Abstract
Objectives:
To explore the rationale and value of consolidative cranial local therapy (CLT) in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) patients with brain metastases (BMs).
Methods:
EGFR-mutant NSCLC patients with baseline BMs who received first-line EGFR-tyrosine kinase inhibitors (TKIs) at two academic centers from May 2015 to June 2020 were retrospectively enrolled. Patterns of tumor response and treatment failure were extensively analyzed in order to explore the rationale of CLT. Cranial lesions with number ⩽3 and largest tumor size ⩽3 cm at baseline and best response to EGFR-TKIs were defined as oligo-BMs and oligo-residual cranial disease (ORCD), respectively. To provide preliminary data supporting CLT, survival outcomes were compared in patients with ORCD, stratified by CLT status.
Results:
Of the 216 patients enrolled, 57.1% had oligo-BMs and 24.5% received first-line osimertinib. At best response to the first-line EGFR-TKIs, intracranial complete response, partial response, and stable disease occurred in 18.5, 31.9, and 44.4% of the whole population, respectively. For patients without CLT (n = 193), ORCD was observed in 78.1% of the 105 patients with baseline oligo-BMs and 10.2% of the 88 patients with baseline multiple-BMs. With a median follow-up of 22.8 months, 107 patients had cranial first progressive disease (PD); more than 60% developed their first PD solely from the residual tumor sites at best response to EGFR-TKIs. Moreover, among patients with ORCD (n = 108), patients who received CLT (n = 17) achieved significantly longer progression-free survival (13.4 versus 8.5 months, p = 0.001) and overall survival (58.9 versus 28.8 months, p = 0.021) than those without CLT. Meanwhile, CLT remained as an independent prognostic factor associated with improved survival after Cox regression analyses.
Conclusions:
Cranial progressive disease developed mostly at the residual cranial lesions in EGFR-mutant NSCLC patients with baseline BMs who received first-line EGFR-TKIs. Consolidative cranial local therapy targeting the oligo-residual cranial tumor lesions may provide survival benefit, which warrants future validation.
Keywords: brain metastasis, consolidative cranial local therapy, failure patterns, oligo-residual cranial disease
Introduction
Lung cancer, the leading cause of cancer-related death worldwide, remains a great threat to public health. As many as 50% of non-small cell lung cancer (NSCLC) patients will develop brain metastasis (BM) during the course of disease,1,2 and the BM prevalence rates are reported even greater for patients who harbor epidermal growth factor receptor (EGFR)-sensitive mutations.2–4 NSCLC patients with BM have poor prognosis with a median survival of 3–6 months. 5 EGFR-tyrosine kinase inhibitors (TKIs), the recommended initial treatment of patients with advanced, recurrent, or metastatic EGFR-mutant NSCLC patients, 6 make it possible to gain longer survival.3,7 However, it seems that the existence of blood–brain–barrier limits the efficacy of EGFR-TKIs to the central nervous system metastasis.
Local therapies, including surgical resection, whole brain radiotherapy (WBRT), and stereotactic radiosurgery (SRS), have been extensively investigated and have historically been considered as a cornerstone in the treatment of brain metastases (BMs).8,9 And previous studies showed EGFR-TKIs plus cranial local therapy could result in longer survival in patients with baseline BMs who receive EGFR-TKI treatment.10–13 However, conflicting results also exist.14,15 Recently, studies from our group10,16 and Miyawaki et al. 17 found that intracranial radiotherapy combining with EGFR-TKIs could significantly improve patient’s survival only among those with limited BMs, but not in those with extensive cranial lesions. However, the optimal timing of adding local therapy for patients with BM remains unknown.
