Abstract
Background
Cystic fibrosis (CF) is an inherited life‐limiting disorder. Over time persistent infection and inflammation within the lungs contribute to severe airway damage and loss of respiratory function. Chest physiotherapy, or airway clearance techniques (ACTs), are integral in removing airway secretions and initiated shortly after CF diagnosis. Conventional chest physiotherapy (CCPT) generally requires assistance, while alternative ACTs can be self‐administered, facilitating independence and flexibility. This is an updated review.
Objectives
To evaluate the effectiveness (in terms of respiratory function, respiratory exacerbations, exercise capacity) and acceptability (in terms of individual preference, adherence, quality of life) of CCPT for people with CF compared to alternative ACTs.
Search methods
We used standard, extensive Cochrane search methods. The latest search was 26 June 2022.
Selection criteria
We included randomised or quasi‐randomised controlled trials (including cross‐over design) lasting at least seven days and comparing CCPT with alternative ACTs in people with CF.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. pulmonary function tests and 2. number of respiratory exacerbations per year. Our secondary outcomes were 3. quality of life, 4. adherence to therapy, 5. cost–benefit analysis, 6. objective change in exercise capacity, 7. additional lung function tests, 8. ventilation scanning, 9. blood oxygen levels, 10. nutritional status, 11. mortality, 12. mucus transport rate and 13. mucus wet or dry weight.
We reported outcomes as short‐term (seven to 20 days), medium‐term (more than 20 days to up to one year) and long‐term (over one year).
Main results
We included 21 studies (778 participants) comprising seven short‐term, eight medium‐term and six long‐term studies. Studies were conducted in the USA (10), Canada (five), Australia (two), the UK (two), Denmark (one) and Italy (one) with a median of 23 participants per study (range 13 to 166). Participant ages ranged from newborns to 45 years; most studies only recruited children and young people. Sixteen studies reported the sex of participants (375 males; 296 females).
Most studies compared modifications of CCPT with a single comparator, but two studies compared three interventions and another compared four interventions. The interventions varied in the duration of treatments, times per day and periods of comparison making meta‐analysis challenging. All evidence was very low certainty.
Nineteen studies reported the primary outcomes forced expiratory volume in one second (FEV1)and forced vital capacity (FVC), and found no difference in change from baseline in FEV1 % predicted or rate of decline between groups for either measure. Most studies suggested equivalence between CCPT and alternative ACTs, including positive expiratory pressure (PEP), extrapulmonary mechanical percussion, active cycle of breathing technique (ACBT), oscillating PEP devices (O‐PEP), autogenic drainage (AD) and exercise. Where single studies suggested superiority of one ACT, these findings were not corroborated in similar studies; pooled data generally concluded that effects of CCPT were comparable to those of alternative ACTs.
CCPT versus PEP
We are uncertain whether CCPT improves lung function or has an impact on the number of respiratory exacerbations per year compared with PEP (both very low‐certainty evidence). There were no analysable data for our secondary outcomes, but many studies provided favourable narrative reports on the independence achieved with PEP mask therapy.
CCPT versus extrapulmonary mechanical percussion
We are uncertain whether CCPT improves lung function compared with extrapulmonary mechanical percussions (very low‐certainty evidence). The annual rate of decline in average forced expiratory flow between 25% and 75% of FVC (FEF25–75) was greater with high‐frequency chest compression compared to CCPT in medium‐ to long‐term studies, but there was no difference in any other outcome.
CCPT versus ACBT
We are uncertain whether CCPT improves lung function compared to ACBT (very low‐certainty evidence). Annual decline in FEF25–75 was worse in participants using the FET component of ACBT only (mean difference (MD) 6.00, 95% confidence interval (CI) 0.55 to 11.45; 1 study, 63 participants; very low‐certainty evidence). One short‐term study reported that directed coughing was as effective as CCPT for all lung function outcomes, but with no analysable data. One study found no difference in hospital admissions and days in hospital for exacerbations.
CCPT versus O‐PEP
We are uncertain whether CCPT improves lung function compared to O‐PEP devices (Flutter device and intrapulmonary percussive ventilation); however, only one study provided analysable data (very low‐certainty evidence). No study reported data for number of exacerbations. There was no difference in results for number of days in hospital for an exacerbation, number of hospital admissions and number of days of intravenous antibiotics; this was also true for other secondary outcomes.
CCPT versus AD
We are uncertain whether CCPT improves lung function compared to AD (very low‐certainty evidence). No studies reported the number of exacerbations per year; however, one study reported more hospital admissions for exacerbations in the CCPT group (MD 0.24, 95% CI 0.06 to 0.42; 33 participants). One study provided a narrative report of a preference for AD.
CCPT versus exercise
We are uncertain whether CCPT improves lung function compared to exercise (very low‐certainty evidence). Analysis of original data from one study demonstrated a higher FEV1 % predicted (MD 7.05, 95% CI 3.15 to 10.95; P = 0.0004), FVC (MD 7.83, 95% CI 2.48 to 13.18; P = 0.004) and FEF25–75 (MD 7.05, 95% CI 3.15 to 10.95; P = 0.0004) in the CCPT group; however, the study reported no difference between groups (likely because the original analysis accounted for baseline differences).
Authors' conclusions
We are uncertain whether CCPT has a more positive impact on respiratory function, respiratory exacerbations, individual preference, adherence, quality of life, exercise capacity and other outcomes when compared to alternative ACTs as the certainty of the evidence is very low.
There was no advantage in respiratory function of CCPT over alternative ACTs, but this may reflect insufficient evidence rather than real equivalence. Narrative reports indicated that participants prefer self‐administered ACTs. This review is limited by a paucity of well‐designed, adequately powered, long‐term studies. This review cannot yet recommend any single ACT above others; physiotherapists and people with CF may wish to try different ACTs until they find an ACT that suits them best.
Keywords: Adolescent; Child; Humans; Infant, Newborn; Middle Aged; Cystic Fibrosis; Cystic Fibrosis/complications; Drainage, Postural; Drainage, Postural/methods; Physical Therapy Modalities; Quality of Life; Respiratory Therapy; Respiratory Therapy/methods
Plain language summary
Conventional (traditional) chest physiotherapy compared to other methods of airway clearance in people with cystic fibrosis
Review question
Is conventional chest physiotherapy (CCPT) better than other methods of airway clearance for people with cystic fibrosis (CF)?
Key messages
Airway clearance techniques (ACTs) are a very important part of therapy in people with CF.
There are now lots of different types of ACTs, but there is still very little known about how good each one is and how they compare to each other.
The focus of this review was to compare CCPT (also known as traditional chest physiotherapy) with alternative ACTs.
What is cystic fibrosis?
CF is a life‐limiting inherited disease affecting between 70,000 and 100,000 people worldwide. People with CF produce mucus in their lungs that can be thick, sticky and difficult to clear. This leads to repeated infections and lung damage. It is important to clear this mucus using medicines and various chest physiotherapy ACTs. CCPTs such as postural drainage, percussion and vibration, huffing and coughing have generally required assistance from someone else during treatments. Alternative ACTs such as active cycle of breathing techniques, forced expiration technique, autogenic drainage, positive expiratory pressure devices, oscillating positive expiratory pressure devices (e.g. Acapella, Aerobika, Flutter, RC‐Cornet) or high‐frequency chest compression and exercise are self‐administered, which increases independence and flexibility.
What did we want to find out?
We wanted to compare how different ACTs affect lung function (how well the lungs work), respiratory exacerbations (flare‐ups of lung disease), a person's preference, adherence (how well the person follows their doctor's advice), quality of life and impact on fitness levels.
What did we do?
We searched medical databases for well‐designed studies comparing CCPT with other ACTs in people with CF. Studies selected people for one treatment or the other in a random or partly random way. We chose studies lasting longer than one week to allow enough time for treatments to have an impact on the outcomes we were interested in.
What did we find?
We found 21 studies with 778 people with CF aged from newborn to 45 years and with all levels of disease severity. The number of people included in each study ranged from 13 to 166. There were more females than males included in all but two studies.
Main results
The studies did not show that any alternative ACTs were better than CCPT in terms of lung function, respiratory exacerbations, a person's preference, adherence, quality of life and impact on fitness level. Studies in which participants had flare‐ups of chest infections showed that lung function improved considerably after treatment, irrespective of the type of ACT. Longer‐term studies showed smaller improvements or a decline in lung function. In 10 studies, participants preferred the chest physiotherapy techniques they could administer themselves, but, since the studies measured preference in different ways, we could not combine the individual study results in an analysis. Side effects from different ACTs tended to be rare, mild and resolved quickly. We cannot recommend any single treatment over another at this time.
What are the limitations of the evidence?
The quality of evidence varied greatly between the studies. We are not sure that the present evidence is able to answer the questions we were asking. We did not find enough evidence to confirm or exclude any differences between CCPT and alternative ACTs in terms of our outcome measures. Furthermore, data from our included studies did not show that any of the alternative ACTs were better than CCPT in people with CF. This may be because the studies did not report enough data for us to analyse, rather than confirming that the ACTs all worked equally well. There were differences in how the studies were run and how the researchers collected data, so this also makes it difficult to compare results. These differences included the number of people in the studies, length of time the studies lasted, location of the studies (e.g. in hospital or at home), types of ACTs used and outcomes measured by the researchers to assess the impact of the different techniques. In future studies it would be better if these factors were more similar to make it easier to compare results.
How up to date is this evidence?
We last searched the databases in June 2022.
Summary of findings
Summary of findings 1. CCPT versus PEP.
| CCPT compared with PEP for cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: CCPT Comparison: PEP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PEP | CCPT | |||||
|
FEV1: change from baseline in FEV1 % predicted Follow‐up: medium‐to‐long term |
There was no difference in the change from baseline in FEV1 % predicted between groups: MD −0.05% (−2.39% to 2.29%). | NA | 169 (6) |
⊕⊝⊝⊝ Very lowa,b |
— | |
|
FVC: change from baseline in FVC % predicted Follow‐up: medium term |
There was no difference in the mean change from baseline in FVC % predicted between groups: MD −0.10 (−3.71 to 3.51). | NA | 169 (6) |
⊕⊝⊝⊝ Very lowa,b |
— | |
|
FEF25–75: change from baseline in FEF25–75 Follow‐up: medium‐to‐long term |
There was no difference in the mean change from baseline in FEF25–75 between groups: MD −0.87 (−4.86 to 3.12). | NA | 87 (4) |
⊕⊝⊝⊝ Very lowb,c |
— | |
|
Respiratory exacerbations: number of respiratory exacerbations per year Follow‐up: medium‐to‐long term |
See comments | None of the included studies reported number of respiratory exacerbations per year. 1 long‐term study reported number of hospital admissions for respiratory exacerbation per year and found no difference between CCPT and PEP (RR 0.85, 95% CI 0.53 to 1.35; P = 0.48) (McIlwaine 1997). 1 further study reported number of days of IV antibiotics for respiratory exacerbations. The data were inconsistent between abstracts of the same study, and we were unable to include them in our analyses (Costantini 2001). |
||||
|
QoL: change in QWB Follow‐up: 2 years |
There was no change in QWB in either the CCPT or PEP group over the 2‐year study (Gaskin 1998). | Not available | 66 (1) |
⊕⊝⊝⊝ Very lowb,d |
There were no data available for this outcome and we narratively reported results directly from the paper (Gaskin 1998). | |
|
Adherence to therapy and individual preference Follow‐up: medium‐to‐long term |
Medium‐term studies 5 medium‐term studies commented favourably on the independence, comfort or ease of use achieved with PEP mask therapy (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tyrrell 1986; van Asperen 1987), although 1 study suggested participants with copious secretions did not consider PEP cleared their secretions fully (Tyrrell 1986), and 1 study found that participants reverted to CCPT during an exacerbation (van Asperen 1987). Long‐term studies Of 3 studies, 1 reported that participants preferred PEP (Costantini 2001), 1 provided no information (Gaskin 1998), and 1 reported slightly better adherence to PEP (96% adherence with PEP vs 92% with CCPT; McIlwaine 1997). |
NA | 224 (8) |
⊕⊝⊝⊝ Very lowe,f |
We were unable to include any of the study data in our analyses and have reported narratively from the original papers. Methods used in the studies to collect this information were varied, ad hoc or mostly anecdotal. |
|
|
Mucus weight Follow‐up: medium term |
3 studies reported no difference in mucus weight (Steen 1991; Tyrrell 1986; van Asperen 1987), and 1 reported greater sputum production with PEP but did not provide details of how this was measured or analysed (McIlwaine 1991). | NA | 75 (4) |
⊕⊝⊝⊝ Very lowb,e |
There were no data available for analysis for this outcome and the methods of reporting and analysis were unclear. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCPT: conventional chest physiotherapy; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; IV: intravenous; MD: mean difference; NA: not applicable; PEP: positive expiratory pressure; QoL: quality of life; QWB: Quality of Wellbeing Scale. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to risk of bias within the studies included in the analysis; 4/6 studies were at high or unclear risk of bias across all domains. The remaining 2 studies were at low risk of bias from the randomisation process and blinding of outcome assessors. The remaining domains were at high or unclear risk of bias. b Downgraded once due to imprecision from small numbers of participants in the individual studies and overall. c Downgraded twice due to risk of bias within the studies included in the analysis; 3/4 studies were at high or unclear risk of bias across all domains. The remaining study was at low risk of bias from the randomisation process and blinding of outcome assessors. The remaining domains were at high or unclear risk of bias. d Downgraded twice for risk of bias in the single study included for this outcome. There was unclear or high risk of bias across all domains. e Downgraded twice due to risk of bias within the studies with most domains across studies being at unclear or high risk of bias. f Downgraded once due to imprecision caused by small number of participants. Although the total number of participants included for this outcome was 224, the individual studies had small numbers of participants. As we could not combine any of the data, the total number is irrelevant.
Summary of findings 2. CCPT versus extrapulmonary mechanical percussion.
| CCPT compared with extrapulmonary mechanical percussion for cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: CCPT Comparison: extrapulmonary mechanical percussion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Extrapulmonary mechanical percussion | CCPT | |||||
|
FEV1: rate of decline in FEV1 % predicted Follow‐up: 1.3–2.8 years |
There was no difference in rate of decline in FEV1 % predicted between groups. | Not available | 151 (1) |
⊕⊝⊝⊝ Very lowa,b |
There were no data available to enter into our analysis for the medium‐ or long‐term studies. We reported narratively from the original long‐term paper (Sontag 2010). 2 short‐term studies reported no difference In FEV1 % predicted (MD −2.10, 95% CI −5.49 to 1.29; P = 0.23) (Arens 1994; Bauer 1994). |
|
|
FVC: rate of decline in FVC % predicted. Follow‐up: 1.3–2.8 years |
There was no difference in rate of decline in FVC % predicted between groups. | Not available | 151 (1) |
⊕⊝⊝⊝ Very lowa,b |
There were no data available to enter into our analysis for the medium‐ or long‐term studies. We reported narratively from the original long‐term paper (Sontag 2010). 2 short‐term studies reported no difference in FVC % predicted (MD −3.86, 95% CI −8.05 to 0.33; P = 0.07) (Arens 1994; Bauer 1994). |
|
|
FEF25–75: rate of decline in FEF25–75 Follow‐up: 1.3–2.8 years |
The annual rate of decline in FEF25–75 was greater with HFCC than with CCPT. | Not available | 151 (1) |
⊕⊝⊝⊝ Very lowa,b |
There were no data available to enter into our analysis for the medium‐ or long‐term studies. We reported narratively from the original long‐term paper (Sontag 2010). 2 short‐term studies reported no difference In FEF25–75, MD 0.49 (95% CI −2.53 to 3.52; P = 0.75) (Arens 1994; Bauer 1994). |
|
|
Respiratory exacerbations: time to first treatment with IV antibiotics Follow‐up: 1.3–2.8 years |
There was no difference in time to first IV antibiotics between groups (P = 0.59). | Not available | 151 (1) |
⊕⊝⊝⊝ Very lowa,b |
Results taken directly from the original paper as no results were available in a format that we could enter into our analyses. 2 short‐term studies included in the review reported the number of days in hospital for respiratory exacerbations and showed that there were fewer days in hospital in the extrapulmonary mechanical percussion group (MD 0.90 days, 95% CI 0.69 to 1.10) (Arens 1994; Bauer 1994). |
|
|
QoL: change in CFQ score Follow‐up: 1.3–2.8 years |
There was no difference between groups for any of the 12 HRQoL domain scores. | Not available | 151 (1) |
⊕⊝⊝⊝ Very lowa,b |
Results reported narratively from the original paper (Sontag 2010). | |
|
Adherence to therapy and individual preference (change in adherence rate and TSS score) Follow‐up: 1.3–2.8 years |
There was no difference in adherence rate between groups (P = 0.09). TSS scores were lower in the CCPT group (P < 0.05). |
Not available | 131 (1) |
⊕⊝⊝⊝ Very lowa,b |
Results reported narratively from the original paper (Sontag 2010). | |
|
Mucus weight Follow‐up: NA |
This outcome was not reported in the medium or long term. | Results of 1 short‐term study showed that sputum production (wet or dry weight) was similar between groups after 24 hours (Arens 1994). | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCPT: conventional chest physiotherapy; CFQ: Cystic Fibrosis Questionnaire; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; HFCC: high‐frequency chest compression; HRQoL: health‐related quality of life; IV: intravenous; MD: mean difference; NA: not applicable; QoL: quality of life; TSS: Treatment Satisfaction Survey. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to risk of bias within the 1 study reporting this outcome. There were particular concerns around incomplete outcome data reporting and withdrawals being biased towards CCPT. b Downgraded once due to imprecision from small numbers of participants from only 1 study.
Summary of findings 3. CCPT versus ACBT.
| CCPT compared with ACBT for cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: CCPT Comparison: ACBT (no studies compared to ACBT directly, but they compared with FET, which is a component of both CCPT and ACBT) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ACBT | CCPT | |||||
|
FEV1: annual decline in FEV1 % predicted Follow‐up: 3 years |
There was no difference between groups in FEV1 % predicted (P = 0.09). | MD 2.80 (−0.39 to 5.99) | 63 (1) |
⊕⊝⊝⊝ Very lowa,b |
1 short‐term study concluded that directed coughing was as effective as CCPT. No data reported (Bain 1988). | |
|
FVC: annual decline in FVC % predicted Follow‐up: 3 years |
There was no difference between groups in FVC % predicted (P = 0.18). | MD 1.80 (−0.83 to 4.43) | 63 (1) |
⊕⊝⊝⊝ Very lowa,b |
1 short‐term study concluded that directed coughing was as effective as CCPT. No data reported (Bain 1988). | |
|
FEF25–75: annual decline in FEF25–75 % predicted Follow‐up: 3 years |
Annual decline in FEF25–75 was worse in the FET only group. | MD 6.00 (0.55 to 11.45) | 63 (1) |
⊕⊝⊝⊝ Very lowa,b |
1 short‐term study r concluded that directed coughing was as effective as CCPT. No data reported (Bain 1988). | |
|
Respiratory exacerbations: time to first exacerbation Follow‐up: 1.3−2.8 years |
See comment. | 1 study reported the number of hospital admissions for an exacerbation and found that 8 participants in the FET group had 15 admissions compared with 5 participants and 8 admissions in the CCPT group (RR 0.61, 95% CI 0.23 to 1.62; P = 0.32) (Reisman 1988). The same study also reported the number of days in hospital for respiratory exacerbations and found that 8 participants in the FET group spent 197 days in hospital compared to 5 participants spending 73 days in hospital. The original paper stated that there was no evidence of a difference (Reisman 1988). |
||||
| QoL: change in CFQ score | — | Not reported | ||||
|
Adherence to therapy and individual preference: self‐reported scoring system. Follow‐up: 1.3–2.8 years |
64/67 participants were consistently compliant with their therapy. | NA | ⊕⊝⊝⊝ Very lowa,b |
No data available for analysis and so results were reported narratively. | ||
|
Mucus weight Follow‐up: NA |
See comment. | No data available for analysis for this outcome. 1 short‐term study reported no difference in mucus weight between CCPT and directed coughing group (Bain 1988). Similarly, a medium‐term study also reported no difference between groups (Steen 1991). |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCPT: conventional chest physiotherapy; CFQ: Cystic Fibrosis Questionnaire; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FET: forced expiration technique; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; IV: intravenous; MD: mean difference; NA: not applicable; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to risk of bias within 1 study reporting on this outcome. There were particular concerns around incomplete outcome data reporting and withdrawals being biased towards CCPT. b Downgraded once due to imprecision from small numbers of participants from only 1 study.
Summary of findings 4. CCPT versus O‐PEP devices.
| CCPT compared with O‐PEP devices for cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: CCPT Comparison: O‐PEP devices (4 studies used the Flutter device and 2 studies used IPV. No studies comparing other O‐PEP devices such as Acapella, Aerobika or RC‐Cornet were included in this review) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| O‐PEP devices | CCPT | |||||
|
FEV1: % predicted at end of study Follow‐up: medium (4–20 weeks) and long term (1.3–2.8 years) |
No difference in FEV1 % predicted between groups in the medium‐ or long‐term studies. | Not available | 185 (3) |
⊕⊝⊝⊝ Very lowa,b |
Only 1 study provided data that we could include in our analyses. The study compared CCPT with IPV and reported no difference between groups (MD 1.12, 95% CI −12.40 to 14.64; P = 0.87) (Homnick 1995). 3 short‐term studies reported this outcome. 1 study compared CCPT to Flutter device and reported no difference between groups (MD 0.70, 95% CI −6.85 to 8.25; P = 0.86) (Homnick 1998). 1 study compared CCPT with IPV and reported no difference in mean change in FEV1 between groups but provided no data (Hare 2002). 1 study compared CCPT to Flutter device and stated there was a greater improvement in FEV1 in the Flutter device group (Gondor 1999). |
|
|
FVC: % predicted at end of study Follow‐up: medium (4–20 weeks) and long term (1.3–2.8 years) |
There was no difference in FVC % predicted between groups in the medium‐ or long‐term studies. | Not available | 185 (3) |
⊕⊝⊝⊝ Very lowa,b |
Only 1 study provided data that we could include in our analyses. The study compared CCPT with IPV and reported no difference between groups (MD 2.00, 95% CI −9.31 to 13.31; P = 0.73) (Homnick 1995). 3 short‐term studies reported this outcome. 1 study compared CCPT to Flutter device and reported no difference between groups (MD 11.30, 95% CI −1.54 to 24.14; P = 0.08) (Homnick 1998). 1 study compared CCPT with IPV and reported no difference in mean change in FVC between groups, but provided no data (Hare 2002). 1 study compared CCPT to Flutter device and stated there was a greater improvement in FVC in the Flutter device group (Gondor 1999). |
|
|
FEF25–75: at end of the study Follow‐up: medium (4–20 weeks) and long term (1.3–2.8 years) |
There was no difference in FEF25–75 between groups in the medium‐ or long‐term studies. | Not available | 171 (2) |
⊕⊝⊝⊝ Very lowa,c |
Only 1 study provided analysable data comparing CCPT with IPV. There was no difference between groups (MD −3.62, 95% CI −20.18 to 12.94; P = 0.67) (Homnick 1995). 3 short‐term studies reported this outcome. 1 study compared CCPT to Flutter device and reported no difference between groups (MD 3.20, 95% CI −7.23 to 13.63; P = 0.55) (Homnick 1998). 1 study compared CCPT with IPV and reported no difference in mean change in FEF25–75 between groups, but provided no data (Hare 2002). 1 study compared CCPT to Flutter device and stated there was a greater improvement in FEF25–75 in the Flutter device group (Gondor 1999). |
|
|
Respiratory exacerbations: number of respiratory exacerbations per year Follow‐up: medium and long term |
See comment. | No studies reported number of exacerbations per year; however, results were presented for number of days in hospital for an exacerbation, number of hospital admissions and number of days of IV antibiotics. 1 study compared CCPT with IPV in the medium term and found no difference in the mean number of hospital days per participant during the study period between groups (MD 1.70 days, 95% CI –3.55 to 6.95; P = 0.53). Similarly, there was no difference in number of admissions to hospital between groups (Homnick 1995). In the longer term, 1 study found no difference between CCPT and Flutter device in time to first treatment with IV antibiotics (P = 0.59) (Sontag 2010). |
||||
|
QoL: change in CFQ score (across 12 domains) Follow‐up: long‐term |
There was no difference in QoL score across domains between CCPT and Flutter device. | Not available | 155 (1) |
⊕⊝⊝⊝ Very lowa,d |
No data available for this outcome and results were reported narratively directly from the paper (Sontag 2010). | |
|
Adherence to therapy and individual preference: TSS score Follow‐up: long term |
There was no difference in self‐reported adherence to treatment between CCPT and Flutter device groups. Participant satisfaction using TSS was lower in the CCPT group than the Flutter device group. |
Not available | 155 (1) |
⊕⊝⊝⊝ Very lowa,d |
We were unable to include any of the study data in our analyses and reported narratively from the original papers. Methods used in the studies to collect this information were varied and mostly subjective. |
|
|
Mucus weight Follow‐up: medium term |
There was no difference in sputum weight (wet and dry) between the CCPT group and the Flutter device group. | Not available | 14 (1) |
⊕⊝⊝⊝ Very lowa,d |
No data available for analysis for this outcome, so we reported directly from the paper (Giles 1996). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCPT: conventional chest physiotherapy; CFQ: Cystic Fibrosis Questionnaire; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; IPV: interpulmonary percussive ventilation; IV: intravenous; MD: mean difference; O‐PEP devices: oscillating positive expiratory pressure devices; QoL: quality of life; SD: standard deviation; TSS: Treatment Satisfaction Survey. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to risk of bias within the studies included in the analysis. All studies were at high or unclear risk of bias across most domains. b Downgraded once due to imprecision caused by small numbers of participants. Although the total number included for this outcome was 185, the individual studies had small numbers of participants and the data could not be combined. c Downgraded once due to imprecision caused by small numbers of participants. Although the total number included for this outcome was 171, the individual studies had small numbers of participants and the data could not be combined. d Downgraded once due to small sample size that did not reach the optimal information size.
Summary of findings 5. CCPT versus AD.
| CCPT compared with AD for cystic fibrosis | ||||||
|
Patient or population: children and adolescents with cystic fibrosis Settings: outpatient Intervention: CCPT Comparison: AD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AD | CCPT | |||||
|
FEV1: % predicted Follow‐up: medium term (8 weeks) and long term (12 months) |
Mean FEV1 % predicted was 1.81% higher in the CCPT group than the AD group (2.52% lower to 6.14% higher). | NA | 54 (2) |
⊕⊝⊝⊝ Very lowa,b |
There was no difference in FEV1 % predicted between groups. | |
|
FVC: % predicted Follow‐up: medium term (8 weeks) and long term (12 months) |
Mean FVC % predicted was 0.39% higher in the CCPT group than the AD group (3.62% lower to 4.40% higher). | NA | 54 (2) |
⊕⊝⊝⊝ Very lowa,b |
There was no difference in FVC % predicted between groups. | |
|
FEF25–75: % predicted Follow‐up: medium term (8 weeks) and long term (12 months) |
Mean FEF25–75 % predicted was 2.23% higher in the CCPT group than the AD group (8.96% lower to 13.42% higher). | NA | 54 (2) |
⊕⊝⊝⊝ Very lowa,b |
There was no difference in FEF25–75 % predicted between groups. | |
|
Respiratory exacerbations: number of respiratory exacerbations per year Follow‐up: long term (12 months) |
— | No studies reported number of respiratory exacerbations per year. 1 long‐term study reported slightly more hospital admissions in the CCPT group than the AD group (MD 0.24, 95% CI 0.06 to 0.42; P = 0.008) (McIlwaine 2010). There was a discrepancy here between our analysis and the results reported in the paper. Our analysis showed a difference favouring the AD group (mean number of admissions was 1 vs 0.76). It was unclear if this is a clinically important difference. |
||||
| QoL | — | Not reported | ||||
|
Adherence to therapy and individual preference Follow‐up: long term (12 months) |
Adherence not reported. Individual preference: there was a preference for AD in all participants who subjectively considered it worked best, gave increased expectoration and gave more independence and freedom. |
Not available | 36 (1) |
⊕⊝⊝⊝ Very lowa,b |
Only the first arm of the study was reported as a high proportion of participants allocated to AD for the first phase either refused to switch to CCPT for the second phase or incorporated AD breathing technique into their CCPT treatment (cross‐over effect). Results reported narratively from the original study paper (McIlwaine 2010). |
|
| Mucus weight | — | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CCPT: conventional chest physiotherapy; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; MD: mean difference; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to risk of bias within the studies. b Downgraded once due to imprecision from small numbers of participants in the individual studies and overall.
Summary of findings 6. CCPT versus exercise.
| CCPT compared with exercise for cystic fibrosis | ||||||
|
Patient or population: children and young adults with cystic fibrosis Settings: inpatient Intervention: CCPT (3 sessions of CCPT) Comparison: exercise (2 sessions of cycle ergometer exercise plus 1 session of CCPT) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise plus CCPT | CCPT | |||||
|
FEV1 Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results Original data provided by the study authors showed a higher FEV1 % predicted in the CCPT group compared to the exercise group (MD 7.05, 95% CI 3.15 to 10.95; P = 0.0004). However, the original paper reported no difference between groups, which is likely to be due to the original analysis accounting for baseline differences (Cerny 1989). |
||||
|
FVC Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results Original data provided by the study authors showed a higher FVC % predicted in the CCPT group compared to the exercise group (MD 7.83, 95% CI 2.48 to 13.18; P = 0.004). However, the original paper reported no difference between groups, which is likely to be due to the original analysis accounting for baseline differences (Cerny 1989). |
||||
|
FEF25–75 Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results Original data provided by the author team showed a higher FEF25–75 % predicted in the CCPT group compared to the exercise group (MD 4.74, 95% CI 1.94 to 7.54; P = 0.0009). However, the original paper reported no difference between groups which is likely to be due to the original analysis accounting for baseline differences (Cerny 1989). |
||||
|
Respiratory exacerbations: number of respiratory exacerbations per year Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results The study reported length of hospital stay during a single admission and found this to be similar between groups (Cerny 1989). |
||||
| QoL | — | Not reported. | ||||
|
Adherence to therapy and individual preference Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results Study authors noted that all CCPT sessions were completed and 96% of exercise sessions (Cerny 1989). |
||||
|
Mucus weight Follow‐up: NA |
This outcome was not measured in the medium or long term. See comments for short‐term results. |
Short‐term results There were no differences in 24‐hour sputum volume or dry weight between groups (Cerny 1989). |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCPT: conventional chest physiotherapy; CI: confidence interval; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; MD: mean difference; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Description of the condition
Cystic fibrosis (CF) is an inherited life‐limiting disease, affecting around 1 in every 2500 babies born and between 70,000 and 100,000 people worldwide (UK CF Trust 2022). Persistent infection and inflammation within the lungs are major contributors to severe airway damage and loss of respiratory function over the years (De Boeck 2020). Continuous production of thick secretions leads to airway obstruction and mucus plugging (Bell 2020). Therefore, removal of airway secretions is an integral part of the management of CF.
Description of the intervention
A variety of methods are used to help remove secretions from the lungs, some of which are physical (e.g. chest physiotherapy) and some chemical (e.g. medications and inhalation therapies). Treatments which improve secretion clearance are considered essential in optimising respiratory status and reducing the progression of CF lung disease. They are normally commenced as soon as the CF diagnosis is made, or taught to families and implemented when symptoms appear.
There are many chest physiotherapy airway clearance techniques (ACTs). Amongst these, conventional chest physiotherapy (CCPT) techniques may include postural drainage (PD), percussion and vibration, huffing and coughing, and generally rely on the assistance of another person such as a physiotherapist, parent or carer. Alternative ACTs such as active cycle of breathing techniques (ACBT), forced expiration technique (FET), autogenic drainage (AD), positive expiratory pressure (PEP) devices, oscillating PEP (O‐PEP) devices (e.g. Acapella, Aerobika, Flutter, RC‐Cornet), or high‐frequency chest compression (HFCC) and exercise are self‐administered, facilitating independence and flexibility. The methods are defined in more detail below.
Regardless of the type of chest physiotherapy, the recommendation is that ACTs are performed regularly (typically twice per day) during stable disease periods and more frequently during exacerbations. However, advice on frequency is currently not evidence‐based and adherence to these treatments is often not optimal, with around 50% of adults and around 30% of children with CF admitting that they do not undertake airway clearance as recommended (Myers 2009; O'Donohoe 2014).
How the intervention might work
Different types of chest physiotherapy are based on respiratory physiological principles thought to facilitate effective airway clearance such as two‐phase gas‐liquid flow, pendelluft flow, collateral ventilation, gravity‐assisted flow and interdependence.
PD, described over a century ago, was one of the earliest gravity‐assisted ACTs for people with chronic respiratory disease (Ewart 1901). In this technique, individuals are placed in specific recumbent or semi‐recumbent positions (based on the anatomy of the bronchial tree) that enable gravity to move mucus from peripheral airways in selected lung segments to more central airways for expectoration. In CCPT, PD or modified PD is often combined with deep breathing and manual interventions such as percussion and vibration, which involve intermittent positive pressures applied to the chest wall, these are transmitted through the lungs to the airways, resulting in a physiological increase and oscillation of the expiratory flow (McCarren 2006). Both the increase and oscillation of the expiratory flow are proposed to assist with secretion clearance.
Some ACTs include specific breathing techniques that do not require equipment (such as ACBT and AD), while others are device‐dependent techniques (such as PEP or O‐PEP devices or HFCC). All of these manipulate air flow and pressure in the lungs, either with or without oscillation of airflow, in a way that is hypothesised to facilitate secretion removal.
Why it is important to do this review
While airway clearance is accepted as a cornerstone of therapy in CF, the performance of chest physiotherapy is burdensome and may be unpleasant, uncomfortable and time‐consuming. Traditional CCPT techniques include PD, percussion and vibration, and, therefore, rely on the assistance of another person, adding to the treatment burden in terms of dependence, inconvenience or embarrassment.
Self‐administered ACTs have more recently been developed that have the potential to mitigate the burden of airway clearance, by improving efficiency, convenience or effectiveness. These include the ACBT, FET, AD, exercise and device‐dependent techniques such as PEP or O‐PEP devices or HFCC. These treatments aim to give people with CF more independence and flexibility in clearing their airway secretions. An increasing number of new ACTs such as the Aerobika, Lung Flute, Shaker and Quake, which are used independently, continue to infiltrate the market and provide a bewildering choice for professionals and people with CF. Despite the expansion in the number of treatment modalities, there remains little evidence supporting their efficacy (Prasad 1998; van der Schans 1996). It is important to undertake this review in order to provide information for people with CF and medical staff, who may be properly guided in their decision‐making by a systematic review of the evidence for ACTs in CF.
The best evidence from many early, typically short‐term crossover design studies, and a minority of longer‐term randomised controlled trials (RCTs) has been synthesised in a number of Cochrane Reviews related to ACTs for CF. The earliest of these reviews compared any form of chest physiotherapy to no chest physiotherapy for people with CF (Warnock 2015). Of the eight included studies (96 participants), six were of single treatments and two were conducted over two days. Conclusions suggested that ACTs could increase mucus transport in the short term. The absence of long‐term studies reflects anxiety in this population about withholding ACTs for any length of time and preclude any conclusions regarding the ongoing mucus transport effects of ACTs in CF.
The remaining Cochrane Reviews of ACTs for CF assessing PEP (McIlwaine 2019), ACBT (Wilson 2023), AD (Burnham 2021), physical exercise training (Heinz 2022; Radtke 2022), and oscillating devices (Morrison 2020) in CF have failed thus far to provide evidence that any ACT technique is superior to another in terms of respiratory function, respiratory exacerbations, individual preference, adherence, quality of life, exercise capacity, sputum weight or other outcomes.
All of these reviews evaluated the certainty of the evidence and risk of bias for the included studies, which varied as a result of differences in length of study, sample sizes, participant dropouts, and methodological detail provided. With some exceptions, included studies tended to be of low quality with high or unclear risk of bias. The lack of clarity on randomisation processes and inability to conceal treatment allocation from participants further increased the risk of bias.
This review compares CCPT with alternative ACTs used for airway clearance in people with CF. This version is an update of a previous version of the review (Main 2005).
Objectives
To evaluate the effectiveness (in terms of respiratory function, respiratory exacerbations, exercise capacity) and acceptability (in terms of individual preference, adherence, quality of life) of CCPT for people with CF compared to alternative ACTs.
Methods
Criteria for considering studies for this review
Types of studies
We considered RCTs or quasi‐RCTs, including those with a cross‐over design. We excluded studies of less than seven days' duration (including single‐treatment studies).
Types of participants
We included people with CF, of any age, with any degree of disease severity, who were diagnosed on the basis of clinical criteria and sweat testing or genotype analysis. We excluded people with CF who had undergone lung transplantation.
Types of interventions
We compared CCPT with alternative ACTs listed below.
In the existing literature and in practical terms, there is variation in the definition and application of different types of ACTs, within and between individuals, practitioners and CF centres worldwide. Therefore, for the purposes of this review it was necessary to group these variations within broad definitions of the treatment modalities. Separate analysis of variations within each technique would have precluded systematic review of this topic.
Conventional chest physiotherapy (CCPT)
This included any combination of the following: PD, percussion, chest wall vibration or shaking, huffing or directed coughing. We excluded any CCPT techniques that were described as including exercise, PEP or other mechanical devices.
Positive expiratory pressure (PEP) therapy
Defined as airway clearance involving breathing through a mouthpiece or oronasal mask interface with a PEP of 10 cmH2O to 25 cmH2O, for example PEP mask, Pari Pep or Thera PEP devices (with or without additional techniques).
High‐pressure positive expiratory pressure (hPEP) therapy
Defined as a modification of PEP that includes a full forced expiration against a fixed mechanical resistance, usually generating pressures ranging between 40 cmH2O and 120 cmH2O (with or without additional techniques).
Active cycle of breathing techniques (ACBT)
Defined as a flexible cycle of specific breathing techniques to enhance airway clearance, comprising relaxation or breathing control, FET, thoracic expansion exercises and may include PD or chest clapping.
Autogenic drainage (AD)
Defined as a three‐phased breathing technique using high‐expiratory flow rates at varying lung volumes to enhance mucus clearance while avoiding airway closure.
Oscillating positive expiratory pressure devices (O‐PEP)
These are device‐dependent ACTs that produce oscillatory PEP effects within the airways (10 Hz to 30 Hz) while breathing through the device. O‐PEP devices include the Flutter, Acapella, RC‐Cornet, Aerobika and intrapulmonary percussive ventilation (IPV). Bottle or bubble PEP is also a form of O‐PEP therapy. Throughout the respiratory cycle, IPV provides continuous oscillation to the airways produced by electrically powered mechanical or acoustic air vibration. The other O‐PEP devices do not require a power source and only produce oscillatory PEP in the airways during the expiratory phase of the breathing cycle, when exhaling. Lung Flute and Quake devices provide theoretically similar O‐PEP effects.
Thoracic oscillating devices (TOD) and mechanical percussive (MP) devices
All TODs provide pulsed high‐frequency external chest wall compressions, usually administered by a snugly fitting inflatable vest over the thorax. The vest is attached to an air pulse‐generating compressor, and chest compressions are transmitted through the chest wall to the airways. Devices include the Thairapy Vest, InCourage system, Smart vest and the Hiyak Oscillator. MP devices operate on a similar principle but are usually handheld and provide chest wall percussion over a limited surface area.
Exercise
As prescribed for the purpose of airway clearance either independently or as an adjunct to other techniques. Airway clearance is theoretically enhanced by the increase in ventilatory demand during exercise, changes in mucus rheology, airway surface hydration and physical body movement.
Types of outcome measures
We planned to assess the following outcome measures in the short‐term (defined as between seven and 20 days), in the medium term (more than 20 days and up to one year) and in the long term (more than one year).
Primary outcomes
-
Pulmonary function tests (measured as z scores or percent (%) predicted (age and height corrected) because of the potential for wide variations in participant age groups)
forced expiratory volume in one second (FEV1)
forced vital capacity (FVC);
average forced expiratory flow between 25% and 75% of FVC (FEF25–75)
-
Number of respiratory exacerbations per year defined by any of the following:
number of days in hospital per year for respiratory exacerbations
number of admissions to hospital per year for respiratory exacerbations
number of intravenous (IV) antibiotics courses per year for respiratory exacerbations
number of IV antibiotics days per year for respiratory exacerbations
time to first respiratory exacerbation
Secondary outcomes
Quality of life (QoL) measures (e.g. standardised questionnaires related to QoL or participation (e.g. Cystic Fibrosis Questionnaire – Revised (CFQ‐R) (Quittner 2009)), or days missed from work or school)
Adherence to therapy, satisfaction and individual preference
Cost–benefit analysis of intervention
Objective change in exercise capacity (e.g. cardiopulmonary exercise test (CPET), incremental shuttle walk test (ISWT), six‐minute walk test (6MWT) and step test)
-
Additional lung function tests, including but not limited to
total lung capacity (TLC)
functional residual capacity (FRC)
Lung Clearance Index (LCI)
Ventilation scanning (radiological or nuclear medicine imaging)
Blood oxygen levels (measured by arterial blood gas, pulse oximetry or transcutaneous oximetry)
Nutritional status as assessed by growth, weight and body composition
Mortality
Mucus transport rate (as assessed by radioactive tracer clearance)
Mucus wet or dry weight
Additional outcomes that have arisen from the review
Adverse events (such as pneumothorax, haemoptysis, deaths or other adverse changes in condition from baseline)
Sputum culture
Other outcomes (see Results)
Search methods for identification of studies
We searched for all relevant published and unpublished studies without restrictions on language, year or publication status.
Electronic searches
The review authors identified relevant studies from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the terms: physiotherapy AND conventional.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the handsearching of two journals – Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website (cfgd.cochrane.org/our-specialised-trials-registers).
Date of the most recent search: 26 May 2022.
The review authors also searched the following databases and trial registers:
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 2002). Searched in 2002 for a previous version of this review. Not updated due to lack of access;
AMED EBSCO (Allied and Complementary Medicine; 1985 to 2002). Searched in 2002 for a previous version of this review. Not updated due to lack of access;
US National Institutes of Health (NIH) Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 29 June 2022);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int/; searched 29 June 2022).
For details of the search strategies, see Appendix 1.
Searching other resources
We screened the bibliographies of included studies and any relevant systematic reviews for further potentially relevant studies. We also contacted authors of included studies, leaders in the field and companies known to be developing and investigating cystic fibrosis transmembrane conductance regulator (CFTR) correctors to identify any studies which may have been missed by the searches.
Data collection and analysis
Selection of studies
Two review authors (EM, SR) independently reviewed all citations and abstracts identified by the search to determine which papers should be included. If there was disagreement about whether they should include a study in the review, we asked an independent author from a third CF centre to review the paper(s) in question.
Data extraction and management
The two review authors (EM, SR) independently extracted data on each of the outcome measures. For all included studies, the review authors recorded the following details: criteria for diagnosis of CF; methods of participant selection; and baseline characteristics of the active and placebo groups including age, sex, genotype and lung function. Where studies were published in insufficient detail, or the review authors could not extract data in the format required, we contacted the study authors to request original data. Where data were lost, or where study design precluded an appropriate comparison, the review authors excluded studies from the meta‐analysis, but included them in the review. One study with a single publication had full text in the Danish language (Tonnesen 1982), and assistance was gratefully received from Dr Connor Brenna in extracting data from the paper for this review. We used Review Manager 5 to compile and analyse the data (Review Manager 2020).
We grouped studies related to specific treatment techniques for the purposes of meta‐analysis (e.g. all studies of CCPT versus PEP). This facilitated comparisons between specific ACTs, as well as comparisons with CCPT. For ease of comparison, and to avoid splitting data to the extent that no comparison was feasible, we also grouped some techniques that had similarities, for example, techniques that involved O‐PEP devices or TODs. In cases where study design incorporated three or more treatment arms (e.g. CCPT versus PEP versus Flutter device), we entered data in both comparisons so that we could compare CCPT to each of the alternative ACTs.
Several studies were published both as abstracts and journal articles, or as more than one journal article with different lead authors. Where a single data set was published more than once, we extracted the data from the final publication whenever possible, and regarded these as the primary references for the studies.
We reported outcomes as short‐term (defined as between seven and 20 days), medium‐term (more than 20 days and up to one year) and long‐term (more than one year).
Assessment of risk of bias in included studies
In earlier versions of this review, authors scored the quality of included studies according to criteria described by Jadad (Jadad 1996). This method allocates five points on the basis of randomisation, double blinding, and the description of withdrawals and dropouts.
For the current version of the review, two review authors (EM, SR) independently assessed the risk of bias for each included study using the RoB 1 tool according to the guidance in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2017). We judged each of the following six criteria to have a low, high or unclear risk of bias: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and any other potential sources of bias. We used published data and additional original data when available in determining whether studies met the criteria.
Measures of treatment effect
For continuous outcomes, we recorded either the mean change from baseline for each group or mean post‐treatment or postintervention values and the standard deviation (SD) or standard error (SE) for each group. They present the results for lung function, number of respiratory exacerbations, and clinical scores etc. as a mean difference (MD) between treatment groups with corresponding 95% confidence intervals (CIs).
For binary outcomes, in order to allow an intention‐to‐treat (ITT) analysis, we collected data on the number of participants with each outcome event by allocated treated group irrespective of compliance and whether the individual was later thought to be ineligible, or otherwise excluded for treatment, or follow‐up. We presented the results for the number of hospital admissions for example, as a binary outcome (whether there was a difference with treatment or not) using the risk ratio (RR) and the corresponding 95% CIs.
Unit of analysis issues
We incorporated data from cross‐over studies into the meta‐analyses using the generic inverse variance (GIV) method, involving expression of data in terms of the paired MDs between treatments and their SE. We calculated these values either from paired individual participant data provided by study authors, or by calculation of MDs between interventions and their SEs, SDs and P values reported in the manuscript (Elbourne 2002). Some authors involved with cross‐over studies provided original individual participant data. For the studies where these data were not available, the review authors elected to use a correlation of zero as the most conservative estimate. In updates of this review, further data and better understanding of mean correlations for these outcomes may allow the use of less‐conservative correlations.
We combined data from parallel‐designed studies with those from cross‐over studies in meta‐analyses. We calculated the SEs in parallel‐designed trials from the MDs between treatments and their CIs and reported these data in the comparison tables.
Dealing with missing data
If we were unable to extract data directly from the publication, we contacted the study authors up to twice to request data for inclusion in the meta‐analyses. If we could not locate the study authors, or the study authors did not send the data, we considered these studies as awaiting classification for potential inclusion in future updates of this review.
Assessment of heterogeneity
Where we were able to include and combine several studies, we examined heterogeneity between the studies. We assessed this visually in the forest plots and using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). The values of the I2 statistic lie between 0% and 100% and we used the following simplified categorisation of heterogeneity (Deeks 2022):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
The importance of the observed value of the I2 statistic depends on the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for the I2 statistic).
Assessment of reporting biases
If we included and combined 10 or more studies, we investigated publication bias by constructing a funnel plot (Page 2022). If the funnel plot was asymmetrical, then we considered reasons other than publication bias (i.e. heterogeneity, small‐study effects and outcome reporting bias).
We planned to investigate potential outcome reporting bias by comparing protocols when available to the final study reports. Where the protocols were unavailable, we compared the methods section to the results section of the final publications and judged potential bias using this in addition to our knowledge of the clinical area.
Data synthesis
Two review authors (EM, SR) analysed the data using a fixed‐effect model (Deeks 2022). If, in the future, we are able to include more studies in the review, and we identify a substantial degree of heterogeneity (as defined above), we will use a random‐effects model in the data analysis.
Subgroup analysis and investigation of heterogeneity
In order to investigate the need for further meta‐analyses, the review authors also examined the potential effects of time according to duration of study. We compared studies undertaken during hospital admissions for pulmonary exacerbations (seven to 21 days' duration) to longer‐term studies during stable disease. We anticipated substantial improvements in respiratory function in hospitalised participants as a result of intensive therapies such as antibiotics, unlike results from longer‐term studies undertaken during stable disease. In such studies, we anticipated a maintenance or slow decline in pulmonary function. It is possible that certain ACTs, which have optimal efficacy during acute exacerbations, may not be appropriate for maintenance therapy and vice versa.
To investigate any heterogeneity we may identify in the future, we will consider performing subgroup analyses based on the following factors:
frequency, quality and duration of therapy;
severity of disease;
exacerbation versus stable disease; and
variability in treatment applications and adjuncts, particularly in relation to CCPT.
Sensitivity analysis
The review authors planned to perform sensitivity analyses based on the risk of bias of the studies, including and excluding quasi‐randomised studies; however, this was not feasible with the data currently included in the review.
Summary of findings and assessment of the certainty of the evidence
In a post hoc change and in line with current Cochrane guidance (Schünemann 2022a; Schünemann 2022b), we presented six summary of findings tables, one for each comparison presented in the review (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). We presented the following outcomes in the tables.
FEV1% predicted (change from baseline) in the medium and long term combined
FVC (change from baseline) in the medium and long term combined
FEF25–75 (change from baseline) in the medium and long term combined
Respiratory exacerbations in the medium and long term combined
QoL measures
Adherence to therapy and individual preference
Mucus wet or dry weight
We calculated the assumed risk as the mean of the effect size of the control group in each study; the corresponding risk being the result of the meta‐analysis as presented in the data tables. We determined the certainty of the evidence using the GRADE approach, where we rated certainty with regard to risk of bias or study limitations, directness, consistency of results, precision, publication bias and effect size. We downgraded the evidence by one level for serious (or by two for very serious) study limitations.
Results
Description of studies
For further details, see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
In this 2022 update, we identified 143 citations (representing 85 original studies) as potentially relevant to this review as shown in the PRISMA flow chart (Figure 1). From these recent searches and the original searches, we included 21 original studies (45 publications) in the review; seven studies were new at the 2022 update (Bain 1988; Giles 1996; Gondor 1999; Hare 2002; Sontag 2010; Steen 1991; Tonnesen 1982). We excluded 69 original studies (112 publications); of these, we identified and excluded eight studies at the 2022 update (Corten 2020; Ghasempour 2019; Hristara‐Papadopoulou 2005; Keller 2001; Klig 1989; Martinez Rodriguez 2017; van Hengstum 1988; Warwick 1991). There are no studies currently awaiting classification or any ongoing studies.
1.
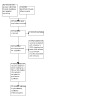
Study flow diagram for 2022 update.
Three changes to studies which the review authors included in the original 2005 review are relevant. First, the Davidson 1992 abstract has been published in full (as McIlwaine 2010) and we have now included this publication as the primary reference for this study. Second, a more recent abstract related to the Dadparvar study was published in 1995 with a larger data set, so we have now included this as the primary reference for this study (Dadparvar 1995). Finally, we excluded one study formerly listed as Kraig 1995; on careful review it was clear that this study did not meet inclusion criteria, although ambiguities in text facilitated its inclusion in the 2005 review (Kirkpatrick 1995). It was also clear that an error in the primary author's name in the printed copy had resulted in this abstract being included with his forename (Kraig) rather than his surname Kirkpatrick (Kirkpatrick 1995). In this update of the review, we amended the study identifier from Kraig 1995 to Kirkpatrick 1995 and excluded it.
We included data in meta‐analysis provided by study investigators or retrieved from the publication (Arens 1994; Bauer 1994; Cerny 1989; Dadparvar 1995; Gaskin 1998; Homnick 1995; Homnick 1998; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Tyrrell 1986; van Asperen 1987). In some cases, the number of participants included in meta‐analysis exceeded those in the publication, when the study continued beyond the date of publication and authors provided the additional original data. We gratefully received original data from several study authors (Arens 1994; Cerny 1989; Dadparvar 1995; Homnick 1995; Homnick 1998; McIlwaine 1991; van Asperen 1987). We additionally requested data from the authors of eight studies (Bain 1988; Costantini 2001; Giles 1996; Gondor 1999; Hare 2002; Sontag 2010; Steen 1991; Tyrrell 1986).
Included studies
In this update, we included 21 studies (42 publications), which recruited 778 participants (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987).
We considered and included seven newly identified studies comprising five full publications (Bain 1988; Gondor 1999; Sontag 2010; Steen 1991; Tonnesen 1982), and two abstracts (Giles 1996; Hare 2002).
Study characteristics
Fifteen studies were conducted in North America; 10 in the USA (Arens 1994; Bauer 1994; Cerny 1989; Dadparvar 1995; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; Sontag 2010), and five in Canada (Gaskin 1998; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Reisman 1988). Of the remaining six studies, two were conducted in Australia (Bain 1988; van Asperen 1987), two in the UK (one in Northern Ireland (Steen 1991) and one in England (Tyrrell 1986)), one in Denmark (Tonnesen 1982), and one in Italy (Costantini 2001).
One study was multicentre, conducted at 20 sites across the USA (Sontag 2010); the remaining studies were all conducted at single centres. Most studies were conducted over 20 years ago, between 1982 and 2000; two studies were more recent (Hare 2002; Sontag 2010).
Methods
Study duration
Fifteen were short‐ or medium‐term studies, with each intervention administered for between two weeks and five months. The shortest studies were typically conducted during hospitalisation for a pulmonary exacerbation (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Gondor 1999; Hare 2002; Homnick 1998). In these studies, participants were usually randomised (or quasi‐randomised) to receive CCPT or another ACT for the duration of their admission, which ranged between 10 and 14 days. Eight studies measured the effects of each intervention over longer periods at home: four weeks (Giles 1996; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), eight weeks (McIlwaine 1991), 12 weeks (Dadparvar 1995), and 20 weeks (Homnick 1995). The remaining six studies were long‐term, evaluating the effects of each intervention over at least one year (Costantini 2001; McIlwaine 1997; McIlwaine 2010), or two years (Gaskin 1998; Reisman 1988; Sontag 2010). There did not seem to be any association between duration of study and risk of bias or overall quality of the study.
Study design
Eleven studies were parallel‐group design RCTs (Arens 1994; Bain 1988: Bauer 1994; Cerny 1989; Costantini 2001; Gaskin 1998; Gondor 1999; Homnick 1995; McIlwaine 1997; Reisman 1988; Sontag 2010), eight were RCTs of cross‐over design (Dadparvar 1995; Giles 1996; McIlwaine 1991; McIlwaine 2010; Steen 1991: Tonnesen 1982; Tyrrell 1986; van Asperen 1987), and the remaining two were quasi‐RCTs (Hare 2002; Homnick 1998); although in Homnick 1998, some participants were admitted to hospital more than once. Of the 19 RCTs that reported participants were randomised to a specific treatment group or order, 13 did not report the method of randomisation (Arens 1994; Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Gondor 1999; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). The remaining six studies used an appropriate method of randomisation, most frequently stratifying participants in each group or order by pulmonary impairment, or age, or gender, or combinations of these (Bain 1988; Homnick 1995; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010).
Participants
Diagnosis
All studies recruited participants with CF, and nine studies specifically stated that CF was diagnosed on the basis of a sweat chloride or genetic testing (Arens 1994; Costantini 2001; Gondor 1999; Homnick 1995; Homnick 1998; McIlwaine 1997; Reisman 1988; Sontag 2010; Steen 1991). Other studies either reported that the diagnosis was "proven" (McIlwaine 2010), or that participants were recruited from a CF centre (Bain 1988; Bauer 1994; Cerny 1989; Gaskin 1998; van Asperen 1987); the remainder did not report diagnostic criteria (Dadparvar 1995; Giles 1996; Hare 2002; McIlwaine 1991; Tonnesen 1982; Tyrrell 1986).
Disease severity and clinical status
Studies reported disease severity in different ways, most often with reference to baseline or threshold FEV1 % predicted or raw scores (Arens 1994; Gaskin 1998; Giles 1996; Homnick 1995; Homnick 1998; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; van Asperen 1987). Two studies reported disease severity simply using the terms "mild, moderate or severe", without clear classification (Steen 1991; Tyrrell 1986); four studies also used these terms, but classified them in terms of specific FEV1 scores, although they were not standard across the studies (Bain 1988; Bauer 1994; Cerny 1989; Gondor 1999). One study applied a multifaceted disease severity scoring system, assigning points on the basis of weight and height, physical activity, presence of clubbed fingers, chest X‐ray, and CF complications (Tonnesen 1982). Four studies did not report disease severity (Costantini 2001; Dadparvar 1995; Hare 2002; McIlwaine 1991). Participants were recruited with a broad range of disease severities, with FEV1 as low as 26% or 28% predicted in studies recruiting participants during acute exacerbations (Bauer 1994; Cerny 1989), or with a mean baseline FEV1 in excess of 73% predicted in research trials recruiting participants during stable clinical periods (Giles 1996; McIlwaine 2010; van Asperen 1987). One study recruited participants with FEV1 between 37% and 115% predicted, testament to the challenges of using FEV1 as a primary outcome in these studies (McIlwaine 1997).
Five studies only recruited participants during periods of 'stable disease' (Dadparvar 1995; Homnick 1995; McIlwaine 1997; Sontag 2010; van Asperen 1987), while seven studies recruited during acute exacerbations (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Gondor 1999; Hare 2002; Homnick 1998); eight studies did not report on clinical status at recruitment (Costantini 2001; Gaskin 1998; Giles 1996; McIlwaine 1991; McIlwaine 2010; Reisman 1988; Steen 1991; Tyrrell 1986).
One study compared colonisation of Staphylococcus aureus, Pseudomonas aeruginosa, Pseudomonas (mucoid strains), Pseudomonas cepacia and Pseudomonas maltophilia, on admission, to ensure they were not different between groups (Arens 1994).
Number of participants
The number of participants with CF in the studies ranged from 13 to 166 participants (median 23 per study). Only one study recruited more than 70 participants (Sontag 2010, which recruited 166 participants) and 13 studies had fewer than 30 participants (Cerny 1989; Costantini 2001; Dadparvar 1995; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987).
Age
Only two studies did not report the age of participants (Giles 1996; McIlwaine 1991). One study only reported mean and SD age of participants (Cerny 1989). The age of participants recruited ranged from newborn babies to adults aged 45 years. This upper age limit may reflect the median age of survival in CF at the time of the latest studies included (approximately 35 years in 2000). One study exclusively recruited babies with CF, within the first few months of life; results from the outcome measures in this unique infant population were not comparable to other included studies (Costantini 2001). Twelve studies recruited children and young people aged between five and 29 years (Arens 1994; Bain 1988; Bauer 1994; Gondor 1999; Homnick 1995; McIlwaine 1991; McIlwaine 1997; Reisman 1988; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). One study recruited only adults aged 18 to 34 years (Dadparvar 1995), while the remaining four studies included heterogeneous paediatric and adult populations between the ages of seven and 45 years (Gaskin 1998; Hare 2002; Homnick 1998; Sontag 2010). In general, studies recruited participants who were old enough to perform spirometry competently, and were compliant with their treatments; it is these criteria that determine the lower age limit for recruitment. A number of studies excluded or withdrew participants who became non‐compliant with treatment.
The mean age of study populations ranged between 10 and 16 years in 14 studies (Bain 1988; Bauer 1994; Cerny 1989; Gondor 1999; Homnick 1995; Homnick 1998; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), and between 20 and 26 years in three studies (Arens 1994; Dadparvar 1995; Gaskin 1998). The remaining four studies did not report mean age.
Sex
In the 16 studies that reported the sex of participants, there were usually more males than females (Arens 1994; Bain 1988; Bauer 1994; Costantini 2001; Dadparvar 1995; Gaskin 1998; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1997; Reisman 1988; Sontag 2010); however this was not the case in two studies (Tonnesen 1982; Tyrrell 1986), and in one, numbers of each sex were matched (McIlwaine 2010). In total, the 16 studies recruited 375 males compared to 296 females.
Interventions
All included studies compared various modifications of CCPT with another intervention. The six studies in this review that were published in abstract form only provided little or no detail on the interventions performed, but all 15 studies that were published as full‐text manuscripts provided reasonable details on the interventions that were being compared. In 18 studies, there was only a single comparator, but in three studies there were more than two intervention groups (McIlwaine 1991; Sontag 2010; Steen 1991). Two studies compared three interventions: CCPT versus Flutter device versus HFCC (Sontag 2010), and CCPT versus PEP versus AD (McIlwaine 1991). The third study compared four different intervention groups (combinations of CCPT, PEP and FET) and added a fifth ad hoc comparison (FET only) at the end of the study period (Steen 1991).
The most common comparison between interventions was CCPT versus PEP (nine studies), where interventions were evaluated over a minimum period of four weeks and a maximum of two years (Costantini 2001; Dadparvar 1995; Gaskin 1998; McIlwaine 1991; McIlwaine 1997; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). The next most common comparison was CCPT versus Flutter device (four studies); two of these were conducted during hospitalisation for acute exacerbation (Gondor 1999; Homnick 1998), one over four weeks (Giles 1996), and one over two years (Sontag 2010). Two studies compared CCPT with IPV, one over two weeks (Hare 2002), and one over five months (Homnick 1995). Two studies compared CCPT with AD, one over eight weeks (McIlwaine 1991) and one for one year (McIlwaine 2010). Two studies compared CCPT with FET, one over four weeks (Steen 1991) and one over two years (Reisman 1988). Two studies compared CCPT with HFCC, one over two weeks (Arens 1994), and one over two years (Sontag 2010). One study compared three sessions of CCPT to two sessions of exercise plus a further session of CCPT ‐ we report this under the comparison of CCPT versus exercise (Cerny 1989).
All seven studies that were conducted during hospitalisation for acute exacerbation involved airway clearance interventions being undertaken either three times daily (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989), or four times daily (Gondor 1999; Hare 2002; Homnick 1998). Studies that were conducted over longer periods and which involved participants undertaking these airway clearance treatments at home, invariably stipulated that they were undertaken at least twice daily (Costantini 2001; Giles 1996; Homnick 1995; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), although four studies did not report how many times per day they performed these interventions (Dadparvar 1995; Gaskin 1998; McIlwaine 1991; Steen 1991).
Five studies did not report the length of each treatment session (four of these were published in abstract form only) (Dadparvar 1995; Gaskin 1998; Hare 2002; Homnick 1995; McIlwaine 1991). The study with five different intervention groups reported that each treatment was undertaken for an 'identical period' (Steen 1991). The remaining 15 studies reported the length of each treatment session, but only 11 of these tried to ensure that the treatment length was similar between groups. In these 11 studies, treatment length was 15 minutes (Giles 1996), 20 minutes (Tyrrell 1986; van Asperen 1987), 30 minutes (Arens 1994; Bauer 1994; Costantini 2001; McIlwaine 2010), and 35 minutes (Bain 1988; Reisman 1988). Two studies reported a treatment length window of between 15 and 20 minutes (Gondor 1999) and 20 to 40 minutes (Sontag 2010). Four studies had different treatment lengths depending on which intervention was being performed: CCPT 30 minutes versus Flutter device 15 minutes (Homnick 1998); CCPT 20 to 40 minutes versus cycle ergometer 15 to 20 minutes (Cerny 1989); CCPT 30 minutes versus PEP 20 minutes (McIlwaine 1997), and CCPT 20 to 30 minutes, once to three times per day versus PEP 15 minutes, three times per day (Tonnesen 1982). However, in Tonnesen 1982, CCPT was given on average 1.6 times daily, and PEP treatment three times daily; the daily treatment time for both treatments was overall the same (CCPT 44 minutes, PEP 45 minutes).
Studies described CCPT variously as in the table below. While PD and percussion were a feature of all CCPT interventions, the interventions varied in multiple other respects, including the number and choice of PD positions, whether vibrations were included, whether the FET was included and whether they were preceded by inhaled bronchodilators. There was no mention of modification to PD positions. Most included studies were undertaken prior to subsequent research which recommended caution with head down tilt PD positions in order to reduce risks of gastro‐oesophageal reflux (GOR). However, even the two most recently published studies did not modify their PD interventions (McIlwaine 2010; Sontag 2010).
| Conventional chest physiotherapy | ||
| Study | Intervention | Frequency |
| Arens 1994 | Aerosolised bronchodilators (albuterol 0.5 mL diluted in 5 mL normal saline) delivered 10–15 minutes before CCPT administration. PD performed in 6 positions (4 lying and 2 sitting), and percussion administered for 4 minutes in every position. |
3 times daily. 30‐minute treatments. |
| Bain 1988 | Nebulised salbutamol administered prior to each physiotherapy treatment. Used standard PD positions. 4 lung segments drained at each treatment based on the latest chest X‐ray. If this showed generalised changes or a clear chest, the segments drained were anterior segments of left and right upper lobes, lateral segment of right lower lobe, lateral segment of left lower lobe and posterior basal segments of both lower lobes. For each drainage position the following treatment was:
Regimen continued until all 4 segments were drained (a minimum of 16 coughs per treatment was required). |
3 times daily. No time limit for this treatment, but it was kept as close to 35 minutes as possible. |
| Bauer 1994 | Each percussion session was performed with cupped hands:
|
3 times daily. Each session performed for 30 minutes. |
| Cerny 1989 | PD was preceded by inhaled beta 2‐receptor agonist bronchodilators. 6 positions, with chest percussion, vibration and forced expiration. |
3 times daily (8 a.m. to 9:30 a.m.; 3 p.m. to 4:30 p.m.; and 7 p.m. to 9 p.m.). Each session was performed for 20–40 minutes (except 1 participant who received 2 afternoon treatments for a total of 4 treatments per day). |
| Gondor 1999 | CCPT immediately after participants received prescribed bronchodilator therapy. Manual clapping over the chest wall, positioned so that gravity could aid mucus drainage from the lobes or segment being percussed. PD with percussion and vibration was performed for 2 minutes in each of 8 standard positions. Following percussion and vibration in each position, participants took deep breaths, coughed and expectorated. |
4 times daily. Sessions lasted 15–20 minutes. |
| Homnick 1995 | Standard aerosol therapy of 2 mL saline or cromolyn sodium with an appropriate amount of albuterol given by compressor and updraft nebuliser. CCPT consisting of manual percussion for about 2 minutes in each of 10 PD positions. |
At least twice daily. |
| Homnick 1998 | Albuterol nebuliser (2.5–5 mg) in either 2 mL normal saline solution or 20 mg (2 mL) cromolyn sodium. Standard manual CCPT administered by registered and trained respiratory therapists (RT) using CF Foundation guidelines that were incorporated into a hospital protocol. |
4 times daily. Sessions lasted 30 minutes. |
| McIlwaine 1997 | PD in each of 5 or 6 positions. In each position, the chest wall was percussed for 3–5 minutes, followed by deep breathing exercises combined with vibration on expiration, forced expirations and vigorous coughing, i.e. 5–7 minutes in each position. | Twice daily. 30 minutes in total. |
| McIlwaine 2010 | PD in each of 5 or 6 positions. In each position, the chest wall was percussed for 3–5 minutes followed by deep breathing exercises combined with vibrations on expiration. Followed by 2–3 huffs. Participant then encouraged to cough and expectorate any mucus produced, followed by a short period of relaxed controlled breathing. The cycle was repeated for each PD position. |
Twice daily (5 positions drained in the morning and 5 in the evening). Each session approximately 30 minutes. |
| Reisman 1988 | CCPT with FET where participants were taught deep breathing with maximal expiratory effort and coughing throughout inhalational therapy, which consisted of beta 2‐bronchodilator inhalation delivered by mask and compressor. CCPT: PD accompanied by percussion (either manual or using a mechanical percussor) with participants instructed in the routine PD positions to be performed each day. Several brands of percussor used, but all had similar stroke force and frequency. FET: 2 maximal inspirations, each followed by a prolonged, controlled, forced expiration, and then 3 normal quiet inspirations, each followed by a prolonged, controlled, forced expiration. A minimum of 3 coughs performed, or coughs performed until there was no more sputum to expectorate. |
Twice daily (although 5 participants did their therapy in a single session). 8 minutes of percussion per position. |
| Sontag 2010 | CCPT was administered by a carer using a wedge provided to assist with appropriate positioning. Positioning, percussion (vibration) and FET with coughing between each of 6 positions. After each position, participants were instructed to do 3 FET and cough. | Twice daily. 20–40 minutes per session. |
| Steen 1991 | CCPT involved a combination of PD (the drainage positions as described by Kendig and Clarwick (Kendig 1977)), percussion, breathing exercises and FET consisting of 1 or 2 forced expirations with an open glottis from mid‐lung volume to low‐lung volume followed by a period of relaxed controlled diaphragmatic breathing. | 4 treatment programmes each 'carried out for an identical period of time'. |
| Tonnesen 1982 | The participants received CCPT by their 'usual therapist' (in 9 it was given by the mother, in 1 by the father, in 5 by a physiotherapist). The treatment consisted of vibrations, percussion, expansion both laying and standing, and PD on a specially made tilt table bed. | 1–3 times daily 20–30 minutes per treatment. |
| Tyrrell 1986 | PD, percussion and coughing. | Twice daily. |
| van Asperen 1987 | Standard PD with manual percussion to all areas followed by FET and coughing. | Twice daily. ≥ 20 minutes per session. |
The PEP interventions varied in a number of respects, including the target PEP level, the number of times participants were required to breathe through the mask and the sequences of FET or relaxed breathing in between cycles of treatment. All eight studies included in this review used an oronasal PEP mask rather than a mouthpiece. The PEP intervention was variously described in four full‐text manuscripts as below.
| Positive expiratory pressure | ||
| Study | Intervention | Frequency |
| McIlwaine 1997 | Used a manometer to create a steady PEP of 10–20 cmH2O during the middle part of expiration in a sitting position. Participant breathed in and out through the mask 15 times (approximately 2 minutes). Tidal volume inspirations and expiration was slightly active against the mask. Participants then removed the mask and performed 2 or 3 forced expirations, followed by a cough to clear secretions that had been mobilised to the central airways. Followed by 1–2 minutes of relaxed controlled breathing. |
Twice daily. Sequence repeated 6 times (approximately 20 minutes). |
| Steen 1991 | Delivered by an Astra or Vitapep mask in the sitting position. The participant sat with elbows resting on a table, applied the face mask firmly and, using a slightly active expiration, the outflow valve was adjusted to give an expiratory resistance of 10–15 cmH2O. Participant breathed through the mask 10–15 times followed by forced expiration and cough, if required. The cycle was then repeated. |
4 treatment programmes each 'carried out for an identical period of time' |
| Tonnesen 1982 | In a sitting position, the participant placed the mask over their nose and mouth and breathed normally through the mask for 15 minutes. During the 15‐minute treatment, the patient took a break every 2 to 3 minutes, during which the mask was removed, for the participants "to collect the secretions." The expiratory resistance, expiratory resistance of 5–20 cmH2O (PEP) was chosen according to the participant's initial tolerance, so that exhalation took place relatively effortlessly and pressure could be scaled up with tolerance. | 3 times daily for all participants. |
| Tyrrell 1986 | Used a manometer such that the participant breathed against a PEP mask pressure of 10–15 cmH2O for 10 breaths through the mask followed by forced expiratory coughing in the sitting position. | Twice daily. Sequence repeated for 20 minutes. |
| van Asperen 1987 | PEP mask to achieve an expiratory pressure of 10–15 cmH2O. 10–15 breaths followed by forced expiration and coughing. |
Twice daily. Sequence repeated for 20 minutes. |
Similarly, the Flutter device interventions varied in a number of respects, including the length of treatment, the number of times participants were required to breathe through the device and the sequences of FET or relaxed breathing associated with cycles of treatment. The Flutter device intervention was variously described in three full‐text manuscripts as follows.
| Flutter device interventions | ||
| Study | Intervention | Frequency |
| Gondor 1999 | Flutter device treatments, supervised by an RT immediately after prescribed bronchodilator therapy. Sitting position with head raised so that the stem of the device was parallel to the floor, placing the cone at a slight tilt away from the participant. After inhaling and holding their breath for 2–3 seconds, participants would slowly exhale through the Flutter valve, causing oscillations of the steel ball inside the cone of the Flutter device. After each series of exhalations, participants were instructed to "huff" and cough, thereby aiding expectoration. |
4 times daily. 3 sets of 15 exhalations performed over 12–20 minutes. |
| Homnick 1998 | Albuterol nebuliser (2.5–5 mg) in either 2 mL normal saline solution or 20 mg (2 mL) cromolyn sodium. Supervised Flutter therapy during hospitalisation administered by registered and trained RTs using manufacturer's recommendations. |
4 times daily. Each session lasted 15 minutes. |
| Sontag 2010 | Self‐administered Flutter device (Scandipharm, Birmingham, Alabama), incorporating Flutter device airway vibration and FET with coughing. Flutter treatment divided into 3 stages: loosening and mobilisation breaths, followed by mucus mobilisation and expectoration. |
Twice daily. Each session 20–40 minutes. |
The FET intervention, used as an alternative to CCPT in two studies differed in terms of the lung volumes from which the forced expiration would occur, and additional breathing sequences included as part of the technique. FET interventions were as below.
| Forced expiration technique | ||
| Study | Intervention | Frequency |
| Reisman 1988 | Participants were taught deep breathing with maximal expiratory effort and coughing throughout inhalational therapy, which consisted of beta2‐bronchodilator inhalation delivered by mask and compressor. FET consisted of 2 maximal inspirations, each followed by a prolonged, controlled, forced expiration, and then 3 normal quiet inspirations, each followed by a prolonged, controlled, forced expiration. A minimum of 3 coughs was performed, or coughs were performed until there was no more sputum to expectorate. |
Twice daily (although 8 in FET group and 5 in CCPT with FET group reported doing all therapy in a single session). |
| Steen 1991 | FET consisted of 1 or 2 forced expirations with an open glottis from mid‐lung volume to low‐lung volume followed by a period of relaxed controlled diaphragmatic breathing. | 4 treatment programmes each "carried out for an identical period of time." |
Other interventions
The IPV, AD and HFCC interventions were only described in one of the two studies including these interventions and these details are provided in the Characteristics of included studies table. In addition, single studies that compared CCPT with other interventions have details included in the same table, if they existed in the publication.
These variations in the way interventions were performed, including the duration of treatments and times per day and periods of comparison make it extremely challenging to perform meta‐analysis.
Outcomes
Primary outcomes
The three most commonly performed spirometric measures (FEV1, FVC and FEF25–75) were the primary outcome measures for this systematic review. Normalised scores (z scores or % predicted) were used because of the potential for wide variations in participant age groups. All but two studies evaluated the effects of CCPT versus alternative ACTs using FEV1 and FVC. One of the exceptions involved a neonatal population, in whom lung function measurements were not easily feasible (Costantini 2001); and the second exception involved primarily younger participants and evaluated FEV25–75 instead of FEV1 (Tyrrell 1986). Fifteen studies also added a third lung function measure, FEF25–75, as part of their standard pulmonary function testing procedures (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Dadparvar 1995; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; van Asperen 1987). The short‐term studies usually compared change in lung function by discrete values before and after interventions, and longer‐term studies used regression analysis of rate of decline (McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010).
We reported the second primary outcome of respiratory exacerbations in terms of 'time to first respiratory exacerbation' or any single or combined reports of number of days in hospital, admissions to hospital, IV antibiotic days or IV antibiotic courses. One study reported the time to first respiratory exacerbation (Sontag 2010). Only three of the longer‐term studies included the number of oral or IV antibiotic courses as an outcome measure (Costantini 2001; Homnick 1995; McIlwaine 2010); longer‐term studies were also more likely to report the number of hospitalisations (Homnick 1995; McIlwaine 1997; McIlwaine 2010; Reisman 1988), or cumulative number of hospital days (Homnick 1995; McIlwaine 2010; Reisman 1988), as outcomes measures. By contrast, short‐term studies sometimes compared length of hospital stay during a single admission for acute exacerbation (Bauer 1994; Homnick 1998).
Secondary outcomes
Only two studies included QoL measures, one using the Quality of Wellbeing (QWB) Scale (Gaskin 1998), and the other using the Cystic Fibrosis Questionnaire (CFQ) (Sontag 2010). No study reported the number of days missed from work or school.
Fifteen studies reported some measure of participant satisfaction or preference (Arens 1994; Bauer 1994; Costantini 2001; Dadparvar 1995; Giles 1996; Hare 2002; Homnick 1995; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Sontag 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). Some studies described prospectively how satisfaction or preferences were measured and compared between groups (Bauer 1994; Giles 1996; Sontag 2010). Others lacked clarity or transparency in their methods to obtain opinions on satisfaction or preference (Arens 1994; Hare 2002; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), potentially reporting ad hoc or spontaneous comments from participants and in some cases investigators concluded that one method was preferred without providing justification (Costantini 2001; Dadparvar 1995). Only one study used a validated Treatment Satisfaction Survey (TSS) (Sontag 2010). Three studies collected participant satisfaction data from one group, precluding comparison of satisfaction between different techniques (Arens 1994; Hare 2002; Homnick 1995).
The seven studies undertaken during hospital admissions did not measure adherence since treatments were supervised. Of the remaining 14 studies, five did not measure or record adherence at all (Costantini 2001; Dadparvar 1995; Giles 1996; McIlwaine 1991; Tonnesen 1982); two mentioned the use of adherence or compliance diaries, but did not report frequency of entries (Gaskin 1998; Reisman 1988); and seven indicated the use of a daily adherence log, diary or telephone diary (Homnick 1995; McIlwaine 1997; McIlwaine 2010; Sontag 2010; Steen 1991; Tyrrell 1986; van Asperen 1987). Three of these studies, undertaken over one month at home, did not explicitly measure adherence, but asked participants to record sputum yield after each treatment and these served as a record of treatment adherence (Steen 1991; Tyrrell 1986; van Asperen 1987). Four studies used findings from these diaries to exclude non‐adherent participants from analysis (Gaskin 1998; McIlwaine 1997; Steen 1991; Tyrrell 1986). Three studies, despite including measures of adherence, did not report results (Gaskin 1998; McIlwaine 2010; van Asperen 1987). One study reported adherence as the number of treatments carried out as a percentage of prescribed treatments during one month (Tonnesen 1982).
No studies used cost analysis as a primary outcome. One argued in the discussion section of their manuscript for Flutter device therapy taking less time to perform than CCPT, and thus being more cost‐effective over time (Homnick 1998). Another suggested that the cost savings of a PEP mask were considerably higher compared to the cost of a physiotherapist to assist with CCPT treatment (Tonnesen 1982).
Nine studies collected and compared a variety of outcomes related to exercise and physical activity. One study compared improvement in 6MWT between groups (Gondor 1999), and three studies compared cardiopulmonary cycle ergometer performance (Cerny 1989; Gaskin 1998; Reisman 1988), although one of these did not report cycle ergometer results (Gaskin 1998), and only one specified the cycle protocol used (Jones Stage 1) (Reisman 1988). Six studies used either physical activity questionnaires (Reisman 1988) or diaries (McIlwaine 1997; McIlwaine 2010; Tyrrell 1986; van Asperen 1987) or exercise diaries (Gaskin 1998; Steen 1991); however, none of these studies reported results from the diaries or questionnaires.
Some studies measured or retorted alternative lung function outcomes such as residual volume (RV), FRC, VC, FEV1/FVC ratio, TLC, RV/TLC ratio, peak expiratory flow rate (PEFR), inspiratory capacity (IC) and FRC, but these were far less consistently measured than the primary pulmonary function measures above (Arens 1994; Bain 1988; Cerny 1989; Dadparvar 1995; Homnick 1998; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). One study measured the Phase III slope of the single breath nitrogen washout test (SP3N2) (Arens 1994). No study reported LCI.
Eleven studies compared chest radiography (Bain 1988; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Homnick 1998; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Steen 1991; Tonnesen 1982), but only five of these used a validated measure for comparison; namely the Brasfield score in three studies (Gaskin 1998; McIlwaine 1997; Reisman 1988) and Chrispin‐Norman in two studies (Steen 1991; Tyrrell 1986). Only one study used radionuclide scanning as their primary outcome measure (Dadparvar 1995).
Four studies measured blood oxygen levels by arterial blood gas, pulse oximetry or transcutaneous oximetry, where participants were admitted to hospital for treatment of an acute exacerbation (Arens 1994; Bain 1988; Cerny 1989; Gondor 1999). One longer‐term study in neonates compared oxygen saturation (SpO2) between groups (Costantini 2001).
Anthropometric measures such as height, weight and body mass index (BMI) were commonly reported, sometimes to reflect clinical improvement in studies undertaken during hospitalisation (Arens 1994; Bain 1988; Cerny 1989; Gondor 1999), and sometimes to ensure that groups were matched in longer‐term studies, or for normalisation of lung function measures, or as a co‐variable in prediction models rather than as discrete outcomes (Homnick 1995; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; Steen 1991; Tyrrell 1986). These measures were rarely used as a direct comparison of efficacy of ACTs. The study involving neonates compared growth in both groups (Costantini 2001), and another compared weight and BMI between groups over five months (Homnick 1995).
No studies used mortality as a comparator between groups, but one study reported a death as reason for loss of follow‐up (Steen 1991).
A variety of outcomes related to mucus or sputum were collected and compared. No studies reported mucus transport rate, but nine studies reported sputum culture (Arens 1994; Bain 1988; Costantini 2001; Gondor 1999; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Steen 1991; Tonnesen 1982). Some studies collected sputum weight or volume over 24 hours (Arens 1994; Reisman 1988), and others over shorter periods following treatment (Bain 1988; Cerny 1989; Giles 1996; Homnick 1998; McIlwaine 1991; Steen 1991; Tyrrell 1986; van Asperen 1987). Seven studies used sputum diaries, which normally related to sputum characteristics including volume, colour, consistency, productivity or any combination of these (Bain 1988; McIlwaine 1997; McIlwaine 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987).
Additional outcomes
Several studies monitored participants for adverse events such as haemoptysis, hypoxaemia, pneumothorax, chest pain and nausea (Arens 1994; Costantini 2001; Hare 2002; Homnick 1998; McIlwaine 1997; Tonnesen 1982). Four studies used diaries to capture participant‐reported cough (McIlwaine 1997; Steen 1991; Tyrrell 1986; van Asperen 1987); and one study attempted to count coughs after each treatment (Cerny 1989). Diaries also captured other features including wheeze, shortness of breath, sleep, appetite, concerns and complications, although these latter items were less frequently measured (Gaskin 1998; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Steen 1991; Tyrrell 1986).
Twelve studies used clinical scores, with the Shwachman score being the most common (Homnick 1995; Homnick 1998; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Steen 1991; Tyrrell 1986). Two of these studies additionally reported the Huang score (McIlwaine 1997; McIlwaine 2010), one study also reported a modified Case‐Western clinical score (Homnick 1998), and a further study used pulmonary function score (Cerny 1989). One study used Cooperman's Cystic Fibrosis Scoring System (Tonnesen 1982). Four studies did not indicate which clinical score they used, or how it was derived (Bain 1988; Costantini 2001; Dadparvar 1995; Hare 2002). As expected, standard blood tests and clinical outcomes such as respiratory rate, were routinely collected during hospital admissions, but not used as comparisons between groups.
The heterogeneity in the outcome measures chosen by investigators and the way data were collected between studies made it difficult to perform meta‐analyses. Even in measures that appeared comparable, such as lung function outcomes, some studies specified that these were undertaken under laboratory conditions according to internationally recognised standards (Arens 1994; Gondor 1999; Homnick 1998; McIlwaine 1997; McIlwaine 2010), whereas another used measures from participants performing simple spirometry at home under poorly standardised conditions (van Asperen 1987).
Excluded studies
We excluded 69 studies (112 publications) (Baldwin 1994; Baran 1977; Bilton 1992; Braggion 1995; Button 2003; Cantin 2006; Chatham 2004; de Boeck 1984; Desmond 1983; Elkins 2005; Falk 1984; Fitzgerald 2005; Giles 1995; Grasso 2000; Hartsell 1978; Hofmeyr 1986; Holsclaw 1977; Keller 2001; Kerrebijn 1982; Kirkpatrick 1995; Klig 1989; Kluft 1996; Konstan 1994; Lannefors 1992; Lorin 1971; Lyons 1992; Maayan 1989; Majaesic 1996; Marks 2004; Maxwell 1979; McCarren 2006; McDonnell 1986; Morris 1982; Natale 1994; Newhouse 1998; Oberwaldner 1986; Orlik 2000; Orlik 2001; Phillips 1998; Placidi 2006; Pryor 1979; Regelmann 1990; Reix 2012; Roos 1987; Rossman 1982; Salh 1989; Samuelson 1994; Sanchez Riera 1999; Scherer 1998; Skopnik 1986; Steven 1992; Stites 2006; Sutton 1985; Tannenbaum 2007; Tonnesen 1984; van der Schans 1991; van Hengstum 1988; Varekojis 2003; Verboon 1986; Warwick 1990; Warwick 1991; Warwick 2004; White 1997; Williams 2001; Wong 2000).
Of these, 53 studies were either single treatment comparison studies or the interventions of interest were applied for less than seven days' duration. Many also had additional reasons for exclusion, including a lack of valid comparison group, lack of randomisation or quasi‐randomisation. We excluded the remaining 16 publications because there was no valid comparison group (Button 2003; Desmond 1983; Fitzgerald 2005; Grasso 2000; Keller 2001; Regelmann 1990), because the study had no conventional randomised or quasi‐randomised design (Klig 1989; Oberwaldner 1986; Orlik 2001; Tonnesen 1984; Warwick 1991), due to insufficient details on the intervention (Ghasempour 2019; Martinez Rodriguez 2017), or because the CCPT group treatments were not as typically described (Corten 2020; Roos 1987; Tannenbaum 2007).
Studies awaiting classification
We found no studies awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
For details of risk of bias assessments using the Jadad system of scoring (Jadad 1996) for earlier versions of this review, please refer to the Published notes.
For this 2022 update of the review, we replaced the Jadad system of scoring with the RoB 1 tool available within Review Manager 5 (Review Manager 2020), and present our findings within this framework (Higgins 2017). The Jadad score is included in the notes section of the Characteristics of included studies table for interest.
We presented a summary of the risk of bias judgements in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Six studies had a low risk of bias for sequence generation (Bain 1988; Homnick 1995; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010). Three of these studies provided detail of the method used to generate the sequence of random allocation, for example "randomly assigned by computer" after stratification (McIlwaine 1997; McIlwaine 2010; Sontag 2010). Three studies used stratified randomisation, without explicitly indicating a method of sequence allocation, and we classified these at low risk of bias on the presumption that the stratification method included random allocation sequence (Bain 1988; Homnick 1995; Reisman 1988). This may not be safe, and we may reconsider this in future updates of this review.
We judged 13 studies at unclear risk of bias because they were described as randomised, but did not clarify which method they used to generate the random allocation sequence. Typically, these studies would simply state that participants were randomly allocated or that the study was a randomised cross‐over design (Arens 1994; Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Gondor 1999; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). We judged two studies at high risk of bias because of their quasi‐randomised design, typically a sequence generation such as alternate assignment (Hare 2002; Homnick 1998).
Concealment of allocation
None of the studies explicitly indicated any method for concealing treatment allocation from the person randomising the participant. Thus, no studies were at low risk of bias.
Nineteen studies did not describe allocation concealment and were at unclear risk of bias (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Gondor 1999; Homnick 1995; McIlwaine 1991; McIlwaine 1997; McIlwaine 2010; Reisman 1988; Sontag 2010; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987).
Two studies were at high risk of bias for this domain as they employed a system of alternate allocation (Hare 2002; Homnick 1998).
Blinding
It was not possible to blind participants or physiotherapists to the treatments allocated in any of the included studies, due to the physical nature of the ACTs and the requirement that these were either reviewed periodically or supervised by healthcare staff. While it is unlikely that outcomes reported in these studies would have been directly influenced by a lack of blinding (e.g. unlikely to affect lung function performance), it is unclear whether outcomes may have been influenced indirectly, for example, by preference of participants (or not) to the treatment they were allocated (Brewin 1989; McPherson 2008; Preference Collaborative Review Group 2008; Silverman 1996; Udell 2011).
It was still potentially possible to blind outcome assessors to the treatment allocation in these studies, but only four studies described blinding of personnel such as radiologists, pulmonary function technologists, exercise physiologists or physicians (Gondor 1999; McIlwaine 1997; McIlwaine 2010; Tonnesen 1982). We judged these studies at low risk of bias in this domain.
There were 15 studies that did not describe any form of blinding for outcome assessors and so we assumed this was unlikely to have been implemented. We considered these studies to have an unclear risk of bias (Arens 1994; Bain 1988; Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Homnick 1995; McIlwaine 1991; Reisman 1988; Sontag 2010; Steen 1991; Tyrrell 1986; van Asperen 1987).
We judged two studies at a high risk of bias for this domain as they employed an open‐label system of allocation, in which all personnel had access to information regarding group allocation (Hare 2002; Homnick 1998).
Incomplete outcome data
One study, judged to have a low risk of attrition bias, explicitly indicated that all participants completed the study and none were lost to follow‐up (Cerny 1989). In four studies, authors were unable to clearly interpret data attrition and these were at unclear risk of bias (Dadparvar 1995; Giles 1996; Hare 2002; Homnick 1998). One analysed 33 hospitalisations from 22 participants (Homnick 1998). Although investigators stated that no participants withdrew from the study, it was unclear if any declined further participation in hospitalisations after their first admission. The other studies failed to provide any explicit detail regarding data attrition (Dadparvar 1995; Giles 1996; Hare 2002).
In eight studies data attrition ranged between 5% and 23% for a variety of reasons, including withdrawal for febrile illness, death, pneumothorax, haemoptysis, participant request, anxiety at stopping a previous treatment, participants moving home or early hospital discharge (Bain 1988; Bauer 1994; Gaskin 1998; Reisman 1988; Steen 1991; Tonnesen 1982). Some studies excluded participants from analysis if they were non‐compliant with the treatment requirements, even if they completed the study (Arens 1994; Bain 1988; Bauer 1994; McIlwaine 1997; Steen 1991). We judged these studies at unclear risk of bias.
Eight studies were at high risk of attrition bias (Costantini 2001; Gondor 1999; Homnick 1995; McIlwaine 1991; McIlwaine 2010; Sontag 2010; Tyrrell 1986; van Asperen 1987). Costantini 2001 reported that 3/26 participants dropped out of the study due to the severity of their reflux symptoms related to CCPT and were not evaluated; these outcomes would have been particularly important to evaluate in neonates and would have provided valuable information. Gondor 1999 stated that 3/23 participants (all from the CCPT group) were excluded from analysis due to early discharge and acknowledged that including them in analysis changed the conclusions of the study. Gondor 1999 also reported that two participants did not perform the 6MWT, but their treatment group was not reported. Homnick 1995 reported 4/20 participants did not finish the study, but investigators provided no reasons for withdrawal or exclusion. Three published abstracts relating to McIlwaine 1991 reported zero data attrition, but in an unpublished draft of the full manuscript for this study it was apparent that 4/18 participants did not complete the study and were excluded from the analysis. McIlwaine 2010 was intended to run over two years, but was terminated at 12 months due to the significant and disproportionate attrition of participants in one treatment group; during the first year, two participants dropped out of the PD group (one was pregnant and one had aspergillosis) and one participant dropped out of the AD group (non‐compliance). Authors further report that 10/17 participants who completed the first period preferred AD and refused to change back to PD (McIlwaine 2010). Sontag 2010, originally intended to run over three years, was terminated early due, in part, to the significant and disproportionate attrition of participants in one treatment group: 51% of CCPT, 16% of Flutter device and 9% of HFCC groups withdrew during the study. Tyrrell 1986 excluded three participants due to non‐compliance, but it is unclear which treatment arm they were in, or what constituted non‐compliance. Finally, van Asperen 1987 reported that 3/13 participants were withdrawn, one electively and two due to infective exacerbations, but it was unclear which treatment arm they were in or when they withdrew from the study.
It was notable that in two studies participants withdrew immediately after randomisation, suggesting that although they agreed to participate initially, they changed their minds because of the treatment group they had been allocated to (Gaskin 1998; Sontag 2010).
Selective reporting
For this domain, we assessed whether studies reported their declared specific outcomes in the publication, whether they reported at all the time points measured and whether they provided numerical results or narrative statements. Three studies were at high risk of reporting bias, two of the potential sources of bias differed in degree of risk (Costantini 2001; Gaskin 1998; Steen 1991).
Six studies declared specific outcomes in the publication and fully reported on all of them. We considered these at low risk of bias for selective reporting (Arens 1994; Bain 1988; Bauer 1994; Gondor 1999; Reisman 1988; Sontag 2010). We considered 10 studies at high risk (Costantini 2001; Cerny 1989; Dadparvar 1995; Gaskin 1998; Giles 1996; Hare 2002; McIlwaine 1991; McIlwaine 1997; Steen 1991; Tyrrell 1986), and five at unclear risk (Homnick 1995; Homnick 1998; McIlwaine 2010; Tonnesen 1982; van Asperen 1987).
We considered longitudinal studies that performed repeated measures, but only presented results between baseline and endpoint, or were unclear about which single time point they were presenting, or reported most but not all outcome measures, at unclear risk of bias in this domain (Costantini 2001; Gaskin 1998; Homnick 1995; Homnick 1998; McIlwaine 2010; Steen 1991; Tonnesen 1982; van Asperen 1987).
Furthermore, we considered any study that did not report on a significant proportion of outcomes measured, or did not provide any numerical data for outcomes in the manuscript, or only briefly summarised results in text without numerical data, or presented results in graphical form from which data could not be extracted, or simply described outcomes in terms of change from baseline or percentage changes, at high risk of bias in this domain (Cerny 1989; Dadparvar 1995; Gaskin 1998; Giles 1996; Hare 2002; McIlwaine 1991; McIlwaine 1997; Steen 1991; Tyrrell 1986). We also considered studies that reported inconsistent findings in different publications of the same data to have a high risk of bias for selective reporting (Costantini 2001).
Other potential sources of bias
There were several other potential sources of bias that affected all the included studies in this review in varying degrees. Overall, six studies were at unclear risk of bias (Arens 1994; Bain 1988; McIlwaine 1997; Reisman 1988; Tonnesen 1982; van Asperen 1987),OK and the remaining 15 studies had a high risk of bias overall (Bauer 1994; Cerny 1989; Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1991; McIlwaine 2010; Sontag 2010; Steen 1991; Tyrrell 1986). We present the full details of the potential sources of bias in the Characteristics of included studies table, but summarise examples of potential bias encountered in more than one study below.
With regard to study design, 13 studies had a sample size of fewer than 25 participants (Cerny 1989; Costantini 2001; Dadparvar 1995; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). In six cross‐over studies there were no washout periods, or they were inadequately described or the washout technique was the same as one of the interventions, leading to a potential risk of carry‐over effect (Dadparvar 1995; Giles 1996; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986). In three studies, the duration of each intervention was not the same, precluding true comparison of interventions and creating the risk that treatment benefit was related to time rather than quality (Giles 1996; Homnick 1998; Tonnesen 1982). In three studies, investigators made post hoc modifications to the original study design (e.g. by adding an extra comparison group with fewer participants) (Steen 1991), or including repeated admissions in only some participants (Bauer 1994; Homnick 1998). A further three studies were terminated early because of disproportionate dropouts in one arm, or difficulty with recruitment, or for reasons not disclosed (McIlwaine 2010; Reisman 1988; Sontag 2010). Five studies were sponsored in whole or in part by manufacturers of airway clearance devices (Gondor 1999; Hare 2002; McIlwaine 1991; Sontag 2010; van Asperen 1987).
With regard to the study participants, three studies included only 'compliant' or 'competent' or 'highly motivated' participants either in the study as a whole or in the analysis of data, raising risk of non‐generalisability of findings or overestimation of effects compared with real‐world usage (Gaskin 1998; McIlwaine 1997; Steen 1991). Two studies did not report the age, gender or numbers of participants allocated to each group (Dadparvar 1995; Hare 2002), and four studies did not account for differences between groups at baseline in the analysis (Cerny 1989; Homnick 1995; Homnick 1998; Steen 1991).
In terms of the interventions, one study presented data on lung function and sputum for only a single supervised physiotherapy session, but it was unclear when in the study this was done or how results were calculated (Tyrrell 1986).
With further regard to study results, in four studies investigators did not undertake any statistical analyses, or there was a lack of clarity on how they analysed data, or they did not present the results clearly (Costantini 2001; Hare 2002; Tyrrell 1986; van Asperen 1987). In four studies there were discrepancies between different publications of the same data set (Costantini 2001; Dadparvar 1995; McIlwaine 2010; Steen 1991). Two studies reported results from only one arm of the study for one outcome (participant satisfaction), precluding a valid comparison (Arens 1994; Homnick 1995); and six studies claim superiority of one intervention over the comparator in terms of preference, safety and efficacy without the necessary rigour in measuring, analysing or quantifying these (Costantini 2001; Dadparvar 1995; Gaskin 1998; Giles 1996; McIlwaine 1991; Steen 1991). Finally, in two studies, investigators included data from repeated hospital admissions (potentially sicker participants) and analysed these alongside single admissions for other participants, potentially over‐representing the less‐well group (Bauer 1994; Homnick 1998).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
We included 21 studies (778 participants randomised in total, 707 participants in the analyses). We present results below comparing the performance of CCPT against each of the alternative ACTs in turn (Comparisons 1 to 6 below). In most cases (18 studies) there was only a single comparator, but in three studies there were more than two intervention groups (McIlwaine 1991; Sontag 2010; Steen 1991). Within each comparison, we report all of their stated outcomes at the different time points, where short‐term studies were between seven and 20 days' duration, medium‐term studies were over 20 days and up to one year and long‐term studies were over one year. We also present results of CCPT compared to alternative ACTs regardless of timescale in the first set of analyses (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6), followed by the individual comparisons by time point (analysis groups 2 to 7 in Data and analyses).
1.1. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 1: Forced expiratory volume in 1 second (FEV 1) (% predicted)
1.2. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 2: Forced vital capacity (FVC) (% predicted)
1.3. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) (% predicted)
1.4. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 4: Number of hospital admissions
1.5. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 5: Number of days in hospital
1.6. Analysis.

