Abstract
Background:
Delivery and use of HIV pre-exposure prophylaxis (PrEP) are suboptimal in the US. Previous reviews of barriers and facilitators have not used an implementation science lens, limiting comprehensiveness and the link to implementation strategies. To summarize the state of the science, we systematically reviewed determinants of PrEP implementation using the updated Consolidated Framework for Implementation Research (CFIR 2.0).
Setting:
PrEP-eligible communities and delivery settings in the US.
Methods:
In January 2021, we searched Ovid MEDLINE, PsycINFO, and Web of Science for peer-reviewed articles related to HIV/AIDS, interventions, implementation, and determinants or strategies. We identified 286 primary research articles published after 1999 about US-based PrEP implementation. Team members extracted discrete “mentioned” and “measured” determinants, coding each by setting, population, valence, measurement, and CFIR 2.0 construct.
Results:
We identified 1,776 mentioned and 1,952 measured determinants from 254 and 239 articles, respectively. Two-thirds of measured determinants were of PrEP use by patients as opposed to delivery by providers. Articles contained few determinants in the inner setting or process domains (i.e., related to the delivery context), even among studies of specific settings. Determinants across priority populations also focused on individual patients and providers rather than structural or logistical factors.
Conclusion:
Our findings suggest substantial knowledge in the literature about general patient-level barriers to PrEP use and thus limited need for additional universal studies. Instead, future research should prioritize identifying determinants, especially facilitators, unique to understudied populations and focus on structural and logistical features within current and promising settings (e.g., pharmacies) that support integration of PrEP into clinical practice.
Keywords: HIV/AIDS, pre-exposure prophylaxis, determinants of implementation, systematic review, implementation science, consolidated framework for implementation research
Introduction
Pre-exposure prophylaxis (PrEP) is a highly-effective preventive intervention for HIV1 and a critical component of ending the HIV epidemic (EHE) in the US2. However, its implementation is suboptimal3. Recent estimates indicate that only 23% of 1.2 million people indicated for PrEP in the US are receiving it4—far below the EHE goal of 50% coverage2. Additionally, adherence and persistence data indicate poor sustainment of the intervention among patients.5,6 Factors believed or shown to influence implementation are known as determinants of implementation, also frequently called barriers and facilitators.7 Multiple studies have examined barriers to PrEP implementation at the provider8 and patient levels.9 Understanding determinants is crucial to selecting and testing implementation strategies that will achieve the implementation success needed to end the epidemic.10
A recent review of HIV-related implementation research funded by the US National Institutes of Health identified a substantial number of studies that were considered “implementation preparation”, defined as studies in preparation for a formal evaluation or test of implementation strategies.11 These commonly included an aim, or focused exclusively, on understanding determinants. Moreover, several previously published reviews of published articles regarding PrEP implementation have shown considerable focus on understanding barriers/facilitators of PrEP, particularly at the individual-level. We identified 23 systematic reviews conducted in the last five years that examined PrEP implementation, of which 16 examined determinants at the patient, provider, and/or systems levels.6,8,12–25 The majority of these focused heavily on barriers, with less attention on facilitators, among US-based samples of specific priority populations (e.g., MSM,12,22–24,26 persons who inject drugs,18 women,17,19 adolescents25,27) or types of providers (e.g., nurse practitioners, pharmacists).16,28 Primary barriers at the patient level included a lack of knowledge about PrEP (e.g., safety, efficacy, indications), HIV stigma, low perceived HIV risk (e.g., self-evaluation of risk behaviors), cost concerns (e.g., insurance status and coverage), concerns about side effects, and access to culturally competent PrEP-related services. Main barriers at the provider level included a lack of knowledge about PrEP and concerns about PrEP costs to patients, patient adherence, and side effects. Facilitators at the patient level included PrEP knowledge and partner and peer support, while facilitators at the provider level included access to data on PrEP efficacy. Despite being implementation-focused systematized reviews, only two reviews used an implementation science framework,18,21 which potentially limits the ability to link findings to the larger implementation science literature on strategies that can overcome barriers and build on facilitators. Reviews not guided by implementation science frameworks could also have resulted in incompleteness due to the diverse array of multilevel determinants. Classifying determinants using a comprehensive implementation science framework is needed to advance both the research on and practice of PrEP implementation.
To fill existing gaps in the PrEP implementation literature, we conducted a systematic review to identify multilevel determinants of PrEP in the US using the updated Consolidated Framework for Implementation Research (CFIR, version 2.0).29–32 In addition to greater specification and relabeling of various constructs, particularly in the outer setting domain, a major change in CFIR 2.0 involves differentiating between implementation determinants, which “capture setting-level barriers and facilitators that predict and/or explain…implementation outcomes,” and innovation determinants, which “capture recipient-level characteristics and/or experiences with the innovation that predict and/or explain innovation outcomes.”30 We conceptualized this distinction as two segments along a continuum of implementation, with the former affecting anticipated or actual adoption, implementation, and sustainment of an innovation by deliverers and the latter affecting uptake, use, adherence, and ultimately effectiveness of an innovation among recipients. (See Appendix A for a list of domains and constructs by implementation target.)
In this article, we aimed to summarize the state of the science on PrEP implementation barriers and facilitators to increase the impact of implementation research in ensuring the population-level utility of PrEP. Our review also identifies areas of determinants research that are saturated—so researchers can turn their attention away from further identifying determinants to instead testing strategies informed by these determinants—as well as areas in need of further investigation on barriers and facilitators. Whereas previous reviews focused on determinants within specific service settings/geographic regions, or for specific populations, our review examines existing literature across diverse key delivery settings and EHE priority target populations.
Methods
The current review on implementation determinants of PrEP focuses on a subset of studies from a larger comprehensive review of determinants of and strategies for implementing evidence-based HIV prevention and care interventions. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)33 flow diagram of the steps in process.
Figure 1.
