Summary
Background
Long-term effects of human mesenchymal stem cell (MSC) treatment on COVID-19 patients have not been fully characterized. The aim of this study was to evaluate the safety and efficacy of a MSC treatment administered to severe COVID-19 patients enrolled in our previous randomized, double-blind, placebo-controlled clinical trial (NCT 04288102).
Methods
A total of 100 patients experiencing severe COVID-19 received either MSC treatment (n = 65, 4 × 107 cells per infusion) or a placebo (n = 35) combined with standard of care on days 0, 3, and 6. Patients were subsequently evaluated 18 and 24 months after treatment to evaluate the long-term safety and efficacy of the MSC treatment. Outcomes measured included: 6-min walking distance (6-MWD), lung imaging, quality of life according to the Short Form 36 questionnaire (SF-36), COVID-19-related symptoms, titers of SARS-CoV-2 neutralizing antibodies, tumor markers, and MSC-related adverse events (AEs).
Findings
Two years after treatment, a marginally smaller proportion of patients had a 6-MWD below the lower limit of the normal range in the MSC group than in the placebo group (OR = 0.19, 95% CI: 0.04–0.80, Fisher's exact test, p = 0.015). At month 18, the general health score from the SF-36 was higher in the MSC group than in the placebo group (50.00 vs. 35.00, 95% CI: 0.00–20.00, Wilcoxon rank sum test, p = 0.018). Total severity score of lung imaging and the titer of neutralizing antibodies were similar between the two groups at months 18 and 24. There was no difference in AEs or tumor markers at the 2-year follow-up between the two groups.
Interpretation
Long-term safety was observed for the COVID-19 patients who received MSC treatment. However, efficacy of MSC treatment was not significantly sustained through the end of the 2-year follow-up period.
Funding
The National Key Research and Development Program of China (2022YFA1105604, 2020YFC0860900, 2022YFC2304401), the specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202216) and the Fund of National Clinical Center for Infectious Diseases, PLA General Hospital (NCRC-ID202105,413FZT6).
Keywords: COVID-19, Mesenchymal stem cell, 2-year follow-up
Research in context.
Evidence before this study
As of January 29, 2023, over 90 clinical trials involving mesenchymal stem cell (MSC) treatment for COVID-19 have been registered at clinicaltrials.gov. Research of PubMed using the terms: “((COVID-19) OR (SARS-CoV-2)) AND (mesenchymal stem cells) AND ((clinical trial) OR (randomized controlled trial)) NOT review”, identified 45 study reports. Most of these trials showed MSC treatment to be safe and beneficial in terms of relieving symptoms, decreasing inflammatory cytokines, accelerating lung recovery, improving oxygenation, and improving survival in COVID-19 patients. However, there remains limited evidence available regarding long-term outcomes (e.g., >12 months) of MSC treatment in patients with severe COVID-19.
Added value of this study
In the present study, safety and efficacy of MSC treatment for COVID-19 were evaluated over a 2-year follow-up period based on our previous randomized controlled trials of MSC treatment. The study also contributes further evidence regarding patient characteristics (e.g., age, BMI) which may increase response rates to MSC treatment.
Implications of all the available evidence
Our results demonstrate long-term safety of MSC treatment over 24 months, yet no significant sustained efficacy of MSC treatment according to 6-MWD data, extent of lung damage and quality of life outcomes. In the future, a well-designed, large-scale phase 3 clinical trial is needed to determine the efficiency of MSC therapy for patients with COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly developed into a global pandemic. As of January 2023, more than 6 million deaths due to COVID-19 have been reported worldwide, and these numbers continue to rise.1 Accumulating evidence further shows that a number of individuals who have recovered from COVID-19 experience at least one long-term COVID-19-related symptom, referred to as a post-COVID-19 condition or long COVID. For individuals with a history of SARS-CoV-2 infection, onset of a long-term related symptom usually manifests three months after the initial onset of COVID-19. Affected individuals exhibit symptoms that cannot be explained by an alternative diagnosis. Common symptoms include fatigue, muscle weakness, joint pain, dyspnea, cough, anxiety and depression, disturbed sleep, and hair loss.2, 3, 4 While the majority of current efforts focus on the development of antiviral drugs5 and eliminating infection through vaccination, suitable interventions to further reduce the mortality of severe COVID-19 and to treat long COVID are still needed.
