Abstract
Rationale
Allergic asthma is linked to impaired bronchial epithelial secretion of IFNs, which may be causally linked to the increased risk of viral exacerbations. We have previously shown that allergen immunotherapy (AIT) effectively reduces asthma exacerbations and prevents respiratory infections requiring antibiotics; however, whether AIT alters antiviral immunity is still unknown.
Objectives
To investigate the effect of house dust mite sublingual AIT (HDM-SLIT) on bronchial epithelial antiviral and inflammatory responses in patients with allergic asthma.
Methods
In this double-blind, randomized controlled trial (VITAL [The Effect of Allergen Immunotherapy on Anti-viral Immunity in Patients with Allergic Asthma]), adult patients with HDM allergic asthma received HDM-SLIT 12-SQ or placebo for 24 weeks. Bronchoscopy was performed at baseline and at Week 24, which included sampling for human bronchial epithelial cells. Human bronchial epithelial cells were cultured at baseline and at Week 24 and stimulated with the viral mimic polyinosinic:polycytidylic acid (poly(I:C)). mRNA expression was quantified using qRT-PCR, and protein concentrations were measured using multiplex ELISA.
Measurements and Main Results
Thirty-nine patients were randomized to HDM-SLIT (n = 20) or placebo (n = 19). HDM-SLIT resulted in increased polyinosinic:polycytidylic acid–induced expression of IFN-β at both the gene (P = 0.009) and protein (P = 0.02) levels. IFN-λ gene expression was also increased (P = 0.03), whereas IL-33 tended to be decreased (P = 0.09). On the other hand, proinflammatory cytokines IL-6 (P = 0.009) and TNF-α (tumor necrosis factor-α) (P = 0.08) increased compared with baseline in the HDM-SLIT group. There were no significant changes in TSLP (thymic stromal lymphopoietin), IL-4, IL-13, and IL-10.
Conclusions
HDM-SLIT improves bronchial epithelial antiviral resistance to viral infection. These results potentially explain the efficacy of HDM-SLIT in reducing exacerbations in allergic asthma.
Clinical trial registered with www.clinicaltrials.gov (NCT 04100902).
Keywords: antiviral immunity, allergic asthma, allergen immunotherapy, bronchial epithelium, airway resistance
At a Glance Commentary
Scientific Knowledge on the Subject
Allergic asthma is linked to impaired production of antiviral IFNs and increased risk of airway infections causing exacerbation. Allergen immunotherapy is a disease-modifying treatment that reduces risk of exacerbation in patients with allergic asthma, indicative of improved airway immunity.
What This Study Adds to the Field
In this randomized, double-blind, placebo-controlled trial of house dust mite sublingual immunotherapy, airway epithelial IFN-β and IFN-λ were significantly enhanced and IL-33 was decreased in response to polyinosinic:polycytidylic acid, indicative of improved innate airway epithelial responses to viral infection. IL-33 and IFN-β were inversely correlated. Overall, these findings suggest a causal link for the preventive effects of allergen immunotherapy observed on exacerbation and respiratory infections in patients with house dust mite allergic asthma.
Acute asthma exacerbations are most frequently caused by respiratory viral infections and are associated with a high burden of morbidity and increased use of healthcare services (1). Therefore, prevention of exacerbations is a key treatment goal in asthma management. Allergen exposure and viral infection greatly increase the risk of acute exacerbation and the need for hospitalization in allergic asthma (2–4). Allergic asthma has been linked with impaired antiviral IFN secretion (5), but the underlying mechanisms remain unclear (6). Inhaled IFN-β increased morning peak flow and improved Asthma Control Questionnaire score after viral infection in predominantly atopic individuals (7). This underscores the importance of IFN-β dynamics in innate antiviral immune functions and the potential role in the prevention of virally induced exacerbation (8). We have recently shown that allergen immunotherapy (AIT) reduces antibiotic-requiring respiratory infections, as well as exacerbations in both seasonal and perennial allergic asthma, which indicates improved airway immunity (9, 10).
The airway epithelium provides the first line of defense to environmental stimuli and plays a dynamic role in the orchestration of innate and adaptive immune responses to pathogens and allergens (11). Both viral infection and proteolytic allergens (e.g., from house dust mites [HDMs]) cause damage to bronchial epithelial cells and induce TLR (Toll-like receptor)–dependent activation of nuclear factor-κB (NF-κB) in the atopic airway (12). This synergistic activation of pattern recognition receptors promotes type 2 (T2) inflammation and may lead to uncontrolled release of epithelium-derived cytokines and alarmins, including TSLP (thymic stromal lymphopoietin) and IL-33, considered of casual importance in asthma exacerbations (13–15).
Recently, we demonstrated that HDM-sensitized patients with asthma have reduced polyinosinic:polycytidylic acid (poly(I:C))–induced IFN-β expression compared with non–HDM-sensitized patients with asthma (16). Allergic inflammation has been experimentally shown to affect IFN responses at many levels of the T2 cascade: upstream alarmin (IL-33) (17), downstream T2 cytokines IL-4/13 (18), and the allergen–IgE axis (19, 20). The link between atopy and aberrant responses to viral infections is further consolidated by the effect of anti-IgE on the risk of virally induced exacerbations in children with asthma (21). AIT is a disease-modifying treatment with a curative potential for IgE-mediated airway diseases and could have a similar effect (22).
The disease-modifying effects of AIT are still not mechanistically fully understood, but the individual interactions of dendritic, T, B, and epithelial cells seem contributory to the development of clinical tolerance (23). AIT enhances immunotolerance in several ways: 1) direct desensitization of mast cells and basophils; 2) suppression of submucosal T2 inflammation through induction of TGF-β (transforming growth factor-β) and tolerogenic IL-10+ regulatory T cells; and 3) induction of IgE-blocking IgG4 antibodies, which has several modes of action that together favor tolerance and resilience (24, 25). In addition, initial therapy has been shown to induce a broad spectrum of inflammatory responses, including IL-8, CXCL-1 (C-X-C motif chemokine ligand 1), CXCL-2, and IL-1α, but the response to viral infections remains undisclosed (23).
