Abstract
The use of therapeutic cannabis preparations in Friuli Venezia Giulia is increasingly expanding. Even if cannabis oil finds its applications in several disorders affecting adults and children, it is not yet a standardized product and, to ensure the quality of the preparation, a quantitative analysis must be carried out before dispensing it to patients. Gas chromatography coupled to mass spectrometry (GC-MS) is a frequently used technique for quantification of cannabinoids, the active compounds of C. sativa. In this context, we developed a GC-MS method for the simultaneous quantification of 7 cannabinoids (CBD, CBDA, CBG, CBN, THCA, THCV and Δ9-THC) that is not time and sample consuming: 10 μL of cannabis oil were used for the sample preparation, that consists in derivatization of analytes through silylation. Calibration curves were built from 0.2 to 2 μg/mL. The percentage of accuracy and precision did not exceed the values recommended by validation guidelines. The limit of detection was 0.01 μg/mL; whereas the lower limit of quantification was 0.2 μg/mL. There was no carry over. The proposed GC-MS method showed good sensitivity, specificity, linearity, accuracy, precision and applicability to therapeutic preparations.
Keywords: Cannabis oil, Cannabinoids, Validation, Gas chromatography, Mass spectrometry
1. Introduction
When we talk about cannabis for medical use, we refer to hemp grown for medical purposes, with plants selected and carefully cultivated with standardized and controlled processes [1]. The inflorescences of Cannabis sativa L. contain several active compounds, including C21 terpeno-phenolic cannabinoids [2]. In detail, cannabinoids are present in their carboxylic acid form in plants, even if they can be readily decarboxylated in their active neutral form through heating processes [3,4].
The most abundant compounds delta-9 tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), that derive from their acid forms tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), have therapeutic effects: psychotropic effects are linked to the use of cannabis with high concentrations of Δ9-THC; whereas preparations with high CBD content have analgesic and antioxidant activity [5,6]. Depending on the varieties of C. sativa, the concentrations of the active compounds vary [2].
Other cannabinoids present in inflorescences and therapeutic cannabis are cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN) and tetrahydrocannabivarin (THCV) [3,7]. Despite evidence from in vitro pharmacological assay on the therapeutic potential of CBC and from its pharmacokinetics [8,9], its efficacy on humans have not yet been examinated, contrary to the other cannabinoids mentioned, whose role has been or is currently investigated [10,11] (clinical trial identifier: NCT05344170).
In Italy, since 2015, it is possible to administer cannabis preparations for therapeutic use for specific clinical indications such as chronic pain related to multiple sclerosis; nausea and vomiting induced by chemotherapy, radiotherapy and antiretroviral therapy; as appetite stimulant in anorexia in cancer patients or those suffering from AIDS; to reduce intraocular pressure in glaucoma; and for the reduction of involuntary movements in Gilles de la Tourette syndrome [[12], [13], [14]]. Furthermore, even if its use in pediatrics is not yet approved in Italy, there is growing evidence about the beneficial effect of therapeutic cannabis in epilepsy (in particular Dravet syndrome), in chemotherapy-induced nausea and vomiting, as well as in neurodevelopmental and neuropsychiatric disorders, after administration in pediatric patients [[15], [16], [17], [18], [19]].
In general, the medical use of cannabis has greatly increased in Italy and in 2021 about 1270 kg of inflorescences were sold. Friuli Venezia Giulia (FVG) is included among the regions with the lowest consumption, with its 22.1 kg used in 2021 [20].
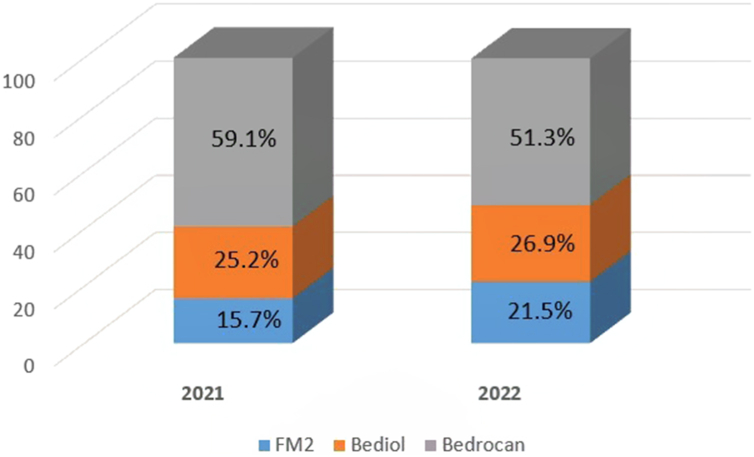
Taking into account that cannabis oil is dispensed free of charge only since August 2021, the use of this formulation in FVG is increasingly expanding and in the first half of 2022 it made up 28% of all cannabis formulations reimbursed [20]. In particular, cannabis oil extracted from inflorescences with high Δ9-THC content (Bedrocan) is the most commonly used, even if its use decreased since 2021 (59.1%) to 2022 (51.3%), because of an increased use of intermediate Δ9-THC content inflorescences (FM2 and Bediol) (Fig. 1) [20].
