Abstract
Acidaminococcus fermentans belongs to the group of strictly anaerobic gram-negative cocci. All previously described Acidaminococcus strains are susceptible to β-lactam antibiotics. An A. fermentans strain (RYC-MR95) resistant to penicillin and expanded-spectrum cephalosporin (amoxicillin and cefotaxime MICs, 64 μg/ml) was isolated from a human perianal abscess. A fragment encoding a β-lactamase from genomic DNA was cloned in Escherichia coli K-12 strain HB101, and the recombinant strain expressed resistance to amoxicillin (MIC, 1,024 μg/ml) and cefotaxime (MIC, 4 μg/ml). Clavulanic acid decreased the MICs to 8 and 0.03 μg/ml, respectively. Analysis of the nucleotide sequence revealed a new class A β-lactamase, ACI-1. In accordance with its biochemical properties, we propose to assign ACI-1 to functional group 2be. The ACI-1 enzyme (estimated pI 4.3) had <50% amino acid identity with any other class A β-lactamases, the closest being ROB-1 from Haemophilus influenzae (44%). ACI-1 was closer to class A β-lactamases from some gram-positive organisms (41 to 44% amino acid identity with Bacillus β-lactamases) than to most class A enzymes from gram-negative organisms (TEM-1, 24.6%). The aci1 gene had a G+C content of 42.1%, in contrast with 56% G+C content for genomic DNA from A. fermentans, thus suggesting that aci1 may have been obtained by horizontal gene transfer.
β-Lactamase-mediated resistance to β-lactams in anaerobic bacteria has been known since the early 1950s (14). During the last two decades, an increasing number of β-lactamases from anaerobes have been described, in particular among gram-negative rods (11, 20, 28, 33). β-Lactamases have been characterized for the genera Bacteroides (30, 34, 35, 38) and Fusobacterium (40). In Prevotella and Porphyromonas, as well as in Bilophila (12, 21, 29), the presence of β-lactamases is known only by positive nitrocefin reactions. Among gram-positive anaerobic bacteria, β-lactamases have been found in Clostridium (3, 17, 19). β-Lactamases from Bacteroides are cephalosporinases and/or penicillinases; all clostridial and fusobacterial β-lactamases are penicillinases. No β-lactamase has been described previously for anaerobic gram-negative cocci (including Veillonella, Acidaminococcus, and Megasphaera). Indeed, all strains described so far are susceptible to β-lactam antibiotics. In this work, cloning and sequencing of the aci1 gene and molecular characterization of the new β-lactamase ACI-1 from a β-lactam-resistant Acidaminococcus fermentans clinical isolate are reported. To our knowledge, this is the first β-lactamase found in anaerobic gram-negative cocci.
MATERIALS AND METHODS
Strains, plasmids, culture conditions, and susceptibility testing procedures.
The ampicillin-resistant A. fermentans strain RYC-MR95 was isolated from a perianal abscess sample from a diabetic male patient. Two susceptible A. fermentans isolates, RYC4093 and RYC4356, were isolated from clinical samples in the same year from different patients. These strains were grown anaerobically at 37°C in brucella agar supplemented with hemin and vitamin K1 (Becton Dickinson, Meylan Cedex, France) and in prereduced brain heart infusion broth supplemented with yeast extract (Oxoid Ltd., Basingstoke, United Kingdom). Strains were identified based on conventional criteria, including detection by gas-liquid chromatography of the typical butyric acid accumulation (18). Antibiotic MICs were measured by the agar dilution method as recommended by the NCCLS (26), using Wilkins & Chalgren agar medium (Difco Laboratories, Detroit, Mich.) supplemented with 5% horse blood. Plates were incubated in an anaerobic chamber (Forma Scientific, Marietta, Ohio) at 37°C for 48 h.
Escherichia coli HB101 [F− Δ(gtp-proA)62 recA13 leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC-mrr) mtl-1 proA2 xyl-5 rpsL20] (5) was the primary host used for cloning experiments. E. coli RYC1000 [F− Δ(argF-lac)U169 araD139 deoC1 flbB5301 pstF25 relA1 rpsL150 Δrib7 thiA gyrA recA56] (15) was used in all subcloning experiments. E. coli JM109 [endA1 hsdR17 gyrA96 Δ(lac-proA) recAB1 relA supE44 thi F′(lacIq lacZΔM15 proAB+ traΔ36)] (42) was used for expression and biochemical characterization of the β-lactamase. Plasmid vectors used in this work were pBGS18− and pBGS19− (Kanr) (39), pACYC184 (Chlr Tetr) (8), and pOGO-295 (Tetr Ampr) (41), as the expression vector.
