Abstract
Our previous studies have shown that riboflavin has activity against Plasmodium falciparum asexual-stage parasites in vitro. In the present study we examine the gametocytocidal activity of riboflavin and the interaction of riboflavin with some commonly used antimalarial drugs against the asexual forms of P. falciparum in vitro. The addition of riboflavin to P. falciparum cultures killed gametocytes at all stages, even those at late stages (III to V), which are not affected by many of the commonly used antimalarials. Combinations of riboflavin with mefloquine, pyrimethamine, and quinine showed a marked potentiation of the activities of these drugs against asexual-stage parasites in vitro. The combination of riboflavin with artemisinin was additive, while that with chloroquine was mildly antagonistic. High doses of riboflavin are used clinically to treat several inborn errors of metabolism with no adverse side effects. Its efficacy in combination with standard antimalarial drugs in treating and preventing the transmission of P. falciparum malaria can therefore be evaluated in humans.
During intraerythrocytic development of malaria parasites, both asexual and sexual parasites are produced. When the extracellular merozoites invade erythrocytes, most of the resulting intracellular parasites develop in the asexual cycle, which comprises three distinct morphological stages known as rings, trophozoites, and schizonts. In the human-malaria species Plasmodium falciparum, this cycle is completed in 48 h. A small proportion of parasites, however, differentiate into sexual-stage parasites, gametocytes that are required for transmission of the disease by the mosquito vector. There are five distinct morphological stages of gametocyte development designated stages I to V. The complete maturation of P. falciparum gametocytes after merozoite invasion takes 10 to 12 days.
The mainstay of malaria management is chemotherapy with antimalarial drugs. Due to the continued appearance of parasites resistant to first-line antimalarial drugs, the therapeutic value of most antimalarials currently in use has been greatly diminished. The difficulty of managing malaria is further compounded by the fact that antimalarial drugs commonly used in countries where malaria is endemic (such as chloroquine, quinine, sulfadoxine-pyrimethamine [SP], and mefloquine) are not effective against the sexual forms of P. falciparum (6, 14, 25). Antifolate drugs such as pyrimethamine and the sulfa drugs, as well as SP, have been reported to raise the proportion of gametocytes in treated patients (4). Treatment of malaria with SP alone results in elevated levels of gametocytes that may increase the potential for malaria transmission from individuals already cured of clinical symptoms (33).
Combinations of antimalarial drugs may be used for two purposes: (i) to enhance activity in the treatment of individual infections and (ii) to delay the appearance of resistance to one or both the associated drugs when they are to be used widely in an area where malaria is endemic (34). To prevent or reduce malaria transmission, there is a need for safe and inexpensive gametocytocidal drugs that can be used in combination with first-line drugs against asexual-stage parasites.
Researchers have previously demonstrated the antimalarial activity of riboflavin against asexual-stage P. falciparum in vitro (1). In the present study, the effect of riboflavin on the sexual-stage parasites and of combinations with standard antimalarial drugs against the asexual-stage parasites (P. falciparum strains) were examined in vitro. Our results show that riboflavin is effective against sexual-stage parasites and potentiates the activity of mefloquine, pyrimethamine, and quinine. Thus, riboflavin used in combination with these drugs could prevent the spread of resistant parasites and also lower malaria transmission by lowering gametocytogenesis.
MATERIALS AND METHODS
Materials.
RPMI 1640 medium was from GIBCO/BRL, and A+ human serum was from Gemini Biological Products (Calabasas, Calif.). Artemisinin, chloroquine diphosphate salt, pyrimethamine, quinine hydrochloride, and riboflavin were from Sigma (St. Louis, Mo.). Mefloquine was a gift from Dennis E. Kyle (Walter Reed Army Institute of Research, Washington, D.C.).
Parasite cultivation and treatment with riboflavin.