Comparison 1: Conventional chest physiotherapy (CCPT) versus specific other treatments, Outcome 6: Schwachman score
Please note: below, we only presented the outcomes and time points for which we had data or narrative information in order to minimise the length of this text section. Missing outcomes indicate investigators did not include them in the study.
Comparison 1: conventional chest physiotherapy versus positive expiratory pressure
The most common comparison reported was CCPT versus PEP (9 studies; 191 participants, 171 analysed), of which six were medium‐term studies (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), and three long‐term studies (Costantini 2001; Gaskin 1998; McIlwaine 1997). See Table 1. The duration of the interventions ranged from four weeks up to two years. Although a mouthpiece can be used to deliver PEP in older children or adults, all nine studies included in this review used an oronasal PEP mask. In seven of nine studies, PEP was the only comparator (including baby PEP in one study (Costantini 2001)). However, one study included a third intervention (AD) (McIlwaine 1991); and a further study included four different intervention groups (combinations of CCPT, PEP and FET) then added a fifth post hoc comparison of FET only at the end of their study period (Steen 1991). We reported the data from these other comparators in the relevant comparison sections below.
Primary outcomes
1. Pulmonary function tests
We evaluated normalised scores (z scores or % predicted) for the stated pulmonary function outcomes. We extracted or obtained pulmonary function data for meta‐analysis in all but one medium‐term study (Steen 1991), and one long‐term study, which did not measure pulmonary function in their neonatal study population (Costantini 2001).
a. Forced expiratory volume in one second
Medium term
Four studies provided data for the meta‐analysis (70 participants, 60 analysed) (Dadparvar 1995; McIlwaine 1991; Tyrrell 1986; van Asperen 1987). Data from two studies were not in a format that could be extracted for analysis and we requested original data from study authors (Steen 1991; Tonnesen 1982). The meta‐analysis found no overall difference in FEV1 % predicted between CCPT and PEP in the medium term (MD 0.53, 95% CI −2.04 to 3.11; P = 0.69; very low‐certainty evidence; Analysis 2.1). The published narrative findings in all six studies also concluded that CCPT was as effective as PEP in terms of FEV1, or that there were no differences in changes between the interventions over the medium term (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). Tonnesen 1982 further concluded that there was no correlation between severity of lung function impairment and treatment effect.
2.1. Analysis.

