Abstract
Background:
Patellar chondral lesions can be particularly challenging to manage in younger and more active populations.
Purpose:
To synthesize, organize, and summarize the results and complication rates of various patellar cartilage restoration techniques.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the Medline, Embase, Scopus, and Cochrane databases. Studies were included that reported on surgical treatment of patellar chondral defects with ≥5 patients and 12 months of follow-up data. Relevant demographic data from the included studies were extracted, and patient-reported outcome scores, visual analog scale for pain results, return-to-sport rate, complications, and concomitant procedures were documented.
Results:
There were 24 studies that met the inclusion criteria, with a total of 575 patients (male, n = 239; female, n = 336). In total, 6 surgical techniques were utilized. In 9 studies, the surgical procedure of choice was osteochondral autograft transplantation (OAT); 8 studies evaluated autologous chondrocyte implantation (ACI); 3 evaluated advanced microfracture/autologous matrix-induced chondrogenesis; 1 evaluated osteochondral allograft transplantation (OCA); 1 evaluated particulate juvenile articulated cartilage; and 2 evaluated a synthetic osteochondral graft. No uniform functional outcome score or assessment was utilized across studies. OAT was predominantly used for smaller chondral lesions (<2 cm2) and demonstrated minimal complication rates and satisfactory outcome scores. Advanced microfracture techniques showed promise, with improvement in outcome scores and zero complications. Matrix-induced ACI consistently exhibited higher mean improvement in the measured outcome scores and resulted in fewer complications when compared with previous generations of ACI.
Conclusion:
OAT and ACI were the most studied procedures for isolated patellar chondral defects. Advanced microfracture techniques showed promise, but indications (ie, size) and variability in techniques need to be elucidated in higher-level studies. Further prospective studies comparing OCA and matrix-induced ACI for larger patellar defects are necessary to determine the superior technique.
Keywords: patella, restoration, cartilage, defects
The articular surface of the patella withstands high forces. Patellofemoral contact pressures reach up to 6.5 times body mass during daily activities with the knee flexed to 90°. 19 Eventually, repetitive compression (eg, high activity level, obesity) or acute trauma may cause injury to the articular surface. Patellar chondral defects, if left untreated, may cause pain and functional disturbance. These defects can prevent the normal force distribution in the patellofemoral joint, which creates the potential for chondral injury and progression to osteochondral injury. 2 Patellar chondral lesions are not uncommon and appear in over one-third of the patients undergoing knee arthroscopy.6,13,40 These problems can be particularly challenging to manage in younger and more active populations. 28
Cartilage restoration techniques historically were stratified into reparative and restorative approaches, although not all approaches fit into a specific pathway. Traditional techniques include microfracture (MF), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OAT), and osteochondral allograft transplantation (OCA). Newer techniques have blended the categories with the goal of filling chondral defects with hyaline-like cartilage. Although there are many surgical options to restore the articular surface of the patella, there is lack of consensus regarding the preferred treatment. 39
The patella and trochlea, while dynamically related, have intrinsic differences that previous systematic reviews failed to distinguish. The trochlea has areas of thin cartilage (range, 2-3 mm), while the patella has the thickest articular cartilage layer in the human body (≤7 mm).21,31,34 The patella has a convex surface and a varying patellar ridge, while the trochlea has a concave surface with varying depth. 11 Access to the patella versus the trochlea may affect surgical treatment options for cartilage-defect management. Given its anatomic specificities, patellar chondral defects should be managed according to outcomes and data exclusively regarding the patella. Two prior systematic reviews attempted to aggregate outcomes specifically related to patellar cartilage defects. The systematic review by Noyes and Barber-Westin 28 did not include all traditional techniques but rather patellofemoral arthroplasty, which involves replacing cartilage rather than restoring it. Yet, the review conducted by Mouzopoulos et al 25 did not include OCA, and 1 of the studies evaluated only tibial tubercle osteotomy (TTO) without cartilage restoration.
During the past 8 years, there have been advances in cartilage restoration options for the knee, such as particulate juvenile articular cartilage (PJAC), augmented/advanced MF (aMF; MF bone marrow stimulation augmented with a collagen matrix and fibrin glue), and the newest generation of ACI: matrix-induced ACI (MACI). Consequently, no updated or inclusive systematic review exists of true cartilage restoration options for the patella. Moreover, previous reviews failed to present results based on the size of the defect. Noyes and Barber-Westin 28 evaluated chondral defects >4 cm2, while Mouzopoulos et al 25 did not control for size of the defect. Previous reviews included arthroplasty and procedures known to produce fibrocartilage.34,42
The purpose of this updated systematic review was to organize and summarize the results and complication rates of various patellar cartilage restoration techniques.
Methods
This systematic review serves to summarize the literature on clinical results of different surgical restoration treatments for patellar cartilage lesions. This review was performed according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses).
Search Strategy
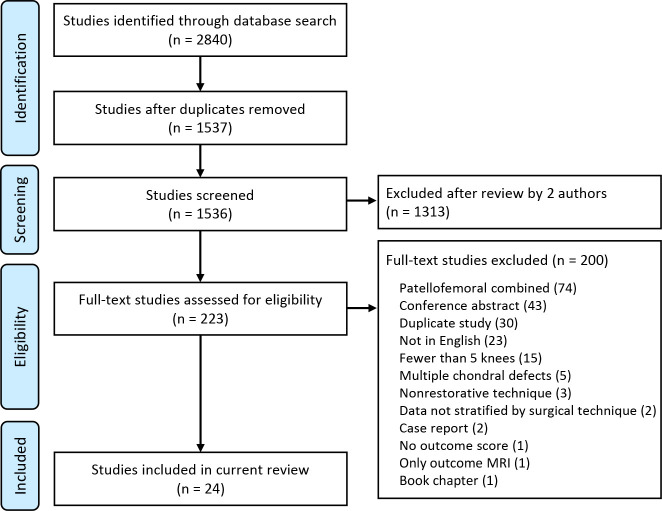
A search strategy was constructed to retrieve the most relevant yield on surgical approaches for patellar cartilage lesions. All searches were conducted on July 19, 2022, by a single author (E.G.) using the Medline, Embase, Scopus, and Cochrane databases (2010 to present). A combination of keywords, with truncation, and indexing terms were used (when available) to combine the 2 concepts of patellar cartilage injury and specific surgical procedure, including but not limited to aMF/AMIC (autologous matrix-induced chondrogenesis), ACI, Novocart (Aesculap), mosaicplasty, OAT, and OCA. No filters or limiters were applied. The results were deduplicated using Covidence software, which facilitated and managed the screening and extraction process. Figure 1 presents a PRISMA diagram of our method to achieve these results.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of study inclusion and exclusion. MRI, magnetic resonance imaging.
Inclusion and Exclusion Criteria
Studies were included if they consisted of patients with isolated patellar cartilage lesions detected by magnetic resonance imaging (MRI), computed tomography (CT), or arthroscopy; conducted a minimum 12-month follow-up; and investigated a surgical cartilage restoration procedure to treat isolated patellar cartilage lesions that included but was not limited to the following techniques: aMF, ACI, OAT, OCA, PJAC, or a synthetic graft. The studies must have reported clinical outcomes, been written in English, had a minimum of 5 knees, and had any level of evidence.
