Abstract
Chromosomally mediated AmpC-type β-lactamases are frequently found among Enterobacteriaceae. Hyperproduction of AmpC β-lactamase results in high-level resistance to β-lactam antibiotics. One striking feature of Salmonella is the absence of the structural ampC gene, encoding AmpC β-lactamase, in contrast with other members in the Enterobacteriaceae family, such as Escherichia, Citrobacter, or Enterobacter. The horizontal acquisition of ampC genes is one of the causes of the increased resistance to extended-spectrum cephalosporins and β-lactamase inhibitors among gram-negative rods. Nevertheless, despite the high number of β-lactam-resistant Salmonella isolates so far described, only two strains expressing resistance to cephalosporin and β-lactamase inhibitors which is mediated by AmpC-type enzymes have been found. In this work, data are provided which support the possibility that the maintenance and expression of the ampC gene may represent an unbearable cost for Salmonella in terms of reduction of some of its lifestyle attributes, such as growth rate and invasiveness. The deleterious AmpC burden can be eliminated by decreasing the production of AmpC when both the regulatory gene, ampR, and ampC are present in Salmonella. Thus, it is suggested that the two genes have to be acquired together by Salmonella, leading to an inducible β-lactam resistance phenotype. AmpC synthesis did not produce major variations in the peptidoglycan composition of Salmonella.
Most members of the Enterobacteriaceae family contain chromosomally mediated AmpC-type β-lactamases. Hyperproduced AmpC β-lactamase results in high-level resistance to β-lactam antibiotics and combinations of β-lactams with commercially available β-lactamase inhibitors. Hyperproduction of these penicillin- and cephalosporin-inactivating enzymes is becoming one of the major problems in antimicrobial chemotherapy. Moreover, ampC genes are found more and more frequently to be harbored by plasmids, which may increase the spread of AmpC β-lactamase-mediated resistance among pathogenic bacteria (9). One striking feature of Salmonella is the apparent absence of the structural gene ampC (2, 26), in contrast with other members in the Enterobacteriaceae family that probably share the same ancestor, such as Escherichia or other related organisms, like Citrobacter or Enterobacter. Interestingly, practically all β-lactamase-positive clinical isolates of Salmonella produce class A enzymes. These class A β-lactamases are, generally, well inhibited by the β-lactamase inhibitors in our antibiotic armamentarium.
Acquisition of DNA sequences relevant for virulence properties, such as pathogenicity islands (16), is a common phenomenon that contributes to the evolution of bacterial pathogens. The gain of functions is relevant for any evolutionary process, but loss of functions also may be important. Deletions of parts of chromosomal DNA can be selected because the functions encoded in these regions can be spared in the new environment. A large deletion (of about 190 kb) of the genome (a “black hole”) has recently been found to be involved in the enhanced virulence properties of Shigella and Escherichia coli (25). Interestingly, this black hole included the deletion of the ampC gene in the chromosomes of Shigella flexneri and Shigella dysenteriae. In the case of other Shigella species and an enteroinvasive E. coli strain, a positive hybridization signal with an ampC-specific oligonucleotide was detected. Nevertheless, AmpC activity remains to be demonstrated. In some pathogens, such as Salmonella, intracellular growth is essential for pathogenesis and requires the expression of special genes in addition to those needed for extracellular growth (23). In this extreme situation, the maintenance of all life functions at a minimal cost is essential.
ampC expression is transcriptionally regulated by the divergently read ampR gene. When cell wall degradation is increased by the action of certain β-lactam antibiotics, the amidase activity of AmpD is overloaded, which results in a transient increase in β-lactamase synthesis known as induction (19). The loss of ampD gene function results in constant hyperproduction of AmpC β-lactamase (stable derepression). The inducible synthesis of these enzymes has been reported for Pseudomonas aeruginosa and many members of the Enterobacteriaceae family. Exceptions are E. coli (which lacks the ampR gene) and Salmonella serotypes (which lack both ampR and ampC genes). Interestingly, at least two Shigella species, S. flexneri and S. dysenteriae, also lack the ampC gene, as demonstrated by Maurelli et al. (25). The presence of the ampC gene, together with its exquisite transcriptional regulation (19) in most enterobacterial species, has suggested that AmpC might be involved in cell wall recycling (3).
In this work, we tried to gain insight about whether the maintenance of the ampC gene may have represented an unbearable cost for Salmonella, in terms of reduction of its lifestyle attributes, and thus the possibility of acquiring AmpC-mediated resistance to cephalosporin and β-lactamase inhibitors was decreased. To address this question, we examined the effect caused by the production of the AmpC β-lactamase enzyme from Enterobacter cloacae in a closely related species, Salmonella enterica serotype Typhimurium, by analyzing the impact of the activity of the enzyme on salient salmonella properties, such as replication and invasiveness. Modifications in S. enterica serotype Typhimurium peptidoglycan structure and composition in the presence of the ampC gene have also been studied. Results obtained when both the structural ampC gene and regulatory ampR gene from E. cloacae were present are also discussed.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 1998.)