We hypothesized that AIT enhances the canonical IFN antiviral resistance and simultaneously improves host tolerance by decreasing the alarmin response to viral stimulation in bronchial epithelial cells from patients with allergic asthma. To explore this hypothesis, we conducted a randomized, double-blind, placebo-controlled trial in patients with asthma and allergy to HDM, randomized to 24 weeks of treatment with either HDM sublingual AIT (HDM-SLIT) or placebo. In this study, we compared the effect of this treatment on the in vitro response to the viral infection mimic poly(I:C) in human bronchial epithelial cells (HBECs).
Methods
Study Design
This randomized, double-blind, placebo-controlled trial of the effect of AIT on antiviral immunity in patients with allergic asthma (VITAL [The Effect of Allergen Immunotherapy on Anti-viral Immunity in Patients with Allergic Asthma]) was conducted at Bispebjerg Hospital in Copenhagen, Denmark, between January 1, 2020, and February 28, 2022. The study was approved by the local scientific ethics committee (H-19052148) and the Danish Medicines Agency (European Union Drug Regulating Authorities Clinical Trials Database: 2019-003261-18) and was monitored according to Good Clinical Practice guidelines by the Danish Good Clinical Practice Unit. The full study protocol is available at ClinicalTrials.gov (NCT04100902). All patients provided informed consent and were randomized 1:1 to 24 weeks of HDM-SLIT (Acarizax 12-SQ daily, ALK-Abelló A/s) or placebo as an add-on therapy for their maintenance asthma treatment. Full clinical and respiratory evaluation was performed at baseline and at Week 24 before bronchoscopy. A full description of all investigations, procedures, and the randomization process are available in the online Supplement.
Primary Outcome
The primary outcome was the change in IFN-β gene expression in HBECs from baseline to Week 24. As a supportive objective, we assessed change in IFN-λ gene expression.
Study Participants
Eligible patients were nonsmoking adults aged 18–65 years with historic or objectively confirmed diagnoses of asthma on a stable inhaled corticosteroid (ICS) regimen and persistent mild or moderate to severe HDM-induced rhinitis according to Allergic Rhinitis and Its Impact on Asthma guidelines (26) and a skin-prick test wheal diameter >3 mm to HDM or HDM-specific IgE >0.7 kUA/l. Major exclusion criteria included recent treatment with oral corticosteroids within the past 8 weeks, antibiotics of any kind within the past 6 weeks, immunosuppressive medications within the past 3 months, immunoglobulin or blood products within the past 30 days, monoclonal antibodies within <6 months or five half-lives (whichever was longer), and prior treatment with any AIT for any duration. All inclusion and exclusion criteria are available in the online supplement.
Bronchial Epithelial Cell Cultures and Stimulations
HBECs were cultured and stimulated with the TLR3 agonist poly(I:C) (10 μg/ml) for 3 and 24 hours, as previously described (16, 27, 28). A full description is available in the online supplement. Seventeen patients in each group were included for in vitro experiments, as 1 patient in each group did not meet the standards for epithelial confluence in the cell culture setup.
RNA Extraction and qRT-PCR
Total RNA was extracted from HBECs using the RNeasy Plus Mini Kit (Qiagen), and 1 μg RNA was reverse transcribed to complementary DNA (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Thermo Fisher Scientific). Amplification was performed using the AriaMx real-time PCR system (Agilent Technologies), as previously described. Target genes are listed in the online supplement. The −ΔΔCt method (29) was then applied for relative quantification using UBC (ubiquitin C)/GAPDH as housekeeping genes and normalized to unstimulated control expression.
Protein Quantification by Multiplex ELISA
Cytokine concentrations were measured in cell-free HBEC supernatants using a Luminex immunoassay according to the manufacturer’s instructions (R&D Systems). Additional information on these measurements is available in the online supplement.
Statistics
The primary outcome was analyzed in the intention-to-treat population. The Mann-Whitney U test for two-group unpaired comparisons and the Wilcoxon signed rank test for paired comparison were used. P values <0.05 were considered to indicate statistical significance. Data were analyzed using Prism 9.0 (GraphPad). For statistics applied to secondary and explorative outcomes, see the online supplement.
Results
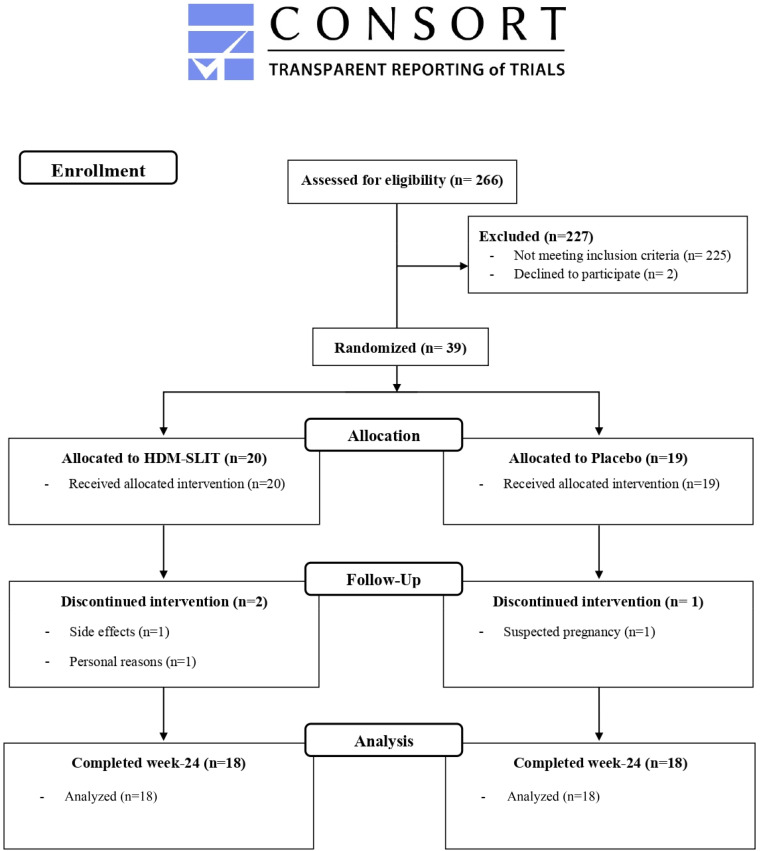
Thirty-nine patients were included and randomized (1:1) to either HDM-SLIT (n = 20) or placebo (n = 19) between February 2020 and February 2022. A Consolidated Standards of Reporting Trials flowchart is provided in Figure 1. Eighteen patients completed the full study protocol in both groups. Patients were equally distributed between the two study arms (Table 1), apart from the daily dose of ICS, which was higher in the placebo group.