Fig. 1.
Percentage of annual prescriptions of cannabis oil by variety of inflorescence.
Among cannabis preparations, cannabis oil is not yet a defined and standardized product and, to ensure the quality of the preparation, a quantitative analysis must be carried out before dispensing it to the patient. Among the techniques used to quantify cannabinoids, gas chromatography (GC-MS) or liquid chromatography (LC-MS) coupled with mass spectrometry are used [[21], [22], [23]]. The GC-MS technique is frequently used to quantify cannabinoids even if, contrary to LC-MS that allows a direct analysis of compounds, derivatization is required [21].
In this study, we propose a validated GC-MS method for the simultaneous quantification of 7 cannabinoids in cannabis oil used for therapeutic purposes.
2. Materials and methods
2.1. Chemicals and reagents
Analytical standards 1000 μg/mL CBD (purity 99.6%), 100 μg/mL CBG (purity 99%), 1000 μg/mL CBN (purity 100%), 1000 μg/mL Δ9-THC (purity 99.2%), 100 μg/mL THCV (purity 95%) in methanol, as well as 100 μg/mL CBDA (purity 98.5%) and 1000 μg/mL THCA (purity 99.2%) in acetonitrile, were purchased from LGC Standards Srl (Milan, Italy). The concentration expressed in molarity of analytical standards is reported in Supplementary materials. Certified internal standards (IS) mixture (CBD-d3, CBN-d3 and Δ9-THC-d3 in methanol) at unknown concentration was purchased from Eureka Lab Division (Ancona, Italy). Solutions were stored at −20 °C.
Diethyl ether, methanol and N,O-bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (BSTFA:TMCS, 99:1, v/v) were purchased from Sigma Aldrich (Milan, Italy).
2.2. Preparation of calibrators and quality controls samples
A working solution (4 μg/mL of CBD, CBDA, CBG, CBN, THCA, THCV and Δ9-THC) for the preparation of the calibrators (CALs) was prepared daily by proper dilution of each analytical standard stock solution. The calibration curve was built from 0.2 to 2 μg/mL (n = 6) on the basis of the expected range of measurements and on the sensibility of the instrument [2].
To avoid biases of estimation, a working solution (4 μg/mL of CBD, CBDA, CBG, CBN, THCA, THCV and Δ9-THC) for the preparation of the quality controls (QCs) was prepared daily from stock solutions and separately from that used for the preparation of CALs. Taking into account the calibration range and the validation guidelines, 4 QCs with different levels of concentration were prepared (0.2, 0.5, 0.7 and 1.2 μg/mL).
Keeping the concentrations of CALs and QCs the same for all the cannabinoids tested limited bias in concentration due to minor impurities in the reference standards and to potential cannabinoid inter-conversion.
2.3. Tested materials
Cannabis oil samples used in this study were leftover from diagnostics routine analysis performed at Advanced Translational Diagnostics Laboratory of IRCCS Burlo Garofolo of Trieste. In particular, they were prepared from pharmacies of FVG area according to the harmonized extraction methods defined by the Italian Society of Compounding Pharmacists (SIFAP) [4]. The protocol of extraction comprises heating cannabis (Bedrocan, FM2 or Bediol varieties) to obtain decarboxylation and maceration in olive oil [4]. Moreover, an internal control, consisting in pure commercial CBD in olive oil (100 mg/mL), prepared from the hospital pharmacy of Burlo Garofolo, was introduced to test daily the accuracy of the analysis.
2.4. Sample preparation
Cannabis oil was centrifuged at 15000×g for 10 s in order to precipitate residual inflorescences. Ten μL of cannabis oil (10.5 mg weighted) were diluted in 990 μL of diethyl ether (1:100 dilution) and vortexed. Again, the sample was diluted 1:100 in diethyl ether and vortexed. This dilution was performed because cannabis oil samples were very concentrated and in order to cope with the high sensibility of the instrument.
