To the Editor:
Patients with acute hypoxemic respiratory failure (AHRF) are at risk of deleterious effects of insufficient, dyssynchronous, or excessively strong respiratory effort. Patients with efforts of the same magnitude as healthy subjects at rest had better outcomes than those with stronger or weaker efforts (1). Frequent dyssynchronies (2) were associated with higher mortality (3) but not in all studies. In an animal model of AHRF, the protective versus injurious effect of dyssynchrony on diaphragm function was determined by the magnitude of effort (4). Therefore, more so than the prevalence of dyssynchrony, the magnitude of effort might play a role.
Noninvasive techniques (e.g., airway occlusion pressure) intermittently estimate the magnitude of synchronous efforts (5). There is no automated technique for quantifying dyssynchronous efforts; therefore the epidemiology of their magnitude remains unknown.
The aims of this study were to 1) develop and validate an automated algorithm on the basis of esophageal pressure (Peso) to generate and quantify muscular pressure (Pmus) for synchronous and dyssynchronous efforts and 2) describe the magnitude and timing of synchronous and dyssynchronous efforts in patients with AHRF under different modes of ventilation and compared with healthy subjects.
Methods
Recordings containing flow, airway pressure, and Peso were obtained from multiple studies with ethics approval and informed consent. Fifty-four patients with moderate and severe AHRF (BEARDS [Incidence of Breathing Efforts in Early ARDS; NCT 03447288] [6]), 9 patients after cardiac surgery during spontaneous breathing trials (EFFORT [Acceptable Range of Inspiratory Effort During Mechanical Ventilation; NCT 02838524]), and 9 healthy subjects at rest and with increased resistance (RegAIN [Effects of Abnormal Respiratory Mechanics and Assisted Mechanical Ventilation on Neuro-Regulation of Respiration; NCT 01818219]) were included either in the derivation or the validation cohort (36 in each). Because exhaustive visual analysis by experts was necessary for algorithm development and validation, a selection of recordings was performed, resulting in 121 recordings (median [interquartile range], 1 [1–2] per subject) containing 22,041 respiratory cycles. Selection was at random for EFFORT and RegAIN. For BEARDS, random selection was performed within specific categories in available recordings (N = 2,991) after removing those technically not acceptable (4%). Categories for random selection were defined by ventilation mode, presence of effort, triggering, and synchrony, to ensure varied representation of events.
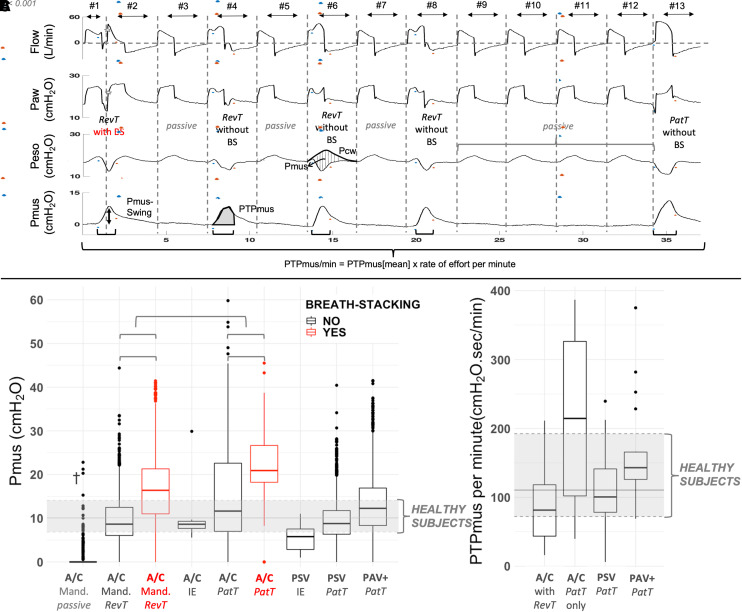
Respiratory cycles were labeled by experts (T. Pham, T. Piraino, R.C., I.T., and L.B.) (for gold-standard definitions, see https://figshare.com/s/0a146e152c25519f1b34, modified from Reference 6) as mandatory passive, mandatory with reverse triggering without or with breath stacking, patient-triggered without or with breath stacking, and ineffective efforts (Figure 1).