The chloroquine-resistant FCB strain of P. falciparum was synchronized with 5% sorbitol and cultivated under standard conditions (13, 32). In the “riboflavin-pulse” experiments, riboflavin was added to the culture for 2 h and then subsequently washed out, and the parasites were cultured in the absence of riboflavin for 3 to 4 h before riboflavin was reintroduced for 2 h. The reintroduction and washing procedure were repeated three times a day for 2 days. The control parasites were treated with or without riboflavin for 48 h, with medium changes after 24 h. Thin blood smears were Giemsa stained to determine parasitemia.
Culturing of gametocytes.
P. falciparum gametocytes (strain 3D7) were cultured as described by Ifediba and Vanderberg (16). Briefly, asexual-parasite cultures were diluted to a 0.2% parasitemia and 6% hematocrit with fresh erythrocytes (day 0). On day 3 the cultures were diluted from a 6% to a 3% hematocrit with RPMI–10% serum and then maintained for the next 18 days with daily medium changes. After day 0, no erythrocytes were added to the cultures. Giemsa-stained slides of the cultures were prepared daily to monitor parasitemia and gametocytogenesis.
Drug combination test.
The antiparasite activities of riboflavin and standard antimalarial drugs were assessed by hypoxanthine incorporation into parasite DNA, essentially as described by Lauer et al. (21). Briefly, parasite suspensions at a 1 to 2% parasitemia and a 1% hematocrit were dispensed into 96-well plates. To determine the effect of one drug on the dose response of the other, 2 concentrations of the test drug were added in triplicate. [3H]hypoxanthine (0.5 μCi/well) was added, and the samples were incubated under culture conditions overnight. The cells were harvested to glass fiber filters and liquid scintillation cocktail was added and counted to determine cell-associated [3H]hypoxanthine. A and B represent the drugs used; IC50 represents the 50% inhibitory concentration. The results were expressed as the sums of the fractional inhibitory concentrations (sum FIC) (5, 7), which were defined as
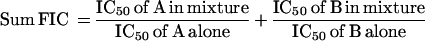 |
Sum FIC values of <1 indicate synergism; values equal to 1 indicate addition; and values of >1 indicate antagonism.
RESULTS AND DISCUSSION
Gametocytocidal activity of riboflavin.
Several studies have shown that higher gametocyte densities lead to increased transmission (11, 23, 28, 31). The antimalarial drugs commonly used to treat blood-stage infections of P. falciparum have little or no activity against mature gametocytes. Hence, malaria may be transmitted from patients who have been successfully cured of asexual-stage infections; thus, drugs that are effective against both asexual- and sexual-stage parasites will be invaluable in our efforts to manage malaria caused by P. falciparum. We have previously shown that riboflavin, which is effective against asexual-stage parasites in vitro, has a profound effect on hemoglobin metabolism by the parasite (1). Since gametocytogenesis results in complete digestion of host hemoglobin, we examined the effects of riboflavin on gametocytogenesis.
Previous studies have demonstrated differences in the metabolisms of early- and late-stage gametocytes (20). Thus, a time course study was done to determine the effect of riboflavin on the entire spectrum of gametocytogenesis. An aliquot was removed once daily from a P. falciparum culture from days 1 to 10 after invasion and exposed to 100 μM riboflavin for the rest of the experiment. Daily blood smears of all cultures were made to determine the number and stage of gametocytes. As shown in Table 1, the addition of riboflavin to cultures resulted in killing of gametocytes at all stages. The effect of riboflavin on gametocytes at each of the different stages was similar. Independent of stage, a decrease in gametocytes was observed 4 days after the addition of riboflavin. The maximum effect on gametocytogenesis occurred 7 days after the introduction of riboflavin. Thus, when riboflavin was initially added on day 5 to the culture (mostly stage I gametocytes), gametocytes were no longer observed by day 15 (Table 1). Addition of riboflavin to the culture (stage II and III gametocytes) on day 10 resulted in a 57% reduction in gametocytes on day 15 and an 88% reduction on day 17. No gametocytes remained on day 18 (Table 1). At these stages (II and III), during the first 2 days of exposure to riboflavin, the gametocytes continued to mature. But after that the number of gametocytes began to decrease, and morphologically abnormal gametocytes were observed in the culture (data not shown). These data demonstrate that riboflavin was effective against both immature and mature P. falciparum gametocytes in vitro.