Studies were excluded if they did not report clinical results; used nonsurgical treatment (ie, injections); were cadaveric, biomechanical, or basic science studies; were editorials, commentaries, case reports, reviews, surgical technique reviews, abstracts, or conference papers; had <5 knees; or were not written in English.
Study Selection
A total of 2840 studies were filtered from all databases. All studies were transferred to covidence.org, and 3 authors (R.M.P, E.G, N.W.) were granted access to screen the studies. Duplicate studies were then removed. This left 1537 studies to be screened by 2 authors (E.G, N.W.), who reviewed the abstracts independently. Any discrepancies between authors were resolved by the senior author (R.M.P.). A total of 223 publications were selected for full-text screening. Of these, 24 studies were eligible to be included in the study. All references from the studies were reviewed to verify that no relevant publications were missing from the systematic review.
The studies were classified by the levels of evidence for therapeutic studies provided by the Centre for Evidence-Based Medicine. 7 All studies were considered to have an evidence level of 3B or 4.
Extracted Data
Relevant demographic data were extracted: author names, year of publication, journal of publication, level of evidence, sample size, patient demographics (age, sex, surgical history, symptoms), details of the surgery (implant diameter, type of surgery, lesion size, location of lesion on the patella, concomitant procedures, number of plugs), preoperative range of motion and imaging, and outcome scores. Relevant follow-up data were extracted as well: follow-up time, second-look arthroscopy, postoperative imaging, return to activity/sports and range of motion, and complications (rate and number).
Results
Overall, there were 24 studies ∥ with a total of 575 patients (239 male, 336 female) treated for patellar chondral defects. Study characteristics are summarized in Table 1. No uniform score or assessment was utilized to assess the functional status of patients. However, all the studies performed a functional postoperative assessment, a pain score assessment, or both. The most utilized functional outcome measures were the International Knee Documentation Committee (IKDC; 13 studies ¶ ), Lysholm (9 studies # ), Tegner (8 studies3,8,11,15,30,35–37), Kujala (7 studies2,3,10,11,15,23,36), and the 36-Item Short Form Health Survey (SF-36) (6 studies2,10,18,20,26,38).
Table 1.
Characteristics of the Included Studies a
| Lead Author (Year) | Operative Procedure | Age, y, Mean ± SD (Range) | No. of Patients (M:F); Knees | Location |
|---|---|---|---|---|
| Astur (2014) 2 | OAT | 37.6 (16-59) | 33 (17:16); 33 | 13 lateral, 13 medial, 5 both, 2 central |
| Astur (2017) 3 | OAT | — (26-45) | 20 (9:11); 20 | 12 lateral, 8 medial |
| Becher (2015) 4 | aMF b | 27 (15-40) | 5 (3:2); 5 | 1 lateral, 3 medial, 1 central |
| Chadli (2017) 8 | Mosaicplasty | 15 (12-17) | 7 (5:2); 8 | 4 central, 3 central, 1 superolateral |
| Cohen (2012) 10 | OAT | 38.06 ± 13.38 | 17 (8:9); 17 | 9 lateral, 7 medial, 1 central |
| Dhollander (2011) 11 | aMF c | 27 (24-45) | 5 (3:2); 5 | — |
| Figueroa (2011) 12 | OAT | 20.2 (15-38) | 10 (10:0); 10 | — |
| Gaweda (2006) 14 | OAT | 25.5 | 19 (—); 19 | — |
| Gigante (2009) 15 | MACI | 31 (25-35) | 12 (6:6); 14 | 5 medial, 7 diffuse |
| Gillogly (2014) 16 | ACI (periosteal) | 31.2 ± 7 | 23 (11:12); 25 | 6 lateral, 3 medial, 3 distal, 13 central/diffuse |
| Gracitelli (2015) 17 | OCA: shell technique | 33.7 (14-64) | 27 (13:14); 28 | — |
| Henderson (2006) 18 | ACI (periosteal) | ▪ ACI + TTO: 32.1 (17-56) | ▪ ACI + TTO: 22; 22 | 9 lateral, 10 medial, 6 distal, 19 proximal/diffuse |
| ▪ ACI: 35.1 (14-55) | ▪ ACI: 22; 22 | |||
| ▪ All: 44 (21:23); 44 | ||||
| Joshi (2012) 20 | Synthetic graft d | 33.6 (17-49) | 10 (4:6); 10 | 2 lateral, 3 medial, 1 distal, 4 diffuse |
| Macmull (2012) 22 | ACI-C (collagen I/II membrane sutured to the defect), MACI | ▪ ACI-C: 34.6 (17-50) | ▪ ACI-C: 25 (5:20); 25 | ▪ All: 13 lateral, 20 medial, 15 multifaceted ▪ ACI-C: 6 lateral, 8 medial, 11 multifaceted ▪ MACI: 7 lateral, 12 medial, 4 multifaceted |
| ▪ MACI: 35 (21-46) | ▪ACI: 23 (9:14); 2 | |||
| ▪ All: 34.8 (17-50) | ▪ All: 48 (14:34); 48 | |||
| Mehl (2019) 23 | ACI | 33.2 ± 10 | 78 (32:46); 78 | 14 lateral, 29 medial, 36 ridge |
| Nho (2008) 26 | OAT (Arthrex) | 30 ± 12 (15-57) | 22 (12:10); 22 | 9 lateral, 5 medial, 6 central, 2 inferior |
| Niemeyer (2008) 27 | MACI (BioSeed-C) | 34.3 ± 10.1 | 70 (—); 70 | — |
| Perdisa (2017) 30 | — | 30 ± 10 | 34 (18:16); 34 | — |
| Sadlik (2017) 32 | aMF e | 36 (22-52) | 12 (7:5); 12 | 3 lateral, 1 medial, 8 central |
| Teo (2013) 35 | ACI (periosteal), BMSC with periosteal patch | 16.8 (12-21) | 23 (19:4); 23 | — |
| Tompkins (2013) 36 | PJAC with fibrin glue (De Novo) | 26.4 ± 9.1 | 13 (6:9); 15 | 6 medial, 3 central, 3 inferior, 3 medial+central |
| Visona (2010) 37 | OAT/mosaicplasty | 20.5 ± 9.2 (14-39) | 6 (4:2); 6 | 2 superolateral, 2 mediolateral, 1 inferolateral, 1 medial |
| von Keudell (2017) 38 | ACI (periosteal) | 32 ± 10 (15-49) | 30 (12:18); 30 | — |
| Yonetani (2019) 41 | OAT (Zimmer) | 38 ± 8.8 (27-51) | 6 (5:1); 6 | — |
a Dashes indicate data not available. ACI, autologous chondrocyte implantation; ACI-C, autologous chondrocyte implantation–collagen; aMF, advanced microfracture; BMSC, bone marrow stem cell; F, female; M, male; MACI, matrix-induced autologous chondrocyte implantation; OAT, osteochondral autograft transplantation; OCA, osteochondral allograft transplantation; PJAC, particulate juvenile articular cartilage; TTO, tibial tubercle osteotomy.
b Microfracture + resorbable textile polyglycolic acid hyaluronan implant (membrane) secured with smart nail (open).
c (Microfracture + collagen matrix to cover the defect + fibrin glue) + platelet-rich plasma.
d Synthetic resorbable osteochondral scaffold plug (TruFit CB) + synthetic resorbable biphasic implants (composite hydrophilic polymer composed of polylactide coglycolide, calcium sulfate, and polyglycolide fibers).
e Microfracture + collagen matrix to cover the defect + fibrin glue (all arthroscopic).
