Abstract
Patients with chronic obstructive pulmonary disease (COPD) may suffer from acute episodes of worsening dyspnea, often associated with increased cough, sputum, and/or sputum purulence. These exacerbations of COPD (ECOPDs) impact health status, accelerate lung function decline, and increase the risk of hospitalization. Importantly, close to 20% of patients are readmitted within 30 days after hospital discharge, with great cost to the person and society. Approximately 25% and 65% of patients hospitalized for an ECOPD die within 1 and 5 years, respectively. Patients with COPD are usually older and frequently have concomitant chronic diseases, including heart failure, coronary artery disease, arrhythmias, interstitial lung diseases, bronchiectasis, asthma, anxiety, and depression, and are also at increased risk of developing pneumonia, pulmonary embolism, and pneumothorax. All of these morbidities not only increase the risk of subsequent ECOPDs but can also mimic or aggravate them. Importantly, close to 70% of readmissions after an ECOPD hospitalization result from decompensation of other morbidities. These observations suggest that in patients with COPD with worsening dyspnea but without the other classic characteristics of ECOPD, a careful search for these morbidities can help detect them and allow appropriate treatment. For most morbidities, a thorough clinical evaluation supplemented by appropriate clinical investigations can guide the healthcare provider to make a precise diagnosis. This perspective integrates the currently dispersed information available and provides a practical approach to patients with COPD complaining of worsening respiratory symptoms, particularly dyspnea. A systematic approach should help improve outcomes and the personal and societal cost of ECOPDs.
Keywords: COPD, differential diagnosis, symptom flare-up, algorithms
At a Glance Commentary
Scientific Knowledge on the Subject
Patients with chronic obstructive pulmonary disease (COPD) frequently have concomitant morbidities that not only increase the risk of subsequent exacerbations of COPD (ECOPDs) but can also mimic or aggravate ECOPDs. A careful search can help detect these and allow for appropriate treatment.
What This Study Adds to the Field
For most morbidities that can mimic or aggravate ECOPDs, recent advances on the basis of a thorough clinical evaluation supplemented by clinical investigations can help the clinician to make precise diagnoses. This perspective integrates the currently dispersed information available and provides clinicians with a practical approach to patients with COPD complaining of worsening respiratory symptoms, particularly dyspnea. A systematic approach should help improve outcomes and the personal and societal cost of ECOPDs.
The course of chronic obstructive pulmonary disease (COPD) is punctuated by acute worsening of dyspnea, often associated with increased cough, sputum volume, and/or purulence (1, 2). These exacerbations of COPD (ECOPDs) worsen health status, accelerate lung function decline, and increase the risk of subsequent ECOPDs, hospitalization, and death (1).
Patients with COPD are usually older (3) and have multiple morbidities, including heart failure (HF), ischemic heart disease (IHD), arrhythmias, interstitial lung diseases (ILDs), bronchiectasis, anxiety, and depression, all of which increase the risk of ECOPDs and death (4). Furthermore, the incidence of pneumonia, pneumothorax, and pulmonary embolism (PE), which can mimic and/or worsen an ECOPD, are frequent in these patients (1, 3–5). Approximately 25% and 65% of patients hospitalized for an ECOPD die within 1 and 5 years, respectively, mainly because of respiratory and cardiovascular complications, with close to 20% readmitted within 30 days after discharge (1, 5, 6). Importantly, close to 70% of these readmissions are because of decompensation of other morbidities (3, 7).
We previously proposed an updated definition of ECOPDs that, in addition to symptom worsening, incorporates the measurement of six objective variables (respiratory and cardiac rate, oxygen saturation, dyspnea, C-reactive protein [CRP], and, when available, arterial blood gases) to determine ECOPD severity (8). In most patients, especially in the milder cases seen in primary care, a classic presentation may be confidently diagnosed as an ECOPD. However, in some, particularly the more severe cases seen in the acute care setting, decompensation of coexisting morbidities (particularly HF) or development of new morbidities (e.g., PE or pneumonia) can be confused with, or contribute to, ECOPDs.
Studies have shown that directed clinical evaluation (pretest probability) complemented with clinical investigations can help precise diagnoses. This review integrates the dispersed information from those studies to provide clinicians with a practical approach to patients with COPD who have worsening respiratory symptoms, particularly dyspnea, not explained by an ECOPD who are seen primarily in the acute care setting. Despite the concerns of potential adverse drug effects, a diagnosis of ECOPD should not alter the treatment of any associated morbidity, which should adhere to the general concept of guideline-directed medical treatment (9).
Methods
This is an expert consensus by the same group that proposed the Rome definition and severity classification of ECOPD (8), expanded to include additional experts from Europe (D.S.) and South America (M.M.d.O.). These 18 experts, representing 10 countries and three continents, were divided into four groups to perform literature reviews, with particular emphasis on guidelines and expert consensus documents. Of the many morbidities that may present with respiratory symptoms resembling ECOPDs, a five-round Delphi process resulted in agreement on 12 (8). These were selected to be included in this practice guide, the contents of which were developed on the basis of reviews of the literature by each group. The groups developed an overarching algorithm and a series of detailed algorithms for the most important of these, intended to be of practical use for healthcare providers. This material was combined by the coordinators (B.R.C. and L.M.F.) and was discussed and agreed on by all members of the project. In this document, patients are defined as confirmed COPD when there is spirometric support (1) and suspected COPD if there is no spirometric confirmation but a history of exposure to tobacco smoke and/or environmental pollutants, chronic respiratory symptoms, and/or patients are receiving pharmacological treatment for COPD (10).
Initial Evaluation
A careful medical history and physical examination complemented by the rational measurement of objective variables can help establish, with a degree of certainty, that an ECOPD is the most likely diagnosis (Table 1), particularly in patients with mild events seen in the outpatient setting. However, in many patients, the signs and symptoms of an ECOPD (especially if more severe) are not specific, and other diseases may mimic an ECOPD and/or aggravate its clinical presentation (Figure 1). Most of these patients are seen in an acute care setting, and we developed an initial practical algorithm to help reach a more precise diagnosis (Figure 2).
Table 1.
The CASE (Complete, Assess, Severity, Establish) Approach to a Patient with Confirmed or Suspected Chronic Obstructive Pulmonary Disease Who Presents with Worsening Respiratory Symptoms
| Complete | A thorough clinical assessment for evidence of underlying COPD and potential respiratory and nonrespiratory concomitant diseases, including consideration of alternative causes for the patient’s symptoms and signs |
| Assess | Symptoms: Severity of dyspnea using a tool such as the dyspnea visual analog scale; documentation of the presence and characteristics of cough and sputum. Presence of pain and its characteristics |
| Signs: Tachypnea, tachycardia, arrhythmia, fever, sputum volume and color, respiratory distress (accessory muscle usage), abnormalities on thoracic examination, and thorough physical examination | |
| Severity | Evaluate by combining symptoms and signs and appropriate additional investigations such as pulse oximetry. If available, obtain laboratory assessments (e.g., CRP), chest X-ray, electrocardiograph, and/or arterial blood gases |
| Establish | Cause of the event (viral, bacterial, environmental, poor treatment adherence, alternative diagnosis, or other) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein.