E. coli strains were grown in Luria-Bertani broth. MICs were determined by microdilution in Mueller-Hinton broth (Difco Laboratories) under aerobic conditions at 37°C for 24 h (27). When β-lactamase inhibitors were studied in combination with β-lactams, a fixed concentration of 2 μg/ml was used. Antibiotics and inhibitors were ampicillin, carbenicillin, and chloramphenicol (Sigma Chemical Co., St. Louis, Mo.), kanamycin and tetracycline (Bio 101 Inc., Vista, Calif.), amoxicillin, ticarcillin, clavulanate, and cloxacillin (SmithKline Beecham Pharmaceuticals, Harlow, United Kingdom), cephalothin (Eli Lilly & Co., Indianapolis, Ind.), cephaloridine and cefotaxime (Hoechst-Roussel, Antony, France), ceftazidime (Glaxo-Wellcome, Verona, Italy), cefepime (Bristol-Myers Squibb, Wallingford, Conn.), cefoxitin and imipenem (Merck Sharp & Dohme Research Laboratories, Rahway, N.J.), sulbactam (Pfizer, Groton, Conn.), and tazobactam (Lederle Wyeth, Pearl River, N.Y.).
DNA isolation and analysis.
A. fermentans genomic DNA from the strains was prepared according to a previously described procedure (1). Plasmid DNA was obtained using Wizard Miniprep (Promega) and High Pure Plasmid (Roche Diagnostics). Restriction enzymes and T4 DNA ligase were purchased from New England Biolabs, Inc., and Roche Diagnostics, respectively. Transformation of plasmid DNA and Southern hybridization analysis were performed as recommended previously (37).
Cloning experiments and recombinant plasmid constructions.
Genomic DNA from A. fermentans RYC-MR95 was digested with EcoRI and ligated to the EcoRI site in pBGS18−. The ligation mixture was transformed into E. coli HB101, and transformants were selected on kanamycin (50 μg/ml) and ampicillin (30 μg/ml). Subcloning experiments were performed in pACYC184 and subsequently in pBGS19−. pOGO-ACI was constructed by digesting plasmid pOGO295 with XbaI and BamHI. The products of this digestion were separated by agarose gel electrophoresis. The XbaI-BamHI fragment corresponding to the pOGO295 replicon (including the tetracycline resistance determinant) was recovered from the gel. The aci1 gene was PCR amplified by using two primers (ACIFX and ACIRB) that were identical to ACIFE and ACIRH (see below) but contained XbaI and BamHI sites instead of EcoRI and HindIII, respectively. The aci1 amplicon was digested with XbaI and BamHI and ligated to the XbaI-BamHI-pOGO295 replicon to obtain the pOGO-ACI hybrid plasmid.
DNA sequencing.
The nucleotide sequence was determined by the dideoxy polymerase chain termination method with a Sequenase, version 2.0, sequencing kit (United States Biochemical Corp.) using an automated DNA sequencing system (model 377; PE/ABI, Foster City, Calif.). The nucleotide sequence and the deduced protein were analyzed by using the software at the website of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). BLASTN and BLASTP programs were applied to search for β-lactamases with homology to the aci1 gene and ACI-1 β-lactamase sequences. Multiple-sequence alignment of the deduced peptide sequence was carried out at the University of Cambridge website using the program ClustalW from the European Bioinformatics Institute (http://www.ebi.ac.uk).
PCR conditions.
Two oligonucleotide primers were synthesized to amplify only the β-lactamase gene: ACIFE (5′-GGGGAATTCAACAGATAGTAGGAGGT-3′) and ACIRH (5′-CGGCAAGCTTGATGCTATCAAGCCCCTT-3′), with EcoRI and HindIII restriction sites, respectively (underlined). Amplifications were carried out in a thermal cycler (Perkin-Elmer 2400) with the following conditions: 30 three-step cycles including denaturation at 94°C for 1 min, annealing at 47°C for 45 s, and extension at 72°C for 2 min. The amplicon was digested with EcoRI and HindIII and subsequently ligated to pBGS18− and transformed into E. coli RYC1000.
β-Lactamase preparation.
The β-lactamase extract was prepared from 4 liters of an overnight culture of the JM109(pOGO-ACI-1) strain grown in Luria-Bertani broth with ampicillin (50 μg/ml) and tetracycline (20 μg/ml). Cells were harvested, washed twice with 50 mM phosphate buffer (pH 7.02), and resuspended in 4 ml of the same buffer. The suspension was sonicated for 10 min with a 2-s pulse (Sonicator Heat Systems-Ultrasonics, Inc.), debris was removed by centrifugation (10,000 × g, 30 min, 4°C), and the supernatant was assayed for β-lactamase activity with nitrocefin. The suspension was ultrafiltered with Centriplus-100 (Amicon, Inc., Beverly, Mass.). Fractions containing β-lactamase activity were pooled and further concentrated with Centriplus-10 (Amicon, Inc.). The final protein concentration was determined by the method of Bradford (6). The pI was determined by using a Pharmacia Phast system (LKB Biotechnology, Uppsala, Sweden) with LKB Ampholine polyacrylamide gel electrophoresis plates, and β-lactamase activity was detected by nitrocefin staining. Extracts of the β-lactamases TEM-1, TEM-3, TEM-15, TEM-24, SHV-1, SHV-3, and CblA from Bacteroides uniformis, ROB-1 from Haemophilus influenzae, and the β-lactamase from Clostridium butyricum were run in parallel. To study the intracellular location of the β-lactamase, cell fractionating procedures were applied (13).
β-Lactamase activity.
