Abstract
In vitro activities of 17 antibiotics against 53 clinical strains of Mycobacterium marinum, an atypical mycobacterium responsible for cutaneous infections, were determined using the reference agar dilution method. Rifampin and rifabutin were the most active drugs (MICs at which 90% of the isolates tested were inhibited [MIC90s], 0.5 and 0.6 μg/ml, respectively). MICs of minocycline (MIC90, 4 μg/ml), doxycycline (MIC90, 16 μg/ml), clarithromycin (MIC90, 4 μg/ml), sparfloxacin (MIC90, 2 μg/ml), moxifloxacin (MIC90, 1 μg/ml), imipenem (MIC90, 8 μg/ml), sulfamethoxazole (MIC90, 8 μg/ml) and amikacin (MIC90, 4 μg/ml) were close to the susceptibility breakpoints. MICs of isoniazid, ethambutol, trimethoprim, azithromycin, ciprofloxacin, ofloxacin, and levofloxacin were above the concentrations usually obtained in vivo. For each drug, the MIC50, geometric mean MIC, and modal MIC were very close, showing that all the strains had a similar susceptibility pattern. Percent agreement (within ±1 log2 dilution) between MICs yielded by the Etest method and by the agar dilution method used as reference were 83, 59, 43, and 24% for minocycline, rifampin, clarithromycin, and sparfloxacin, respectively. Reproducibility with the Etest was low, in contrast to that with the agar dilution method. In conclusion, M. marinum is a naturally multidrug-resistant species for which the agar dilution method is more accurate than the Etest for antibiotic susceptibility testing.
Mycobacterium marinum is an atypical photochromogenic mycobacterium belonging to group I of Runyon's classification (18). This mycobacterium was successively named M. piscium, M. marinum (1), M. platypoecilus, M. anabanti, and M. balnei. Comparative sugar fermentative reaction data together with published morphological, cultural, and pathogenic data suggested that they were all synonymous with M. marinum (17). M. marinum inhabits fresh and salt water and causes disease in many fish species and occasionally in humans (24, 28). Human infections are generally limited to cutaneous diseases and are referred to as “swimming pool granuloma” and “fish tank granuloma” in reference to the epidemiology and the inoculation mode (24, 28). The frequency of M. marinum in bacteriology laboratories is low, since less than 1% of the mycobacterial clinical isolates belong to this species (11). Susceptibility data on M. marinum are scarce and rely upon the small numbers of strains and antibiotics tested (20, 23, 25). As a consequence, intrinsic antibiotic susceptibilities of M. marinum are not well defined, and methods for their routine determination have not been evaluated.
In this study we looked for the antibiotic susceptibilities of 53 clinical isolates of M. marinum by determining the MICs of 17 antibiotics using the agar dilution method. Antibiotics tested were tetracyclines, rifampin, and cotrimoxazole, which were reported to be effective for treating M. marinum infections (8), and antimycobacterial antibiotics active against Mycobacterium tuberculosis (isoniazid, rifabutin, ethambutol, and aminoglycosides) or against atypical mycobacteria (clarithromycin, azithromycin, and imipenem). In addition, we tested fluoroquinolones, since new derivatives (levofloxacin, sparfloxacin, and moxifloxacin) appear particularly active against mycobacteria (15). We compared the reference (but cumbersome) method used to a more practical and routine method of antibiotic susceptibility testing, the new stable gradient method known as Etest.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The study involved 53 clinical strains of M. marinum that were isolated over a period of 3 years (1995 to 1997) in bacteriology laboratories located in all parts of France. These strains were referred to the National Reference Centre for the Surveillance of Mycobacterial Infections and their Resistance to Antituberculous Agents (Laboratory of Bacteriology, Groupe Hospitalier Pitié-Salpêtrière, Paris, France), working for the present study in collaboration with the AZAY-Mycobacterium Group of the university hospitals of France. The strains of M. marinum were identified on the basis of phenetic characters as described previously (4). Strains were stored at −80°C in Youmans broth supplemented with 20% fetal bovine serum until the MICs were determined. M. marinum ATCC 927, Mycobacterium smegmatis ATCC 19420 and mc2 155, and Escherichia coli ATCC 25922 were used as controls for MIC determination. Mycobacteria were grown in Middlebrook 7H9 broth for 3 to 5 days (2 days for M. smegmatis), and the culture suspension was adjusted with additional sterile distilled water to equal a McFarland 1.0 turbidity standard (approximately 108 CFU per ml).
