Abstract
RECA (Radiotherapy enhanced with Cherenkov photo-activation) is a proposed treatment where the anti-cancer drug psoralen is photo-activated in situ by UVA (Ultraviolet A, 320–400 nm) Cherenkov light (CL) produced directly by the treatment beam itself. In this study, we develop a UVA-imaging technique to quantify relative UVA CL produced by bulk tissues and other phantoms upon clinical x-ray megavoltage irradiation. UVA CL emission (320–400 nm) was quantified in tissue samples of porcine and poultry and in two kinds of solid waters (SW): brown (Virtual Waters, Standard Imaging, WI) and white (Diagnostic Therapy, CIRS, VA), and in 1% agarose gels variously doped with absorbing dye. Quantification was achieved through cumulative imaging of the samples placed in a dark, light-blocking chamber during irradiation on a Varian 21 EX accelerator. UVA imaging required a specialized high-sensitivity cooled camera equipped with UVA lenses and a filter. At 15MV, white SW emitted 66±5%,64±5% and 76±3% less UVA than chicken breast, pork loin and pork belly, respectively. Similar under-response was observed at 6MV. Brown SW had 21±8% less UVA emission than white SW at 15MV, and negligible emission at 6MV. Agarose samples (1% by weight) doped with 250ppm India ink exhibited equivalent UVA CL emission to chicken breast (within 8%). The results confirm that for the same absorbed dose, SW emits less UVA light than the tissue samples, indicating that prior in vitro studies utilizing SW as the CL-generating source may have underestimated the RECA therapeutic effect. Agarose doped with 250ppm India ink is a convenient tissue-equivalent phantom for further work.
Keywords: Cherenkov, radiation therapy, UVA imaging, RECA, psoralen
1. Introduction
Psoralens (Schmitt et al 1995a, Bethea et al 1999) are naturally occurring phototoxic compounds that can absorb UV radiation and damage the function of DNA and other cell constituents. In the absence of Ultraviolet A (UVA light, 320–400 nm), psoralen molecules are biologically inert and intercalate into DNA base pairs. Unactivated psoralens are ultimately excreted as metabolites in the urine within 24 h (Oldham et al 2016). Upon absorbing UVA radiation, they form photoadducts and crosslinks with DNA strands and photomodify cell proteins and lipids, suppressing their function and inducing cell apoptosis. In addition, psoralens are observed to improve cellular immunogenicity with an increased expression of major histocompatibility complex I (MHCI) (Moor etal 1995, Schmitt et al 1995b, Gasparro and Schmitt 1997). 8-Methoxypsoralen (8-MOP) and 5-Methoxypsoralen (5-MOP) are FDA approved drugs (UVADEX, Therakos Inc., New Jersey) that are widely used in Psoralen and Ultraviolet A (PUVA) therapies to treat skin diseases, such as psoriasis, vitiligo and cutaneous T-cell lymphoma (Pathak and Fitzpatrick 1992). Despite their excellent anti-cancer and immunogenic properties, psoralens have been limited to treating superficial diseases due to the short penetration depth of UVA-light in tissue.
The efficacy of psoralen-based drugs to treat deep-seated tumors was investigated through a new treatment approach, x-ray psoralen activated cancer (X-PACT) therapy (Oldham et al 2016), which uses kilovoltage x-ray beams incident on phosphor particles (which absorb x-rays and re-emit UVA photons) to activate psoralen in situ. The potential of this treatment regimen was demonstrated by in vitro and in vivo studies. Through flow cytometry, in vitro experiments showed increased apoptosis and cytotoxicity in X-PACT exposed cells versus psoralen or radiation therapy alone. In vivo studies showed a slower rate of tumor growth in the mice treated with X-PACT. This regimen, however, poses two challenges in a clinical setting: intratumoral injection of phosphors, and the use of Kv x-rays to activate phosphors (which deliver a high skin and bone radiation dose).
Yoon et al proposed an alternative to X-PACT, radiation therapy enhanced with Cherenkov photo-activation (RECA) (Yoon et al 2018), which combines conventional external beam radiation therapy with psoralen phototherapy, without the use of phosphor intermediaries. Psoralen activation is achieved through Cherenkov emission in the tissue, which is produced when the megavoltage beam traversing the tissue produces electrons with relativistic energies (see section 2). RECA has the potential to add a systemic anti-cancer aspect to local radiation therapy modality. RECA may also be effective against hypoxic tumours (Yoon 2018). This is because psoralenDNA crosslinking requires no oxygen for activation, only UVA photons. Furthermore, chronic hypoxia downregulates NER (nucleotide excision repair) and HR (homologous repair) pathways. Blocking either the NER or HR pathway is known to have increased sensitivity to crosslinking agents (such as psoralens) (Hall 2006). Figure 1 shows the spectrum of Cherenkov light generated from RT beams in water has a greater overlap with the psoralen (8-MOP) absorbance spectrum as compared to the UVA lamp output used for PUVA therapy.
