Abstract
Anticoagulation and antiplatelet therapy are individually mainstays of treatment for multiple cardiovascular conditions. Antiplatelet therapy, most commonly with dual agents, is vital in the setting of coronary artery disease with acute coronary syndrome requiring percutaneous coronary intervention to prevent in-stent complications. A multitude of cardiovascular conditions with increased thromboembolic risk also require anticoagulation, including atrial fibrillation, venous or arterial thrombosis, and prosthetic heart valves to name a few. There is often an overlap in comorbidities as our patient population ages and becomes more complex, frequently necessitating a combination of both anticoagulation and antiplatelet agents, known as “triple therapy”. To reduce or treat thromboembolic disease states as well as reduce platelet aggregation for coronary stent protection, many patients are placed at an increased bleeding risk without compelling evidence of reduction in major adverse cardiac events. With this comprehensive review of the existing literature, we aim to analyse different strategies and durations of triple therapy medication regimens.
Keywords: triple therapy, anticoagulation, antiplatelet therapy, coronary artery disease, atrial fibrillation
Introduction
Antiplatelet therapy is the standard of care following percutaneous coronary intervention (PCI) with drug-eluting stent (DES) placement, to reduce in-stent complications such as acute thrombosis [1, 2]. Dual antiplatelet therapy (DAPT) involves the usage of aspirin with an additional agent (P2Y12 inhibitors) such as ticagrelor, clopidogrel, or prasugrel, which provides additional suppression of platelet aggregation for coronary stent protection [1, 3–5].
Oral anticoagulation (OAC) therapy is common in clinical practice, where patients are managed with one of many available oral or intravenous anticoagulants, such as unfractionated or low-molecular-weight heparin, vitamin K antagonists (VKA), or direct acting oral anticoagulants (DOAC), for the treatment of a multitude of thromboembolic disease states [6–8]. Similarly to antiplatelet regimens, there is an increased bleeding risk with OAC, which needs to be weighed against the potential clinical benefit when prescribing these agents alone or in combination with other medications [6].
Because both antiplatelet therapy and anticoagulation play a large role in the treatment of certain cardiovascular diseases, the use of these medications in combination poses potential challenges, especially an increase in adverse bleeding events. In the aging adult population, many patients over time are diagnosed with multiple comorbid conditions requiring complex medical regimens. Due to shared risk factors and at times a causal relationship, these patients undergoing PCI present with conditions that increase thromboembolic risk, necessitating a multi-pronged approach to therapy. Atrial fibrillation (AF) is commonly diagnosed in patients with coronary artery disease, which unsurprisingly increases patient morbidity [9]. Current literature suggests that between 5% and 12% of patients with coronary artery disease (CAD) also have concomitant non-valvular AF [10–13]. Other indications for anticoagulation as an addition to antiplatelet therapy include left ventricular (LV) thrombus and prosthetic heart valves [14]. LV thrombus is frequently seen in patients who sustain anterior myocardial infarction (MI) and/or have significantly decreased ejection fraction, which may further be complicated by DES placement for the management of CAD [14, 15].
Literature review
Triple therapy and major adverse cardiac events
DAPT therapy in addition to anticoagulation was initially poorly understood, where many patients were placed on VKAs and DAPT without a clear timeline or strategy. The goal of initiating triple therapy (TT) after PCI is to theoretically reduce in-stent complications and downstream major adverse cardiac events (MACE). Whether there is a significant improvement in outcomes is, however, controversial. Early data suggested TT was associated with a decrease in the occurrence of cardiovascular events and mortality when compared to dual antithrombotic therapy alone [16, 17]. However, registry data from 2015 including close to 5000 AF patients > 65 years of age who underwent PCI, showed no significant difference in MACE with TT containing warfarin as compared to DAPT. This was redemonstrated in clinical trials in subsequent years, including those conducted with TT containing DOACs. In a large meta-analysis including the PIONEER-AF PCI, RE-DUAL PCI, AUGUSTUS, and ENTRUST-AF PCI trials, dual antithrombotic therapy (OAC plus P2Y12 inhibitor) compared to TT showed similar rates of all-cause mortality, cardiovascular death, or stroke; however, the absence of TT did show a slight increase in MI and stent thrombosis (ST) with borderline statistical significance (p = 0.07) [18].