Sonication of the sample for 5 min three times after dilution was also tested to evaluate if it could help the dissolution of analytes.
Fifty μL of IS mixture were added to 100 μL of cannabis oil sample/calibrator/QC and were evaporated to dryness under a gentle stream of nitrogen. Once dryness was obtained, 100 μL of BSTFA:TMCS and 30 μL of pyridine were added to the dried residue and vortexed for 30 s. The solution was heated on a thermoblock at 60 °C for 20 min to obtain derivatization of the analytes through silylation. The same procedure without the addition of pyridine and with different time of incubation was tested.
2.5. Instrumentation
Analyses were performed with a HP6890 gas chromatograph (Hewlett-Packard, Roma, Italia) combined with a 7683 series Injector (Agilent, Milan, Italy) coupled with a HP 5973 quadrupole mass spectrometer (Hewlett-Packard, Roma, Italia).
2.6. Method development
2.6.1. GC conditions
Two μL were injected in the instrument with a split ratio of 1:10. The temperature of the injection port and of the transfer line was set at 280 °C. A capillary column supplied by J&W Scientific (30 m × 0.25 mm I.D., 0.25 μm film thickness with 5% phenylmethylsiloxane) was used to achieve separation. Helium, the carrier gas, was set at a constant flow of 1 mL/min for the total run time (26 min). Moreover, the oven temperature was initially set at 60 °C and rose with a temperature ramp of 10 °C/min till 300 °C; this temperature was held for 2 min.
2.6.2. MS conditions
The temperature of the ion source and the quadrupole was set respectively at 220 and 150 °C. The electron ionization mode was positive. Solvent delay was set at 5.30 min. The mass spectrometer operated in selected ion monitoring.
2.7. Method validation
Validation of the analytical method was performed according to the most recent International Council for Harmonisation (ICH) guidelines (ICH guideline M10 on bioanalytical method validation and ICH guideline Q2(R2) on validation of analytical procedures).
2.8. Data analyses
Data were analyzed using Agilent G1701DA GC/MSD ChemStation (Agilent, Milan, Italy).
3. Results and discussion
3.1. Method development
3.1.1. GC conditions
Comparing to other analytical methods available in literature that have exploited LC-MS technique [[23], [24], [25], [26]], the proposed one is based on the separation of the derivatized analytes through GC-MS. Comparing to LC-MS technique, GC-MS require derivatization for making analytes more volatile, allowing to analyse the acidic forms of cannabinoids [27].
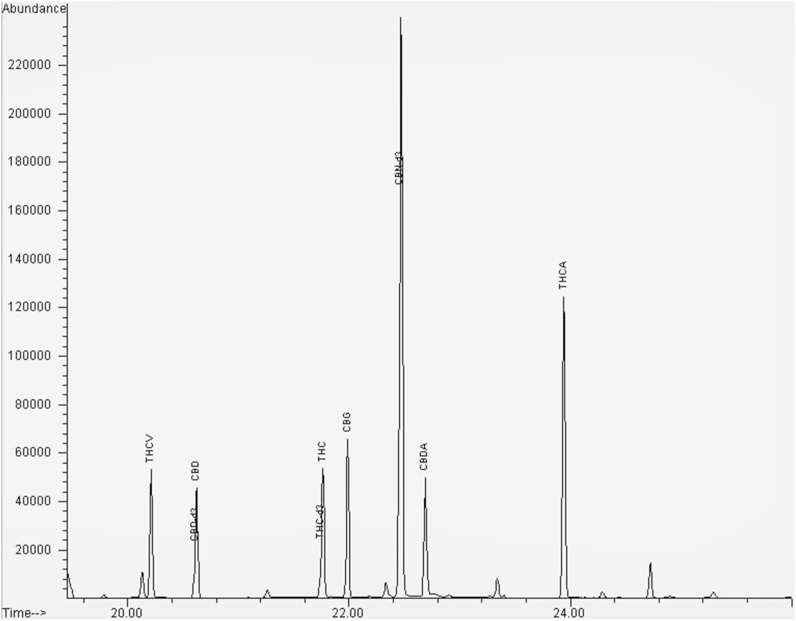
An example of chromatogram is represented in Fig. 2. In particular, the cannabinoids tested were 7 (CBD, CBDA, CBG, CBN, THCA, THCV and Δ9-THC), contrary to other studies that focused their work only on those cannabinoids whose therapeutic activity is largely recognized (CBD and Δ9-THC) and to their acid forms [22,24]. The method was set-up also for measuring CBG, CBN and THCV since there is evidence of their presence in inflorescences of cannabis and of their interest in therapeutic apllications [10,11] (clinical trial identifier: NCT05344170). Measuring the concentrations of the other cannabinoids, in addition to CBD, Δ9-THC and their acid precursors, could allow the identification of inter-conversion reactions [21].