Figure 1.
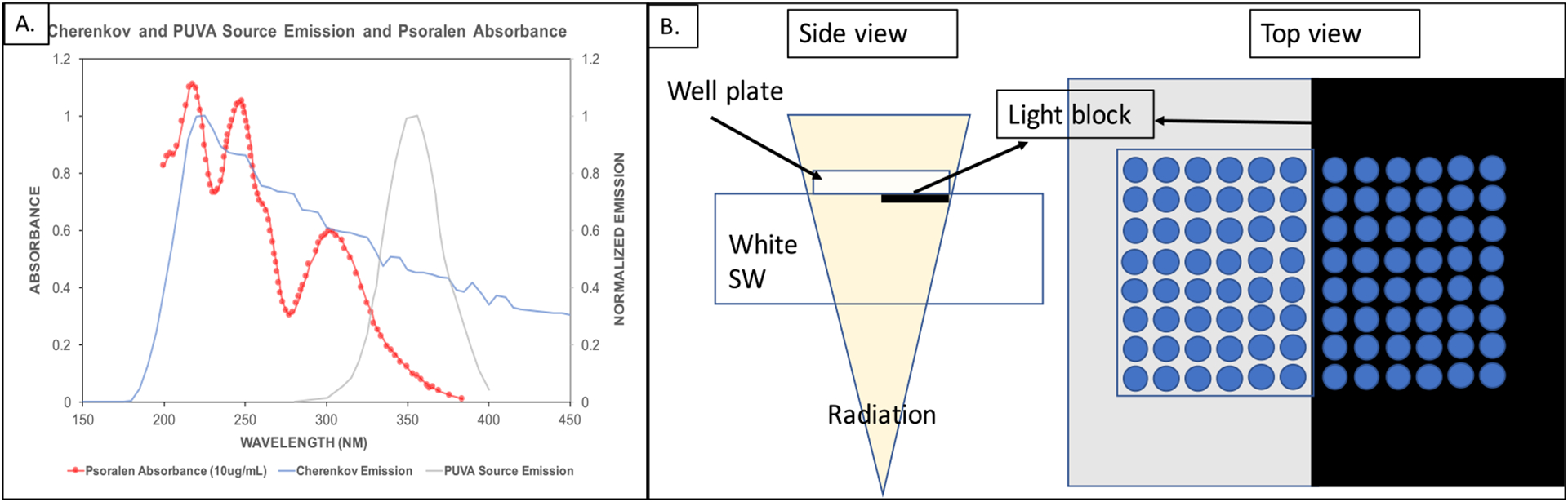
(A): Relative psoralen (8-Methoxypsoralen) absorbance spectrum at 10μgml(−1), normalized PUVA source emission and Cherenkov emission from a 15MV photon beam traversing through water (obtained from a Monte Carlo simulation by Shrock et al (2016)). (B): Experimental setup for irradiation and UVA light block implementation (adapted from Yoon et al (2018)).
Recent in vitro RECA trials on 4T1 and B16 cell lines indicated an increased MHC1 expression, greater tumor cell cytotoxicity and decreased tumor cell viability in cells treated with RECA as compared to the cells treated with RT alone (Yoon et al 2018). The irradiation setup used a CIRS diagnostic therapy plastic water slab (Inc C I R S 2003) (white SW) as a surrogate for tissue to produce the CL. Psoralen-labelled cells were plated on 96-well and 12-well black polystyrene, clear-bottom plates (i.e. the plate walls were opaque, but the base had 90% transmittance down to 300 nm). These plates were placed on top of the white SW and irradiated with a posterior-anterior (PA) photon beam. A UVA-absorbing black aluminum sheet was used to cover half of the SW, such that the wells placed directly above did not receive any psoralen activating UVA-light from the SW. Some CL was observed to be generated in the well-plate base by MV radiation traversing the plate, however measurements indicated this to be a relatively small component at 20%. The study observed decreased tumor cell viability, increased MHC1 expression and increased cytotoxicity in the cells treated with RECA versus RT alone (no UVA psoralen activation). The main assumption in this setup was that white SW closely simulates in vivo conditions, meaning that it has similar CL emission in the UVA range to that from human tissue when irradiated with clinical MV beams, which then travels from the SW surface through to the transparent cell medium in the wells.