Fourteen studies** were prospective while 10 studies8,16,17,23,27,35–38,41 were retrospective. AppendixTable A1 provides information regarding outcome scores and complications.
Diagnostic Assessment of Cartilage
Most of the studies (17/24; 71%) utilized MRI as a part of the advanced imaging evaluation. Three studies2,3,15 (12.5%) used diagnostic arthroscopy for evaluation of the cartilage lesion, and another 3 studies15,16,35 (12.5%) used conventional CT preoperatively. One study 37 (4.1%) performed MRI and CT for evaluation of chondral injuries.
Type of Surgical Treatment
In total, 6 surgical techniques were utilized. In 9 studies,2,3,8,10,12,14,26,37,41 the surgical procedure of choice was OAT for patellar cartilage lesions. Eight studies15,16,18,22,23,27,35,38 evaluated the ACI technique on the patella. Of these, 4 studies16,18,35,38 analyzed first-generation ACI; 1 study 15 examined MACI; 2 studies23,27 reviewed all generations without differentiation; and 1 study 22 compared second-generation ACI with MACI. No study solely evaluated second-generation ACI.
Patellar chondral defects are commonly treated via an open approach in which a parapatellar arthrotomy is made, and the patella is everted to gain access. However, some techniques are amenable to arthroscopic intervention. Typically, these are augmented or aMF techniques. aMF was evaluated in 3 studies.4,11,32
Last, 1 study 17 evaluated OCA; 1 study 36 evaluated PJAC; and 2 studies20,30 evaluated a synthetic osteochondral graft. Of the 575 operations performed, 230 were conducted with concomitant procedures. In most of the studies, no further information was provided; thus, we were unable to differentiate the results from solitary cartilage restoration.
Advanced Microfracture
Three studies4,11,32 reviewed aMF for the treatment of patellar chondral defects. In all 3 studies, scores on the postoperative IKDC, Knee injury and Osteoarthritis Outcome Score (KOOS), and visual analog scale (VAS) all showed improvement as compared with their preoperative baselines. Two of the 3 studies did not provide a mean lesion size; however, Dhollander et al 11 reported a median lesion size of 2 cm2 (range, 1-3 cm2). Sadlik et al 32 evaluated 12 patients who underwent MF augmented with a collagen matrix combined with fibrin glue (ie, the AMIC technique) to address the patellar cartilage defect arthroscopically. The mean age was 36 years (range, 22-52 years), and the mean follow-up was 38 months (range, 24-70 months). Five patients had a concomitant procedure, including 2 with a TTO. The size of the defect was not provided, and the authors cited zero complications. Becher et al 4 examined 5 patients with a mean age of 27 years (range, 15-40 years) who were treated via the open approach using aMF with subsequent overlay of a cell-free chondrotissue polyglycolic acid–hyaluronan implant. The mean size of the defect was 4 cm2 (range, 3-5 cm2). There were no complications at the mean follow-up of 21 months (range, 11-31 months).
Dhollander et al 11 reported on 5 patients treated via aMF combined with a platelet-rich plasma gel, which was held in place by sutures. The mean age was 27 years (range, 24-45 years). At the 24-month follow-up, intralesional osteophytes were observed in 3 of the 5 patients. Two patients (40%) developed hypertrophy of the repair tissue, and 1 of these 2 patients underwent an arthroscopy because of catching attributed to the hypertrophy. Three patients (60%) had incomplete filling of the defect after 12 months.
First-Generation ACI
Four studies16,18,35,38 evaluated first-generation ACI. Teo et al 35 did not report on lesion size, while the mean lesion size for the 3 other studies ranged from 2.92 to 6.4 cm2. Gillogly and Arnold 16 evaluated 23 patients (25 knees; mean ± SD age, 31.2 ± 7 years) treated by ACI with concomitant procedures. The mean follow-up was 7.6 years (range, 5.1-11.4 years), and the mean lesion size was 6.4 cm2. All postoperative outcome scores showed improvement versus baseline and a reoperation rate of 40% (10/25 knees), with 9 (36%) of the reoperations being necessitated by periosteal graft hypertrophy. One patient experienced a failure at 5.9 years postoperatively and underwent patellofemoral arthroplasty. Henderson and Lavigne 18 examined 44 patients treated with ACI. Half of the patients (n = 22; group A) underwent a concomitant TTO while the other half (group B) underwent patellar ACI only. The mean follow-up was 26.2 months (range, 9-52 months) and 28.9 months (range, 11-55 months), respectively, and the mean lesion sizes were 2.92 and 3.22 cm2. The mean ages were 32.1 years (range, 17-56) and 35.1 years (range, 14-55). At 24 months, all postoperative outcome scores showed improvement when compared with baseline, and the group with concomitant procedures demonstrated better improvement in their IKDC scores (36.2 vs 22.3; P < .006). The total reoperation rate was 52.2% (23 of 44). Group A had 10 reoperations while group B had 13.
Teo et al 36 reported on 23 young patients with a mean age of 16.8 years (range, 12-21 years); all had osteochondritis dissecans and were treated with solitary ACI. The mean follow-up was 6 years (range, 2-11 years), and the mean lesion size was not indicated. These patients had improvement in every outcome score postoperatively and an 8% complication rate (2 patients) attributed to graft hypertrophy. von Keudell et al 38 studied 30 patients with a mean age of 32 ± 10 years (range, 15-49 years) treated with ACI and concomitant TTO for a mean follow-up of 7.3 years (range, 2-14.6 years). The mean lesion size was 4.7 cm2 (range, 2.2-30 cm2). They found improvement in every outcome score postoperatively and a complication rate of 60% (18 patients). Additionally, after 2007, an unspecified number of patients involved in this study were treated with second-generation ACI, involving a collagen membrane (ACI-C), although these patients were not differentiated in the study. For these patients, a standardized collagen membrane (Biogide; Geistlich Pharma) was used. The periosteal graft hypertrophy rate in this study was 18.8% for first-generation ACI and 12.5% for second-generation ACI-C.
In all of the first-generation ACI studies reviewed, periosteal graft hypertrophy/extrusion appeared as the most common complication (Table 1).