Since the start of the COVID-19 outbreak, over 90 clinical trials involving mesenchymal stem cell (MSC) treatment have been registered at clinicaltrials.gov. These trials have utilized different sources for obtaining MSCs, including umbilical cord, bone marrow, menstrual blood, placenta, and adipose tissue. Some of these clinical trials have showed MSC treatment to be safe and beneficial for relieving symptoms, decreasing inflammatory cytokines, accelerating lung recovery, improving oxygenation, and improving survival in COVID-19 patients.6, 7, 8, 9, 10, 11, 12, 13, 14 However, most of these studies mainly focused on short-term outcomes (e.g., ≤12 months) in patients with severe COVID-19.
In March 2020, we established one of the largest randomized, double-blind, placebo-controlled trials to evaluate the safety and efficacy of human umbilical cord-derived MSCs for severe COVID-19 in Wuhan, China (NCT 04288102). Enrolled patients were at the convalescent stage but could not be discharged due to severe lung damage. Initially, when the COVID-19 patients were evaluated 28 days and 1-year after treatment, improvements in lung lesion recovery and symptoms were observed with good tolerance.15,16 For the present study, the aim was to evaluate the safety and efficacy of MSC in this previously established cohort of severe COVID-19 patients at 18 and 24 months after treatment.
Methods
Study design and participants
In our previous phase 2 double-blind, randomized, placebo-controlled trial of MSC treatment for severe COVID-19 patients (NCT04288102),15,16 a total of 101 patients were enrolled between March 6, 2020 and March 20, 2020. A total of 100 patients finally received either MSC (n = 65) or placebo (n = 35) combined with standard of care on days 0, 3, and 6. Key inclusion criteria were: hospitalized patients with severe COVID-19 confirmed by real-time reverse transcription PCR assay, either man or woman, aged 18–75 years old, and pneumonia combined with lung damage confirmed by chest computed tomography (CT). Major exclusion criteria were: shock, organ failures, invasive ventilation, malignant tumor, pregnancy, lactation, or co-infection with other pathogens. Severe COVID-19 was defined as any of the following conditions: dyspnea with respiratory rate ≥30/min; oxygen saturation ≤93% on room air; arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen ≤300 mmHg; or lung lesion on CT progressed >50% in 24–48 h.15 Sex information was collected from the Hospital Information System, which was recorded according to the participant's identity card. Follow-up was conducted at day 28, month 3, month 6, month 9, and month 12, results been reported previously.15,16 After the 28-day follow-up for the primary endpoint, the trial was unblinded on June 23, 2020. For the present study, data from additional follow-up visits conducted at month 18 and month 24 (between September 4, 2021, and May 31, 2022) at the outpatient clinic of General Hospital of Central Theater Command (Wuhan, Hubei, China) were collected to evaluate the long-term safety and efficacy of MSC treatment. Written informed consent was obtained from all of the participants. This study was approved by the Ethics Committee of the Fifth Medical Center, Chinese PLA General Hospital (2020-013-D).
Outcomes
The outcomes evaluated in this study included: (1) distance walked in 6-min (6-MWD) and the proportion of patients with a 6-MWD below the lower limit of the normal range (LLN)17,18; (2) lung imaging according to the percentages of normal CT images and total severity score (TSS)19, 20, 21; In our previous report,16 lung lesions were measured by centralized imaging interpretation based on both lung radiologist analyses and Lung Imaging Artificial intelligence system software (LIAIS). After 1 year, LIAIS was not sensitive enough to segment the lesion area and distinguish injured area from normal lung tissue along with lung repair. Herein, we evaluated the lung lesions only by radiologist analyses at the 18- and 24-month follow-ups. (3) quality of life according to the Short Form 36 (SF-36) questionnaire; (4) COVID-19-related symptoms measured based on symptom questionnaires and numerical rating scales (NRS); (5) titers of SARS-CoV-2 neutralizing antibody (NAb); and (6) MSC-related adverse events (AEs) and tumor markers.
Considering about 1/3 of the patients refused to undergo pulmonary function test during 1-year follow-up and no significant difference was found,16 we didn't perform pulmonary function test at the 18- and 24-month follow-ups.
Procedures
As previously described,15,16 allogeneic MSCs derived from human umbilical cord were supported by VCANBIO Cell & Gene Engineering Corp (Tianjin, China). MSC (or placebo) was infused intravenously three times at 3-day intervals, with (or without) each dose of 4.0 × 107 cells in a volume of 100 ml/bag. Standard of care was provided according to the 7th edition of guidelines for the diagnosis and treatment of COVID-19 issued by the Chinese National Health Commission.22
During follow-up visits, patients were physically examined by trained physicians and completed SF-36, NRS, and symptom (e.g., appetite, sleep difficulties, pain or discomfort, fatigue or muscle weakness, anxiety or depression, and usual activity) questionnaires. In addition, a high-resolution CT (HRCT) of the chest, a standardized 6-MWD test, routine blood and biochemical tests, tumor markers, and SARS-CoV-2 NAb assays were performed.