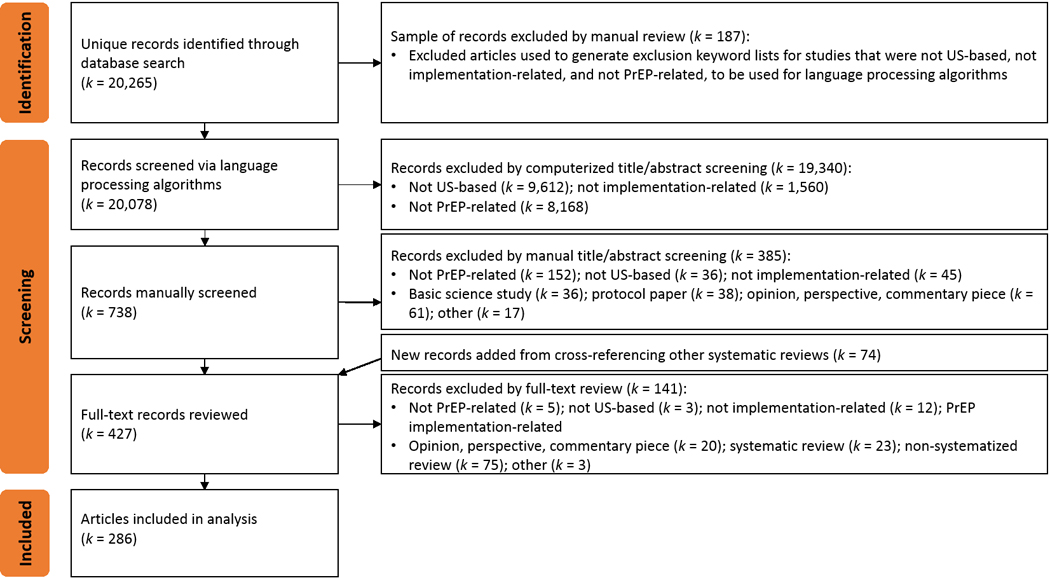
PRISMA flowchart for articles related to PrEP implementation
Identification: Comprehensive search of the HIV implementation research literature
Between November 2020 and January 2021, our team developed a broad search strategy to capture implementation-related studies for all evidence-based interventions along the HIV prevention and care continua.34,35 The protocol for this search is registered with the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42021233089). Following the Problem–Intervention–Comparison–Outcome (PICO) framework,36 a clinical informationist (author CM) searched Ovid MEDLINE [1946—January 19th, 2021], PsycINFO (EBSCOhost), and Web of Science (Clarivate Analytics) [2007–2021] for peer-reviewed articles published in English that contained the following main words and related terms (examples in parentheses) in their titles or abstracts: (a) HIV (e.g., AIDS, human immunodeficiency virus, sexually transmitted disease); (b) intervention (e.g., HIV prevention, HIV treatment, antiretroviral therapy, pre-exposure prophylaxis, post-exposure prophylaxis, linkage to care, patient navigation, testing, medication adherence, retention in care, condom); (c) implementation (e.g., adoption, uptake, utilization, delivery, quality improvement, health services, program evaluation); and either (d) determinant (e.g., barrier, facilitator, factor, context) or (e) strategy (e.g., implementation intervention, support, monitor, implementation approach, facilitation, training, adapt, technical assistance, partnership). After record deduplication, we used a computer algorithm to remove records that were tagged as books or conference proceedings, records that were published before 2000, and records that did not contain specifically “HIV” or “AIDS” in the title or abstract.
Screening: Domestic PrEP implementation research
To identify only those articles focused on domestic PrEP implementation that also met our other inclusion/exclusion criteria, we conducted a three-phase screening procedure: (1) semi-automated computerized study exclusion, (2) double-screening of titles and abstracts, and (3) full-text review. In Phase 1, we used language processing algorithms to first exclude records that were (a) studies conducted outside the US or (b) not implementation-related studies. For each criterion (e.g., US- vs. non-US-based study), we created a pair of dictionaries to specify keywords for inclusion (US cities, states, counties, demonyms) and exclusion (non-US cities, countries, continents, country demonyms). Records that contained any exclusion terms and zero inclusion terms were excluded. Then, we further excluded records that did not contain the keywords “PrEP,” “preexposure,” “pre-exposure,” or “prophylaxis” in the title or abstract.
After the computerized exclusions, senior researchers on our team (authors DHL and JDS) trained five screeners with masters-level training in health research to screen titles and abstracts against inclusion and exclusion criteria using Covidence software (Phase 2).37 We included records if they were related to PrEP; based in the US; and considered dissemination or implementation research, defined as studies on how evidence-based practices are spread, translated, or used in real-world settings. We included studies of patient-oriented strategies that support patients’ use of PrEP (e.g., patient navigation, case management, medication adherence programs) as implementation research even if they were described as effectiveness studies. We excluded records if they were basic science research; protocol papers; opinion, perspective, or commentary pieces; studies about research recruitment; or studies solely focused on comorbidities among people with HIV. Two screeners reviewed each record, and discrepancies were reconciled by team members more experienced with IS. Records deemed “Maybe” by both screeners were automatically included for full-text review. Senior team members conducted a random-sample audit of records at this stage with a 5% threshold for misclassification. The audit identified erroneously excluded records above this threshold, prompting rescreening of all excluded records by senior team members.
Before full-text review in Phase 3, we identified additional records for potential inclusion by examining the citation lists of previous systemized reviews on PrEP. Then, in Phase 3, we obtained the full text for all remaining articles, to which the screeners applied the inclusion and exclusion criteria. Additionally, screeners excluded other review articles and studies that did not contain implementation determinants.
Extraction and coding
We developed two data extraction tools: a Microsoft Form to capture study-level information (e.g., setting, target populations) and a Microsoft Excel spreadsheet to capture discrete implementation determinants of PrEP. The lead author trained four extractors (who previously served as screeners) on these tools and on how to identify determinants in article text and tables; he also monitored extraction quality and provided feedback throughout this process. Informed by the multilevel domains of CFIR, extractors noted all conditions, characteristics, states, and traits presented in articles as influencing either the provision/delivery of PrEP by the health system or the uptake and sustained use of PrEP by patients (Round 1 identification and coding). We distinguished between determinants that were “mentioned” in the introduction or discussion sections from those that were formally “measured” using quantitative, qualitative, or mixed/multi methods as part of the reported study. This distinction between measured and mentioned is important for guiding focus to those determinants based on empirical evidence rather than on theory, anecdotes, or citing previous research where it is unclear whether the determinant was measured or not. For the former, extractors coded the level of supporting evidence provided (i.e., no citation, citation of a model, citation of prior empirical studies), and for both sets, they coded the valence of the determinants (i.e., barrier, facilitator, both, neither, unspecified/unsure).
A second coding team, comprising four implementation researchers familiar with CFIR 2.0, coded each extracted determinant to a construct from the framework, differentiating between implementation and innovation targets (Round 2 coding). Coding challenges were flagged for group discussion and reconciliation.
Analysis and synthesis
We tabulated the number of discrete determinants and articles by mentioned and measured CFIR 2.0 constructs using Microsoft Excel. Using data from the Microsoft Form, we further stratified determinants by common PrEP delivery settings (HIV, infectious disease, and LGBT specialty or primary care, hereafter “HIV specialty clinics”; non-HIV primary care, including STI and family planning clinics; pharmacies; and substance use treatment centers) and CDC priority target populations (gay, bisexual, and other men who have sex with men [GBMSM]; Black or African Americans; Latinx or Hispanic individuals; people who use/inject drugs; and transgender individuals). Studies that included multiple settings or target populations were included in counts for all relevant categories.
Results
Our broad search strategy for the comprehensive review identified 20,265 unique records, which computerized methods using exclusion keyword lists based on 187 manually excluded records reduced to 8,906 records about domestic implementation of all HIV interventions and then to 738 specifically about PrEP (Figure 1). Manual title/abstract and full-text screening resulted in 286 articles included in this review (see Appendix B for a complete list).