MATERIALS AND METHODS
Bacterial strains.
Two different derivatives of serotype Typhimurium strain LT2 were used. Serotype Typhimurium LB5010, a galE derivative of LB5000 (8) which is deficient in all three restriction-modification systems, was used as the mediator to transform plasmid DNA into serotype Typhimurium strain SL1344, a mouse-virulent strain (17) used for all infection experiments. The E. coli K-12 strains used were HB101 and MI1443 (4).
Plasmid DNA and genetic manipulation.
All DNA manipulations were performed as described previously (32). To overcome the restriction of the extracted E. coli DNA by serotype Typhimurium strain SL1344, a previous passage through serotype Typhimurium strain LB5010 was needed. Plasmid pBGMHN1 is a pBGS18− (33) hybrid derivative containing the ampC gene from E. cloacae MHN1 (27). The extended-spectrum TEM-24 β-lactamase was used as a control in all the experiments. Plasmid pBGTEM-24 was constructed by subcloning the appropriate restriction fragments from pBGTEM-2 (containing the blaTEM-2 gene), pBGTEM-17 (containing the blaTEM-17 gene) (6), and pBGTEM-5 (containing the blaTEM-5 gene) (7). To ascertain whether the hybrid blaTEM-24 gene was obtained, the whole gene was sequenced to verify that it contained the five changes expected for TEM-24 (Gln39Lys, Glu104Lys, Arg164Ser, Ala237Thr, and Glu240Lys). The expected ceftazidime (CAZ) resistance phenotype was also verified. The ampR-ampC region from E. cloacae MHN1 was PCR amplified and cloned on plasmid pBGS18− to yield plasmid pBGAMPC-R (both genes were resequenced to discard the introduction of mutations during the PCR process). The presence of ampR and ampC allowed the Salmonella strain to display a β-lactam-inducible profile (not shown). Transformants of strain SL1344 containing either pBGTEM-24 or pBGS18− were used as controls. In all cases, transformants were selected on blood agar plates containing kanamycin (KAN, 30 μg/ml) alone or in combination with CAZ (32 μg/ml). To study the effect of the overproduction of the AmpC enzyme from E. coli in Salmonella, the ampC gene from E. coli K-12 strain MC4100 was also PCR amplified, cloned in plasmid pBGS18− to obtain plasmid pBGAMPC-Ec, and introduced into serotype Typhimurium strains. Additionally, all the above-cited plasmids were introduced into E. coli strains HB101 (ampC+) and MI1443 (ampC defective) (4) to observe the effect of multiple ampC copies on E. coli.
Media and antimicrobial agents.
Plates containing 5% sheep blood agar were used in transformation experiments. KAN was purchased from Sigma Chemical Co., St. Louis, Mo. CAZ and gentamicin (GEN) were kindly provided by Glaxo Group Research Ltd. (Greenford, United Kingdom) and by Schering-Plough Research (Bloomfield, N.J.), respectively.
Epithelial cell cultures and bacterial infections.
Madin-Darby canine kidney (MDCK) cells (GIBCO) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS) purchased from BioWhittaker (Walkersville, Md.).
All experiments were performed using freshly obtained serotype Typhimurium SL1344(pBGMHN1). Transformants were plated on blood agar plates containing CAZ (32 μg/ml) and KAN (30 μg/ml). The same antibiotic selection was used for transformants expressing either TEM-24 or AmpC under the control of AmpR. Bacterial cells were resuspended in phosphate-buffered saline (PBS) and used for infection experiments as described below. Monolayers for invasion assays were prepared by seeding MDCK cells in 24-well tissue culture plates and grown overnight to 80% confluence. On the following day, epithelial cells were washed with PBS, and fresh DMEM–10% FBS containing CAZ (32 μg/ml) and KAN (30 μg/ml) was then added. Five microliters (7 × 104 to 8 × 104 bacteria) of the bacterial suspension was then added to infect the cells contained in each well. In parallel, appropriate dilutions of the bacterial suspensions were plated for counting viable bacteria. After 2 h of infection at 37°C in a 5% CO2 atmosphere, epithelial cells were washed twice with PBS, and DMEM–10% FBS containing CAZ (32 μg/ml) and KAN (30 μg/ml) was added. A 2-h treatment with GEN (100 μg/ml) was performed to kill the remaining extracellular salmonellae. After washing of the monolayers with PBS, the invasion level was determined by lysing the infected cells with 0.2 ml of 1% Triton X-100 for 5 to 10 min. The number of viable intracellular bacteria relative to the number added was then calculated by making appropriate dilutions in Luria-Bertani (LB) broth of released viable intracellular bacteria and plating them on LB agar.
Intracellular replication assays were performed as described above, except that after the 2-h GEN treatment cells were washed with PBS and incubated with fresh DMEM–10% FBS for 24 h. CAZ (32 μg/ml) was added to overcome the possible loss of the ampC-containing plasmid, and GEN (10 μg/ml) was added to kill any remaining extracellular bacteria. The intracellular replication level was expressed as the ratio of viable intracellular bacteria after 24 h of GEN treatment and the initial intracellular bacterial inoculum, determined after the 2-h GEN treatment. In all cases, assays were performed in triplicate.