Figure 1.
Event categories and breathing effort according to each category and mode of ventilation. (A) Recording on pressure-control ventilation illustrating different event categories. From top to bottom flow, airway pressure, esophageal pressure (Peso), and muscular pressure (Pmus) tracings are shown. The start and end of each respiratory cycle (breath) are defined by the zero crossing of flow (vertical dashed lines). An inspiratory effort is defined by the positive deflection in Pmus (horizontal black lines). The start and end of each effort are marked with blue and red dots, respectively. Reverse-triggering (RevT) events are seen on breaths #1, #2, #4, #6, and #8, interposed with mandatory passive events (#3, #5, and #7) in a 1:2 ratio. The first RevT event (breaths #1 and #2) results in breath stacking (BS) (star). A series of mandatory passive events is seen from breaths #9–#12, where Pmus is zero (flat line). A patient-triggered (PatT) event is seen at the end (breath #13). Instantaneous calculation of Pmus is represented with vertical dashed lines on breath #6 in the Peso tracing (i.e., difference between chest wall recoil pressure [Pcw] and Peso). Pcw is the expected increase in Peso during a hypothetical passive inflation with the same Vt, calculated as the product of chest wall elastance and Vt (see similarity between Pcw in breath #6 and Peso in breaths #5 and #7, where inflation is passive but Vt is smaller). The black vertical arrow in the Pmus tracing (breath #2) illustrates the maximum positive deflection in Pmus during each inspiratory effort (Pmus swing), and the gray area (breath #4) illustrates the pressure–time product. (B) Pmus swings according to the type of event and mode of ventilation in patients with acute hypoxemic respiratory failure. Median and interquartile range of Pmus swing in healthy subjects breathing at rest without inspiratory assistance are displayed as gray horizontal full and dashed lines, respectively (comparison with healthy subjects at rest: RevT without BS and PaT on pressure support, P > 0.05; PatT in A/C, ineffective efforts in assist-control mode [A/C] and pressure support mode [PSV], P < 0.05, PaT during proportional-assist ventilation with adjustable gain factors [PAV+], P < 0.01; passive, RevT, and PaT with BS on A/C, P < 0.001). Events with BS are seen in red, showing a higher Pmus swing versus corresponding events without BS. Numbers of events were as follows: A/C mandatory breath (Mand.) passive, n = 10,017; A/C Mand. RevT without BS, n = 1,387; A/C Mand. RevT with BS, n = 455; A/C ineffective effort (IE), n = 27; A/C PatT without BS, n = 1,334; A/C PatT with BS, n = 58; PSV IE, n = 23; PSV PatT, n = 3,212; and PAV+ PatT, n = 2,572. Twelve breathing efforts with Pmus swing higher than 60 cm H2O were removed to improve data visualization. (C) Pressure–time product per minute for recordings during different modes of ventilation and containing different types of events in patients with acute hypoxemic respiratory failure. The reference median and interquartile range of PTPmus per minute for recordings of healthy subjects breathing at rest without inspiratory assistance are displayed as gray horizontal full and dashed lines, respectively (comparison with healthy subjects at rest: A/C with RevT, PSV, and PAV+, P > 0.05; A/C with only PaT, P < 0.05). Numbers of recordings (breathing efforts) were as follows: A/C with RevT, n = 18 (n = 2,333); A/C only PatT, n = 4 (n = 887); PSV PatT, n = 21 (n = 3,029); and PAV+ PatT, n = 24 (n = 2,564). †Sixty-three efforts out of 10,017 mandatory passive events were erroneously detected by the algorithm because of an artifact in the Peso signal in passive breaths (rate of false positives < 1%), resulting in measurement of effort (Pmus swing). Paw = airway pressure; PTPmus = pressure–time product measured according to the algorithm; PTPmus[mean] = mean pressure–time product measured according to the algorithm for one recording.