The activity of the β-lactamase preparation obtained from E. coli JM109 harboring the recombinant plasmid pOGO-ACI-1 was measured spectrophotometrically (Uvikon-940 spectrophotometer) against different β-lactam antibiotics. Km and Vmax values were obtained by double-reciprocal (Lineweaver-Burk) plots of the initial steady-state rates at different substrate concentrations. Inhibition studies were carried out by incubating the β-lactamase extract at various concentrations of inhibitors at 25°C for 10 min; then, 100 μM nitrocefin was added as the substrate. The IC50 was defined as the concentration of the inhibitor required to reach a 50% inhibition of the β-lactamase activity (4).
Comparison of β-lactamase sequences and phylogenetic analysis.
Both the nucleotide sequence of the aci1 gene and its deduced ACI-1 protein sequence from A. fermentans were compared with those of 35 other class A β-lactamases, including those from Actinomadura sp. strain R39, Bacillus amyloliquefaciens, Bacillus cereus, Bacillus licheniformis, Bacillus mycoides, Bacillus subtilis, Bacteroides fragilis, B. uniformis, Bacteroides vulgatus, Burkholderia cepacia, Citrobacter diversus, Enterobacter cloacae, E. coli MEN-1, E. coli TEM-1, H. influenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Mycobacterium tuberculosis, Nocardia farcinica, Nocardia lactamdurans, Pseudomonas aeruginosa PSE-1, Proteus vulgaris, Staphylococcus aureus, Streptomyces badius, Streptomyces cacaoi, Streptomyces clavuligerus, Streptomyces fradiae, Serratia fonticola, Serratia marcescens, and Yersinia enterocolitica. Sequences were classified by distance similarity and systematized by parsimony approach using the PAUP program (version 4; Sinauer Associates, Sunderland, Mass.). A matrix of pairwise mean distances (corrected for absolute distance for missing characters) among β-lactamases was computed and values were grouped in a dendrogram by the UPGMA linkage method. Parsimony analysis was carried out with the bootstrapping option for DNA sequences and the step matrix option for protein sequences. Amino acid sequences were analyzed in this way using a step matrix, which specifies the cost of changing from one amino acid to another, with the PROTPARS program of PHYLIP (version 3.4; J. Felsenstein, University of Washington, Seattle).
Nucleotide sequence accession number.
The aci1 nucleotide sequence is available at the EMBL database, with the accession number AJ007350.
RESULTS
Isolation of A. fermentans RYC-MR95.
Strain RYC-MR95 was isolated in 1995 from a perianal abscess of a diabetic male patient who was admitted to the emergency room of the Hospital Ramón y Cajal (Madrid, Spain). Before admission, the patient was initially treated with a full course of amoxicillin and then, when the patient did not improve, a new treatment with ciprofloxacin was started, but signs and symptoms of infection continued. The isolate was obtained on brucella agar supplemented with hemin and vitamin K1. Routine microdilution susceptibility testing revealed resistance to penicillin, amoxicillin, piperacillin, and tetracycline and susceptibility to amoxicillin-clavulanate, cefoxitin, and imipenem in addition to clindamycin, erythromycin, metronidazole, and chloramphenicol. A positive nitrocefin disk test revealed the production of a β-lactamase.
Cloning and characterization of the β-lactamase aci1 gene.
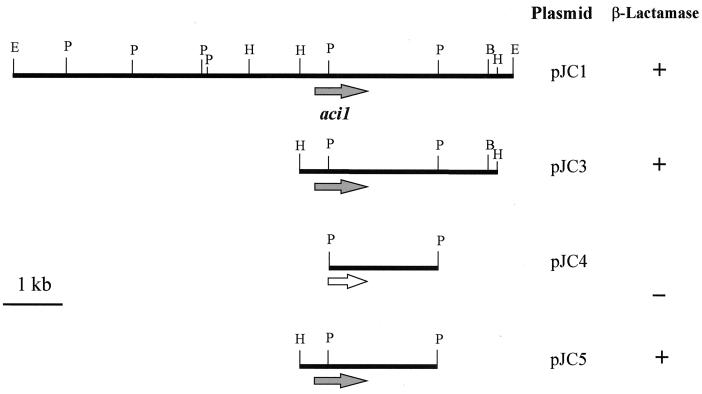
Genomic DNA from RYC-MR95 was digested with EcoRI and ligated to the EcoRI site in pBGS18−. The ligation mixture was transformed into E. coli HB101, and transformants were selected on plates containing kanamycin (50 μg/ml) and ampicillin (30 μg/ml). The recombinant plasmid, pJC1, harbored an EcoRI fragment of 8.3 kb (Fig. 1). Plasmid DNA was not found in the wild-type RYC-MR95 strain when different plasmid extraction methods were used (such as alkaline lysis and the Nakamura technique [25]). The plasmid pJC1 was digested with HindIII and ligated to the HindIII site in pACYC184 and then transformed into E. coli RYC1000, using chloramphenicol (30 μg/ml) and ampicillin (50 μg/ml) as selectors for transformants. The new recombinant plasmid pJC3, harboring a HindIII fragment of 3.3 kb, was isolated. Finally, the plasmid pJC3 was digested with either PstI or HindIII plus PstI, ligated to pBGS19−, and transformed into E. coli RYC1000. A recombinant plasmid, pJC4, harboring a PstI fragment of 1.6 kb, provided no ampicillin resistance to the vector strain. However, the plasmid pJC5, which contained the 1.6-kb PstI fragment plus an additional 0.5-kb HindIII-PstI fragment, was able to confer ampicillin resistance. This 2.1-kb fragment was sequenced on both strands. Analysis of coding regions revealed an open reading frame (ORF) of 852 bp encoding a 284-amino-acid polypeptide (estimated size, 32 kDa). A BLAST search of the deduced polypeptide sequence against the GenBank database from the National Center for Biotechnology Information showed the presence of a single β-lactamase with homology to class A β-lactamases from gram-positive bacteria.