TABLE 1.
Gametocytocidal action of riboflavin on P. falciparum 3D7 clone in vitroa
| Day on which riboflavin was added | No. of gametocytes per 500 erythrocytes after the following days in culture:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Culture alone | 0 | 0 | 0 | 0 | 0 | 2 | 23 | 56 | 69 | 61 | 53 | 48 | 46 | 36 | ||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 4 | 0 | 1 | 8 | 6 | 9 | 2 | 2 | 0 | 0 | 0 | ||||||||
| 5 | 2 | 10 | 22 | 21 | 16 | 5 | 0 | 0 | 0 | |||||||||
| 6 | 12 | 38 | 40 | 16 | 6 | 1 | 3 | 1 | ||||||||||
| 7 | 44 | 50 | 28 | 10 | 1 | 2 | 1 | |||||||||||
| 8 | 59 | 52 | 17 | 3 | 1 | 0 | ||||||||||||
| 10 | 51 | 49 | 22 | 6 | 0 | |||||||||||||
| Gametocyte stages | I=II | I=II | 1<II | I<II>III | II>III | II<III>IV | III<IV=V | III<IV>V | ||||||||||
Aliquots of parasites were taken from a control culture, and riboflavin (100 μM) was added on the days indicated. Giemsa-stained smears were made each day and analyzed to determine the number of gametocytes per 500 erythrocytes. The gametocyte stages present each day are indicated at the bottom in Roman numerals.
Artemisinin and its derivatives are also effective against early-stage gametocytes as well as asexual parasites. They have been shown to reduce total parasitemia and gametocyte load in clinical trials (25, 33). Administration of artemisinin derivatives in combination with mefloquine to treat malaria on the Thai-Burmese border resulted in the reduction of malaria transmission by 50% from 1994 to 1996 (25). Since riboflavin is effective against both immature and mature gametocytes in vitro, it may also have the potential to block gametocytogenesis in vivo, which may lead to a reduction in P. falciparum transmission from treated patients.
Combination of riboflavin with standard antimalarial drugs.
Riboflavin has a short half-life in animals and humans (2 to 6 h) (17, 18). Since micromolar concentrations are required to kill asexual and sexual parasites in vitro, it may not be suitable for use as a single agent to treat malaria. It may be useful, however, when combined with standard antimalarial drugs to treat drug-resistant malaria. Antimalarial-drug combinations may slow the emergence of drug-resistant strains and prolong the effectiveness of each drug in the treatment of infections. Several drug combinations have been tested in vitro and in vivo for their use in malaria chemotherapy (7–9, 25–27, 30, 33, 34).