Peptidoglycan analysis of serotype Typhimurium SL1344 overproducing AmpC.
Peptidoglycan of serotype Typhimurium SL1344 transformed with pBGMHN1, pBGTEM-24, or pBGS18− was purified, in all cases, from agar plate-grown bacteria. Bacteria were collected, suspended in 3 ml of PBS, mixed immediately in a 1:1 (vol/vol) proportion with a boiling solution of 8% sodium dodecyl sulfate (SDS) (Bio-Rad), and maintained at 100°C for 18 h. The SDS-insoluble material containing peptidoglycan was processed for high-pressure liquid chromatography analysis as described previously (28) and finally washed until it was free of SDS by successive suspensions in distilled water and high-speed centrifugation (300,000 × g, 10 min) at room temperature. Peptidoglycan was further processed by muramidase (20 μg/ml) digestion at 37°C for 18 h, using the muramidase Cellosyl (Hoechst). The reaction was stopped by incubating the samples in boiling water for 5 min. After this treatment, peptidoglycan was totally digested to muropeptides. Insoluble material was removed by centrifugation (10,000 × g, 10 min), and soluble muropeptides were reduced with NaH4B and frozen at −70°C. Muramidase-digested samples were analyzed by high-pressure liquid chromatography as described previously (14) by using a Hypersil RP18 column (250 by 4 mm; particle diameter, 3 μm) (Teknochroma). Elution buffers were 50 mM sodium phosphate (pH 4.35) (buffer A) and 15% (vol/vol) methanol in 75 mM sodium phosphate (pH 4.95) (buffer B). Elution conditions were as described elsewhere (31), with a flow rate of 0.5 ml/min and a column temperature of 37°C.
Serological tests.
Somatic (O) and flagellar (H) antigenic profiles were determined for serotype Typhimurium SL1344 carrying different genetic constructions in order to detect any possible variation or even loss of any of these antigens. Tests were performed as previously described (12) using Bacto Salmonella O antisera and Spicer-Edwards Salmonella H antisera purchased from Difco Laboratories (Detroit, Mich.).
Determination of β-lactamase specific activity.
The β-lactamase specific activities of serotype Typhimurium SL1344(pBGMHN1), E. coli MI1443 (pBGMHN1), E. coli HB101(pBGMHN1), and E. cloacae RYC12991-2 (a clinical isolate expressing derepressed AmpC synthesis) were determined as described elsewhere (10, 11). Serotype Typhimurium SL1344 was used as the negative control (no β-lactamase production). In each case, cell lysates were obtained by ultrasonication of exponentially growing cultures at 37°C in LB broth. The specific enzyme activity of each extract was determined by measuring the hydrolysis of a 100 μM cephaloridine (Glaxo Group Research Ltd.) solution prepared in 0.1 M phosphate buffer (pH 7.2), monitored at 25°C, with a Uvikon-940 spectrophotometer at a wavelength of 255 nm. Protein concentration was measured by the method of Lowry et al. (24). The values below, expressed as micromoles of cephaloridine hydrolyzed per minute per milligram of protein, were obtained in triplicate experiments.
Effect of cell tissue culture medium and Triton X-100 on serotype Typhimurium SL1344 viability.
Known amounts of AmpC- and TEM-24-expressing serotype Typhimurium cells were inoculated in plates containing MDCK cells in DMEM with serum under the same conditions as described for the invasion experiments. Two hours later, bacterial cells were recovered from the culture medium, and the number of CFU was determined. To test the susceptibility of AmpC- and TEM-24-expressing S. enterica serotype Typhimurium to Triton X-100, known amounts of bacterial cells were incubated in PBS–Triton X-100 under the same conditions as used for recovering bacteria from inside MDCK cells. However, in this control experiment, incubation with the detergent was prolonged to 1 h instead of the 15-min incubation time used for the invasion tests.
RESULTS
Effect of AmpC production on Salmonella colony morphology and cell size.
Figure 1 shows the colony morphology of serotype Typhimurium SL1344 producing either the TEM-24 or AmpC β-lactamase. Bacterial cells producing TEM-24 formed colonies indistinguishable from those of serotype Typhimurium SL1344 harboring the control plasmid pBGS18− alone (not shown). Nevertheless, when bacteria produced the AmpC β-lactamase, colonies were flattened and rough. When the regulatory ampR gene was introduced into the cell together with ampC, colonies recovered a single and stable morphology, even without antibiotic pressure (data not shown). In addition, when cells obtained from an LB liquid culture (see next paragraph) were Gram stained and microscopically observed, those expressing TEM-24 exhibited the size and cell shape expected for this strain (identical to cells containing the vector pBGS18− alone). In the case of bacteria containing ampC, cells were larger and in many cases appeared as “diplobacilli,” suggesting that septation and/or segregation of daughter cells was affected. Results obtained with Salmonella strains containing the ampC gene from E. coli strain MC4100 were identical to those for strains containing ampC from E. cloacae, i.e., colonies were flattened and rough and cells were larger and appeared as diplobacilli.