FIG. 1.
Physical map of plasmid pJC1 and subcloning strategy. The solid arrow represents the aci1 β-lactamase gene, and the open arrow represents the truncated gene. Restriction sites: E, EcoRI; B, BamHI; H, HindIII; P, PstI. The enzymatic activity was detected by nitrocefin reaction.
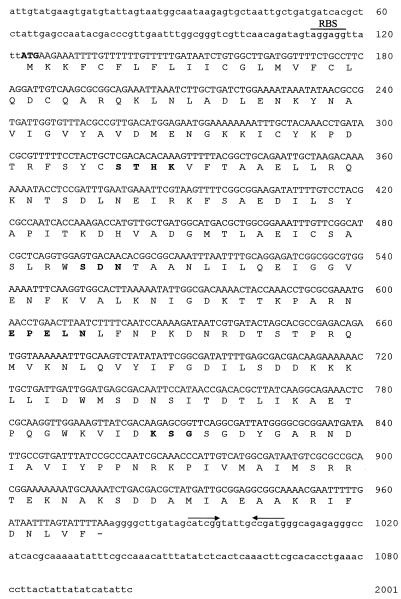
The nucleotide sequence of this ORF is shown below in Fig. 3. The G+C content of this sequence was 42%, quite dissimilar to the 56% overall G+C content of A. fermentans chromosomal DNA (36). A possible Shine-Dalgarno ribosome-binding site (AGGAGG) was identified 5 bp prior to the start codon.
FIG. 3.
Nucleotide sequence of the A. fermentans aci1 β-lactamase gene and its flanking regions. The predicted amino acid sequence is shown below the nucleotide sequence. The putative initiation codon and conserved motifs are shown in bold. The pair of arrows indicates the putative transcription terminator. The predicted Shine-Dalgarno sequence is also shown as RBS.
To isolate the putative gene, two primers, ACIFE and ACIRH, were designed and synthesized to amplify the ORF, including its putative Shine-Dalgarno site. The amplification product, 0.8 kb, was cloned into pBGS18−, yielding a plasmid, pJC10, which conferred resistance to ampicillin in E. coli RYC1000. In summary, the β-lactamase found in the resistant A. fermentans clinical isolate corresponded to a new member of the class A β-lactamases that we propose to name ACI-1 (for “Acidaminococcus”).
Antimicrobial susceptibility pattern.
The MICs of different β-lactam antibiotics for the ACI-1-positive and -negative A. fermentans strains, as well as for the E. coli strains harboring (or not) the recombinant plasmids, are shown in Table 1. The results showed that organisms harboring this new class A β-lactamase displayed resistance to penicillins (penicillin, amoxicillin, and ticarcillin) and expanded-spectrum cephalosporins (cefotaxime and ceftazidime). The cefotaxime MIC was 128-fold higher for the original resistant strain than for the susceptible Acidaminococcus strains. A similar decrease in susceptibility occurred in the E. coli strains harboring the recombinant ACI-1-encoding plasmids, compared with the strains harboring no plasmids or plasmids encoding a truncated enzyme. The presence of clavulanic acid (2 μg/ml) strongly reduced the MICs of both penicillins and cephalosporins. The presence of ACI-1 in E. coli strains slightly increased the MICs of cefepime but not of imipenem or cefoxitin.
TABLE 1.
MICs of β-lactams for A. fermentans RYC-MR95, E. coli harboring recombinant plasmids which produce β-lactamase ACI-1, and reference strainsa
| Antibiotic | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| RYC-MR95 | RYC4093 | RYC4356 | RYC1000/(pJC10) | RYC1000/(pJC4) | RYC1000 | |
| Penicillin | 128 | ≤0.5 | ≤0.5 | |||
| Amoxicillin | 64 | 1 | ≤0.5 | 1,024 | 8 | 8 |
| Amoxicillin-Cla | ≤0.03 | ≤0.03 | ≤0.03 | 8 | 4 | 4 |
| Ticarcillin | 256 | 1 | ≤0.5 | ≥1,024 | 4 | 4 |
| Ticarcillin-Cla | 0.25 | 0.5 | ≤0.03 | 16 | 4 | 4 |
| Cephalothin | 4 | ≤0.5 | ≤0.5 | 16 | 2 | 2 |
| Cephalothin-Cla | 2 | 0.5 | 0.25 | 4 | 2 | 2 |
| Ceftazidime | 32 | 2 | 2 | 16 | 0.12 | 0.12 |
| Ceftazidime-Cla | 2 | 2 | 0.5 | 0.5 | 0.12 | 0.12 |
| Cefotaxime | 64 | 0.5 | 0.5 | 4 | 0.03 | 0.03 |
| Cefotaxime-Cla | 0.25 | 0.5 | 0.25 | 0.03 | 0.03 | 0.03 |
| Cefepime | 1 | 0.015 | 0.03 | |||
| Cefepime-Cla | 0.015 | 0.03 | ||||
| Cefoxitin | 4 | 1 | 1 | 1 | 0.5 | 0.5 |
| Imipenem | 0.5 | 0.5 | 0.5 | 0.12 | 0.06 | 0.06 |
RYC-MR95 is wild-type A. fermentans producing ACI-1. RYC4093 and RYC4356 are β-lactam-susceptible A. fermentans strains. RYC1000 harboring recombinant plasmid pJC10 produced the ACI-1 β-lactamase. RYC1000 harboring recombinant plasmid pJC4 contained a truncated aci1 gene. Cla, clavulanic acid at a fixed concentration of 2 μg/ml.