Fig. 2.
Chromatogram representing the separation of the derivatized cannabinoids.
Furthermore, the IS used comprised only CBD-d3, CBN-d3 and Δ9-THC-d3, and not the deuterated acid forms, since cannabis oils were prepared according to SIFAP procedure, and therefore through decarboxylation, process that avoid the abundance of acid forms [4].
3.1.2. MS conditions
Optimized compound dependent MS parameters are reported in Table 1.
Table 1.
Optimized compound dependent MS parameters. RT retention time TW time window. QT quantification QL qualification.
| Compound | RT (min) | TW (min) | Dwell time (ms) | QT ion (m/z) | QL ion (m/z) |
|---|---|---|---|---|---|
| CBD-d3 | 20.6 | 20.35–21.00 | 100 | 393 | NA |
| CBN-d3 | 22.4 | 22.10–23.40 | 80 | 370 | NA |
| Δ9-THC-d3 | 21.7 | 21.00–22.10 | 80 | 374 | NA |
| CBD | 20.6 | 20.35–21.00 | 100 | 390 | 458 |
| CBDA | 22.7 | 22.10–23.40 | 80 | 491 | 453 |
| CBG | 22.0 | 21.00–22.10 | 80 | 337 | NA |
| CBN | 22.4 | 22.10–23.40 | 80 | 367 | 368 |
| THCA | 23.9 | 23.40–26.00 | 80 | 487 | 488 |
| THCV | 20.2 | 19.50–20.35 | 100 | 343 | 358 |
| Δ9-THC | 21.7 | 21.00–22.10 | 80 | 371 | 386 |
3.1.3. Sample preparation
There is evidence about the use of several organic solvents for dissolving cannabis oil [4,21,22]. Given the chemicals readily available in laboratory and that diethyl ether resulted to be used both in the phase of extraction and preparation of the sample due to its capability of making cannabinoids soluble, this solvent was chosen [22,28].
Moreover, sonication for 5 min three times after dilution of the sample was tested in order to evaluate if it could help the dissolution of analytes. Comparing the concentrations of cannabinoids in QCs with and without this step, the accuracy was between 100 ± 15% of the nominal value, suggesting that sonication did not enhance the yield of the preparation, as previously reported [22].
Also, the yield of preparation was not enhanced increasing the time of incubation at 60 °C, temperature used to derivatize the cannabinoids and the IS, indicating that all cannabinoids underwent the complete process of derivatization after 20 min.
Interestingly, the addition of pyridine, as adjuvant for derivatization, resulted to be fundamental to derivatize the acid forms of CBD and Δ9-THC (CBDA and THCA). When pyridine was not added, QCs concentrations were not accurate and exceeded the threshold value of 100 ± 15% of the nominal value. Another work, that did not take in consideration and optimize the analytical method for CBDA and THCA, did not use pyridine, obtaining a good accuracy only of CBD, CBN and Δ9-THC [22].
A previous study tested different approaches of derivatization of cannabinoids [21]. In particular, since it was fully clarified that other processes, such as diazomethane-mediated methylation or esterification, increase the signal-to-noise ratio and require time for chemical synthesis of compounds, silylation was chosen and confirmed as convenient process of derivatization for cannabinoids.
As a whole, the proposed GC-MS method did not require much sample (10 μL of cannabis oil) and time for preparation, as other methods already published did [21,23,24].
3.2. Method validation
3.2.1. Selectivity and specificity
Selectivity was evaluated on olive oil, the matrix usually used for the extraction of cannabinoids from C. sativa. After injection of 6 blank samples of olive oil, we assessed that blanks’ signals were not more than 20% of those of cannabinoids at the lower limit of quantification (LLOQ) and than 5% of that of IS at the corresponding retention times.
Furthermore, there was no significant response, different from the analytes and the IS, associated to interfering components present in the matrix.
