Abstract
Globally, the deterioration of drinking water quality is a major public health problem that contributes to the spread of disease and causes death. Therefore, it is important to have regular quality control monitoring. This study aimed to assess the level of physicochemical and bacteriological quality of household drinking water and its contributing factors in flood-prone settlements of South Gondar Zone, Ethiopia. A community-based cross-sectional study was conducted in flood-prone settings of Northwest Ethiopia from January 17 to March 30, 2021. Structured questionnaires were used to gather the sociodemographic, environmental, and behavioral data. A total of 675 drinking water samples were collected from water storage containers of selected households. Logistic regression models were used for both univariate and multivariable studies. The survey included a total of 675 households. The mean values of pH (5.9 ± 1.03), turbidity (6.7 ± 2.21 NTU), and free residual chlorine (0.02 ± 0.01 mg/l) did not meet the WHO recommended limits for drinking water. The prevalence of fecal contamination of drinking water in the study area was 62.2% with [95% CI (53–60%)]. Family size [AOR = 2.205, 95% CI (1.375–3.536), absence of latrine [AOR = 3.449, 95% CI (1.349–8.823)], and lack of a separate container to draw water from its storage [AOR = 0.454, 95% CI (0.249–0.827)] were significant predictors for fecal contamination of household drinking water. In conclusion, the water quality in terms of pH, turbidity, residual chlorine, and bacteriological parameters was poor and not suitable for consumption. High prevalence of fecal contamination of water was found, and it was significantly associated with family size, the absence of a latrine, and the lack of a separate cap to take water from the storage. Therefore, continuous chlorination and monitoring its concentration, educating the community on how to use stored water, educating the advantage of having a latrine, and promoting point-of-use treatments such as filtration and boiling are needed.
Keywords: Drinking water quality, Risk factors, Fecal coliform, Flood-prone settlements
1. Introduction
Water is the essence of life and safe drinking water is a basic human right essential to all, and for sustainable development [[1], [2], [3]]. Water quality is a critical factor affecting human health and welfare [4,5]. Access to safe water is an important global public health concern. Improving access to safe drinking water can result in tangible health benefits, can boost countries’ economic growth, and contribute greatly to poverty reduction [6]. In many nations, the decline in the quality of drinking water is a significant problem that may be caused by a variety of interrelated biological, physical, and chemical variables [7].
The primary pollutants in water sources include human excreta, animal waste, effluent agricultural practices, and floods as well as a lack of knowledge among end-users about hygiene and environmental cleanliness; storage and disposal must be taken into account for the protection of water resources [8,9]. Even if the source is clean, the process of collecting, transporting, storing, and drawing water in the household can all lead to fecal contamination [10]. The consumption of contaminated water may be hazardous to health and transmit diseases unless it is properly treated before consumption. Consumption of contaminated water may be responsible for the spread of illnesses like cholera, dysentery, diarrhea, hepatitis A, typhoid, polio [6], and hydatid disease [11]. Children are more susceptible to microbiological pollutants and develop an illness due to their immature immune systems [6] and due to their consumption of more water per body mass than adults do [12].
According to estimates from the World Health Organization (WHO), unclean water, inadequate sanitation, and poor hygiene are responsible for up to 80% of diseases, 3.1% of fatalities (1.7 million), and 3.7% of disability-adjusted life years (54.2 million) worldwide [2,4]. Worldwide waterborne diseases are the cause of death and suffering of millions of people, especially children in developing countries [2,13]. In sub-Saharan Africa, over 275 million people rely on unsafe drinking water sources from lakes, rivers, and open wells. Consequently, many water-borne diseases are responsible for significant morbidity and mortality among children [14].
The majority of rural populations get their water from tainted or questionable sources, exposing them to a variety of water-borne ailments [8,9], and such conditions are constant within flood-prone areas. People living in flood-prone areas are faced with so many challenges such as poor sanitary systems; overflowing latrines, and poor drainage which drastically alter the drinking water quality and affect their lives negatively [15,16]. In Ethiopia, poor environmental health conditions resulting from subpar water quality and inadequate hygiene and sanitation standards are responsible for more than 60% of infectious diseases [10,17]. About 80% of the rural and 20% of the urban population of Ethiopia have no access to clean water, which is the least of any country on the continent. Children in the country suffer from communicable diseases caused by deprived environmental conditions, particularly poor water quality, and sanitary conditions, which account for three-quarters of all child health issues [9,10].
In rural locations where water sources are communally shared and exposed to several fecal-oral transmission channels in their local boundaries, fecal contamination of drinking water is a primary cause of water-borne diseases [18]. This could be detected by examining the presence of potential indicator organisms such as fecal coliforms [19,20]. Even though there are studies conducted on water quality in urban and rural parts of Ethiopia [4,7,9,[21], [22], [23], [24]], to date, no studies have been carried out to determine the household drinking water quality and associated factors in flood-prone settings of the country. Therefore, the main purposes of this study are to (1) evaluate the physicochemical quality of household drinking water, (2) examine the bacteriological quality of household drinking water, and (3) identify the factors associated with bacteriological quality of drinking water among households located in flood-prone areas of Northwest Ethiopia. As a result, this research may help the community in evaluating the drinking water quality and its predictors in flood-prone settings of Northwest Ethiopia.