FIG. 1.
Colony morphology of serotype Typhimurium SL1344 producing either TEM-24 (left) or AmpC (right) after 48 h of incubation in blood agar plates at 37°C.
Effect of AmpC production on Salmonella growth rate.
Serotype Typhimurium SL1344 growth rates, measured by changes either in optical density at 600 nm or in viable count, were nearly identical for the variants harboring either the vector plasmid pBGS18− or the same plasmid with the TEM-24 β-lactamase-encoding gene (Fig. 2A). Thus, the hyperproduction of a TEM-derived β-lactamase had no apparent biological cost for Salmonella under the present experimental conditions. The behavior of Salmonella in complex media is probably more predictive of the clinical situation than its behavior in minimal media, where the production of TEM-derived β-lactamases may have a certain cost (F. Baquero and J. Blázquez, unpublished results). The fact that many Salmonella strains are TEM β-lactamase producers in the clinical setting suggests the absence of cost, even though the presence of a compensatory mutation cannot be ruled out.
FIG. 2.
Growth curves represented by both optical density (OD) at 600 nm (filled symbols) and viable counts (open symbols) of serotype Typhimurium SL1344 harboring pBG18− (⧫) or pBGTEM-24 (■) (A) and pBGMHN1 (▴) or pBGAMPC-R (●) (B). Values on the x axes are hours of incubation.
Interestingly, when the ampC gene was introduced in place of blaTEM-24 in the plasmid pBGS18−, a dramatic drop in the viable cell count of serotype Typhimurium SL1344 occurred, apparently starting at the late exponential phase. Growth, as measured by the optical density of cultures, stopped when the decrease in cell viability started (Fig. 2B). The absence of reduction in optical density when viable counts became low indicates the absence of lysis and the eventual formation of a certain number of filaments, cells with increased size, and diplobacilli (which can be visualized with conventional microscopy). Nevertheless, it is hard to conclude that these phenomena can account for the observed huge reduction in viable counts. It seems more likely that reduction of viable counts (CFU) may be due to massive death (without cell lysis) or to an unrecoverable loss of the ability to grow under our experimental conditions. A similar behavior was observed when experiments were conducted in medium containing CAZ, indicating that during this period of time bacteria express the ampC gene. These effects should be attributed to the production of AmpC β-lactamase in Salmonella, since with the construction harboring both the ampC and regulatory ampR genes and hence producing smaller amounts of AmpC, the cultures behaved as the control cultures (Fig. 2B).
Effect of AmpC production on Salmonella invasion rates and intracellular replication.
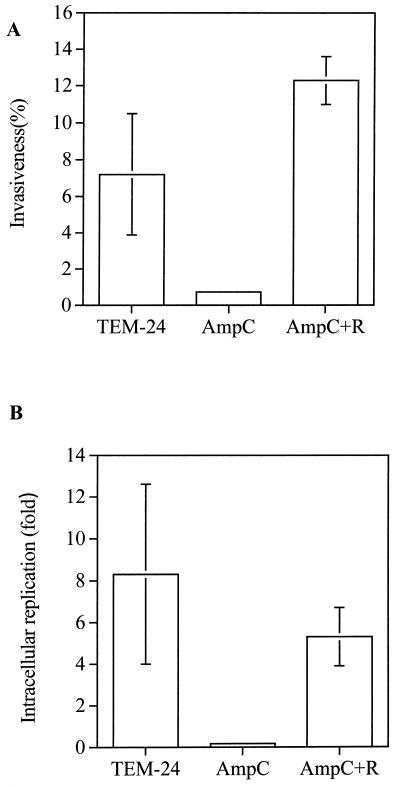
The influence of AmpC on growth and viable count could be an effect occurring only in artificial culture media. To gain some insight into the influence of ampC expression on the natural lifestyle of Salmonella, the invasion rates and intracellular replication of serotype Typhimurium SL1344, producing either AmpC or TEM-24, were studied in MDCK cells. The results, shown in Fig. 3, demonstrate that the expression of ampC in serotype Typhimurium SL1344 clearly produced a decrease in invasion rate compared with that of the same strain expressing blaTEM-24 (mean ± standard deviation, 0.73% ± 0.18% versus 7.19% ± 3.31%). A significant decrease in intracellular replication was also observed when ampC was present, compared to that observed when blaTEM-24 was present (0.17- ± 0.06-fold versus 8.3- ± 4.3-fold). The addition of the regulatory gene ampR in ampC-containing Salmonella restored the normal behavior of the strain (invasion rate, 12.3% ± 1.3%; intracellular replication, 5.3- ± 1.4-fold). Serotype Typhimurium SL1344 carrying both genes displayed the same β-lactam AmpC-inducible profile as other members of the Enterobacteriaceae.
FIG. 3.