Figure 1.
CONSORT flowchart. CONSORT = Consolidated Standards of Reporting Trials; HDM-SLIT = house dust mite sublingual allergen immunotherapy.
Table 1.
Baseline Demographic and Clinical Characteristics in the Intention-to-Treat Population
| Placebo (n = 19) |
HDM-SLIT (n = 20) |
Total (n = 39) |
|
|---|---|---|---|
| Age, yr | 28 ± 8.8 | 28 ± 7.3 | 28 ± 8.0 |
| Female | 12 (63) | 13 (65) | 25 (64) |
| BMI, kg/m2 | 24 ± 4.7 | 23 ± 3.8 | 24 ± 4.2 |
| Ex-smoker | 8 (42) | 5 (25) | 13 (33) |
| Pack-years | 1 (1–8) | 2.5 (1–6) | 1.5 (1–8) |
| ACQ-6 score | 1.8 ± 0.65 | 1.7 ± 0.64 | 1.8 ± 0.64 |
| RQLQ score | 1.74 ± 0.8 | 1.83 ± 1.02 | 1.78 ± 0.91 |
| Prebronchodilator FEV1, L | 3.7 ± 0.71 | 3.6 ± 0.82 | 3.7 ± 0.76 |
| Prebronchodilator FEV1, % predicted | 95 ± 12 | 90 ± 13 | 93 ± 12 |
| FEV1/FVC | 0.80 ± 0.077 | 0.77 ± 0.078 | 0.79 ± 0.077 |
| FEV1 reversibility, % increase | −0.30 ± 5.1 | 4.2 ± 6.0 | 2.0 ± 6 |
| FeNO, ppb | 17 (6–59) | 18 (5–69) | 18 (5–69) |
| Mannitol test, positive | 10 (53) | 13 (65) | 23 (59) |
| PD15 mannitol, mg | 241 (82–461) | 297 (43–584) | 249 (43–584) |
| Monosensitized, HDM | 5 (26) | 6 (30) | 11 (28) |
| Polysensitized | 14 (74) | 14 (70) | 28 (72) |
| Total IgE, kUa ⋅ L−1 | 122 (22.0–361) | 138 (12.0–628) | 130 (12.0–628) |
| IgE Der-p, kUa ⋅ L−1 | 7.12 (0.36–55.7) | 11.6 (0.34–58.1) | 9.13 (0.34–58.1) |
| IgE Der-f, kUa ⋅ L−1 | 7.12 (0.38–73.9) | 12.4 (0.34–150) | 9.46 (0.34–150) |
| Blood eosinophils, ×109 ⋅ L−1 | 0.14 (0.02–0.46) | 0.16 (0.04–0.05) | 0.15 (0.02–0.53) |
| Blood eosinophils ⩾ 0.25 × 109 ⋅ L−1 and/or sputum eosinophils ⩾ 3% | 5 (26) | 5 (25) | 10 (26) |
| ICS total equivalent budesonide dose, μg | 1,020 (400–1,600)* | 736 (400–1,600) | 862 (400–1,600) |
| Asthma severity, high ICS d.d.d. | 8 (42) | 4 (20) | 12 (31) |
| Adherence to primary controller in past 365 days, % | 77 (12–100) | 74 (9–100) | 75 (9–100) |
| More than one exacerbation in ⩽12 mo | 2 (11) | 0 (0) | 2 (5.1) |
| ICS | 5 (26) | 4 (20) | 9 (23) |
| ICS/LABA | 18 (95) | 16 (80) | 34 (87) |
| INCS | 13 (68) | 14 (70) | 27 (69) |
| Antihistamine | 17 (90) | 15 (75) | 32 (36) |
| Ocular antihistamine | 6 (32) | 8 (40) | 14 (36) |
| SABA as needed, puffs/d | 1.3 ± 1.11 | 0.86 ± 1.2 | 1.05 ± 1.14 |
Definition of abbreviations: ACQ-6 = Asthma Control Questionnaire; BMI = body mass index; d.d.d. = daily dose delivered; FeNO = fractional exhaled nitric oxide; HDM = house dust mite; ICS = inhaled corticosteroid; INCS = intranasal corticosteroid; LABA = long-acting β2 agonist; PD15 = provoking dose of mannitol causing a 15% reduction in FEV1; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SABA = short-acting β2 agonist; SLIT = sublingual allergen immunotherapy.
Data are presented as mean ± SD, n (%), or geometric mean (range).
Statistically significant.