2. Materials and methods
2.1. Study design and area
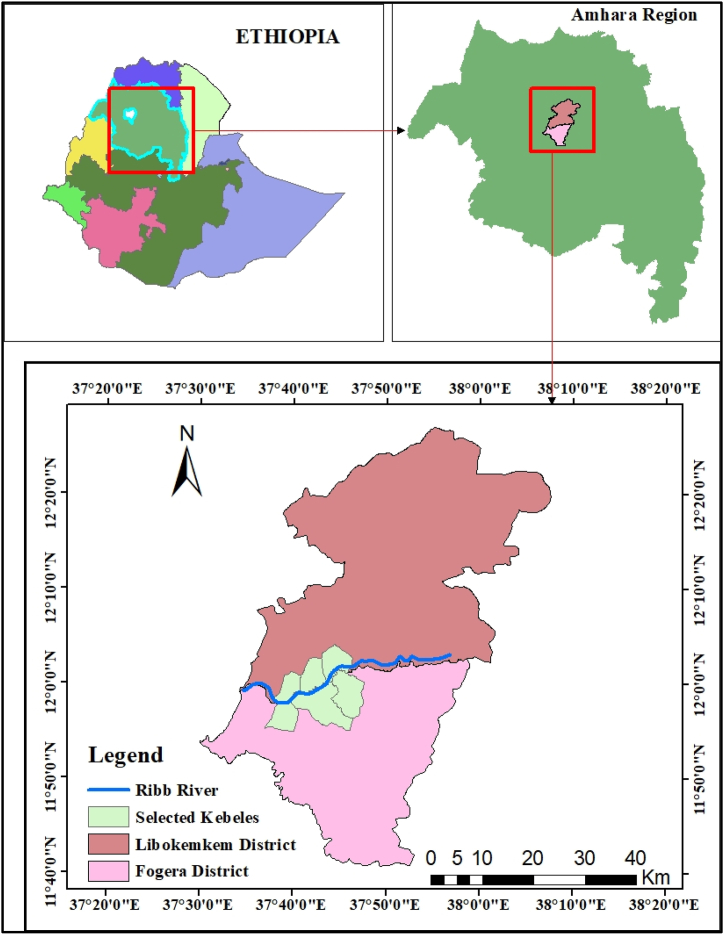
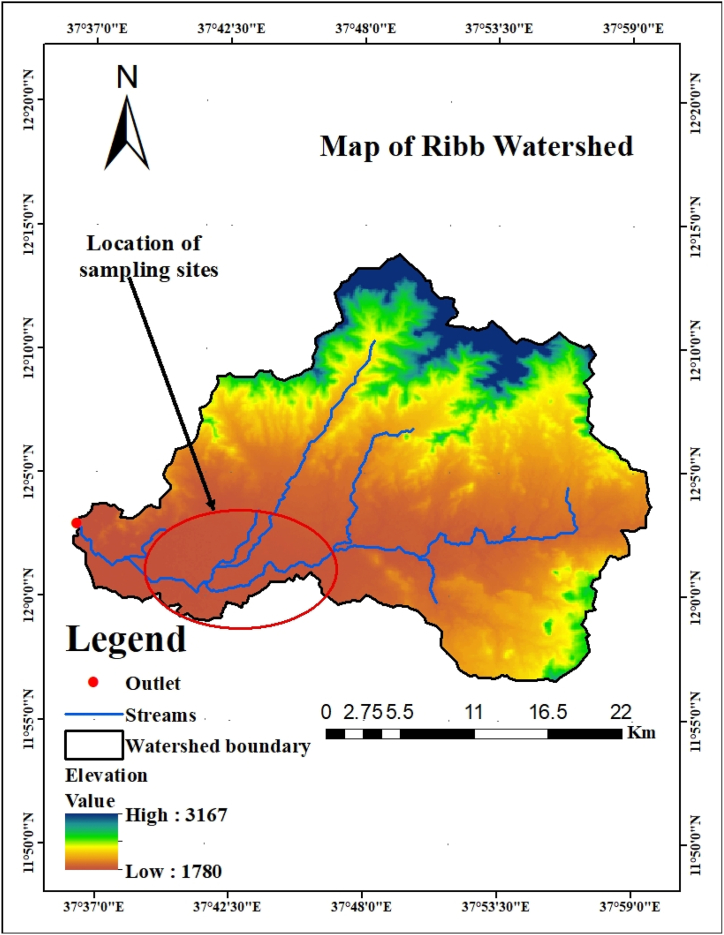
A community-based cross-sectional study was employed in flood-prone settings of the Fogera and Libokemkem districts of the south Gondar administrative Zone from January 17 to March 30, 2021, upon the complete return of the flood-displaced population to their households. These areas are located about 650 km northwest of the Ethiopian capital Addis Ababa, about 70 km from Bahir Dar town, the capital of the Amhara region (Fig. 1). According to the Ethiopia National Metrology Agency, the annual rainfall of the Fogera and Libo Kemkem districts ranges from 1103 to 1336 mm and 900 to 1200 mm, respectively. These districts are located in the catchment of the Ribb River at an altitude of 2000 m above sea level (Fig. 2) and repeatedly suffer from floods due to the overflow of the Ribb River. According to the 2009 census, the population of Fogera and Libo Kemkem was 226,595 and 198,374 respectively; and also have 44 and 33 kebeles respectively [25]. According to the Amhara mass media agency report on September 15, 2020; about 2439 households from Fogera and 1750 households from Libo Kemkem districts were affected and displaced by the recent flooding.
Fig. 1.
Map of the study area.
Fig. 2.
Location of study area on Ribb watershed.
2.2. Study population
All households in flood-prone settlements in Fogera and Libo Kemkem districts were the source population. All households located in purposively selected kebeles of the districts were the study population. A family head or a family member 18 and above years old present during data collection time was interviewed for the study.
2.3. Sample size and sampling technique
The sample size was determined using single population proportion formula with the following assumptions: 30% of households in rural areas had faecally contaminated drinking water sources [26], with a 5% margin of error (d) and 95% confidence interval.
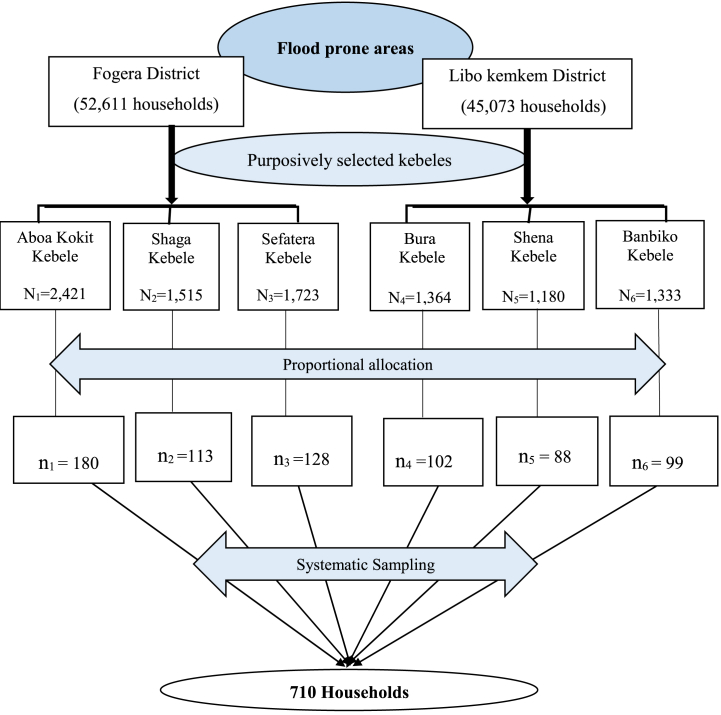
By taking 10% of the non-response rate and design effect of 2 (because the study has employed two-stage sampling), then the total sample size was 710. Three kebeles in each of the two districts Aboa Kokit, Shaga, and Sefatera kebeles from Fogera district; and Bura, Shena, and Banbiko kebeles from Libo Kemkem district are selected purposively based on their proximity to Ribb River and Suffers with the common flooding and overflow of the river. Then households are allocated proportionally and selected using systematic sampling from each of the six kebeles to have a total sample size of 710 households (Fig. 3).