Antimicrobial agents.
Rifampin, ofloxacin, and levofloxacin (Hoechst Marion Roussel, La Défense, France), rifabutin (Pharmacia & Upjohn, Rueil Malmaison, France), ethambutol (Lederle, Paris La Défense, France), amikacin (Bristol-Myers Squibb, Paris La Défense, France), imipenem (Merck Sharp & Dohme Chibret, Paris, France), minocycline (Wyeth Lederle, La Défense, France), doxycycline (Elerte, Aubervilliers, France), azithromycin (Pfizer, Orsay, France), clarithromycin (Abbott, Saint Rémy sur Avre, France), ciprofloxacin and moxifloxacin (Bayer Pharma, Puteaux, France), sparfloxacin (Rhône Poulenc Rorer, Vitry sur Seine, France), and isoniazid, sulfamethoxazole, and trimethoprim (Roche, Fontenay sous Bois, France) were kindly provided by the manufacturers.
Etest strips containing either rifampin, clarithromycin, sparfloxacin, or minocycline were obtained from AB Biodisk, BMD, Marne-la-Vallée, France.
Determination of the MICs by the agar dilution method.
The agar dilution method was performed on Mueller-Hinton agar (Difco, Serlabo, Bonneuil sur Marne, France) supplemented with 5% Middlebrook OADC (oleic acid, albumin, dextrose and catalase [OSI, Elancourt, France]). The 5% (vol/vol) ratio of OADC was found optimal for M. marinum growth by inoculating in preliminary tests 16 strains in duplicate on Mueller-Hinton agar prepared with 0% OADC (8 of 16 grew normally), 2.5% (14 of 16 grew normally), 5% (16 of 16 grew normally), and 10% (16 of 16 grew normally).
Twofold dilutions of the antibiotics were added in order to obtain the following final concentrations: amikacin, 0.25 to 64 μg/ml; imipenem, 0.06 to 32 μg/ml; rifampin, 0.015 to 32 μg/ml; rifabutin, 0.001 to 4 μg/ml; ethambutol, 0.03 to 8 μg/ml; azithromycin, 0.015 to 64 μg/ml; ofloxacin and ciprofloxacin, 0.06 to 128 μg/ml; sparfloxacin, levofloxacin, and moxifloxacin, 0.03 to 128 μg/ml; minocycline, doxycycline, and clarithromycin, 0.06 to 32 μg/ml; sulfamethoxazole and trimethoprim, 1 to 512 μg/ml; and isoniazid, 0.5 to 32 μg/ml. A 1/100 dilution of a McFarland 1.0 turbidity standard suspension was inoculated with a Steers replicator delivering approximately 104 CFU per spot. Plates were incubated at 30°C in a 5% CO2 incubator. MICs for E. coli ATCC 25922, M. smegmatis mc2 155, and M. smegmatis ATCC 19420 were determined after 24 and 48 h of incubation, respectively. MICs for M. marinum strains were determined after 7 days (i.e., when half of the strains had grown) or 11 days (i.e., when all of the strains had grown) of incubation. The MIC was defined as the lowest concentration of antibiotic resulting in complete inhibition of growth or in growth of fewer than 10 colonies (<1% of the inoculum).
To evaluate the reproducibility with the method, independent tests were performed for M. marinum ATCC 927 (six tests) and 37 clinical strains (two tests each).