Accumulating evidence has revealed the survival benefits of extracranial consolidative local therapy in metastatic EGFR-mutant NSCLC patients with oligo-metastatic extracranial lesions.18,19 However, studies investigating the clinical utility of consolidative cranial local therapy (CLT) in patients with oligo-residual BMs remain poorly understood. We hypothesized that CLT may be feasible and beneficial for patients with BMs for the following reasons. Firstly, some patients with baseline multiple-BMs that are not suitable for the less neuro-toxic stereotactic radiosurgery/radiotherapy (SRS/SRT) could eventually harbor oligo-residual cranial disease (ORCD) after EGFR-TKI treatment. 13 Therefore, there may be more patients eligible for SRS/SRT at the time of best response to EGFR-TKI than at the time of disease diagnosis. Secondly, SRS/SRT targeting smaller BMs using smaller target volumes could possibly lead to less neuro-toxicities.11,20,21 Herein, we performed a two-center retrospective analysis of patients with EGFR-mutant NSCLC who developed BMs and received EGFR-TKIs as first-line therapy to evaluate the rationale and clinical value of CLT, especially in patients with ORCD.
Materials and methods
Patients
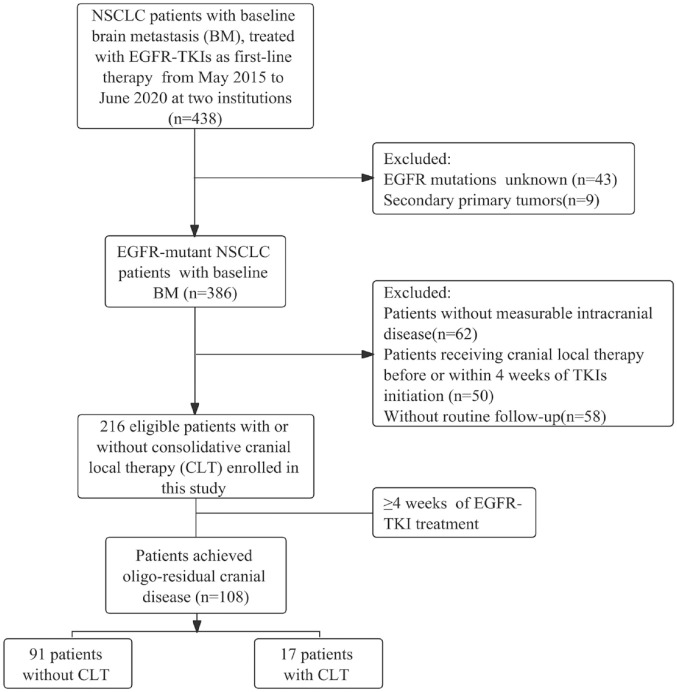
The baseline characteristics and clinical outcomes of EGFR-mutant NSCLC patients with baseline BMs, who received first-line EGFR-TKIs treatment, including first-generation EGFR-TKIs and osimertinib, at two academic centers from May 2015 to June 2020 were retrospectively collected. Patients diagnosed with BMs while receiving first-line EGFR-TKIs treatment were included in this study. Of note, only patients with measurable cranial disease and adequate regular radiographic follow-up were included. Patients who received intracranial local therapy prior to or within 4 weeks of EGFR-TKIs initiation were excluded. Patients’ clinical data at the time of disease diagnosis, including gender, age, smoking status, Karnofsky Performance Status, EGFR mutation status, intracranial tumor size and number, and EGFR-TKI treatment were retrospectively collected from electronic medical records. The Graded Prognostic Assessment (GPA) score 22 was assigned to each patient. The flowchart for patient selection is shown in Figure 1.
Figure 1.
Patients’ selection flowchart.
Treatment and follow-up
EGFR-TKIs were administered with standard dosage (osimertinib 80 mg once daily, gefitinib 250 mg once daily, or erlotinib 150 mg once daily) as sole systemic therapy in all patients. Patients were divided into two groups based on whether they received CLT; CLT was defined as consolidative cranial local therapy, including surgical resection, SRS/SRT, and WBRT, performed at least 4 weeks after the initiation of EGFR-TKIs, and disease was clinically confirmed to respond to EGFR-TKIs. In general, BMs were confirmed based on brain magnetic resonance imaging (MRI, T1-weighted with gadolinium contrast) or contrast-enhanced computed tomographic (CT) imaging, positron emission tomography (PET)–CT of the brain in combination with corresponding clinical signs and/or symptoms. 21 Pathologic confirmation could only be obtained in a small percentage of patients. And CT of the chest and upper abdomen and bone scintigraphy were performed at the same time. PET/CT was not mandatory. Radiographic follow-up was generally performed regularly 4 weeks after EGFR-TKI initiation and every 8–12 weeks thereafter.