Third-Generation ACI (MACI)
Two studies15,22 reported on MACI and/or MACI differentiated from other ACI generations studied. The mean lesion size ranged from 4 to 4.7 cm2. Gigante et al 15 evaluated 12 patients (14 knees) who were treated with MACI and TTO with a mean lesion size of 4 cm2 (range, 3-9 cm2). The mean age was 31 years (range, 25-35 years), and the mean follow-up was 36 months. All postoperative outcome measures showed improvement. Two knees (14%) required screw removal but with no complications specific to the cartilage treatment. Macmull et al 22 examined 48 patients using 2 generations of ACI. Of these, 25 patients were treated with ACI-C and 23 with MACI. The mean ages were 34.6 years (range, 17-50 years) and 35 years (range, 21-46 years), respectively. The mean follow-up was 45 and 35.3 months, and the mean lesion sizes were 4.7 cm2 (range, 1-8.7 cm2) and 4.6 cm2 (range, 1-10.5 cm2). The mean postoperative modified Cincinnati and VAS scores showed improvement for both groups pre- to postoperatively, and no statistically significant difference was observed between ACI-C and MACI.
Two studies23,28 evaluated all 3 generations of ACI and found no differentiation in outcomes and results among the technique types. The range of lesion sizes for these 2 studies was 4.3 to 4.8 cm2. Niemeyer et al 27 evaluated 70 patients with various generations of ACI. The mean age was 34.3 ± 10.1 years, and the mean follow-up was 38.4 months (range, 14-64 months). The mean size of the lesion was 4.8 ± 2.2 cm2. The authors concluded that despite different techniques being utilized, the postoperative functional scores were similar in all groups. Nine severely abnormal cases were considered failures (12.9%; 9/70). Furthermore, 3 cases (4.3%) had complications, 2 of which required arthroscopic debridement for graft hypertrophy. Mehl et al 23 assessed 78 patients treated with various generations of ACI. Of these, 40 patients had a concomitant procedure for patellar instability or malalignment. The mean age was 33.2 ± 10.7 years, and the mean follow-up was 78 ± 40.8 months. The mean lesion size was 4.3 ± 1.6 cm2. Although the data were not stratified by ACI generation, the authors found no postoperative differences in IKDC and Kujala scores between the solitary cartilage restoration group and the group that received a concomitant procedure. There were 6 (7.7%) failures: 1 revision ACI and 5 requiring total knee arthroplasty.
Osteochondral Autograft Transplantation
OAT for the patella was reported in 9 studies.2,3,8,10,12,14,26,37,41 Of these, 6 studies3,8,12,26,37,41 provided their mean lesion size, which ranged from 0.88 to 9.7 cm2. Astur et al 2 presented 33 patients who underwent OAT for the patella with a median follow-up of 30.2 months. The size of lesions treated ranged from 1 to 2.5 cm2. Of the 33 knees, 28 had a single 1.5-cm plug while the remaining 5 knees required 2 plugs. The mean age was 37.6 years (range, 16-59 years). The group showed statistically significant improvements in functional outcome scores (P < .001). Three patients had postoperative arthrofibrosis that was treated with manipulation under anesthesia (MUA) for a complication rate of 9%. In another study by Astur et al, 3 a different cohort of 20 patients aged <45 years were followed for 2 years after OAT of the patella. Their mean follow-up was 24 months, and their mean lesion size was 1.16 cm2 (range, 1-1.18 cm2). The authors cited normal gaits for all patients, a significant decrease in swelling (P < .05), a statistically significant increase in muscle strength (P < .05), and a mean reduction in VAS score (improvement) of 4.7 points. After 2 years, the only complication was thigh hypotrophy in 11 (55%) participants.
Two larger studies, conducted by Cohen et al 10 (17 patients) and Gaweda et al 14 (19 patients), appreciated an improvement in every outcome score with no complications in either group after a mean follow-up of 19.8 months (range, 12-33 months) and 24 months, respectively. The mean ages of the participants were 38.06 ± 13.38 years (range, 16-59 years) and 25.5 years. The mean lesion size was not indicated for either study, but Cohen et al used a 1.5-cm plug for every patient and Gaweda et al utilized a mean 3 plugs (size not specified). Chadli et al 8 studied 8 cases of osteochondritis dissecans of the patella treated with mosaicplasty in adolescents with a mean age of 15 years (range, 12-17 years). After a mean follow-up of 28.6 months (range, 16-50 months), they appreciated an improvement in all outcome scores. The mean lesion size was 9.7 ± 3.7 cm2. Figueroa et al 12 examined 10 patients with a mean follow-up of 37.3 months (range, 24-70 months). The mean age was 20.2 years (range, 15-38), and the mean lesion size was 1.2 cm2 (range, 0.9-2). All the outcome scores improved postoperatively, and no complications were noted. Nho et al 26 evaluated 22 patients for a mean 28.7 months (range, 17.7-57.8 months). The mean age was 30 ± 12 years (range, 15-57 years), and the mean lesion size was 1.65 ± 1.27 cm2. While the mean IKDC outcome score improved with statistical significance postoperatively (P < .028), the SF-36 outcome score improved as well yet failed to reach significance (P < .059). Three patients needed hardware removal after a mean 9.7 months (range, 5-14 months).
Two studies with 6 patients each were performed by Visona et al 37 and Yonetani et al. 41 The mean follow-up was 26 months (range, 10-68 months) and 51 months (range, 24-101 months), respectively, and the mean age was 20.5 years (range, 14-39 years) and 38 years (range, 27-51 years). The mean lesion sizes were 0.88 ± 0.47 cm2 and 1.3 cm2 (range, 0.78-2.2 cm2). All postoperative outcome scores in the Visona et al 38 study improved, and no complications were noted. Yonetani et al 42 solely tracked the Lysholm score, which improved postoperatively with statistical significance (P < .06), and reported a 33.3% complication rate (2 patients with arthrofibrosis addressed with MUA).
Particulate Juvenile Articulated Cartilage
Tompkins et al 36 studied PJAC (Denovo NT Natural Tissue Graft; Zimmer) for cartilage restoration of patellar defects. They reported on 13 patients (15 knees) with a mean age of 26.4 ± 9.1 years and a mean follow-up of 28.8 ± 10.2 months. The mean lesion size was 2.4 ± 1.2 cm2. Five patients had concomitant procedures for patellar instability. Only postoperative outcome scores were provided. In a subgroup analysis of solitary PJAC as compared with a combined procedure, no statistically significant differences were found in the combined procedure group in terms of postoperative mean Tegner scores, but a significantly higher VAS score was found for the concomitant procedure cohort (2.5 ± 1.4 vs 0.8 ± 0.6; P < .01). Seven complications (46.7%) were noted, including 3 of the 15 knees requiring arthroscopic debridement for graft hypertrophy (20%) and 2 of the 15 knees requiring a MUA for arthrofibrosis (13.3%).
Osteochondral Allograft Transplantation
Gracitelli et al 17 performed OCA on 28 knees in 27 patients with a mean age of 33.7 years (range, 14-64 years) and a mean follow-up of 9.7 years (range, 1.8-30.1 years). While the mean lesion size was not provided, the mean implant diameter was 10.1 cm2 (range, 4-18 cm2). Ten patients had a concomitant procedure performed during the index surgery. Overall, patients showed improvement in postoperative outcome scores when compared with preoperative scores. Although 19 knees (70%) required further surgery and 8 knees (28.6%) failed, 89% of the patients were ultimately satisfied. Additionally, the overall survival of the allograft used in this technique was 78.1% at 5- and 10-year follow-up and 55.8% at 15-year follow-up (P < .05).