Fig. 3.
Schematic representation of sampling procedures.
2.4. Study variables
Dependent variables were the physicochemical and bacteriological quality of household drinking water. The physicochemical quality was measured by selected parameters of water, namely the pH, turbidity, electrical conductivity, total dissolved salts (TDS), and free residual chlorine and it is compared with WHO guidelines for drinking water. The bacteriological quality of water was measured by using fecal coliforms as indicator organisms (positive/negative).
Independent variables for the bacteriological quality of household drinking water were the sociodemographic characteristics of the respondents such as age, marital status, religion, education level, occupation, family size, and family monthly; the environmental factors such as household floor type, household roof cover, household cleanliness, presence latrine, presence of child feces on the house, type of the source of drinking water, daily water access, separate cap to draw water from the storage, and animal access to water storage; and behavioral factors such as solid waste disposal mechanism, liquid waste disposal mechanism, regular cleaning of the house, presence of a cover of the storage container, critical time hand wash, and hand washing material.
2.5. Data collection procedures and tools
The sociodemographic, environmental, and behavioral data were collected using interviewer-administered questionnaires containing observational checklists (see Supplementary file) that were prepared by reviewing literatures [14,[27], [28], [29], [30]].
The sociodemographic characteristics of the respondents were collected using the questionnaire. Moreover, trained environmental health professionals have also collected the environmental and behavioral factors using observational checklists of the questionnaire. Data collectors and supervisors received three days of training on data-gathering processes, techniques, and methods. Before the actual data collection, the data collecting tools were pretested on a neighboring district that was not part of the real data collection. Moreover, during the data collection, there was constant and tight supervision.
2.5.1. Collection and transportation of water sample
Drinking water samples from storage containers of systematically selected households were collected in sterilized glass bottles to conduct the physicochemical and bacteriological examinations. Trained laboratory technicians took all of the water samples. The American Public Health Association (APHA) recommendations for drinking water quality assessment were followed when collecting drinking water samples from selected households [31]. The selected physicochemical parameters, namely pH, turbidity, electrical conductivity, total dissolved salts (TDS), and free residual chlorine were examined onsite using portable devices. In parallel, the collected water samples for bacteriological analysis were dechlorinated with sodium thiosulphate, held at a temperature of 4 °C, and transported to the laboratory of the Environmental and Occupational Health and Safety department, at the University of Gondar. Before analysis, the necessary laboratory equipment and culture media were sterilized. Furthermore, blank samples were prepared and examined using the same technique to confirm the authenticity of the results.
2.5.2. Physicochemical and bacteriological analysis of water samples
The APHA (2005) standard techniques were used to determine all physicochemical assessments of water [31]. The pH, turbidity, electrical conductivity, TDS, and free residual chlorine were measured in situ. The pH was analyzed using a portable digital pH meter (Wagtech model, UK). The pH meter was calibrated just before analysis using PH 4.0, PH 7.0, and PH 10.0. Conductivity, TDS, and temperature were analyzed using a portable digital conductivity meter (wagtech model, UK). The analysis of conductivity was done after calibration of the conductivity meter using 0.1 M of potassium chloride. Concerning turbidity, it was measured using a portable microprocessor turbidity meter (wagtech model, UK) after calibrating with the standard solutions of 800, 100, 20, and 0.02NTU. A free residual chlorine test was done using a portable spectrophotometer (wagtech model 1700, UK) through DPD (diethyl para-phenylenediamine) method [31,32].
The analysis of fecal coliform (FC) was done within 4 h of sample collection using the membrane filtration (MF) method according to standard methods for the examination of water [31]. Membrane lauryl sulfate broth was prepared and autoclaved at 121 °C for 15 min before being inoculated with membrane filters. Then, a 100 ml water sample was drawn and filtered with a sterile membrane filter of 0.45 μm and the filter membrane was then placed aseptically on reusable aluminum Petri dish with an absorbent pad soaked with membrane lauryl sulfate broth. Then the plate was incubated at 44.5 °C for 24 h to determine the fecal coliform bacteria. Then, all yellow colonies were counted as FC [33]. Then, when the CFU/100 ml of water was zero, the results were classified as “Negative,” and when it was larger than zero, they were classified as “Positive".
2.6. Data processing and analysis
The SPSS version 25.0 software was used to evaluate the data after it had been entered using Epi-Info version 7.1. The mean values of physicochemical parameters of water samples were computed and compared with WHO standards for drinking water. Multicollinearity among predictor variables was checked by variance inflation factors (VIF) and the tolerance test. Only non-collinear covariates (VIF <5 and tolerance below 0.2) were included in binary logistic regression to assess the possible association between each independent and dependent variable. Then, the variables that have a p-value less than 0.25 in the bivariate binary logistic regression analysis were also considered for the multivariable analysis to control all possible confounders and determine the significant predictors of the outcome variable. In the multivariable logistic regression analysis, a p-value of less than 0.05 was used to declare the association between predictor factors with the bacteriological quality of household drinking water. The Hosmer-Lemeshow goodness of fit test (p-value >0.05) was also used to test the fitness of the model. The p-value of the multivariable logistic regression model was 0.477 which confirmed model fitness.
3. Results
Socio-demographic characteristics of respondents: From a total of 675 respondents of households who participated, the mean (±SD) age was 27.8 ± 5.5 years, and about 288 (42.7%) respondents were aged less than 25 years. The majority, 592 (87.7%), of the participants were married, and more than half of the respondents 369 (54.7%) were unable to read and write. Most of the respondents, 654 (96.9%), were Orthodox Christians. The majority, 468 (69.3%), of the respondents had a family size of fewer than five individuals and 369 (54.7%) of the respondents had income less than 1000 ETB (Table 1).
Table 1.
Distribution of socio-demographic features of respondents in flood-prone settlements of the south Gondar zone (Fogera and Libokemkem districts), Ethiopia, 2021 (n = 675).
| Variables | Frequency | Percent | |
|---|---|---|---|
| Age (years) | ≤25 | 288 | 42.7 |
| 26–30 | 225 | 33.3 | |
| 31–35 | 108 | 16.0 | |
| ≥36 | 54 | 8.0 | |
| Marital status | Married | 592 | 87.7 |
| Single | 19 | 2.8 | |
| Divorced | 47 | 7.0 | |
| Widowed | 17 | 2.5 | |
| Religion | Orthodox | 654 | 96.9 |
| Muslim | 15 | 2.2 | |
| Protestant | 6 | .9 | |
| Education level | Not read and write | 369 | 54.7 |
| Read and write | 180 | 26.7 | |
| Primary education | 99 | 14.7 | |
| Secondary | 27 | 4.0 | |
| Occupation | Farmer | 612 | 90.7 |
| Merchant | 63 | 9.3 | |
| Family size | ≤5 | 468 | 69.3 |
| >5 | 207 | 30.7 | |
| Family monthly income | <1000 ETB | 369 | 54.7 |
| 1000-2000 ETB | 162 | 24.0 | |
| >2000 ETB | 144 | 21.3 |
N.B: ETB = Ethiopian Birr (1 ETB = 0.023$, on July 07, 2021).