Definition and response assessment of BM status
Oligo-BMs and ORCD were defined as cranial tumor lesions with a number of ⩽3 and largest tumor size of ⩽3 cm, assessed at baseline and best response to EGFR-TKIs, respectively. And cranial lesions with number >3 and/or tumor size >3 cm, which were evaluated at baseline and best response to EGFR-TKIs, were designated as multiple-BMs and multiple-residual cranial disease, respectively. Treatment and response with respect to both extracranial and intracranial tumor lesions was assessed according to the response evaluation criteria in solid tumors (RECIST) 1.1 and AURA3 clinical trial as described previously, 23 including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Statistics analysis
The initial patterns of cranial treatment failure were assessed using regular brain MRI scans in this study. Brain MRI was reviewed by two independent experienced radiologists to determine whether the progressive disease was derived from preexisted tumor lesions or newly arising lesions, or both. Cranial disease progression occurred only from residual tumor lesions at the best response to EGFR-TKIs and was defined as original site progressive disease (OPD). Cranial disease progression occurring solely from newly emergent tumor lesions that did not exist at the best response to EGFR-TKIs (which may exist at baseline before EGFR-TKI initiation but disappeared after EGFR-TKIs) was defined as new site progressive disease (NPD). Cranial disease developed at both sites and was named as original-new progressive disease (ONPD). Overall survival (OS) was calculated from the date of diagnosis of advanced NSCLC to death from any cause or the day of last follow-up. Progression-free survival (PFS) was calculated from the date of diagnosis of advanced NSCLC to initial disease progression or death result from any cause, or censored at the day of last follow-up. The Kaplan-Meier method was used to calculate the survival curve; and log-rank test was used to compare the survival differences. A Cox proportional hazards regression model was conducted to figure out prognostic factors associated with OS and PFS of patients with ORCD. Two-sided p value less than 0.05 was considered as statistical significance.
Results
Patient characteristics
Of the 216 patients included in this study, 44% had oligo-BMs and 56% had multiple-BMs at baseline. EGFR 19del mutation and L858R mutation were detected in 52.3 and 38.4% of the patients, respectively. There were respective 75.5 and 24.5% of the patients who received first-generation EGFR-TKIs and osimertinib as their first-line treatment. Patient characteristics and treatment details for the whole population are shown in Table 1.
Table 1.
Characteristics and treatment details in the whole population.
| Characteristics | N = 216 | % |
|---|---|---|
| Age | ||
| <60 | 90 | 41.7 |
| ⩾60 | 126 | 58.3 |
| Gender | ||
| Women | 138 | 63.9 |
| Men | 78 | 36.1 |
| GPA | ||
| <2 | 100 | 46.3 |
| ⩾2 | 116 | 53.7 |
| KPS | ||
| <70 | 30 | 13.9 |
| ⩾70 | 186 | 86.1 |
| Smoking | ||
| No | 174 | 80.6 |
| Yes | 21 | 9.7 |
| Unknown | 21 | 9.7 |
| EGFR mutation | ||
| 19del | 113 | 52.3 |
| L858R | 83 | 38.4 |
| Others | 20 | 9.3 |
| Baseline oligo-BM | ||
| No | 121 | 56.0 |
| Yes | 95 | 44.0 |
| TKI generations | ||
| 1st | 163 | 75.5 |
| 3rd | 53 | 24.5 |
BM, brain metastasis; GPA, Graded Prognostic Assessment; KPS, Karnofsky Performance Status; TKIs, tyrosine kinase inhibitors.