The 6-MWD test was conducted according to practical guidelines of the American Thoracic Society.17 The results are expressed as distance walked in 6-min. LLN was additionally calculated by subtracting 153 m from the predicted value for men, and by subtracting 139 m for women.18 Lung lesions were evaluated based on HRCT images and imaging interpretations provided by three independent radiologists evaluating the outcome of lung damage. All radiologists were blinded to the treatment allocation during analysis, and final outcomes were determined by consensus. CT findings were assessed based on distribution, density, morphology, internal structure of the lesions, and mediastinum. TSS was used to quantify anatomic involvement (Appendix 1).19, 20, 21 Levels of SARS-CoV-2 NAb were detected according to the manufacturer's instructions at month 24 (Appendix 2). Through the follow-up period, AEs were defined as abnormal symptoms, signs, laboratory tests, and tumorigenesis among the patients with severe COVID-19 or comorbidities after receiving treatment.15
Statistics
This study had no predefined hypotheses. Thus, statistical tests, confidence intervals (CIs), and p values were used for description rather than inference. Continuous variables were reported as median [interquartile range (IQR)] or mean [standard deviation (SD)] values and were compared across groups using the Wilcoxon rank sum test or Student's t-test. The Hodges-Lehmann estimation was used to determine the 95% CI of the median differences for the non-normally distributed variables. Categorical variables were reported as n/N (%) and compared across groups using the Chi-square test, the continuity-adjusted Chi-square test or Fisher's exact test. A logistic regression model was used to estimate odds ratios (ORs). Additionally, a post-hoc subgroup analysis was performed according to age (<65 y vs. ≥ 65 y) and body mass index (BMI) (≤24 kg/m2 vs. > 24 kg/m2) to explore heterogeneity of the therapeutic benefits of MSC. No adjustment for multiple testing was applied. The modified intention-to treat population was used as the analysis population. Missing values were not included. Statistical analyses were performed using SAS software (version 9.4; Cary, NC, USA). Figures were generated using GraphPad Prism software (version 8.0, San Diego, CA, USA).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Follow-up and baseline characteristics
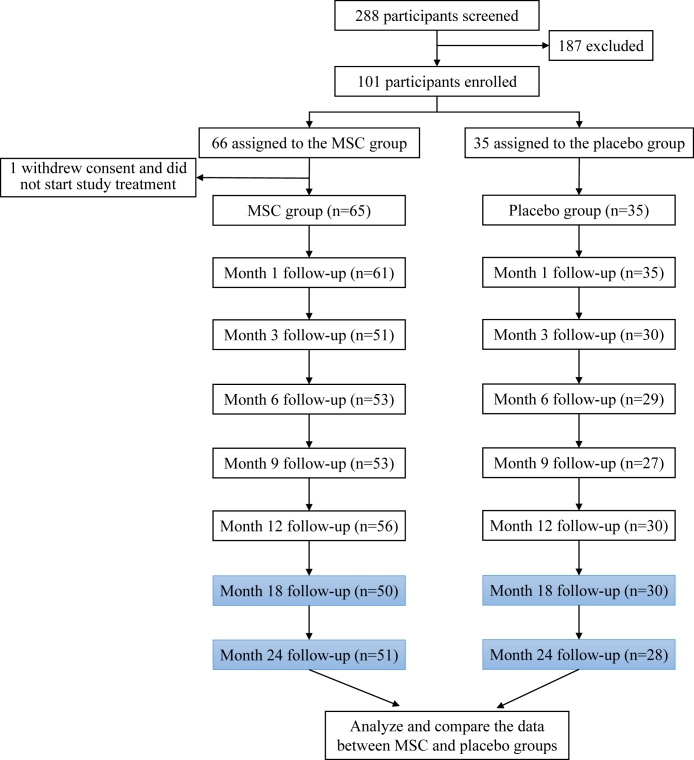
During the previously established phase 2 trial conducted to evaluate MSC treatment, a 62-year-old man in the placebo group died of liver cancer at month 3, and a 64-year-old man in the MSC group died for an unknown reason at month 18. Individuals included in the follow-up analyses conducted at month 18 and 24 in the present study are highlighted in Fig. 1. There were 65 patients in the MSC group and 35 patients in the placebo group who were followed for a median of 546.0 days (IQR: 543–548) at month 18 and 771 days (IQR: 765–781) at month 24. Eighty patients were assessed at month 18 (50/65 in the MSC group, 30/35 in the placebo group) and 79 patients were assessed at month 24 (51/65 in the MSC group, 28/35 in the placebo group).