Pmus is the pressure generated by the respiratory muscles to displace the chest wall from its resting position: it becomes positive when Peso deviates from the expected increase during passive insufflation (chest wall recoil pressure), making it easy to recognize efforts during assisted ventilation (Figure 1). Methodological details are available at https://figshare.com/s/0a146e152c25519f1b34.
The algorithm automatically recognized efforts and their start and end by using the first- and second-derivative difference approximations of Pmus. Then, each respiratory cycle (defined by zero crossings of flow) was associated with an effort when present and classified into one of the categories shown in Figure 1, using a decision tree based on the timing of effort versus the respiratory cycle.
Each effort was characterized with maximum positive deflection (Pmus swing), the integral of Pmus over the whole effort (PTPmus), and effort per minute (PTPmus/min) (Figure 1).
A sample size of 40 recordings including 7,600 events was necessary for algorithm validation to detect efforts and classify reverse triggering with a sensitivity of >89%, specificity of >94%, α of 0.05, and power of 0.8, considering a rate of effort of >50% and reverse triggering of 15% (6, 7).
Descriptive statistics are expressed as proportions, mean (SD), or median (interquartile range); normality was assessed using the Shapiro-Wilk test.
Accuracy for the detection of events was compared with the gold standard in the validation data set. PTPmus was compared with the classical pressure–time product (time references were based on flow) (8) for purely spontaneous or synchronous breaths (end of effort simultaneous to the end of insufflation).
We used mixed-effects regression models to account for repeated measures (random intercept for participants) with Tukey adjustment for pairwise comparisons between effort in different event categories.
MATLAB version R2018b (The MathWorks) was used for algorithm development and R version 4.1.2 (www.R-project.org) for statistical analyses.
Results
Characteristics of subjects and diagnostic accuracy of the algorithm are displayed in Table 1. Accuracy was 95% for effort detection and 97% for reverse triggering. Bias in the measurement of PTPmus per breath versus classical pressure–time product (8) was low: 0.4 cm H2O ⋅ s (limits of agreement, 2.9 to −2.1 cm H2O ⋅ s).
Table 1.
Subjects’ Clinical Characteristics, Clinical Data Simultaneous with Recordings, and Algorithm’s Diagnostic Accuracy
| Subjects’ Clinical Characteristics | Acute Hypoxemic Respiratory Failure |
Cardiac: SBT |
Healthy Subjects |
|||
|---|---|---|---|---|---|---|
| DERIV (n = 26) | VALID (n = 28) | DERIV (n = 5) | VALID (n = 4) | DERIV (n = 5) | VALID (n = 4) | |
| Age, yr, mean (SD) | 61.7 (13.7) | 65.4 (11.7) | 64.0 (6.48) | 62.2 (13.9) | 23.8 (3.50) | 24.6 (3.51) |
| Female, n (%) | 5 (18) | 8 (31) | 0 (0) | 2 (40) | 0 (0) | 0 (0) |
| Height, cm, mean (SD) | 171 (8.12) | 169 (9.23) | 172 (12.2) | 172 (10.7) | 175 (8.81) | 180 (3.96) |