Bleeding risk
The biggest consideration in patients receiving TT is the incremental bleeding risk. Identifying those at an increased risk of bleeding is critical. Utilizing clinical criteria such as the HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding, labile INR, elderly, and drugs/alcohol) can provide an accurate bleeding risk and has been found to significantly predict spontaneous bleeding events in patients on TT [19]. The administration of TT in patients with CAD and concomitant AF has been increasing over the years and appears to have increased adverse risks despite delineating which type of OAC is used. Further understanding of the average bleeding risk of commonly used regimens is important.
Dual antiplatelet therapy + vitamin K antagonist
VKAs such as warfarin are well established and indicated for both treatment and prevention of venous thromboembolic phenomena, especially for indications such as valvular AF, where there is a paucity of data regarding the use of DOACs [20, 21]. However, TT using VKAs and DAPT has been shown to increase the risk of both major and minor bleeding [14, 22, 23].
In a study of 127 patients who underwent DES or bare metal stent (BMS) placement, Rogacaka et al. concluded that 4.7% of their study population experienced major bleeding within the first month of the indexed procedure without evidence of increased MACE [22]. All patients were on DAPT and warfarin for a mean of 5.6 months for recent PCI and known history of AF, LV thrombus, prosthetic heart valves, pulmonary embolism (PE), deep venous thrombosis (DVT), or coronary aneurysm, respectively. Furthermore, no significant difference was found in major bleeding events between DES and BMS placement, and only one patient sustained definite ST within 30 days of PCI.
Within the TRANSLATE-ACS trial, Jackson et al. evaluated 11,756 patients who were treated with ADP receptor inhibitors post-PCI and who were also on OAC therapy (93% on VKA) [23]. The authors analysed bleeding risk and need for hospitalization due to bleeding based on the Bleeding Academic Research Consortium in patients on aspirin + anticoagulation + clopidogrel (triple-C), aspirin + anticoagulation + prasugrel (triple-P), aspirin + clopidogrel (dual-C), or aspirin + prasugrel (dual-P). The triple-C cohort was associated with higher risk of bleeding compared to the dual-C cohort (28.7% vs. 19.7%), with similar findings when comparing the triple-P and dual-P groups (38.5% vs. 26.7%). In total comparison, the triple-P cohort was associated with the highest percentage of bleeding between all groups, with no statistical difference in the composite risk of MACE between all groups. The authors noted that the increased bleeding was patient reported, and there were no significant differences in bleeding requiring rehospitalization between the triple-P and triple-C groups.
Porter et al. studied the effect of using a shorter duration of VKA-based TT [14]. They conducted a retrospective study analysing 180 patients on a TT regimen including aspirin, VKA, and a thienopyridine/P2Y12 inhibitor (clopidogrel or ticlopidine). All patients were previously on VKA-guided anticoagulation for indications including LV thrombus, AF, prosthetic heart valves, and thrombophilia. Nearly 87% of patients underwent urgent PCI with most patients sustaining ST-elevation myocardial infarction (STEMI). Post-PCI, TT was continued for 30 days, during which 9% of patients developed bleeding complications; however, many bleeding episodes were noted during unfractionated heparin-warfarin bridging. Also notably, most bleeding events in this series were deemed to be minor, and the authors’ conclusions were not prohibitive to the use of short-term TT in the setting of clear VKA indication.
Dual antiplatelet therapy + direct oral anticoagulant
Over the years, DOACs such as apixaban or rivaroxaban have been found to be non-inferior to warfarin in the management of AF or LV thrombus, with an additional decrease in bleeding risk, as well [24–26]. Compared to VKAs, DOACs may provide decreased adverse bleeding events without increasing future risk of MACE [12, 27–29].
