acad Sci USA. 1992;89:6535–6539. doi: 10.1073/pnas.89.14.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNicholas P M, Najarian D J, Mann P A, Hesk D, Hare R S, Shaw K J, Black T A. Evernimicin binds exclusively to the 50S ribosomal subunit and inhibits translation in cell-free systems derived from both gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:1121–1126. doi: 10.1128/aac.44.5.1121-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier A, Kirschner P, Springer B, Steingrube V A, Brown B A, Wallace R J, Jr, Böttger E C. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381–384. doi: 10.1128/aac.38.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moazed D, Noller H F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989;57:585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- 16.Muralikrishna P, Cooperman B S. Ribosomal components neighboring the 2475 loop in Escherichia coli 50S subunits. Biochemistry. 1995;34:115–121. doi: 10.1021/bi00001a014. [DOI] [PubMed] [Google Scholar]
- 17.Prammananan T, Sander P, Springer B, Böttger E C. RecA-mediated gene conversion and aminoglycoside resistance in strains heterozygous for rRNA. Antimicrob Agents Chemother. 1999;43:447–453. doi: 10.1128/aac.43.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander P, Prammananan T, Meier A, Frischkorn K, Böttger E C. The role of RNAs in macrolide resistance. Mol Microbiol. 1997;26:469–480. doi: 10.1046/j.1365-2958.1997.5811946.x. [DOI] [PubMed] [Google Scholar]
- 19.Sigmund C D, Morgan E A. Erythromycin resistance due to a mutation in a ribosomal RNA operon of Escherichia coli. Proc Natl Acad Sci USA. 1982;79:5602–5606. doi: 10.1073/pnas.79.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A M, Klugman K P. “Megaprimer” method of PCR-based mutagenesis: the concentration of megaprimer is a critical factor. BioTechniques. 1997;22:438–442. doi: 10.2144/97223bm13. [DOI] [PubMed] [Google Scholar]
- 21.Stern S, Moazed D, Noller H F. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- 22.Tate W P, Schulze H, Nierhaus K H. The importance of the Escherichia coli ribosomal protein L16 for the reconstitution of the peptidyl-tRNA hydrolysis activity of peptide chain termination. J Biol Chem. 1983;258:12810–12815. [PubMed] [Google Scholar]
- 23.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace R J, Jr, Meier A, Brown B A, Zhang Y, Sander P, Onyi G O, Böttger E C. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother. 1996;40:1676–1681. doi: 10.1128/aac.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein M J, Wagman G H, Oden E M, Luedemann G M, Sloane P, Murawski A, Marquez J. Purification and biological studies of everninomicin B. 1966. pp. 821–827. . Antimicrob. Agents Chemother. 1965. [PubMed] [Google Scholar]
- 26.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]