| APACHE III score | 74.9 (25.0) | 92.8 (34.1) | — | — | — | — |
| Comorbidities, n (%) | ||||||
| Hypertension | 12 (43) | 13 (50) | 4 (80) | 4 (100) | 0 (0) | 0 (0) |
| COPD | 7 (25) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diabetes | 10 (36) | 11 (42) | 4 (80) | 1 (25) | 0 (0) | 0 (0) |
| Chronic kidney disease | 5 (18) | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Left ventricular failure | 6 (21) | 1 (4) | 1 (20) | 0 (0) | 0 (0) | 0 (0) |
| Oncohematologic | 2 (7) | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Etiology of hypoxemia, n (%) | ||||||
| Pneumonia | 20 (71) | 20 (77) | — | — | — | — |
| Aspiration | 2 (7) | 6 (23) | — | — | — | — |
| Trauma | 3 (11) | 1 (4) | — | — | — | — |
| Nonpulmonary sepsis | 3 (11) | 3 (11) | — | — | — | — |
| Drug toxicity | 0 (0.0) | 2 (8) | — | — | — | — |
| Clinical data simultaneous with recordings | ||||||
| Days on MV up to the recording, median (IQR) | 6 (4–7) | 5 (3–8) | — | — | — | — |
| Prone-position recordings, n (%) | 4 (7) | 0 (0) | — | — | — | — |
| SAS, median (range) | 3 (1–5) | 2 (1–4) | — | — | — | — |
| P0.1, cm H2O, median (IQR) (recordings with patient-triggered breaths) | 2.9 (1.8–4.2) | 2.1 (1.5–3.8) | — | — | — | — |
| PaO2:FiO2 mm Hg, median (IQR) | 220.0 (162.0–268.0) | 184.0 (159.9–221.1) | — | — | — | — |
| pH, median (IQR) | 7.37 (7.32–7.42) | 7.39 (7.36–7.43) | — | — | — | — |
| PaCO2 mm Hg, median (IQR) | 44.0 (38.0–50.0) | 44.0 (40.2–53.3) | — | — | — | — |
| Midazolam-infusion recordings, n (%) | 18 (32) | 13 (46) | — | — | — | — |
| Propofol-infusion recordings, n (%) | 15 (26) | 8 (29) | — | — | — | — |
| Opioid-infusion recordings, n (%) | 45 (79) | 23 (82) | — | — | — | — |
| NMBA-infusion recordings, n (%) | 10 (18) | 5 (18) | — | — | — | — |
| Algorithm performance (VALID) Total 7,471 respiratory cycles plus 50 IEs |
Effort detection: Acc = 0.95, Se = 0.93, Sp = 0.98, PPV = 0.99, NPV = 0.87 PatT vs. Mand: Acc = 0.99, Se = 0.98, Sp = 0.99, PPV = 0.99, NPV = 0.98 BS: Acc = 1.00, Se = 0.96, Sp = 1.00, PPV = 0.94, NPV = 1.00 Overall RevT: Acc = 0.97, Se = 0.90, Sp = 0.98, PPV = 0.89, NPV = 0.99 RevT without BS: Acc = 0.98, Se = 0.88, Sp = 0.99, PPV = 0.87, NPV = 0.99 RevT with BS: Acc = 1.00, Se = 0.95, Sp = 1.00, PPV = 0.96, NPV = 1.00 PatT with BS: Acc = 1.00, Se = 0.79, Sp = 1.00, PPV = 0.66, NPV = 1.00 IEs: Acc = 1.00, Se = 0.69, Sp = 1.00, PPV = 0.70, NPV = 1.00 |
|||||
Definition of abbreviations: Acc = accuracy; APACHE = Acute Physiology and Chronic Health Evaluation; BS = breath stacking; COPD = chronic obstructive pulmonary disease; DERIV = derivation data set; IE = ineffective effort; IQR = interquartile range; Mand = mandatory; MV = mechanical ventilation; NMBA = neuromuscular-blocking agents; NPV = negative predictive value; P0.1 = airway occlusion pressure; PatT = patient triggered; PPV = positive predictive value; RevT = reverse triggered; SAS = sedation agitation score; SBT = spontaneous breathing trial; Se = sensitivity; Sp = specificity; VALID = validation data set.