Fig. 1.
Overview of patient enrollment for the original phase 2 trial and for the current follow-up at 18 and 24 months.
Baseline characteristics for the MSC and placebo groups were similar according to sex, age, time from symptom onset, comorbidities, concomitant medication, lung lesion proportion, etc (Appendix 3).15 A post-hoc subgroup analysis according to age included 63 patients aged <65 y and 37 patients aged ≥65 y. The mean (SD) age for the patients aged <65 y in the MSC and placebo groups were 55.50 (7.21) y and 55.10 (5.15) y, respectively. The mean (SD) age for the patients aged ≥65 y in the MSC and placebo groups were 69.60 (3.31) y and 68.20 (3.08) y, respectively. Patients were also categorized into subgroups according to BMI, with 39 patients with BMI ≤24 kg/m2 and 53 patients with BMI >24 kg/m2. The mean (SD) BMI for these two groups are reported in Appendix 3. Baseline characteristics of the post-hoc subgroups that were analyzed were matched according to age (<65 y, ≥65 y) and BMI (≤24 kg/m2, >24 kg/m2) between the MSC and placebo groups.
Efficacy outcomes
To assess exercise capacity and integrated reserve capability after MSC treatment, 6-MWD was evaluated at months 18 and 24. In the MSC group, the distances achieved were 447.00 m (IQR: 417.00, 480.00) and 450.00 m (IQR: 417.00, 480.00); in the placebo group, 448.00 m (IQR: 408.00, 471.00) and 450 m (IQR: 400.00, 500.00), respectively in each case (Appendix 4). The proportion of LLN was 5.9% (3/51) in the MSC group and 25.0% (7/28) in the placebo group at month 24 (OR = 0.19, 95% CI: 0.04–0.80, Chi-square, p = 0.015) (Table 1).
Table 1.
The proportion of patients had a 6-MWD below the LLN in the MSC and placebo groups at months 18 and 24.
| MSC groupa | Placebo groupa | OR (95% CI)b | p value | |
|---|---|---|---|---|
| Month 18 | 6/50 (12.0) | 4/30 (13.3) | 0.89 (0.23, 3.44) | 0.861c |
| Age <65 y | 5/31 (16.1) | 4/21 (19.1) | 0.82 (0.19, 3.48) | |
| Age ≥65 y | 1/19 (5.3) | 0/9 (0.0) | NA | |
| BMI ≤24 | 2/19 (10.5) | 2/13 (15.4) | 0.65 (0.08, 5.29) | |
| BMI >24 | 3/30 (10.0) | 2/15 (13.3) | 0.72 (0.11, 4.86) | |
| Month 24 | 3/51 (5.9) | 7/28 (25.0) | 0.19 (0.04, 0.80) | 0.015c |
| Age <65 y | 3/33 (9.1) | 6/20 (30.0) | 0.23 (0.05, 1.07) | |
| Age ≥65 y | 0/18 (0.0) | 1/8 (12.5) | NA | |
| BMI ≤24 | 1/18 (5.6) | 4/13 (30.8) | 0.13 (0.01, 1.37) | |
| BMI >24 | 2/32 (6.3) | 3/13 (23.1) | 0.22 (0.03, 1.53) |
The available values of 6-MWD were 49 in the MSC group and 30 in the placebo group at month 18, and 51 in the MSC group and 27 in the placebo group at month 24.
The p values provided are for descriptive purposes only. LLN, lower limit of the normal range; OR, odds ratio; NA, not applicable.
Data are n/N (%).
Calculated by the logistic regression model.
Group difference assessed by Chi-square test.
Radiological outcomes were evaluated based on CT images obtained at months 18 and 24 after MSC treatment (Table 2). Normal CT images were observed in 28.8% (23/80) and 31.7% (25/79) of the enrolled patients at months 18 and 24, respectively. Fibrous stripes, ground-glass opacity, and reticular opacity were most commonly observed in the abnormal CT images (Appendix 5). There were no significant differences in pulmonary fibrosis, the TSS scores and the numbers of normal lung imaging scans between the MSC and placebo groups at 18- and 24-month follow-ups.
Table 2.