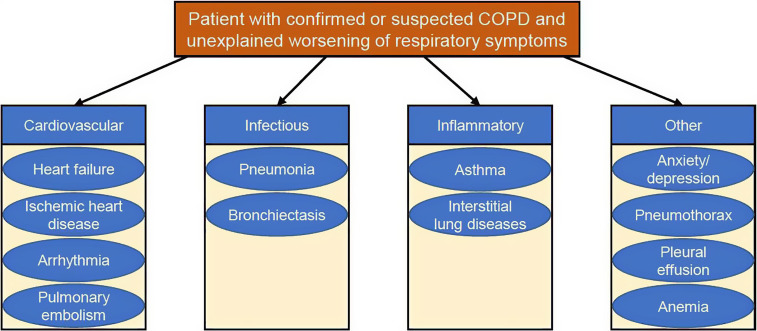
Figure 1.
Diseases in patients with chronic obstructive pulmonary disease (COPD) presenting with acute worsening of dyspnea/chest discomfort that may mimic or contribute to the clinical presentation of an exacerbated COPD.
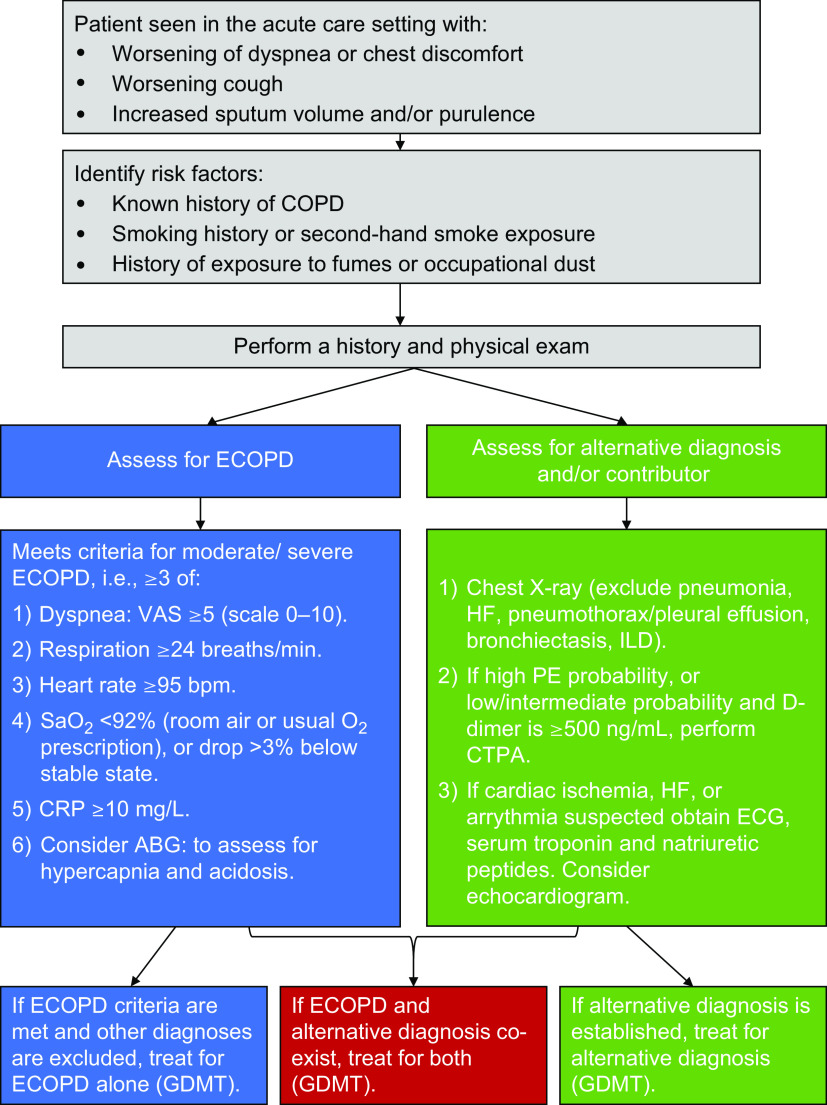
Figure 2.
A practical algorithm to help direct evaluation of a patient with chronic obstructive pulmonary disease (COPD) presenting with symptoms consistent with an exacerbated COPD. Recommendations are on the basis of the most recent available guidelines, consensus documents, and/or original studies referenced in the manuscript. ABG = arterial blood gases; CRP = C-reactive protein; CTPA = computed tomography pulmonary angiogram; ECG = electrocardiogram; ECOPD = exacerbation of chronic obstructive pulmonary disease; GDMT = guideline-directed medical treatment; HF = heart failure; ILDs = interstitial lung diseases; PE = pulmonary embolism; VAS = visual analog scale.
Cardiovascular Morbidities
Cardiovascular morbidities are frequently present in patients experiencing ECOPDs, and thus a patient history is important to assess their presence or impact (11, 12). Chest pain/discomfort, fluid retention, or irregular pulse suggest a cardiovascular cause as an alternative or contributing diagnosis to an ECOPD.
Acute HF
Acute HF is defined by worsening of dyspnea at rest (particularly while supine) or during exertion and/or fatigue, together with signs of fluid retention such as pulmonary congestion, increased jugular vein distension, or ankle swelling, with objective evidence of an abnormality of the structure or function of the heart (13). Some of these clinical findings can make distinguishing ECOPD from acute HF difficult (14). In addition, COPD is present in up to 50% of patients with HF, and HF occurs in roughly 20% of individuals with COPD because of overlapping risk factors (aging and smoking) and underlying mechanisms (systemic inflammation, endothelial dysfunction, and muscular atrophy). Furthermore, an HF diagnosis is often difficult to confirm in patients with preserved ejection fraction, which is common in patients with COPD (12, 14). Hypoxemia and tachycardia in ECOPD can promote decompensation in HF, whereas pulmonary fluid retention in HF can worsen airway obstruction (12). In addition, wheezing is common in acute HF (approximately 30%) (15), whereas COPD can mask the presence of basilar crackles. However, their presence, coupled with evidence of leg edema, elevated jugular venous pressure, and a third heart sound increases the likelihood of HF being the cause of, or a contributor to, the acute event (15).