Synthetic Graft
Two studies20,30 used a synthetic graft, and the mean lesion sizes ranged from 2.1 to 2.64 cm2. Joshi et al 20 studied a synthetic resorbable chondral or osteochondral scaffold plug (TruFit CB; Smith & Nephew). The plug is a synthetic resorbable biphasic implant made of a hydrophilic polymer composed of polylactide coglycolide, polyglycolide fibers, and calcium sulfate. Ten patients with a mean age of 33.6 years (range, 17-49 years) and a mean lesion size of 2.64 cm2 (range, 1-5 cm2) were reviewed for treatment of patellar defects. The mean follow-up was 24 months. Outcome scores demonstrated variable results with a minimal improvement in the KOOS and a deterioration in SF-36 scores. At the last follow-up, the group saw a reoperation rate of 70% (7 patients). Perdisa et al 30 reported on 34 patients with a mean age of 30 ± 10 years who underwent a procedure with a synthetic graft. The scaffold implant was a biphasic cell-free collagen-hydroxyapatite scaffold (Maioregen; Fin-Ceramica Faenza SpA). The mean lesion size was 2.1 ± 1 cm2, and the mean follow-up was 24 months. Sixteen patients (47%) had concomitant procedures. The authors found improvement in every outcome score collected. No differences were found between patients with a solitary procedure and patients with a concomitant procedure at the final follow-up. The group did not have any surgical failures at the last follow-up (ie, no reoperation). Two patients (5.9%) did not achieve clinical improvement, and both cases were considered clinical failures.
Discussion
A total of 24 studies reporting on 7 surgical techniques were reviewed. No uniform outcome score was utilized, but the IKDC score was most commonly assessed (13 studies). Furthermore, variability in lesion size, concomitant osteotomies, and reported data made it difficult to compare the surgical techniques. OAT (n = 9) and ACI (n = 8) were the most frequently studied procedures for isolated patellar chondral defects, while fewer studies were performed on OCA (n = 1), PJAC (n = 1), and synthetic grafts (n = 2).
While many systematic reviews and meta-analyses have been conducted on various aspects of cartilage treatment pertaining to the knee, only 2 studies25,28 focused solely on the patella. The reviews by Noyes and Barber-Westin 28 and Mouzopoulos et al 25 reported on various techniques, including nonrestorative procedures such as arthroplasty, periosteal transplantation, and isolated tibial tubercle osteotomies. Furthermore, traditional techniques such as OCA and newer techniques such as PJAC and synthetic grafts were not discussed. Zamborsky and Danisovic 42 performed a systematic review of 21 randomized controlled trials (evidence level 1) to present surgical treatment options for knee cartilage defects, and they found that patient outcome scores and rates of return to activity were superior for those who underwent cartilage restoration techniques (ie, ACI and OAT) than for those who underwent MF. Shanmugaraj et al 33 conducted a systematic review of 28 studies (evidence levels 2-4) on cartilage restoration techniques for the patellofemoral joint. The authors could not conclude on 1 superior treatment since all cartilage treatment techniques noted clinical improvement from baseline. The review reemphasized a long-term trend toward improved functional outcome scores with ACI as compared with MF in the treatment of patellofemoral joint defects. There was no differentiation of the patella versus the trochlea. The authors concluded that ACI, particularly the third generation, was the most common restoration technique used in the past decade. 33
As mentioned previously, the anatomic and biomechanical differences of the patella, as compared with the trochlea and condyles, make treatment challenging. Studies with stratified results for patellar cartilage restoration are limited, and management may oftentimes be based on results from condylar cartilage restoration outcomes. It is important to note that ACI for full-thickness cartilage defects of the patella has a lower satisfaction rate (∼66%) when compared with ACI for the femoral condyles (75%-85%).4,11,23 Similarly, Brittberg et al 5 evaluated 213 patients with ACI and found good to excellent results in 90% of patients with femoral condyle lesions, as opposed to 69% of patients with patellar lesions.
During this review, it became evident that the definition of failure provided by the study investigators varied widely. Some authors considered a failure a conversion to arthroplasty, whereas others considered any reoperation as a failure. Clinical failures were also reported when postoperative outcome scores did not improve over preoperative or baseline scores. We used each study’s criteria for failure when provided. Therapies requiring ingrowth or maturation from a cell or collagen-based matrix (ACI, PJAC, aMF) dealt with complications related to graft hypertrophy or overgrowth and were susceptible to arthroscopic debridement. While OAT had no failures, the OCA failure rate was 28.6% based on 1 study (Gracitelli et al 17 ), which included conversion to arthroplasty, revision OCA, and patellectomy. ACI failure rates ranged from 4% to 12.9% with the result most commonly being a conversion to arthroplasty. Complication rates and reoperation rates were notably higher for first-generation ACI vs third generation (MACI). Last, for synthetic grafts, Joshi et al 20 did not report a failure rate but noted a 70% reoperation rate, while Perdisa et al 30 cited a 5.9% clinical failure rate.
Noyes and Barber-Westin 28 commented on patellar lesions >4 cm2, and their preferred method of treatment was ACI/MACI in their systematic review of patellofemoral cartilage lesions in patients <50 years old. In our review, OCA, MACI, and OAT were all used to treat lesions >4 cm2. The concern with OAT or a mosaicplasty would be donor-site morbidity in larger lesions. Thus, between OCA and MACI, while both are viable techniques with promising results, more comparative and higher-level studies are needed to discern the superior technique.
In contrast, while OCA and MACI demonstrated promising results for smaller lesions as well, they are both cost-intensive procedures that require more long-term planning with multiple procedures. Alternatively, OAT provides improved postoperative outcomes and the benefit of being a cost-effective technique for smaller cartilage defects.
In lesions of ∼2 cm, aMF resulted in consistent improvements in the measured outcome scores, while 2 other techniques demonstrated less favorable results and higher complication rates. PJAC, despite being a single-stage off-the-shelf procedure that lacks donor-site morbidity, had a high complication rate, which may outweigh the benefits of the procedure. The second technique, synthetic osteochondral graft, was utilized in 2 studies and the results were conflicting. Further research is warranted before the routine use of synthetic grafts for treatment of patellar cartilage lesions. Nevertheless, with timing being of considerable importance, the off-the-shelf availability of PJAC and synthetic graft justifies additional exploration of their clinical use.
OCA had the longest follow-up (9.7 years) for patellar cartilage treatment. Long-term follow-up remains critical in effectively evaluating the treatment options. In the Gracitelli et al 17 study, the overall survival of OCA was 78.1% at 5- and 10-year follow-up and 55.8% at 15-year follow-up. A systematic review of OCA in the patellofemoral joint by Chahla et al 9 reported the long-term survival rates of OCA at 5 and 10 years as 87.9% and 77.2%, respectively. However, Assenmacher et al 1 performed a systematic review of generalized knee OAT and found a 72% success rate at 10-year follow-up. Overall, 70% at 10 years appears a reasonable benchmark for cartilage treatment of patellar defects.