The use of white SW to mimic human tissue in the in vitro studies has been questioned, since it may not resemble tissue in its optical properties, such as refractive index, absorption and scattering coefficients (Pratx and Kapp 2018). SW may scintillate and has been observed to do so on being irradiated by kV photon beams. SW scintillation may have led to an exaggerated amount of UVA light reaching the cells as compared to in vivo conditions, suggesting the observed RECA therapeutic effect may have been overestimated.
The aim of the study was to develop an imaging technique to quantify UVA Cherenkov light (CL) emission from tissues and phantoms, and use it to evaluate SW as a tissue surrogate for UVA CL production. A secondary aim was to use the same imaging technique to identify a gel-based phantom with tissue-equivalent CL production, which could potentially be used for future in vitro studies. While both Cherenkov and scintillation processes could contribute to UVA emission, it is non-trivial to distinguish between them. However, the equivalence of UVA emission from SW to that from tissue is more pertinent to this study, irrespective of the processes that contributed to it.
2. Theory: Cherenkov emission
2.1. Threshold energy for Cherenkov production
Cherenkov emission is produced when charged particles move at a velocity greater than the phase velocity of light in a dielectric medium (Čerenkov 1937). The minimum kinetic energy required for Cherenkov production in tissue can be derived by considering an electron travelling at a velocity v comparable to the speed of light, c. Due to the high speed of the electron, we must consider relativistic effects and the Lorentz factor, γ,
| (1) |
where, vp = c/n is the phase velocity of the relativistic electron. The kinetic energy is then given by,
| (2) |
and substituting the expression for vp in (2) and placing it in (3) gives the following threshold energy for Cherenkov production,
| (3) |
To estimate this threshold, m is substituted with the rest mass of the electron (511keV) and n is substituted with a estimate for the refractive index of tissue in the UVA range, 1.35–1.4 (Ding et al 2006, Giannios et al 2016). This gives an energy threshold of 219keV-250keV. In plastic materials, this threshold drops to 143keV(n=1.6 (Sultanova et al 2009)). Thus, at clinical MV beams, the electrons produced do satisfy the threshold for Cherenkov production in both tissues and plastics (Glaser et al 2015).
2.2. Cherenkov anisotropy
Cherenkov emission is produced in a conical wave, which has a well-defined angle depending on the energy of the charged particle and the refractive index of the material Therefore, it is well established that Cherenkov emission is highly anisotropic.
However, RT photon beams produce fast-moving electrons with varying energies and directions in the medium, producing their own cones of Cherenkov in different directions (Glaser 2015). Moreover, tissue is a strong and heterogeneous scatterer of light. Jacques (2013) present two equations to model the reduced scattering coefficients and corresponding reduced scattering lengths (distance after which the photon’s direction is completely random) for IR and visible range photons in tissue. These are,
| (4) |
where the wavelength λ is normalised to 500 nm and scaled to a factor b and a=μ_ŝ’ (λ=500 nm), and
| (5) |
where and correspond to the contributions from Rayleigh and Mie scattering, respectively.
Using these two models and data provided for different tissue types in the study (Jacques 2013), the values obtained for reduced scattering lengths for 400 nm are less than 1 mm. This means that light loses directionality completely within a millimeter in tissue. Even though the two models diverge in the UV range, both suggest that the reduced scattering length value would be even smaller in the UV range, since the reduced scattering coefficient increases at lower wavelengths. Therefore, for biomedical UVA imaging of tissue samples, anisotropy can be ignored.
3. Methods
3.1. UVA-imaging system
A low-noise camera (iKon-M934, Andor, Belfast, UK; with 10%−40% quantum efficiency in the UVA range (OxfordInstruments 2010)), deep-cooled to −85 °C with a back-illuminated CCD type sensor was used to image the Cherenkov emission from the samples (figure 2). It was further equipped with a UVA-transparent lens assembly crafted from two plano-convex lenses (ThorLabs, Inc., 50%–100% transmittance at 300–400 nm (ThorLabs 1999)) and a band-pass filter (FF01–390/SP25, 60%–95% transmission, Semrock, Rochester, New York) to exclude UVB, visible and infrared light (OD > 6).
Figure 2.

(A): Camera equipped with UVA lens and filter assembly. (B): Sensitivity characteristics of the imaging components and the overall system.
Although PMT may be used to conduct these experiments, we chose to use the cooled CCD camera equipped with quartz lens for the following reasons: (1) compared to standard PMT, cooled CCD is temporally more stable, making it easy to obtain multiple measurements simultaneously (single image versus multiple acquisitions). This provides better spatial and temporal consistency. (2) Deep-cooled CCD is best suited for low-light measurement as its noise floor is significantly lower than PMT. (3) Even if cooled PMT is used as the detector, quartz lens would still be necessary to improve coupling efficiency and to avoid radiation interference with the electronics of PMT.