Environmental features of households of respondents: The majority, 666 (98.7%), of the respondents had households with mud floor types. Most of the respondents 639 (94.7%) have no latrine and practice open defecation, and nearly half of the respondents 324 (48%) used protected wells and springs as a source of potable water. About 144 (21.3%) of the participants reported as animals have access to water storage containers in the household (Table 2).
Table 2.
Environmental features of households of respondents in flood-prone settlements of the south Gondar zone (Fogera and Libokemkem districts), Ethiopia, 2021 (n = 675).
| Variables | Frequency | Percent | |
|---|---|---|---|
| Household floor type | Mud | 666 | 98.7 |
| Cement | 9 | 1.3 | |
| Household roof cover | Thatched | 243 | 36.0 |
| Corrugated iron sheet | 432 | 64.0 | |
| Household cleanliness | Good | 171 | 25.3 |
| Moderate | 423 | 62.7 | |
| Poor | 81 | 12.0 | |
| Presence latrine | Yes | 36 | 5.3 |
| No | 639 | 94.7 | |
| Child feces on the house | Yes | 342 | 50.7 |
| No | 333 | 49.3 | |
| Source of drinking water | Protected well/spring | 324 | 48.0 |
| Unprotected well/spring | 351 | 52.0 | |
| Daily water access | Yes | 495 | 73.3 |
| No | 180 | 26.7 | |
| Separate can/cap to take water | Yes | 288 | 42.7 |
| No | 387 | 57.3 | |
| Animal access to water storage | Yes | 144 | 21.3 |
| No | 531 | 78.7 | |
Behavioral characteristics of respondents: The vast majority of respondents, 639 (94.7%), dispose of their solid trash in a field that is not fenced in. Similarly to this, 630 respondents (93.3%) discarded their liquid waste in an open area. The majority of respondents (432, or 64%) do regular housecleaning. About 360 of the respondents (53.3%), cleaned their hands using water only (Table 3).
Table 3.
Behavioral features of respondents in flood-prone settlements of the south Gondar zone (Fogera and Libokemkem districts), Ethiopia, 2021 (n = 675).
| Variables | Frequency | Percent | |
|---|---|---|---|
| Solid waste disposal mechanism | Pit | 9 | 1.3 |
| Burning | 27 | 4.0 | |
| Open dump | 639 | 94.7 | |
| Liquid waste disposal mechanism | Dispose of absorption pit | 45 | 6.7 |
| open field | 630 | 93.3 | |
| Regular cleaning of the house | Yes | 243 | 36.0 |
| No | 432 | 64.0 | |
| Cover of Storage | Covered | 396 | 58.7 |
| not covered | 279 | 41.3 | |
| Critical time hand wash | Before eating | 315 | 46.7 |
| After latrine use | 63 | 9.3 | |
| After handling the baby's diaper/feces | 18 | 2.7 | |
| After eating | 108 | 16.0 | |
| Before feeding child | 27 | 4.0 | |
| Before food preparation | 108 | 16.0 | |
| Wash under all conditions | 450 | 66.67 | |
| Hand washing material | only water | 360 | 53.3 |
| soap and water | 297 | 44.0 | |
| Ash and water | 18 | 2.7 | |
3.1. Physicochemical and bacteriological quality of drinking water
The mean pH of household drinking water samples in the study area was 5.9 ± 1.03. The mean level of fecal coliforms found in the household drinking water samples was 220.45 CFU per 100 ml with a range of 0 CFU/100 ml to 750 CFU/100 ml. The prevalence of positive fecal coliforms was found to be 62.2% in water samples examined from households in flood-prone villages in the south Gondar zone (Table 4).
Table 4.
Physicochemical and bacteriological quality of household drinking water samples in flood-prone settlements of the south Gondar zone (Fogera and Libokemkem districts), Ethiopia, 2021 (N = 675).
| Physicochemical parameters | Units | Mean ± SD | WHO standards [2] |
|---|---|---|---|
| pH | – | 5.9 ± 1.03 | 6.5–8.5 |
| Turbidity | NTU | 6.7 ± 2.21 | <5 |
| Free residual chlorine | mg/l | 0.02 ± 0.01 | 0.2–0.5 |
| Conductivity | μs/cm | 122.0 ± 59.0 | 1500 |
| TDS | ppm | 75.0 ± 21.0 | 1000 |
| Bacteriological parameters | N (percentage) | ||
| Fecal coliform | Positive | 420 (62.2%) | Zero (Must not be detected) |
| Negative | 255 (37.8%) | ||
Note: NTU=Nephlometric Turbidity Unit.
3.2. Factors associated with fecal contamination of drinking water
In the multivariable binary logistic regression analysis, the presence of fecal coliform in household drinking water was significantly associated with family size, the absence of a latrine, and the lack of a separate can to draw water from its storage.
There was a 2.2-fold increase in the likelihood of fecal-contaminated household drinking water among respondents with families larger than five [AOR = 2.205, 95% CI (1.375–3.536)]. The respondents who did not use separate cans to take water from storage have a 55.6% higher likelihood of fecal coliform contamination in their household drinking water than those who did use a separate can [AOR = 0.454, 95% CI (0.249–0.827)]. The likelihood of fecal coliform contamination in household drinking water was 3.449 times greater in respondents who had latrines [AOR = 3.449, 95% CI (1.349–8.823)]. (Table 5).
Table 5.