This systematic review includes newer cartilage restoration techniques and focuses on solitary patellar chondral defects. Cartilage restoration can be influenced by multiple factors, such as location of the defect, cost, compliance, comorbidities, number of defects, concomitant procedures, and whether the defect is contained or uncontained. No single study within this systematic review provided all this information. Within the studies considered, many factors influenced the success and viability of the techniques that were used. Given the considerable inconsistency and variability in the reported data, no conclusive statement can be made on the ideal surgical restoration technique for the patella.
Limitations
This systematic review has its own set of strengths and weaknesses, in addition to those of the studies, and is level 4 evidence given the composition of the studies and the criteria provided by the Centre for Evidence-Based Medicine. These studies did not use a common outcome score, which made it difficult to directly compare all techniques; in fact, 5 outcome scores were used, with the IKDC being the most common (13 of 24 studies). Only 4 studies15,16,23,27 gave general information regarding the patients’ return to activities/sports, and no study gave information regarding cost-efficiency. These limitations prevented the ability to synthesize and analyze the data in a true meta-analysis. As a result, this systematic review serves to provide an organized and comprehensive approach to the most modern surgical techniques for restoration of patellar chondral lesions. Additionally, complication rates and follow-up lengths were provided for most, if not all, techniques. Another factor not considered or presented in these studies is the status of the underlying bone, which may direct management. Osteochondral allografts or autografts that address the chondral and underlying osseous abnormalities may prove beneficial in lesions with subchondral cysts or bone marrow edema. Alternatively, cell- or chondral-based therapies may require appropriate bone grafting (single or 2 stage) for success.24,29
Last, cartilage restoration in any part of the knee requires a thorough understanding of bony alignment and subsequent correction of any malalignment. Most of the studies in this review did not stratify their results based on the presence of a concomitant TTO. The presence of an osteotomy can affect complication rates, but the absence of an osteotomy can affect outcome scores.
Conclusion
From the studies reviewed, 1 technique (OAT) was predominantly used for smaller chondral lesions (<2 cm2) and demonstrated minimal complication rates and satisfactory outcome scores. aMF techniques show promise with improvement in outcome scores and zero complications, but indications (ie, size) and variability in techniques need to be elucidated in higher-level studies. MACI consistently exhibited higher mean improvement in the measured outcome scores and resulted in fewer complications when compared with previous generations of ACI. Further prospective studies comparing OCA and MACI for larger patellar defects are necessary to determine the superior technique.
Appendix
Table A1.
Patient-Reported Outcome Scores and Complications a
| Lead Author (Year) | Procedure (No. of Knees at Follow-up) | Outcome | Score, Mean ± SD (Range) | Complications (No. [%]) | Concomitant Procedures | |
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | |||||
| Astur (2014) 2 | OAT (33) | Lysholm | 57.27 ± 19.97 | 80.76 ± 12.26 | Arthrofibrosis (3 [9]) | — |
| Fulkerson | 54.24 ± 18.89 | 80.42 ± 10.20 | ||||
| Kujala | 54.76 ± 17.61 | 75.18 ± 12.47 | ||||
| SF-36 (physical) | 45.91 ± 13.31 | 63.64 ± 29.11 | ||||
| Astur (2017) 3 | OAT (20) | Kujala | 55.9 | 76.9 | Thigh hypotrophy (11 [55]) | — |
| Tegner | 0-5 | 5-9 (7) | ||||
| Becher (2015) 4 | aMF (5) | KOOS | — | 73 ± 19 (40-90) | None | 1 MPFL, 1 MPFL + TTO |
| Chadli (2017) 8 | Mosaicplasty (8) | IKDC | 49.9 (34.5-57.5) | 86.1 (70-100) | None | — |
| Lysholm | 53.8 (42-80) | 88.5 (69-100) | ||||
| Tegner | 4.5 (3-7) | 6.2 (4-7) | ||||
| Cohen (2012) 10 | OAT (17) | Lysholm | 54.59 ± 25.99 (9-98) | 75.76 ± 18.89 (36-100) | None | 1 MPFL, 6 lateral release |
| Fulkerson | 52.53 ± 25.80 (2-93) | 78.41 ± 18.76 (21-100) | ||||
| Kujala | 49.82 ± 22.04 (12-81) | 73.47 ± 17.66 (43-100) | ||||
| SF-36 (physical) | 45.88 ± 15.02 | 63.53 ± 30.09 | ||||
| Dhollander (2011) 11 | aMF (5) | KOOS | 65 (38-76) | 93 (62-97) | Hypertrophy (2 [40]), incomplete filling of defect (3 [60]) | 2 AMZ, 1 AMZ + MPFL |
| Tegner | 2 (1-3) | 3 (2-3) | ||||
| Kujala | 38 (30-55) | 71 (53-82) | ||||
| Figueroa (2011) 12 | OAT (10) | IKDC | — | 93.6 ± 1.74 (92-96) | None | MPFL, MF |
| Lysholm | 73.8 ± 8.36 (66-86) | 95 ± 4.47 (90-100) | ||||
| Gaweda (2006) 14 | OAT (19) | Marshall | 36.3 ± 2.1 | 46.2 ± 1.8 | — | AMZ + lateral release |
| Gigante (2009) 15 | MACI (14) | Lysholm | 55 (47-74) | 92.5 (85-99) | Pain (screw removal) (2 [14.2]) | AMZ |
| Kujala | 52 (43-69) | 88.5 (85-95) | ||||
| Mod Cincinnati | 2 (2-4) | 8 (6-10) | ||||
| Tegner | 1 (1 -1) | 4 (4-5) | ||||
| Gillogly (2014) 16 | ACI (25) | IKDC | 42.5 | 75.7 | Graft hypertrophy (8 [33]), failed (PFA) (1 [4]) | AMZ + lateral release |
| Mod Cincinnati | 3 | 7 | ||||
| Lysholm | 40.2 | 79.3 | ||||
| SF-12 | 41.2 | 47.6 | ||||
| Gracitelli (2015) 17 | OCA (28) | MA-P (18-point) | 12 | 15.2 | Debridement (9 [32.1]), hardware removal (6 [21.4]), ACLR (1 [3.5]), PF realignment (1 [3.5]), MUA (1 [3.5]), loose body removal (1 [3.5]), failed (8 [28.5]; 4 TKR, 2 PFR, 1 revision, 1 patellectomy | 7 lateral release, 3 realignment (vastus medialis imbrication, AMZ, MPFL) |
| IKDC | 36.5 | 66.5 | ||||
| KS-F | 64.4 | 80.5 | ||||
| Henderson (2006) 18 | ACI (44) | IKDC | 42.3 ± 17.2 | 68.1 ± 23 | 23 (52.2). Group A (ACI + TTO): 10 patients; 9 lesions with periosteal patch hypertrophy or extrusion, 2 with removal of an internal fixation device. Group B (ACI): 13 patients; 15 lesions with periosteal patch hypertrophy or extrusion, 1 new chondral defect. No group specified: 1 meniscectomy, 1 fat pad adhesion | 22 ACLR, AMZ with lateral release |