Being very sensitive to stray UV light within the room, the camera was shielded by sources of UV noise by placing the whole setup in a black, light-blocking chamber. To image the phantoms during MV irradiation, the camera was placed on the LINAC couch, and was connected using a 15 m long cable to a computer that allowed remote operation from the treatment console. The exposure time was set to the total duration of the irradiation. For example, to deliver 1000 MU at a dose rate of 400MUmin–1, the exposure time was set to 2.5 min. The image acquisition and irradiation were begun simultaneously.
3.1. Experiment 1: comparing UVA emission from meats and solid water
Tissue samples of chicken breast, pork loin and pork belly were cut roughly into 3×3×2.5 cm3 slices and were imaged along with white (CIRS (Inc C I R S 2003)) and brown (standard imaging (Inc S I 0000)) SWs. The setup is shown in figure 3. The gantry was rotated to 270∘ and the phantoms were irradiated laterally, with the camera positioned approximately 45∘ from the axis of irradiation (so it does not lie within the radiation beam). Using this orientation, we increased the distance between the LINAC head (identified as the main source of radiation background, see appendix) and the camera, and decreased background noise in the images.
Figure 3.
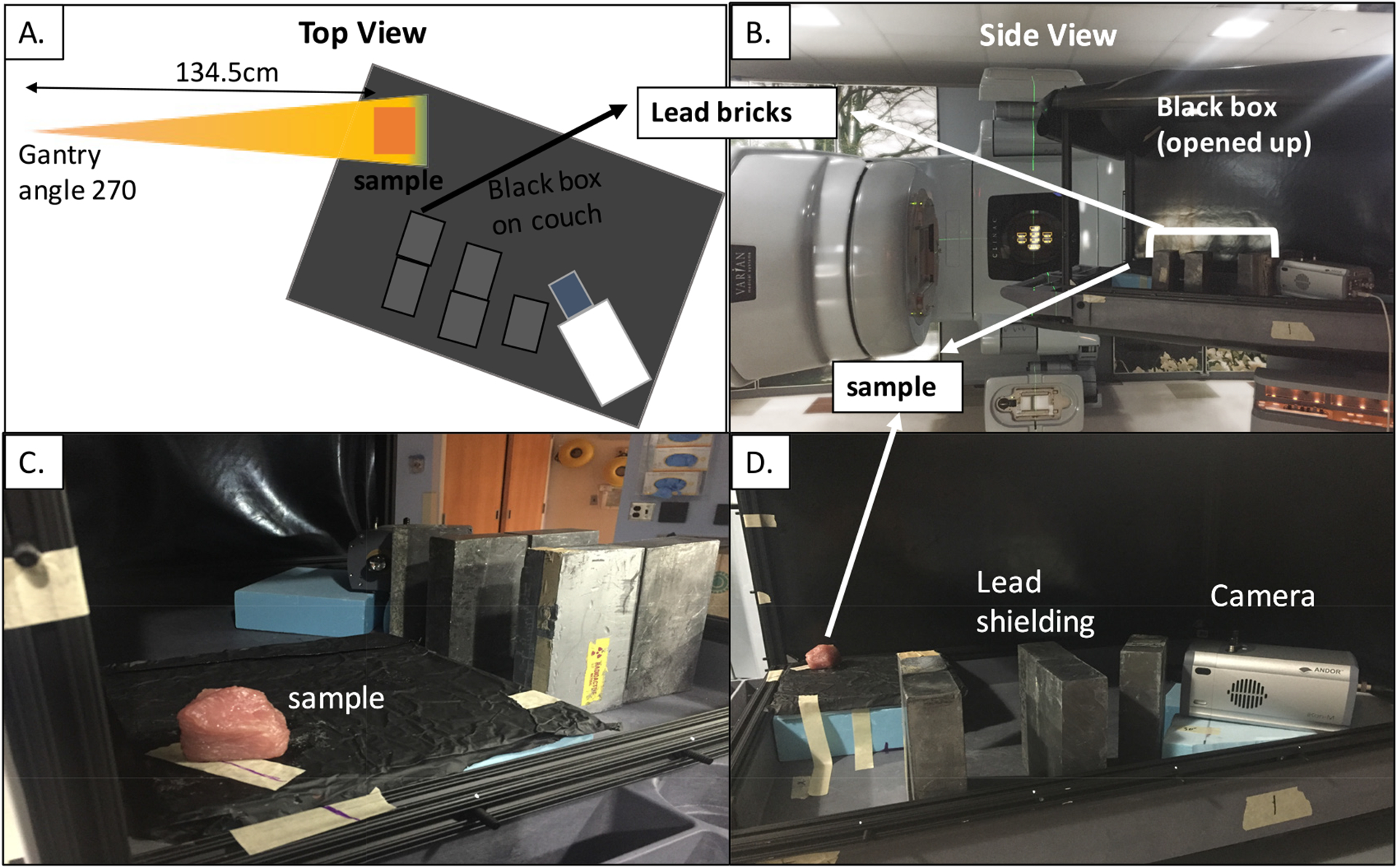
(A): Schematic of experimental setup on the LINAC. (B): Real-life experimental image. (C)& (D): Phantom orientation with respect to camera and lead shielding.