Comparison 2: Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP), Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
One study reported FEV25–75 instead of FEV1 because of the predominance of younger participants (Tyrrell 1986). In this study, although investigators measured lung function before, and 20 and 90 minutes after a single treatment under supervision, they did not clarify which post‐treatment measures were used in analysis (Tyrrell 1986). In contrast to the other studies included in this analysis, the FEV25–75 data from the Tyrrell 1986 clearly favoured CCPT over PEP (MD 7.82, 95% CI 0.31 to 15.33; P < 0.05; very low‐certainty evidence; Analysis 2.1); however, the narrative conclusions from the published paper were not consistent with conclusions from the meta‐analysis in this review (Tyrrell 1986).
Long term
Two long‐term studies comparing CCPT with PEP reported data for FEV1 % predicted (106 participants, 97 analysed) (Gaskin 1998; McIlwaine 1997). One study (36 participants) favoured PEP over CCPT (McIlwaine 1997), but pooled data showed no difference between groups (MD −3.08, 95% CI −11.69 to 5.54; P = 0.48; very low‐certainty evidence; Analysis 2.1). Statistical heterogeneity was 'considerable' for these two studies (I2 = 79%), representing inconsistency in the results that is greater than might be expected by chance (Deeks 2022), but we could not identify any obvious potential sources of difference between studies. The published narrative findings concurred with those in the analyses for both studies; one study concluded that CCPT was as effective as PEP in terms of FEV1 % predicted over the long term (Gaskin 1998), while the second found that PEP was superior to CCPT in terms of change in slope (rate of decline) of FEV1 % predicted over one year (McIlwaine 1997).
b. Forced vital capacity
Medium term
We analysed FVC data from the same four studies as for FEV1 (70 participants, 60 analysed) (Dadparvar 1995; McIlwaine 1991; Tyrrell 1986; van Asperen 1987), but found no difference between CCPT and PEP in the medium term (MD 1.06, 95% CI –3.48 to 5.61; P = 0.65; very low‐certainty evidence; Analysis 2.2). Statistical heterogeneity was substantial for these four studies (I2 = 55%), representing inconsistency in their results that is greater than might be expected by chance, but we could not identify any obvious potential sources of difference between the studies. The published narrative findings in all six medium‐term studies concluded that CCPT was as effective as PEP in terms of FVC or that there were no differences in changes between the interventions, which is consistent with conclusions from the meta‐analysis in this review (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). Tonnesen 1982 further concluded that there was no correlation between severity of lung function impairment and treatment effect.
2.2. Analysis.