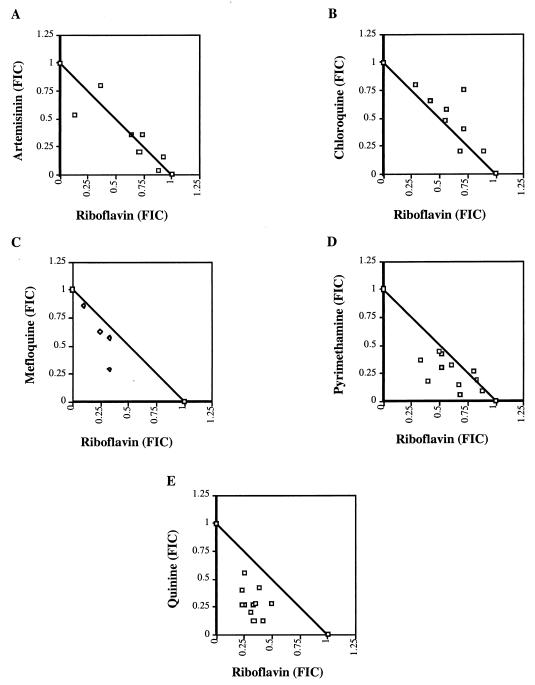
Mefloquine, SP, and quinine remain first-line drugs for treatment of malaria in areas of endemicity. However, declining efficacy due to the emergence of resistance to these drugs has prompted the search for suitable combination partners to treat resistant parasites (25, 33). In addition to the problem of drug resistance, these drugs have no significant activity against P. falciparum gametocytes. As shown in Table 2 and Fig. 1C to E, riboflavin interacts synergistically with mefloquine, pyrimethamine, and quinine in vitro. This suggests that riboflavin might be a suitable combination partner in vivo against the asexual-stage parasites and also provide gametocytocidal activity necessary to prevent or reduce transmission from drug-treated individuals.
TABLE 2.
In vitro efficacies of riboflavin in combination with standard antimalarial drugs against P. falciparum clone FCB
| Drug | Sum FIC | Effect |
|---|---|---|
| Artemisinin | 1.04 ± 0.07 | Additive |
| Chloroquine | 1.11 ± 0.06 | Mildly antagonistic |
| Mefloquine | 0.84 ± 0.08 | Synergistic |
| Pyrimethamine | 0.85 ± 0.05 | Synergistic |
| Quinine | 0.61 ± 0.04 | Synergistic |
FIG. 1.
Isobolograms of the interactions of riboflavin with artemisinin (addition), chloroquine (weak antagonism), mefloquine (synergism), pyrimethamine (synergism), and quinine (synergism). The axes represent normalized FICs.
Due to the relatively high recrudescence rates observed when artemisinin and its derivatives are used clinically to treat P. falciparum infections (22, 34), artemisinin compounds are used in combination with other drugs (in particular, those with a long half-life) for improved action against asexual-stage parasites. The in vitro antimalarial additive activity of riboflavin and artemisinin shown in Fig. 1A, and Table 2 suggest that riboflavin and artemisinin or artemisinin's derivatives could be used together in vivo to more effectively reduce transmission of malaria caused by P. falciparum. However, since both riboflavin and artemisinin have short half-lives, a third drug with a longer half-life may also be needed to effectively clear asexual parasites. The interaction of riboflavin with chloroquine was mildly antagonistic against asexual-stage parasites. This suggests that chloroquine may not be a suitable combination partner for riboflavin in vivo.
Inhibition of parasite growth by short pulses of riboflavin.
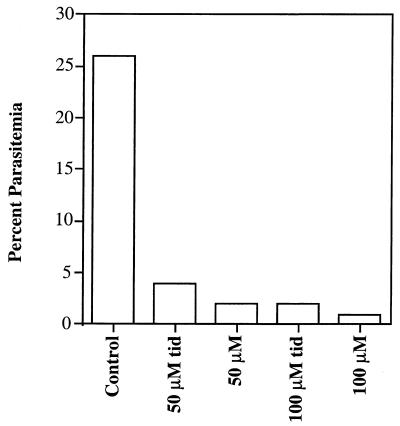
The concentration of riboflavin required to inhibit asexual- and sexual-stage parasites (25 to 100 μM) can be achieved in vivo only by administration of high doses of riboflavin (50 to 400 mg/day). When high doses of riboflavin are administered orally to humans, the maximum concentration in serum is reached by 2 h and then declines to basal levels 4 to 6 h later (18). We therefore examined whether short pulses of riboflavin similar to what may occur in vivo after administration of high-dose riboflavin were effective in killing malaria parasites in vitro. These experiments were done by treating infected cultures with or without riboflavin for 2 h, once, twice, or three times a day (t.i.d.) for a total of 2 days. The effect of treatment on parasite growth was measured by examining Giemsa-stained blood smears. As shown in Fig. 2, administration of riboflavin (50 or 100 μM) (t.i.d.) inhibited parasite growth by 80 to 92%. The administration of 25 μM riboflavin t.i.d., on the other hand, resulted in a 45% inhibition (Table 3). Administration of 50 μM riboflavin twice a day resulted in only a 26% inhibition, while administration of 50 or 100 μM riboflavin once a day had no significant effect (Table 3 and data not shown). These results suggest that a brief elevation of riboflavin concentration in vitro, similar to the increase in the serum concentration of riboflavin that occurs after the administration of high oral doses in vivo, is effective in killing asexual-stage P. falciparum. However, riboflavin may bind tightly to many proteins in the cell as a cofactor for many oxidative reactions. Thus, the fluctuations in its concentration after the administration of high doses in vivo may be less severe than those caused by our in vitro washing procedure, a difference that could affect its antimalarial activity in vivo. The data are consistent with the hypothesis that the administration of high doses of riboflavin several times a day may be effective in killing P. falciparum in vivo. However, riboflavin binds serum proteins, and its uptake in vivo exhibits saturable kinetics. It is therefore possible that the administration of high doses in vivo may not produce sufficient concentrations to kill P. falciparum in one generation, as occurs in vitro. The need to administer riboflavin three times a day may hamper its development as an antimalarial because of problems with compliance. However, multiple dosing is not unusual in malaria chemotherapy; for example, the standard regimen of quinine and tetracycline to treat uncomplicated malaria is given in multiple doses (quinine every 8 h for 3 days with tetracycline every 6 h for 7 days) (34).
FIG. 2.
Effect of short pulses of riboflavin on parasite growth. Ring-stage parasites were cultured with 50 or 100 μM riboflavin for 2 h t.i.d. or continuously incubated for 48 h. Parasitemia was determined by counting the number of parasites per 1,000 erythrocytes.
TABLE 3.
Effect of short pulses of riboflavin on asexual-parasite growth in vitroa
| Riboflavin regimens | % Inhibition of parasite growth at different riboflavin concns (μM):
|
|
|---|---|---|
| 25 | 50 | |
| Present 48 h | 90 | 93 |
| For 2 h t.i.d. | 45 | 76 |
| For 2 h twice a day | 12 | 26 |
| For 2 h once a day | 0 | 13 |
Ring-stage parasites were cultured with the indicated concentration of riboflavin for 2 h once or twice daily or t.i.d. for 2 days. Parasitemia was determined by counting the number of parasites per 1,000 erythrocytes on Giemsa-stained blood smears.
The data presented here strongly suggest that riboflavin could be an effective agent in treating and blocking the transmission of malaria caused by P. falciparum. High doses of riboflavin are used to treat patients with several inborn errors of metabolism (2, 3, 10, 12, 15, 19, 24, 29), in some cases up to 2 years, with no adverse side effects. Since the safety of riboflavin is well established, it should be possible in clinical trials to test its efficacy in combination with standard antimalarials against malaria caused by P. falciparum.
ACKNOWLEDGMENTS
We were supported by NIH grant AI39071 and Burroughs Wellcome New Initiatives in Malaria Awards (to K.H.) and NIH grant AI40592 (to K.W.).