| SF-36 (physical) | 48.3 ± 16.2 | 61.4 ± 16.9 | ||||
| Joshi (2012) 20 | Synthetic graft (10) | KOOS | 64.7 (38-81) | 69.9 (5-90) | Pain, inflammation (7 [70]; 2 arthroplasty, 5 removal) | — |
| SF-36 | 64.1 (60-71) | 61.3 (52.4-80) | ||||
| Macmull (2012) 22 | ACI-C (25) MACI (23) |
Mod Cincinnati (6-100) | ▪ ACI-C: 42.12 (18-60) | ▪ ACI-C: 48.76 (11-83) | — | — |
| ▪ MACI: 48.39 (22-75) | ▪ MACI: 61.39 (8-100) | |||||
| ▪ All: 45.13 (18-75) | ▪ All: 54.81 (11-100) | |||||
| Stanmore (0-4) | ▪ ACI-C: 3.04 (1-4) | ▪ ACI-C: 2.44 (0-4) | ||||
| ▪ MACI: 2.78 (1-4) | ▪ MACI: 2.09 (0-4) | |||||
| ▪ All: 2.92 (0-4) | ▪ All: 2.27 (0-4) | |||||
| Mehl (2019) 23 | ACI (78) | IKDC | — | 64.7 ± 20.2 | Revision ACI (1 [1.2]), TKA (5 [6.4]) | 40 AMZ, lateral release |
| Kujala | 67.7 ± 20.2 | |||||
| Nho (2008) 26 | OAT (22) | IKDC | 47.2 ± 14 | 74.4 ± 12.3 | Hardware removal (3 [13.6]; of 9 concomitant TTO), chondromalacia (debridement; 1 [4.5]) | 9 AMZ, 13 lateral release, 3 proximal realignment |
| ADL | 60.1 ± 16.9 | 84.7 ± 8.3 | ||||
| SF-36 | 64 ± 14.8 | 79.4 ± 15.4 | ||||
| Niemeyer (2008) 27 | ACI (70) | Lysholm | — | 73 ± 22.4 | Transplant hypertrophy (2 [2]), wound healing (1 [1.4]), severely abnormal cases (9 [12.9]) | — |
| IKDC | — | 62 ± 21.5 | ||||
| Cincinnati | 34.4 ± 33.9 | 61.5 ± 21.5 | ||||
| Perdisa (2017) 30 | Synthetic graft (34) | IKDC | 39.5 ± 14.5 | 67.6 ± 17.4 | Realignment (2 [5.9]) | 9 realignment (tibial tubercle anteromedialization), 3 removal of posttraumatic calcification, 1 lateral release, 1 MPFL reconstruction, 1 MAT, 1 patellar tendon repair |
| Tegner | 1.8 ± 1 | 3.3 ± 1.1 | ||||
| Sadlik (2017) 32 | aMF (12) | KOOS | 50.3 (17.3-83.9) | 90.1 (77.4-100) | — | 2 AMZ, 1 MPFL, 1 HTO |
| IKDC | 37.4 (4.6-90.8) | 79.4 (42.5-100) | ||||
| Teo (2013) 35 | ACI (23) | IKDC | 45 (5.8-63) | 75 (40.2-96.6) | Periosteal hypertrophy (asymptomatic; 2 [8]) | — |
| Lysholm | 50 (11-79) | 70 (48-100) | ||||
| Tegner | 2.5 (0-5) | 4 (2-7) | ||||
| Tompkins (2013) 36 | PJAC (15) | IKDC | — | 73.3 ± 17.6 | Mild graft hypertrophy (1 needed debridement; 3 [20]), gross graft hypertrophy (debridement; 2 [13.3]), arthrofibrosis (MUA; 2 [13.3]) | 2 MPFL, 3 MPFL + AMZ |
| KOOS | 88.9 ± 12.9 | |||||
| Kujala | 79 (55-99) | |||||
| Tegner | 5 (3-9) | |||||
| Visona (2010) 37 | OAT/mosaicplasty (6) | IKDC | — | 66.3 (36.8-88.5) | None | 1 MPFL + AMZ |
| Lysholm | 85 (69-100) | |||||
| Tegner | 5.7 (4-9) | |||||
| von Keudell (2017) 38 | ACI (30) | Mod Cincinnati | 3.1 ± 1 | 5.7 ± 1.5 | Failed graft (3 [10]; 2 PF arthroplasty, 1 bicompartmental arthroplasty), graft hypertrophy (7 [23]), chondroplasty (5 [16]), arthrofibrosis (4 [13]), hardware removal (2 [6]) | 19 AMZ, 28 lateral release, 5 tracheoplasty |
| KSS | 55.7 ± 12.8 | 73 ± 14.7 | ||||
| WOMAC | 52.2 ± 16.9 | 27.9 ± 23.6 | ||||
| SF-36 | 40 ± 8.2 | 47 ± 10 | ||||
| Yonetani (2019) 41 | OAT (6) | Lysholm | 67 ± 8.8 (54-80) | 90 ± 13 (79-100) | Arthrofibrosis (MUA; 2 [33.3]) | — |
a Dashes indicate data not available. ACI, autologous chondrocyte implantation; ACI-C, autologous chondrocyte implantation–collagen; ACLR, anterior cruciate ligament reconstruction; ADL, Activities of Daily Living; aMF, advanced microfracture; AMZ, anteromedialization; HTO, high tibial osteotomy; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; KS-F, Knee Society–function; KSS, Knee Society Score; MACI, matrix-induced autologous chondrocyte implantation; MA-P, Merle d’Aubigné and Postel score; MAT, meniscal allograft transplant; MF, microfracture; Mod, modified; MPFL, medial patellofemoral ligament; MUA, manipulation under anesthesia; OAT, osteochondral autograft transplantation; OCA, osteochondral allograft transplantation; PF, patellofemoral; PFA, patellofemoral arthroplasty; PFR, patellofemoral replacement; PJAC, particulate juvenile articular cartilage; SF-36, 36-Item Short Form Health Survey; TKA, total knee arthroplasty; TKR, total knee replacement; TTO, tibial tubercle osteotomy; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Footnotes
Final revision submitted October 16, 2022; accepted October 26, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: R.M.P. has received consulting fees from Ceterix, Daiichi Sankyo, and Smith & Nephew; speaking fees from Sanofi-Aventis and Smith & Nephew; hospitality payments from Arthrosurface; and honoraria from Fidia Pharma. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1.Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160–2168. [DOI] [PubMed] [Google Scholar]
- 2.Astur DC, Arliani GG, Binz M, Macalena JA, Stuart MJ, Krych AJ. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816–823. [DOI] [PubMed] [Google Scholar]
- 3.Astur DC, Bernardes A, Castro S, et al. Functional outcomes after patellar autologous osteochondral transplantation. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3084–3091. [DOI] [PubMed] [Google Scholar]
- 4.Becher C, Ettinger M, Ezechieli M, Kaps C, Ewig M, Smith T. Repair of retropatellar cartilage defects in the knee with microfracture and a cell-free polymer-based implant. Arch Orthop Trauma Surg. 2015;135(7):1003–1010. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 6.Brophy RH, Wojahn RD, Lamplot JD. Cartilage restoration techniques for the patellofemoral joint. J Am Acad Orthop Surg. 2017;25(5):321–329. [DOI] [PubMed] [Google Scholar]
- 7.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chadli L, Cottalorda J, Delpont M, Mazeau P, Thouvenin Y, Louahem D. Autologous osteochondral mosaicplasty in osteochondritis dissecans of the patella in adolescents. Int Orthop. 2017;41(1):197–202. [DOI] [PubMed] [Google Scholar]
- 9.Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Amaro JT, Fernandes RS, et al. Osteochondral autologous transplantation for treating chondral lesions in the patella. Rev Bras Ortop. 2012;47(3):348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhollander AA, De Neve F, Almqvist KF, et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):536–542. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa D, Melean P, Calvo R, Gili F, Zilleruelo N, Vaisman A. Osteochondral autografts in full thickness patella cartilage lesions. Knee. 2011;18(4):220–223. [DOI] [PubMed] [Google Scholar]