Extractors identified Nmentioned = 1,776 mentioned determinants from 254 of the articles: 1,280 determinants were coded as barriers; 355 as facilitators; 49 as both barriers and facilitators; and 92 as unspecified. Approximately 20% of mentioned determinants did not reference a previous study or theoretical model. For measured determinants, extractors identified Nmeasured = 1,952 determinants from 239 articles: 1,112 barriers, 563 facilitators, 64 both, 96 neither (measured but found to have no effect), and 117 unspecified/unsure. Among measured determinants, more were measured in quantitative studies (n = 1,069 from 179 articles) than qualitative ones (n = 824 from 82 articles), and 59 (7 articles) were assessed using mixed/multi-method approaches.
Table 1 presents the distribution of mentioned and measured determinants by CFIR 2.0 constructs, along with examples of common barriers and facilitators. About a third of measured determinants (n = 714; 36.6%) explicitly impacted delivery of PrEP while the other two-thirds (n = 1,238; 63.4%) were determinants of PrEP use. The domains with explicit individual patient foci (i.e., patient characteristics and their perspectives about PrEP in innovation determinants) together accounted for 46.6% (n = 909) of total measured determinants, whereas the equivalent domains for individual providers comprised 18.3% (n = 357). The most studied constructs were other personal attributes of individual patients (n = 462), sociological characteristics (n = 247), and innovation characteristics that did not fit other constructs (n = 123)—all innovation determinants—followed by outer setting patient characteristics (implementation determinant; n = 92), other personal attributes of individual providers (implementation determinant; n = 88), and patient and provider knowledge and attitudes about the innovation (ns = 79 each). Table 2 presents the measured determinants by method of data collection.
Table 1.
Number (n), proportion, and examples of mentioned and measured determinants by CFIR 2.0 construct among K = 286 articles related to PrEP implementation
| Target, Domain, and Construct | Mentioned | Measured | Examples | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | k | n | % | k | Barriers | Facilitators | |||
| Total | 1776 | 254 | 1952 | 239 | ||||||
| Implementation determinants | Innovation characteristics (deliverers’ perceptions) | Source | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A |
| Evidence base | 25 | 1.4% | 20 | 28 | 1.4% | 17 | • Uncertainty around PrEP real-world effectiveness in different populations • Discomfort with prescribing drugs for new indications |
• Additional clinical efficacy data for different populations • Belief that PrEP is efficacious and has public health benefit |
||
| Relative advantage | 4 | 0.2% | 4 | 10 | 0.5% | 8 | • Belief that that behavioral interventions are safer and more effective | • Belief that PrEP is more effective than condoms | ||
| Adaptability | 2 | 0.1% | 2 | 0 | 0.0% | 0 | N/A | • Belief that PrEP can be adopted by a range of subspecialties and clinical environments • Belief that PrEP can be integrated with other effective HIV-prevention tools |
||
| Trialability | 0 | 0.0% | 0 | 1 | 0.1% | 1 | • Reduced trialability of long-acting injectable PrEP | N/A | ||
| Complexity | 33 | 1.9% | 24 | 29 | 1.5% | 19 | • Concern about supporting patient adherence to daily pill • Clinical and lab monitoring requirements |
• Required regular follow-up visits allow providers to assess ongoing HIV risk and provide ongoing adherence counseling | ||
| Design | 3 | 0.2% | 3 | 2 | 0.1% | 2 | • Lack of culturally specific or tailored marketing and education materials | N/A | ||
| Cost | 19 | 1.1% | 19 | 18 | 0.9% | 18 | • Cost to patient is too high, leading to provider discontinuing prescription • Cost of PrEP to the system is too high |
• PrEP can be cost-saving or cost-effective from a public health perspective | ||
| Other innovation characteristic that does not fit above | 52 | 2.9% | 30 | 61 | 3.1% | 28 | • Concerns about side effects, increasing drug resistance, risk compensation, impact on incidence of other STIs | • Belief that PrEP could empower women who are unable to negotiate condom use | ||
| Domain subtotal | 138 | 7.8% | 63 | 149 | 7.6% | 47 | ||||
| Outer setting | Critical incidents | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | |
| Recipient characteristics | 63 | 3.5% | 41 | 92 | 4.7% | 40 | • Beliefs that certain patients (e.g., women) do not need PrEP • Beliefs that certain patients (e.g., people who use drugs, youth) have reduced capacity to adhere • Populations with high risk but low PrEP use affect models of public health impact |
• Patient discloses HIV risk factors (e.g., being a man who has sex with men, being in a sero-discordant relationship) • Patient requests PrEP prescription |
||
| Socioecological characteristics | 50 | 2.8% | 36 | 43 | 2.2% | 27 | • Complexity of the medical system • Provider deserts, built environment • Resources for marginalized groups are not prioritized • Lack of culturally competent provider training and services for populations indicated for PrEP |
• Shift in HIV prevention toward biomedical intervention • Metropolitan area • Safety net health systems |
||
| Partnerships and connections | 11 | 0.6% | 5 | 13 | 0.7% | 7 | • Poor coordination between local healthcare organizations | • Formality of partnerships | ||
| Market forces | 6 | 0.3% | 4 | 5 | 0.3% | 5 | • Active discouragement of clients to utilize services of other organizations | • Perceived norm of PrEP prescription among peer providers | ||
| Policies | 106 | 6.0% | 82 | 26 | 1.3% | 19 | • Fragmentation of HIV services, impacting sero-discordant couples • Lack of FDA approval for minors (at the time) |
• Prescription guidelines from top normative agencies (e.g., CDC, WHO) | ||
| Performance goals | 2 | 0.1% | 2 | 0 | 0.0% | 0 | N/A | • Goals to scale up PrEP as part of local Ending the HIV Epidemic plans | ||
| Financing | 19 | 1.1% | 14 | 22 | 1.1% | 21 | • Uncertainty about financial coverage or reimbursement for PrEP-related costs | • Medicaid approval for PrEP coverage | ||
| Domain subtotal | 257 | 14.5% | 137 | 201 | 10.3% | 75 | ||||
| Inner setting | Structural characteristics | 12 | 0.7% | 11 | 17 | 0.9% | 12 | • HIV providers see relatively few patients • Primary care physicians need a large patient base to keep up to date with high-quality PrEP care |
• Larger organizations can offer more comprehensive HIV services and have more resources to engage the most vulnerable populations | |
| Relational connections | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Communications | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Culture | 4 | 0.2% | 2 | 6 | 0.3% | 4 | • Organization is not sensitive to patients’ needs and priorities unrelated to HIV | • Institutional willingness to implement new clinical protocols | ||
| Tension for change | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Compatibility | 35 | 2.0% | 25 | 22 | 1.1% | 15 | • Purview paradox: disagreement over which providers should prescribe PrEP • Staff are used to focusing only on the needs of clients living with HIV |
• Methadone maintenance programs already require routine counseling, care coordination, and drug dispensing • Pharmacy hours extend beyond typical business hours • Belief that HIV prevention is essential to family planning clinic operations |
||
| Relative priority | 2 | 0.1% | 2 | 5 | 0.3% | 5 | • Need to manage competing health concerns during clinical encounters | • Perception that PrEP is a high priority for the organization | ||
| Incentive systems | 1 | 0.1% | 1 | 0 | 0.0% | 0 | • Incentives (or lack thereof) for pharmacists to prescribe PrEP | N/A | ||
| Mission alignment | 4 | 0.2% | 4 | 3 | 0.2% | 3 | ||||
| Leadership engagement | 5 | 0.3% | 4 | 8 | 0.4% | 7 | • Lack of leadership support | • Leadership buy-in for offering PrEP • Leaders are involved in administrative decisions |
||
| Available resources | 54 | 3.0% | 36 | 44 | 2.3% | 31 | • Concerns about time constraints during clinical encounters • Lack of personnel to manage increase administrative burden |