Invasion rate (invasiveness) and intracellular replication 24 h after invasion by serotype Typhimurium SL1344 expressing TEM-24, AmpC, or AmpC and AmpR (AmpC+R). Standard deviations for AmpC were below the scale of the drawing.
Under the experimental conditions of the present study and in the absence of antibiotic pressure, part of the population of serotype Typhimurium SL1344 harboring ampC (CAZ MIC, 128 μg/ml) reverted to lower CAZ resistance levels (CAZ MIC, 8 to 4 μg/ml) and even to complete loss of β-lactam resistance (CAZ MIC, 0.06 μg/ml). To prevent such variation, invasion rate and intracellular replication experiments were always performed in the presence of CAZ, as described in Materials and Methods.
Effect of AmpC production on Salmonella peptidoglycan composition.
The peptidoglycan composition of serotype Typhimurium SL1344 carrying plasmid pBGS18−, pBGTEM-24, or pBGMHN1 is detailed, for each case, in Table 1. The muropeptide composition of peptidoglycan in resting serotype Typhimurium SL1344 cells was almost identical to that reported for E. coli cells in stationary phase (30), as would be expected for cells growing on plates. Specific peptidoglycan alterations shared by S. enterica serotype Typhimurium and E. coli cells under these specific growth conditions were found: high relative percentage of cross-linked muropeptides (37.2%), high relative proportion of l-d peptide cross-linked bridges (9.0%), and high content of lipoprotein-bound muropeptides (12.1%). No major qualitative differences were found in the peptidoglycan from the strain, in spite of the production of AmpC β-lactamase. However, some small quantitative changes (Table 1) in the levels of l-d dimers (7.3%), lipoprotein-bound muropeptides (8.3%), and anhydrous muropeptides (4.9%) were consistently found when the SL1344 strain produced the AmpC enzyme. Also, an increase in the level of cross-linked muropeptides (39.9%) was found in the TEM-24 β-lactamase-producing strain. These small changes found in the level of some peptidoglycan constituents of Salmonella producing AmpC are not easily attributable to the recently confirmed dd-carboxypeptidase activity of AmpC (J. Ayala, unpublished data), but such an effect cannot be ruled out.
TABLE 1.
Muropeptide composition of peptidoglycan from serotype Typhimurium strain SL1344 carrying pBGS18− and derivatives
| Sampleb | Relative abundance (mol%)a
|
Cross-linkage (%)c | |||||
|---|---|---|---|---|---|---|---|
| Monomers | Dimers
|
Trimers | Lpp | Anhydrous forms | |||
| d-d | l-d | ||||||
| SL1344(pBGS18−) | 65.5 ± 0.6 | 22.7 ± 0.6 | 9.0 ± 0.5 | 2.8 ± 0.4 | 12.1 ± 0.6 | 3.9 ± 0.3 | 37.2 ± 0.3 |
| SL1344(pBGTEM-24) | 63.4 ± 0.3 | 23.4 ± 0.3 | 9.9 ± 0.2 | 3.3 ± 0.2 | 13.1 ± 0.3 | 9.1 ± 0.4 | 39.9 ± 0.4 |
| SL1344(pBGMHN1) | 68.1 ± 0.5 | 22.6 ± 0.4 | 7.3 ± 0.3 | 1.9 ± 0.3 | 8.3 ± 0.6 | 4.9 ± 0.5 | 33.8 ± 0.5. |
Muropeptides are grouped according to structural similarities as described previously (14). Values are means and standard deviations from three independent experiments. d-d, cross-linked by a d-d peptide bridge; l-d, cross-linked by an l-d peptide bridge; Lpp, muropeptides bound to the C-terminal dipeptide (Arg-Lys) of Braun's lipoprotein.
Peptidoglycan of serotype Typhimurium SL1344 transformed with the indicated plasmids.
Proportion of cross-linked peptide chains.
Effects of cell tissue culture medium and Triton X-100 on serotype Typhimurium SL1344 viability.
The inability of ampC-expressing S. enterica serotype Typhimurium to invade cells might be due to a reduced viability of those cells, either in the cell tissue culture medium prior to infection or in the PBS–Triton X-100 solution used to recover viable bacteria from inside epithelial MDCK cells. To test these possibilities, three control experiments were performed. In the first one, no differences in invasiveness were observed between AmpC- and TEM-24-producing bacteria. This finding indicates that the low invasion yields observed for AmpC-producing S. enterica serotype Typhimurium are not the consequence of a defective behavior in cell culture medium during the steps previous to invasion. Alternatively, ampC-expressing bacteria could be more susceptible to detergents than blaTEM-24-expressing bacteria. Nevertheless, neither AmpC- nor TEM-24-producing S. enterica serotype Typhimurium showed any decrease in viability upon incubation with the detergent for prolonged periods of time. This result demonstrates that ampC-expressing S. enterica serotype Typhimurium cells are not more susceptible to detergents than blaTEM-24-expressing ones. On the other hand, ampC-expressing S. enterica serotype Typhimurium might be more susceptible to detergents only under invasion conditions. To analyze this possibility, invasion experiments were performed, and the number of CFU recovered from MDCK cells was determined after 10 min of incubation with Triton X-100 solution and upon 1 h of further incubation in the presence of the detergent. No changes in CFU were observed in any case, indicating that cell invasion does not induce a detergent-susceptible phenotype for ampC-expressing S. enterica serotype Typhimurium. Altogether, these results indicate that the low invasion yield of ampC-expressing S. enterica serotype Typhimurium is not an artifactual result due to the methods used for testing invasiveness.