Multivariable analysis of factors associated with the presence of fecal coliform in household drinking water in flood-prone settings of south Gondar zone (Fogera and Libokemkem districts), Ethiopia, 2021 (n = 675).
| Variables | Fecal contamination |
Crude OR (95% C.I.) | AOR (95% C.I.) | |
|---|---|---|---|---|
| Positive | Negative | |||
| Marital status | ||||
| Married | 371 (55.0%) | 221 (32.7%) | 1 | 1 |
| Single | 12 (1.8%) | 7 (1.0%) | 0.979 (0.380–2.524) | 0.550 (0.173–1.749) |
| Divorced | 29 (4.3%) | 18 (2.7%) | 1.042 (0.565–1.920) | 1.538 (0.731–3.237) |
| Widowed | 7 (1.0%) | 10 (1.5%) | 2.398 (0.900–6.391 | 2.593 (0.857–7.842) |
| Educational status | ||||
| Unable to read and write | 239 (35.4%) | 130 (19.3%) | 1.088 (0.475–2.490) | 0.979 (0.374–2.560) |
| read and write | 114 (16.9%) | 66 (9.8%) | 1.158 (0.492–2.724) | 1.883 (0.735–4.824) |
| Primary | 48 (7.1%) | 51 (7.5%) | 2.125 (0.871–5.185) | 2.092 (0.811–5.400) |
| Secondary and above | 18 (2.7%) | 9 (1.3%) | 1 | 1 |
| Family size | ||||
| ≤5 | 305 (45.2%) | 163 (24.1%) | 1 | 1 |
| >5 | 114 (16.9%) | 93 (13.8%) | 1.526 (1.094–2.131) | 2.205 (1.375–3.536)*** |
| Presence of a latrine to use | ||||
| Yes | 27 (4.0%) | 9 (1.3%) | 1 | |
| No | 392 (58.1%) | 247 (36.6%) | 1.980 (0.874–4.087) | 3.449 (1.349–8.823)** |
| Source of drinking water | ||||
| Protected well/spring | 192 (28.4%) | 132 (19.6%) | 0.795 (0.582–1.085) | 0.721 (0.413–1.258) |
| Unprotected well/spring | 227 (33.6%) | 124 (18.4%) | 1 | 1 |
| Is there a separate can to take water from the storage | ||||
| Yes | 158 (23.4%) | 130 (19.2%) | 0.587 (0.428–0.804) | 0.454 (0.249–0.827)** |
| No | 261 (38.7%) | 126 (18.7%) | 1 | 1 |
| Animal access to the water storage | ||||
| Yes | 105 (15.6%) | 39 (5.8%) | 1 | 1 |
| No | 314 (46.5%) | 217 (32.1%) | 1.861 (1.239–2.793) | 1.753 (0.991–3.103) |
| The condition of water storage | ||||
| Covered | 195 (28.9%) | 84 (12.4%) | 1 | 1 |
| Not covered | 224 (33.2%) | 172 (25.5%) | 1.783 (1.289–2.464) | 0.734 (0.426–1.265) |
Note: Hosmer and Lemeshow test = 0.477 showed that the model fitted well.
**Significant at P value < 0.01, ***Significant at P value < 0.00.
4. Discussion
Drinking water is a potential source of human disease when it contains chemicals and microbes [34]. Therefore, this study aimed to assess the physicochemical and bacteriological quality of drinking water and predictor factors in flood-prone settings of Northwest, Ethiopia.
4.1. Physico-chemical quality of drinking water
Analysis of the pH of drinking water was conducted in the study areas as a physicochemical quality parameter. Since it impacts the physical and chemical properties of water, pH is one of the most important water quality factors [35]. In this study, the mean pH of the drinking water samples was found lower (5.9 ± 1.03) than WHO limits of 6.5–8.5. The pH was also lower than the findings reported from previous studies conducted in western Ethiopia [36], southern Ethiopia [37], and northwest Ethiopia [38]. This lower pH might be linked to the geological makeup of the study area in which acidic ions may be leaked to the water bodies through infiltration during the flood season. This indicated that exposure to such low pH values causes corrosive, irritation to the eyes, skin, and mucous membranes for persons [39]. Additionally, low pH values indirectly harm people by allowing heavy metals to enter the water supply through the corrosion of metallic networks, which has a cumulatively negative effect [40]. It might also impart a sour taste to the water and make it unpleasant for consumption [41].
The mean turbidity of drinking water samples was 6.7 ± 2.21NTU which was higher than the WHO guideline for drinking water, i.e. less than 5NTU [2]. This could be linked to the entrance of turbidity causing dissolved particles and suspended solids to the water body due to repeated flooding in the study area. The higher turbidity may mask the microbes during disinfection and minimize the effectiveness of disinfection which may lead to poor microbial quality of the drinking water [42]. Moreover, it might result in aesthetic consequences for drinking-water provisions. Thus, turbidity could represent a key issue regarding the microbiological quality and disinfection of water.
The results showed that the mean TDS readings were 75.0 ± 21.0 ppm, below both the WHO norm of 1000 ppm and the proposed TDS level of 600 ppm for the tastiness of drinking water [43]. This implies the TDS levels of household drinking water in the study area were in the acceptable range that may not alter the taste of the water and don't cause aesthetic problems among the consumers.
Comparing the mean value of free residual chlorine in the drinking water samples to the WHO guideline (0.2–0.5 ppm) [2], it was found to be lower. Due to its rapid and extensive levels of bacterial and viral destruction, free chlorine residue is the most popular and effective residual disinfectant [44]. The structure of pipelines, and wall materials, such as corrosion level and pipe age, may have an impact on such low free residual chlorine concentration [45]. As a result, free chlorine residuals might not last very long in the water. Free chlorine, on the other hand, has significant potential as a stand-in signal of pathogenic infection.
According to the current study, the mean conductivity value of water samples was 122.0 ± 59.0 μs/cm. This result was lower than the WHO standard (1500 μs/cm) [2]. This implies that the mineral contents of the water samples were low and safe for consumption. The conductivity indicates the water mineralization which varies by the concentration of dissolved salts and is habitually subjective by temperature [46].
4.2. Bacteriological quality of drinking water
The primary factor that should be considered in any water quality inspection is the bacteriological quality of the water. Particularly, fecal coliform serves as a marker for the presence of feces from warm-blooded animals and can be utilized as a bacteriological substitute for testing water quality [47]. The magnitude of fecal coliforms found in water samples taken from the present study setting was about 220.45 CFU per 100 ml, which is higher than the findings reported from rural communities of Ethiopia [[48], [49], [50]]. This could be due to the present study area repeatedly suffering from flooding that may destroy sanitary facilities and water supply systems; that may also lead to fecal contamination of water sources and poor water quality at the point of use. This finding has also shown that 62.2% of households’ drinking water samples were contaminated by fecal coliform. This is contrary to WHO guidelines for drinking water quality [2]. This finding was also lower than similar previous studies conducted in Eastern Ethiopia (83.3%) [48], India (80%) [51], and Ghana (83%) [52]. However, this outcome was comparable to past research carried out in several regions of Ethiopia (58%) [53], and (55%) [54].; while it was higher than studies done in rural areas of North Gondar Zone, Ethiopia (56.5%) [55], in rural parts of Ethiopia (58%) [56], households in Hyderabad, India (36%) [57], Uganda [28]. This difference could be due to variation in the study settings, most of the above-mentioned studies have been conducted in areas that were not affected by the flood, whereas the present study was conducted in flood-affected areas. Moreover, these studies have included both point-of-use samples and water samples at sources, while the present study only included water samples at the point of use. This could be linked to the high level of fecal contamination found in the present study area.