- 13.Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42(3):626–634. [DOI] [PubMed] [Google Scholar]
- 14.Gaweda K, Walawski J, Wegłowski R, Drelich M, Mazurkiewicz T. Early results of one-stage knee extensor realignment and autologous osteochondral grafting. Int Orthop. 2006;30(1):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gigante A, Enea D, Greco F, et al. Distal realignment and patellar autologous chondrocyte implantation: mid-term results in a selected population. Knee Surg Sports Traumatol Arthrosc. 2009;17(1):2–10. [DOI] [PubMed] [Google Scholar]
- 16.Gillogly S, Arnold R. Autologous chondrocyte implantation and anteromedialization for isolated patellar aticular cartilage lesions: 5- to 11-year follow-up. Am J Sports Med. 2014;42(4):912–20. [DOI] [PubMed] [Google Scholar]
- 17.Gracitelli GC, Meric G, Pulido PA, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879–884. [DOI] [PubMed] [Google Scholar]
- 18.Henderson IJP, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13(4):274–279. [DOI] [PubMed] [Google Scholar]
- 19.Huberti HH, Hayes WC. Patellofemoral contact pressures: the influence of Q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66(5):715–724. [PubMed] [Google Scholar]
- 20.Joshi N, Reverte-Vinaixa M, Díaz-Ferreiro EW, Domínguez-Oronoz R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am J Sports Med. 2012;40(6):1289–1295. [DOI] [PubMed] [Google Scholar]
- 21.Loudon JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11(6):820–830. [PMC free article] [PubMed] [Google Scholar]
- 22.Macmull S, Jaiswal PK, Bentley G, et al. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int Orthop. 2012;36(7):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehl J, Huck J, Bode G, et al. Clinical mid- to long-term outcome after autologous chondrocyte implantation for patellar cartilage lesions and its correlation with the geometry of the femoral trochlea. Knee. 2019;26(2):364–373. [DOI] [PubMed] [Google Scholar]
- 24.Minas T, Ogura T, Headrick J, Bryant T. Autologous chondrocyte implantation “sandwich” technique compared with autologous bone grafting for deep osteochondral lesions in the knee. Am J Sports Med. 2018;46(2):322–332. [DOI] [PubMed] [Google Scholar]
- 25.Mouzopoulos G, Borbon C, Siebold R. Patellar chondral defects: a review of a challenging entity. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):1990–2001. [DOI] [PubMed] [Google Scholar]
- 26.Nho SJ, Foong Foo L, Green DM, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med. 2008;36(6):1101–1109. [DOI] [PubMed] [Google Scholar]
- 27.Niemeyer P, Steinwachs M, Erggelet C, et al. Autologous chondrocyte implantation for the treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg. 2008;128(11):1223–1231. [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR, Barber-Westin SD. Advanced patellofemoral cartilage lesions in patients younger than 50 years of age: is there an ideal operative option? Arthroscopy. 2013;29(8):1423–1436. [DOI] [PubMed] [Google Scholar]
- 29.Orgura T, Merkely G, Bryant T, Winalski CS, Minas T. Autologous chondrocyte implantation “segmental-sandwich” technique fordeep osteochondral defects in the knee. Orthop J Sports Med. 2019;7(5):2325967119847173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perdisa F, Filardo G, Sessa A, et al. One-step treatment for patellar cartilage defects with a cell-free osteochondral scaffold: a prospective clinical and MRI evaluation. Am J Sports Med. 2017;45(7):1581–1588. [DOI] [PubMed] [Google Scholar]
- 31.Porteous A, Sullivan N, Murray J, Eldridge J. How thick is the patella? A reproducible measure of patella width: thickness from adult MRI [abstract]. Orthopaedic Proceedings. 2013;95B(suppl 14):46. [Google Scholar]
- 32.Sadlik B, Puszkarz M, Kosmalska L, Wiewiorski M. All-arthroscopic autologous matrix–induced chondrogenesis-aided repair of a patellar cartilage defect using dry arthroscopy and a retraction system. J Knee Surg. 2017;30(9):925–929. [DOI] [PubMed] [Google Scholar]
- 33.Shanmugaraj A, Coughlin RP, Kuper GN, et al. Changing trends in the use of cartilage restoration techniques for the patellofemoral joint: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):854–867. [DOI] [PubMed] [Google Scholar]
- 34.Strauss EJ, Galos DK. The evaluation and management of cartilage lesions affecting the patellofemoral joint. Curr Rev Musculoskelet Med. 2013;6(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teo BJ, Buhary K, Tai BC, Hui JH. Cell-based therapy improves function in adolescents and young adults with patellar osteochondritis dissecans. Clin Orthop Relat Res. 2013;471(4):1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661–1670. [DOI] [PubMed] [Google Scholar]
- 37.Visona E, Chouteau J, Aldegheri R, Fessy MH, Moyen B. Patella osteochondritis dissecans end stage: the osteochondral mosaicplasty option. Orthop Traumatol Surg Res. 2010;96:543–548. [DOI] [PubMed] [Google Scholar]
- 38.von Keudell A, Han R, Bryant T, Minas T. Autologous chondrocyte implantation to isolated patella cartilage defects: two- to 15-year follow-up. Cartilage. 2017;8(2):146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Belkin NS, Burge AJ, et al. Patellofemoral cartilage lesions treated with particulated juvenile allograft cartilage: a prospective study with minimum 2-year clinical and magnetic resonance imaging outcomes. Arthroscopy. 2018;34(5):1498–1505. [DOI] [PubMed] [Google Scholar]
- 40.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. [DOI] [PubMed] [Google Scholar]
- 41.Yonetani Y, Tanaka Y, Kanamoto T, et al. Autologous osteochondral transplantation in full-thickness patella chondral lesion: a case series. J Orthop Case Rep. 2019;9(1):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamborsky R, Danisovic L. Surgical techniques for knee cartilage repair: an updated large-scale systematic review and network meta-analysis of randomized controlled trials. Arthroscopy. 2020;36(3):845–858. [DOI] [PubMed] [Google Scholar]