To address and compare VKAs to DOACs within TT, the PIONEER AF-PCI trial in 2016 assessed bleeding risks in patients on TT that included DOACs [12]. The investigators analysed 2124 patients with nonvalvular AF who required OAC and also underwent PCI. Groups were assigned into low-dose rivaroxaban (15 mg once daily) in addition to a P2Y12 inhibitor for 12 months (group 1), very-low-dose rivaroxaban (2.5 mg twice daily) in addition to DAPT for 1, 6, and 12 months (group 2), or standard therapy with VKA plus DAPT for 1, 6, or 12 months (group 3). The study found that the 2 groups receiving rivaroxaban had significantly lower bleed rates based on the Thrombolysis in Myocardial Infarction (TIMI) bleeding criteria or bleeding requiring medical attention, compared to VKA plus DAPT therapy (group 3) (16.8% in group 1, 18.0% in group 2, and 26.7% in group 3). The study further concluded that the rates of death from cardiovascular causes, MI, or stroke were similar in all 3 groups. Overall, Gibson et al. concluded that either low-dose rivaroxaban in addition to a single antiplatelet inhibitor or very-low-dose rivaroxaban in addition to DAPT was less likely to cause clinically significant adverse events of bleeding without increasing the risk of future MACE.
The authors of the RE-DUAL PCI trial conducted a multicentre study including 2725 patients with a history AF who also had undergone PCI [27]. The groups within their study included a triple therapy cohort (warfarin, clopidogrel/ticagrelor, and aspirin for 1–3 months) and a dual therapy cohort (dabigatran 110 mg or 150 mg twice daily and clopidogrel/ticagrelor). The primary endpoint (major or clinically relevant bleeding) was found in 15.4% in the 110 mg dual-therapy dabigatran cohort compared to 26.9% in the triple-therapy VKA group. Additionally, major bleeding was noted in 20.2% of the 150 mg dual-therapy dabigatran group compared to 25.7% in the corresponding triple-therapy VKA group. They further concluded that dual therapy was noninferior compared to triple therapy in respect to thrombotic events. Overall, dual therapy with dabigatran and a single antiplatelet agent provided a significantly lower risk of bleeding complications without sacrificing an increase in ST.
Sindet-Pedersen et al. conducted a study that included 3222 patients and compared several groups under standard medication dosing: VKA + single antiplatelet therapy (SAPT), DOAC + SAPT, VKA + DAPT, and DOAC + DAPT [28]. Outcomes investigated included all-cause mortality, major bleeding leading to hospitalization, ischaemic stroke, and MI. The investigators found the highest absolute risk of bleeding in the VKA + DAPT after 3-month follow-up, compared to the lowest absolute risk of bleeding in DOAC + DAPT, with a significantly decreased risk of bleeding comparing DOAC + DAPT to VKA + DAPT. Similar associations were found at 12-month follow-up, as well. When further comparing VKA + DAPT to DOAC + DAPT, no difference in 3-month and 12-month risk of MI, ischaemic stroke, and all-cause mortality were found between the 2 groups.
Duration of triple therapy
An additional concern for TT has revolved around adequate duration of all 3 medications and/or the timeline of when to discontinue part of the regimen [30]. The ISAR-Triple trial in 2015 was conducted to investigate the optimal duration of triple therapy following PCI. Fiedler et al. observed 614 patients at 3 European centres, assessing 6-weeks versus 6-months of clopidogrel therapy post DES placement in addition to concomitant aspirin and OAC. The primary endpoint included death, MI, ST, stroke, or thrombolysis in myocardial infarction (TIMI) major bleeding. Secondary endpoints included the incidence of ischaemic or bleeding complications. Between the 6-week and 6-month group, no difference was found in primary endpoints, which occurred in 9.8% in the 6-week group and 8.8% in the 6-month group. It was concluded that 6 weeks of triple therapy (aspirin, clopidogrel, OAC) was not superior to 6 months of therapy regarding differences in net clinical outcomes, ischaemic complications, or major bleeding.