3.2.2. Linearity
According to ICH guidelines, linearity was assessed constructing calibration curves in three different analytical runs. The concentrations of CAL were respectively 0.2, 0.4, 0.8, 1.0, 1.5 and 2.0 μg/mL for all the cannabinoids tested. Depending on the calibrators, the proposed GC-MS method allow to quantify concentrations of cannabis oils at least between 2 and 20 mg/mL. Calibration curves were built plotting the areas of each analyte normalized on the IS of reference: in particular CBD-d3 was the IS for CBD and CBDA, CBN-d3 for CBN and Δ9-THC-d3 for CBG, THCA, THCV and Δ9-THC. However, no statistical weight was applied and curves with R2 < 0.99 were excluded.
The inter-day percentages of accuracy (AC%) for each cannabinoid at the CALs analyzed are reported in Table 2. Moreover, intra-day accuracy, equation of calibration curves and R2 used to evaluate linearity are reported in Supplementary materials.
Table 2.
Inter-day percentages of accuracy of CALs.
| CALs (μg/mL) | CBD (AC%) | CBDA (AC%) | CBG (AC%) | CBN (AC%) | THCA (AC%) | THCV (AC%) | Δ9-THC (AC%) |
|---|---|---|---|---|---|---|---|
| 0.2 | 111.73 | 102.76 | 98.53 | 115.08 | 102.70 | 108.66 | 110.87 |
| 0.4 | 96.89 | 100.85 | 103.08 | 96.18 | 96.18 | 97.02 | 95.62 |
| 0.8 | 96.51 | 97.66 | 102.82 | 96.10 | 99.90 | 96.34 | 98.26 |
| 1.0 | 100.42 | 99.66 | 106.30 | 99.84 | 100.37 | 100.78 | 99.55 |
| 1.5 | 100.67 | 100.63 | 103.25 | 100.77 | 101.91 | 101.39 | 101.11 |
| 2.0 | 100.14 | 100.16 | 99.83 | 100.32 | 99.26 | 99.74 | 99.87 |
3.2.3. Sensitivity
To assess the sensitivity of the analytical method, dilutions of the CAL1 (0.2 μg/mL) were done to identify the LLOQ and the limit of detection (LOD). In particular, diluting CAL1, the percentage of accuracy exceeded the threshold value of 100 ± 20% of the nominal value, suggesting that CAL1 (0.2 μg/mL) was LLOQ.
Furthermore, the evaluation of LOD was also done diluting until there was no peak detectable from the background. In particular, LOD of all analytes was 0.01 μg/mL. Interestingly, LOD values were lower than those of another GC-MS method already published [21]. Depending on these results, the proposed GC-MS method allows not only to quantify concentrations until 2 mg/mL, but also to detect concentration until 0.1 mg/mL, value of interest with respect to the Δ9-THC limit content in legal C. sativa in Italy [29].
3.2.4. Accuracy and precision
According to ICH guidelines, the accuracy and precision, referred as the coefficient of variation (CV%), of QCs (4 levels of concentration: 0.2 (LLOQ), 0.5 (low QC: LQC), 0.7 (medium QC: MQC) and 1.2 μg/mL (high QC: HQC)) were evaluated analysing them 5 times in three different analytical runs. Comparing to a previous work [21], we obtained better intra-day accuracy according to the most recent guidelines and injecting more times QCs daily.