Absence of aci1 in ampicillin-susceptible A. fermentans strains.
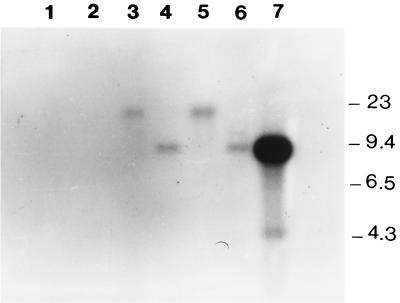
To verify that aci1 was not present in ampicillin-susceptible A. fermentans strains, we developed the Southern blot hybridization shown in Fig. 2. The 8.3-kb EcoRI chromosomal fragment present in plasmid pJC1 was labeled and used as a probe against genomic DNAs from A. fermentans RYC-MR95 and RYC4093 that were previously digested with EcoRI or BamHI. The results suggest that A. fermentans RYC-MR95 harbors only one copy of the β-lactamase gene and flanking regions into genomic DNA, while the susceptible strain RYC4093 did not show any hybridization signal. Similarly, the aci1 gene was not detected by PCR in another susceptible A. fermentans RYC4356 clinical isolate (data not shown).
FIG. 2.
Southern hybridization. The A. fermentans 8.3-kb EcoRI DNA fragment was labeled and used as a probe for Southern blot analysis. Lane 1, RYC4093 genomic DNA digested with BamHI; lane 2, RYC4093 genomic DNA digested with EcoRI; lane 3, RYC-MR95 genomic DNA digested with BamHI; lane 4, RYC-MR95 genomic DNA digested with EcoRI; lanes 5 and 6, same as lanes 3 and 4, respectively, with slightly higher concentrations of DNA; lane 7, plasmid pJC1 digested with EcoRI. Molecular sizes are shown, in kilobases.
Structural characteristics of ACI-1.
Within the deduced protein sequence, all characteristic motifs of penicillin-binding proteins were found. A bla active-site (STHK) tetrad was detected at positions 65 to 68 (positions 70 to 73 of TEM-1 β-lactamase in the numbering scheme of Ambler [2]). An SDN motif at positions 123 to 125 (positions 130 to 132 of TEM-1) and a KSG motif at positions 226 to 228 (positions 234 to 236 of TEM-1) were also found. In addition, a specific feature of class A β-lactamases was found: the Ω loop region (EPELN) at positions 159 to 163 (Fig. 3).
Amino acid analysis showed 41 to 44% identity with class A penicillinases from some gram-positive bacteria, particularly with those of B. licheniformis (43.3%), B. amyloliquefaciens (44%), B. cereus (40.8%), and S. aureus (43%) and with ROB-1 from H. influenzae (44%). A lower homology with common class A β-lactamases from gram-negative bacteria (24.6% with TEM-1 and 28.2% with SHV-1) was found. The sequence of the ACI-1 enzyme showed higher homology with non-TEM and non-SHV extended-spectrum β-lactamases, such as CTX-M-3 from Salmonella enterica serovar Typhimurium (35.2%) (16).
Biochemical properties of ACI-1 β-lactamase.
The isoelectric point of ACI-1 was studied on preparations of the wild-type RYC-MR95 and recombinant RYC1000(pJC10) strains. In both cases, two bands were observed when the polyacrylamide gel was stained with nitrocefin. The main band showed a pI of 4.3, and a second band appeared around pI 6.7. When cell fractionating procedures were applied, the pI 4.3 band was found only in the periplasmic extract. Conversely, in the cytoplasmic extract only the pI 6.7 band was detected. The ACI-1 β-lactamase from E. coli strain JM109(pOGO-ACI-1) was partially purified. ACI-1 is a broad-spectrum β-lactamase which hydrolyzes both penicillins (penicillin, carbenicillin, ticarcillin, and cloxacillin) and cephalosporins (cephaloridine and cefotaxime). The highest hydrolysis rate corresponded to penicillin, but cefotaxime was also efficiently hydrolyzed, better than cephaloridine. The β-lactamase affinity for cefotaxime was the highest among the tested compounds (Table 2). The clavulanate IC50 for ACI-1 was 0.018 μM, lower than that for TEM-1 (0.08 μM). The IC50 obtained for sulbactam was 0.008 μM (TEM-1, 8 μM), and that for tazobactam was 0.007 μM (TEM-1, 0.16 μM). The ACI-1 hydrolytic effect was not inhibited by EDTA. These results suggest that the ACI-1 enzyme is a β-lactamase that may belong in the group 2be β-lactamases of the Bush classification (7).