The camera was shielded from background radiation using 10 cm-thick lead bricks, in the optimum geometry identified through trial and error to minimize radiation from the treatment head (see appendix). The phantoms were imaged one at a time, so that the smallest possible field size (3 × 3 cm2) could be used, further reducing radiation noise. Each phantom was placed at a distance of 134.5 cm SSD, and irradiated with 6 MV and 15 MV photon beams to 2000 MU.
Optically stimulated luminescence (OSL) detectors were used to measure dose. OSLs are known to be supralinear at higher doses (close to 10 Gy (Yukihara and McKeever 2008)) so they could not be irradiated to 2000 MU that were delivered to the phantoms. Instead, an OSL was placed on the distal surface of each phantom and irradiated to 200 MU. The measured dose was then scaled by a factor of 10 to obtain the dose delivered by 2000 MU. This dose was then used to normalize the UVA counts detected per region of interest (ROI) in each image.
3.3. Experiment 2: devising a tissue-equivalent optical phantom for UVA CL production
1% agarose gels doped with 100 ppm, 150 ppm, 200 ppm, 250 ppm and 1000 ppm India ink concentrations (figure 4) were imaged. To make the gels, 1 g of dry agarose (Agarose, Standard, Low Electroendosmosis) was mixed with 100 ml water. 10 μl, 15 μl, 20 μl and 25 μl India ink, measured through a pipette, was added to the agarose in 100 ml water solutions. They were heated until the agarose dissolved completely and then poured out in a plastic container and placed in the refrigerator to set. All the phantoms were 2.1 cm thick.
Figure 4.

(A): Flatbed scan of agarose gel samples with varying India ink concentrations (B): (left) image of the sample taken by the camera under exposure to light from a UVA lamp. (right) corresponding UVA Cherenkov image upon 6 MV irradiation.
Chicken breast was used as a control to check for tissue equivalence. The phantoms were imaged one at a time in the same geometry as that of Experiment 1 (figure 3). They were irradiated with a 6 MV beam to 1000 MU. Dose measurements were made using OSLs, irradiated to 200 MU and later scaled by a factor of five.