Poly(I:C)-induced HBEC Expression of Type I and III IFNs Is Increased after HDM-SLIT in Patients with Allergic Asthma
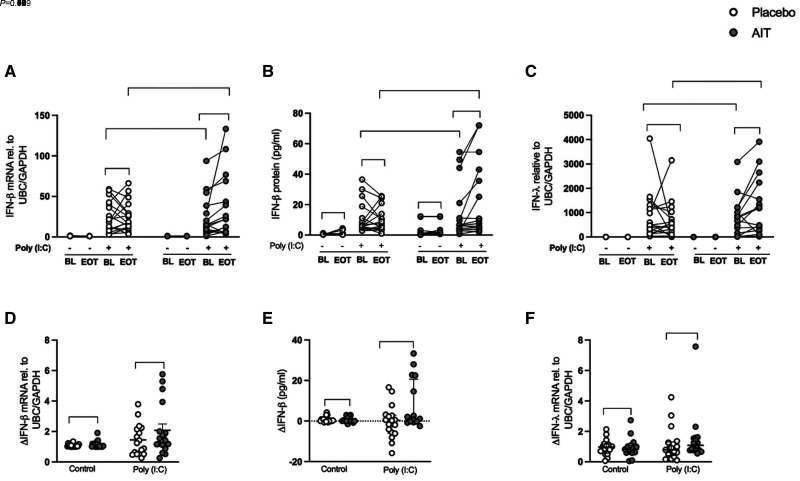
HBECs obtained at baseline (before treatment) had a similar response to the viral mimic poly(I:C) in the placebo and HDM-SLIT arms in terms of type I and III IFN expression (Figures 2A–2C). At Week 24, the induction of IFN-β (type I IFN) after poly(I:C) stimulation was higher in response to HDM-SLIT therapy compared with the baseline at both the gene (P = 0.009) and protein (P = 0.020) levels (Figures 2A and 2B); this result was not found in the placebo treatment group (Figures 2A and 2B). As shown in Figure 2C, type III IFN (i.e., IFN-λ) expression followed a very similar pattern, with a higher induction in response to poly(I:C) after HDM-SLIT (P = 0.030) that was absent in the placebo group (Figure 2C). When comparing the relative increase in expression of IFN-β and IFN-λ in the HDM-SLIT and placebo arms, they were not significantly different (Figures 2D and 2F), but for IFN-β protein release, there was a trend (P = 0.07) (Figure 2E).
Figure 2.
House dust mite sublingual allergen immunotherapy (AIT) increased polyinosinic:polycytidylic acid (poly(I:C))–induced human bronchial epithelial cell IFN expression in patients with allergic asthma. Bronchial epithelial cells from patients with allergic asthma were stimulated with poly(I:C) 10 μg/ml for 24 hours. IFN mRNA expression was quantified using qRT-PCR, and protein release was measured using multiplex ELISA. (A–F) mRNA expression of IFN-β in fold change (poly(I:C) was normalized to respective control) (A), protein release (absolute value) (B), mRNA expression of IFN-λ in fold change (poly(I:C) was normalized to respective control) (C), relative change of IFN-β mRNA fold induction (end of treatment [Week 24] [EOT] − baseline [BL]) (D), relative change of IFN-β protein release (EOT − BL) (E), and relative change of IFN-λ mRNA fold induction (EOT − BL) (F). Relative change (Δ) values are presented as median with interquartile range; placebo (n = 17) and AIT (n = 17). P values <0.05 were considered to indicate statistical differences. rel. = relative; UBC = ubiquitin C.
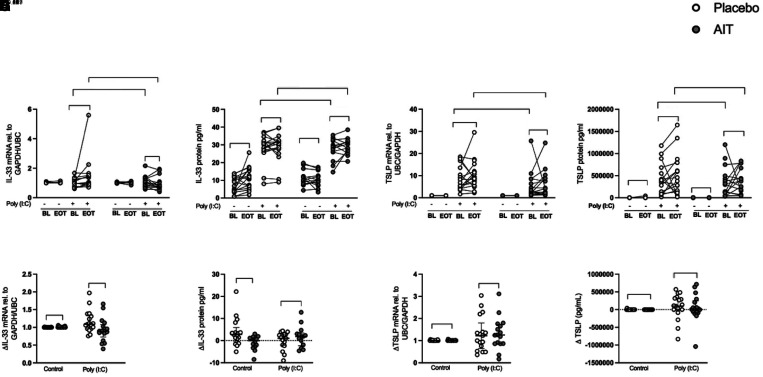
Poly(I:C)-induced Alarmin IL-33 Expression in HBECs Was Reduced after HDM-SLIT in Patients with Allergic Asthma
Stimulation with poly(I:C) induced IL-33 expression in HBECs from patients collected before treatment (Figures 3A and 3B). The induction of IL-33 gene expression tended to decrease in the HDM-SLIT arm (P = 0.09) compared with baseline (Figure 3A) and decreased significantly compared with the placebo arm (Figure 3E). Also, IL-33 protein release was reduced (P = 0.009) in unstimulated HBECs from HDM-SLIT compared with placebo (Figure 3F). There was no change in the poly(I:C)-induced expression of TSLP (both gene and protein) between baseline and Week 24 either within or between the groups (Figures 3C, 3D, 3G, and 3H). Protein release of the alarmin IL-25 in the absence and presence of poly(I:C) was undetectable (data not shown).
Figure 3.
House dust mite sublingual allergen immunotherapy (AIT) reduced polyinosinic:polycytidylic acid (poly(I:C))–induced human bronchial epithelial cell (HBEC) alarmin IL-33 expression in patients with allergic asthma. HBECs from patients with allergic asthma were stimulated with 10 μg/ml poly(I:C) for 3 and 24 hours. IL-33 and TSLP (thymic stromal lymphopoietin) mRNA expression was quantified using qRT-PCR, IL-33 protein release was measured using multiplex ELISA, and TSLP protein release was measured using the S-PLEX kit (Meso Scale Discovery) (A–F), relative change of TSLP mRNA fold induction (EOT-BL) (G), and relative change of TSLP protein release (EOT-BL) (H). (A–F) IL-33 mRNA expression in fold change (poly(I:C) was normalized to respective control) (A), IL-33 protein release (absolute value) (B), TSLP mRNA expression in fold change (poly(I:C) was normalized to respective control) (C), TSLP protein release (absolute value) (D), relative change of IL-33 mRNA fold induction (end of treatment [Week 24] [EOT] − baseline [BL]) (E), relative change of IL-33 protein release (EOT − BL) (F), relative change of TSLP mRNA fold induction (EOT − BL) (E), and relative change of TSLP protein release (EOT − BL) (F). Relative change (Δ) values are presented as median with interquartile range; placebo (n = 17) and AIT (n = 17) for gene expression, and placebo (n = 17) and AIT (n = 14–17) for protein release. P values <0.05 were considered to indicate significant differences. rel. = relative; UBC = ubiquitin C.