Etest method.
A suspension, equal to a McFarland 1.0 turbidity standard suspension, was applied onto the surface of a 5% sheep blood Mueller-Hinton agar plate (Sanofi Diagnostic Pasteur; 15 by 15 mm) using a sterile cotton swab. The plate was incubated at 30°C in 5% CO2 as described above. The MIC was read at the point where the zone of inhibition intersected the MIC scale on the strip, taking into account even faint growth as recommended for other mycobacteria (3, 27).
To evaluate the reproducibility with the method, 10 independent tests were performed for M. marinum ATCC 927. In addition, Etests were performed by two distinct operators, one operating under research conditions and one under routine conditions, for 39 clinical strains.
Result analysis.
The reproducibility was evaluated on the basis of the MIC differences in log2 dilution between the tests with the same strain. The reproducibility value was defined as the percentage of strains which yielded the same MIC within ±1 log2 dilution. The agreement between agar dilution and Etest methods was the percentage of strains which yielded the same MIC value within ±1 log2 dilution by the two methods. Category discrepancies were evaluated using the breakpoints for determining susceptibility and resistance categories recommended by the NCCLS for aerobic bacteria (14). A very major interpretive discrepancy was defined as resistance by the reference agar dilution method and susceptibility by the Etest method, a major interpretive discrepancy was defined as resistance by the Etest method and susceptibility by the agar dilution method, and a minor discrepancy was defined as intermediate susceptibility by one method and susceptibility or resistance by the other method.
RESULTS
Agar dilution method.
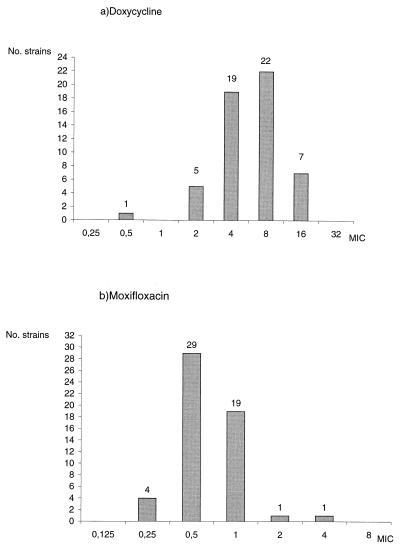
The reproducibility of results with the agar dilution method was good (>80% agreement) for all antibiotics except for sulfamethoxazole (<50% reproducibility). The MIC results are presented in detail in Table 1. MICs of rifampin and rifabutin (MICs at which 90% of the isolates were inhibited [MIC90s], 0.5 and 0.06 μg/ml, respectively) were far lower than those of other antibiotics. The MIC90s of minocycline (4 μg/ml), doxycycline (16 μg/ml), clarithromycin (4 μg/ml), imipenem (8 μg/ml), and amikacin (4 μg/ml) were close to the breakpoints. MICs of isoniazid (MIC90, 8 μg/ml), ethambutol (MIC90, 4 μg/ml), trimethoprim (MIC90, 128 μg/ml), and azithromycin (MIC90, 128 μg/ml) were above the breakpoints. Among the fluoroquinolones tested, MICs of sparfloxacin (MIC90, 2 μg/ml) and moxifloxacin (MIC90, 1 μg/ml) were four- to eightfold lower than those of ciprofloxacin (MIC90, 8 μg/ml), ofloxacin (MIC90, 16 μg/ml), and levofloxacin (MIC90, 8 μg/ml). For each antibiotic, the MICs were distributed in a narrow range (see the examples of doxycycline and moxifloxacin in Fig. 1) with an overall standard deviation comprised of between 1.5 and 2.6 log2 dilution. As a consequence, modal MICs were equal to or within 1 log2 dilution of MIC50s and were close to geometric mean MICs (Table 1). The MICs for the reference strain, ATCC 927, were within 0 to 1 dilutions of the modal MIC for 53 clinical strains.