Degrees of effort for the different types of breaths and modes of ventilation in patients with AHRF are shown in Figure 1.
Discussion
Our approach for accurate detection and quantification of the magnitude of synchronous and dyssynchronous efforts using Pmus allows to characterize all types of efforts in patients with AHRF and to compare them with those of healthy subjects. We deliberately targeted a specific population. Patients with hypoxemic respiratory failure requiring prolonged mechanical ventilation are among the most vulnerable ICU patients, in whom attention to the interaction with the ventilator in the first days may be essential.
Previous techniques for dyssynchrony detection did not quantify the magnitude of effort and were restricted to specific dyssynchronies, modes of ventilation, or ventilators (9). Our approach measures instantaneous effort and effort per minute. Pressure–time product per minute could not be obtained during dyssynchronies using classical methods (8), because of the lack of reference time points in the flow. Pressure–time product per minute correlates with the oxygen cost of breathing and quantifies power (i.e., energy per unit of time) applied to the lung by the respiratory muscles. Several dyssynchronies exist under the category of patient-triggered events (2). However, we focus here on the comparison between patient-triggered and reverse-triggered breaths, given the high prevalence of these two in the first days of mechanical ventilation for patients with AHRF (7). Reverse triggering is recognized as a frequent event that can generate breath stacking, excessive effort, or volume and its magnitude can be quantified by the algorithm, thus offering an estimate of its protective versus injurious effects (4). Effort during reverse triggering without breath stacking was similar to that in healthy subjects at rest. Stronger efforts were observed overall during breath stacking in assist-control mode, being potentially injurious to the lung and diaphragm, with large individual variations. Surprisingly, patient triggering in assist-control mode showed the highest effort, probably due to high drive in moderate and severe AHRF combined with limited peak flow, Vt, and/or insufflation time. This concerning finding will need further exploration.
The need for Peso and the calculation/estimation of chest wall recoil pressure impose limitations. However, if the clinical relevance of the magnitude of synchronous and dyssynchronous effort is further demonstrated, this might justify using Peso for monitoring more often. The algorithm can be implemented in real time. We did not aim here to describe the epidemiology of breathing efforts, and analysis of BEARDS will offer an epidemiological description and evaluation of the clinical consequences of different types of effort.
Acknowledgments
BEARDS investigators: The study would not have been possible without the work of all participants, and as such, they must be cited as collaborators. The BEARDS sites and investigators and their affiliations are as follows: Laurent Brochard, Irene Telias, Felipe Damiani, Cesar Santis, Tài Pham, Thomas Piraino, Michael Sklar, Audery Kim, and Megan Abbott, Saint Michael’s Hospital, Unity Health Toronto, Toronto, Ontario, Canada. Tommaso Mauri, Elena Spinelli, Giacomo Grasselli, Nadia Corcione, and Francesca Dalla Corte, Dipartimento di Anestesia, Rianimazione ed Emergenza-Urgenza, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy. Savino Spadaro and Carlo Alberto Volta, Department of Morphology, Surgery and Experimental Medicine, Section of Intensive Care Unit, University of Ferrara, Sant’Anna Hospital, Ferrara, Italy. Francesco Mojoli, Anesthesia and Intensive Care, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Policlinico San Matteo, Pavia, Italy. Dimitris Georgopoulos, Eumorfia Kondili, and Stella Soundoulounaki, Department of Intensive Care Medicine, University Hospital of Heraklion and School of Medicine, University of Crete, Heraklion, Crete, Greece. Tobias Becher, Norbert Weiler, and Dirk Schaedler, Department of Anesthesiology and Intensive Care Medicine, University Medical Center Schleswig-Holstein, Campus Kiel, Kiel, Germany. Oriol Roca