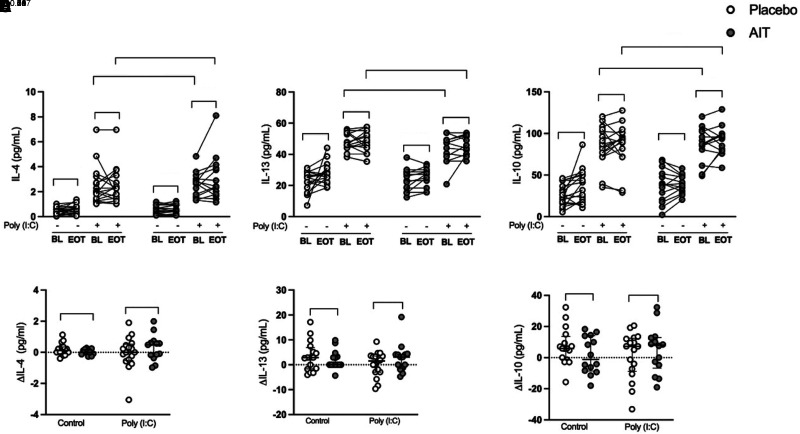
HDM-SLIT Does Not Alter Poly(I:C)-induced Epithelial T2 Cytokines in Patients with Allergic Asthma
HBECs stimulated with poly(I:C) had increased concentrations of the T2 cytokines IL-4, IL-13, and IL-10 at 24 hours compared with unstimulated cells (Figures 4A–4C), but there were no differences and no change within or between the groups (Figures 4A–4F) at Week 24.
Figure 4.
House dust mite sublingual allergen immunotherapy (AIT) does not alter polyinosinic:polycytidylic acid (poly(I:C))–induced epithelial type 2 cytokines in patients with allergic asthma. Human bronchial epithelial cells from patients with allergic asthma were stimulated with 10 μg/ml poly(I:C) for 24 hours. Cytokines were measured in cell-free supernatants using multiplex ELISA. (A–C) Protein release (absolute values) of IL-4 (A), IL-13 (B), and IL-10 (C). (D–F) Relative changes of protein release (end of treatment [Week 24] [EOT] − baseline [BL]) of IL-4 (D), IL-13 (E), and IL-10 (F). Relative change (Δ) values are presented as median with interquartile range; placebo (n = 17) and AIT (n = 14–17) for protein release. P values <0.05 were considered to indicate significant differences.
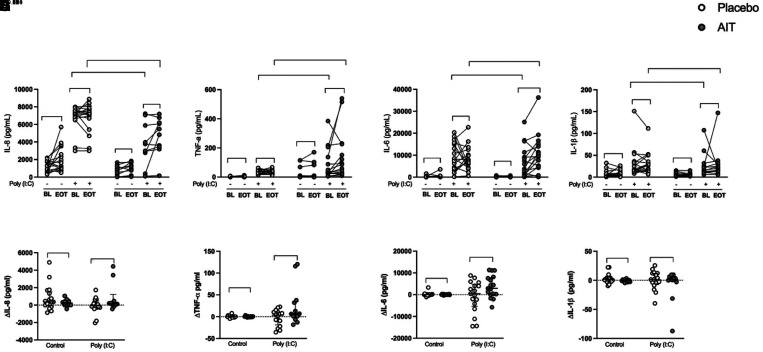
HDM-SLIT Increased Poly(I:C)-induced HBEC Proinflammatory Cytokines IL-6 and TNF-α in Patients with Allergic Asthma
As expected, poly(I:C) stimulation induced the proinflammatory cytokines IL-8, TNF-α (tumor necrosis factor-α), IL-6, and IL-1β (Figures 5A–5H). The induction was generally not altered in the placebo group, but HDM-SLIT treatment increased the induction of IL-6 (P = 0.009) (Figures 5C and 5G) and to some extent TNF-α (P = 0.08) (Figures 5B and 5F) and IL-8 (P = 0.05) in HBECs stimulated with poly(I:C) (Figures 5A and 5E).
Figure 5.
House dust mite sublingual allergen immunotherapy (AIT) increased polyinosinic:polycytidylic acid (poly(I:C))–induced human bronchial epithelial cell (HBEC) proinflammatory cytokines IL-6 and TNF-α (tumor necrosis factor-α) in patients with allergic asthma. HBECs from patients with allergic asthma were stimulated with 10 μg/ml poly(I:C) for 24 hours. Cytokines were measured in cell-free supernatants using multiplex ELISA. (A–D) Protein release absolute values of IL-8 (A), TNF-α (B), IL-6 (C), and IL-1β (D). (E–H) Relative changes of protein release ([Week 24] EOT − BL) of IL-8 (E), TNF-α (F), IL-6 (G), and IL-1β (H). Relative change (Δ) values are presented as median with interquartile range; placebo (n = 17) and AIT (n = 14–17) for protein release. P values <0.05 were considered to indicate significant differences. BL = baseline; EOT = end of treatment.