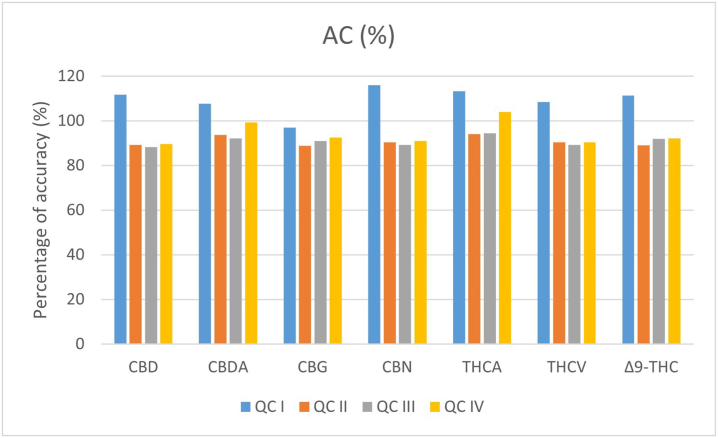
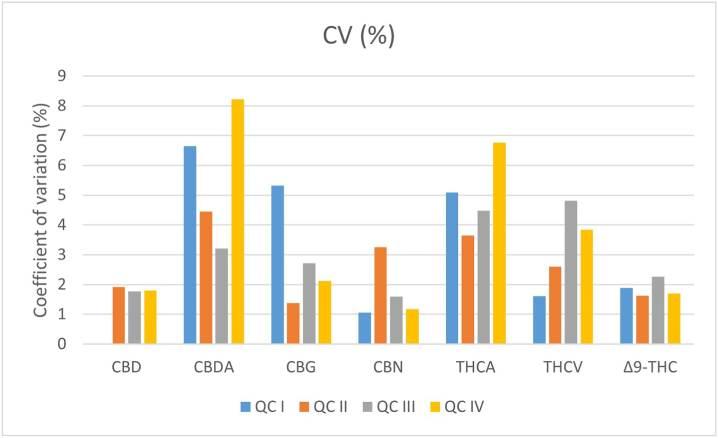
However, percentages of accuracy did not exceed the threshold values of 100 ± 20% of the nominal value for the LLOQ and of 100 ± 15% for the other QCs; also, CV% was not >15%. Inter-day accuracy and precision for the 4 QCs for each cannabinoid is represented in Fig. 3, Fig. 4 respectively; intra-day accuracy and precision are reported in Supplementary materials.
Fig. 3.
Inter-day percentage of accuracy values (means ± standard error) for QCs.
Fig. 4.
Inter-day coefficient of precision values (means ± standard error) for QCs.
3.2.5. Carry over
In order to evaluate a carry over effect, after the CAL with the highest concentration, a blank was injected three times. Blanks’ signals were lower than 20% and 5% of those of cannabinoids at LLOQ and IS respectively at the retention times.
3.2.6. Application to samples
Examples of tested cannabis oil samples are shown in Table 3. In particular, they consist in 1 internal control, pure commercial CBD in olive oil (100 mg/mL) prepared from the hospital pharmacy, and in 11 cannabis oils prepared from pharmacies of FVG area according to the harmonized extraction methods defined by SIFAP [4].
Table 3.
Cannabinoid content (mean ± CV) of tested cannabis oil samples.
| Sample | CBD (mg/mL) | Δ9-THC (mg/mL) |
|---|---|---|
| Internal control diluted 1:10 | 10.3 ± 0.02 | – |
| Bedrocan 1 | – | 17.4 ± 0.02 |
| Bedrocan 2 | – | 16.6 ± 0.03 |
| Bedrocan 3 | – | 17.1 ± 0.03 |
| Bedrocan 4 | – | 18.5 ± 0.02 |
| FM2 1 | 8 ± 0.01 | 6 ± 0.07 |
| FM2 2 | 8.5 ± 0.04 | 5.3 ± 0.01 |
| FM2 3 | 7.2 ± 0.03 | 5 ± 0.03 |
| FM2 4 | 12.7 ± 0.02 | 6.5 ± 0.07 |
| Bediol 1 | 8 ± 0.01 | 5 ± 0.10 |
| Bediol 2 | 9.2 ± 0.02 | 4.9 ± 0.01 |
| Bediol 3 | 7.5 ± 0.02 | 4 ± 0.04 |
Interestingly, the measurement of the internal control with known concentration of CBD was very accurate and precise (AC% = 103%; CV% = 2%).
Furthermore, the measurements of cannabis oils derived from extraction of different varieties of C. sativa (Bedrocan, FM2 or Bediol varieties) were repeatable in different session of analysis (CV% ≤ 4%) and in line with the expectations [2,4]. In particular, in accordance to an already published manuscript [30], after SIFAP method of extraction, total Δ9-THC content was about 14.9 ± 0.34 mg/mL (mean ± standard deviation) in Bedrocan; whereas FM2 and Bediol presented a lower Δ9-THC content (5.6 ± 0.11 and 4.4 ± 0.09 respectively) but also a significant CBD content (8.8 ± 0.18 and 6.9 ± 0.15 respectively) [30].
Interestingly, after decarboxylation, we did not detect the acid forms of CBD and Δ9-THC, suggesting that they are present in lower quantity than 0.1 mg/mL (taking into consideration LOD value). These data were also in line with the manuscripts by Dei Cas and Casiraghi [4,30], where CBDA and THCA were far below 0.5 mg/mL. As acid forms were not abundant, SIFAP extraction procedure is successful in converting them in their neutral forms, making cannabinoids more easily absorbed through the intestine [31]. The other cannabinoids tested, CBG, CBN and THCV, were not detected in the samples of cannabis oils, indicating that these could be not responsible for the therapeutic effects after administration of these galenic preparations. Similarly, CBN was also previously not detected in tested samples [26,30].