Lung lesion imaging in the MSC and placebo groups at months 18 and 24.
| MSC group | Placebo group | Difference/OR (95% CI) | p value | |
|---|---|---|---|---|
| TSS scorea | ||||
| Month 18 | 3.50 (0.00, 10.00) | 5.00 (0.00, 10.00) | 0.00 (−3.00, 2.00)b | 0.801d |
| Age <65 y | 4.00 (0.00, 10.00) | 5.00 (0.00, 10.00) | 0.00 (−4.00, 2.00)b | |
| Age ≥65 y | 3.00 (0.00, 10.00) | 5.00 (0.00, 9.00) | 0.00 (−5.00, 4.00)b | |
| BMI ≤24 | 2.00 (0.00, 11.00) | 5.00 (0.00, 6.00) | 0.00 (−4.00, 5.00)b | |
| BMI >24 | 4.50 (0.00, 8.00) | 4.00 (0.00, 10.00) | 0.00 (−4.00, 3.00)b | |
| Month 24 | 5.00 (0.00, 9.00) | 4.00 (0.00, 9.50) | 0.00 (−2.00, 2.00)b | 0.942d |
| Age <65 y | 6.00 (2.00, 10.00) | 5.50 (0.00, 10.00) | 0.00 (−3.00, 3.00)b | |
| Age ≥65 y | 4.50 (0.00, 8.00) | 11.50 (9.50, 17.50) | 0.00 (−4.00, 4.00)b | |
| BMI ≤24 | 5.00 (0.00, 9.00) | 4.00 (0.00, 8.00) | 0.00 (−4.00, 4.00)b | |
| BMI >24 | 5.00 (0.00, 9.00) | 4.00 (0.00, 8.00) | 0.00 (−3.00, 4.00)b | |
| No. normal chest CT images | ||||
| Month 18 | 14/50 (28.0) | 9/30 (30.0) | 0.91 (0.34, 2.46)c | 0.848e |
| Age <65 y | 9/31 (29.0) | 6/21 (28.6) | 1.02 (0.30, 3.48)c | |
| Age ≥65 y | 5/19 (26.3) | 3/9 (33.3) | 0.71 (0.13, 3.99)c | |
| BMI ≤24 | 6/19 (31.6) | 4/13 (30.8) | 1.04 (0.23, 4.77)c | |
| BMI >24 | 8/30 (26.7) | 5/15 (33.3) | 0.73 (0.19, 2.79)c | |
| Month 24 | 15/51 (29.4) | 10/28 (35.7) | 0.75 (0.28, 2.00)c | 0.565e |
| Age <65 y | 8/33 (24.2) | 8/20 (40.0) | 0.48 (0.14, 1.59)c | |
| Age ≥65 y | 7/18 (38.9) | 2/8 (25.0) | 1.91 (0.30, 12.26)c | |
| BMI ≤24 | 6/18 (33.3) | 5/13 (38.5) | 0.80 (0.18, 3.54)c | |
| BMI >24 | 9/32 (28.1) | 5/13 (38.5) | 0.63 (0.16, 2.43)c | |
Data are n/N (%) or median (IQR), unless otherwise specified.
The p-values provided are for descriptive purposes only. OR, odds ratio.
The available values of lung lesion imaging were 50 in the MSC group and 30 in the placebo group at month 18, and 51 in the MSC group and 28 in the placebo group at month 24.
Differences are expressed as Hodges–Lehmann estimator and 95% confidence interval (CI).
Calculated by the logistic regression model.
Group difference assessed by Wilcoxon rank sum test.
Group difference assessed by Chi-square test.
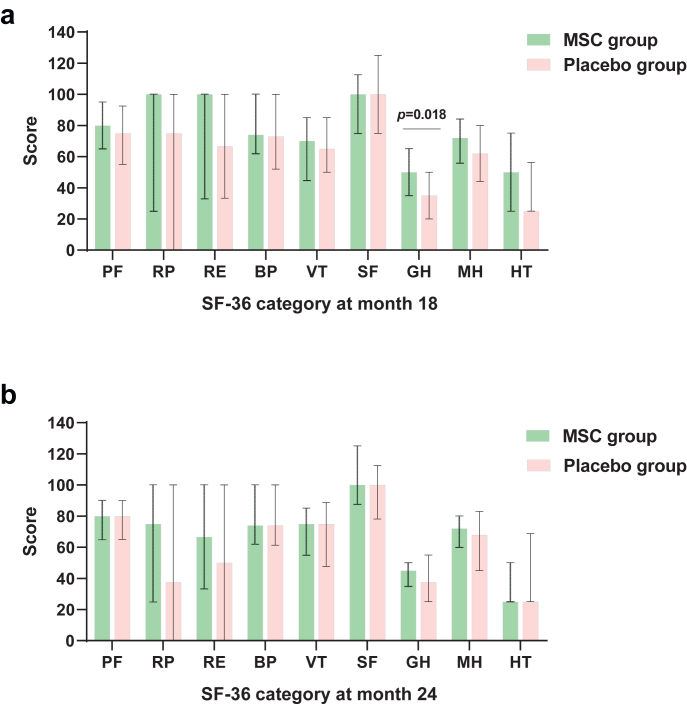
To evaluate quality of life, the SF-36 questionnaire was completed at months 18 and 24 (Fig. 2, Appendix 6 and 7). At month 18, general health (GH) category was 50.00 (IQR: 35.00, 65.00) in the MSC group and 35.00 (IQR: 20.00, 50.00) in the placebo group, respectively, with a difference of 10.00 (95% CI: 0.00–20.00, Wilcoxon, p = 0.018). The other categories assessed by the SF-36 did not differ between the two groups.