Clinical Response
Pulmonary metrics, biochemistry, and questionnaires are shown in Tables E1 and E2 in the online supplement. The provoking dose of inhaled mannitol causing a 15% reduction in FEV1 increased significantly in the HDM-SLIT group (P < 0.001) and the placebo group (P = 0.024), but there was no difference between the groups (P = 0.43); FEV1 increased in both groups before/after bronchodilator treatment, but there were no differences between the groups. There was no change in fractional exhaled nitric oxide. Both groups improved in Asthma Control Questionnaire, Asthma Quality of Life Questionnaire, and Rhinoconjunctivitis Quality of Life Questionnaire scores, with no difference between the groups. As expected, HDM-SLIT increased total IgE (P < 0.001), IgE Der-p (P < 0.001), and IgE Der-p (P < 0.001) (see Table E1) as well as blocking IgG4 (see Figure E1) and IgE-blocking factor (see Figure E2).
Sensitivity Analysis
The HDM-SLIT group was stratified on the basis of delta increase versus decrease of IFN-β mRNA expression, but no meaningful baseline characteristics could be identified (see Table E3). Similarly, we stratified IL-33 mRNA expression increase versus decrease, but again no clinically relevant characteristics could be identified. However, stratification of in vitro outcomes on the basis of decreased IL-33 mRNA expression identified patients with enhanced IFN-β mRNA expression (see Figure E3) (Spearman’s r = −0.88; P = 0.002).
Safety
Three serious adverse events (SAEs) were recorded during the study. Two SAEs were recorded in the HDM-SLIT group: one participant developed angioedema of the tongue and lips after initiation of the investigational medicinal product, and one developed pleuritis and was admitted after bronchoscopy. One SAE was recorded in the placebo group, due to pyelonephritis, and this subject was admitted for treatment with antibiotics. The number of SAEs did not differ significantly between the groups.
Discussion
In this study, we have demonstrated that 24 weeks of treatment with HDM-SLIT significantly enhanced poly(I:C)-induced bronchial epithelial gene expression of IFN-β, IFN-λ, and IL-6, as well as the release of IFN-β protein. Simultaneously, the gene expression of IL-33 tended to be reduced in the epithelial cells challenged with poly(I:C). This suggests that HDM-SLIT enhances antiviral resistance IFNs but has mixed responses regarding epithelial inflammatory tolerance to viral infection.
Although most evidence concerning AIT has focused on the changes related to the adaptive immunology, here we report novel modification of the innate antiviral immune defense in the bronchial epithelium induced by HDM-SLIT. This is the first trial to investigate the effect of HDM-SLIT on measures of human bronchial epithelial antiviral and inflammatory responses to viral infection. In general, our findings align with recent findings showing that AIT modulates a broad array of airway inflammatory responses: in children with allergic rhinitis, AIT reduced IL-33 in sera and nasal lavage (30); in nasal specimens, AIT initially increased a broad spectrum of inflammatory responses, including IL-8, IL-36G, CXCL-1, CXCL-2, IL-1α, and IFN-γ, after three years (31); and not surprisingly, there was no change in the virally induced T2 cytokines IL-4, IL-13, and IL-10, as they are secreted primarily by other sources (32). Indirectly demonstrated in epidemiologic studies, our findings align with our recent findings showing enhanced resistance to viral and bacterial airway infections measured by the preventive effect of AIT observed on exacerbation and respiratory infections requiring antibiotics (9, 10). These findings were also confirmed in the hitherto largest real-world efficacy study of AIT (33). Recently, our group demonstrated that HDM impaired epithelial antiviral responses via TLR3 by targeting the receptor glycosylation (28). This study now indicates that HDM-SLIT modulates the negative effects on the bronchial epithelium induced by HDM sensitization and exposure and ameliorates antiviral airway resistance and tolerance with 24 weeks of therapy.
The primary outcome of this study was partially met, although the treatment group significantly increased in IFN expression, but the ΔIFN-β compared with placebo was insignificant. The sample size assumed a fold increase in IFN-β of 1.55, with an SD of 0.55, but the actual mean increase was 1.50 with an SD of 2.35, so we could not demonstrate significantly increased poly(I:C)-induced ΔIFN-β, but ΔIL-33 was significantly decreased compared with placebo. This is despite performing double determination for all outcomes and normalizing gene expressions to the housekeeping genes UBC/GAPDH, thus indicating a grossly underpowered study to compare with placebo (for additional details, see section E2.5 in the online supplement). In addition, the trial had a much shorter treatment duration than a standard course of immunotherapy (usually 3 yr), which potentially limits the margin of relative changes between groups.
To make a large and feasible study, we examined HBECs’ responses to the viral infection mimic poly(I:C) in vitro. We used submerged HBEC cultures instead of air–liquid interface cultures because we did not aim to study epithelial barrier function, airway mucous and remodeling, or other features of asthma physiology. Submerged HBEC cultures have proved to be a solid model to replicate innate immune responses, and the use of poly(I:C) as a viral infection mimic is widely accepted because of the induction of physiologically relevant proinflammatory and antiviral mediators in bronchial epithelial cell cultures (34, 35). Hence, a limitation of this study is the isolated innate antiviral response from airway epithelial cells as a single compartment without considering the contributing effects of plasmacytoid dendritic cells, conventional dendritic cells, and macrophages. In vivo inoculation of patients with rhinovirus followed by bronchoscopy could potentially more precisely elucidate the bronchial antiviral response and the modulatory effects of AIT on virally induced asthma exacerbation.
Patients were included according to a relatively wide classification of HDM-induced allergic rhinitis (mild and moderate to severe persistent), introducing increased heterogeneity to the study population; however, this reflects to a high degree real-life patients in outpatient clinics, where AIT may be considered. Optimally, we would have liked to include only patients with HDM monosensitization, but this would have decreased feasibility and clinical translation, as most patients are polysensitized and because HDM sensitization often precedes and predisposes to the development of polysensitization (22). It could be hypothesized that polysensitization and relevant exposure could increasingly impair innate immunity, but we avoided planning bronchoscopies during seasonal allergen peaks in patients with concomitant birch and or grass pollen allergy. Indeed, it has been demonstrated that the efficacy of single-allergen sublingual AIR is sustained in patients with polysensitization (36). At inclusion, we assessed ICS adherence by prescription filling and thus did not include a run-in period. Improvement in placebo groups is a well-known phenomenon in clinical trials, but the improvement in airway hyperresponsiveness indicates that the initial adherence assessment, despite being high, was insufficient. The improvements in clinical parameters and questionnaire scores, at least to some extent, are to be attributed to improved ICS adherence.