REFERENCES
- 1.Akompong T, Ghori N, Haldar K. In vitro activity of riboflavin against the human malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother. 2000;44:88–96. doi: 10.1128/aac.44.1.88-96.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antozzi C, Garavaglia B, Mora M, Rimoldi M, Morandi L, Ursino E, DiDonato S. Late-onset riboflavin-response myopathy with combined multiple acyl coenzyme A dehydrogenase and respiratory chain deficiency. Neurology. 1994;44:2153–2158. doi: 10.1212/wnl.44.11.2153. [DOI] [PubMed] [Google Scholar]
- 3.Arts W, Scholte H, Bogaard J, Kerrebijn K, Luyt-Houwen I. NADH-CoQ reductase deficient myopathy: successful treatment with riboflavin. Lancet. 1983;ii:581–582. doi: 10.1016/s0140-6736(83)90618-9. [DOI] [PubMed] [Google Scholar]
- 4.Barkakaty B N, Sharma G K, Chakravorty N K. Studies on efficacy of treatment with sulfamethoxazole + trimethoprim and sulfalene + pyrimethamine combinations in Plasmodium falciparum malaria of known and unknown resistant status. J Commun Dis. 1988;20:165–174. [PubMed] [Google Scholar]
- 5.Berenbaum M C. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 6.Bruce-Chwatt L J, Black R H, Canfield C J, Clyde D F, Peters W, Wernsdorfer W H. Fundamental aspects of chemotherapy of malaria. In: Bruce-Chwatt L J, editor. Chemotherapy of malaria. 2nd ed. Geneva, Switzerland: World Health Organization; 1981. pp. 21–55. [Google Scholar]
- 7.Canfield C J, Pudney M, Gutteridge W E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 8.Chawira A N, Warhurst D C. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J Trop Med Hyg. 1987;90:1–8. [PubMed] [Google Scholar]
- 9.Doherty J F, Sadiq A D, Bayo L, Alloueche A, Olliaro P, Milligan P, von Seidlein L, Pinder M. A randomized safety and tolerability trial of artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone for the treatment of uncomplicated malaria in Gambian children. Trans R Soc Trop Med Hyg. 1999;93:543–546. doi: 10.1016/s0035-9203(99)90376-0. [DOI] [PubMed] [Google Scholar]
- 10.Folkers K, Wolaniuk A, Vadhanavikit S. Enzymology of the response of the carpal tunnel syndrome to riboflavin and to combined riboflavin and pyridoxine. Proc Natl Acad Sci USA. 1984;81:7076–7078. doi: 10.1073/pnas.81.22.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves P M, Burkot T R, Carter R, Cattani J A, Lagog M, Parker J, Brabin B J, Gibson F D, Bradley D J, Alpers M P. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988;96:251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- 12.Gregersen N, Wintzensen H, Christensen S K, Christensen M F, Brandt N J, Rasmussen K. C6- C10-dicarboxylic aciduria: investigations of a patient with riboflavin responsive multiple acyl-CoA dehydrogenation defects. Pediatr Res. 1982;16:861–868. doi: 10.1203/00006450-198210000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Haldar K, Ferguson M A, Cross G A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J Biol Chem. 1985;260:4969–4974. [PubMed] [Google Scholar]
- 14.Heppner D G, Ballou W R. Malaria in 1998: advances in diagnosis, drugs and vaccine development. Curr Opin Infect Dis. 1998;11:519–530. [PubMed] [Google Scholar]
- 15.Hirano M, Matsuki T, Tanishima K, Takeshita M, Shimizu S, Nagamura Y, Yoneyama Y. Congenital methaemoglobinaemia due to NADH methaemoglobin reductase deficiency: successful treatment with oral riboflavin. Br J Haematol. 1981;47:353–359. doi: 10.1111/j.1365-2141.1981.tb02802.x. [DOI] [PubMed] [Google Scholar]
- 16.Ifediba T, Vanderberg J P. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 17.Jusko W J, Levy G. Absorption, metabolism, and excretion of riboflavin-5′-phosphate in man. J Pharm Sci. 1967;56:58–62. doi: 10.1002/jps.2600560112. [DOI] [PubMed] [Google Scholar]
- 18.Jusko W J, Levy G. Absorption, protein binding, and elimination of riboflavin. In: Rivlin S R, editor. Riboflavin. New York, N.Y: Plenum Press; 1975. pp. 99–151. [Google Scholar]