3.4. Image processing and analysis
Regions of interests (ROI) were manually identified to measure average CCD counts from each sample using the image processing package, Fiji (ImageJ) (Schindelin et al 2012) (figure 5). A small ROI was placed in the brightest region of the signal and a line profile was plotted through it. A linear fit was used to estimate the background and the net signal was obtained by subtracting the average value in the ROI and the average background corresponding to those pixels estimated from the linear fit. The error on the signal was set to the standard deviation on pixel intensities in the ROI (obtained from Fiji measurements).
Figure 5.
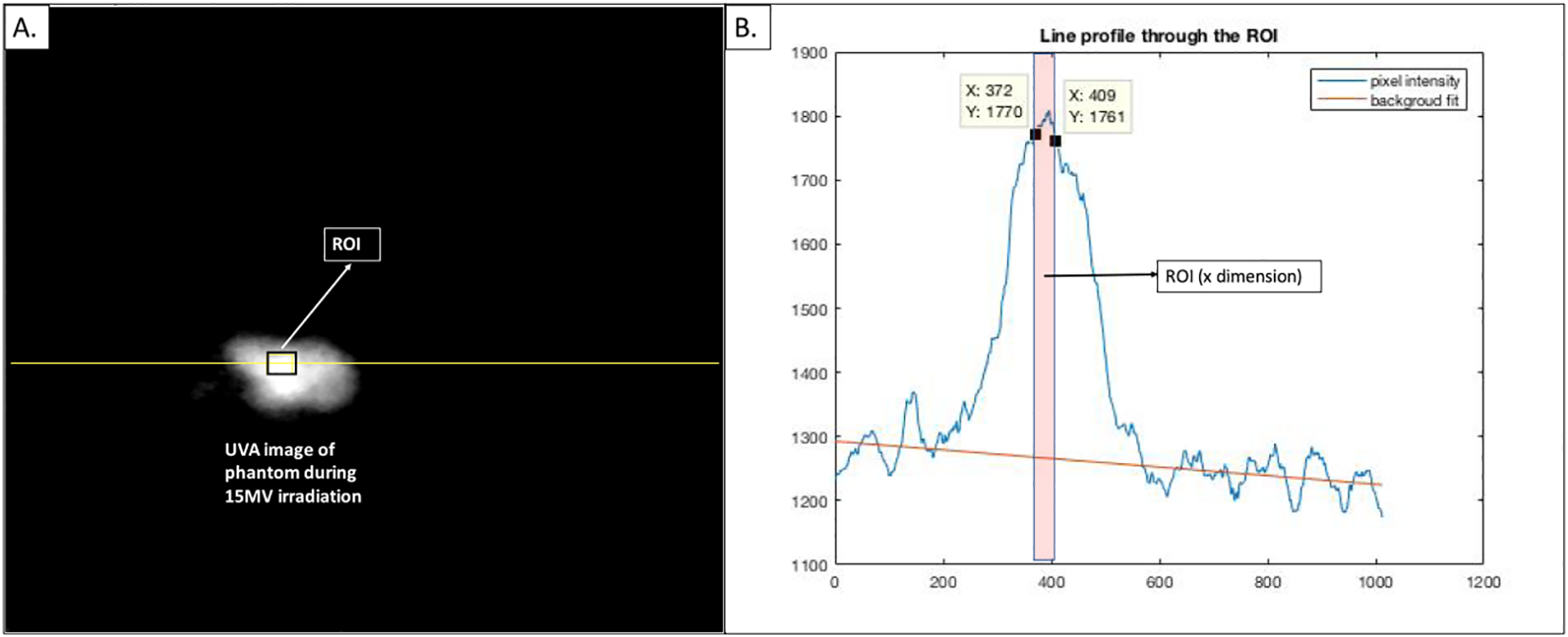
(A): UVA image of a chicken breast sample on 15 MV irradiation, with the ROI (to estimate signal) and line (to estimate background) marked. (B): MATLAB image of corresponding line profile with linear fit to estimate the background.
4. Results
4.1. Experiment 1: UVA emission from solid water and bulk tissue samples
The UVA images obtained upon 15 MV irradiation of bulk tissues and solid water samples are displayed in figure 6. The background subtracted counts per Gray for the 6 MV and 15 MV irradiations are also shown in figure 6.
Figure 6.

(left) UVA images taken by the camera during 15 MV irradiation of (A): pork loin, (B): chicken breast, (C): pork belly and (D): white solid water. (right) Background subtracted UVA intensity per dose from all the samples at 6 MV and 15 MV for the same size ROI in each image. There was negligible emission from brown solid water at 6 MV.
4.2. Experiment 2: UVA emission from agarose gel samples
This experiment was conducted to find the optimum agarose and India ink combination that has equivalent UVA CL to that from tissue upon 6 MV irradiation, using a chicken breast sample as the control. The UVA counts emitted per Gray from the the four agarose phantoms and chicken breast are shown in figure 7.
Figure 7.
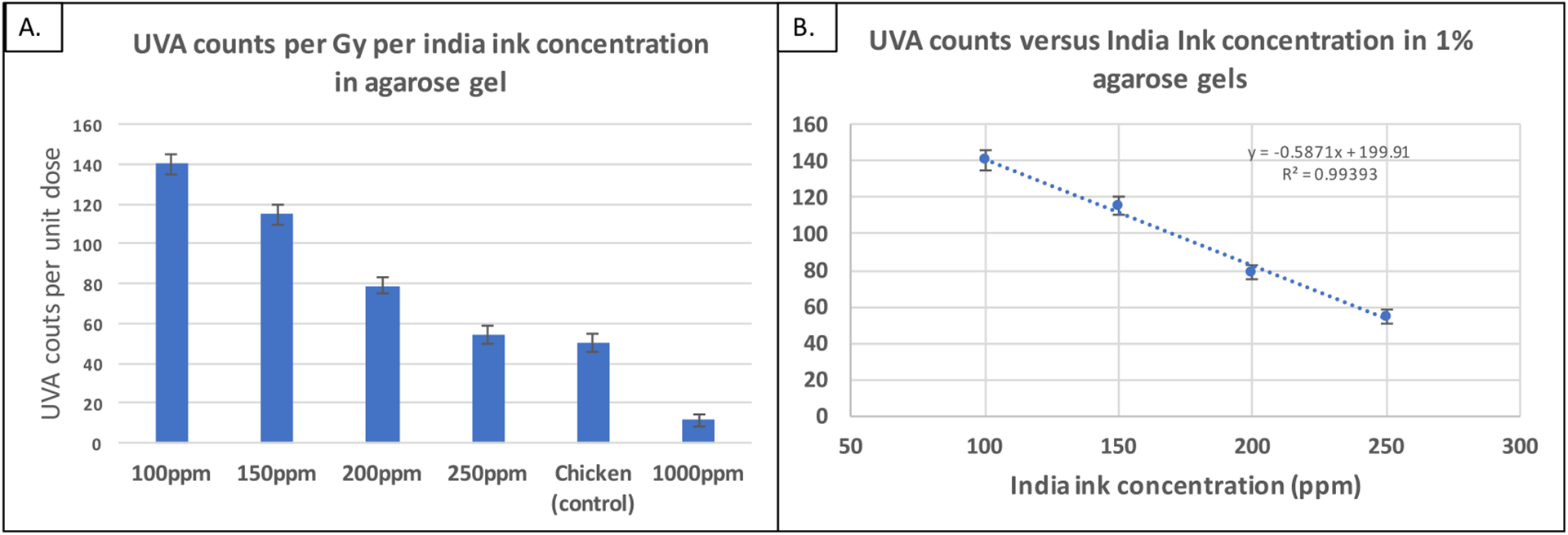
(A): Background subtracted UVA intensities normalized by measured dose for all the samples imaged. (B): Linear fit through UVA signal versus India ink concentration.