TABLE 2.
Hydrolytic activity of ACI-1 enzyme against different β-lactam antibiotics
| β-Lactam agent | Km (μM) | Relative Vmaxa | Relative Vmax/Km |
|---|---|---|---|
| Penicillin | 3 ± 0.4 | 100 | 100 |
| Ticarcillin | 13.3 ± 3 | 27 ± 1 | 6.7 |
| Carbenicillin | 116 ± 26 | 30 ± 2 | 6.5 |
| Cloxacillin | 29 ± 9 | 33 ± 4 | 2.7 |
| Cephaloridine | 6 ± 0.6 | 64 ± 8 | 36 |
| Cefotaxime | 2.1 ± 0.2 | 26 ± 2 | 42.2 |
| Imipenem | 747 ± 65 | 0.4 ± 0.1 | 3 |
Penicillin was a substrate reference, set at 100. Data are the means and standard deviations from three independent experiments.
ACI-1 sequence comparison with other class A β-lactamases.
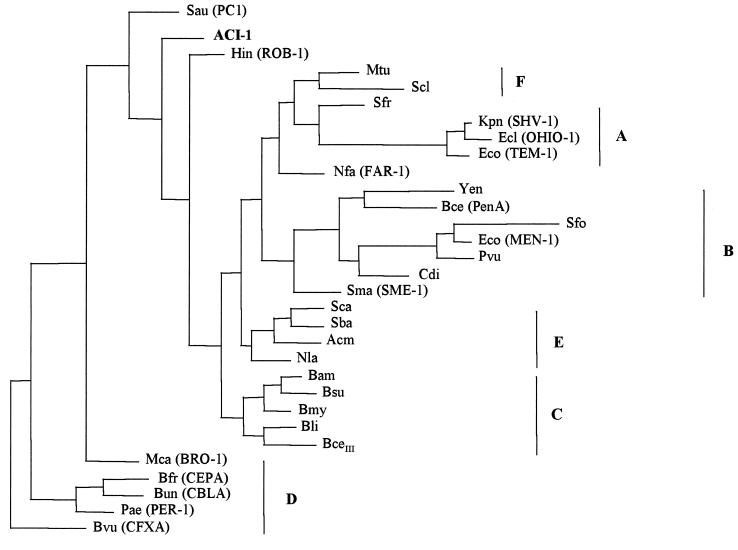
The alignment of amino acid sequences generated 408 protein positions that served to compare ACI-1 with other class A β-lactamases. Although alignment of the nucleotide sequence was also done, scarce resolution in any tree was obtained by using such sequences. No significant cophenetic correlation between sets of data was detected. Therefore, the tree in Fig. 4 only expresses relationships among protein sequences, because of its higher resolution and consistency. This figure suggests evolutionary relationships based on parsimony criteria in which the outgroup was considered paraphyletic. The tree shows that only the β-lactamase BRO-1 from M. catarrhalis could be considered apart. The ACI-1 enzyme from A. fermentans was consistently found in or near the basal node of the main phylogenetic class A β-lactamase group. That was coincident to distance analysis criteria (tree not shown). A similar situation was found for ROB-1 from H. influenzae, with both constituting the possible origin of independent monophyletic lines.
FIG. 4.
Phylogenetic trees obtained for 31 class A β-lactamases, according to the parsimony criteria. Abbreviations: Sau, S. aureus PC1 (P00807); Hin, H. influenzae ROB-1 (P33949); Mtu, M. tuberculosis (Q10670); Scl, S. clavuligerus (Z54190); Nfa, N. farcinica FAR-1 (AF024601); Acm, Actinomadura sp. strain R39 (X53650); Sca, S. cacaoi (P14560); Sba, S. badius (P35391); Nla, N. lactamdurans (Q06316); Bam, B. amyloliquefaciens (Q44674); Bsu, B. subtilis (P39824); Bmy, B. mycoides (P28018); Bli, B. licheniformis (P00808); BceIII, B. cereus β-lactamase type III (P06548); Yen, Y. enterocolitica (Q01166); Bce, B. cepacia (U85041); Sfo, S. fonticola (P80545); Eco (MEN-1), E. coli (P28585); Cdi, C. diversus (P22390); Pvu, P. vulgaris (P52664); Sma, S. marcescens Sme-1 (P52682); Sfr, S. fradiae (P35392); Kpn, K. pneumoniae SHV-1 (P23982); Ecl, E. cloacae (OHIO-1) (P18251); Eco (TEM-1), E. coli (P00810); Mca, M. catarrhalis (BRO-1) (Q59514); Bvu, B. vulgatus (CfxA) (P30899); Bfr, B. fragilis (CepA) (L13472); Bun, B. uniformis (CblA) (P30898); Pae, P. aeruginosa (PER-1) (P37321). The codes in parentheses correspond to listed accession numbers (see reference 23 and the European Bioinformatics Institute website [http://www.ebi.ac.uk]).