In 2020, the American College of Cardiology created an expert consensus to guide TT in patients who required treatment, recommending shorter duration of therapy (less than 30 days), < 100 mg of daily aspirin, addition of a proton pump inhibitor, preference of DOAC over VKA, and discontinuation of all antiplatelet agents within 6–12 months [13]. In a recent Letter to the Editor in the Journals of the American College of Cardiology, author Davide Capodanno further discusses how the new consensus for TT probably includes the lowest therapeutic dosing for OAC (preferably DOAC), a P2Y12 inhibitor (preferably clopidogrel) for 6–12 months, and aspirin for 1–7 days or until hospital discharge, with an option to maintain aspirin therapy for up 30 days if patients are high risk for thrombotic events [31].
Special considerations
Anticoagulation in the periprocedural period for coronary interventions
Managing patient’s long-term anticoagulants immediately prior to and during PCI for urgent coronary interventions poses a great clinical challenge [32]. In a small observational study by Rubboli et al., chronic VKA therapy was held in most patients at a median of 4 days prior to PCI while patients were either pre-treated with no additional medications (7%), aspirin only (69%), DAPT (17%), or intravenous unfractionated heparin and aspirin (7%). No patient sustained MACE during the periprocedural period, and most complications were due to bleeding secondary to TT at an average of 32 days post-PCI [32]. Due to concerns of thromboembolic phenomena in several cardiovascular disease states, the ideal strategy would include discontinuation of OAC with subsequent administration of intravenous unfractionated heparin [33]. Furthermore, DOACs can probably be resumed once appropriate haemostasis is obtained, and VKAs would require appropriate bridging until desired INR is reached [33]. To minimize bleeding, radial access during cardiac catheterization is proposed to minimize overall bleeding and haematoma risk for patients on combined OAC and antithrombotic therapy [33–35].
Differences by stent type
There is a significant paucity of data specifically analysing different stent types and how their differences alter TT outcomes [36]. In the ISAR-TRIPLE trial, most patients received different types of DES (biolimus, everolimus, paclitaxel, sirolimus, zotarolimus) and only 2 patients received BMS placement in both the 6-week and 6-month cohort; however, the study concluded that there was not significant enough power to further analyse the stent type [30]. Traditionally, DES placement is favoured over BMS placement to avoid in-stent restenosis [37]; therefore, we would extrapolate that most guidelines would be based on the DES usage over BMS.
The COBRA-REDUCE trial is a relatively new study that was aimed at assessing differences between the Cobra Polyzene-F (Pzf) stent compared to traditional FDA-approved DES [38]. The Cobra Pzf stent is a cobalt chromium alloy stent that does not release anti-stenotic drugs; it is instead optimized to bind to albumin rather than fibrinogen to prevent increased platelet activity [38]. The study design included patients who are previously on OAC (VKA or DOAC) and were divided into two groups: Cobra Pzf stent placement with indefinite treatment of OAC, aspirin, and 14 days of clopidogrel compared to standard FDA-approved DES placement followed by indefinite treatment of OAC, aspirin, and clopidogrel for 3–6 months [38]. As per the Society of Cardiovascular Angiography & Interventions, the trial failed to demonstrate decreased bleeding risk when compared to standard DES and DAPT regimens and also failed to show non-inferiority in regard to thrombotic and thromboembolic events [39].
Challenges and future directions
With increasing prevalence of multiple cardiovascular comorbidities within several patient populations, TT poses several challenges in different subsets of patients. Despite DOACs appearing to have a safer bleeding profile, VKA therapy may be preferred over DOAC in certain scenarios, such as prosthetic heart valves, antiphospholipid syndrome, or high-risk gastrointestinal bleeding [40]. Additionally, there is an absence of literature on the effects of the type of DES placed when TT is needed. Despite recent guidelines emerging to guide TT, differences in patient comorbidities, demographics, and clinical presentation can create multiple challenges. New advances in stent technology and duration of chronic medications may alter the way we approach TT in the future.