A linear decrease in UVA counts is seen with the increasing India ink concentrations. The UVA emission from the 250 ppm India ink agarose sample and the chicken breast sample was within 8%, making it the most equivalent to chicken breast. The less absorbing 200 ppm phantom would likely be representative of tissue that generates more UVA, such as, pork belly.
5. Discussion
The results (figure 6) show that SW had the lowest UVA emission among all the samples studied. This indicates in vivo conditions would generate a higher UVA fluence than was generated by the white SW used in prior studies (Yoon et al 2018). Putting into clinical perspective, this means RECA’s therapeutic response in the in vitro studies was likely underestimated due to the lower UV production and subsequent psoralen activation. RECA may have an even higher therapeutic potential in an in vivo setting than demonstrated in prior work.
There is also a greater UVA Cherenkov emission with increasing energy. This is owed to the fact that at higher energies, the charged particles have longer path lengths along which they can produce Cherenkov. There is also higher emission from the meat samples that had higher fat content (pork belly) than the ones with higher muscle content (chicken breast and pork loin).
Theoretically, Cherenkov output should increase with increasing refractive index at the same beam energy (section 3). Plastics have higher refractive index than tissue (n = 1.59 for plastic and n = 1.41 for tissue), yet our experiments show less Cherenkov emission from plastics. This may be attributed to the optical absorption and scattering of UVA light within the material, which affects the emission that can escape the phantom and get detected by the camera. Glaser et al (2015), in a simulation study, reported 3.5–3.5 × 10 3/cm2/Gyanda 50–5 × 104/cm2/Gy Cherenkov output per radiation dose with and without absorption in tissue, respectively. This result shows that optical absorption and scatter can drastically change Cherenkov light output. Even though more Cherenkov photons are generated in the plastic, not all of these make it out of the material and into the detector.
While the doped agarose hydrogel (1% by weight with 250 ppm India ink) can achieve tissue equivalent parity for MV generated CL emission, there are other factors such as India ink contamination and toxicity to the cells which may need to be investigated. Future studies may also benefit from more sophisticated technique to minimize UVA contamination from CL production in the cell plates (for example, an ex vivo approach for cell-plating may be considered).
Appendix. Radiation background reduction in UVA images
The sources of background affecting the UVA image noise were identified as (i) high-energy leakage from the LINAC head (ii) scatter radiation from the walls, ceiling and the phantom itself and (iii) stray UV-photons from the room lighting. 10 cm thick lead bricks were strategically placed around the camera to shield it from background sources. The irradiation was carried out with a posterior-anterior beam of 6 MV photons to 200 MU, with no phantom in place so as to solely quantify the background radiation for different shielding geometries. The general setup is shown in figure A1.
The average background counts were measured from the images using Fiji (ImageJ) (Schindelin et al 2012). These measurements corresponding to the lead brick geometries are plotted in figure A1. There was a 25 % reduction in background radiation as compared to the control, achieved by primarily shielding the camera from the LINAC head (placing lead bricks in front of the camera, directly above the LINAC head).
After reducing the background, a 3% by weight gelatin phantom was placed within the beam to test whether any signal could be observed. Furthermore, a UVA-reflecting mirror was placed behind the phantom (so that it was in the field of view of the lens but did not lie in the radiation beam) to boost the signal from the phantom. The image acquired is shown in figure A2.
Figure A1.

(A): Setup for camera shielding with lead bricks and imaging a gelatin phantom. Background images were taken without the phantom in place. (B): Background measurements corresponding to the various lead shielding geometries.