• Having a private room to ensure privacy and facilitate PrEP counseling • Resources to compensate patients to come back for follow-up |
||
| Access to knowledge and information | 16 | 0.9% | 11 | 35 | 1.8% | 16 | • No protocols for PrEP referral or initiation and maintenance | • Access to previous training or continuing education about PrEP • Availability of on-site support |
||
| Domain subtotal | 133 | 7.5% | 57 | 140 | 7.2% | 45 | ||||
| Characteristics of individuals (deliverers) | General knowledge and attitudes about the innovation | 80 | 4.5% | 56 | 79 | 4.0% | 41 | • Lack of awareness of PrEP • Uncertainty around how to determine patient eligibility |
• Belief that PrEP is good for public health • Knowledge about insurance/financial resources for PrEP |
|
| Self-efficacy, comfort, and prior experience delivering PrEP | 29 | 1.6% | 21 | 33 | 1.7% | 19 | • Discomfort prescribing PrEP • Belief that they cannot help patients stay adherent |
• Prior experience prescribing PrEP • Perceived skills (among pharmacists) to counsel patients on PrEP |
||
| Individual stage of change, interest, and willingness to implement PrEP | 12 | 0.7% | 10 | 8 | 0.4% | 7 | • Resistance to PrEP | • Willingness to prescribe PrEP • Desire for more education on PrEP |
||
| Individual identification with organization | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Other personal attributes | 27 | 1.5% | 19 | 88 | 4.5% | 36 | • Lack of/variations in provider training and competence about PrEP • Disapproval of patients’ motivations for seeking PrEP |
• Comfort discussing sexual behavior and health with patients • Having liberal values |
||
| Domain subtotal | 148 | 8.3% | 67 | 208 | 10.7% | 64 | ||||
| Process | Teaming | 2 | 0.1% | 2 | 2 | 0.1% | 2 | N/A | • Pharmacies forming partnerships with PrEP-prescribing physicians | |
| Assessing | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Planning | 1 | 0.1% | 1 | 3 | 0.2% | 2 | N/A | • Preparing staff for biomedical HIV prevention • Determining how to obtain reimbursement for clinic services provided by pharmacists |
||
| Engaging | 5 | 0.3% | 5 | 6 | 0.3% | 4 | • Challenges accessing and engaging individuals at high risk for HIV acquisition not currently in care • Lack of effective patient–provider communication around PrEP |
• Engaging community partners and peers to disseminate information and link individuals to PrEP services • Assessing and utilizing preferred communication methods |
||
| Doing | 5 | 0.3% | 3 | 5 | 0.3% | 4 | • Paucity of evidence on (strategies for) implementing PrEP | • Providing lab results for monitoring PrEP drug levels perceived as useful | ||
| Reflecting and evaluating | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Adapting | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | ||
| Domain subtotal | 13 | 0.7% | 2 | 16 | 0.8% | 2 | ||||
| Implementation determinants subtotal | 689 | 38.8% | 186 | 714 | 36.6% | 115 | ||||
| Innovation determinants | Innovation characteristics (recipients’ perceptions) | Source | 1 | 0.1% | 1 | 1 | 0.1% | 1 | • Mistrust of pharmaceutical industry | N/A |
| Evidence base | 21 | 1.2% | 20 | 29 | 1.5% | 26 | • Doubts that PrEP can provide complete protection • Newness of the drug |
• Belief in the efficacy/effectiveness of PrEP | ||
| Relative advantage | 14 | 0.8% | 10 | 41 | 2.1% | 20 | • Ambivalence about integrating PrEP into existing HIV prevention practices • Use of PEP as an alternative |
• Acknowledgement of the value of using PrEP in addition to condoms • Viewed as the only woman-controlled HIV prevention method • Varying preferences for different forms of PrEP (e.g., injectable, daily oral) |
||
| Adaptability | 0 | 0.0% | 0 | 3 | 0.2% | 2 | N/A | • Allows for individualized approaches to routine integration and adherence | ||
| Trialability | 0 | 0.0% | 0 | 1 | 0.1% | 1 | N/A | N/A | ||
| Complexity | 57 | 3.2% | 40 | 60 | 3.1% | 39 | • Frequency of required clinic visits as a barrier to uptake • Challenges adhering to daily medication and over time |
• Attending regular PrEP visits supports adherence | ||
| Design | 5 | 0.3% | 4 | 15 | 0.8% | 8 | N/A | • Perception that the pharmaceutical feel of pill makes it more powerful | ||
| Cost | 61 | 3.4% | 53 | 64 | 3.3% | 56 | • Concern about paying for PrEP • Out-of-pocket costs for necessary labs and visit copays |
• Perception that PrEP was offered at no cost to patients | ||
| Other innovation characteristic that does not fit above | 88 | 5.0% | 60 | 123 | 6.3% | 61 | • Concerns about side effects, toxicity, interactions with other medications or illicit drugs • Concern that PrEP allows for or pressures one into more sexual promiscuity and risk • Perception that the promotion of PrEP distracts from finding a cure for HIV |
• Increased access to health care and social services while on PrEP • PrEP alleviates anxiety about acquiring HIV through sex, especially in sero-discordant relationships • Perception of more empowerement in sexual decision making |
||
| Domain subtotal | 247 | 13.9% | 105 | 337 | 17.3% | 104 | ||||
| Outer setting | Critical incidents | 0 | 0.0% | 0 | 0 | 0.0% | 0 | N/A | N/A | |
| Provider characteristics | 15 | 0.8% | 13 | 19 | 1.0% | 13 | • Provider’s lack of knowledge or support of PrEP • Perception that PrEP should be obtained from a specialist (vs. a primary care physician) |
• Positive provider relationship • Receiving PrEP from an attending physician (vs. a trainee or mid-level provider) |
||
| Socioecological characteristics | 355 | 20.0% | 122 | 247 | 12.7% | 94 | • Stigma or discrimination related to PrEP, HIV, LGBTQ identity, sexual behavior • Mistrust of the healthcare system • Social determinants of health (e.g., poverty, unemployment, unstable housing, lack of insurance) • Complexity of the medical system • Concerns about availability and/or accessibility of PrEP |
• Convenient, familiar, and accessible clinic locations • Access to mass transit allowing for routine clinic visits • Having a primary care provider • Cultural values of caballerismo |
||
| Community connections | 27 | 1.5% | 20 | 34 | 1.7% | 24 | • Lack of partner support for taking PrEP • Lack of communication about health issues among community members |
• Endorsement of PrEP by community members • Tangible support for PrEP adherence from partners and family • Having a higher proportion of individuals with HIV in one’s social network |
||
| Policies | 3 | 0.2% | 3 | 2 | 0.1% | 2 | • Segregated healthcare for sero-discordant couples • Programmatic regulations (e.g., expiring patient assistance program, requirement for updated lab tests) |
N/A | ||
| Financing | 20 | 1.1% | 17 | 27 | 1.4% | 20 | • Insufficient insurance coverage for lab tests and medical visits • Being on a parent’s insurance |
• Government- and pharmaceutical-sponsored payment assistance programs | ||
| Domain subtotal | 420 | 23.6% | 133 | 329 | 16.9% | 110 | ||||
| Characteristics of individuals (recipients) | General knowledge and attitudes about the innovation | 85 | 4.8% | 68 | 79 | 4.0% | 54 | • Low levels of PrEP awareness and knowledge | • Positive attitudes about the effects of PrEP | |
| Self-efficacy, comfort, and prior experience using PrEP | 9 | 0.5% | 8 | 18 | 0.9% | 17 | • Discomfort talking to providers about PrEP • Low medical decision-making agency |
• Belief in ability to adhere to daily dosing • High self-efficacy to navigate PrEP-related medical care |
||
| Individual stage of change, interest, and willingness to use PrEP | 11 | 0.6% | 9 | 13 | 0.7% | 11 | • Low readiness for a PrEP regimen • Lack of motivation to take PrEP |
• Interest in and willingness to use PrEP | ||
| Other personal attributes | 315 | 17.7% | 121 | 462 | 23.7% | 131 | • Self-perception as being low HIV risk • Competing health priorities • Being in a relationship • Poor mental health and/or substance use • Difficulty integrating PrEP into changing routine |
• Motivation to stay healthy • Fear of HIV acquisition • Having discussed having sex with men with a healthcare provider • Being in a sero-discordant relationship • Identifying as gay |
||
| Domain subtotal | 420 | 23.6% | 150 | 572 | 29.3% | 151 | ||||
| Innovation determinants subtotal | 1087 | 61.2% | 187 | 1238 | 63.4% | 179 | ||||
Table 2.