Patterns of intracranial tumor treatment and response
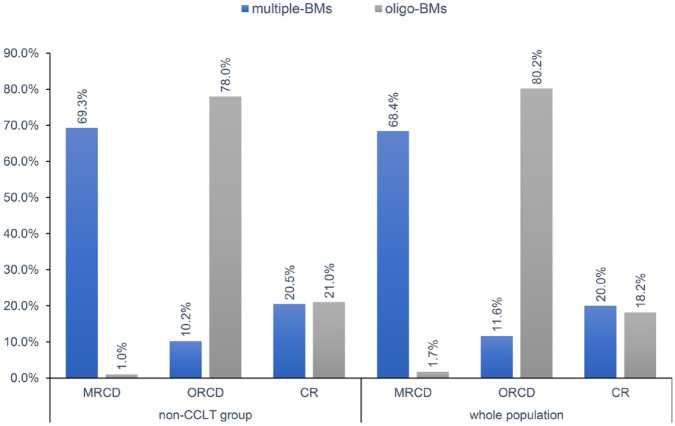
In the whole cohort, the median PFS of patients who received first-generation EGFR-TKIs and osimertinib were 8.5 (95% CI: 7.7–9.3 months) and 11.4 (95% CI: 6.8–16.0 months) months, respectively. Meanwhile, the median time to best cranial response was 3.0 months, at which no one had extracranial progressive disease, and 40 (18.5%), 69 (31.9%), and 96 (44.4%) patients achieved intracranial CR (iCR), intracranial PR (iPR), and intracranial SD (iSD), respectively, in the whole population (n = 216). No significant difference of the rates of iCR, iPR, and iSD was observed between patients receiving first-generation EGFR-TKIs and osimertinib (p = 0.74). Of the 193 patients without CLT, 88 (45.6%) patients had baseline multiple-BMs and 105 (54.4%) patients had baseline oligo-BMs. Among the patients with baseline multiple-BMs and without CLT, 18 (20.5%) patients achieved iCR and 9 (10.2%) patients achieved ORCD. For the patients with baseline oligo-BMs and without CLT, 22 (21.0%) patients achieved iCR and 82 (78.1%) remained with oligo-BMs. Details of tumor response patterns in the entire population as well as in patients without CLT are shown in Figure 2 and Supplemental Table S3. Correlations between clinicopathological parameters, including age, gender, GPA, EGFR-TKIs, smoking status, size and number of baseline BMs, and baseline oligo-BM status, were analyzed. We found that baseline BM status (oligo-BM versus multiple-BM) was associated with the development of ORCD (p < 0.001), among patients without CLT.
Figure 2.
Treatment–response patterns. Patterns of treatment and response of patients in the whole population and in the non-CLT group. Blue bars indicate patients with baseline multiple-BMs, and gray bars indicate patients with baseline oligo-BMs.
BMs, brain metastases; CR, complete response; MRCD, multiple-residual cranial disease; ORCD, oligo-residual cranial disease.
Patterns of treatment failure
With a median follow-up of 22.8 months (range: 1.1–78.9 months), the median time to intracranial treatment failure for first-generation EGFR-TKIs and osimertinib was 15.7 (95% CI: 13.2–18.1 months) and 34.7 (95% CI: 10.0–59.4 months) months, respectively. A total of 107 patients experienced intracranial PD: 69 (64.5%) patients had OPD and 25 (23.4%) patients developed NPD. Among the 93 patients without CLT who experienced intracranial PD, 59 (63.5%) patients had OPD and 23 (24.7%) patients developed NPD. Moreover, among the 46 patients who achieved ORCD without CLT and experienced intracranial PD, 29 (63.0%) patients had OPD and 11 (23.9%) patients developed NPD. Detailed data about patterns of treatment failure were summarized in Figure 3 and Supplemental Table S3; and more than 60% of patients developed OPD regardless of the BM status at the best response to EGFR-TKIs, highlighting the potential role of CLT targeting residual intracranial tumor lesions at the best response to EGFR-TKIs.
Figure 3.