Family size, absence of a latrine, and lack of a separate can to take water were recognized as predictors of the bacteriological quality of drinking water among study subjects.
In contrast to families with a smaller family size, families with a large family size had a greater rate of fecal pollution in their drinking water (AOR = 2.205, 95% CI (1.375–3.536)). Studies conducted in Debre Tabor town of Ethiopia [58], rural Vietnam [59], and Pakistan [60] support this finding. This could be because having a large family makes it difficult to maintain family hygiene standards and causes feces to contaminate drinking water.
The odds of fecal coliform contamination of drinking water samples were higher among households lacking a latrine compared to those having a latrine [AOR = 3.449, 95%CI (1.349–8.823)]. This suggests that the lack of a latrine may increase the risk of fecal contamination of water since it causes poor human feces management that is released to water sources and open fields. This implies that the low latrine access (5.3%) in the present study area could be the reason for the high fecal contamination of household drinking water. The respondents stated that the repeated flooding in the area causes the collapse and destruction of constructed latrines and resulted in the prevalent open defecation practice. Therefore, encouraging the community to construct flood-proof latrines with modified designs is recommended to be more resilient to flooding. This finding is consistent with studies conducted in Ethiopia [58], Kenya [61], Ghana [62], rural Mali [63], and rural villages of Lesotho [18] that reported inadequate access to sanitation facilities and open defecation are linked to the fecal contamination of drinking water. Therefore, the provision of latrines is one aspect of sanitation promotion, which works to protect public health by safely containing feces and preventing their release into private and public areas [64].
The likelihood of fecal coliform contamination of the drinking water samples taken from the households that used separate cans/containers to draw water from the storage was 55.6% greater than those samples that were taken from the households that used the same cans/containers to draw water from the storage and to do other activities (hand washing and cleaning after latrine use) [AOR = 0.454, 95% CI (0.249–0.827)]. This could be because families who do not have separate cans to take water from the storage may use contaminated cans, especially when dipping water from the storage, which could give a supply of fecal-contaminated water. This finding is in line with studies done in Tehuledere woreda, northeast Ethiopia [21].
4.3. Strengths and limitations of the study
This study is the first to be carried out in flood-affected areas of the country after a flood season, and it may provide information to governmental and non-governmental organizations that deal with emergencies and disaster relief. A large number of samples (about 675 water samples) were examined to generate reliable information about drinking water quality in the study area.
Since the study is cross-sectional, causation is not possible. Due to limited resources, the study did not include a control group (households situated distant from the river). This study was also likewise conducted during the dry season and lacked seasonal adjustment. Limited numbers of physicochemical parameters were considered due to resource constraints.
5. Conclusion
This finding indicated that there are problems with drinking water quality based on physicochemical and microbiological parameters in the study area. The mean value of pH (5.9 ± 1.03) and free residual chlorine (0.02 ± 0.01 mg/l) were below the WHO recommended values, while the level of turbidity (6.7 ± 2.21 NTU) and a load of fecal coliform (220.45 CFU per 100 ml) were higher than the WHO standard for drinking water. Therefore, the water quality in terms of pH, turbidity, residual chlorine, and bacteriological parameters was poor and not suitable for consumption. Nearly three-fifths of the drinking water samples were contaminated by fecal coliform. The family size, the absence of a latrine, and the lack of a separate cap to take water from the storage were significantly associated with the bacteriological quality of drinking water. Therefore, the local and regional health offices and water supply organizations should work on the continuous application of chlorine and monitor its concentration to control microbiological contamination of drinking water. Furthermore, to supply safe drinking water, those stakeholders should educate the community on how to use stored water, educate the advantage of having a latrine and promote point-of-use treatments such as filtration and boiling.
Ethics declarations
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Ethical approval
The Institutional Review Board of the University of Gondar granted ethical permission (Ref. no: V/P/RCS/05/544/2020). From the district's health offices, letters of permission were given. Privacy and secrecy were upheld throughout the interview. Therefore, the tools used to collect data only included code. Additionally, those who wished to refrain from taking part in the study did so at their own choice.
Consent to participate
The authors provide their consent for the research to be published.
Consent for publication
The study participants were included in the study based on their willingness to take part in the study.
Author contribution statement
Tsegaye Adane Birhan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bikes Destaw Bitew, Henok Dagne, Dagnachew Eyachew Amare, Jember Azanaw, Zewudu Andualem, Awrajaw Dessie, Gebisa Guyasa, Alem Getaneh, Ayenew Addisu, Mengesha Genet, Garedew Tadege Engdaw, Amensisa Hailu Tesfaye,
Tigist Kibret Asmare, Tarekegn Fentie Yimer: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding
This research was funded by the University of Gondar.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgments
The authors are grateful for the funding support from the University of Gondar. We are also delighted to acknowledge the health offices of the districts, the study participants, data collectors, and supervisors for their dedication and active involvement.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15072.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ondieki J.K., Akunga D., Warutere P., et al. Bacteriological and physico-chemical quality of household drinking water in Kisii Town, Kisii County, Kenya. Heliyon. 2021;(5):7. doi: 10.1016/j.heliyon.2021.e06937. e06937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2006. Guidelines For Drinking-Water Quality [Electronic Resource] : Incorporating First Addendum. Vol. 1, Recommendations. – 3rd ed. World Health Organization (WHO) [Google Scholar]