DISCUSSION
The antibiotic susceptibility of the different gram-negative anaerobic cocci isolated from humans remains largely unknown. The isolation of such microorganisms from clinical samples is relatively infrequent, and among them, Acidaminococcus has been very rarely reported. Most general studies on anaerobes do not distinguish Acidaminococcus from Veillonella and Megasphaera; others deal only with strains of veterinary origin (32). Penicillin resistance in Veillonella has previously been reported by our group, but the strains were in all cases β-lactamase negative (M. Reig, N. Mir, and F. Baquero, Letter, Antimicrob. Agents Chemother. 41:1210, 1997). To date, Acidaminococcus has been considered fully susceptible to β-lactam antibiotics. β-Lactamases are the main mechanism of resistance in anaerobic gram-negative rods (33), but these enzymes had never been found among gram-negative anaerobic cocci. In this work, the presence of a novel class A β-lactamase (ACI-1) was detected in an A. fermentans clinical strain resistant to penicillins and cephalosporins. The nucleotide sequence of ACI-1 revealed a closer relationship with class A β-lactamases from some gram-positive bacteria than with many enzymes from gram-negative bacteria. The ACI-1 enzyme shares all characteristics common with those of class A β-lactamases. The highest homology was found with β-lactamases from Bacillus and with ROB-1 from H. influenzae. The phylogenetic analysis of sequences strongly suggests that ACI-1, like ROB-1, is located in an independent monophyletic line, very near the basal node that constitutes the common root of most class A β-lactamases.
What is the origin of the aci1 β-lactamase gene found in the chromosome of A. fermentans? No plasmids were found in the resistant isolate. The G+C content of 42% for the aci1 structural gene was very dissimilar to that of A. fermentans chromosomal DNA (56%). The flanking regions of the aci1 gene have a 41.8% G+C content upstream and a 52.4% G+C content downstream. Moreover, the 8.3-kb fragment containing the aci1 gene was not detected in two β-lactam-susceptible A. fermentans strains. Altogether, these data strongly suggest that the β-lactamase gene could be part of a transposable element, as has also been proposed for the origin of the ROB-1-encoding gene in Pasteurella (22). The higher similarity of ACI-1 was found with class A β-lactamases from some gram-positive organisms. Members of the family Veillonellaceae, which includes Veillonella, A. fermentans, and Megasphaera elsdenii, are anaerobic gram-negative cocci but may stain weakly as gram positive. On the other hand, the 16S rRNA gene sequences of these three genera have allowed their classification within cluster IX (Sporomusa subbranch) of the low-G+C-content Bacillus/Clostridium gram-positive subphylum (10). The taxonomic position of Acidaminococcus may explain the presence of a β-lactamase similar to those of gram-positive bacteria. The consequences of a broad-spectrum β-lactamase in Acidaminococcus are difficult to evaluate. These organisms are part of the resident microbiota of the gastrointestinal tract in humans and animals, although their prevalence and density are low compared with those of B. vulgatus or Fusobacterium prausnitzii (24). Even though Acidaminococcus is rarely involved in clinical infections, it has been isolated from abdominal and pulmonary abscesses (9) and in bacteremia (31), always as part of a mixed anaerobic flora. The results from this study suggest that A. fermentans may have an indirect effect on human health and may contribute to the origin or spreading of resistance genes encoding both penicillin- and cefotaxime-hydrolyzing β-lactamases in one of the most complex microbial ecosystems known.
ACKNOWLEDGMENTS
We thank Constantino Cespón for assistance with the biochemical characterization of this enzyme and Luis de Rafael for his critical comments.
J. C. Galán is the recipient of a fellowship from the F.I.S.S. (BEFI 98/9060).
REFERENCES
- 1.Alonso R, Nicholson R, Pitt T. Rapid extraction of high purity chromosomal DNA from Serratia marcescens. Lett Appl Microbiol. 1993;16:77–79. doi: 10.1111/j.1472-765x.1993.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson F W. A standard numbering scheme for the Class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum P C, Spangler S K, Pankuch G A, Philippon A, Jacobs M R, Shiman R, Goldstein E J C, Citron D M. Characterization of a β-lactamase from Clostridium clostridioforme. J Antimicrob Chemother. 1994;33:33–40. doi: 10.1093/jac/33.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Blazquez J, Baquero M-R, Canton R, Alos I, Baquero F. Characterization of a new TEM-type β-lactamase resistant to clavulanate, sulbactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1993;37:2059–2063. doi: 10.1128/aac.37.10.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee B D, Chakraborti C K. Non-sporing anaerobes in certain surgical group of patients. J Indian Med Assoc. 1995;93:333–339. [PubMed] [Google Scholar]
- 10.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 11.Edwards R, Greenwood D. An investigation of β-lactamases from clinical isolates of Bacteroides species. J Med Microbiol. 1992;36:89–95. doi: 10.1099/00222615-36-2-89. [DOI] [PubMed] [Google Scholar]
- 12.Finegold S, Summanen P, Gerardo S H, Baron E. Clinical importance of Bilophila wadsworthia. Eur J Clin Microbiol Infect Dis. 1992;11:1058–1063. doi: 10.1007/BF01967799. [DOI] [PubMed] [Google Scholar]