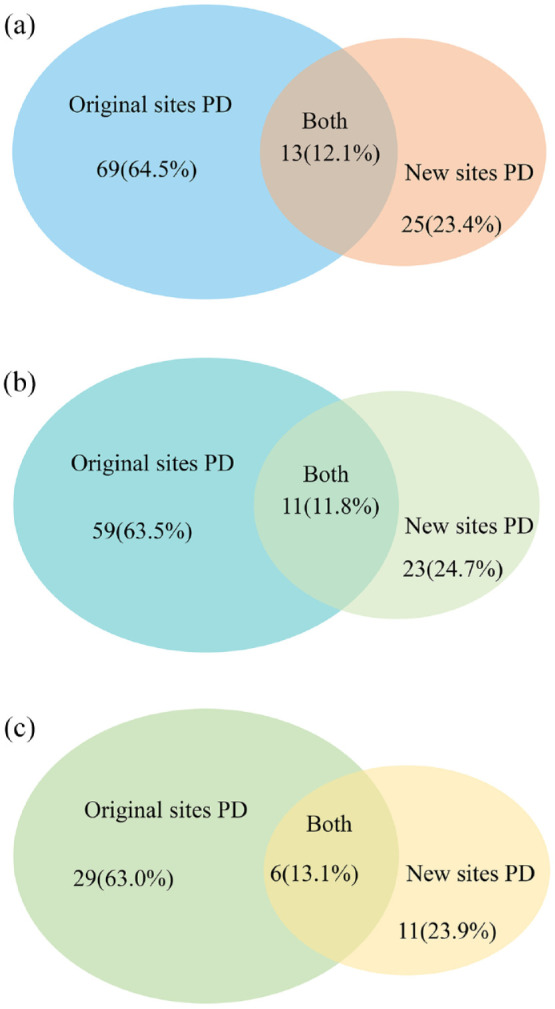
Failure patterns. Patterns of intracranial disease failure of patients (a) in the whole population, (b) non-CLT cohort, and (c) patients with oligo-residual cranial disease in the non-CLT population. Failure patterns were defined as original sites progressive disease (PD), new sites PD, and both.
Clinical value of CLT for patients with ORCD
At the best response to EGFR-TKIs, 108 patients achieved ORCD, including 17 (15.7%) patients receiving CLT and 91 (84.3%) patients without CLT. Baseline characteristics of patients with or without CLT were generally balanced (Supplemental Table S1). In terms of patients with CLT, 15 (88.2%) patients received SRS, while WBRT and neurosurgery were performed in each patient, respectively. By the time of data cut-off, 8 (53.3%) of the 15 patients who received consolidative SRS had developed their initial intracranial PD, of which 6 (75.0%) patients developed oligo-progressive intracranial disease which was still eligible for salvage SRS/SRT.
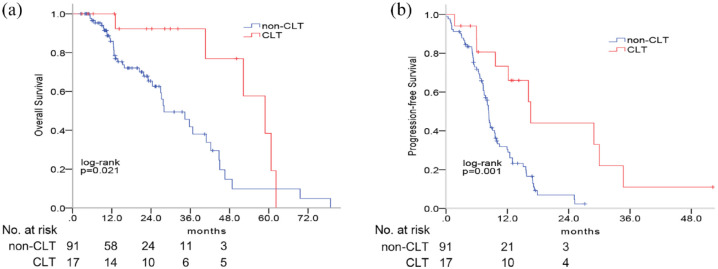
By now, 94 (43.5%) patients had died. Among the 108 patients with ORCD, the median PFS and OS were 8.5 months (95% CI: 7.4–9.6 months) and 36.7 months (95% CI: 29.3–44.2 months), respectively. Patients with CLT achieved significantly longer PFS (13.4 versus 8.5 months; hazard ratio (HR) = 0.51, 95% CI: 0.34–0.76; p = 0.001) and OS (58.9 versus 28.8 months, HR = 0.42, 95% CI: 0.25–0.70, p = 0.021) than those without CLT (Figure 4). Univariate analysis showed that EGFR L858R (HR = 0.35, 95% CI: 0.14–0.89, p = 0.03) and CLT (HR = 0.36, 95% CI: 0.15–0.88, p = 0.03) were associated with better OS; GPA ⩾ 2 (HR = 1.76, 95% CI: 1.09–2.83, p = 0.02) and CLT (HR = 0.29, 95% CI: 0.13–0.64, p = 0.002) were associated with better PFS. Multivariate analysis revealed that CLT remained as an independent prognostic factor for improved PFS (HR = 0.31, 95% CI: 0.14–0.68, p = 0.004) and OS (HR = 0.37, 95% CI: 0.15–0.93, p = 0.03) (Supplemental Table S2).