Fig. 2.
Nine SF-36 category scores in the MSC and placebo groups at months 18 and 24. Nine SF-36 category scores in the MSC and placebo groups at follow-up month 18 (a) and month 24 (b). The available values of SF-36 were 50 in the MSC group and 30 in the placebo group at month 18, and 51 in the MSC group and 28 in the placebo group at month 24, except for 1 missing value of PF category in the control group and 1 missing value of GH category in both groups at month 18. I bars indicate Q1 (denotes the first quartile), Q3 (the third quartile), and points indicate the median. Group difference assessed by Wilcoxon rank sum test. SF-36, Short-Form 36; BP, bodily pain; GH, general health; HT, reported health transition; MH, mental health; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; VT, vitality. Data are median (IQR).
Regarding COVID-19-related symptoms, 80.0% (64/80) of the enrolled patients at month 18, and 86.1% (68/79) of the enrolled patients at month 24, reported at least one symptom since disease onset. Symptom reporting was similar between the MSC and placebo groups at month 18 (80.0% (40/50) and 80.0% (24/30), respectively; Chi-square, p = 1.000) and month 24 (84.3% (43/51) and 89.3% (25/28), respectively; Chi-square, p = 0.738). Incidence of loss of appetite, sleep difficulties, pain or discomfort, fatigue or muscle weakness, decreased usual activity, and anxiety or depression were also similar between the MSC and placebo groups (Appendix 8). NRS scores and severity did not differ between the two groups at month 18 or month 24 (Appendix 9). Titers of SARS-CoV-2 NAb were similar between the two groups at both months 18 and 24 (Appendix 10).
The descriptive results of post-hoc subgroup analysis according to age and BMI were presented on Table 1, Table 2 and Appendices 4 and 6–10.
Safety outcomes
To evaluate the long-term safety of the MSC therapy administered, serum levels of tumor markers were assayed at month 24. Most of the tumor markers examined remained within normal ranges and there were no differences observed between the two groups (Table 3). In addition, tumorigenesis was not detected during the 2-year follow-up period, except for one patient in the placebo group who died of liver cancer at month 3. The total incidence of AEs between the 1-year and 2-year follow-up time points was 6.2% in the MSC group and 2.9% in the placebo group (Appendix 11), which does not represent a statistically significant difference. There were no MSC-related AEs reported at the 18- or 24-month follow-ups.
Table 3.
Tumor markers at month 24.
| Tumor Marker | MSC group (n = 51) |
Placebo group (n = 28) |
p value |
|---|---|---|---|
| Abnormal n/N (%) | Abnormal n/N (%) | ||
| Total prostate-specific antigen | 6/51 (11.8) | 2/28 (7.1) | 0.705 |
| Carcinoembryonic antigen | 1/51 (2.0) | 0/28 (0.0) | 1.000 |
| Free prostate-specific antigen | 6/51 (11.8) | 0/28 (0.0) | 0.084 |
| Carbohydrate antigen 125 | 0/51 (0.0) | 0/28 (0.0) | NA |
| Carbohydrate antigen 15-3 | 0/51 (0.0) | 0/28 (0.0) | NA |
| Alpha-fetoprotein | 0/51 (0.0) | 0/28 (0.0) | NA |
| Carbohydrate antigen 19-9 | 0/51 (0.0) | 0/28 (0.0) | NA |
| Carbohydrate antigen 24-2 | 0/51 (0.0) | 0/28 (0.0) | NA |
| Neuron-specific enolase | 8/51 (15.7) | 5/28 (17.9) | 1.000 |
| Squamous cell carcinoma antigen | 1/51 (2.0) | 0/28 (0.0) | 1.000 |
| Free beta-subunit of human chorionic gonadotropin | 0/51 (0.0) | 0/28 (0.0) | NA |
Group differences assessed according to Fisher's exact test. The p values provided are for descriptive purposes only.