TABLE 1.
MICs (μg/ml) of 17 antibiotics for 54 strains of M. marinum, determined by the agar dilution method
| Antibiotic | MIC50 | MIC90 | Modal MIC | Geometric mean MIC | Geometric standard deviation | Range |
|---|---|---|---|---|---|---|
| Rifampin | 0.25 | 0.5 | 0.25 | 0.24 | 1.7 | 0.125–4 |
| Rifabutin | 0.06 | 0.06 | 0.06 | 0.06 | 1.8 | 0.015–1 |
| Isoniazid | 4 | 8 | 4 | 5.6 | 1.5 | 4–16 |
| Ethambutol | 2 | 4 | 2 | 1.7 | 1.6 | 1–4 |
| Amikacin | 2 | 4 | 4 | 1 | 1.7 | 1–8 |
| Doxycycline | 8 | 16 | 8 | 5.7 | 2 | 0.5–16 |
| Minocycline | 2 | 4 | 2 | 2.9 | 1.7 | 0.5–8 |
| Clarithromycin | 1 | 4 | 2 | 1.2 | 2.3 | 0.5–32 |
| Azithromycin | 32 | 128 | 32 | NAa | NA | 8–>128 |
| Ofloxacin | 4 | 16 | 4 | 6.1 | 1.7 | 2–32 |
| Ciprofloxacin | 4 | 8 | 4 | 3.8 | 1.8 | 1–16 |
| Levofloxacin | 4 | 8 | 4 | 4.5 | 1.7 | 2–32 |
| Sparfloxacin | 1 | 2 | 1 | 1 | 1.8 | 0.5–4 |
| Moxifloxacin | 0.5 | 1 | 0.5 | 0.6 | 1.7 | 0.25–4 |
| Sulfamethoxazole | 8 | 128 | 8 | NA | NA | 4–>128 |
| Trimethoprim | 64 | 128 | 128 | 67.4 | 2.3 | 16–512 |
| Imipenem | 2 | 8 | 2 | 2.6 | 2.6 | 0.5–16 |
NA, not applicable (upper MICs were above the highest concentration tested).
FIG. 1.
MIC distribution for doxycycline (a) and moxifloxacin (b) for 54 strains of M. marinum.
Etest method.
We determined by Etest the MICs of rifampin, clarithromycin, minocycline, and sparfloxacin, which were the most active drugs against M. marinum as determined by the agar dilution method and for which Etest strips were available. For these four antibiotics, the ellipse inhibition zone was clear, without trailing growth, and thus reading the MIC was not ambiguous. The reproducibility with the Etest method for the reference strain was 100% in the case of minocycline (10 out of the 10 tests yielded the same MICs ±1 log2) and 70% for rifampin and clarithromycin but only 40% for sparfloxacin. The results of duplicate tests performed by independent operators are detailed in Table 2. Again, reproducibility of results was high for minocycline (83%), low for clarithromycin and rifampin (48% and 46%, respectively), and very low for sparfloxacin (21%).
TABLE 2.
Reproducibility of results with the Etest as evaluated by the determination of MICs by two different operators for 39 clinical strains of Mycobacterium marinum
| Antibiotic | No. of results within log2 concentration difference of:
|
% Agreementa | ||||||
|---|---|---|---|---|---|---|---|---|
| >−2 | −2 | −1 | 0 | 1 | 2 | >2 | ||
| Rifampin | 1 | 3 | 4 | 7 | 6 | 1 | 17 | 44 ± 7.8 |
| Minocycline | 1 | 4 | 8 | 16 | 8 | 1 | 1 | 82 ± 5.9 |
| Clarithromycin | 5 | 3 | 4 | 12 | 3 | 8 | 4 | 46 ± 7.9 |
| Sparfloxacin | 4 | 3 | 1 | 3 | 4 | 11 | 13 | 21 ± 6.4 |
Percentage of isolates within duplicate MICs ± log2 dilution (± standard error).