Figure 4.
Survival outcomes of patients with oligo-residual cranial disease in the consolidative cranial local therapy (CLT) cohort and non-CLT cohort. Patients in the CLT could obtain greater overall survival (a) and progression-free survival (b).
Discussion
To the best of our knowledge, this is the first study investigating the rationale and clinical value of consolidative cranial local therapy in first-line EGFR-TKI treated NSCLC patients who had BMs at baseline. It showed that a considerable percentage of patients developed ORCD after first-line EGFR-TKI treatment; and the majority of patients with ORCD suffered cranial progressive disease at the residual cranial lesions, highlighting the potential role of CLT targeting the oligo-residual cranial lesions. In addition, as a proof-of-concept study, we found that CLT significantly improved patient’s survival among those who achieved ORCD, which warrants future validation in prospective studies with larger sample sizes.
Although EGFR-TKIs have been shown to be effective for patients with advanced EGFR-mutant NSCLC and BMs, the dynamic changes of cranial tumor lesions and patterns of cranial tumor response are largely unknown. In our study, the majority (78.1%) of patients with baseline oligo-BM and 10.2% of patients with baseline multiple-BMs who received first-line EGFR-TKIs alone achieved ORCD, indicating that a clinically relevant percentage (about 30–50%) of patients could develop ORCD with the treatment of first-line EGFR-TKI, since the prevalence of baseline oligo-BMs generally ranged from 40 to 60% in the previous studies.10,16,21,24 Future studies are urgently needed to examine the exact frequency of baseline oligo-BMs in metastatic EGFR-mutant NSCLC patients and to comprehensively investigate the spatial temporal changes of cranial tumor lesions after first-line EGFR-TKIs with larger sample size.
The majority of cranial PD originated solely from the residual cranial tumor lesions in our study, both in patients treated with osimertinib and first-generation EGFR-TKIs, which generally collaborated with other studies and provided preliminary rationale for CLT. Previous studies found that about 59–76.9% of BM initially developed from the original sites in untreated EGFR-mutant NSCLC patients with BMs who received osimertinib or first-generation EGFR-TKIs.10,16 In this study, about 60% of patients, regardless of the status of residual cranial tumors, experienced intracranial OPD. Miyawaki et al. 17 also reported that most disease progression developed from residual lesions after EGFR-TKIs treatment. Mechanically, it was found that spatially and temporally separated BM sites were genetically homogenous, whereas great genetic heterogeneity was identified between BM and primary tumors or extracranial metastatic lesions.12,25,26 These findings indicate that the initial few BMs might serve as the ‘seeds’ for potential disseminated BMs and recurrence. Moreover, drug resistance develops largely as a result of the regrowth of TKI-resistant clones that often arise within the sites of persistent disease.27,28 Therefore, preemptive local therapy targeting the residual cranial disease may significantly delay the disease progression in the brain, and our previous studies have found that upfront cranial radiotherapy could significantly reduce the risk of intracranial PD for first-line EGFR-TKI treated NSCLC with BMs.10,16
Consolidative local therapy has a crucial role in the treatment of advanced NSCLC patients. Numerous studies reported that the benefit of extracranial consolidative local therapy in patients with limited metastatic NSCLC patients.19,29–31 In the present study, CLT was shown to significantly prolong patient’s survival among those who reached ORCD; and it remained as an independent prognostic factor associated with improved survival, which provided proof-of-concept evidence supporting the clinical utility of CLT, especially consolidative SRS/SRT. Meanwhile, by the time of data-cutoff, only 2 (25%) of the 8 patients who received consolidative SRS and experienced initial intracranial PD, developed multiple intracranial progression diseases. Salvage SRS when patients experienced intracranial PD may be still suitable for the other 75% of patients who received consolidative SRS. Since numerical studies have found that SRS/SRT is the superior choice as a safer and more effective treatment for NSCLC patients with limited BMs,21,32,33 the above findings provide another supportive argument for CLT.