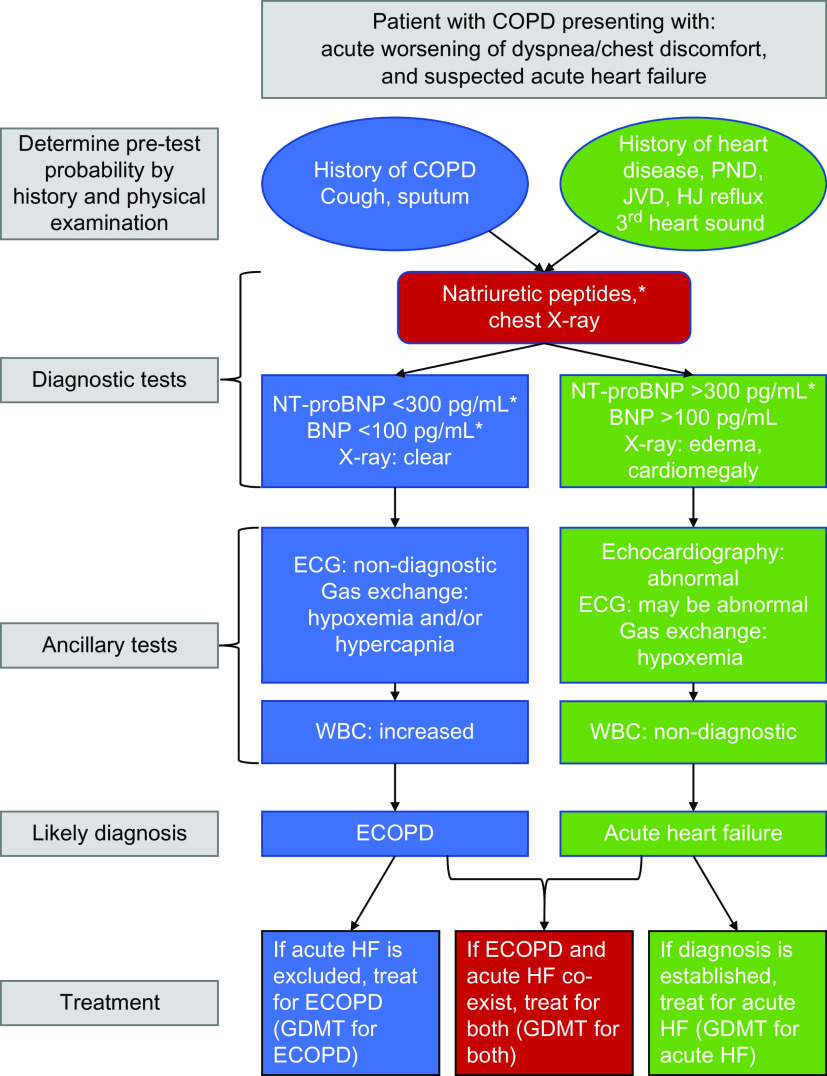
A chest X-ray can help distinguish an ECOPD from acute HF, with cardiomegaly supporting structural heart damage and HF likely present if there is pulmonary edema (16). The best laboratory markers of HF are elevation of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic peptide) (17–19), with thresholds supportive of acute HF, consistent with European Society of Cardiology Guidelines on HF, shown in Figure 3 (20). Importantly, natriuretic peptides are commonly elevated in patients with stable COPD or pneumonia and increase further in patients with ECOPD even without acute HF (17, 19, 20). As such, higher thresholds are needed to accurately diagnose concomitant acute HF and ECOPD. More informative is the presence of low peptide concentrations, which have excellent negative predictive value for acute HF, even in patients with an ECOPD (17). Patients with elevated natriuretic peptides during an ECOPD have a worse prognosis, including a risk of death (21). Finally, point-of-care ultrasonography can help differentiate ECOPD from acute HF, although more research is required to establish their impact on health outcome benefits (19, 20). A diagnostic algorithm to evaluate a patient with suspected acute HF is shown in Figure 3.
Figure 3.
Algorithm reflecting the clinical features of a patient with chronic obstructive pulmonary disease (COPD) presenting with increased respiratory symptoms and suspected acute heart failure. Recommendations are on the basis of the most recent European and American Guidelines on heart failure (19, 20). *The thresholds for N-terminal pro-brain natriuretic peptide (NT-proBNP) and BNP are intended for the diagnosis of acute heart failure (HF) (20) and may need correction with age. Thresholds for new onset stable HF are much lower (i.e., NT-proBNP >125 pg/ml and BNP >35 pg/ml) (19) and should be used outside the context of an exacerbation of COPD. Note that the presence of COPD may raise these values. Please see text for further details. ECG = electrocardiogram; ECOPD = exacerbation of chronic obstructive pulmonary disease; GDMT = guideline-directed medical treatment; HJ reflux = hepatojugular reflux; JVD = jugular vein distention; PND = paroxysmal nocturnal dyspnea; WBC = white blood cell count.
IHD
Underlying IHD is associated with more prolonged ECOPDs (22). Myocardial injury is common and clinically significant during ECOPDs, particularly in patients with pre-existing IHD (23), and an ECOPD significantly increases the risk of myocardial infarction and stroke, not only in the first 30 days after an ECOPD (by 4–10 times) but for up to 1 year after the event (24, 25). Moreover, one-third of patients with IHD have COPD, which is associated with more frequent emergency room visits (26), with COPD independently increasing IHD risk and heightening morbidity and mortality (12, 27).
In outpatients presenting with an ECOPD, a clinical history (established IHD), current symptoms (chest pain/discomfort and/or palpitations), and signs of systemic atherosclerosis (carotid bruit and/or reduced peripheral pulses) or those consistent with acute myocardial ischemia (diaphoresis and/or third heart sound), suggest concomitant IHD. Symptoms plus an abnormal resting electrocardiogram (ECG) support the presence of acute myocardial ischemia. Nevertheless, the diagnosis of myocardial injury or infarction, either as an independent or contributing morbidity of an ECOPD, should follow the fourth universal definition criteria (28). This requires elevation in blood troponin at admission and/or increase during follow-up, plus at least one other factor (symptoms and/or new ECG changes). COPD can be associated with chronic (usually stable) elevations in troponin concentrations, which can increase during an ECOPD. The diagnostic approach for a patient with suspected IHD is shown in Figure E1 in the online data supplement (29). No troponin thresholds are quoted because of variability across laboratories and methodology. When the clinical suspicion of IHD is high, a guideline-directed evaluation is generally warranted. Furthermore, given the high prevalence of IHD with ECOPD, evaluation for atherosclerosis and associated diseases should always be considered (29, 30).
Arrhythmias
The prevalence of cardiac arrhythmias, especially atrial fibrillation (AF), is increased in patients with COPD. Furthermore, during an ECOPD, the risk of AF and other arrhythmias doubles (12, 31), and, importantly, AF during an ECOPD increases morbidity and mortality (31). AF presence can be ascertained by atypical chest discomfort, palpitations, lightheadedness, and dyspnea, as well as an irregular pulse on palpation. Unlike other morbidities, there is less diagnostic dilemma between AF and ECOPD. Standard ECG and heart monitoring can detect the presence of chronic, acute, or intermittent arrhythmias (32). The role of new-onset AF as a cause of acute dyspnea in a patient with COPD is still unknown, so the unresolved clinical question is whether AF was a pre-existing or new-onset event (1, 32). The diagnostic approach to a patient with suspected AF is shown in Figure E2.