and Manel Santafe, Critical Care Department, Vall d’Hebron University Hospital, Vall d’Hebron Research Institute and Ciber Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain. Jordi Mancebo and Nuria Rodriguez, Intensive Care Medicine, Hospital de Sant Pau, Barcelona, Spain. Leo Heunks and Heder de Vries, Department of Intensive Care, Amsterdam UMC Location VUmc, Amsterdam, the Netherlands, and Amsterdam Cardiovascular Sciences Research Institute, Amsterdam, the Netherlands. Chang Wen Chen, National Cheng Kung University Hospital, College of Medicine, National Cheng-Kung University, Tainan, Taiwan. Jian-Xin Zhou and Guang-Qiang Chen, Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, China. Nuttapol Rittayamai, Division of Respiratory Diseases and Tuberculosis, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. Norberto Tiribelli, Servicio de Kinesiología, Unidad de Medicina Crítica y Terapia Intensiva, Complejo Médico de la Policía Federal Argentina Churruca Visca, Buenos Aires, Argentina. Sebastian Fredes, Sanatorio de la Trinidad Mitre, Buenos Aires, Argentina. Ricard Artigas Mellado and Carlos Ferrando Ortolá, Hospital Clinic, Barcelona, Spain. François Beloncle and Alain Mercat, Medical Intensive Care Unit, University Hospital of Angers, Angers, France. Jean-Michel Arnal, Service de Réanimation Polyvalente, Hôpital Sainte Musse, Toulon, France. Jean-Luc Diehl and Romy Younan, Medical Intensive Care Unit, Hôpital Européen Georges Pompidou, Assistance Publique–Hôpitaux de Paris, Paris, France. Alexandre Demoule, Martin Dres, and Quentin Fossé, AP-HP, Groupe Hospitalier Pitié-Salpêtrière Charles Foix, Service de Pneumologie, Médecine Intensive – Réanimation (Département “R3S”), Paris, France. Sébastien Jochmans and Jonathan Chelly, Groupe Hospitalier Sud Ile-De-France, Centre Hospitalier de Melun, Melun, France. Nicolas Terzi and Claude Guérin, Médecine Intensive Réanimation, CHU de Grenoble-Alpes, Grenoble, France. Elias Baedorf Kassis, Division of Pulmonary and Critical Care, Beth Israel Deaconess Medical Center and Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts. Jeremy Beitler, Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University College of Physicians and Surgeons, NewYork-Presbyterian Hospital, New York, New York. Davide Chiumello and Erica Ferrari Luca Bolgiaghi, Department of Anesthesia and Intensive Care, Sao Paolo Hospital, University of Milano, Milan, Italy. Arnaud W. Thille and Rémi Coudroy, Médecine Intensive Réanimation, Centre Hospitalier Universitaire de Poitiers, Poitiers, France. Laurent Papazian and C. Guervilly, Assistance Publique–Hôpitaux de Marseille, Hôpital Nord, Médecine Intensive Réanimation; Aix-Marseille Université, Faculté de Médecine, Centre d’Etudes et de Recherches sur les Services de Santé et Qualité de Vie EA 3279, Marseille, France. Ewan C. Goligher and Niall D. Ferguson, Division of Respirology, Department of Medicine, University Health Network and Sinai Health System, Toronto, Ontario, Canada.
Footnotes
A complete list of BEARDS Investigators members may be found before the beginning of the References.
Supported by a salary support grant from the Canadian Institutes for Health Research in the form of a Post-Doctoral Fellowship (I.T.), the Keenan Chair in Critical Care and Acute Respiratory Failure (L.B.).
Author Contributions: I.T., M.M., and L.B. designed the study, analyzed all data together, and interpreted the results. I.T., T. Pham, T. Piraino, R.C., and L.B. labeled the recordings (gold standard). I.T., T. Pham, M.C.S., and L.B. conducted the EFFORT study. I.T., T. Pham, T. Piraino, R.C., M.C.S., E.K., S.S., T.B., C.-W.C., T.M., L.B., and the BEARDS investigators conducted the BEARDS study. L.P. and L.B. conducted the RegAIN study. I.T. and L.B. drafted the manuscript. All authors had full access to all of the study data, contributed to drafting the manuscript or critically revised it for important intellectual content, approved the final version of the manuscript, and took responsibility for the integrity of the data and the accuracy of the data analysis. The Pleural Pressure Working Group is an international group dedicated to the study of applied physiology in the critically ill.