- 3.Daramola J., Ekhwan T.M., Adepehin E.J., et al. Seasonal quality variation and environmental risks associated with the consumption of surface water: implication from the Landzun Stream, Bida Nigeria. Heliyon. 2019:5. doi: 10.1016/j.heliyon.2019.e02121. (7): p. e02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werkneh A.A., Medhanit B.Z., Abay A.K., et al. Physico-chemical analysis of drinking water quality at Jigjiga City, Ethiopia. Am. J. Environ. Protect. 2015;4(1):29–32. [Google Scholar]
- 5.Li P., Wu J. Drinking water quality and public health. Exp. Health. 2019;11(2):73–79. [Google Scholar]
- 6.WHO . 2020. Drinking water: key facts. 2019 July 30, 2020.https://www.who.int/news-room/fact-sheets/detail/drinking-water Available from: [Google Scholar]
- 7.Garoma B., Kenasa G., Jida M. Drinking water quality test of shambu town (Ethiopia) from source to household taps using some physico-chemical and biological parameters. Res. Rev.: J. Ecol. Environ. Sci. 2018:6. (4) [Google Scholar]
- 8.Amenu D., Menkir S., Gobena T. Microbiological quality of drinking water sources in rural communities of dire dawa administrative council. Science. Techn. Arts Res. J. 2012;1(4):33–37. [Google Scholar]
- 9.Meride Y., Ayenew B. Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia. Environ. Sys. Res. 2016;5(1):1. [Google Scholar]
- 10.Amenu D., Menki S., Gobena T. Assessment of water handling practices among rural communities of dire dawa administrative council, dire dawa, Ethiopia. Science. Techn. Arts Res. J. 2013;2(2):75–82. [Google Scholar]
- 11.Wang D., Wu J., Wang Y., et al. Finding high-quality groundwater Resources to Reduce the hydatidosis Incidence in the shiqu County of sichuan province, China: analysis, assessment, and management. Exp. Health. 2020;12(2):307–322. [Google Scholar]
- 12.EPA . 2020. Environments and Contaminants: Drinking Water Contaminants. 2015 [cited july 30.https://www.epa.gov/sites/production/files/2015-10/documents/ace3_drinking_water.pdf Available from: [Google Scholar]
- 13.Mulamattathil S.G., Bezuidenhout C., Mbewe M. Analysis of physico-chemical and bacteriological quality of drinking water in Mafikeng, South Africa. J. Water Health. 2015;13(4):1143–1152. doi: 10.2166/wh.2015.273. [DOI] [PubMed] [Google Scholar]
- 14.Too J.K., Kipkemboi Sang W., Ng'ang'a Z., et al. Fecal contamination of drinking water in Kericho District, Western Kenya: role of source and household water handling and hygiene practices. J. Water Health. 2016;14(4):662–671. doi: 10.2166/wh.2016.137. [DOI] [PubMed] [Google Scholar]
- 15.Abu M., Codjoe S.N.A. Experience and future perceived risk of floods and diarrheal disease in urban poor communities in Accra, Ghana. Int. J. Environ. Res. Publ. Health. 2018;15(12):2830. doi: 10.3390/ijerph15122830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun R., An D., Lu W., et al. Impacts of a flash flood on drinking water quality: case study of areas most affected by the 2012 Beijing flood. Heliyon. 2016:2. doi: 10.1016/j.heliyon.2016.e00071. (2): p. e00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . World Health Organization; Geneva: 2004. Water, Sanitation and Hygiene Links to Health, Facts and Figures. [Google Scholar]
- 18.Gwimbi P., George M., Ramphalile M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: exposures through neighbourhood sanitation and hygiene practices. Environ. Health Prev. Med. 2019;24(1):33. doi: 10.1186/s12199-019-0790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladokun O.A., Oni S.O. Physico-chemical and microbiological analysis of potable water in Jericho and Molete areas of Ibadan metropolis. Adv. Biol. Chem. 2015;5(4):197. [Google Scholar]
- 20.Berhanu A., Hailu D. Bacteriological and physicochemical quality of drinking water sources and household water handling practice among rural communities of Bona District, sidama zone-Southern, Ethiopia. Sci. J. Publ. Health. 2015;3(5):782. [Google Scholar]
- 21.Mereta S., Legesse W., Endale H., et al. Factors affecting drinking water quality from source to home in tehuledere woreda, northeast Ethiopia. Eth. J. Heal. Sci. 2018:13. [Google Scholar]
- 22.Feleke H., Medhin G., Kloos H., et al. Household-stored drinking water quality among households of under-five children with and without acute diarrhea in towns of Wegera District, in North Gondar, Northwest Ethiopia. Environ. Monit. Assess. 2018;190(11):669. doi: 10.1007/s10661-018-7033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addisie M.B. Cornell University; 2012. Assessment of Drinking Water Quality and Determinants of Household Potable Water Consumption in Simada District, Ethiopia. [Google Scholar]
- 24.Tiku S., Legesse W., Endale H., et al. Factors affecting drinking water quality from source to home in Tehuledere Woreda, Northeast Ethiopia. Eth. J. Heal. Sci. 2003:13. (2) [Google Scholar]
- 25.Herrador Z., Sordo L., Gadisa E., et al. Cross-sectional study of malnutrition and associated factors among school aged children in rural and urban settings of Fogera and Libo Kemkem districts, Ethiopia. PLoS One. 2014:9. doi: 10.1371/journal.pone.0105880. (9): p. e105880-e105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsega N., Sahile S., Kibret M., et al. Bacteriological and physico-chemical quality of drinking water sources in a rural community of Ethiopia. Afr. Health Sci. 2013;13(4):1156–1161. doi: 10.4314/ahs.v13i4.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gizachew M., Admasie A., Wegi C., et al. Bacteriological contamination of drinking water supply from protected water sources to point of use and water handling practices among beneficiary households of boloso sore woreda, wolaita zone, Ethiopia. Int. J. Microb. 2020;2020:5340202. doi: 10.1155/2020/5340202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agensi A., Tibyangye J., Tamale A., et al. Contamination potentials of household water handling and storage practices in kirundo subcounty, kisoro district, Uganda. Journal of J. Environ. Pub. HealthEnvironmental and Public Health. 2019;2019:7932193. doi: 10.1155/2019/7932193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma H.R., Worku W., Hassen M., et al. Water handling practices and level of contamination between source and point-of-use in Kolladiba Town, Ethiopia. Environ. W.: Int. J. Sci. Technol. 2013;8:25–35. [Google Scholar]
- 30.WHO/UNICEF Core questions on water, sanitation and hygiene for household surveys, WHO/UNICEF joint monitoring programme for water supply, Sanit. Hyg. 2018 https://washdata.org/sites/default/files/documents/reports/2019-03/JMP-2018-core-questions-for-household-surveys.pdf [Google Scholar]
- 31.APHA . Washington; DC, USA: 2005. Standard Methods for the Examination of Water and Wastewater. American Public Health Association (APHA) [Google Scholar]
- 32.Hailu B. Addis Ababa University Addis Ababa; Ethiopia: 2017. Physicochemical and Microbial Quality of Drinking Water from Source to Household Taps; the Case of Legedadi Reservoir. [Google Scholar]
- 33.Eliku T., Sulaiman H. Assessment of physico-chemical and bacteriological quality of drinking water at sources and household in Adama Town, Oromia Regional State, Ethiopia. Afr. J. Environ. Sci. Techn. 2015;9(5):413–419. [Google Scholar]