Conclusion
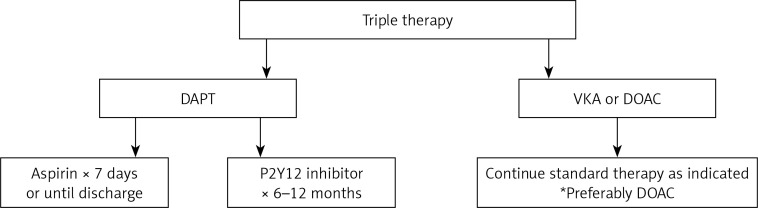
As TT continues to possess clinical challenges in cardiovascular care, studies have shown that shorter duration of therapy probably decreases adverse bleeding risk and may not increase overall MACE risk. Long-term TT probably does not show any benefit in cardiovascular mortality and only increases adverse bleeding risk. If TT is required for patients with an anticoagulation indication and recent PCI, non-VKA-guided anticoagulation is preferable to further decrease bleeding risk. In general, the ideal TT regimen should include a DOAC for chronic anticoagulation, aspirin for up to 7 days or until hospital discharge, and a P2Y12 inhibitor for 6 to 12 months, depending on thrombotic risk following PCI (Figure 1).
Figure 1.
Triple therapy
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371: 2155-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valgimigli M, Cao D, Makkar RR, et al. Design and rationale of the XIENCE short DAPT clinical program: An assessment of the safety of 3-month and 1-month DAPT in patients at high bleeding risk undergoing PCI with an everolimus-eluting stent. Am Heart J 2021; 231: 147-56. [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Frigoli E, Heg D, et al. Dual Antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med 2021; 385: 1643-55. [DOI] [PubMed] [Google Scholar]
- 4.Kinlay S. Post-PCI antithrombotic treatment with high bleeding risk. J Am Coll Cardiol 2022; 80: 1238-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045-57. [DOI] [PubMed] [Google Scholar]
- 6.Umerah C, Momodu II. Anticoagulation. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 7.Stults BM, Dere WH, Caine TH. Long-term anticoagulation. Indications and management. West J Med 1989; 151: 414-29. [PMC free article] [PubMed] [Google Scholar]
- 8.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (6 Suppl): 454S-545S. [DOI] [PubMed] [Google Scholar]
- 9.Kudaiberdieva G, Gorenek B. Post PCI atrial fibrillation. Acute Card Care 2007; 9: 69-76. [DOI] [PubMed] [Google Scholar]
- 10.Hussain A, Minhas A, Sarwar U, Tahir H. Triple antithrombotic therapy (triple therapy) after percutaneous coronary intervention in chronic anticoagulation: a literature review. Cureus 2022; 14: 21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bainey KR, Morais J, Zeymer U, Welsh RC. Atrial fibrillation with percutaneous coronary intervention: navigating the minefield of antithrombotic therapies. Atherosclerosis 2019; 289: 118-25. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016; 375: 2423-34. [DOI] [PubMed] [Google Scholar]
- 13.2020 ACC Expert Consensus Pathway for Anticoagulant and Antiplatelet Therapy. American College of Cardiology. Available at: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2020/12/17/21/04/2020-acc-expert-consensus-anticoagulant-antiplatelet. Accessed December 26, 2022. [Google Scholar]
- 14.Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68: 56-61. [DOI] [PubMed] [Google Scholar]
- 15.Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012; 98: 1743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedler KA, Byrne RA, Schulz S, et al. Rationale and design of The Intracoronary Stenting and Antithrombotic Regimen – Testing of a six-week versus a six-month clopidogrel treatment Regimen In Patients with concomitant aspirin and oraL anticoagulant therapy following drug-Eluting stenting (ISAR-TRIPLE) study. Am Heart J 2014; 459–65.e1. [DOI] [PubMed] [Google Scholar]
- 17.Zhao HJ, Zheng ZT, Wang ZH, et al. “Triple therapy” rather than “triple threat”: a meta-analysis of the two antithrombotic regimens after stent implantation in patients receiving long-term oral anticoagulant treatment. Chest 2011; 139: 260-70. [DOI] [PubMed] [Google Scholar]
- 18.Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019; 40: 3757-67. [DOI] [PubMed] [Google Scholar]
- 19.Smith JG, Wieloch M, Koul S, et al. Triple antithrombotic therapy following an acute coronary syndrome: prevalence, outcomes and prognostic utility of the HAS-BLED score. EuroIntervention 2012; 8: 672-8. [DOI] [PubMed] [Google Scholar]