Comparison 2: Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP), Outcome 2: Forced vital capacity (FVC) % predicted
One cross‐over study was reported in two abstracts; the first in 1990 with preliminary data on 13 participants and the second in 1995 with 20 participants (Dadparvar 1995). While the first publication showed no difference between CCPT and PEP in terms of FVC, the 1995 abstract concluded that FVC was significantly better in the second PEP group following the cross‐over point in the study, but was unable to demonstrate the same benefit in the PEP group during the initial period preceding the cross‐over point. This inconsistent finding is unlikely to be of importance.
Long term
The same two long‐term studies (106 participants, 97 analysed) comparing CCPT with PEP provided data for FVC % predicted (Gaskin 1998; McIlwaine 1997). One study favoured PEP over CCPT (McIlwaine 1997), but pooled data showed no difference between groups in the long term (MD –3.01, 95% CI –13.05 to 7.03; P = 0.56; very low‐certainty evidence; Analysis 2.2). Statistical heterogeneity was considerable for these two studies (I2 = 85%), representing inconsistency in the results that is greater than might be expected by chance (Deeks 2022); we could not identify any obvious potential sources of difference between studies. The published narrative findings concurred with those in the meta‐analysis for both studies. Individually, one study concluded that CCPT was as effective as PEP in terms of FVC % predicted over two years (Gaskin 1998), while the second found that PEP was superior to CCPT in terms of change in FVC % predicted slope (rate of decline) over one year (McIlwaine 1997).
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Medium term
Only three of the six medium‐term studies in this comparison provided data for analysis of FEF25–75 (51 participants, 44 analysed) (Dadparvar 1995; McIlwaine 1991; van Asperen 1987). Pooled data showed no difference between CCPT and PEP (MD −0.51, 95% CI −5.37 to 4.36; P = 0.84; very low‐certainty evidence; Analysis 2.3). Statistical heterogeneity was substantial for these four studies (I2 = 53%), representing inconsistency in their results that is greater than might be expected by chance and review authors could not identify any obvious potential sources of difference between studies. The published narrative findings in these three studies also concluded that CCPT was as effective as PEP in terms of FEF25–75 or that there were no differences in changes between the interventions and thus were consistent with conclusions from the meta‐analysis in this review (Dadparvar 1995; McIlwaine 1991; van Asperen 1987).
2.3. Analysis.