Serological tests.
It can be speculated that a variation in antigenic components derived from the presence of the ampC gene or its product would condition a lower invasive capacity of these Salmonella strains. Indeed, many lipopolysaccharide-deficient (rough) pathogens frequently lose their virulence. We tested whether AmpC-producing strains of serotype Typhimurium SL1344 failed to attach O antigen to the lipopolysaccharide. No variation in either the somatic or flagellar antigenic profile was observed for any serotype Typhimurium SL1344 strains carrying the different ampC genetic constructions (pBGS18−, pBGMHN1, and pBGAMPC-R). The antigenic formula was the same in all cases as for the wild-type serotype Typhimurium SL1344 strain: 4,5:i:1,2, which corresponds to serotype Typhimurium.
β-Lactamase specific activity.
In our assays, the observed effects of AmpC β-lactamase production on Salmonella may be considered to be of an unspecific nature. Abnormally high protein production may collapse the transcriptional-translational and/or bacterial export machinery, resulting in multiple unspecific deleterious effects. In our case, this high AmpC production may depend on the high copy number of plasmid pBGS18−. In an attempt to correlate the level of AmpC production with its putative role in phenotype variation and to compare this level with that of E. coli K-12 strains HB101(pBGMHN1) and MI1443(pBGMHN1) and a stable clinical β-lactamase hyperproducer E. cloacae strain, we determined the respective specific β-lactamase activities. The specific AmpC β-lactamase activities of serotype Typhimurium SL1344(pBGMHN1), E. coli HB101(pBGMHN1), E. coli MI1443 (pBGMHN1), and E. cloacae RYC12991-2 were, respectively, 103 ± 5, 174 ± 4, 118 ± 6, and 367 ± 15 μmol min−1 mg−1. E. cloacae RYC12991-2 showed a β-lactamase specific activity three times higher than those of serotype Typhimurium SL1443(pBGMHN1) and E. coli MI1443(pBGMHN1) and two times higher than that of E. coli HB101(pBGMHN1). Despite this high β-lactamase production, E. cloacae strain RYC12991-2 showed a colony morphology, cell size, and growth rate that were indistinguishable from those of its repressed isogenic strain (data not shown). Similarly, E. coli strains HB101(pBGMHN1) and MI1443(pBGMHN1), with AmpC specific activities very close to that of serotype Typhimurium SL1443(pBGMHN1), showed no differences in these parameters from their isogenic strains, HB101(pBGS18−) and MI1443(pBGS18−), which lack the ampC-MHN1 gene.
DISCUSSION
The above results show that expression of ampC (cloned from either E. cloacae MNH1 or E. coli MC4100) affected Salmonella colony morphology, cell size, and growth rate. Variations in invasion rates and intracellular replication were also observed when Salmonella cells expressed ampC from E. cloacae. These effects were fully reversed when the regulatory gene ampR was introduced into serotype Typhimurium SL1344 together with ampC. ampC expression did not affect significantly the peptidoglycan composition or the surface antigen profile. Our data also show that these results cannot be considered methodological artifacts.
The effects of AmpC β-lactamase production on colony morphology, cell size, and growth rate were not detected in E. coli K-12 strains HB101 and MI1443, containing plasmid pBGMHN1 or pBGAMPC-Ec (data not shown). Moreover, a very high production of AmpC [over three times that of serotype Typhimurium SL1344(pBGMHN1)] in a derepressed strain of E. cloacae did not significantly affect these parameters. These results indicate that these phenomena are produced exclusively in S. enterica serotype Typhimurium.
A large deletion (black hole) in the genomes of Shigella and enteroinvasive E. coli has recently been described as being responsible for their enhanced virulence properties (25). This deletion eliminated some genes, including cadA, whose product inhibits Shigella virulence. In the adaptive process, it is expected that deletions would be favored if they eliminate not only one specific detrimental gene but also other genes in the same region whose products may also be detrimental for the pathogenic lifestyle. In this regard, it should also be possible to identify small specific deletions that include one or a small number of detrimental genes. This may be the case for the ampC gene in Salmonella. Thus, we now propose the existence of “black points,” or small deletions, in contrast to the large deletions, or black holes.