The percentages of agreement between the MICs yielded by Etest and those yielded by agar dilution are shown in Table 3. Etest MICs were in good agreement with agar dilution MICs for minocycline only (83% agreement). Agreement was lower for rifampin (59%), sparfloxacin (43%), and clarithromycin (24%). When agar dilution and Etest MICs were converted into interpretative categories using NCCLS breakpoints, there was about 90% agreement for rifampin, minocycline, and clarithromycin and 58% agreement for sparfloxacin (Table 4). No very major discrepancy and very few major discrepancies were observed for clarithromycin, although Etest regularly underestimated by 2 to 3 dilutions the MIC of this drug. In the case of sparfloxacin, Etest tended to overestimate or at the opposite to underestimate the MICs by 2 to 3 dilutions. Since sparfloxacin MICs were close to the susceptibility breakpoint for most of the strains, this led to minor (28%) or in some cases major (15%) discrepancies.
TABLE 3.
Comparison of MICs yielded by Etest and those by agar dilution for 54 strains of M. marinum
| Antibiotic | No. of Etest MICs within indicated log2 dilution of agar dilution MICs
|
% Agreementa | ||||||
|---|---|---|---|---|---|---|---|---|
| >−2 | −2 | −1 | 0 | 1 | 2 | >2 | ||
| Rifampin | 0 | 7 | 11 | 13 | 8 | 9 | 6 | 59 ± 6.7 |
| Minocycline | 1 | 6 | 20 | 23 | 2 | 0 | 2 | 83 ± 5.2 |
| Clarithromycin | 18 | 22 | 9 | 3 | 1 | 0 | 1 | 24 ± 5.8 |
| Sparfloxacin | 9 | 5 | 6 | 11 | 6 | 5 | 12 | 43 ± 6.7 |
Percentage of isolates with MICs within ± log2 dilution (± standard error).
TABLE 4.
Distribution of category discrepancies by a comparison of Etest results to agar dilution results for 54 strains of M. marinum
| Antibiotic (breakpoints [μg/ml])a | No. of category discrepancies
|
% Agreementb | ||
|---|---|---|---|---|
| Very major | Major | Minor | ||
| Rifampin (1–4) | 0 | 2 | 3 | 91 ± 3.9 |
| Minocycline (4–16) | 0 | 1 | 5 | 89 ± 4.3 |
| Clarithromycin (2–8) | 0 | 1 | 5 | 89 ± 4.3 |
| Sparfloxacin (1–4) | 0 | 8 | 15 | 57 ± 6.7 |
MIC interpretive breakpoints as defined by the NCCLS.
Percentage of isolates within the accuracy limits of the test ± standard error.
DISCUSSION
Our primary objective was the determination of in vitro susceptibilities of M. marinum, since published studies on antibiotic susceptibility are scarce, involving 10 to 20 strains per study. Moreover, very few data are available on new antimycobacterial agents, such as new macrolides, imipenem, and fluoroquinolones (19). To date no method has been recommended as the standard for determining the in vitro susceptibility of M. marinum. In some studies, the methods used were those designed for slowly growing mycobacteria, such as the proportion method on solid media and in BACTEC liquid media (10). In contrast, in other studies, the methods used for antibiotic susceptibility testing of M. marinum were those designed for rapidly growing mycobacteria, such as broth microdilution (14), disk diffusion, agar disk elution (22), or agar dilution using a Steers replicator (20, 25). M. marinum grows rapidly enough, indeed, to be tested by the method used for rapidly growing mycobacteria, despite the fact that it belongs to the slowly growing mycobacteria on the basis on genetic and mycolic acid analysis (5, 21). We confirmed the rapid growth of M. marinum and showed that its growth required a lower level of OADC supplementation than is required for other slowly growing mycobacteria. Consequently, we used as a reference the agar dilution method using a Steers replicator (25) for its convenience and because of the large worldwide experience of this method of testing antibiotic activity that has been acquired with nonfastidious organisms.