Comparison 2: Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP), Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
Long term
Only one study provided long‐term data for FEF25–75 (40 participants, 36 analysed) (McIlwaine 1997). Results showed that while FEF25–75 declined in the CCPT group and improved in the PEP group, the changes were not significant (MD −3.56, 95% CI −13.30 to 6.18; P = 0.47; very low‐certainty evidence; Analysis 2.3).
2. Number of respiratory exacerbations
None of the six medium‐term studies comparing CCPT with PEP reported data for any of our measures for respiratory exacerbations (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987). One study did indicate that antibiotic consumption was the same for each treatment, but did not provide data (Tyrrell 1986). One further study (28 participants) noted that seven participants had a total of 12 hospital admissions during the 16‐week study period and this was not altered from the four months preceding the study, but investigators did not elaborate on which treatment groups the hospital admissions were from (Steen 1991).
b. Number of admissions to hospital per year for respiratory exacerbations
Long term
One study (40 participants, 36 analysed) reported that the numbers of hospital admissions for respiratory exacerbations in one year were not different between groups (11 for the CCPT group and 13 for the PEP group; RR 0.85, 95% CI 0.53 to 1.35; P = 0.48; very low‐certainty evidence; Analysis 2.4) (McIlwaine 1997). The original narrative conclusions from the investigators were consistent with conclusions from the meta‐analysis.
2.4. Analysis.