PE
The prevalence of PE in patients with ECOPD ranges from 3.3% to 29.4% (33), being more common in patients with atypical or unexplained ECOPD and requiring specific and prompt management to improve outcomes (34). PE should be considered in patients with increased dyspnea in the context of risk factors such as a history of venous thromboembolic disease, hospitalization for HF, or malignancy (34, 35). Although patients with either PE or ECOPD characteristically complain of worsened dyspnea, those with PE commonly report pleuritic chest pain, hemoptysis, and signs of right HF and are less likely to have a cough with increased or purulent sputum.
Two studies have considered practical approaches to evaluating PE frequency in hospitalized patients with ECOPD (36, 37). The first used the revised Geneva score to assess the pretest clinical probability of PE in 740 patients, whether PE was suspected or not (36). Patients with a high clinical probability of PE (score of at least 11) proceeded to computed tomography (CT) pulmonary angiogram (CTPA) and leg ultrasound. For patients scoring under 11, D-dimer testing was performed, with PE excluded (and no subsequent testing) if the D-dimer value was less than 500 ng/ml. Of 17 patients with high pretest probability, 5 had positive CTPA. In the 442 with low pretest probability and D-dimer of at least 500 μg/L, 36 had a positive CTPA. Importantly, 3-month mortality when PE or deep-vein thrombosis (DVT) was excluded was 5.5%, compared with 27% and 20% in patients with confirmed PE or isolated DVT, respectively.
In the second study, usual care plus an active strategy for diagnosing PE was compared with usual care alone in 746 patients not suspected of having a PE by the physician in charge who were hospitalized because of an ECOPD (37), the primary outcome being a composite index of nonfatal venous thromboembolism, COPD readmission, or death within 90 days. Patients in the intervention group underwent D-dimer testing, with a negative D-dimer value (less than 500 ng/ml) used to exclude PE. A CTPA was performed only in patients with a positive D-dimer value. The primary outcome was similar in the two groups (29.7% and 29.2% in the intervention and control groups, respectively).
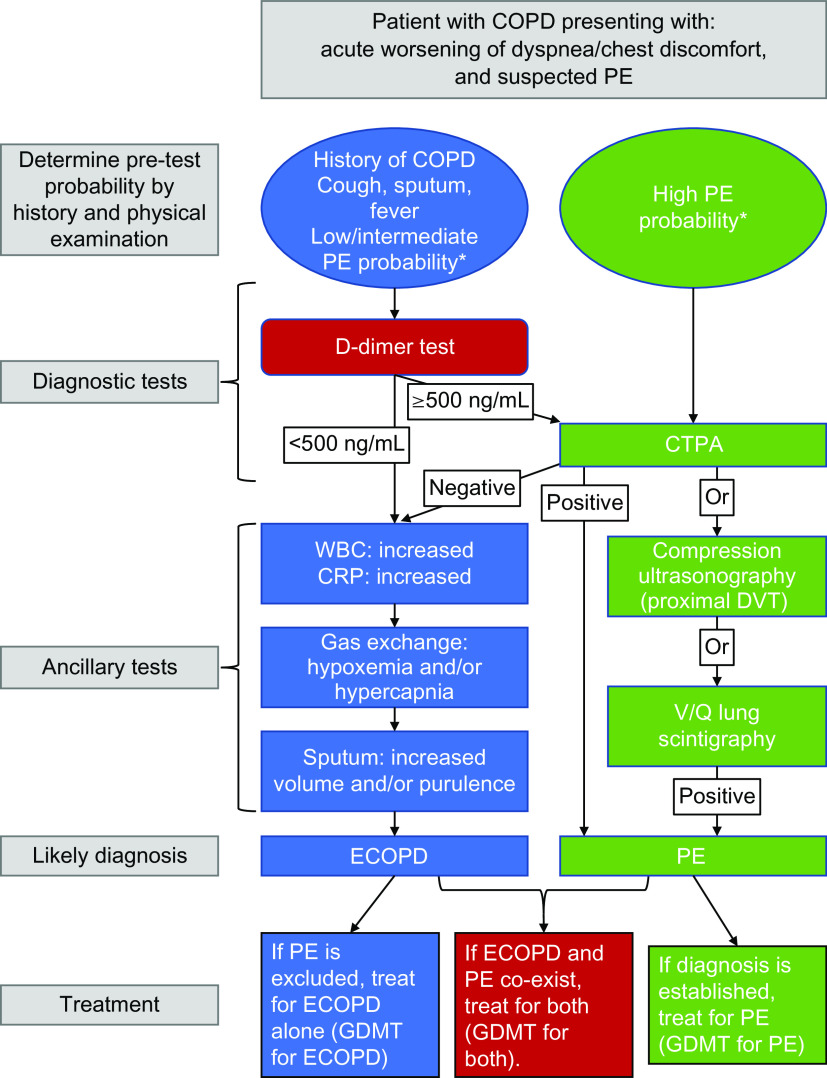
These studies show that in patients hospitalized with an ECOPD, overall PE prevalence is around 4–6% (36, 37). In patients with suspected PE, the prevalence of venous thromboembolism was 11.7% and 4.3% among those in whom PE was not suspected (36). Given the high mortality with PE, evidence supports CTPA imaging in patients with a high clinical PE pretest probability (Geneva score of at least 11) and an atypical clinical presentation for ECOPD, with no need to assess D-dimer concentrations. If the pretest probability is low (Geneva score less than 11), a D-dimer less than 500 ng/ml would support ECOPD as the diagnosis and help avoid imaging testing. In addition, lower-limb compression ultrasonography (CUS) can diagnose DVT in patients with a negative CTPA (or when CTPA is contraindicated) (35). In the setting of suspected PE, CUS can be limited to a simple four-point examination (bilateral groin and popliteal fossa) (35). The probability of a positive proximal CUS in suspected PE is higher in patients with signs and symptoms related to the leg veins than in asymptomatic patients (38). The diagnostic approach for a patient with suspected PE is shown in Figure 4.
Figure 4.
Algorithm reflecting the clinical features of a patient with COPD presenting with increased respiratory symptoms and suspected pulmonary embolism (PE). Recommendations are on the basis of Konstantinides and colleagues (35) and Couturaud and colleagues (36). *PE probability assessed using Geneva or Wells scores. COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; CTPA = computed tomography pulmonary angiogram; DVT = deep-vein thrombosis; ECOPD = exacerbation of chronic obstructive pulmonary disease; GDMT = guideline-directed medical treatment; WBC = white blood cell count.