Figure A2.

UVA emission upon 6 MV irradiation of the gelatin phantom.
References
- Bethea D et al. 1999. Psoralen photobiology and photochemotherapy: 50 years of science and medicine J. Dermatol. Sci 19 78–88 [DOI] [PubMed] [Google Scholar]
- Čerenkov PA 1937. Visible radiation produced by electrons moving in a medium with velocities exceeding that of light Phys. Rev 52 378–9 [Google Scholar]
- Ding H, Lu J and Wooden W 2006. Refractive indices of human skin tissues at eight wavelengths and estimated dispersion relations between 300 and 1600 nm Phys. Med. Biol 51 1479–89 [DOI] [PubMed] [Google Scholar]
- Gasparro F and Schmitt AFM 1997. Psoralen photobiology: the relationship between dna damage, chromatin structure, transcription, and immunogenic effects Risk Progression Factors Carcinogenesis 143 101–27 [DOI] [PubMed] [Google Scholar]
- Giannios P. et al. Visible to near-infrared refractive properties of freshly-excised human-liver tissues: marking hepatic malignancies. Sci. Rep. 2016;6:27910. doi: 10.1038/srep27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser AK 2015. Applications of Cherenkov light emission for dosimetry in radiation therapy PhD Thesis (Hanover, NH: Thayer School of Engineering, Dartmouth College) [Google Scholar]
- Glaser AK. et al. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015;60:6701. doi: 10.1088/0031-9155/60/17/6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ 2006. Radiobiology for the Radiobiologists (Philadelphia, PA: Lippincott Williams & Wilkins; ) [Google Scholar]
- Inc C I R S. Plastic water—model pw. 2003. www.cirsinc.com/file/Products/PW/PWDS011714(1).pdf.
- Inc S I. Virtual water. 0000. www.standardimaging.com/phantoms/virtual-water.
- Jacques S 2013. Optical properties of biological tissues: a review Phys. Med. Biol 58 R37–61 [DOI] [PubMed] [Google Scholar]
- Moor AC et al. 1995. Treatment with 8-MOP and UVA enhances MHC class I synthesis in RMA cells: preliminary results J. Photochem. Photobiol. B 29 193–8 [DOI] [PubMed] [Google Scholar]
- Oldham M. et al. X-ray psoralen activated cancer therapy (x-pact) PLoS One. 2016;11:e0162078. doi: 10.1371/journal.pone.0162078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OxfordInstruments. Andor IKON-M-934. 2010. https://andor.oxinst.com/products/ikon-xl-and-ikon-large-ccd-series/ikon-m934#product-information-tabs.
- Pathak MA and Fitzpatrick TB 1992. The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD J. Photochem. Photobiol 14 3–22 [DOI] [PubMed] [Google Scholar]
- Pratx G and Kapp DS 2018. In regard to Yoon et al Int. J. Radiat. Oncol 101 494–5 [DOI] [PubMed] [Google Scholar]
- Schindelin J et al. 2012. Fiji: an open-source platform for biological-image analysis Nat. Methods 9 676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt IM, Chimenti S and Gasparro FP 1995a. Psoralen-protein photochemistry: a forgotten field J. Photochem. Photobiol. B 27 101–7 [DOI] [PubMed] [Google Scholar]
- Schmitt IM et al. 1995b. Increased surface expression of class I MHC molecules on immunogenic cells derived from the xenogenization of P815 mastocytoma cells with 8 methoxypsoralen and long-wavelength ultraviolet radiation Tissue Antigens 45 45–9 [DOI] [PubMed] [Google Scholar]
- Shrock Z. et al. Modelling Cerenkov emissions from medical linear accelerators: a Monte Carlo study. Med. Phys. 2016;43:3580. doi: 10.1002/mp.12927. [DOI] [PubMed] [Google Scholar]
- Sultanova N, Kasarova S and Nikolov I 2009. Dispersion properties of optical polymers Acta Phys. Pol 116 585–7 [Google Scholar]
- ThorLabs. UV-fused silica plano-convex spherical lenses. 1999. www.thorlabs.com/navigation.cfm?guide_id=2241.
- Yoon SW 2018. An exploration of the feasibility of combining radiation therapy with psoralen phototherapy PhD Thesis (Durham, NC:Duke University Medical Physics Graduate Program; ) [Google Scholar]
- Yoon SW et al. 2018. Enhancing radiation therapy through Cherenkov light-activated phototherapy Int. J. Radiat. Oncol 100 794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukihara EG and McKeever SWS 2008. Optically stimulated luminescence (OSL) dosimetry in medicine Phys. Med. Biol 53 R351. [DOI] [PubMed] [Google Scholar]