Comparison 2: Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP), Outcome 4: Number of hospital admissions
d. Number of intravenous antibiotics days for respiratory exacerbations
Long term
The three abstracts describing Costantini 2001 included data on number of "days/pt/year" on antibiotic therapy, but did not present data in a format that could be included in a meta‐analysis. The 1998 abstract presented data from 12 participants, while both abstracts from 2001 reported data from a further 14 infants, giving 26 in total. The data related to number of days on antibiotics were not consistent across the three publications, and it is unclear from the abstracts how these were calculated or whether the differences were considered significant. In the 1998 abstract, Costantini found that number of "days/pt/year" on antibiotic therapy were higher for infants using PEP over 12 months (29.5 days with PEP versus 18.2 days with CCPT), but did not specify whether these were IV or oral courses of antibiotics, or comment on the significance of these values. In the 2001 abstracts, oral antibiotic requirements per participant per year were 20.3 days with CCPT versus 21.3 days with PEP days in one abstract and 20.3 days with CCPT versus 22.9 days with PEP in the second. IV antibiotic use per participant per year were 1.8 days with CCPT versus 6.2 days with PEP in both abstracts.
Secondary outcomes
1. Quality of life
There is likely to be a degree of overlap between perceived QoL and individual satisfaction and preference with therapy, especially when expressed in terms of convenience, independence, ease of use, control and flexibility over treatment times and interruption to daily living. Unless a specific QoL instrument was used, we report sentiments relating to satisfaction, preference and adherence in the section below (see 'Additional outcomes that have arisen from the review).
Long term
Only one study assessed QoL using the QWB scale (Gaskin 1998). Baseline QWB scores were similar between the groups and reported no change in either group over the duration of the study; there were no data or details of analysis provided.
2. Adherence to therapy, satisfaction and individual preference
Medium term
All six medium‐term studies provided information on this outcome, although no data could be analysed (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987).
Dadparvar 1995 used a "standardised written questionnaire", but investigators did not name or describe this, or provide data; it is unclear how they assessed or analysed this (e.g. whether the preference was unanimous, in each subscore, or a simple majority). Participants were reported to prefer the PEP mask for convenience, independence and ease of use.
While McIlwaine 1991 did not formally assess adherence, satisfaction or preference, all three abstracts concluded that PEP could be 'done independently with less discomfort' than CCPT and participants 'reported a greater sense of control with less interruption in their daily life using PEP.' There is no indication in any of the abstracts whether these conclusions were reached via ad hoc conversations with a subset of participants or a formal survey involving all participants and unclear how data were analysed or interpreted.
Steen 1991 asked participants "to note their general impressions" about each treatment, which appear to have been collected in an ad hoc manner, but which reflected satisfaction or preference. Participants reported that they enjoyed the increased independence of PEP and FET combined; they found it more sociably acceptable, while still efficient. All considered that PEP on its own did allow clearance of secretions, but if used long term, it would require an increase in treatment times; examples are given in Table 7.
1. Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP): examples of 'impressions' of treatment from participants – Steen 1991.
| Comments |
| 1 participant with substantial secretion production asked to be withdrawn because "PEP alone was failing to clear secretions." |
| "Four mildly affected patients who were non‐sputum producers became sputum producers on introducing PEP with CCPT and this alone made them more compliant with their chest physiotherapy." |
| A few participants "altered the allotted treatment programmes in some small way to suit their personal requirements." |
| "7 patients, while carrying out CCPT only, developed exacerbations of their chest condition', and 'unable to clear secretions adequately and fearing hospital admission, each patient re‐introduced a combination of CCPT and PEP for a mean period of 3 days (range 2–6 days)' which resulted in clinical improvement and the patients then returned to CCPT for the remainder of the allotted period." |
| "Parents of the younger children reported that they had mixed feelings about PEP alone or PEP with FET. They welcomed the increased independence but were concerned about handing over total control to the child and felt close supervision would still be required." |
| "On completing the study, 23 of the 24 patients chose PEP mask in combination with FET as their long‐term chest physiotherapy programme. When asked why they had chosen this treatment programme, they replied that this combination improved their independence and, at the same time, was still an effective form of chest physiotherapy." |
CCPT: conventional chest physiotherapy; FET: forced expiratory technique; PEP: positive expiratory pressure.
Tonnesen 1982 reported that all 14 participants preferred the mask treatment and would continue using it over the long term because they appreciated the independence it afforded. Nine participants considered the PEP mask was most effective, four considered both treatments were equally effective and one found CCPT most effective. Adherence was described as 88% for CCPT and 89% for PEP treatments.
Tyrrell 1986 did not formally assess individual preference, but indicated that comments about the PEP mask were generally favourable. Given the choice, six months after completion of the study, 9/16 participants were using PEP exclusively, 4/16 were using it in addition to CCPT and 3/14 participants considered they had no benefit from PEP. Study authors "gained the impression that children producing moderate or large amounts of sputum were least happy with the PEP mask, complaining that their chests did not always feel clear after treatment."
van Asperen 1987 also did not formally assess individual preference, but indicated from ad hoc communications that participants "commented favourably" on the independence achieved with PEP mask therapy, allowing more freedom in planning their lifestyle; however, "the majority tended to revert to postural drainage during acute exacerbations."
Long term
All three abstracts relating to Costantini 2001 reported that infants and their parents "greatly preferred" PEP to CCPT. It is unclear how this was assessed or analysed or interpreted, particularly in infants. There was no indication in any of the abstracts whether these conclusions were reached through ad hoc conversations with a subset of families or via formal survey involving all participants.
Gaskin 1998 asked participants to keep compliance diaries for the duration of the study, but did not provide data or narrative details on their findings.
McIlwaine 1997 asked participants to keep a daily record of treatment adherence. A level of less than 85% compliance with performance of twice‐daily physiotherapy over a one‐month period was considered non‐compliance and a reason for the individual to be removed from the study. Two participants from each group dropped out due to non‐compliance with treatment or non‐attendance at clinic. The levels of compliance as recorded by the participants who completed the study were 92% for CCPT and 96% for PEP.
Participants in McIlwaine 1997 also completed a monthly questionnaire that recorded how they were feeling, their impression of the physiotherapy technique and summarised compliance or reasons for non‐compliance with physiotherapy. Investigators reported that participants in the PEP group, who had performed CCPT as their prestudy ACT, reported on the questionnaire that they preferred using PEP as they considered PEP mobilised greater quantities of mucus than CCPT and was easier to perform. However, investigators did not provide any details of analysis, or the proportions of participants who contributed these impressions. In addition, they provided no data or narrative details on findings in relation to participants' impressions of CCPT, reasons for compliance or non‐compliance with either therapy, or how participants in either group reported feeling during the study.
4. Objective change in exercise capacity
Medium term
Three of the six medium‐term studies reported this outcome (Steen 1991; Tyrrell 1986; van Asperen 1987). Two studies included daily diaries that reflected changes in exercise tolerance (Steen 1991) and daily activity (Tyrrell 1986), but provided no data or narrative findings. van Asperen 1987 used daily diaries that included a daily activity score, but investigators concluded that "all patients were fully active during the treatment periods and so activity score was not used for comparison.".
Long term
Two studies reported this outcome (Gaskin 1998; McIlwaine 1997). Gaskin 1998 asked participants to keep exercise diaries for the two‐year duration of the study and assessed exercise capacity using cycle ergometry, but provided no data or narrative details on any findings. McIlwaine 1997 instructed participants to maintain their prestudy level of physical activity throughout the one‐year study period and asked them to complete a monthly questionnaire that recorded physical activity levels. They provided no data or any narrative details on any findings related to this outcome measure.
5. Additional lung function tests
Medium term
Dadparvar 1995 reported that all "other PFT values did not change significantly," but did not specify what these were. McIlwaine 1991 reported no difference in FEV1/FVC between groups. Three studies assessed PEFR, which did not change after either treatment (Steen 1991; Tyrrell 1986; van Asperen 1987).
6. Ventilation scanning or lung imaging
Medium term
Four of the six medium‐term studies reported this outcome (Dadparvar 1995; Steen 1991; Tonnesen 1982; Tyrrell 1986).
One cross‐over study, reported in two abstracts (the first in 1990 with preliminary data on 13 participants and the second in 1995 with 20 participants), used 99mTc‐DTPA aerosol ventilation scanning to calculate the half‐life clearance in the whole lung (WL) or peripheral lung (PL) at baseline, and three months and six months following completion of each study arm (Dadparvar 1995). The 1990 abstract reported no difference between CCPT and PEP for clearance of either WL or PL. The second abstract concluded that after three months only WL clearance was better in the PEP group and after six months only PL clearance was better in the PEP group, with all other comparisons of half‐life clearance being equivalent. At both three and six months, regional ventilation in the right middle lobe was better in the CCPT group. These findings should be interpreted with caution; data were only provided as P values in the 1995 abstract and the arbitrary and unsustained nature of these findings mean it is unlikely that any valuable meaning can be extracted from these data. In Group 1, participants received CCPT first and PEP second, while Group 2 received PEP first and CCPT second. While there was better PL clearance in the PEP arm (Group 1) at six months, this was not demonstrated in the other PEP arm (Group 2) at three months; the PEP WL clearance superiority at three months was not sustained at six months; and the regional ventilation only improved in the right middle lobe and no other regions of the lung for the CCPT group compared with PEP. Investigators also performed chest radiography at baseline for clinical scoring, but did not use this as an outcome measure (Dadparvar 1995).
Two studies assessed Chrispin‐Norman scores (Steen 1991; Tyrrell 1986); this system scores lung X‐rays from 0 (no changes) to 38 (maximum number of changes). Steen 1991 found no change between any of the five treatment groups. Tyrrell 1986 reported scores ranging from 3 to 20 (mean 6.12), but these were only performed at baseline to describe the severity of disease, rather than as an outcome measure for comparison.
One study provided narrative findings on paired chest radiographs by a blinded assessor and concluded there was no definite difference between the two treatments, and neither CCPT nor PEP alone were enough to prevent new X‐ray changes from occurring (Tonnesen 1982).
Long term
All three studies performed chest X‐rays (Costantini 2001; Gaskin 1998; McIlwaine 1997).
In the neonate study, chest X‐rays were taken at baseline, and six and 12 months (Costantini 2001). Findings were worryingly inconsistent between abstracts; the 1998 abstract (12 infants) reported that lung deterioration, measured by "increase in bronchial markings" was found more often in the CCPT group than the PEP group (80% of cases with CCPT versus 28% of cases with PEP), but that 74% of participants treated with PEP showed "increased hyperinflation in the chest" (data for CCPT group not stated). By contrast, in both of the 2001 abstracts (26 infants), authors indicated that there were lung deterioration, measured by "increase in bronchial markings," in both groups (100% with CCPT versus 93% with PEP), and this time, 54% of participants in the CCPT group showed "increased hyperinflation in the chest" (data for the PEP group not stated) (Costantini 2001).
Gaskin 1998 included the Brasfield Chest radiographic score, but provided no data or narrative findings.
McIlwaine 1997 took a chest X‐ray at the beginning and end of the study, and a radiologist blinded to the participant allocation reported results. There were no differences between groups with respect to prestudy and poststudy results, but they provided no data. Two clinic physicians in consultation with the radiologist scored chest X‐rays using the Brasfield system; all were blinded to the participant's name, date of X‐ray and intervention. There was no difference in Brasfield scores between the two groups at baseline or the mean change in score after the study (0.37 (SD 1.80) with CCPT versus 0.37 (SD 1.86) with PEP; P = 1.0).
7. Blood oxygen levels
Long term
Costantini 2001 reported SpO2, which was monitored at baseline, and six and 12 months. The 1998 abstract (12 infants) reported that there were non‐significant changes in saturation before and after each treatment. In the first of the 2001 abstracts (26 infants), study authors indicated that SpO2 were lower in the CCPT group compared with the PEP group at the "run‐in evaluation" (95% with CCPT versus 98.5% with PEP). In the second 2001 abstract (26 infants), authors reported that SpO2 was higher in the PEP group at every evaluation (96.7% with CCPT versus 98.1% with PEP; P = 0.049).
8. Nutritional status as assessed by growth, weight and body composition
Medium term
Only two medium‐term studies assessed this outcome (Steen 1991; Tyrrell 1986).
Steen 1991 included daily diaries that reflected change in appetite, but provided no data or narrative findings. There were also no changes in Shwachman score, which would have included nutritional domain questions. Investigators found no difference in growth between any of the five treatment groups, but provided no data.
Tyrrell 1986 measured the Shwachman score, which would have included basic nutritional domain questions, but did not report any information.
Long term
Costantini 2001 monitored growth at baseline, and six and 12 months. They provided no data or narrative findings in the 1998 abstract, but both 2001 abstracts reported that growth was in the normal range for CCPT and PEP groups.
Gaskin 1998 and McIlwaine 1997 measured the Shwachman score, which would have included basic nutritional domain questions, but did not report any information.
9. Mortality
While none of the medium‐term studies explicitly reported this outcome (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tyrrell 1986; van Asperen 1987), Steen 1991 did report the death of one 18‐year‐old participant. None of the long‐term studies reported this outcome (Costantini 2001; Gaskin 1998; McIlwaine 1997).
11. Mucus wet or dry weight
Medium term
Four studies reported at least some information for this outcome (McIlwaine 1991; Steen 1991; Tyrrell 1986; van Asperen 1987). One of the McIlwaine abstracts concluded that 3/18 participants produced more sputum with PEP, but it was unclear how they collected, analysed or interpreted these data and investigators did not provide details on the method and over what period sputum were collected (McIlwaine 1991). Steen 1991 found no differences in sputum weight between any of the five treatment groups. Tyrrell 1986 measured sputum weight expectorated after a single treatment under supervision at the end of each treatment period (mean sputum production: 8.2 g with CCPT versus 8.1 g with PEP; range 0 g to 44.7 g). van Asperen 1987 reported the mean sputum volume in the hour from the commencement of each treatment and found no difference between the treatments (22.0 (SE 14.1) g with CCPT versus 11.5 (SE 4.0) g with PEP).
Long term
Only one long‐term study assessed this outcome (McIlwaine 1997). Participants completed a monthly questionnaire that recorded amount of cough and sputum productivity, but the study authors provided no data or narrative findings.
Additional outcomes that have arisen from the review
Steen 1991 collected daily diary data on cough, sputum characteristics, wheeze and shortness of breath, but provided no data or narrative findings. Tyrrell 1986 used diary cards to record symptoms: sleep, cough, wheeze, activity and sputum production, with a maximum composite score of 13. The mean daily diary scores were 3.5 (range 0 to 9.4) during the physiotherapy month and 3.7 (range 0 to 9) during the PEP month; there was no difference between groups. van Asperen 1987 used diary cards to evaluate cough score (0 to 3) day and night, activity score (0 to 3), sputum volume in the hour after each treatment and PEFR using a Wright mini‐peak flowmeter recorded one hour after treatment. There were no differences between the mean daily scores for sputum volume, cough or PEFR during PEP mask therapy compared with CCPT, paper states, "All patients were fully active during the treatment periods and so activity score was not used for comparison."
Adverse events
Medium term
Tonnesen 1982 reported that there were no signs of pneumothorax in any participants after one month of using a PEP mask. One participant using PEP reported a headache that was related to sinusitis.
Long term
Two studies reported adverse events (Costantini 2001; McIlwaine 1997).
Costantini 2001 monitored adverse effects at baseline, and six and 12 months. The 1998 abstract to the Costantini study (12 participants) reported that adverse effects were rare, with one infant in the PEP group demonstrating GOR, and another, also in the PEP group, developing atelectasis (complete or partial collapse of the lung) that had a rapid recovery. In both of the 2001 abstracts relating to this study (26 participants), authors also reported that adverse effects were rare; they did not report atelectasis, but reported that five participants in the PEP group demonstrated mild GOR compared to four participants in the CCPT group, of which three dropped out of the study because of the severity of their GOR symptoms and were not evaluated.
McIlwaine 1997 reported there were no adverse effects from use of the PEP mask; no participants in either group had pneumothorax during the study.
Clinical score
Medium term
Two medium‐term studies did not report on these outcomes (Dadparvar 1995; van Asperen 1987), although one of these included a baseline "clinical score," which was not named and provided no data (Dadparvar 1995). Two studies reported that they calculated Shwachman clinical scores; Steen 1991 found no difference between groups, but provided no data, and Tyrrell 1986 reported their participants had mild‐to‐moderate disease with Shwachman scores ranging from 47 to 85 (mean 62, normal = 100). Only one of the three published abstracts for McIlwaine 1991 made reference to clinical scores; these were not different between groups, but they did not report the score used or provide data. Tonnesen 1982 used Cooperman's Cystic Fibrosis Scoring system to indicate baseline disease severity, but did not compare changes between groups after treatment.
Long term
McIlwaine 1997 gave participants a full clinical assessment, including Shwachman and Huang scores, at baseline and at three‐monthly intervals. There were no differences between the groups with respect to prestudy and poststudy scores, but they provided no data.
Sputum culture
Medium term
Two medium‐term studies reported sputum culture in describing participants, but the outcome was not used to compare interventions. Steen 1991 reported 6/24 participants had Staphylococcus aureus colonisation and 18 participants had chronic Pseudomonas aeruginosa colonisation. Tonnesen 1982 reported 12/14 participants had chronic pulmonary P aeruginosa infections.
Long term
Two long‐term studies assessed sputum cultures (Costantini 2001; McIlwaine 1997). Costantini 2001 monitored sputum cultures at baseline, and six and 12 months but only reported them in the 2001 abstracts (26 participants). Both abstracts reported P aeruginosa colonisation in 9% of the CCPT group compared to 13.3% of the PEP group; however, the abstracts were inconsistent with respect to the reports on S aureus colonisation stating in one abstract this was 63.6% in the CCPT group compared to 73.3% in the PEP group, but in the second abstract that this was 54.5% in the CCPT group compared to 60% in the PEP group. Reasons for this inconsistency were unclear.
McIlwaine 1997 obtained sputum specimens from each participant for bacteriological culture at baseline and at three‐monthly intervals. There were no differences between the groups with respect to changes in bacteriological cultures, but they provided no data.
Comparison 2: conventional chest physiotherapy versus extrapulmonary mechanical percussion
Three studies compared CCPT with therapies involving extrapulmonary MP (274 participants, 252 analysed); this was HFCC in two studies (Arens 1994; Sontag 2010) and MP in one study (Bauer 1994). Two short‐term studies conducted during hospitalisation for an acute exacerbation compared CCPT with a single comparator (Arens 1994; Bauer 1994). The third, a long‐term study, compared CCPT with HFCC and the Flutter device; in this section, we review only the CCPT versus HFCC comparison (Sontag 2010). This long‐term study was terminated early, but reported interim analyses at the termination of study. See Table 2.
Primary outcomes
1. Pulmonary function tests
a. Forced expiratory volume in one second
Short term
Two studies (108 participants, 101 analysed) provided data for analysis of FEV1 % predicted (Arens 1994; Bauer 1994). There was no difference between CCPT and HFCC or CCPT and MP intervention groups (MD −2.10, 95% CI −5.49 to 1.29; P = 0.23; very low‐certainty evidence; Analysis 3.1). The published narrative findings concurred with those in the meta‐analysis for both studies.
3.1. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
Long term
The long‐term study reported interim analyses at termination of study, by which time participants had participated for at least 1.3 years and at most 2.8 years (166 participants, 151 analysed) (Sontag 2010). There were no differences between CCPT and HFCC in the rate of decline for FEV1 % predicted.
b. Forced vital capacity
Short term
Both short‐term studies (108 participants, 101 analysed) provided data for FVC % predicted (Arens 1994; Bauer 1994). There was no difference between CCPT and HFCC or CCPT and MP intervention groups, although there was a small overall tendency towards improved FVC in favour of extrapulmonary MP (MD −3.86, 95% CI −8.05 to 0.33; P = 0.07; very low‐certainty evidence; Analysis 3.2). The published narrative findings concurred with those in the meta‐analysis for both studies.
3.2. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 2: Forced vital capacity (FVC) % predicted
Long term
The long‐term study (166 participants, 151 analysed) reported no differences between CCPT and HFCC (and Flutter device) in the rate of decline for FVC (Sontag 2010).
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Short term
Two studies (108 participants, 101 analysed) provided data for FEF25–75 in the short term (Arens 1994; Bauer 1994). There was no difference between CCPT and HFCC or CCPT and MP intervention groups (MD 0.49, 95% CI −2.53 to 3.52; P = 0.75; very low‐certainty evidence; Analysis 3.3). The published narrative findings concurred with those in the meta‐analysis for both studies.
3.3. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
Long term
The long‐term study (166 participants, 151 analysed) reported the annual rate of decline for FEF25–75 was greater in those using HFCC than those using CCPT. Authors also concluded that HFCC may be associated with worse flow at low lung volumes, and different physiological effects in the small airways in people with CF (Sontag 2010).
2. Number of respiratory exacerbations
Respiratory exacerbations were defined by 'time to first respiratory exacerbation' or any single or combined reports of number of days in hospital, admissions to hospital, IV antibiotic courses or IV antibiotic days.
a. Number of days in hospital for respiratory exacerbations
Short term
Both short‐term studies reported the length of hospital stay during a single admission for an acute exacerbation (Arens 1994; Bauer 1994). Pooled analysis of the data showed fewer days in hospital for the extrapulmonary MP group (MD 0.90 days, 95% CI 0.69 to 1.10; very low‐certainty evidence; Analysis 3.4). However, statistical heterogeneity was considerable for these two studies (I2 = 93%), representing inconsistency in the results that was greater than might be expected by chance (Deeks 2022). Comparison of CCPT versus HFCC showed no difference between the groups in Arens 1994 (MD 0.20 days, 95% CI −0.22 to 0.62; very low‐certainty evidence), but the Bauer 1994 results strongly favoured MP over CCPT (MD 1.10 days, 95% CI 0.87 to 1.33; very low‐certainty evidence; Analysis 3.4). Findings from the pooled analysis were not consistent with those from the published papers, which both reported non‐significant differences between groups (Arens 1994; Bauer 1994). The heavily weighted Bauer 1994 involved cross‐over analysis for a subgroup of participants who were readmitted to hospital during the study period. The original paper concluded there was no difference in duration of hospitalisation between the groups, either in the total population of participants or in the cross‐over group (Bauer 1994). The fact that the study authors analysed these data as if they were from a parallel study may account for the discrepancy in the results both for the individual study and in terms of the pooled results.
3.4. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 4: Number of days in hospital for respiratory exacerbations
e. Time to first respiratory exacerbation
Long term
The long‐term study that compared CCPT with HFCC found that the risk of developing a pulmonary exacerbation (based on the time to first treatment with IV antibiotics) was not different between groups (P = 0.59), but did not present data in a format that was compatible with meta‐analysis (Sontag 2010).
Secondary outcomes
1. Quality of life
Long term
Sontag 2010 administered the CFQ at selected visits. The 12 health‐related quality of life (HRQoL) domains were not different at baseline and there were no differences between CCPT and HFCC after correcting for multiple comparisons in the CFQ analyses.
2. Adherence to therapy, satisfaction and individual preference
Short term
Arens 1994 reported that three participants originally assigned to receive HFCC, and one participant assigned to the CCPT regimen, were not compliant and were excluded from the study. Furthermore, study authors reported that 22 participants (88%) in the HFCC group expressed satisfaction with this technique of chest physiotherapy and requested HFCC therapy in the management of future acute pulmonary exacerbations; there was no indication that satisfaction was assessed in the CCPT group. It was unclear whether these conclusions were reached via ad hoc conversations or via a structured survey methodology involving all participants, and it is also unclear how data were analysed or interpreted.
Before the start of Bauer 1994, participants were given questionnaire cards and results showed that 26% preferred MP, 37% preferred CCPT and 37% had no preference. After the study, follow‐up telephone responses were obtained from 38/51 participants at which time 47% preferred MP, 26% preferred CCPT and 26% had no preference. Of the 28 respondents who received MP during the study, 57% preferred MP, 11% preferred manual percussion (CCPT) and 32% had no preference. Furthermore, three participants dropped out of the study because of dissatisfaction with the mechanical percussor, one because MP was painful, and two because of perceived lack of benefit. Although these figures might suggest a preference for MP, authors concluded that the data did not support a general preference for one form of percussion over the other, but that preference was subject to significant individual variability and probably depended on a combination of perceived comfort, convenience and benefit (Bauer 1994).
Long term
Participants withdrew from Sontag 2010 at higher rates than expected; 15 participants withdrew within 60 days of randomisation and 11 on the day of randomisation. The withdrawal rate was significantly higher in the CCPT group than in the HFCC group, and was higher in older age groups. Satisfaction with the therapy was an independent predictor of withdrawal.
Sontag 2010 used a validated TSS to evaluate four factors: effectiveness, convenience, comfort and overall satisfaction. The mean scores were not different at baseline, reflecting satisfaction with participants' ACT prior to randomisation. The last TSS recorded for each participant was lower in CCPT than HFCC across all factors (Table 8). TSS differences persisted across age groups. The last reported scores from the TSS were associated with participant withdrawal from the study, indicating individuals with lower TSS were more likely to withdraw (odds ratio (OR) 0.44, 95% CI 0.34 to 0.73). Study authors suggested these findings might mean that if an individual dissatisfied with an ACT may be more likely to discontinue treatment entirely compared to those who are satisfied with their ACT.
2. Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC): adherence to therapy, participant satisfaction and individual preference – Sontag 2010.
| Domain | CCPT mean (SD) score | HFCC mean (SD) score |
| Effectiveness (possible score 5–25) |
16.3 (0.8) | 19.4 (0.4) |
| Convenience (possible score 5–25) |
12.1 (0.9) | 17.7 (0.6) |
| Discomfort (possible score 5–25) |
18.7 (0.6) | 21.2 (0.5) |
| Overall satisfaction (possible score 2–10) |
5.1 (0.3) | 8.5 (0.3) |
CCPT: conventional chest physiotherapy; HFCC: high‐frequency chest compression; SD: standard deviation.
Sontag 2010 measured adherence in 131 participants using a daily phone diary. Mean adherence was never greater than 65%, with a mean of 50%, which, according to the study authors was consistent with the broader adherence literature and provides insight into real‐life practice patterns in CF. Both therapies showed improvement in adherence from baseline to the first visit postrandomisation (CCPT (42 participants) increased by 16% (interquartile range (IQR) 0% to 38%) and HFCC (55 participants) improved by 19% (IQR 0% to 50%)). However, there were no differences between the different interventions in the improvement in adherence at this time point (P = 0.56). The improvement in adherence from baseline to the fifth assessment was slightly greater in the HFCC group, although still non‐significant between the groups (P = 0.09) (CCPT (23 participants) improved by 6% (IQR 25% to 38%) and HFCC (47 participants) improved by 25% (IQR 0% to 56%)).
There were no differences in adherence rates in participants who withdrew from the study early and those who did not withdraw, but individuals who withdrew from the study were not satisfied with their assigned therapy. Adherence was one of the factors tested as a covariate in the FEV1% predicted decline model, but this was not significant. Study authors postulated that the parallel rates of decline suggested regular airway clearance may be more important than differences in the ACTs themselves. In addition, that therapy choice is dynamic over an individual's lifetime, given developmental and disease‐related changes. Teenagers might need therapies allowing a shift towards greater independence, while adults reaching end‐stage disease might want the adaptability of CCPT provided by a trained carer. Flexibility in prescribing ACTs may encourage long‐term adherence to airway clearance.
5. Additional lung function tests
Short term
Only one of the short‐term studies performed a number of additional lung function tests including FRC, RV, RV/TLC and as a measure of uniformity of ventilation distribution, the slope of Phase III of the single breath nitrogen washout test (SP3N2) (Arens 1994). After 14 days, there were non‐significant changes in RV % predicted, RV/TLC and SP3N2 % predicted (Table 9); there were no data for FRC.
3. Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC): additional lung function outcomes – Arens 1994.
| Measurement | CCPT mean (SD) | HFCC mean (SD) |
| RV % predicted | −12.5 (3.3) | −15.2 (2.8) |
| RV/TLC | −11.4 (2.6) | −14.4 (1.8) |
| SP3N2 % predicted | −27.9 (5.7) | −15.2 (7.5) |
CCPT: conventional chest physiotherapy; HFCC: high‐frequency chest compression; RV: residual volume; SD: standard deviation; SP3N2: phase 3 of single‐breath nitrogen washout; TLC: total lung capacity.
7. Blood oxygen levels
Short term
Arens 1994 compared CCPT to HFCC and measured SpO2 before pulmonary function testing while participants were breathing room air. In addition, they measured SpO2 in all participants during both CCPT and HFCC as well as 30 minutes and one hour following treatments. After 14 days, there were no differences in SpO2 between groups (MD 0.10, 95% CI −0.29 to 0.49; very low‐certainty evidence; Analysis 3.5).
3.5. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 5: Blood oxygen levels
Bauer 1994 recorded each session in the hospital chart, noting oximetry readings, but provided no data or narrative findings in the manuscript.
8. Nutritional status as assessed by growth, weight and body composition
Short term
Arens 1994 reported a significant improvement in mean weight gain within both groups during the hospital admission and no differences between groups. However, meta‐analysis of these data in this review suggests differences in favour of HFCC after 14 days (3.3% (SD 1.1%) with CCPT versus 4.4% (SD 1.0%) with HFCC). The cross‐over study by Bauer 1994 reported no difference in weight gain during hospitalisation between the groups (mean weight gain 1.0 (SE 0.2) kg with CCPT versus 0.9 (SE 0.2) kg with MP). Combining these results in our analysis showed an overall lower change in weight in the short term for CCPT compared to HFCC/MP (MD −0.47 kg, 95% CI −0.87 to −0.07; very low‐certainty evidence; Analysis 3.6). However, we noted a high degree of heterogeneity (I2 = 88%) in these findings and could not identify any obvious potential sources of difference between the studies.
3.6. Analysis.