Infectious Diseases
Pneumonia
In patients with COPD, the symptoms of pneumonia and ECOPD may be similar (worsened cough, sputum production, dyspnea, and fever) (39). Physical examination may help in the differentiation, with signs of consolidation (localized crackles, crepitus, and dullness on percussion) more frequent in patients with pneumonia. However, because a diagnosis of COPD increases pneumonia risk (by 1.3- to 13.5-fold) and severity (40), pneumonia should always be considered in the differential diagnosis of patients with symptoms of ECOPD.
If the clinical assessment deems the ECOPD to be mild or moderate and there are no signs of lung consolidation, no further diagnostic testing to differentiate the two diseases is indicated. However, if the episode is severe and/or prolonged (41), and there are abnormal chest exam findings, a proper diagnosis of pneumonia should be undertaken. Mortality after hospitalized pneumonia is higher than after hospitalized ECOPD (42). Although most patients without severe ECOPD can be safely treated on the basis of chest X-ray results alone, opacities not visible on a chest X-ray may be seen with more sensitive CT scans (43, 44).
Serum biomarkers are becoming increasingly available as point-of-care tests and are readily obtainable in emergency rooms or hospitals. Higher CRP values are more likely to support a diagnosis of pneumonia, particularly of bacterial etiology, rather than ECOPD (45). The role of procalcitonin is less clear, with evidence suggesting that in patients with a low pretest probability for pneumonia, a low procalcitonin value supports a nonbacterial etiology for the process (46, 47). Other potentially useful biomarkers include IL-6, white blood cell count, and platelets, although no cut-off values have yet been validated (48).
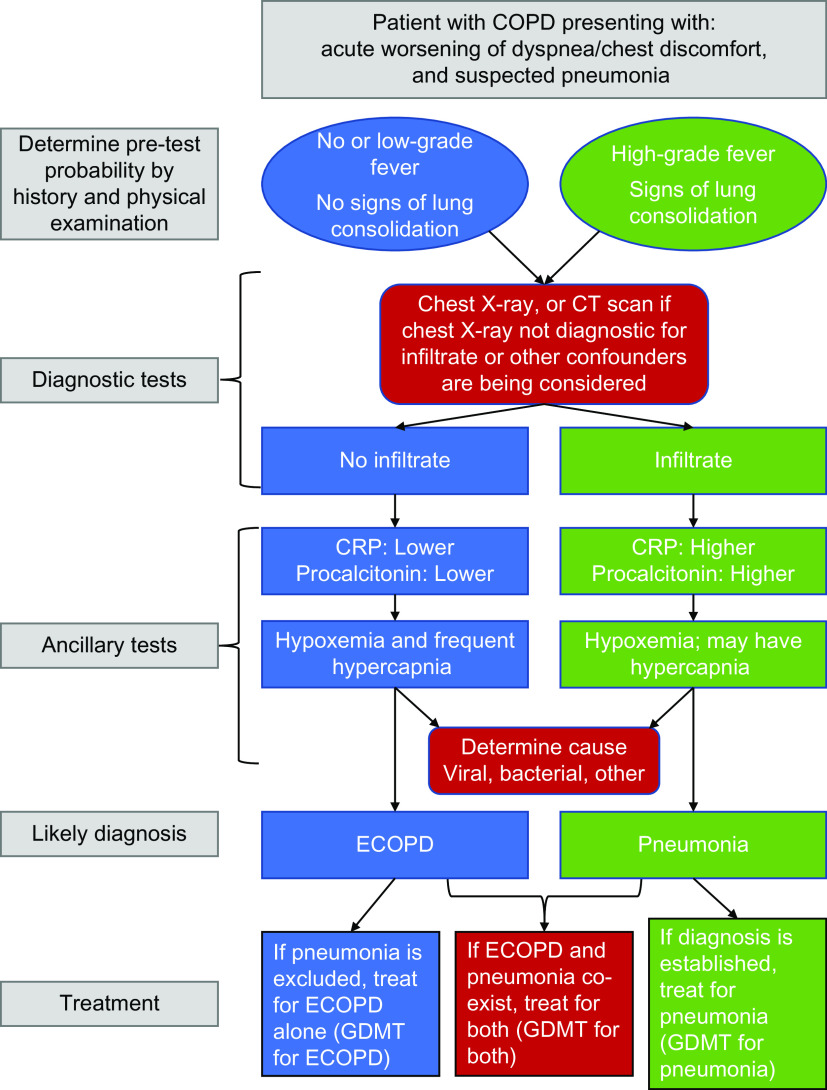
In a patient with an ECOPD, respiratory syncytial virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]), or influenza as the causing agent should be considered, on the basis of the prevalence of these viruses in the community and the season, as specific treatments are available and patients with COPD are good candidates for these interventions (49). Sputum purulence is a clinical biomarker that supports bacterial infection and predicts the benefit of treatment with antibiotics (50). In patients with severe ECOPD or pneumonia, sputum gram staining and cultures and molecular point-of-care testing (51) can help guide antibiotic therapy, as difficult-to-treat bacterial pathogens can often be present in these patients. The presence of pneumonia should also prompt urine tests for pneumococcal and Legionella antigens. The diagnostic approach for a patient with suspected pneumonia is shown in Figure 5.
Figure 5.
Algorithm reflecting the clinical features of a patient with chronic obstructive pulmonary disease presenting with increased respiratory symptoms and when potential pneumonia is considered. Recommendations are on the basis of European guidelines (Woodhead and colleagues [43]), consensus documents, and/or relevant original studies quoted in the text. COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; CT = computed tomography; ECOPD = exacerbation of chronic obstructive pulmonary disease; GDMT = guideline-directed medical treatment.
Bronchiectasis
Bronchiectasis prevalence in patients with COPD varies from 8% to 69%, being higher in those with more severe airflow obstruction (52). Patients with overlapping COPD and bronchiectasis have more exacerbations and symptoms, a higher risk of pneumonia, and increased mortality than patients with either diagnosis. This increased exacerbation risk may be because of more frequent isolation of pathogenic bacteria, including Pseudomonas aeruginosa (53).
The diagnosis of COPD is primarily physiological, whereas that of bronchiectasis is radiological. However, patients with severe and/or extensive bronchiectasis can have poorly-reversible airflow obstruction, meeting spirometric criteria for COPD. Diagnostic criteria for COPD/bronchiectasis overlap include appropriate ROSE (radiology, airflow obstruction, symptoms, and exposure) (54). In these patients, it is impossible to differentiate exacerbation of the bronchiectasis from ECOPD. Bronchiectasis guidelines recommend searching for an alternative treatable underlying cause (such as antibody deficiency) (55), and it is logical to use CT scans to exclude bronchiectasis in patients with frequent ECOPDs (1).
Inflammatory Morbidities
Asthma Exacerbation
The prevalence of comorbid bronchial asthma in patients with COPD ranges from 3.8% to 38.8% depending on the definitions used (56), with a family history of asthma, younger age, and the presence of atopy or childhood asthma suggesting comorbid asthma in a patient with COPD. Patients with both diseases are more symptomatic, have poorer lung function, and are at increased risk of exacerbation and hospitalization compared with patients with either disease (56, 57).