Type I and III IFNs, in this study represented by IFN-β and IFN-λ, are hallmark cytokines produced for early innate immune responses for all classes of pathogens, including viruses and bacteria (37). Impaired secretion of type I and III IFNs upon viral infection has been reported mostly in atopic asthma, and multiple mechanisms have been proposed to be involved, including the inversed correlation between T2 cytokines and innate epithelial IFN-β and IFN-λ1 (18). In this study, we demonstrated an inverse correlation between decreased IL-33 mRNA expression and increased IFN-β mRNA expression, which was shown previously with TSLP and IFN-β by Uller and colleagues (13). Recently, Menzel and colleagues showed a novel correlation between bronchial epithelial oxidative stress and impaired IFN-β secretion (38). Proteolytic allergens such as HDM are known to both induce epithelial oxidative stress and impair epithelial IFN secretion (28, 39). A potential mechanism for AIT has recently been shown, decreasing epithelium-derived IL-25 and hence alleviating epithelial barrier dysfunction and epithelial oxidative stress (30); unfortunately, IL-25 could not be detected in our setup.
AIT induces a variety of immunological changes, starting with the rapid desensitization of mast cells and basophils (24). This is believed to be followed by the induction of tolerance by IL-10–secreting regulatory T and B cells, which skews T2 inflammation back toward a more balanced T-helper cell type 1/T-helper cell type 2 submucosal environment. Consequently, mucosal concentrations of T2 cytokines (IL-4, IL-5, and IL-13) and eosinophils are decreased (40). The first months of AIT induce plasma cell production of allergen-specific IgG4, which “captures” free allergen and blocks for binding to IgE and subsequent cross-linking of IgE bound to the high-affinity receptor FcὲRI on the surface of basophils and mast cells (41). IgG4 also blocks IgE–allergen immune complex formation and thereby inhibits interaction with the low-affinity IgE receptor FcὲRII, also known as CD23 (cluster of differentiation 23) (42). IgG4 is known to inhibit IgE-facilitated antigen presentation via CD23, resulting in a decrease in T-helper cell type 2 polarization and subsequent T2 cytokine production (43). The role of CD23 expressed on epithelia is thus also likely affected by IgG4, although the effect of this is not understood. In summary, the mechanisms by which AIT exerts its disease-modifying effect are complex and remain only partly understood. It is likely that especially inhibition of the IgE axis and the dampened T2 inflammatory submucosal milieu is crucial to restoring a more normally functioning innate antiviral response (44). However, further studies are needed to elucidate the exact mechanisms involved, though linking innate and adaptive immune mechanisms has proved difficult to investigate (45).
In this study, we demonstrate that HDM-SLIT improves airway resistance and tolerance to viral stimulation and hence provide a novel mechanistic rationale for the preventive and sustained effects of AIT observed on exacerbation and respiratory infections requiring antibiotics in the hitherto largest real-world efficacy study of AIT (33). AIT is still underused in allergic asthma, and patients with recurrent respiratory tract infections could benefit from its preventive effects. This study warrants further studies of AIT for seasonal allergens and the combination with HDM-AIT to elucidate the impact on the respiratory epithelium.
Conclusions
HDM-SLIT significantly improved bronchial epithelial expression of IFN-β and IFN-λ and decreased IL-33 in response to poly(I:C), suggesting improved airway resistance and tolerance to viral infection. However, only IL-33 was significantly changed compared with placebo, because of a relatively larger SD in IFN-β expression than accounted for in the power calculation. In a subanalysis, IL-33 and IFN-β were inversely correlated. Overall, these findings support the use of AIT for the prevention of exacerbation and respiratory infections in patients with HDM-allergic asthma and warrant further investigations of AIT in patients with seasonal allergic asthma.
Acknowledgments
Acknowledgment
The authors acknowledge all subjects for participating and making this study possible. Furthermore, the authors acknowledge research nurses Charlotte Bernhoff, Mette Boye, and Anne Arentoft Bayer for their particularly qualified participation in this trial, as well as the nurses in the endoscopy and recovery wards for taking very good care of the patients.
Footnotes
Supported by ALK Abelló A/s, Harboefonden, Danmarks Lungeforening, Savvaerksejer Jeppe Juhl og Ovita Juhls Mindelegat, Pharmaxis Ltd., Medicinska Forskningsrådet grants 2020-00922_VR and 2017-00806_VR, and Hjärt-Lungfonden grant 20180207_HLF.