In the MSC group, a 35-year-old woman became pregnant 15 months after receiving MSC treatment, when her chest CT finding returned to normal. She gave birth to a healthy female infant (birth weight: 2850 g; length: 49 cm) on April 22, 2022 at 38 weeks gestation. By December 2022, the infant exhibited normal growth and development.
Discussion
Based on our previous 1-year follow-up data obtained, promising benefits and good safety for MSC treatment of severe COVID-19 patients were observed.15,16 In the present study, long-term safety and efficacy of the MSC treatment were evaluated over a 2-year follow-up period. There were no MSC treatment-related AEs were reported. We also observed a lower LLN proportion of the 6-MWD data at month 24, as well as a higher GH score on SF-36 at month 18, with marginal differences detected between the MSC and placebo groups. Nevertheless, there were no differences between the two groups in terms of lung CT images and titers of SARS-CoV-2 Nab. Taken together, these findings demonstrate that the MSC treatment administered exhibited long-term safety, yet not significant sustained efficacy during the 2-year follow-up period.
A longitudinal study with a large cohort recently reported that 7–9% of COVID-19 survivors had a 6-MWD lower than LLN at 2 years, independent of disease severity.23 In the MSC group, a smaller proportion of 6-MWD lower than LLN was observed compared with the placebo group at month 24. MSC treatment possibly accelerates exercise capacity rehabilitation and provides physiologic improvements in severe COVID-19 patients via anti-fibrosis and regenerative pathways derived from the initial biological effects mediated by MSC therapy.24, 25, 26
A few studies have reported 2-year follow-up CT outcomes for COVID-19 patients. In a longitudinal cohort study, 17.5% (10/57) of COVID-19 survivors with abnormal CT results at 12 months achieved complete imaging restoration at 24 months.23 In our previous study, MSC treatment was found to markedly accelerate the resolution of lung lesions one year after treatment. In the present study, 31.7% of the patients had normal chest CT images obtained at month 24. However, no significant difference in the proportion of normal CT images was observed between the MSC and placebo groups at month 24. It is possible that the improvements in lung lesions that were observed in both groups in the present study were due to spontaneous recovery that occurred over time. Thus, the duration of MSC-mediated effects on lung lesions appears to have lasted only one year, and not up to two years. In addition, the assessment method of CT images was partly different from our earlier study, which might lead to the neutral findings. The present results support the need for additional studies of long-term imaging resolution in severe COVID-19 patients.
Patients in the MSC group also exhibited a higher GH score on SF-36 compared with the placebo group. In another study of MSC treatment with 1-year follow-up, a significantly reduced rate of fatigue relief was observed compared with the control group, possibly due to a reduction in malondialdehyde production and regulation of oxidative stress.13 It is also possible that the mechanism(s) mediating symptom alleviation and improved quality of life involve a reduction in neuroinflammation and promotion of regeneration in organs such as the lungs, brain, and heart.
Recent studies further explored whether potential heterogeneity of the patient enrolled contributed to the findings of MSC treatment. In this study, we presented descriptive subgroup results according to age and BMI, which were of exploratory nature. In another randomized controlled trial that was conducted by Bowdish et al., patients younger than 65 y with moderate to severe ARDS exhibited a greater 30-day survival benefit.27 These findings may provide additional preliminary evidence and interesting clues for future research.
It is also important to note that no tumorigenesis or AEs related to MSC treatment were observed in our clinical trial. Moreover, there was a woman in our cohort who gave birth to a healthy baby after undergoing MSC treatment. Thus, our findings indicate that MSC treatment exerted good safety among the severe COVID-19 patients who were examined. We plan to continuously monitor tumorigenesis of this cohort in the future.
There were several limitations associated with the present study. First, despite being one of the largest randomized controlled trials of MSC treatment, future clinical trials with larger sample sizes are needed to validate these preliminary findings. Second, given the large number of tests performed, the drawbacks of multiple comparisons must be fully appreciated. Third, a longer follow-up period inevitably involves the challenge of retaining all of the randomized patients in a cohort. Loss to follow-up can reduce statistical power and bias results, thereby leading to treatment safety or efficacy being overestimated. Therefore, the preliminary findings of the present study need to be interpreted carefully, and exact conclusions remain to be verified in prospectively designed studies with adequate statistical power.
In conclusion, and to the best of our knowledge, the present study represents the longest follow-up of MSC therapy in patients with severe COVID-19. Our results demonstrate long-term safety, yet no significant sustained efficacy, of MSC treatment over 24 months. We plan to continue our follow-up of this cohort. In addition, a well-designed, large-scale phase 3 clinical trial and related mechanism research are needed to determine the efficiency of MSC therapy for patients with COVID-19 or other types of pneumonia.