The present study allowed us to delineate the susceptibility pattern of M. marinum towards antituberculous drugs and new drugs which have been shown to be active against other antimycobacterial species. Clearly, M. marinum is susceptible to rifampin, rifabutin, and amikacin but resistant to isoniazid and ethambutol. With regard to susceptibilities to fluoroquinolones, on one hand M. marinum is resistant to ofloxacin, ciprofloxacin, and levofloxacin, and on the other hand the majority of the strains were susceptible to moxifloxacin and sparfloxacin (MIC50 and geometric mean MIC, 0.5 and 1 μg/ml, respectively). As described previously for M. tuberculosis and atypical mycobacteria (12), fluoroquinolones were arranged from that with the lowest MIC to that with the highest MIC as follows: moxifloxacin, sparfloxacin, levofloxacin, ciprofloxacin, and ofloxacin. The results also confirmed the moderate susceptibility of M. marinum to tetracyclines (20, 23, 25) and the higher activity of minocycline than of doxycycline (MICs being constantly twofold lower). A majority of strains were susceptible to clarithromycin, to sulfamethoxazole, and to imipenem, but modal MICs of these drugs were close to the breakpoints. Finally, M. marinum was found to be resistant to azithromycin and to trimethoprim.
The geometric mean MIC, the modal MIC, and the MIC50 for each antibiotic taken separately were close, and the geometric standard deviations were very low (Table 1), strongly suggesting a homogeneous susceptibility pattern for the M. marinum species. This fact was confirmed by the narrow MIC distribution for each antibiotic, as shown for doxycycline and moxifloxacin in Fig. 1. The susceptibility pattern of M. marinum described herein likely corresponds to the wild-type susceptibility pattern. Until now, no relapse due to the selection of a resistant mutant has been reported, and acquired resistance is not known for M. marinum.
Therefore, on the basis of in vitro susceptibilities, candidates for treatment of M. marinum can be chosen. Cases of success have seldom been reported after treatment of M. marinum infections by rifampin (7, 8), and MICs of the rifamycins and rifampin and rifabutin are indeed the lowest and are close to those found for M. tuberculosis. Minocycline and to a lesser extent doxycycline, clarithromycin, imipenem, and amikacin are serious candidates for the treatment of M. marinum infections, since their MICs are in the range of blood levels. Moreover, these MICs are close to those found for other atypical mycobacteria, such as M. avium, and rapidly growing mycobacteria, for which in vitro activity has been correlated with in vivo efficacy (6, 26). The activities of sparfloxacin and moxifloxacin, even if higher than those of other fluoroquinolones, remained lower than those against M. tuberculosis, and thus in vivo efficacy should be carefully assessed. Pharmacokinetic considerations might also influence the therapeutic value of the antibiotics. However, all the antibiotics with good in vitro activity cited above also have a very high intracellular penetration and extravascular distribution.
Our second objective was the evaluation of a routine method for susceptibility testing of M. marinum. Etest has been demonstrated to be an accurate and precise method of MIC determination for bacteria other than mycobacteria (2). The results yielded by the Etest method were shown to agree with those yielded by the agar dilution method for rapidly growing and some slowly growing mycobacterial species (3, 9, 13, 16, 27). In the present study on M. marinum, the level of agreement between results for Etest and those for agar dilution was high only for minocycline (83% agreement) but in contrast was low for rifampin, sparfloxacin, and clarithromycin. The low agreement rates found for M. marinum were not expected, since more than 70% agreement was reported between results for Etest and those for agar dilution for M. fortuitum and M. chelonae (3) and for M. avium (16), and Etest MICs determined in the present study were close to those reported by Flynn et al. (9). If reproducibility of results with the Etest was excellent for minocycline, it was poor for clarithromycin, rifampin, and sparfloxacin. Consequently, we cannot recommend the Etest method for antibiotic susceptibility testing of M. marinum. Nevertheless, no acquired resistance has been described so far, and routine susceptibility testing seems unnecessary except for relapse cases, as for other atypical mycobacteria (24).