Comparison 3: Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP), Outcome 6: Weight (change from baseline) (kg)
Long term
Sontag 2010 tested BMI as a covariate in the FEV1 and FVC % predicted decline models, and BMI percentile at baseline was the only significant covariate (P < 0.01) in the models, reflecting that individuals with higher BMI percentiles had higher FEV1 and FVC % predicted. Study authors also described an association of BMI percentile and age with time to exacerbation but did not report any specific results.
11. Mucus wet or dry weight
Short term
Arens 1994 collected sputum for 24 hours after beginning CCPT or HFCC and measured the first hour and 24‐hour wet sputum collection samples. They measured dry sputum weight after slow desiccation in a commercial microwave for a period of 10 to 60 minutes. There was an increase in wet sputum production during the first hour after treatment with HFCC compared with CCPT. However, these differences were not observed in dry sputum weight after one hour. Sputum production (wet or dry weight) was also similar in CCPT and HFCC after the 24‐hour collection (Table 10).
4. Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC): sputum weight – Arens 1994.
| Weight | Time point | CCPT mean (SD) | HFCC mean (SD) | Significance |
| Wet weight | 1 hour | 6.0 (1.8) g | 14.6 (2.9) g | P < 0.035 |
| 24 hours | 30.6 (9.4) g/day | 51.3 (6.4) g/day | Non‐significant | |
| Dry weight | 1 hour | 0.8 (0.2) g | 1.4 (0.4) g | Non‐significant |
| 24 hours | 3.4 (0.9) g/day | 5.3 (0.9) g/day | Non‐significant |
CCPT: conventional chest physiotherapy; HFCC: high‐frequency chest compression; SD: standard deviation.
Bauer 1994 recorded each session in the hospital chart, noting secretion assessments, but provided no data or narrative findings in the manuscript.
Additional outcomes that have arisen from the review
The short‐term study, comparing CCPT to HFCC reported haemoglobin, white blood cell count, absolute neutrophil count, serum protein and albumin concentrations, which were drawn on admission and at days seven and 14 of hospitalisation; there were no differences after 14 days (Arens 1994).
The long‐term study, comparing CCPT to HFCC reported no differences in rates of new prescriptions for dornase alfa (8/47 (17%) participants with CCPT versus 9/57 (16%) participants with HFCC; P = 0.90) or tobramycin solution for inhalation (21/47 (45%) participants with CCPT versus 29/57 (51%) participants with HFCC; P = 0.46) (Sontag 2010).
Adverse events
Short term
In Arens 1994, two participants in the CCPT group and one participant in the HFCC group developed mild haemoptysis during the study; treatments were discontinued for 24 hours and then continued with no further haemoptysis. Some participants in the HFCC group reported occasional mild chest pain and nausea, particularly during the first two to three days of treatment, which resolved.
Bauer 1994 recorded each session in the hospital chart, noting any complications, but provided no data or narrative findings in the manuscript.
Clinical score
Short term
Only Bauer 1994 reported data relevant to this outcome. On the date of admission, investigators used NIH scores to classify the severity of illness, and found no difference in NIH scores between groups, but those with more severe illness had greater improvement in lung function during hospitalisation.
Sputum culture
Short term
Arens 1994 measured sputum cultures at baseline only.
Long term
Sontag 2010tested P aeruginosa colonisation as a covariate in the FEV1% predicted decline model, and found no differences between groups.
Comparison 3: conventional chest physiotherapy versus active cycle of breathing techniques
CCPT is typically a composite intervention that includes any combination of the following components: PD, percussion, chest wall vibration or shaking, huffing (or FET) or directed coughing. Sometimes FET is described as the most important component of both CCPT and ACBT ACTs. There were no eligible studies comparing CCPT with ACBT directly, but three studies compared CCPT with specific components of both CCPT and ACBT, namely FET (Reisman 1988; Steen 1991), and directed huffing and coughing (Bain 1988). See Table 3.
Primary outcomes
1. Pulmonary function tests
a. Forced expiratory volume in one second
Short term
Bain 1988 compared CCPT with directed coughing in people with CF admitted to hospital for an acute pulmonary exacerbation and provided FEV1 data for analysis (46 participants, 38 analysed). They concluded that directed coughing was as effective an ACT as CCPT.
Medium term
Steen 1991 compared CCPT with five different intervention groups, each over four weeks (combinations of CCPT, PEP and FET); the fifth group (FET only) was added as a comparison at the end of their study period and involved only five participants. Data for FEV1 for these five participants could not be extracted for meta‐analysis and we requested them from the study authors. The publication concluded that four of five participants undertaking FET in isolation for four weeks were not confident that it would provide adequate airway clearance over the long term and said they preferred FET in combination with other treatments.
Long term
Reisman 1988 conducted over more than two years compared CCPT combined with FET to FET alone (67 participants, 63 analysed). They provided FEV1 data for meta‐analysis, which showed that although there was no difference in FEV1 % predicted, the difference approached significance in favour of CCPT (MD 2.80, 95% CI −0.39 to 5.99; P = 0.09; very low‐certainty evidence; Analysis 4.1). The publication also reported a non‐significant tendency for the annual decline in FEV1 to be worse in the FET alone group, but authors suggested "strongly" that the long‐term course of pulmonary function was adversely affected when CCPT was abandoned; this was on the basis of outcomes other than FEV1 (Reisman 1988).
4.1. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
b. Forced vital capacity
Short term
Bain 1988, comparing CCPT with directed coughing in people with CF admitted to hospital for an acute pulmonary exacerbation, provided FVC data for analysis (46 participants, 38 analysed) and concluded that directed coughing was as effective an ACT as CCPT.
Medium term
Steen 1991, comparing CCPT with FET only, found that four of five participants undertaking FET in isolation for four weeks were not confident that it would provide adequate airway clearance over the long term and said they preferred FET in combination with other treatments. We could not extract FVC data for these five participants for meta‐analysis and we requested data from study authors.
Long term
Reisman 1988, comparing CCPT combined with FET to FET alone, found no differences between groups in FVC % predicted (67 participants, 63 analysed) (MD 1.80, 95% CI −0.83 to 4.43; P = 0.18; very low‐certainty evidence; Analysis 4.2). This analysis concurred with FVC findings within the manuscript, but study authors suggested "strongly" that the long‐term course of pulmonary function was adversely affected when CCPT was abandoned; this was on the basis of outcomes other than FVC.
4.2. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 2: Forced vital capacity (FVC) % predicted
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Short term
Bain 1988, comparing CCPT with directed coughing in people with CF during hospitalisation for an acute pulmonary exacerbation, provided FEF25–75 data for analysis (46 participants, 38 analysed) and concluded that directed coughing was as effective an ACT as CCPT.
Long term
Reisman 1988, comparing CCPT combined with FET to FET alone, demonstrated differences in annual decline between groups after two years in favour of CCPT for FEF25–75 % predicted (67 participants, 63 analysed) (MD 6.00, 95% CI 0.55 to 11.45; P = 0.03; very low‐certainty evidence; Analysis 4.3). This analysis concurred with the findings within the manuscript, in which the annual decline in FEF25–75 % predicted was worse in the FET only group. Study authors concluded that CCPT should remain a standard component of therapy in CF and suggested "strongly" that the long‐term course of pulmonary function was adversely affected when CCPT was abandoned.
4.3. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
2. Number of respiratory exacerbations
Respiratory exacerbations were defined by 'time to first respiratory exacerbation' or any single or combined reports of number of days in hospital, admissions to hospital, IV antibiotic courses or IV antibiotic days per year.
Neither the short‐term study that compared CCPT with directed coughing (Bain 1988), nor the medium‐term study that compared CCPT with FET (Steen 1991), reported respiratory exacerbations.
There was one long‐term study that compared CCPT with FET and it reported number of hospital admissions during the study period and days in hospital during the study period (Reisman 1988). The mean duration of follow‐up was similar in both groups (2.4 (SD 0.4) years with CCPT versus 2.4 (SD 0.4) with FET). Only five (17%) participants in the CCPT group and nine (27%) participants in the FET group were hospitalised for pulmonary exacerbations. The differences in number of participants, number of hospitalisations and days spent in the hospital were not significant.
a. Number of days in hospital for respiratory exacerbations
Long term
Reisman 1988 (63 participants) reported that five participants in the CCPT group spent 73 days in hospital compared with eight (plus one) participants in the FET group who spent 197 (plus 150) days in hospital during the study. The values for one extraordinary participant in the FET group was reported in parentheses because her case was extreme, and her 15 admissions were for treatment of respiratory illness complicated by social problems. Two other participants in the FET group accounted for 43 and 60 days, with three admissions each. Although the differences in number of days spent in the hospital were reported as not significant there appear, on face value, to be significantly greater days in hospital associated with the FET group. Data were not presented in a format that was compatible with meta‐analysis.
b. Number of admissions to hospital per year for respiratory exacerbations
Long term
Reisman 1988 (63 participants) reported that five participants in the CCPT group had eight hospital admissions compared to eight (plus one) participants in the FET group who had 15 (plus 15) hospital admissions during the study period. The values for one extraordinary participant in the FET group was reported in parentheses. Although the differences in number of admissions to hospital were reported as not significant there appears, on face value, to be greater numbers of admissions hospital associated with the FET group. Data were not presented in a format that was compatible with meta‐analysis. However, data were available for the number of individuals requiring at least one hospital admission; there was no difference between groups (RR 0.61, 95% CI 0.23 to 1.62; P = 0.32; very low‐certainty evidence; Analysis 4.4).
4.4. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 4: Number of participants per group requiring ≥ 1 hospital admission
Secondary outcomes
2. Adherence to therapy, satisfaction and individual preference
Short term
While Bain 1988 did not specifically report on this outcome, one participant was withdrawn from the study due to a lack of compliance and a further participant was withdrawn at parent's request; there was no further information about which group or for which reasons.
Medium term
Steen 1991 did not use a recognised assessment tool for any of these outcomes, but asked participants to note their general impressions about each treatment. The publications reported some perceptions (from participants and parents) that reflected satisfaction or preference, and appeared to have been collected in an ad hoc and inconsistent manner.
Long term
Reisman 1988 asked participants to keep a diary reporting adherence to their physiotherapy regimen and evaluated compliance annually with the scoring system of Passero and colleagues (Passero 1981). All but three participants were judged to be consistently compliant with their therapy. The two participants in the FET group who were sporadically non‐compliant had declining pulmonary function and the one consistently non‐compliant participant in the CCPT group had stable pulmonary function throughout the study.
4. Objective change in exercise capacity
Medium term
Steen 1991 included daily diaries that reflected change in exercise tolerance, but provided no data or narrative findings on this outcome.
Long term
All participants in Reisman 1988 were advised to participate in as much physical activity and sport as they could and this was monitored by the use of an annual questionnaire. Both intervention groups performed a mean level of physical activity (11 to 15 hours per week) with no difference between the groups. Low, mean and high activity levels were used in analysis as a possible risk factor for decline in FEV1, but these were not different.
At baseline and after at least one year, participants performed a Jones stage 1 graded exercise test with the use of an electronically braked cycle ergometer. Investigators recorded maximum work capacity and monitored heart rate and respiratory rate throughout exercise (Table 11). Some younger participants were unwilling to complete the exercise test. A total of 25 participants underwent initial and follow‐up graded exercise challenge tests, and there was no difference between groups (P = 0.10).
5. Conventional chest physiotherapy (CCPT) versus forced expiratory technique (FET): objective change in exercise capacity – Reisman 1988.
| Wmax % predicteda | CCPT mean (SD) | FET mean (SD) |
| Baseline | 98 (30) % predicted | 109 (24) % predicted |
| Follow‐up | 99 (23) % predicted | 94 (19) % predicted |
CCPT: conventional chest physiotherapy; FET: forced expiration technique; SD: standard deviation; Wmax: maximal work capacity. anormal values reported by Godfrey 1971 were used for the calculations.
5. Additional lung function tests
Short term
Bain 1988 reported TLC and RV calculated from measurements of thoracic gas volume (TGV) made in an integrated flow, pressure compensated total body plethysmograph. Maximum expiratory flow–volume curves were performed in the body plethysmograph and expiratory flow was measured at 70%, 60% and 40% of TLC and corrected for lung volume by dividing by TLC and expressed as TLC/s. Maximum static inspiratory and expiratory pressures at the mouth (PImax and PEmax) were measured. Comparison of the differences between day 1 and day 15 between CCPT and the directed coughing group showed no difference.
Medium term
Steen 1991 reported that PEFR did not change after any treatment.
6. Ventilation scanning or lung imaging
Short term
Bain 1988 reported that on admission all participants had a chest X‐ray, but this appeared to be used primarily to classify disease severity rather than as an outcome measure.
Medium term
Steen 1991 reported Chrispin‐Norman scores and found no change between any of the treatment groups.
Long term
Reisman 1988 obtained posteroanterior and lateral chest X‐rays every six months and scored them using the Brasfield scoring system; there were no data or narrative findings in the publication.
7. Blood oxygen levels
Short term
Bain 1988 measured ear oximetry using a Hewlett Packard ear oximeter. There was an improvement in oximetry in both treatment groups, but there was no difference in changes from baseline at day 15 between the CCPT and the directed coughing group (MD −0.20%, 95% CI −1.49% to 1.09%; very low‐certainty evidence; Analysis 4.5).
4.5. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 5: Blood oxygen levels (%)
8. Nutritional status as assessed by growth, weight and body composition
Short term
On admission, all participants in Bain 1988 had height and weight measured, but these data were not used for comparison of treatments, or to measure growth during the study.
Medium term
Steen 1991 included daily diaries that reflected change in appetite, but provided no data or narrative findings. Shwachman score, which would have included nutritional domain questions, was assessed but again no results were reported. Investigators reported change in growth and found no difference between any of the five treatment groups, but they provided no data.
Long term
Reisman 1988 measured height and weight in all participants to calculate % predicted results and define participant characteristics, but data were not used for comparison of treatments, or to measure growth during the study.
11. Mucus wet or dry weight
Short term
Bain 1988 collected sputum after the first physiotherapy treatment on day one and the last treatment on day 15. It was weighed, and the volume measured; in addition, the colour and consistency was noted and graded on a scale of one to five. For colour, one was light yellow and five was dark green or brown; for consistency one was thin and five was thick and tenacious (Bain 1988). Analysis of the sputum collected showed an improvement from baseline in both treatments for the total cohort, but there was no difference in a comparison of the change from baseline values between CCPT and the directed coughing group.
Medium term
Steen 1991 reported no difference in sputum weight between any of the treatment groups.
Long term
Reisman 1988 monitored sputum production at each clinic visit by asking participants to collect all their sputum for the 24 hours before their clinic visit. There were no data provided and no further narrative findings on these outcomes included in the publication. Sputum production (up to 5 mL/day, 5 mL/day and over) was used in analysis as a possible risk factor for decline in FEV1, but this was not significant.
Additional outcomes that have arisen from the review
Steen 1991 collected daily diary data on cough, sputum characteristics, wheeze and shortness of breath, but provided no data or narrative findings on these outcomes.
Clinical score
Medium term
Steen 1991 reported calculating Shwachman clinical scores, which were not different between groups, but did not provide data that could be analysed.
Long term
At each clinic visit Reisman 1988 calculated a Shwachman clinical score, incorporating the most recent X‐ray score. Changes in mean Shwachman score (units per year) were worse after FET (MD 3.90, 95% CI 1.52 to 6.28; Analysis 4.6). This was not consistent with the publication which reported no between‐group differences.
4.6. Analysis.

Comparison 4: Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET), Outcome 6: Shwachman score
Comparison 4: conventional chest physiotherapy versus oscillating positive expiratory pressure devices
O‐PEP devices included those that produced oscillatory PEP effects within the airways (10 Hz to 30 Hz) while breathing through the device, such as the Acapella, Aerobika, Flutter device, RC‐cornet and IPV. There were six studies comparing CCPT with O‐PEP devices in this review (259 participants, 237 analysed); four studies included the Flutter device as a comparator (225 participants, 207 analysed) (Giles 1996; Gondor 1999; Homnick 1998; Sontag 2010), and two studies compared CCPT to IPV (34 participants, 30 analysed) (Hare 2002; Homnick 1995). See Table 4.
Primary outcomes
1. Pulmonary function tests
a. Forced expiratory volume in one second
Short term
There were three short‐term studies (59 participants, 56 analysed) comparing CCPT with O‐PEP devices; two studies included Flutter device (45 participants, 42 analysed) (Gondor 1999; Homnick 1998) and one IPV (14 participants, 14 analysed) (Hare 2002). Two were quasi‐randomised studies, using alternate assignment (Hare 2002; Homnick 1998), while the third study used an RCT design (Gondor 1999).
Homnick 1998 provided data for our analysis of FEV1 % predicted. There were no differences between treatment groups (MD 0.70, 95% CI −6.85 to 8.25; P = 0.86; very low‐certainty evidence; Analysis 5.1). The three published manuscripts relating to this study concluded that CCPT was as effective as IPV or the Flutter device during hospital admissions on the basis that there were no differences in FEV1 improvements between the groups. Investigators added that although the Flutter device appeared to be useful for independent, cost‐effective and safe administration of CCPT in their pilot study, a much larger clinical trial would be necessary to make definitive conclusions (Homnick 1998). Hare 2002 concluded that the mean change in FEV1 from admission to discharge was not different between groups, but provided no data. Gondor 1999 stated that both the CCPT and Flutter device groups showed improvement in FEV1 % predicted over the two‐week treatment period with a greater improvement in the Flutter device group.
5.1. Analysis.

Comparison 5: Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices, Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
Medium term
Two medium‐term studies (34 participants, 30 analysed) reported data for FEV1 % predicted; Giles 1996 compared CCPT with the Flutter device over four weeks (14 participants, 14 analysed) and Homnick 1995 compared CCPT to IPV over 20 weeks (20 participants, 16 analysed). Only Homnick 1995 reported data that could be analysed, showing no difference between treatment groups (MD 1.12, 95% CI ‐12.40 to 14.64; P = 0.87; very low‐certainty evidence; Analysis 5.1). The cross‐over study from Giles 1996 presented data for FEV1 but not in a format that could be analysed. The mean score for the whole cohort at baseline was 78 (SD 5) % predicted, after the Flutter device 84 (SD 7) % predicted, after the washout period 79 (SD 6) % predicted and after CCPT 82 (SD 6) % predicted. Both published manuscripts concluded that CCPT was as effective as the Flutter device or IPV on the basis that there were no differences in FEV1 changes between the groups. Homnick 1995 also concluded that although IPV appeared to be an acceptable alternative to standard aerosol and CCPT in people with CF, the optimum frequency of oscillatory pulses, inspiratory to expiratory ratio of oscillations, and optimal peak pressures needed to be determined and larger and longer‐term studies comparing IPV to other therapies or combining it with other methods such as FET techniques or mucolytic agents would be necessary to further define its usefulness.
Long term
One long‐term study compared CCPT with the Flutter device and although it was terminated early, investigators reported interim analyses at the termination of the study, by which time participants had participated for at least 1.3 years and at most 2.8 years (166 participants, 155 analysed) (Sontag 2010). Investigators concluded that there were no differences between CCPT and Flutter device in the rate of decline for FEV1, and that the parallel rates of decline suggest regular ACT may be more important than differences in the therapies themselves.
b. Forced vital capacity
Short term
Three short‐term studies (59 participants, 56 analysed) reported FVC, two comparing CCPT with the Flutter device (45 participants, 42 analysed) (Gondor 1999; Homnick 1998) and one comparing CCPT to IPV (14 participants, 14 analysed) (Hare 2002). Only Homnick 1998 reported analysable data, which showed no difference between treatment groups, although there was a tendency towards FVC differences being higher in the CCPT group (MD 11.30, 95% CI −1.54 to 24.14; P = 0.08; very low‐certainty evidence; Analysis 5.2). The three published manuscripts relating to this study concluded that CCPT was as effective as IPV or the Flutter device during hospital admissions on the basis that there were no differences between the groups in changes in FVC. Investigators added that although the Flutter device appeared to be useful for independent, cost‐effective and safe administration of CCPT in their pilot study, a much larger clinical trial would be necessary to make definitive conclusions (Homnick 1998). Hare 2002 reported that the mean change in FVC from admission to discharge was not different between groups, but provided no data. Gondor 1999 stated that both the CCPT and Flutter device groups showed significant improvement in FVC % predicted over the two‐week treatment period with a greater improvement in the Flutter device group.
5.2. Analysis.

Comparison 5: Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices, Outcome 2: Forced vital capacity (FVC) % predicted
Medium term
Two medium‐term studies (34 participants, 30 analysed) reported data for FVC after comparing CCPT with the Flutter device over four weeks (14 participants, 14 analysed) (Giles 1996) and IPV over 20 weeks (20 participants, 16 analysed) (Homnick 1995). Homnick 1995 provided analysable data that showed no difference between treatment groups (MD 2.00, 95% CI −9.31 to 13.31; P = 0.73; Analysis 5.2). The cross‐over study from Giles 1996 presented data for FVC but not in a format that could be analysed. The mean score for the whole cohort at baseline was 89 (SD 5) % predicted, after the Flutter device 95 (SD 6) % predicted, after the washout period 89 (SD 4) % predicted and after CCPT 92 (SD 4) % predicted. Both published manuscripts concluded that CCPT was as effective as Flutter device or IPV on the basis that there were no differences in FVC changes between the groups. Investigators concluded that although IPV appeared to be an acceptable alternative to standard aerosol and CCPT, longer‐term studies would be necessary to further define its usefulness (Homnick 1995).
Long term
Sontag 2010, comparing CCPT with the Flutter device (166 participants, 155 analysed), reported FVC analyses at the termination of study concluding that there were no differences between groups in the rate of decline for FVC.
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Short term
Three short‐term studies (59 participants, 56 analysed) reported this outcome; two comparing CCPT with the Flutter device (45 participants, 42 analysed) (Gondor 1999; Homnick 1998) and one with IPV (14 participants, 14 analysed) (Hare 2002). Homnick 1998 reported analysable data that showed no difference between treatment groups (MD 3.20, 95% CI −7.23 to 13.63; P = 0.55; very low‐certainty evidence; Analysis 5.3). The three published manuscripts relating to this study concluded that CCPT was as effective as IPV or Flutter device during hospital admissions on the basis that there were no differences in FEF25–75 improvements between the groups. Investigators added that although the Flutter device appeared to be useful for independent, cost‐effective and safe administration of CCPT in their pilot study, a much larger clinical trial would be necessary to make definitive conclusions (Homnick 1998). Hare 2002 reported that the mean change in FEF25–75 from admission to discharge was not different between groups, but provided no data. Gondor 1999 stated that both the CPT and Flutter device groups showed significant improvement in FEF25–75 over the two‐week treatment period with a greater improvement in the Flutter device group.
5.3. Analysis.