Number (n) and proportion of measured determinants by CFIR 2.0 domain and data collection method among K = 286 articles related to PrEP implementation
| Qualitative | Quantitative | Mixed/Multi-Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target and Domain | n | % | k | n | % | k | n | % | k | |
| Total | 824 | 82 | 1069 | 179 | 59 | 7 | ||||
| Implementation determinants | Innovation characteristics (providers’ perceptions) | 66 | 8.0% | 17 | 81 | 7.6% | 28 | 2 | 3.4% | 2 |
| Outer setting | 81 | 9.8% | 29 | 118 | 11.0% | 45 | 2 | 3.4% | 2 | |
| Inner setting | 70 | 8.5% | 22 | 60 | 5.6% | 25 | 10 | 16.9% | 1 | |
| Characteristics of individuals (providers) | 56 | 6.8% | 23 | 143 | 13.4% | 45 | 9 | 15.3% | 2 | |
| Process | 11 | 1.3% | 1 | 4 | 0.4% | 0 | 1 | 1.7% | 1 | |
| Innovation determinants | Innovation characteristics (patients’ perceptions) | 212 | 25.7% | 57 | 122 | 11.4% | 47 | 3 | 5.1% | 2 |
| Outer setting | 185 | 22.5% | 53 | 131 | 12.3% | 58 | 13 | 22.0% | 4 | |
| Characteristics of individuals (patients) | 143 | 17.4% | 50 | 410 | 38.4% | 109 | 19 | 32.2% | 5 | |
Delivery settings
For articles that focused on a particular delivery setting or patient population, Table 3 presents the distribution of measured determinants in each CFIR 2.0 domain stratified by common settings and CDC priority target populations. We identified similar distributions of determinants across domains for HIV specialty clinics and non-HIV primary care, with high percentages in the characteristics of individual providers and patients as well as the implementation outer setting domains. However, differences arose in specific facilitators and barriers (data not displayed). For instance, articles characterized HIV specialty clinics as generally having PrEP training and high provider knowledge about PrEP (facilitators) but also found that those providers believed PrEP to be better suited for general primary care settings (barrier). Articles about non-HIV primary care settings identified lower PrEP knowledge, lack of training and clinical guidelines, more frequent stigma and discrimination, and competing health priorities as challenges but reported providers’ ability to link clients to other services and willingness to learn new protocols to provide preventive care as facilitators. Time and personnel constraints were common barriers in both settings.
Table 3.
Number (n) and proportion of measured determinants by CFIR 2.0 domain from articles (k) examining specific delivery settings and CDC priority target populations
| By Setting | By Population | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV/ID/LGBT specialty or primary care | Non-HIV primary care, including STI and family planning clinics | Pharmacies | Substance use treatment center | GBMSM | Black/African American | Latinx/ Hispanic |
PWUD/PWID | Transgender | ||||||||||||||||||||
| Target and Domain | n | % | k | n | % | k | n | % | k | n | % | k | n | % | k | n | % | k | n | % | k | n | % | k | n | % | k | |
| Total | 582 | 70 | 613 | 80 | 61 | 7 | 109 | 10 | 876 | 120 | 434 | 53 | 200 | 27 | 153 | 24 | 236 | 30 | ||||||||||
| Implementation determinants | Innovation | 85 | 14.6% | 23 | 56 | 9.1% | 18 | 11 | 18.0% | 2 | 11 | 10.1% | 4 | 18 | 2.1% | 14 | 2 | 0.5% | 2 | 1 | 0.5% | 1 | 12 | 7.8% | 5 | 5 | 2.1% | 4 |
| Outer setting | 103 | 17.7% | 34 | 80 | 13.1% | 28 | 3 | 4.9% | 2 | 6 | 5.5% | 2 | 59 | 6.7% | 23 | 15 | 3.5% | 11 | 11 | 5.5% | 9 | 15 | 9.8% | 9 | 19 | 8.1% | 6 | |
| Inner setting | 62 | 10.7% | 15 | 59 | 9.6% | 20 | 13 | 21.3% | 5 | 6 | 5.5% | 4 | 24 | 2.7% | 8 | 10 | 2.3% | 3 | 8 | 4.0% | 2 | 5 | 3.3% | 3 | 12 | 5.1% | 3 | |
| Individuals (providers) | 114 | 19.6% | 32 | 102 | 16.6% | 32 | 17 | 27.9% | 3 | 6 | 5.5% | 3 | 20 | 2.3% | 10 | 10 | 2.3% | 7 | 8 | 4.0% | 5 | 8 | 5.2% | 4 | 13 | 5.5% | 3 | |
| Process | 5 | 0.9% | 0 | 4 | 0.7% | 1 | 3 | 4.9% | 1 | 0 | 0.0% | 0 | 5 | 0.6% | 1 | 4 | 0.9% | 1 | 2 | 1.0% | 0 | 1 | 0.7% | 0 | 2 | 0.8% | 0 | |
| Innovation determinants | Innovation | 65 | 11.2% | 24 | 85 | 13.9% | 24 | 5 | 8.2% | 1 | 24 | 22.0% | 7 | 194 | 22.1% | 61 | 125 | 28.8% | 27 | 59 | 29.5% | 9 | 35 | 22.2% | 12 | 57 | 24.2% | 10 |
| Outer setting | 49 | 8.4% | 0 | 74 | 12.1% | 33 | 4 | 6.6% | 1 | 30 | 27.5% | 9 | 180 | 20.5% | 61 | 109 | 25.1% | 31 | 54 | 27.0% | 17 | 30 | 19.6% | 12 | 57 | 24.2% | 18 | |
| Individuals (patients) | 99 | 17.0% | 34 | 153 | 25.0% | 41 | 5 | 8.2% | 2 | 26 | 23.9% | 6 | 376 | 42.9% | 91 | 159 | 36.3% | 41 | 57 | 28.5% | 18 | 48 | 31.4% | 12 | 71 | 30.1% | 20 | |
ID = infectious disease. LGBT = lesbian, gay, bisexual, transgender. GBMSM = gay, bisexual, and other men who have sex with men. PWUD/PWID = persons who use/inject drugs.