Some hypotheses can be drawn from this observation. The regulatory ampR gene, probably present in E. coli and Salmonella ancestors, may have been lost, possibly by a homologous recombination event (18) at the time of the divergence of the Escherichia-Salmonella group from the rest of Enterobacteriaceae. At this stage, another mechanism controlling AmpC production (attenuation) was sufficient to maintain the production of the β-lactamase at low cost. Divergence between E. coli and Salmonella took place nearly 100 million years ago (22) with the acquisition of the SPI-1 pathogenicity island (15), enabling Salmonella to exploit new habitats. Invasion of novel habitats can result in rapid rates of evolutionary divergence (29), including the acquisition of novel pathogenicity determinants and/or the deletion of parts of the chromosome (25). Under these new conditions, a functional interference between AmpC production and pathogenicity may have occurred, with evolutionary loss of the β-lactamase-encoding gene. An additional burden is the physiological overexpression of ampC in certain growth phases (20, 28). The almost absolute absence of published reports about natural isolates of Salmonella that express AmpC β-lactamases supports our results. Only two communications describing the presence of an ampC gene in clinical strains of S. enterica serotype Enteritidis (13) and S. enterica serotype Senftenberg (21) have been published. In all cases, the type C β-lactamases were plasmid encoded. One of them indicated the inducible nature of AmpC production and demonstrated the presence of a regulatory ampR gene on the plasmid; such circumstances cannot be ruled out in the other case.
A search of the S. enterica serotype Typhi DNA sequence database (Sanger Center), using the known sequence of all pbp-like genes in E. coli, has shown that all of the sequences were present (the degree of identity at the nucleotide level was in all cases higher than 80%) in the former microorganism, with the exception of ampC. The presence of an ampC locus near min 94 in the genetic map of Sanderson et al. (32a) was expected, due to homology between the microorganisms and the presence of chromosomal β-lactamases of class C in other enterobacteria, but the analysis of the nucleotide sequence clearly contradicts this prediction. When the search of pbp-like genes was conducted in the unfinished genomes at the Washington University Salmonella Project, no gene homologous to E. coli ampC was found in any of the following related microorganisms: S. enterica serotype Typhimurium, S. enterica serotype Paratyphi, and Klebsiella pneumoniae. As sequencing of these genomes is not yet finished, we interpret this result cautiously. However, all other genes homologous to E. coli pbp1a, -1b, -2, -3, -4, -4*, -5, -6, and -6b and ampH were found in serotype Typhimurium. Also, related genes at the dcw cluster of E. coli (ftsW, ftsZ, and mraW) were found in serotype Typhimurium, serotype Paratyphi, and K. pneumoniae.
The molecular mechanisms involved in AmpC interference with Salmonella replication and intracellular penetration remain to be explored. The observed larger cells, the diplobacilli, and the filaments produced by ampC overexpression may reduce the abilities for cell internalization. The same mechanism that produces a reduction in viable cells at late exponential phase in LB cultures may be responsible for the observed reduction in intracellular replication.
Lack of the ampC gene may also have important implications for antibiotic treatment of Salmonella infections. Chromosomally mediated AmpC-type β-lactamases are frequently found among Enterobacteriaceae, and hyperproduced AmpC β-lactamase results in high-level resistance to β-lactam antibiotics. Inhibitors of β-lactamases (clavulanic acid, sulbactam, and tazobactam) are being increasingly used in clinical practice. Unfortunately, class C chromosomally mediated enzymes, such as AmpC β-lactamase, are poorly inhibited by these compounds. Indeed, hyperproduction of these penicillin- and cephalosporin-inactivating enzymes is one of the major problems in antimicrobial chemotherapy. Moreover, plasmids carrying ampC genes are being found more and more frequently and this may increase the spread of AmpC β-lactamase-mediated resistance among pathogenic bacteria (9). Interestingly, practically all (see above) β-lactamase-positive clinical isolates of Salmonella produce class A enzymes.
Acquisition of antibiotic resistance determinants might have some fitness cost for bacteria (1). In fact, antibiotic-resistant serotype Typhimurium was less virulent in an in vivo model (5). Herein, we demonstrate that acquisition of AmpC-encoding plasmids also produces a biological cost for S. enterica serotype Typhimurium in an in vitro system.
Salmonella must cope not only with antimicrobial selective pressure but also with the selection pressure imposed by its particular intracellular lifestyle. Because a high production of AmpC is deleterious, in the absence of regulation Salmonella has to acquire the ampR and ampC genes together. It is expected that both genes are less easily acquired than one of the abundant blaA genes, encoding class A β-lactamases. Again, the almost absolute absence of published communications on natural isolates of Salmonella producing AmpC β-lactamases supports our hypothesis. Thus, the β-lactam–β-lactamase inhibitor combination is expected to be active in most cases. Nevertheless, because of the high genetic variability of bacteria, we cannot discard the emergence of virulent Salmonella variants containing compensatory mutations enabling them to produce AmpC enzymes in the absence of AmpR regulation under β-lactam–β-lactamase inhibitor pressure. In fact, one such variant has been obtained in our laboratory after successive passages of serotype Typhimurium SL1344(pBGMHN1) in media containing β-lactam antibiotics. This variant is currently under study in our laboratory to determine the molecular mechanism involved in the AmpC deleterious effect.