- 19.Kaplan J, Chirouze M. Therapy of recessive congenital methaemoglobinaemia by oral riboflavin. Lancet. 1978;ii:1043–1044. doi: 10.1016/s0140-6736(78)92357-7. [DOI] [PubMed] [Google Scholar]
- 20.Lang-Unnasch N, Murphy A D. Metabolic changes of the malaria parasite during the transition from the human to the mosquito host. Annu Rev Microbiol. 1998;52:561–590. doi: 10.1146/annurev.micro.52.1.561. [DOI] [PubMed] [Google Scholar]
- 21.Lauer S A, Rathod P K, Ghori N, Haldar K. A membrane network for nutrient import in red cells infected with the malaria parasite. Science. 1997;276:1122–1125. doi: 10.1126/science.276.5315.1122. [DOI] [PubMed] [Google Scholar]
- 22.Looareesuwan S. Overview of clinical studies on artemisinin derivatives in Thailand. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):S9–S11. doi: 10.1016/0035-9203(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 23.Mulder B, Tchuinkam T, Dechering K, Verhave J P, Carnevale P, Meuwissen J H, Robert V. Malaria transmission-blocking activity in experimental infections of Anopheles gambiae from naturally infected Plasmodium falciparum gametocyte carriers. Trans R Soc Trop Med Hyg. 1994;88:121–125. doi: 10.1016/0035-9203(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 24.Penn A M W, Lee J W K, Thuillier P, Wagner M, Maclure K M, Menard M R, Hall L D, Kennaway N G. MELAS syndrome with mitochondrial tRNA(Leu)(UUR) mutation: correlation of clinical state, nerve conduction, and muscle 31P magnetic resonance spectroscopy during treatment with nicotinamide and riboflavin. Neurology. 1992;42:2147–2152. doi: 10.1212/wnl.42.11.2147. [DOI] [PubMed] [Google Scholar]
- 25.Price R N, Nosten F, Luxemburger C, ter Kuile F O, Paiphun L, Chongsuphajaisiddhi T, White N J. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 26.Price R N, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White N J. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–577. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 27.Sabchareon A, Attanath P, Chanthavanich P, Phanuaksook P, Prarinyanupharb V, Poonpanich Y, Mookmanee D, Teja-Isavadharm P, Heppner D G, Brewer T G, Chongsuphajaisiddhi T. Comparative clinical trial of artesunate suppositories and oral artesunate in combination with mefloquine in the treatment of children with acute falciparum malaria. Am J Trop Med Hyg. 1998;58:11–16. doi: 10.4269/ajtmh.1998.58.11. [DOI] [PubMed] [Google Scholar]
- 28.Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102(Part 1):27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- 29.Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. Neurology. 1998;50:466–470. doi: 10.1212/wnl.50.2.466. [DOI] [PubMed] [Google Scholar]
- 30.Skinner-Adams T, Davies T E M. Synergistic in vitro antimalarial activity of omeprazole and quinine. Antimicrob Agents Chemother. 1999;43:1304–1306. doi: 10.1128/aac.43.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchuinkam T, Mulder B, Dechering K, Stoffels H, Verhave J P, Cot M, Carnevale P, Meuwissen J H, Robert V. Experimental infections of Anopheles gambiae with Plasmodium falciparum of naturally infected gametocyte carriers in Cameroon: factors influencing the infectivity to mosquitoes. Trop Med Parasitol. 1993;44:271–276. [PubMed] [Google Scholar]
- 32.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 33.von Seidlein L, Milligan P, Pinder M, Bojang K, Anyalebechi C, Gosling R, Coleman R, Ude J I, Sadiq A, Duraisingh M, Warhurst D, Alloueche A, Targett G, McAdam K, Greenwood B, Walraven G, Olliaro P, Doherty T. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet. 2000;355:352–357. doi: 10.1016/S0140-6736(99)10237-X. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Practical chemotherapy of malaria. WHO Tech Rep Ser. 1990;805:24–51. [PubMed] [Google Scholar]