- 20.Kuruvilla M, Gurk-Turner C. A review of warfarin dosing and monitoring. Baylor University Medical Center Proceedings 2001; 14: 305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly SJ, Karthikeyan G, Ntsekhe M, et al. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med 2022; 387: 978-88. [DOI] [PubMed] [Google Scholar]
- 22.Rogacka R, Chieffo A, Michev I, et al. Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation. JACC Cardiovasc Interv 2008; 1: 56-61. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LR 2nd, Ju C, Zettler M, et al. Outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention receiving an oral anticoagulant and dual antiplatelet therapy: a comparison of clopidogrel versus prasugrel from the TRANSLATE-ACS study. JACC Cardiovasc Interv 2015; 8: 1880-9. [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981-92. [DOI] [PubMed] [Google Scholar]
- 25.Abdelnabi M, Saleh Y, Fareed A, et al. Comparative study of oral anticoagulation in left ventricular thrombi (No-LVT Trial). J Am Coll Cardiol 2021; 77: 1590-2. [DOI] [PubMed] [Google Scholar]
- 26.Van Mieghem NM, Unverdorben M, Hengstenberg C, et al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med 2021; 385: 2150-60. [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017; 377: 1513-24. [DOI] [PubMed] [Google Scholar]
- 28.Sindet-Pedersen C, Lamberts M, Staerk L, et al. Combining oral anticoagulants with platelet inhibitors in patients with atrial fibrillation and coronary disease. J Am Coll Cardiol 2018; 72: 1790-800. [DOI] [PubMed] [Google Scholar]
- 29.Khan SU, Osman M, Khan MU, et al. Dual versus triple therapy for AF after PCI: review and meta-analysis. Ann Internal Med 2020; 172: 474-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiedler KA, Maeng M, Mehilli J, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol 2015; 65: 1619-29. [DOI] [PubMed] [Google Scholar]
- 31.Capodanno D. Triple therapy, dual therapy, and modulation of anticoagulation intensity. JACC Cardiovasc Interv 2021; 14: 781-4. [DOI] [PubMed] [Google Scholar]
- 32.Rubboli A, Colletta M, Herzfeld J, Sangiorgio P, Di Pasquale G. Periprocedural and medium-term antithrombotic strategies in patients with an indication for long-term anticoagulation undergoing coronary angiography and intervention. Coron Artery Dis 2007; 18: 193-9. [DOI] [PubMed] [Google Scholar]
- 33.Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation 2021; 143: 583-96. [DOI] [PubMed] [Google Scholar]
- 34.Dewilde W, Verheugt FWA, Breet N, Koolen JJ, Ten Berg JM. “Ins” and “outs” of triple therapy: optimal antiplatelet therapy in patients on chronic oral anticoagulation who need coronary stenting. Neth Heart J 2010; 18: 444-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agostoni P, Biondi-Zoccai GGL, De Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures. J Am Coll Cardiol 2004; 21: 349-56. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Nodar JM, Marín F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51: 818-25. [DOI] [PubMed] [Google Scholar]
- 37.Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of coronary stent technology and implications for duration of dual antiplatelet therapy. Prog Cardiovasc Dis 2018; 60: 478-90. [DOI] [PubMed] [Google Scholar]
- 38.Colleran R, Joner M, Cutlip D, et al. Design and rationale of a randomized trial of COBRA PzF stenting to REDUCE duration of triple therapy (COBRA-REDUCE). Cardiovasc Revasc Med 2022; 34: 17-24. [DOI] [PubMed] [Google Scholar]
- 39.Chadi Alraies M. Randomized trial of cobra PzFTM stenting to reduce duration of triple therapy: final results of cobra-reduce trial—coverage of CRT 2022.
- 40.Wadsworth D, Sullivan E, Jacky T, Sprague T, Feinman H, Kim J. A review of indications and comorbidities in which warfarin may be the preferred oral anticoagulant. J Clin Pharm Ther 2021; 46: 560-70. [DOI] [PubMed] [Google Scholar]