Note. Settings and populations are not mutually exclusive. For example, determinants from an article that examined both HIV and non-HIV clinics would be included in both respective columns. Similarly, determinants from an article that included both GBMSM and transgender individuals would be included in both columns.
Articles about pharmacy settings focused more heavily on individual provider characteristics (27.9%), inner setting (21.3%), and providers’ perspectives about PrEP (18.0%). They indicated that pharmacies currently have limited staff knowledge, capacity, and experience with PrEP and counseling about sexual health in general, but the long operating hours and frequent interactions with patients (as they seek refills) were seen as positive, patient-centered attributes. In contrast, studies on substance use treatment centers found poor infrastructure and/or administrative capacity to integrate PrEP delivery into those settings despite providers’ familiarity with the intervention. Additionally, negative experiences with healthcare and stigma were key innovation barriers. Concerns about cost, side effects, behavioral disinhibition, drug resistance, and patients’ ability to adhere cut across all settings at both the patient and provider levels.
Target populations
Across priority target populations, studies were heavily focused on determinants of PrEP uptake and use (73.2–90.6%), divided closely among the three domains: characteristics of individual patients (28.5–42.9%), patients’ perceptions about PrEP (22.1–29.5%), and outer setting (19.6–27.0%). Barriers to PrEP delivery and use that were common to all populations included low levels of PrEP awareness and knowledge among both providers and patients; HIV stigma; out-of-pocket cost; inadequate insurance coverage and other forms of socioeconomic instability (e.g., housing, transportation); concerns about side effects; substance use and mental health issues; and observed challenges with adherence. Common facilitators included provider awareness of PrEP and PrEP protocols; having the cost of PrEP covered by insurance or other means; active provider engagement in linkage and retention to PrEP; social support for patients from partners, friends, family, and other PrEP users; and patients’ recognition of their own behaviors that increase HIV risk (e.g., sero-discordant relationship, multiple partners, condomless anal intercourse, injection drug use). Because there was substantial overlap in studies on target populations (e.g., a paper on Black and Latinx GBMSM and transwomen appears in four columns), we highlight determinants mostly unique to each group below.
Studies involving GBMSM identified barriers related to internalized stigma, homophobia, and lack of access to LGBT-affirming care as challenges for PrEP implementation for this population. Difficulty integrating PrEP into daily routines and perceiving that PrEP promotes promiscuity were also frequent patient-level barriers. Conversely, studies identified greater recognition of HIV risk within the community, more knowledge and favorable attitudes about PrEP, and feelings of responsibility to protect one’s self and others from HIV as unique facilitators. Determinants for transgender individuals were similar but included additional patient-level barriers concerning hormone therapy (e.g., drug interactions, prioritization when resources are limited). Fear of adverse interactions with illicit substances or substance use treatment (e.g., methadone) was also a barrier among people who use drugs, as were heightened concerns among both providers and patients about the ability to adhere to a daily regimen; however, reports on provider perspectives showed general support for PrEP as appropriate for this population.
Studies involving Black/African American individuals identified racial discrimination, experiences of trauma and violence, mistrust of medical/pharmaceutical systems, ambivalence toward integrating PrEP with other prevention efforts (e.g., condoms), and discomfort with going to gay-focused health centers for PrEP as barriers but highlighted having PrEP-support services (e.g., support groups, text message reminders, one-on-one counseling, access to free testing) as key facilitators. The few studies involving Latinx/Hispanic individuals identified cultural norms of machismo (aggressiveness/power) and caballerismo (family values/chivalry) as unique barriers and facilitators, respectively.38
Discussion
Aiming to catalogue our current understanding of implementation determinants for HIV PrEP, our systematic review identified over 1,900 measured determinants from 239 peer-reviewed articles using CFIR 2.0. An interactive dashboard and database of these studies and their coded determinants, to be updated over time, is available at http://HIVimpsci.northwestern.edu. Given the historical focus on individual characteristics in HIV research39 and lack of focus on delivery systems in research in general, it is unsurprising that innovation determinants comprised over 61% of both mentioned and measured. This also reflects the findings of previous PrEP systematic reviews we identified,12,22–27,40 which likely included many of the same studies. Understanding such determinants is critical for designing strategies to support individuals’ uptake and sustained use of PrEP, and our stratified examination by CDC target populations identified key barriers and facilitators that are common to almost all groups indicated for it.
However, while additional research to explicate innovation determinants unique to specific subpopulations may still be warranted (e.g., we found few studies on Latinx/Hispanic individuals), future inquiries around determinants of PrEP implementation should primarily focus on system-level factors that influence provision of PrEP in existing and new settings, coupled with the determinants of the populations they serve. Provider characteristics and their perspectives on PrEP comprised 18% of measured determinants in our review, and constructs from the inner setting, outer setting for implementation, and process domains comprised only 7.2%, 10.3%, and 0.8% of measured determinants, respectively. These latter areas correspond to the structural supports and logistical considerations for adopting and integrating PrEP into current practice, and the relative lack of research, particularly in new but promising contexts (e.g., pharmacies, substance use treatment centers), may limit the development of effective implementation strategies that can support actual delivery and reach to individuals vulnerable to HIV. Relatedly, the majority of mentioned and measured determinants were barriers (72.1% and 57.0%, respectively), again reflecting previous reviews but also suggesting opportunities for future studies to more thoroughly examine facilitators.
Determinants that were measured quantitatively came from over double the number of articles as those measured qualitatively, but the relative difference in number of discrete determinants identified was far smaller. This matches the purpose and strengths of each design, with qualitative methods more often used to explore or expand on concepts and quantitative methods to evaluate relationships. Many of the determinants identified through the latter were covariates in tests of factors associated with PrEP use or of support interventions to increase use (e.g., PrEP navigation), which contributed to the density of innovation determinants in our review. Only 59 determinants from 7 articles were identified as using a mixed/multi-method approach, which is intended to provide a better understanding of a topic than either design alone.41 It is possible that multi-method articles used each method complementarily to examine different and unique sets of determinants, which our coding at the determinant level would have counted as strictly qualitative or quantitative. This may also be an artifact of qualitative and quantitative findings from the same project being published separately, but our review did not match articles at the project level to be able to examine this further.