In conclusion, we described the wild-type susceptibility pattern of M. marinum for 17 antibiotics. Among these antibiotics, rifampin, rifabutin, tetracyclines (particularly minocycline), amikacin, imipenem, and clarithromycin are good candidates for testing in vivo efficacy.
ACKNOWLEDGMENTS
We thank for their technical assistance Pascale Bonafous, Claudine Wichlacz, Lysiane Jeanson, Murielle Renard, and Michel Szpytma. We also thank Jacques Grosset and Nacer Lounis for their helpful advice. The protocol was defined with the collaboration of Olivier Chosidow (Service de Medecine Interne, Hôpital Pitié-Salpêtrière) and Eric Caumes (Service de Maladies Infectieuses, Hôpital Pitié-Salpêtrière). The study was organized with the collaboration of the members of the AZAY-Mycobacterium Group (a group of mycobacteriologists working in university hospitals). The following laboratories (and microbiologists) kindly sent the strains of M. marinum included in the study: CHU Amiens (H. Laurans), CHU Angers (E. Carpentier), CHG Arles (B. Hautefort), Centre Medico-chirurgical de Bligny (C. Boval-Gallet), CHU Brest (M. L. Abalain), CHU Caen (M. Fines and B. Malbruny), Laboratoire Lescaroux Chateauroux (M. Drochon), CHU Clermont Ferrand (J. Sirot), CHU Lille (C. Savage), CHU Limoges (C. Martin), CHG Nancy (A. Didion), CHU Nice (A. Gouby), CHU Paris-Cochin (G. Paul), CHU Paris-Henri Mondor (L. Desforges), CHU Paris-Pitié-Salpêtrière, CHU Reims (O. Bajolet-Laudinat), CHU Saint Etienne (A. Carricajo), and CHU Toulouse (R. Bauriaud).
This work was supported by a grant from Association Claude Bernard.
REFERENCES
- 1.Aronson J. Spontaneous tuberculosis in salt water fish. J Infect Dis. 1926;39:315–320. [Google Scholar]
- 2.Baker C N, Stocker S H, Culver D H, Thornsberry C. Comparison of Etest to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J Clin Microbiol. 1991;29:533–538. doi: 10.1128/jcm.29.3.533-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biehle J, Cavalieri S, Saubolle M, Gestsinge L. Evaluation of Etest for susceptibility testing of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:1760–1764. doi: 10.1128/jcm.33.7.1760-1764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cernoch P L, Enns R K, Saubolle M A, Wallace R J., Jr . Cumitech 16A. Laboratory diagnosis of the mycobacterioses. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 5.Daffe M, Laneelle M, Lacave C. Structure and stereochemistry of mycolic acids of Mycobacterium marinum and Mycobacterium ulcerans. Res Microbiol. 1991;142:397–403. doi: 10.1016/0923-2508(91)90109-n. [DOI] [PubMed] [Google Scholar]
- 6.Dautzenberg B, Truffot-Pernot C, Legris S, Meyohas M C, Berlie H C, Mercat A, Chevret S, Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with acquired immune deficiency syndrome. Am Rev Respir Dis. 1991;144:584–589. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- 7.Donta S T, Smith P W, Levitz R E, Quintiliani R. Therapy of Mycobacterium marinum infections. Use of tetracyclines vs. rifampin. Arch Intern Med. 1986;146:902–904. [PubMed] [Google Scholar]
- 8.Edelstein H. Mycobacterium marinum skin infections. Arch Intern Med. 1994;154:1359–1364. doi: 10.1001/archinte.154.12.1359. [DOI] [PubMed] [Google Scholar]
- 9.Flynn C, Kelly C, Barrett M, Jones R. Application of the Etest to the antimicrobial susceptibility testing of Mycobacterium marinum clinical isolates. J Clin Microbiol. 1997;35:2083–2086. doi: 10.1128/jcm.35.8.2083-2086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsgren A. Antibiotic susceptibility of Mycobacterium marinum. Scand J Infect Dis. 1993;25:779–782. doi: 10.3109/00365549309008579. [DOI] [PubMed] [Google Scholar]