3.2.7. Stability
In order to test the stability of the cannabinoids in oil, measurements of cannabis oil samples were repeated after a week, the maximum time in which analyses are usually carried out for diagnostics, after being stored at +4 °C. Percentages of accuracy of the measurements did not exceed the values of accuracy recommended by validation guidelines, suggesting they are stable for at least 1 week at +4 °C. Interestingly, previous studies investigated the stability of cannabinoids in oil [26,32]. Regarding short-term stability, cannabinoids resulted to be stable at +4 °C for 24 h [26]. Instead, long-term storage of cannabis oils at +4 °C caused a significant decrease of cannabinoids [32].
4. Conclusion
In conclusion, we developed and validated a GC-MS method for the simultaneous quantification of 7 cannabinoids in cannabis oil and tested its applicability on therapeutic cannabis oil samples derived from Italian pharmacies in Friuli Venezia Giulia, Italy. Besides showing appropriate selectivity, specificity, linearity, sensitivity, accuracy and precision, our GC-MS method is not time and sample consuming.
The analytical method resulted to be applicable to cannabis oil samples. Our data were in line with the ones previously reported, confirming that the main abundant cannabinoids after SIFAP method extraction were CBD and Δ9-THC; whereas their acidic forms were not detected probably because of decarboxylation and sensibility of the method.
Noteworthy, it could be useful for measuring the concentration of cannabinoids, and in particular CBD, in cannabis oil, which will be administered to pediatric patients in trials, thus monitoring the concentrations of the active ingredients prior to administration and comparing them to clinical efficacy and potential toxicities. Indeed, even if scientific evidence in this field is limited, medical cannabis resulted to be useful to control treatment-resistant pain and epilepsy in 6 pediatric palliative care patients [16].
Interestingly, our GC-MS method could be also implemented in diagnostics for quantification of cannabinoids in therapeutic cannabis preparations already approved for clinical indication and intended for consumption by adult patients. Indeed, the use of cannabinoids have a complex toxicological profile, even if their pharmacological properties in adults affected by several diseases are largely proved [14].
Moreover, since cannabis oil preparation is not yet a standardized product and there are evidences about its instability [32], measuring the concentration of its active compounds prior the administration in patients would be essential.
Author contribution statement
Martina Franzin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rachele Ruoso, Rossella Del Savio, Eugenia Akhavan Niaki: Performed the experiments; Wrote the paper.
Aba Pettinelli: Analyzed and interpreted the data; Wrote the paper.
Giuliana Decorti, Gabriele Stocco, Riccardo Addobbati: Conceived and designed the experiments; Contributed reagents, materials, analysis tools and data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding and acknowledgements
This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy (RC 56/22).
Additional information
Supplementary content related to this article has been published online at [URL].
Notes
Informed consent Samples for routine analyses leftover were used for evaluating the applicability of the analytical method.