Originally Published in Press as DOI: 10.1164/rccm.202211-2086LE on February 27, 2023
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
on behalf of the BEARDS and PLUG Investigators:
Laurent Brochard, Irene Telias, Felipe Damiani, Cesar Santis, Tài Pham, Thomas Piraino, Michael Sklar, Audery Kim, Megan Abbott, Tommaso Mauri, Elena Spinelli, Giacomo Grasselli, Nadia Corcione, Francesca Dalla Corte, Savino Spadaro, Carlo Alberto Volta, Francesco Mojoli, Dimitris Georgopoulos, Eumorfia Kondili, Stella Soundoulounaki, Tobias Becher, Norbert Weiler, Dirk Schaedler, Oriol Roca, Manel Santafe, Jordi Mancebo, Nuria Rodriguez, Leo Heunks, Heder de Vries, Chang Wen Chen, Jian-Xin Zhou, Guang-Qiang Chen, Nuttapol Rittayamai, Norberto Tiribelli, Sebastian Fredes, Ricard Artigas Mellado, Carlos Ferrando Ortolá, François Beloncle, Alain Mercat, Jean-Michel Arnal, Jean-Luc Diehl, Romy Younan, Alexandre Demoule, Martin Dres, Quentin Fossé, Sébastien Jochmans, Jonathan Chelly, Nicolas Terzi, Claude Guérin, Elias Baedorf Kassis, Jeremy Beitler, Davide Chiumello, Erica Ferrari Luca Bolgiaghi, Arnaud W. Thille, Rémi Coudroy, Laurent Papazian, C. Guervilly, Ewan C. Goligher, and Niall D. Ferguson
References
- 1. Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med . 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 2. Pham T, Telias I, Piraino T, Yoshida T, Brochard LJ. Asynchrony consequences and management. Crit Care Clin . 2018;34:325–341. doi: 10.1016/j.ccc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 3. Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med . 2015;41:633–641. doi: 10.1007/s00134-015-3692-6. [DOI] [PubMed] [Google Scholar]
- 4. Damiani LF, Engelberts D, Bastia L, Osada K, Katira BH, Otulakowski G, et al. Impact of reverse triggering dyssynchrony during lung-protective ventilation on diaphragm function: an experimental model. Am J Respir Crit Care Med . 2022;205:663–673. doi: 10.1164/rccm.202105-1089OC. [DOI] [PubMed] [Google Scholar]
- 5. Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med . 2020;201:1086–1098. doi: 10.1164/rccm.201907-1425OC. [DOI] [PubMed] [Google Scholar]
- 6. Pham T, Montanya J, Telias I, Piraino T, Magrans R, Coudroy R, et al. BEARDS Study Investigators Automated detection and quantification of reverse triggering effort under mechanical ventilation. Crit Care . 2021;25:60. doi: 10.1186/s13054-020-03387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodrigues A, Telias I, Damiani LF, Brochard L. Reverse triggering during controlled ventilation: from physiology to clinical management. Am J Respir Crit Care Med . 2023;207:533–543. doi: 10.1164/rccm.202208-1477CI. [DOI] [PubMed] [Google Scholar]
- 8. Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med . 1997;155:906–915. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- 9. Sinderby C, Liu S, Colombo D, Camarotta G, Slutsky AS, Navalesi P, et al. An automated and standardized neural index to quantify patient-ventilator interaction. Crit Care . 2013;17:R239. doi: 10.1186/cc13063. [DOI] [PMC free article] [PubMed] [Google Scholar]