- 34.Malek A., Kahoul M., Bouguerra H. Groundwater’s physicochemical and bacteriological assessment: case study of well water in the region of Sedrata, North-East of Algeria. J. Water Land Dev. 2019;41(IV–VI)):91–100. doi: 10.2478/jwld-2019-0032. [DOI] [Google Scholar]
- 35.Water C.D. 2014. Guidelines for Canadian Drinking Water Quality. [Google Scholar]
- 36.Duressa G., Assefa F., Jida M. Assessment of bacteriological and physicochemical quality of drinking water from source to household tap connection in Nekemte, Oromia, Ethiopia. J. Environ. Pub. Health. 2019:2019. doi: 10.1155/2019/2129792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amanial H. Assessment of physicochemical quality of spring water in Arbaminch, Ethiopia. J. Environ. Anal. Chem. 2015;2(157):2380–2391. [Google Scholar]
- 38.Tabor M., Kibret M., Abera B. Bacteriological and physicochemical quality of drinking water and hygiene-sanitation practices of the consumers in bahir dar city, Ethiopia. Ethiop. J. Heal. Sci. 2011;21(1):19–26. doi: 10.4314/ejhs.v21i1.69040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambica A. Groundwater quality characteristics study by using water quality index in Tambaram area, Chennai, Tamil Nadu. Middle East J. Sci. Res. 2014;20(11):1396–1401. [Google Scholar]
- 40.Sorlini S., Palazzini D., Sieliechi J.M., et al. Assessment of physical-chemical drinking water quality in the Logone Valley (Chad-Cameroon) Sustainability. 2013;5(7):3060–3076. [Google Scholar]
- 41.Ibrahim M.N. Assessing groundwater quality for drinking purpose in Jordan: application of water quality index. J. Ecolog. Eng. 2019:20. (3) [Google Scholar]
- 42.WHO . 2017. Water Quality and Health-Review of Turbidity: Information for Regulators and Water Suppliers. [Google Scholar]
- 43.WHO . vol. 1. World Health Organization; 2004. Guidelines for Drinking-Water Quality. [Google Scholar]
- 44.Gray N.F. Elsevier; 2014. Free and Combined Chlorine, in Microbiology of Waterborne Diseases. 571–590. [Google Scholar]
- 45.Neff M.R. Michigan Technological University; 2018. Optimizing Chlorine Disinfection by Chlorine Injection Location and Pipe Diameter Selection in A Water Distribution System. [Google Scholar]
- 46.Benrabah S., Attoui B., Hannouche M. Characterization of groundwater quality destined for drinking water supply of Khenchela City (eastern Algeria) J. Water Land Dev. 2016:13–20. (30) [Google Scholar]
- 47.Motlagh A.M., Yang Z. Detection and occurrence of indicator organisms and pathogens. Water Environ. Res. 2019;91(10):1402–1408. doi: 10.1002/wer.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alemeshet Asefa Y., Alemu B.M., Baraki N., et al. Bacteriological quality of drinking water from source and point of use and associated factors among households in Eastern Ethiopia. PLoS One. 2021:16. doi: 10.1371/journal.pone.0258806. (10): p. e0258806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amenu D., Menkir S., Gobena T. Assessing the bacteriological quality of drinking water from sources to household water samples of the rural communities of dire dawa administrative council, eastern Ethiopia. Science. Techn. Arts Res. J. 2013;2(3):126–133. [Google Scholar]
- 50.Yasin M., Ketema T., Bacha K. Physico-chemical and bacteriological quality of drinking water of different sources, Jimma zone, Southwest Ethiopia. BMC Res. Notes. 2015;8(1):541. doi: 10.1186/s13104-015-1376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roopavathi C., Mamatha S. R. NS, Assessment of physicochemical and bacteriological drinking water quality of different sources of HD Kote town, Mysore district. Int. J. Eng. Res. Afr. 2016;6(7):45–51. [Google Scholar]
- 52.Boateng D., Tia-Adjei M., Adams E.A. Determinants of household water quality in the Tamale Metropolis, Ghana. J. Environ. Earth Sci. 2013;3(7):70–77. [Google Scholar]
- 53.Usman M., Gerber N., Pangaribowo E. Determinants of household drinking water quality in rural Ethiopia. ZEF - Discuss. Pap. Dev. 2016;220:33. doi: 10.22004/ag.econ.241763. [DOI] [Google Scholar]
- 54.Asfaw H.S., Reta M.A., Yimer F.G. High enteric bacterial contamination of drinking water in Jigjiga city, Eastern Ethiopia. Ethiop. J. Health Dev. 2016;30(3):118–128. [Google Scholar]
- 55.Getachew A., Tadie A., Chercos D.H., et al. Level of faecal coliform contamination of drinking water sources and its associated risk factors in rural settings of North Gondar zone, Ethiopia: a cross-sectional community based study. Eth. J. Heal. Sci. 2018;28(2):227–234. doi: 10.4314/ejhs.v28i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usman M.A., Gerber N., Pangaribowo E.H. Drivers of microbiological quality of household drinking water – a case study in rural Ethiopia. J. Water Health. 2017;16(2):275–288. doi: 10.2166/wh.2017.069. [DOI] [PubMed] [Google Scholar]
- 57.Eshcol J., Mahapatra P., Keshapagu S. Is fecal contamination of drinking water after collection associated with household water handling and hygiene practices? A study of urban slum households in Hyderabad, India. J. Water Health. 2008;7(1):145–154. doi: 10.2166/wh.2009.094. [DOI] [PubMed] [Google Scholar]
- 58.Gebremichael S.G., Yismaw E., Tsegaw B.D., et al. Determinants of water source use, quality of water, sanitation and hygiene perceptions among urban households in North-West Ethiopia: a cross-sectional study. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0239502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anh V.T., Mølbak K., Cam P.D., et al. In: Factors Associated with Faecal Contamination of Household Drinking Water in a Rural Area, Vietnam, in Sustainability in Food and Water: an Asian Perspective. Sumi A., et al., editors. Springer Netherlands; Dordrecht: 2010. pp. 123–136. [Google Scholar]
- 60.Rauf S., Bakhsh K., Hassan S., et al. Determinants of a household's choice of drinking water source in Punjab, Pakistan. Pol. J. Environ. Stud. 2015;24(6):2751–2754. [Google Scholar]
- 61.Okullo J.O., Moturi W.N., Ogendi G.M. Open defaecation and its effects on the bacteriological quality of drinking water sources in isiolo county, Kenya. Environ. Health Insights. 2017;11 doi: 10.1177/1178630217735539. 1178630217735539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakobie N., Ibrahim A., Duwiejuah A. Sanitation practices and microbial quality of drinking water in open defaecation free and open defaecation communities in the Savelugu Municipality. Ghana J. Sci. 2020;61(2):1–12. [Google Scholar]
- 63.Harris M., Alzua M.L., Osbert N., et al. Community-level sanitation coverage more strongly associated with child growth and household drinking water quality than access to a private toilet in rural Mali. Environ. Sci. Technol. 2017;51(12):7219–7227. doi: 10.1021/acs.est.7b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali S.I. Alternatives for safe water provision in urban and peri-urban slums. J. Water Health. 2010;8(4):720–734. doi: 10.2166/wh.2010.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.