- 13.Fraipont C, Adam M, Nguyen-Disteche M, Keck W, Van Beeumen J, Ayala J A, Granier B, Hara H, Ghuysen J M. Engineering and overexpression of periplasmic forms of the penicillin-binding protein 3 of Escherichia coli. Biochem J. 1994;298:189–195. doi: 10.1042/bj2980189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrod L P. Sensitivity of four species of Bacteroides to antibiotics. Br Med J. 1955;ii:1529–1531. doi: 10.1136/bmj.2.4955.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genilloud O, Garrido M C, Moreno F. The transposon Tn5 carries a bleomycin-resistance determinant. Gene. 1984;32:225–233. doi: 10.1016/0378-1119(84)90050-7. [DOI] [PubMed] [Google Scholar]
- 16.Gniadkowski M, Schneider I, Paucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart C A, Barr K, Makin T, Brown P, Cooke R W I. Characterization of a β-lactamase produced by Clostridium butyricum. J Antimicrob Chemother. 1982;10:31–35. doi: 10.1093/jac/10.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Holdeman L V, Cato E P, Moore W E C, editors. Anaerobe laboratory manual. 4th ed. Blacksburg: Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- 19.Kesado T, Lindqvist L, Hedberg M, Tunér K, Nord C E. Purification and characterization of a new β-lactamase from Clostridium butyricum. Antimicrob Agents Chemother. 1989;33:1302–1307. doi: 10.1128/aac.33.8.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King A, Downes J, Nord C E, Phillips I on behalf of a European Study Group. Antimicrobial susceptibility of non-Bacteroides fragilis group anaerobic gram-negative bacilli in Europe. Clin Microbiol Infect. 1999;5:404–416. doi: 10.1111/j.1469-0691.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 21.Könöen E, Nyfors S, Mätö J, Asikainen S, Jousimies-Somer H. β-Lactamase production by oral pigmented Prevotella species isolated from young children. Clin Infect Dis. 1997;25:S272–S274. doi: 10.1086/516208. [DOI] [PubMed] [Google Scholar]
- 22.Livrelli V, Peduzzi J, Joly B. Sequence and molecular characterization of the ROB-1 β-lactamase gene from Pasteurella haemolytica. Antimicrob Agents Chemother. 1991;35:242–251. doi: 10.1128/aac.35.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob Agents Chemother. 1998;42:1–17. doi: 10.1128/aac.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura M, Sato S, Ohya T, Suzuki S, Ikeda S. Plasmid profile analysis in epidemiological studies of animal Salmonella typhimurium infection in Japan. J Clin Microbiol. 1986;23:360–365. doi: 10.1128/jcm.23.2.360-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria. 3rd ed. Approved standard M11-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 28.Nyfors S, Könönen E, Takala A, Jousimies-Somer H. β-Lactamase production by oral anaerobic gram-negative species in infants in relation to previous antimicrobial therapy. Antimicrob Agents Chemother. 1999;43:1591–1594. doi: 10.1128/aac.43.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajukanta R, Asikainen S, Forsblom B, Saarela M, Jousimies-Somer H. β-Lactamase production and in vitro antimicrobial susceptibility of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 1993;6:241–244. doi: 10.1111/j.1574-695X.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 30.Parker A C, Smith C J. Genetic and biochemical analysis of a novel Ambler class A β-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993;37:1028–1036. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peraino V A, Cross S A, Goldstein E J C. Incidence and significance of anaerobic bacteremia in a community hospital. Clin Infect Dis. 1993;16:S288–S291. doi: 10.1093/clinids/16.supplement_4.s288. [DOI] [PubMed] [Google Scholar]
- 32.Piriz S, Cuenca R, Valle J, Vadillo S. Susceptibilities of anaerobic bacteria isolated from animals with ovine foot rot to 28 antimicrobial agents. Antimicrob Agents Chemother. 1992;36:198–201. doi: 10.1128/aac.36.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24:S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers M B, Parker A C, Smith C J. Cloning and characterization of the endogenous cephalosporinase gene, cepA, from Bacteroides fragilis reveals a new subgroup of Ambler class A β-lactamases. Antimicrob Agents Chemother. 1993;37:2391–2400. doi: 10.1128/aac.37.11.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogosa M. Family I. Veillonellaceae. In: Krieg N R, editor. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 680–685. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Smith C J, Bennett T K, Parker A C. Molecular and genetic analysis of the Bacteroides uniformis cephalosporinase gene, cblA, encoding the species-specific β-lactamase. Antimicrob Agents Chemother. 1994;38:1711–1715. doi: 10.1128/aac.38.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spratt B G, Hedge P J, te Heesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 40.Tunér K, Lindqvist L, Nord C E. Purification and properties of a novel β-lactamase from Fusobacterium nucleatum. Antimicrob Agents Chemother. 1985;27:943–947. doi: 10.1128/aac.27.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usher K C, Blaszczak L C, Weston G S, Shoichet B K, Remington S J. Three-dimensional structure of AmpC β-lactamase from Escherichia coli bound to a transition-state analogue: possible implications for the oxyanion hypothesis and for inhibitor design. Biochemistry. 1998;37:16082–16092. doi: 10.1021/bi981210f. [DOI] [PubMed] [Google Scholar]
- 42.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]