No publication has addressed whether exacerbations in patients with comorbid COPD and asthma are different from exacerbations of asthma or ECOPD, and guidelines define asthma exacerbations and ECOPDs similarly (1, 57). The most frequent cause of both ECOPDs and asthma exacerbations are viral infections and/or pollutants, and although sputum/purulence, one of the typical features of ECOPD, could help distinguish the two events, sputum/purulence has also been reported in asthma exacerbations. Thus, the management of concomitant COPD and asthma is similar to that of asthma (1, 57).
ILDs
ILDs occur in approximately 8% of individuals with tobacco smoke exposure (58). Despite marked differences in static respiratory mechanics between ILDs and COPD, patients with both diseases characteristically describe dyspnea and dry cough as their major symptoms, with dyspnea likely because of an uncoupling between the effort needed to breathe and the effective respired volume of inspired air (59). As in COPD, the natural course of ILDs (especially idiopathic pulmonary fibrosis [IPF]) is punctuated by exacerbations, usually severe, which are associated with clinical deterioration, although IPF exacerbations have a worse prognosis than ECOPDs (60).
Some patients may present with combined pulmonary fibrosis and emphysema (61). When these patients experience an exacerbation, it may be difficult to differentiate an ECOPD from an IPF exacerbation (62). The evidence of new widespread alveolar abnormalities (ground-glass) in imaging studies in a patient with symptoms consistent with ECOPD supports an important role of an ILD exacerbation in the patient’s event (60).
Miscellaneous Morbidities
Anxiety and Depression
In patients with ECOPD, the prevalence of depression and anxiety ranges between 9% and 58%, depending on the assessment method (63). Furthermore, patients with COPD who have depression and/or anxiety are at increased risk of ECOPD and hospitalizations compared with nondepressed individuals (64). In addition, nearly 10% of current and former smokers have concomitant anxiety and depression, which independently predict the risk of exacerbations (65). This vicious cycle worsens with every exacerbation, as each episode leads to symptom worsening, aggravates muscle weakness, and decreases functional capacity (limiting activity), consequently worsening anxiety and depression (63, 64, 66).
The causal role of anxiety/depression in ECOPDs has not been systematically studied. Panic attacks and anxiety may cause tachypnea that, in COPD, may result in hyperinflation and culminate in acute hypercapnic respiratory failure (67). Furthermore, hypercapnia triggers panic attacks because of the activation of specific brainstem reflexes (68), causing hyperventilation, hyperinflation, and aggravating panic symptoms (67). In patients with COPD who have a panic attack, the lack of increase in cough and sputum in the absence of either an inflammatory burst or another explanatory morbidity for dyspnea and tachypnea can help establish a diagnosis.
Pneumothorax
Pneumothorax is a potentially life-threatening event that occurs in some patients with COPD, resembles an ECOPD, and requires prompt recognition. Subpleural blebs and bullae are frequent in older males with more severe emphysema and cigarette or marijuana smoke exposure (69, 70). In patients with COPD who present with pneumothorax, dyspnea is associated with chest pain, often accompanied by hypoxemia, and occasionally, acute respiratory failure (71). A pneumothorax diagnosis is usually confirmed by chest X-ray, being careful to avoid misinterpretation with bullae. Lung ultrasound may also be diagnostic and more readily available in the ICU or emergency room settings (72).
Pleural Effusion
Large pleural effusions may contribute to worsening dyspnea in patients with COPD (73), as they alter lung and chest wall elastic equilibrium, creating a restrictive ventilatory defect. If the effusion is large and associated with elevated natriuretic peptides, the causes are more likely cardiovascular in origin, particularly acute HF (74), whereas if it is associated with high CRP concentrations, it likely reflects a parapneumonic effusion or empyema because of underlying infection (71, 74) and occasionally lung cancer (71). Chest X-rays, ultrasound imaging, and chest CT are all useful in determining the presence, size, and complexity of pleural effusion in a patient with ECOPD (71, 75).
Anemia
Hemoglobin assessment is important in patients with COPD. Polycythemia can indicate untreated hypoxemia, whereas anemia, a frequent concomitant morbidity in stable COPD with a prevalence of 5–30% (76), may contribute to breathlessness. Patients with anemia tend to be older, with worse airflow limitation and a higher burden of cardiac and metabolic comorbidities than nonanemic patients (76). There is an association between anemia and elevated CRP concentration, a cytokine often augmented during an ECOPD (77). Although there does not appear to be a relationship between the presence of anemia and ECOPD incidence, it seems logical that coexistent anemia may worsen the dyspnea that is characteristic of such episodes and so impact ECOPD severity. Patients with comorbid COPD and anemia tend to have longer hospital stays and are at increased risk of death, perhaps through association with comorbidities (77).
Conclusions
Combining a thorough clinical history and examination with currently available clinical biomarkers, most patients with COPD who have a classic presentation may be diagnosed with reasonable confidence as having an ECOPD. However, in other patients, acute decompensations of coexisting morbidities or the development of acute events such as acute HF, pneumonia, or PE can contribute to or cause those events. Thus, all patients with COPD who have worsening respiratory symptoms, particularly dyspnea without infective evidence, should be investigated for the frequent morbidities that may complicate those events. It is extremely important that trial planners adopt a more objectively supported system of determining the cause of admissions labeled as COPD exacerbations, given that prior trials may have incorrectly labeled hospitalized deteriorations as COPD exacerbations when, in fact, these events were driven by other comorbidities. With the knowledge and resources available to us, the time for precise diagnosis of these episodes is now (78).
Acknowledgments
Acknowledgment
Writing support (in the form of editing content for grammar and journal style) was provided by David Young of Young Medical Communications and Consulting Ltd. This support was funded by Chiesi Farmaceutici SpA.
Footnotes
Supported by Chiesi Farmaceutici.