The study also has some limitations. First, selection bias existed due to the retrospective nature of the analysis, thus the survival benefit of CLT was just hypothesis-generating, which should be interpreted with caution and needed to be tested in randomized clinical trials. Second, information about treatment-related toxicities as well as salvage therapies were not gathered in our study. The safety profiles of intracranial SRS/SRT in combination with EGFR-TKIs needs to be further clarified. Furthermore, the safety and efficacy of cranial local therapy to more than 3 BMs in EGFR-mutant NSCLC patients are currently under investigations and prospective trials, such as NCT05378633, NCT04905550, and NCT03497767, are ongoing. Hence, further investigations examining the feasibility and clinical value of CLT in patients with more than three cranial residual tumor lesions are warranted.
In conclusion, the majority of EGFR-mutant NSCLC patients who had BMs and received EGFR-TKIs as first-line treatment would develop cranial progressive disease at the residual cranial lesions, indicating the potential value of consolidative cranial local therapy. Moreover, consolidative cranial local therapy targeting the oligo-residual cranial tumor lesions may provide survival benefit, which warranted future validation.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231169975 for Rationale and value of consolidative cranial local therapy in EGFR-mutant non-small cell lung cancer patients with baseline brain metastasis treated with first-line EGFR-TKIs by Ya Zeng, Xi Su, Yang Zhao, Yue Zhou, Tiantian Guo, Xiao Chu, Li Chu, Xi Yang, Jianjiao Ni and Zhengfei Zhu in Therapeutic Advances in Medical Oncology
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Zhengfei Zhu  https://orcid.org/0000-0001-7537-3619
https://orcid.org/0000-0001-7537-3619
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ya Zeng, Department of Radiation Oncology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
Xi Su, Department of Radiation Oncology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Yang Zhao, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Yue Zhou, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Tiantian Guo, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Xiao Chu, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Li Chu, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Xi Yang, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Jianjiao Ni, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, 270 Dong An Road, Shanghai 200032, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Zhengfei Zhu, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, 270 Dong An Road, Shanghai 200032, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China; Institute of Thoracic Oncology, Fudan University, Shanghai, China.
Declarations
Ethics approval and consent to participate: The required informed consent was waived due to the retrospective nature of the study. This study was approved by the institutional review boards of Fudan University Shanghai Cancer Center (2012228-4) and Shanghai Chest Hospital (KS1716).
Consent for publication: Not applicable.
Author contribution(s): Ya Zeng: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Xi Su: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Yang Zhao: Data curation; Formal analysis; Writing – review & editing.
Yue Zhou: Data curation; Formal analysis; Writing – review & editing.
Tiantian Guo: Data curation; Formal analysis; Writing – review & editing.
Xiao Chu: Investigation; Writing – review & editing.
Li Chu: Investigation; Writing – review & editing.
Xi Yang: Investigation; Writing – review & editing.
Jianjiao Ni: Conceptualization; Methodology; Writing – review & editing.
Zhengfei Zhu: Conceptualization; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Hui Lan Public Welfare (No. HL-HS2020-46) to Jianjiao Ni and the CSCO Foundation (No. Y-2019AZMS-1045) to Zhengfei Zhu.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data generated and analyzed during this study are included in this provided article (as well as in the supplementary information files).