Contributors
FSW and LS contributed to the conception and design of the study. BZ, HF, LH, ZX, XY and JLF were responsible for acquisition of data. TTL, BZ, YYL, ZRW, ZYZ and MQY were responsible for analysis and interpretation of data. FSW, LS, BZ, HF, TTL, WFX and YGL verified the underlying data. BZ, HF, WQY, CZ, JWS, YZ, TYD, RDB and LLZ was responsible for laboratory testing and assay development. JMC, JHD and JZZ performed image analysis. TTL drafted the manuscript. FSW, LS and MS critically revised the manuscript. All authors revised and approved the final version of the manuscript for submission.
Data sharing statement
After approval from the Human Genetic Resources Administration of China, this trial data can be shared with qualifying researchers who submit a proposal with a valuable research question. A contract should be signed.
Declaration of interests
HF, WQY, YZ and RDB are current employees of Wuhan Optics Valley Zhongyuan Pharmaceutical Co.,Ltd. TYD and LLZ are current employees of Wuhan Optics Valley Vcanbio Cell & Gene Technology Co., Ltd. All authors declare no other conflicts of interest.
Acknowledgements
We thank Le Song, Junli He, Ning Zheng and Guiyue Shen for their excellent work in follow-up of all enrolled patients. We also thank independent statisticians (Yongji Wang, Jing Hu, Qingqing Wang) and academic secretarie (Haibo Dong) for their services. We thank Medjaden Inc. for scientific editing of this manuscript. This study was supported by the National Key Research and Development Program of China (2022YFA1105604, 2020YFC0860900, 2022YFC2304401), the specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202216) and the Fund of National Clinical Center for Infectious Diseases, PLA General Hospital (NCRC-ID202105,413FZT6).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104600.
Contributor Information
Lei Shi, Email: shilei302@126.com.
Fu-Sheng Wang, Email: fswang302@163.com.
Appendix A. Supplementary data
References
- 1.World Health Organization (WHO) Coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Yang X., Hou C., Shen Y., et al. Two-year health outcomes in hospitalized COVID-19 survivors in China. JAMA Netw Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.31790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taquet M., Sillett R., Zhu L., et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatr. 2022;9(10):815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin H., Liu X., Sun H., et al. Sustained abnormality with recovery of COVID-19 convalescents: a 2-year follow-up study. Sci Bull. 2022;67(15):1556–1561. doi: 10.1016/j.scib.2022.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami N., Hayden R., Hills T., et al. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2022:1–15. doi: 10.1038/s41581-022-00642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L., Jiang Y., Zhu M., et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng F., Xu R., Wang S., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Targeted Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaffash Farkhad N., Sedaghat A., Reihani H., et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther. 2022;13(1):283. doi: 10.1186/s13287-022-02920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghayan H.R., Salimian F., Abedini A., et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment. Stem Cell Res Ther. 2022;13(1):365. doi: 10.1186/s13287-022-02953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregoire C., Layios N., Lambermont B., et al. Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.932360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karyana M., Djaharuddin I., Rif'ati L., et al. Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther. 2022;13(1):134. doi: 10.1186/s13287-022-02812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L., Zheng Y., Cheng Z., et al. One-year follow-up study after patients with severe COVID-19 received human umbilical cord mesenchymal stem cells treatment. Stem Cell Res Ther. 2022;13(1):321. doi: 10.1186/s13287-022-02972-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebelatto C.L.K., Senegaglia A.C., Franck C.L., et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13(1):122. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L., Huang H., Lu X., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Targeted Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L., Yuan X., Yao W., et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. eBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 19.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francone M., Iafrate F., Masci G.M., et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New coronavirus pneumonia prevention and control program. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml 7th ed.
- 23.Huang L., Li X., Gu X., et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863–876. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L., Wang L., Xu R., et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Targeted Ther. 2021;6(1):339. doi: 10.1038/s41392-021-00754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R., Feng Z., Wang F.S. Mesenchymal stem cell treatment for COVID-19. eBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H., Li X., Huang L., et al. Lung-function trajectories in COVID-19 survivors after discharge: a two-year longitudinal cohort study. eClinicalMedicine. 2022;54 doi: 10.1016/j.eclinm.2022.101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowdish M.E., Barkauskas C.E., Overbey J.R., et al. A randomized trial of mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome from COVID-19. Am J Respir Crit Care Med. 2023;207(3):261–270. doi: 10.1164/rccm.202201-0157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.