Author Contributions: B.R.C. and L.M.F. developed the original concept for this work, including the organization of the methodology on which the content is based. All authors contributed to the literature searches and the virtual discussions. The first draft of the manuscript was written by B.R.C., with all authors then providing intellectual input. All authors approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202209-1795CI on January 26, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. 2023. https://goldcopd.org/2023-gold-report-2/
- 2. Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA . 2019;321:786–797. doi: 10.1001/jama.2019.0131. [DOI] [PubMed] [Google Scholar]
- 3. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 4. Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. BODE Collaborative Group Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 5. MacLeod M, Papi A, Contoli M, Beghé B, Celli BR, Wedzicha JA, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology . 2021;26:532–551. doi: 10.1111/resp.14041. [DOI] [PubMed] [Google Scholar]
- 6. García-Sanz MT, Cánive-Gómez JC, Senín-Rial L, Aboal-Viñas J, Barreiro-García A, López-Val E, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis . 2017;9:636–645. doi: 10.21037/jtd.2017.03.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobs DM, Noyes K, Zhao J, Gibson W, Murphy TF, Sethi S, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thorac Soc . 2018;15:837–845. doi: 10.1513/AnnalsATS.201712-913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med . 2021;204:1251–1258. doi: 10.1164/rccm.202108-1819PP. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi S, Kohno T, Goda A, Shiraishi Y, Kawana M, Saji M, et al. West Tokyo Heart Failure Registry Investigators Multimorbidity, guideline-directed medical therapies, and associated outcomes among hospitalized heart failure patients. ESC Heart Fail . 2022;9:2500–2510. doi: 10.1002/ehf2.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diab N, Gershon AS, Sin DD, Tan WC, Bourbeau J, Boulet LP, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 11. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis . 2018;12:1753465817750524. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med . 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 13. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the universal definition of heart failure. J Card Fail . 2023;Mar:381–383. doi: 10.1016/j.cardfail.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 14. Čelutkienė J, Balčiūnas M, Kablučko D, Vaitkevičiūtė L, Blaščiuk J, Danila E. Challenges of treating acute heart failure in patients with chronic obstructive pulmonary disease. Card Fail Rev . 2017;3:56–61. doi: 10.15420/cfr.2016:23:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renier W, Winckelmann KH, Verbakel JY, Aertgeerts B, Buntinx F. Signs and symptoms in adult patients with acute dyspnea: a systematic review and meta-analysis. Eur J Emerg Med . 2018;25:3–11. doi: 10.1097/MEJ.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 16. Allen CJ, Guha K, Sharma R. How to improve time to diagnosis in acute heart failure – clinical signs and chest X-ray. Card Fail Rev . 2015;1:69–74. doi: 10.15420/cfr.2015.1.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawkins NM, Khosla A, Virani SA, McMurray JJV, FitzGerald JM. B-type natriuretic peptides in chronic obstructive pulmonary disease: a systematic review. BMC Pulm Med . 2017;17:11. doi: 10.1186/s12890-016-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation . 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 19. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation . 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 20. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Scientific Document Group 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J . 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 21. Stolz D, Breidthardt T, Christ-Crain M, Bingisser R, Miedinger D, Leuppi J, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest . 2008;133:1088–1094. doi: 10.1378/chest.07-1959. [DOI] [PubMed] [Google Scholar]
- 22. Patel ARC, Kowlessar BS, Donaldson GC, Mackay AJ, Singh R, George SN, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;188:1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med . 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 24. Kunisaki KM, Dransfield MT, Anderson JA, Brook RD, Calverley PMA, Celli BR, et al. SUMMIT Investigators Exacerbations of chronic obstructive pulmonary disease and cardiac events: a post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med . 2018;198:51–57. doi: 10.1164/rccm.201711-2239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest . 2010;137:1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 26. Franssen FME, Soriano JB, Roche N, Bloomfield PH, Brusselle G, Fabbri LM, et al. Lung function abnormalities in smokers with ischemic heart disease. Am J Respir Crit Care Med . 2016;194:568–576. doi: 10.1164/rccm.201512-2480OC. [DOI] [PubMed] [Google Scholar]
- 27. Rothnie KJ, Smeeth L, Herrett E, Pearce N, Hemingway H, Wedzicha J, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart . 2015;101:1103–1110. doi: 10.1136/heartjnl-2014-307251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth universal definition of myocardial infarction (2018) Circulation . 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 29. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Scientific Document Group 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J . 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 30. Hawkins NM, Peterson S, Ezzat AM, Vijh R, Virani SA, Gibb A, et al. Control of cardiovascular risk factors in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2022;19:1102–1111. doi: 10.1513/AnnalsATS.202104-463OC. [DOI] [PubMed] [Google Scholar]
- 31. Ye J, Yao P, Shi X, Yu X. A systematic literature review and meta-analysis on the impact of COPD on atrial fibrillation patient outcome. Heart Lung . 2022;51:67–74. doi: 10.1016/j.hrtlng.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 32. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. ESC Scientific Document Group 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J . 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 33. Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysis. Chest . 2017;151:544–554. doi: 10.1016/j.chest.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 34. Kahn SR, de Wit K. Pulmonary embolism. HN Engl J Med . 2022;387:45–57. doi: 10.1056/NEJMcp2116489. [DOI] [PubMed] [Google Scholar]
- 35. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. ESC Scientific Document Group 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J . 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 36. Couturaud F, Bertoletti L, Pastre J, Roy PM, Le Mao R, Gagnadoux F, et al. PEP Investigators Prevalence of pulmonary embolism among patients with COPD hospitalized with acutely worsening respiratory symptoms. JAMA . 2021;325:59–68. doi: 10.1001/jama.2020.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiménez D, Agustí A, Tabernero E, Jara-Palomares L, Hernando A, Ruiz-Artacho P, et al. SLICE Trial Group Effect of a pulmonary embolism diagnostic strategy on clinical outcomes in patients hospitalized for COPD exacerbation: a randomized clinical trial. JAMA . 2021;326:1277–1285. doi: 10.1001/jama.2021.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med . 1998;129:1044–1049. doi: 10.7326/0003-4819-129-12-199812150-00009. [DOI] [PubMed] [Google Scholar]
- 39. Crisafulli E, Manco A, Ferrer M, Huerta A, Micheletto C, Girelli D, et al. Pneumonic versus nonpneumonic exacerbations of chronic obstructive pulmonary disease. Semin Respir Crit Care Med . 2020;41:817–829. doi: 10.1055/s-0040-1702196. [DOI] [PubMed] [Google Scholar]