Author Contributions: C.W., A.S., L.U., and C.P. conceptualized the trial protocol. C.W., S.R., A.S., J.J.N.-F., S.V.-M., S.C., A.P., L.M.A., L.L.E., N.D.-P., D.K.K., M.M., and C.P. were involved with data collection. C.W., S.R., J.J.N.-F., S.H., and L.U. carried out data analysis, and all authors contributed to interpretation. C.W., S.R., A.S., L.U., and C.P. contributed to the original manuscript. All authors reviewed and appraised the manuscript. All authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202209-1708OC on January 26, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med . 2017;17:74. doi: 10.1186/s12890-017-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax . 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soto-Quiros M, Avila L, Platts-Mills TAEE, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol . 2012;129:1499–1505.e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ . 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston SL. IFN therapy in airway disease: is prophylaxis a new approach in exacerbation prevention? Am J Respir Crit Care Med . 2020;201:9–11. doi: 10.1164/rccm.201909-1850ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol . 2017;140:909–920. doi: 10.1016/j.jaci.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, et al. INTERCIA Study Group The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med . 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson A, Spalluto CM, McCrae C, Cellura D, Burke H, Cunoosamy D, et al. Dynamics of IFN-β responses during respiratory viral infection insights for therapeutic strategies. Am J Respir Crit Care Med . 2020;201:83–94. doi: 10.1164/rccm.201901-0214OC. [DOI] [PubMed] [Google Scholar]
- 9. Woehlk C, von Bülow A, Kriegbaum M, Backer V, Porsbjerg C. Allergic asthma is associated with increased risk of infections requiring antibiotics. Ann Allergy Asthma Immunol . 2018;120:169–176.e1. doi: 10.1016/j.anai.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 10. Woehlk C, Von Bülow A, Ghanizada M, Baastrup Søndergaard M, Hansen S, Porsbjerg C. Allergen immunotherapy effectively reduces the risk of exacerbations and lower respiratory tract infections in both seasonal and perennial allergic asthma: a nationwide epidemiological study. Eur Respir J . 2022;60:2200446. doi: 10.1183/13993003.00446-2022. [DOI] [PubMed] [Google Scholar]
- 11. Frey A, Lunding LP, Ehlers JC, Weckmann M, Zissler UM, Wegmann M. More than just a barrier: the immune functions of the airway epithelium in asthma pathogenesis. Front Immunol . 2020;11:761. doi: 10.3389/fimmu.2020.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med . 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 13. Uller L, Leino M, Bedke N, Sammut D, Green B, Lau L, et al. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-β in bronchial epithelial cells from donors with asthma. Thorax . 2010;65:626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 14. Jackson DJ, Makrinioti H, Rana BMJ, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med . 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med . 2017;377:936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 16. Cerps S, Sverrild A, Ramu S, et al. House dust mite sensitization and exposure affects bronchial epithelial anti-microbial response to viral stimuli in patients with asthma. Allergy . 2021;2022:1–11. doi: 10.1111/all.15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynch JP, Werder RB, Simpson J, Loh Z, Zhang V, Haque A, et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening antiviral immunity. J Allergy Clin Immunol . 2016;138:1326–1337. doi: 10.1016/j.jaci.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 18. Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy . 2015;70:910–920. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 19. Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol . 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol . 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol . 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy . 2019;74:855–873. doi: 10.1111/all.13749. [DOI] [PubMed] [Google Scholar]
- 23. Zissler UM, Jakwerth CA, Guerth FM, Pechtold L, Aguilar-Pimentel JA, Dietz K, et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. EBioMedicine . 2018;36:475–488. doi: 10.1016/j.ebiom.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durham SR, Shamji MH. Allergen immunotherapy: past, present and future. Nat Rev Immunol . 2022 doi: 10.1038/s41577-022-00786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-β and functional regulatory T cells. Am J Respir Crit Care Med . 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 26. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. World Health Organization; GA(2)LEN; AllerGen Allergic Rhinitis and Its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy . 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 27. Porsbjerg C, Nieto-Fontarigo JJ, Cerps S, Ramu S, Menzel M, Hvidtfeldt M, et al. Phenotype and severity of asthma determines bronchial epithelial immune responses to a viral mimic. Eur Respir J . 2022;60:2102333. doi: 10.1183/13993003.02333-2021. [DOI] [PubMed] [Google Scholar]
- 28. Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy Eur J Allergy Clin Immunol . 2017;2018:1–11. doi: 10.1111/all.13378. [DOI] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods . 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30. Yuan X, Wang J, Li Y, He X, Niu B, Wu D, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25-induced endoplasmic reticulum stress in asthma. Sci Rep . 2018;8:7950. doi: 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zissler UM, Schmidt-Weber CB. Predicting success of allergen-specific immunotherapy. Front Immunol . 2020;11:1826. doi: 10.3389/fimmu.2020.01826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity . 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 33. Fritzsching B, Contoli M, Porsbjerg C, Buchs S, Larsen JR, Elliott L, et al. Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: results from the REACT study, a retrospective cohort study. Lancet Reg Health Eur . 2022;13:100275. doi: 10.1016/j.lanepe.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med . 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lever AR, Park H, Mulhern TJ, Jackson GR, Comolli JC, Borenstein JT, et al. Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model. Physiol Rep . 2015;3:1–11. doi: 10.14814/phy2.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciprandi G, Cadario G, Di Gioacchino GM, Gangemi S, Gasparini A, Isola S, et al. Sublingual immunotherapy in children with allergic polysensitization. Allergy Asthma Proc . 2010;31:227–231. doi: 10.2500/aap.2010.31.3337. [DOI] [PubMed] [Google Scholar]
- 37. Ali S, Mann-Nüttel R, Schulze A, Richter L, Alferink J, Scheu S. Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol . 2019;10:778. doi: 10.3389/fimmu.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menzel M, Ramu S, Calvén J, Olejnicka B, Sverrild A, Porsbjerg C, et al. Oxidative stress attenuates TLR3 responsiveness and impairs anti-viral mechanisms in bronchial epithelial cells from COPD and asthma patients. Front Immunol . 2019;10:2765. doi: 10.3389/fimmu.2019.02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan TK, Tan WSD, Peh HY, Wong WSF. Aeroallergens induce reactive oxygen species production and DNA damage and dampen antioxidant responses in bronchial epithelial cells. J Immunol . 2017;199:39–47. doi: 10.4049/jimmunol.1600657. [DOI] [PubMed] [Google Scholar]
- 40. Kucuksezer UC, Ozdemir C, Cevhertas L, Ogulur I, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol Int . 2020;69:549–560. doi: 10.1016/j.alit.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 41. Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy . 2021;76:3627–3641. doi: 10.1111/all.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Engeroff P, Vogel M. The role of CD23 in the regulation of allergic responses. Allergy . 2021;76:1981–1989. doi: 10.1111/all.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holm J, Willumsen N, Würtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol . 2011;127:1029–1037. doi: 10.1016/j.jaci.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 44. Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med . 2017;196:985–992. doi: 10.1164/rccm.201701-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity . 2021;54:291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]