The relationship between measured and mentioned determinants is complex—as measured determinants in earlier articles may subsequently be mentioned in later articles—and outside the scope of this review. But, that there were very similar distributions for mentioned and measured determinants across CFIR constructs suggests that although it is critical for research to build upon prior studies, this process could also lead to a narrowing of the focus in the field. Mentioning a determinant serves as an implicit endorsement of its importance, which may subsequently influence what future researchers concentrate on. While such determinants are nonetheless meaningful to consider, they may not always be the most significant in terms of impacting implementation outcomes.
Challenges and limitations
We encountered a number of challenges in conducting this review of implementation determinants (broadly defined), which reflects the nascency of the field of implementation science and its formal use in PrEP research. First, titles and abstracts often did not contain much information about implementation, which necessitated full-text review of many of the records. Within the full text, details on mentioned and measured determinants were often scant; in particular, articles often lacked information on valence and explanations as to why or how a determinant might impact implementation. We expected this based on our previous review of NIH-funded grant abstracts,42 but future studies of implementation determinants could benefit from reporting guidelines similar to those for implementation strategies (e.g., TIDieR checklist,43 Proctor et al.44).
Second, extraction and coding of determinants was challenging even with masters-level staff (Round 1 identification and description) and highly-trained implementation researchers (Round 2 classification to CFIR 2.0). Despite substantial training for Round 1 extractors, there was a steep learning curve to thinking about determinants at the level of systems rather than individuals, and close monitoring and feedback by the lead author was critical. Round 2 coding required extensive prior knowledge of implementation science and familiarity with CFIR 2.0. Despite having coders with such expertise, the lack of detail provided in some articles hindered the differentiation between certain CFIR constructs that have subtle, nuanced differences. For example, patients’ and providers’ perceptions of PrEP characteristics (their own domains) often overlapped with their knowledge and attitudes about PrEP and other personal attributes, including motivations (individual characteristics); barriers around cost (in innovation characteristics), coverage by insurance (under financing in outer setting), and lack of insurance (a socioecological characteristic in outer setting) were related and could affect PrEP prescription (implementation determinant) or uptake (innovation determinant) depending on context; and stigma from providers could be described at either the socioecological level (in outer setting) or among individual providers (an individual characteristic). Future researchers who conduct similar reviews should build in ample time for training, reconciliation, and consensus building. We also recommend coding directly on the full text using qualitative coding software (e.g., Dedoose) rather than first extracting determinants into a separate form; our Round 2 coders frequently had to return to the articles for additional context in order to differentiate between similar CFIR codes.
Third, we identified determinants that were not sufficiently captured or differentiated by CFIR 2.0 at the construct level, despite newly added constructs and the distinction between patient and delivery agent. Many determinants were related to peripheral consequences of PrEP (e.g., side effects, drug interactions, additional benefits gained from health and support services paired with PrEP care like frequent HIV testing), which we coded into an “other” category within the two innovation characteristics domains. These categories ultimately had the highest number of mentioned and measured determinants in those domains. Similarly, the constructs of other personal attributes, outer setting patient characteristics, and socioecological characteristics became catchalls for numerous related but diverse concepts that would require further disentangling before informing the selection of appropriate implementation strategies. As CFIR 2.0 continues to evolve through application in studies like this one, additional pragmatic categories of determinants may be incorporated in future iterations.
Our findings should be interpreted with some additional methodological caveats. First, we examined determinants for a broad definition of PrEP implementation, including not only delivery of PrEP from clinics and providers but also awareness, acceptability, uptake, and adherence by patients. We did not separate determinants by step along the PrEP cascade,35 but CFIR 2.0’s differentiation between innovation and implementation determinants facilitates making theoretical links to patient- and provider-level PrEP implementation outcomes.45 Second, we did not separate determinants by PrEP formulation (e.g., long-acting injectable, oral pill) or dosing regimen (e.g., daily, on-demand/event-driven). However, none of the 286 articles examined actual implementation or use of any type of PrEP other than the daily oral pill. The few articles that examined other types focused on perceived acceptability, and identified determinants were concentrated in the intervention characteristics domains. As different types of PrEP become implemented outside research settings, future studies should begin to explore potential differences in their determinants in different delivery systems.
Conclusion
The findings of this review fill an important gap in the literature by synthesizing the determinants of PrEP implementation and uptake through the lens of a widely used implementation science framework. In doing so, it positions the field of HIV prevention to draw on the broader implementation science literature to identify appropriate implementation strategies to address these determinants. Our findings suggest that as a field, we have achieved a substantial generalized knowledge of patient-level PrEP barriers, operationalized in three domains of innovation determinants in our application of CFIR 2.0. Any further research in these areas—precluding local assessments in support of planning implementation efforts—should prioritize understanding determinants, especially facilitators, unique to populations that have been understudied or that sit at the intersections of multiple marginalized identities. More research is also needed on the relationship between determinants and the implementation strategies that are effectively used to achieve better implementation outcomes.46 Structural, policy, and logistical factors, especially those in the CFIR process domain, have been less frequently studied. Development of effective implementation strategies to support scale-up and scale-out 47 of PrEP in primary and specialty care and other settings will require a better understanding of these systems-level factors that are more central to the delivery of PrEP. These needs should shape future HIV implementation research in this area.
Supplementary Material
Source of Funding
This work was supported by a supplement grant to the Third Coast Center for AIDS Research, an NIH-funded center (P30 AI117943; PI: D’Aquila; Supplement PIs: Mustanski & Benbow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CFAR or the National Institutes of Health. The sponsor had no involvement in the conduct of the research or the preparation of the article.
Footnotes
Conflicts of Interest:
The authors declare that they have no conflicts of interest.
References
- 1.Spinner CD, Boesecke C, Zink A, et al. HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection. 2016;44(2):151–158. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. HIV National Strategic Plan for the United States: A Roadmap to End the Epidemic 2021–2025. In. Washington, DC.2021. [Google Scholar]
- 3.Pinto RM, Lacombe-Duncan A, Kay ES, Berringer KR. Expanding Knowledge About Implementation of Pre-exposure Prophylaxis (PrEP): A Methodological Review. AIDS Behav. 2019;23(10):2761–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2019. 2021.