- 11.Good R, Snider D. Isolation of nontuberculous mycobacteria in the United States, 1979. J Infect Dis. 1980;146:829–833. doi: 10.1093/infdis/146.6.829. [DOI] [PubMed] [Google Scholar]
- 12.Guillemin I, Jarlier V, Cambau E. Correlation between quinolones susceptibility patterns and sequences in the A and B subunits of DNA gyrase in mycobacteria. Antimicrob Agents Chemother. 1998;42:2084–2088. doi: 10.1128/aac.42.8.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffner S, Klintz L, Olsson-Liljequist B, Bolmström A. Evaluation of Etest for susceptibility testing of Mycobacterium chelonei and M. fortuitum. J Clin Microbiol. 1994;32:1846–1849. doi: 10.1128/jcm.32.8.1846-1849.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inderlied C B, Nash K A. Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biological fluids. In: Lorian V, editor. Antibiotics in laboratory medecine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 127–176. [Google Scholar]
- 15.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebrun L, Onody C, Vincent V, Nordmann P. Evaluation of the Etest for rapid susceptibility testing of Mycobacterium avium to clarithromycin. J Antimicrob Chemother. 1996;37:999–1003. doi: 10.1093/jac/37.5.999. [DOI] [PubMed] [Google Scholar]
- 17.Ross A, Earp B, Wood J. Mycobacterial infections in adult salmon and steelhead trout returning to the Columbia River Basin and other areas in 1957. U.S. Fish and Wildlife Service special scientific report on fisheries, handbook no. 332. 1959. [Google Scholar]
- 18.Runyon E. Anonymous mycobacteria in pulmonary disease. Med Clin N Am. 1959;43:273–290. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 19.Saito H, Tomioka H, Sato K, Dekio S. In vitro and in vivo antimycobacterial activities of a new quinolone, DU-6859a. Antimicrob Agents Chemother. 1994;38:2877–2882. doi: 10.1128/aac.38.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders W, Wolinsky E. In vitro susceptibility of Mycobacterium marinum to eight antimicrobial agents. Antimicrob Agents Chemother. 1980;18:529–531. doi: 10.1128/aac.18.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl D, Urbance J. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990;172:116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone M, Wallace R, Swenson J, Thornsberry C, Christensen L. Agar disk elution method of susceptibility testing of Mycobacterium marinum and Mycobacterium fortuitum complex to sulfonamides and antibiotics. Antimicrob Agents Chemother. 1983;24:486–493. doi: 10.1128/aac.24.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres J, Sands M, Sanders C. In vitro sensitivity of Mycobacterium marinum to minocycline and doxycycline. Tubercle. 1978;59:193–195. doi: 10.1016/0041-3879(78)90026-0. [DOI] [PubMed] [Google Scholar]
- 24.Wallace R J, Glassroth J, Griffith D E, Olivier K N, Cook J L, Gordin F the American Thoracic Society. Diagnostic and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 25.Wallace R J, Jr, Wiss K. Susceptibility of Mycobacterium marinum to tetracyclines and aminoglycosides. Antimicrob Agents Chemother. 1981;20:610–612. doi: 10.1128/aac.20.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace R J, Jr, Tanner D, Brennan P J, Brown B. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482–486. doi: 10.7326/0003-4819-119-6-199309150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Wanger A, Mills K. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampicin, and streptomycin by using Etest. J Clin Microbiol. 1996;34:1672–1676. doi: 10.1128/jcm.34.7.1672-1676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]