References
- 1.Moro-Sibilot D, Smit E, Carpeño JC, et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: analysis from the European FRAME study. Lung Cancer 2015; 90: 427–432. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 2004; 22: 2865–2872. [DOI] [PubMed] [Google Scholar]
- 3.Shin DY, Na L, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014; 9: 195–199. [DOI] [PubMed] [Google Scholar]
- 4.Iuchi T, Shingyoji M, Itakuraet M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015; 20: 674–679. [DOI] [PubMed] [Google Scholar]
- 5.Kelly K, Bunn PA., Jr.Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer 1998; 20: 85–91. [DOI] [PubMed] [Google Scholar]
- 6.Khozin S, Blumenthal GM, Jiang XP, et al. U.S. Food and drug administration approval summary: erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (l858r) substitution mutations. Oncologist 2014; 19: 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu F, De Caluwe A, Anderson D, et al. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer 2016; 96: 101–107. [DOI] [PubMed] [Google Scholar]
- 8.Johung KL, Yao XP, Lie FY, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res 2013; 19: 5523–5532. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Saad S, Qureshi Y, et al. Does lung cancer mutation status and targeted therapy predict for outcomes and local control in the setting of brain metastases treated with radiation? Neuro Oncol 2015; 17: 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu F, Ni J, Zeng W, et al. Clinical value of upfront cranial radiation therapy in osimertinib-treated epidermal growth factor receptor-mutant non-small cell lung cancer with brain metastases. Int J Radiat Oncol Biol Phys 2021; 111: 804–815. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi AM, Schroeder J, Angelov L, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg 2017; 126: 735–743. [DOI] [PubMed] [Google Scholar]
- 12.Jiang T, Fang ZY, Tang SJ, et al. Mutational landscape and evolutionary pattern of liver and brain metastasis in lung adenocarcinoma. J Thorac Oncol 2021; 16: 237–249. [DOI] [PubMed] [Google Scholar]
- 13.Koba T, Kijima T, Takimotoet T, et al. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790m mutation. Medicine 2017; 96: e6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang T, Su CX, Li XF, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 2016; 11: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 15.Thomas NJ, Myall NJ, Sun FD, et al. : Brain metastases in EGFR- and ALK-positive NSCLC: outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol 2022; 17: 116–129. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Li S, Yang X, et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant nonsmall cell lung cancer with brain metastasis. Int J Cancer 2022; 150: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 17.Miyawaki T, Kenmotsu H, Kodama H, et al. Association between oligo-residual disease and patterns of failure during EGFR-TKI treatment in EGFR-mutated non-small cell lung cancer: a retrospective study. BMC Cancer 2021; 21: 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016; 17: 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu QH, Zhou F, Liu H, et al. Considative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol 2018; 13: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 20.Miller JA, Bennett EE, Xiao R, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys 2016; 96: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 21.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 2017; 35: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 22.Gong X, Li X, Jiang T, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol 2017; 12: 1085–1097. [DOI] [PubMed] [Google Scholar]
- 23.Wu YL, Ahn M, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018; 36: 2702–2709. [DOI] [PubMed] [Google Scholar]
- 24.Lin CY, Chang CC, Su PL, et al. Brain MRI imaging characteristics predict treatment response and outcome in patients with de novo brain metastasis of EGFR-mutated NSCLC. Medicine 2019; 98: e16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 2015; 5: 1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik PK, Shen R, Won H, et al. Next generation sequencing of stage IV squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov 2015; 5: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini D, Rose FD, Cozzi S, et al. The use of radiation therapy for oligoprogressive/oligopersistent oncogene-driven non small cell lung cancer: state of the art. Crit Rev Oncol Hematol 2020; 148: 102894. [DOI] [PubMed] [Google Scholar]
- 28.Chan OSH, Lam K, Li JYC, et al. ATOM: a phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of nsclc after EGFR TKI. Lung Cancer 2020; 142: 41–46. [DOI] [PubMed] [Google Scholar]
- 29.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer a phase 2 randomized clinical trial. JAMA Oncol 2018; 4: e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez DR, Tang C, Zhang JJ, et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional,phase II, randomized study. J Clin Oncol 2019; 37: 1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng Y, Ni J, Yu F, et al. The value of local consolidative therapy in osimertinib-treated non-small cell lung cancer with oligo-residual disease. Radiat Oncol 2020; 15: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang WX, Song ZB, Zhang YP. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell lung cancer patients who develop brain metastasis. Arch Med Sci 2018; 14: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014; 15: 387–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231169975 for Rationale and value of consolidative cranial local therapy in EGFR-mutant non-small cell lung cancer patients with baseline brain metastasis treated with first-line EGFR-TKIs by Ya Zeng, Xi Su, Yang Zhao, Yue Zhou, Tiantian Guo, Xiao Chu, Li Chu, Xi Yang, Jianjiao Ni and Zhengfei Zhu in Therapeutic Advances in Medical Oncology