- 5.Spinelli MA, Buchbinder SP. Pre-exposure Prophylaxis Persistence Is a Critical Issue in PrEP Implementation. Clinical Infectious Diseases. 2020;71(3):583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidebottom D, Ekström AM, Strömdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV – how can we improve uptake and adherence? BMC Infectious Diseases. 2018;18(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsen P, Bernhardsson S. Context matters in implementation science: a scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv Res. 2019;19(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleuhs B, Quinn KG, Walsh JL, Petroll AE, John SA. Health Care Provider Barriers to HIV Pre-Exposure Prophylaxis in the United States: A Systematic Review. AIDS Patient Care and STDs. 2020;34(3):111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer KH, Agwu A, Malebranche D. Barriers to the Wider Use of Pre-exposure Prophylaxis in the United States: A Narrative Review. Advances in Therapy. 2020;37(5):1778–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JD, Li DH, Rafferty MR. The Implementation Research Logic Model: a method for planning, executing, reporting, and synthesizing implementation projects. Implementation Science. 2020;15(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JD, Li DH, Hirschhorn LR, et al. Landscape of HIV Implementation Research Funded by the National Institutes of Health: A Mapping Review of Project Abstracts. AIDS Behav. 2020;24(6):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholl E. Improving outpatient implementation of preexposure prophylaxis in men who have sex with men. Journal of the American Association of Nurse Practitioners. 2016;28(8):446–452. [DOI] [PubMed] [Google Scholar]
- 13.Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP Implementation Through Multilevel Interventions: A Synthesis of the Literature. AIDS Behav. 2018;22(11):3681–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldfield BJ, Edelman EJ. Addressing Unhealthy Alcohol Use and the HIV Pre-exposure Prophylaxis Care Continuum in Primary Care: A Scoping Review. AIDS Behav. 2021;25(6):1777–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, McMahon J, Fiscella K, et al. HIV Pre-Exposure Prophylaxis Implementation Cascade Among Health Care Professionals in the United States: Implications from a Systematic Review and Meta-Analysis. AIDS Patient Care STDS. 2019;33(12):507–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Mitchell W, Xue Y, LeBlanc N, Liu Y. Understanding the role of nurse practitioners, physician assistants and other nursing staff in HIV pre-exposure prophylaxis care in the United States: a systematic review and meta-analysis. BMC Nurs. 2020;19(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey JL, Molino ST, Vega AD, Badowski M. A Review of HIV Pre-Exposure Prophylaxis: The Female Perspective. Infect Dis Ther. 2017;6(3):363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazzi AR, Drainoni ML, Biancarelli DL, et al. Systematic review of HIV treatment adherence research among people who inject drugs in the United States and Canada: evidence to inform pre-exposure prophylaxis (PrEP) adherence interventions. BMC Public Health. 2019;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley E, Forsberg K, Betts JE, et al. Factors Affecting Pre-Exposure Prophylaxis Implementation for Women in the United States: A Systematic Review. Journal of women’s health. 2019;28(9):1272–1285. [DOI] [PubMed] [Google Scholar]
- 20.Koechlin FM, Fonner VA, Dalglish SL, et al. Values and Preferences on the Use of Oral Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among Multiple Populations: A Systematic Review of the Literature. AIDS Behav. 2017;21(5):1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer KH, Chan PA, R RP, Flash CA, Krakower DS. Evolving Models and Ongoing Challenges for HIV Preexposure Prophylaxis Implementation in the United States. J Acquir Immune Defic Syndr. 2018;77(2):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannaford A, Lipshie-Williams M, Starrels JL, et al. The Use of Online Posts to Identify Barriers to and Facilitators of HIV Pre-exposure Prophylaxis (PrEP) Among Men Who Have Sex with Men: A Comparison to a Systematic Review of the Peer-Reviewed Literature. AIDS and Behavior. 2018;22(4):1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezennia O, Geter A, Smith DK. The PrEP Care Continuum and Black Men Who Have Sex with Men: A Scoping Review of Published Data on Awareness, Uptake, Adherence, and Retention in PrEP Care. AIDS Behav. 2019;23(10):2654–2673. [DOI] [PubMed] [Google Scholar]
- 24.Golub SA, Myers JE. Next-Wave HIV Pre-Exposure Prophylaxis Implementation for Gay and Bisexual Men. AIDS Patient Care and STDs. 2019;33(6):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner MR, Miele P, Carter W, et al. Preexposure Prophylaxis for Prevention of HIV Acquisition Among Adolescents: Clinical Considerations, 2020. MMWR Recomm Rep. 2020;69(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remy L, Enriquez M. Behavioral Interventions to Enhance PrEP Uptake Among Black Men Who Have Sex With Men: A Review. J Assoc Nurses AIDS Care. 2019;30(2):151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velloza J, Kapogiannis B, Bekker L-G, et al. Interventions to improve daily medication use among adolescents and young adults: what can we learn for youth pre-exposure prophylaxis services? AIDS. 2021;35(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill LA, Ballard C, Cachay ER. The Role of the Clinical Pharmacist in the Management of People Living with HIV in the Modern Antiretroviral Era. AIDS reviews. 2019;21(4):195–210. [DOI] [PubMed] [Google Scholar]
- 29.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science. 2009;4(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damschroder LJ, Reardon CM, Opra Widerquist MA, Lowery J. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR Outcomes Addendum. Implementation science : IS. 2022;17(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damschroder LJ. Introduction and application of version 2 of the consolidated framework for implementation research (CFIR). Presented at: 14th Annual Conference on the Science of Dissemination and Implementation in Health, December 14–16, 2021; virtual. [Google Scholar]
- 32.Damschroder LJ. Introducing Version 2 CFIR Updates. Presented at: Prevention Science and Methodology Group Virtual Grand Rounds, February 8, 2022; virtual. https://cepim.northwestern.edu/calendar-events/2022-02-08-damschroder.
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Advisory Panel on HIVCCO. IAPAC Guidelines for Optimizing the HIV Care Continuum for Adults and Adolescents. Journal of the International Association of Providers of AIDS Care. 2015;14 Suppl 1:S3–S34. [DOI] [PubMed] [Google Scholar]
- 35.Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute JB. Joanna Briggs Institute reviewers’ manual: 2014 edition. Australia: The Joanna Briggs Institute. 2014. [Google Scholar]
- 37.Covidence systematic review software [computer program]. 2018.
- 38.Rivera DB, Brady JP, Blashill AJ. Traditional Machismo, Caballerismo, and the Pre-Exposure Prophylaxis (PrEP) Cascade Among a Sample of Latino Sexual Minority Men. J Sex Res. 2021;58(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson BT, Redding CA, DiClemente RJ, et al. A network-individual-resource model for HIV prevention. AIDS Behav. 2010;14(Suppl 2):204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazzi AR, Biancarelli DL, Childs E, et al. Limited Knowledge and Mixed Interest in Pre-Exposure Prophylaxis for HIV Prevention Among People Who Inject Drugs. AIDS Patient Care and STDs. 2018;32(12):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins CS, Ware NC, dosReis S, Willging CE, Chung JY, Lewis-Fernandez R. Dialogues on mixed-methods and mental health services research: anticipating challenges, building solutions. Psychiatr Serv. 2008;59(7):727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JD, Li DH, Hirschhorn LR, et al. Landscape of HIV Implementation Research Funded by the National Institutes of Health: A Mapping Review of Project Abstracts. AIDS Behav. 2020;24(6):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 44.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation science : IS. 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JD, Li DH, Rafferty MR. The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implementation Science. 2020;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell BJ, Fernandez ME, Williams NJ, et al. Enhancing the Impact of Implementation Strategies in Healthcare: A Research Agenda. Front Public Health. 2019;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aarons GA, Sklar M, Mustanski B, Benbow N, Brown CH. “Scaling-out” evidence-based interventions to new populations or new health care delivery systems. Implementation science : IS. 2017;12(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