ACKNOWLEDGMENTS
We thank J. C. Galán and M. C. Negri for helpful discussions, F. García del Portillo for providing the SL1344 and LB5010 Salmonella strains, and L. de Rafael and J. Andrew for English corrections.
This work was supported in part by a grant from Lilly Spain.
REFERENCES
- 1.Andersson D I, Levin B R. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 2.Bergström S, Lindberg F P, Olsson O, Normark S. Comparison of the overlapping frd and ampC operons of Escherichia coli with the corresponding DNA sequences in other gram-negative bacteria. J Bacteriol. 1983;155:1297–1305. doi: 10.1128/jb.155.3.1297-1305.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop R E, Weiner J H. Coordinate regulation of murein peptidase activity and AmpC β-lactamase synthesis in Escherichia coli. FEBS Lett. 1992;304:103–108. doi: 10.1016/0014-5793(92)80598-b. [DOI] [PubMed] [Google Scholar]
- 4.Bishop R E, Weiner J H. Complementation of growth defect in an ampC deletion mutant of Escherichia coli. FEMS Microbiol Lett. 1993;114:349–354. doi: 10.1111/j.1574-6968.1993.tb06597.x. [DOI] [PubMed] [Google Scholar]
- 5.Björkman J, Hughes D, Andersson D I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:3949–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazquez J, Morosini M-I, Negri M-C, Gonzalez-Leiza M, Baquero F. Single amino acid replacements at positions altered in naturally occurring extended-spectrum TEM β-lactamases. Antimicrob Agents Chemother. 1995;39:145–149. doi: 10.1128/aac.39.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blázquez J, Negri M-C, Morosini M-I, Gómez-Gómez J M, Baquero F. A237T as a modulating mutation in naturally occurring extended-spectrum TEM-type β-lactamases. Antimicrob Agents Chemother. 1998;42:1042–1044. doi: 10.1128/aac.42.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullas L R, Ryu J-I. Salmonella typhimurium LT2 strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K. Characterization of β-lactamases. Antimicrob Agents Chemother. 1989;33:259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush K, Sykes R B. Methodology for the study of β-lactamases. Antimicrob Agents Chemother. 1986;30:6–10. doi: 10.1128/aac.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewing W H. Edwards and Ewing's identification of Enterobacteriaceae. 4th ed. New York, N.Y: Elsevier Science Publishing Co.; 1986. [Google Scholar]
- 13.Gaillot O, Clément C, Simonet M, Philippon A. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother. 1997;39:85–87. doi: 10.1093/jac/39.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Glauner B, Holtje J V, Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 15.Groisman E A, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5:343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 16.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Honoré N, Nicolas M H, Cole S T. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 1986;13:3709–3714. doi: 10.1002/j.1460-2075.1986.tb04704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs C, Frère J M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 20.Jaurin B, Grundström T, Edlund T, Normark S. The E. coli β-lactamase attenuator mediates growth rate-dependent regulation. Nature. 1981;290:221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- 21.Koeck J L, Arlet G, Philippon A, Basmaciogullari S, Thien H V, Buisson Y, Cavallo J D. A plasmid-mediated CMY-2 β-lactamase from an Algerian clinical isolate of Salmonella senftenberg. FEMS Microbiol Lett. 1997;152:255–260. doi: 10.1111/j.1574-6968.1997.tb10436.x. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence J G, Ochman H. Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci USA. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung K Y, Finlay B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Maurelli A T, Fernández R E, Bloch C A, Rode C K, Fasano A. “Black holes” and bacterial pathogenicity: a large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc Natl Acad Sci USA. 1998;95:3943–3948. doi: 10.1073/pnas.95.7.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotic. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 27.Morosini M I, Negri M C, Shoichet B, Baquero M R, Baquero F, Blázquez J. An extended-spectrum AmpC-type β-lactamase obtained by in vitro antibiotic selection. FEMS Microbiol Lett. 1998;165:85–90. doi: 10.1111/j.1574-6968.1998.tb13131.x. [DOI] [PubMed] [Google Scholar]
- 28.Normark S, Grundström T. Initiation of translation makes attenuation of ampC in E. coli dependent on growth rate. Mol Gen Genet. 1985;198:411–415. doi: 10.1007/BF00332931. [DOI] [PubMed] [Google Scholar]
- 29.Orr M R, Smith T B. Ecology and speciation. Trends Ecol Evol. 1998;13:502–506. doi: 10.1016/s0169-5347(98)01511-0. [DOI] [PubMed] [Google Scholar]
- 30.Pisabarro A G, de Pedro M A, Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985;161:238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintela J C, De Pedro M A, Zöllner P, Allmaier G, García del Portillo F. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol Microbiol. 1997;23:693–704. doi: 10.1046/j.1365-2958.1997.2561621.x. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32a.Sanderson K E, Hessel A, Rudd K E. Genetic map of Salmonella typhimurium, edition VIII. Microbiol Rev. 1995;59:241–303. doi: 10.1128/mr.59.2.241-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spratt B G, te Hedge P J, Heesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]