Ethic committee Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15479.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brunetti P., et al. Herbal preparations of medical cannabis: a vademecum for prescribing doctors. Medicina (Kaunas) 2020;56(5) doi: 10.3390/medicina56050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palermiti A., et al. Analysis of cannabinoids concentration in cannabis oil galenic preparations: harmonization between three laboratories in Northern Italy. Pharmaceuticals. 2021;14(5) doi: 10.3390/ph14050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casiraghi A., et al. Extraction method and analysis of cannabinoids in cannabis olive oil preparations. Planta Med. 2018;84(4):242–249. doi: 10.1055/s-0043-123074. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci C., et al. Neurological aspects of medical use of cannabidiol. CNS Neurol. Disord.: Drug Targets. 2017;16(5):541–553. doi: 10.2174/1871527316666170413114210. [DOI] [PubMed] [Google Scholar]
- 6.Russo E.B., McPartland J.M. Cannabis is more than simply delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2003;165(4):431–432. doi: 10.1007/s00213-002-1348-z. author reply 433-4. [DOI] [PubMed] [Google Scholar]
- 7.Elsohly M.A., Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Udoh M., et al. Cannabichromene is a cannabinoid CB2 receptor agonist. 2019;176(23):4537–4547. doi: 10.1111/bph.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters E.N., et al. Pharmacokinetics of cannabichromene in a medical cannabis product also containing cannabidiol and Δ9-tetrahydrocannabinol: a pilot study. Eur. J. Clin. Pharmacol. 2022;78(2):259–265. doi: 10.1007/s00228-021-03232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo E.B., et al. Survey of patients employing cannabigerol-predominant cannabis preparations: perceived medical effects, adverse events, and withdrawal symptoms. Cannabis Cannabinoid Res. 2022;7(5):706–716. doi: 10.1089/can.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abioye A., et al. Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020;2(1):6. doi: 10.1186/s42238-020-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salute M.d. 2015. DECRETO 9 novembre 2015.https://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg Available from: [Google Scholar]
- 13.Pichini S., et al. The challenge of clinical application of FM2 cannabis oil produced in Italy for the treatment of neuropathic pain. Eur. Rev. Med. Pharmacol. Sci. 2018;22(4):863–865. doi: 10.26355/eurrev_201802_14363. [DOI] [PubMed] [Google Scholar]
- 14.Solimini R., et al. Neurological disorders in medical use of cannabis: an update. CNS Neurol. Disord.: Drug Targets. 2017;16(5):527–533. doi: 10.2174/1871527316666170413105421. [DOI] [PubMed] [Google Scholar]
- 15.Campbell C.T., Phillips M.S., Manasco K. Cannabinoids in pediatrics. J. Pediatr. Pharmacol. Therapeut. 2017;22(3):176–185. doi: 10.5863/1551-6776-22.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divisic A., et al. The use of medical cannabis in pediatric palliative care: a case series. Ital. J. Pediatr. 2021;47(1):229. doi: 10.1186/s13052-021-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treves N., et al. Efficacy and safety of medical cannabinoids in children: a systematic review and meta-analysis. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-02770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong S.S., Wilens T.E. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140(5) doi: 10.1542/peds.2017-1818. [DOI] [PubMed] [Google Scholar]
- 19.Kwan Cheung K.A., Mitchell M.D., Heussler H.S. Cannabidiol and neurodevelopmental disorders in children. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.643442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salute M.d. 2022. La Distribuzione Della Cannabis Ad Uso Medico.https://www.salute.gov.it/portale/medicinaliStupefacenti/dettaglioContenutiMedicinaliStupefacenti.jsp?lingua=italiano&id=5066&area=sostanzeStupefacenti&menu=organismo Available from: [Google Scholar]
- 21.Cardenia V., et al. Development and validation of a fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018;26(4):1283–1292. doi: 10.1016/j.jfda.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández N., et al. Quantification of cannabinoids in cannabis oil using GC/MS: method development, validation, and application to commercially available preparations in Argentina. Planta Med. Int. Open. 2020;7(2):e81–e87. [Google Scholar]
- 23.McRae G., Melanson J.E. Quantitative determination and validation of 17 cannabinoids in cannabis and hemp using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020;412(27):7381–7393. doi: 10.1007/s00216-020-02862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Q., et al. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellati F., et al. New methods for the comprehensive analysis of bioactive compounds in cannabis sativa L. (hemp) Molecules. 2018;23(10) doi: 10.3390/molecules23102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citti C., et al. Medicinal cannabis: principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016;128:201–209. doi: 10.1016/j.jpba.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Pourseyed Lazarjani M., et al. Methods for quantification of cannabinoids: a narrative review. J. Cannabis Res. 2020;2(1):35. doi: 10.1186/s42238-020-00040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei T., Wang T. Comparative extraction of cannabinoids and terpenoids from Cannabis sativa L. using three solvents. J. Am. Oil. Chem. Soc. 2022;99(6):525–533. [Google Scholar]
- 29.Sgrò S., et al. Delta9-THC determination by the EU official method: evaluation of measurement uncertainty and compliance assessment of hemp samples. Anal. Bioanal. Chem. 2021;413(13):3399–3410. doi: 10.1007/s00216-021-03283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dei Cas M., et al. vol. 11. 2020. (Phytocannabinoids Profile in Medicinal Cannabis Oils: the Impact of Plant Varieties and Preparation Methods). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baratta F., et al. Development of standard operating protocols for the optimization of cannabis-based formulations for medical purposes. Front. Pharmacol. 2019;10:701. doi: 10.3389/fphar.2019.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacifici R., et al. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018;56(4):94–96. doi: 10.1515/cclm-2017-0758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.