- 40. Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax . 2015;70:984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calverley PMA, Stockley RA, Seemungal TAR, Hagan G, Willits LR, Riley JH, et al. Investigating New Standards for Prophylaxis in Reduction of Exacerbations (INSPIRE) Investigators Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest . 2011;139:505–512. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 42. Vestbo J, Waterer G, Leather D, Crim C, Diar Bakerly N, Frith L, et al. Salford Lung Study Investigators Mortality after admission with pneumonia is higher than after admission with an exacerbation of COPD. Eur Respir J . 2022;59:2102899. doi: 10.1183/13993003.02899-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect . 2011;17:E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Debray MP, Carette MF, Loubet P, Pasquet B, Houhou Fidouh N, Benjoar M, et al. ESCAPED study group CT features of community-acquired pneumonia at the emergency department. Respir Med Res . 2022;81:100892. doi: 10.1016/j.resmer.2022.100892. [DOI] [PubMed] [Google Scholar]
- 45. Bafadhel M, Clark TW, Reid C, Medina MJ, Batham S, Barer MR, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest . 2011;139:1410–1418. doi: 10.1378/chest.10-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA . 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 47. Pulia MS, Lindenauer PK. Web exclusive. Annals for hospitalists inpatient notes - a critical look at procalcitonin testing in pneumonia. Ann Intern Med . 2021;174:HO2–HO3. doi: 10.7326/M21-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karakioulaki M, Stolz D. Biomarkers and clinical scoring systems in community-acquired pneumonia. Ann Thorac Med. 2019;14:165–172. doi: 10.4103/atm.ATM_305_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2021;203:24–36. doi: 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Soler N, Esperatti M, Ewig S, Huerta A, Agustí C, Torres A. Sputum purulence-guided antibiotic use in hospitalised patients with exacerbations of COPD. Eur Respir J . 2012;40:1344–1353. doi: 10.1183/09031936.00150211. [DOI] [PubMed] [Google Scholar]
- 51.Poole S, Tanner AR, Naidu VV, Borca F, Phan H, Saeed K, et al. Molecular point-of-care testing for lower respiratory tract pathogens improves safe antibiotic de-escalation in patients with pneumonia in the ICU: results of a randomised controlled trial. J Infect. 2022;85:625–633. doi: 10.1016/j.jinf.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 52. Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis . 2017;12:1401–1411. doi: 10.2147/COPD.S132961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du Q, Jin J, Liu X, Sun Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One . 2016;11:e0150532. doi: 10.1371/journal.pone.0150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Traversi L, Miravitlles M, Martinez-Garcia MA, Shteinberg M, Bossios A, Dimakou K, et al. ROSE: radiology, obstruction, symptoms and exposure - a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC airways working group. ERJ Open Res . 2021;7:00399-2021. doi: 10.1183/23120541.00399-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J . 2017;50:1700629. doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 56. Barrecheguren M, Pinto L, Mostafavi-Pour-Manshadi SMY, Tan WC, Li PZ, Aaron SD, et al. CanCOLD Collaborative Research Group and the Canadian Respiratory Research Network Identification and definition of asthma-COPD overlap: the CanCOLD study. Respirology . 2020;25:836–849. doi: 10.1111/resp.13780. [DOI] [PubMed] [Google Scholar]
- 57.Global Initiative For Asthma. 2022. https://ginasthma.org/gina-reports/
- 58. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med . 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med . 2016;193:299–309. doi: 10.1164/rccm.201504-0841OC. [DOI] [PubMed] [Google Scholar]
- 60. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med . 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 61. Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr Opin Pulm Med . 2012;18:418–427. doi: 10.1097/MCP.0b013e328356803b. [DOI] [PubMed] [Google Scholar]
- 62. Zantah M, Dotan Y, Dass C, Zhao H, Marchetti N, Criner GJ. Acute exacerbations of COPD versus IPF in patients with combined pulmonary fibrosis and emphysema. Respir Res . 2020;21:164. doi: 10.1186/s12931-020-01432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med . 1996;154:6–17. doi: 10.1164/ajrccm.154.1.8680700. [DOI] [PubMed] [Google Scholar]
- 64. Quint JK, Baghai-Ravary R, Donaldson GC, Wedzicha JA. Relationship between depression and exacerbations in COPD. Eur Respir J . 2008;32:53–60. doi: 10.1183/09031936.00120107. [DOI] [PubMed] [Google Scholar]
- 65. Iyer AS, Parekh TM, O’Toole J, Bhatt SP, Eakin MN, Krishnan JA, et al. COPDGene and SPIROMICS Investigators Clinically significant and comorbid anxiety and depression symptoms predict severe respiratory exacerbations in smokers: a post hoc analysis of the COPDGene and SPIROMICS cohorts. Ann Am Thorac Soc . 2022;19:143–146. doi: 10.1513/AnnalsATS.202103-240RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis . 2014;9:315–330. doi: 10.2147/COPD.S53255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oleynick C. Recurrent episodes of hypercapnic respiratory failure triggered by panic attacks in a patient with chronic obstructive pulmonary disease. Respir Med Case Rep . 2020;30:101044. doi: 10.1016/j.rmcr.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goossens L, Leibold N, Peeters R, Esquivel G, Knuts I, Backes W, et al. Brainstem response to hypercapnia: a symptom provocation study into the pathophysiology of panic disorder. J Psychopharmacol . 2014;28:449–456. doi: 10.1177/0269881114527363. [DOI] [PubMed] [Google Scholar]
- 69. Cheng YL, Huang TW, Lin CK, Lee SC, Tzao C, Chen JC, et al. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg . 2009;138:192–195. doi: 10.1016/j.jtcvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 70. Mishra R, Patel R, Khaja M. Cannabis-induced bullous lung disease leading to pneumothorax: case report and literature review. Medicine (Baltimore) . 2017;96:e6917. doi: 10.1097/MD.0000000000006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feller-Kopman D, Light R. Pleural disease. N Engl J Med . 2018;378:740–751. doi: 10.1056/NEJMra1403503. [DOI] [PubMed] [Google Scholar]
- 72. Zanobetti M, Scorpiniti M, Gigli C, Nazerian P, Vanni S, Innocenti F, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest . 2017;151:1295–1301. doi: 10.1016/j.chest.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 73. Muruganandan S, Azzopardi M, Thomas R, Fitzgerald DB, Kuok YJ, Cheah HM, et al. The pleural effusion and symptom evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur Respir J . 2020;55:1900980. doi: 10.1183/13993003.00980-2019. [DOI] [PubMed] [Google Scholar]
- 74. Alotaibi NM, Chen V, Hollander Z, Hague CJ, Murphy DT, Leipsic JA, et al. Phenotyping COPD exacerbations using imaging and blood-based biomarkers. Int J Chron Obstruct Pulmon Dis . 2018;13:217–229. doi: 10.2147/COPD.S152484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chiumello D, Sferrazza Papa GF, Artigas A, Bouhemad B, Grgic A, Heunks L, et al. ERS statement on chest imaging in acute respiratory failure. Eur Respir J . 2019;54:1900435. doi: 10.1183/13993003.00435-2019. [DOI] [PubMed] [Google Scholar]
- 76. Putcha N, Fawzy A, Paul GG, Lambert AA, Psoter KJ, Sidhaye VK, et al. SPIROMICS investigators Anemia and adverse outcomes in a chronic obstructive pulmonary disease population with a high burden of comorbidities. An analysis from SPIROMICS. Ann Am Thorac Soc . 2018;15:710–717. doi: 10.1513/AnnalsATS.201708-687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Markoulaki D, Kostikas K, Papatheodorou G, Koutsokera A, Alchanatis M, Bakakos P, et al. Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructivepulmonary disease. Eur J Intern Med. 2011;22:103–107. doi: 10.1016/j.ejim.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Agusti A, Faner R, Celli B, Rodriguez-Roisin R. Precision medicine in COPD exacerbations. Lancet Respir Med. 2018;6:657–659. doi: 10.1016/S2213-2600(18)30296-0. [DOI] [PubMed] [Google Scholar]