Comparison 5: Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices, Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
Medium term
Only one medium‐term study reported data for FEF25–75 after comparing CCPT with IPV over 20 weeks (20 participants, 16 analysed) (Homnick 1995). Data showed no difference between treatment groups (MD −3.62, 95% CI −20.18 to 12.94; P = 0.67; very low‐certainty evidence; Analysis 5.3). This concurred with the published manuscript, which concluded that CCPT was as effective as IPV over 20 weeks on the basis that there were no differences in FEF25–75 changes between groups. Investigators concluded that although IPV appeared to be an acceptable alternative to standard aerosol and CCPT, longer‐term studies would be necessary to further define its usefulness (Homnick 1995).
Long term
Sontag 2010 comparing CCPT with the Flutter device (166 participants, 155 analysed) reported FEF25–75 analyses at the termination of the study and concluded that there were no significant differences between CCPT and the Flutter device in the rate of decline for FEF25–75. This was different to the annual rate of decline for FEF25–75 in the third arm of this study (HFCC), which was greater than either CCPT or the Flutter device. Investigators concluded that HFCC may be associated with worse flow at low lung volumes, and different physiological effects in the small airways in people with CF compared to CCPT.
2. Number of respiratory exacerbations
Respiratory exacerbations were defined by 'time to first respiratory exacerbation' or any single or combined reports of number of days in hospital, admissions to hospital, IV antibiotic courses or IV antibiotic days per year.
a. Number of days in hospital for respiratory exacerbations
Short term
All three‐short term studies comparing CCPT with O‐PEP devices reported length of hospital stay during a single admission for acute exacerbation (Gondor 1999; Hare 2002; Homnick 1998), but only two provided data (45 participants, 42 analysed) (Gondor 1999; Homnick 1998). Both studies reported that mean days of hospitalisation for the two groups were similar; in Gondor 1999, these were 16.6 (SD 6.8) days in the CCPT group and 17.9 (SD 5.1) days in the Flutter device group and, in Homnick 1998, these were 8.8 (SD 2.4) days in the CCPT group and 8.9 (SD 2.5) days in the Flutter device group. The combined data were consistent with the individual findings (MD −0.22 days, 95% CI −1.81 to 1.36; P = 0.78; very low‐certainty evidence; Analysis 5.4). Hare 2002 simply reported that length of hospitalisation was not different between groups, but provided no data.
5.4. Analysis.

Comparison 5: Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices, Outcome 4: Number of days in hospital
Medium term
Homnick 1995 (16 participants) reported days of hospitalisation during the study period. Investigators found no difference in the mean number of hospital days per participant during the study period (20 weeks) when comparing CCPT with IPV (5.6 (SD 6.1) days with CCPT versus 3.9 (SD 4.5) days with IPV; P = 0.55). When data were combined there was no difference between groups (MD 1.70 days, 95% CI −3.55 to 6.95; P = 0.53; very low‐certainty evidence; Analysis 5.4).
b. Number of admissions to hospital per year for respiratory exacerbations
Medium term
Homnick 1995 (16 participants) reported in the abstract that there was no difference in the number of hospitalisations during the study period (20 weeks) when comparing CCPT with IPV, but provided no further data in the manuscript.
d. Number of intravenous antibiotics days for respiratory exacerbations
Medium term
Homnick 1995 (16 participants) found no difference in the number of oral or IV antibiotics administered to the CCPT or IPV groups over the 20‐week study. Participants in the CCPT group experienced a mean of 33.3 (SD 25.3) days of oral antibiotic use compared to 46.3 (SD 25.9) days in the IPV group (P = 0.33). Similarly, the mean days of IV antibiotic use were 14.3 (SD 15.7) days for the CCPT group and 15.9 (SD 14.4) days for the IPV group (P = 0.83). Analysis showed no difference in the number of days of IV antibiotics between groups (MD −1.60 days, 95% CI −16.36 to 13.16; P = 0.83; very low‐certainty evidence; Analysis 5.5).
5.5. Analysis.

Comparison 5: Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices, Outcome 5: Number of days of intravenous antibiotics for respiratory exacerbations
e. Time to first respiratory exacerbations
Long term
Sontag 2010 compared CCPT with the Flutter device and found that the risk of developing a pulmonary exacerbation, using the time to first treatment with IV antibiotics, demonstrated no differences between groups (P = 0.59). Data were not presented in a format that was compatible with meta‐analysis.
Secondary outcomes
1. Quality of life
Long term
Sontag 2010 administered the CFQ at selected visits. CFQ results across the 12 domains were not different at baseline between CCPT and the Flutter device; after correcting for multiple comparisons in the CFQ analyses, there were no differences in HRQoL between CCPT and the Flutter device.
2. Adherence to therapy, satisfaction and individual preference
Short term
Hare 2002 noted that participants in the IPV group were 'generally satisfied with the device' (PercussiveTech HF). It was unclear how this was assessed or analysed, and there was no mention of any attempt to assess satisfaction in the CCPT group or compare satisfaction between treatment groups. Gondor 1999 did not report this outcome but postulated that the main advantage of using the Flutter device was that it allows independence.
Medium term
Giles 1996 asked participants to fill out a treatment preference questionnaire; they did not describe the questionnaire and provided no data. Study authors concluded that the Flutter device was "preferred by the patients and their families overall" with reasons for this given as "more confidence that the lungs were kept less congested" and "convenience for caregiver." It is unclear how this was assessed or analysed, for example whether the preference was unanimous, or a simple majority.
Homnick 1995 asked eight participants in the IPV group to provide a satisfaction index to be completed after each 30‐day period of using the device. Responses were generally positive and participants on IPV stated that they would continue to use the IPV if given the opportunity. However, there was no mention of any attempt to assess satisfaction in the CCPT group or compare satisfaction between treatment groups.
Long term
Participants in Sontag 2010 withdrew at higher rates than expected. A total of 15 participants withdrew within 60 days of randomisation, 11 on the day of randomisation. The withdrawal rate was significantly higher in CCPT than in the Flutter device group, and was higher in older age groups. Satisfaction with the therapy was an independent predictor of withdrawing.
Investigators administered a validated TSS evaluating each of four factors (effectiveness, convenience, comfort and overall satisfaction) (Sontag 2010). The mean scores were not different at baseline, reflecting satisfaction with ACT prior to randomisation. The last TSS score recorded for each participant showed that satisfaction was significantly lower in CCPT than Flutter device groups across all factors except effectiveness (Table 12). Differences in TSS persisted across age groups. Scores at the last TSS indicated that individuals with lower TSS were more likely to withdraw (OR 0.44, 95% CI 0.34 to 0.73). Study authors suggested these findings might mean that individuals dissatisfied with an ACT may be more likely to discontinue treatment entirely compared to those who are satisfied with their ACT.
6. Conventional chest physiotherapy (CCPT) versus Flutter device: treatment satisfaction scores – Sontag 2010.
| Domain | CCPT mean (SD) | Flutter device mean (SD) |
| Effectiveness (5–25) |
16.3 (0.8) | 17.9 (0.8) |
| Convenience (5–25) |
12.1 (0.9) | 22.5 (0.4) |
| Discomfort (5–25) |
18.7 (0.6) | 22.3 (0.3) |
| Overall satisfaction (2–10) |
5.1 (0.3) | 7.9 (0.4) |
CCPT: conventional chest physiotherapy; SD: standard deviation.
Sontag 2010 measured adherence using the daily telephone diary in 131 participants. Mean adherence was never greater than 65%, with a mean of 50%, which, according to the study authors was consistent with the broader adherence literature and provides insight into real‐life practise patterns in CF. Both therapies showed an improvement from baseline to the first and fifth visit postrandomisation, but there was no difference between CCPT and flutter (Table 13).
7. Conventional chest physiotherapy (CCPT) versus Flutter device: change in adherence score from baseline – Sontag 2010.
| Visit | CCPT | Flutter device | HFCWO | P value | |||
| Mean change (IQR) | n | Mean change (IQR) | n | Mean change (IQR) | n | ||
| 1 | 16% (0% to 38%) | 42 | 16% (0% to 38%) | 42 | 19% (0% to 50%) | 55 | 0.56 |
| 5 | 6% (−25% to 38%) | 23 | 16% (−19% to 38%) | 30 | 25% (0 to 56%) | 47 | 0.09 |
CCPT: conventional chest physiotherapy; HFCWO: high‐frequency chest wall oscillation; IQR: interquartile range; n: number. P values describe differences between all 3 therapies.
There were no differences in adherence rates in participants who withdrew from the study early and those who did not withdraw, but individuals who withdrew from the study were not satisfied with their assigned therapy. Adherence was one of the factors tested as a covariate in the FEV1% predicted decline model, but this was not significant.
3. Cost–benefit analysis of intervention
Short term
Homnick 1998 reported this outcome and found that CCPT therapy was labour‐intensive in the hospital setting, requiring one‐to‐one attendance by trained RTs and entailing significant costs. The mean cost per hour for an RT salary and benefits was reported at about USD 18.50, thus the mean cost savings of one therapist administering 30 minutes of CCPT compared to supervising 15 minutes of Flutter device therapy would be 50% over the duration of hospitalisation. Further, if one therapist administered CCPT individually to three people with CF and only one therapist was required to supervise three people with CF administering their own Flutter device therapy simultaneously four times per day, the respiratory care cost savings over a 10‐day hospitalisation would approach 85%:
RT salary CCPT (for three people): 30 minutes' treatment at USD 9.50 multiplied by three (for each person) multiplied by four CCPT sessions per day for 10 days = USD 1140.00;
RT salary Flutter device (for three people): 15 minutes' treatment at USD 4.25 split between three people (performing Flutter device simultaneously and supervised by one RT) multiplied by four Flutter device sessions per day for 10 days = USD 170.00.
4. Objective change in exercise capacity
Short term
Gondor 1999 reported this outcome when 18 participants performed the 6MWT on admission, then again on days 7 and 14 of hospitalisation (two participants refused to walk). All participants were able to walk continuously for each test and the mean walk distance improved significantly and similarly over the two‐week period for both groups; CCPT group improved from 428 (SD 106) m to 481 (SD 73) m and the Flutter device group improved from 403 (SD 88) m to 461 (SD 105) m. However, there were no differences between groups for the increases in walk distance.
5. Additional lung function tests
Short term
Hare 2002 measured RV and concluded that mean improvements in RV from admission to discharge were not different between groups, but provided no data.
Additional lung function tests recorded and analysed by Homnick 1998 included, FEV1/FVC ratio, TLC, RV and RV/TLC ratio from admission to discharge showed significant improvements with CCPT and Flutter device in all parameters studied except TLC for Flutter device and TLC and FEV1/FVC ratio for CCPT. However, there were no differences between groups (Table 14).
8. Conventional chest physiotherapy (CCPT) versus Flutter device: mean % change from baseline in additional lung function outcomes – Homnick 1998.
| Measurement | CCPT | P value | Flutter device | P value |
| FEV1/FVC (%) | 4.7 | 0.1396 | 12 | 0.0045 |
| TLC (L) | 2.9 | 0.1916 | 2.4 | 0.2602 |
| RV (L) | −15.3 | 0.0099 | −18.2 | 0.0007 |
| RV/TLC (%) | −18.4 | 0.0004 | −20.7 | 0.0001 |
CCPT: conventional chest physiotherapy; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; RV: residual volume; SD: standard deviation; TLC: total lung capacity.
7. Blood oxygen levels
Short term
Gondor 1999 measured oxygen saturation of arterial blood (SaO2) prior to pulmonary function testing, while participants were breathing room air. Both treatment groups showed improvement in resting SaO2 by the end of the two‐week treatment period, but there were no differences between groups. The paper only provided data for the baseline assessments.
8. Nutritional status as assessed by growth, weight and body composition
Short term
Gondor 1999 measured height and weight, but provided only baseline data and used these primarily to calculate and express pulmonary function data in % of predicted values rather than for comparison between groups.
Medium term
Homnick 1995 noted that BMI was higher in the IPV group than in the CCPT group at baseline, but there were no changes in weight and BMI in either group between 30 and 180 days.
Long term
Sontag 2010 tested BMI as a covariate in the FEV1 and FVC % predicted decline models, and BMI percentile at baseline was the only significant covariate (P < 0.01) in the models, reflecting that individuals with higher BMI percentiles had higher FEV1 and FVC % predicted. Study authors also described an association of BMI percentile and age with time to exacerbation but did not report any specific results.
11. Mucus wet or dry weight
Medium term
Giles 1996 (cross‐over study) collected sputum at the last treatment of each intervention period and compared wet and dry (lyophilised) sputum weights. There was no difference in the mean amount of sputum recovered with CCPT (6.5 (SD 4.8) g wet and 1.2 (SD 0.7) g dry) compared to the Flutter device (7.5 (SD 4.8) g wet and 1.3 (SD 0.5) g dry).
Additional outcomes that have arisen from the review
In terms of additional pulmonary therapies, Sontag 2010 found no differences in rates of new prescriptions for dornase alfa (8/47 (17%) with CCPT versus 9/47 (19%) with Flutter device; P = 0.90) or tobramycin solution for inhalation (21/47 (45%) with CCPT versus 27/47 (57%) with Flutter device; P = 0.46).
Adverse events
Short term
Two studies monitored participants for complications, including haemoptysis, hypoxaemia and pneumothorax, but there were no complications in either study (Hare 2002; Homnick 1998).
Medium term
Homnick 1995 asked participants about adverse effects every 30 days during the study. One nine‐year‐old boy experienced acute, minor haemoptysis during the fourth week of using the IPV device; this was accompanied by the usual signs of an acute exacerbation of P aeruginosa bronchitis, and he was treated with IV antibiotics. The IPV treatments were stopped for 10 days until all gross haemoptysis had resolved when the boy restarted IPV therapy and completed the study without additional haemoptysis. There were no episodes of acute pulmonary air leak or other complications during the study.
Clinical score
Short term
Hare 2002 determined clinical scores at admission and discharge; there was no difference in Shwachman score at baseline or discharge between groups, but they provided no data. Homnick 1998 determined Shwachman scores and modified Case‐Western Clinical scores at the time of hospital admission and at discharge. There were significant improvements from admission to discharge in clinical score in both groups but no difference in change in clinical score between CCPT and Flutter device groups.
Medium term
Homnick 1995 measured Shwachman clinical score as part of the randomisation procedure, but did not provide data on changes after treatment.
Sputum culture
Short term
Gondor 1999 measured prevalent organisms colonising the respiratory tracts of participants, but used these to characterise populations rather than compare groups.
Long term
Sontag 2010 tested P aeruginosa colonisation as a covariate in the FEV1% predicted decline model, but did not use data for the comparison of groups.
Comparison 5: conventional chest physiotherapy versus autogenic drainage
Two randomised cross‐over studies (54 participants, 47 analysed) compared CCPT with AD (McIlwaine 1991; McIlwaine 2010). McIlwaine 1991 (18 participants, 14 analysed) compared three interventions (CCPT, AD and PEP) with each intervention lasting two months; we presented the CCPT and AD data in this comparison. McIlwaine 2010 was intended to run over two years, but was terminated after 12 months (36 participants, 33 analysed). See Table 5.
Primary outcomes
1. Pulmonary function tests
When combining both studies there were no overall differences between CCPT or AD in terms of FEV1 (MD 1.81, 95% CI −2.52 to 6.14; very low‐certainty evidence; Analysis 1.1), FVC (MD 0.39, 95% CI −3.62 to 4.40; very low‐certainty evidence; Analysis 1.2), or FEF25–75 (MD 2.23, 95% CI −8.96 to 13.42; very low‐certainty evidence; Analysis 1.3).
a. Forced expiratory volume in one second
Medium term
None of the three abstracts published for McIlwaine 1991 presented any data for this outcome. The study authors provided original data for FEV1 % predicted that were included in the meta‐analysis; there was no difference between interventions at eight weeks (MD 1.29, 95% CI ‐4.07 to 6.65; P = 0.64; very low‐certainty evidence; Analysis 6.1). The published narrative findings concurred with those in the review's meta‐analysis.
6.1. Analysis.

Comparison 6: Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD), Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
Long term
McIlwaine 2010 reported data for FEV1 % predicted at the early termination of the study (at the cross‐over point at 12 months), and found no difference between interventions. The review's analysis also found no difference between interventions (MD 2.79, 95% CI −4.54 to 10.12; P = 0.46; very low‐certainty evidence; Analysis 6.1).
b. Forced vital capacity
Medium term
None of the three abstracts published for McIlwaine 1991 presented any data for this outcome. The study authors provided original data for FVC % predicted that were included in the meta‐analysis; there was no difference between interventions at eight weeks (MD 1.36, 95% CI −4.00 to 6.72; P = 0.62; very low‐certainty evidence; Analysis 6.2). The published narrative findings concurred with those in the meta‐analysis.
6.2. Analysis.

Comparison 6: Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD), Outcome 2: Forced vital capacity (FVC) % predicted
Long term
McIlwaine 2010 reported data for FVC % predicted at the early termination of the study (at the cross‐over point at 12 months). We found no difference between interventions (MD −0.84, 95% CI −6.88 to 5.20; P = 0.79; very low‐certainty evidence; Analysis 6.2).
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Medium term
None of the three abstracts published for McIlwaine 1991 presented any data for this outcome. The study authors provided original data for FEF25–75 data that were included in the meta‐analysis; there was no difference between interventions at eight weeks (MD −2.13, 95% CI −7.49 to 3.23; P = 0.44; very low‐certainty evidence; Analysis 6.3). The published narrative findings concurred with those in the meta‐analysis.
6.3. Analysis.

Comparison 6: Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD), Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
Long term
McIlwaine 2010 reported data for FEF25–75 at the early termination of the study (at the cross‐over point at 12 months). We found no difference between interventions (MD 9.71, 95% CI −3.35 to 22.77; P = 0.15; very low‐certainty evidence; Analysis 6.3).
2. Number of respiratory exacerbations
Respiratory exacerbations were defined by 'time to first respiratory exacerbation' or any single or combined reports of number of: days in hospital, admissions to hospital, IV antibiotic courses or IV antibiotic days. Only the long‐term study reported number of hospitalisations and number of hospital days (McIlwaine 2010).
a. Number of days in hospital for respiratory exacerbations
Long term
McIlwaine 2010 indicated that duration of hospitalisations were recorded throughout the study, but did not report these data.
b. Number of admissions to hospital per year for respiratory exacerbations
Long term
McIlwaine 2010 (36 participants, 33 analysed) reported that the mean number of hospital admissions between groups during the study were not different (1.00 (SD 0.32) with CCPT versus 0.76 (SD 0.18) with AD). This was not consistent with the meta‐analysis, which found that there were fewer admissions in the AD group (MD 0.24, 95% CI 0.06 to 0.42; P = 0.008; very low‐certainty evidence; Analysis 6.4). In 12 months of the study, there were 16 hospitalisations (amongst 16 participants) for pulmonary exacerbations in CCPT, compared to 13 hospitalisations (amongst 17 participants) for pulmonary exacerbations in the AD group.
6.4. Analysis.

Comparison 6: Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD), Outcome 4: Number hospital admissions
We also analysed the proportion of admissions per number of participants in each group, but found no difference between groups (RR 1.29, 95% CI 0.98 to 1.71; P = 0.07; very low‐certainty evidence; Analysis 6.5).
6.5. Analysis.

Comparison 6: Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD), Outcome 5: Proportion of admissions per number of participants
Secondary outcomes
2. Adherence to therapy, satisfaction and individual preference
Medium term
McIlwaine 1991 did not formally assess any of these outcomes. All three published abstracts concluded that AD could be "done independently with less discomfort" than CCPT and participants "reported a greater sense of control with less interruption in their daily life using AD." There was no indication in any of the abstracts whether these conclusions were reached via ad hoc conversations with a subset of participants or via a rigorous survey methodology involving all participants; it is also unclear how they analysed or interpreted data. Study authors reported that participants with "hyperreactive airways consistently responding best to AD," although it is unclear how they assessed hyper‐reactive airways or how they analysed or interpreted responses.
Long term
McIlwaine 2010 closely monitored adherence with therapy throughout the long‐term study. Participants kept daily diaries recording the number of treatments performed, time length of treatments, activity level and sputum production. The physiotherapist telephoned each participant monthly to check adherence, health status and to answer any concerns.
One participant withdrew from the study due to non‐compliance (treatment group not stated). During the second year, only 7/17 participants who had completed the first year of AD agreed to return to CCPT for the second year. The remaining 10 participants withdrew from the study, expressing a strong preference to continue performing AD. In the seven who agreed to return to CCPT, there was a cross‐over effect, with participants incorporating the AD breathing technique into CCPT (number not provided). Therefore, only results from the first year of the study were analysed and reported. Investigators wrote there was a "massive stated preference for AD by all patients in the study" with many participants refusing to go back to performing CCPT. Participants subjectively felt it "worked the best" and they reported an increased expectoration with AD. In addition, AD also gave them more independence in performing their treatment, and a greater amount of freedom since it could be performed anywhere. There was no indication in the publications how the study authors obtained these preferences, for example whether these conclusions were from ad hoc conversations with some participants or a survey method involving all.
There was a discrepancy in the data from earlier published abstracts of this study (Included studies), with both the 1992 abstracts by Davidson and McIlwaine indicating that 10 participants (not seven) agreed to return to CCPT for the second year (8/18 participants using AD refused to revert to CCPT). They also specified that five participants who had agreed to return to CCPT were using AD to augment their treatments.
4. Objective change in exercise capacity
Long term
Participants kept daily diaries recording, amongst other things, activity level; however, there were no data or narrative findings in the publication (McIlwaine 2010).
5. Additional lung function tests
Medium term
McIlwaine 1991 reported no significant difference in FEV1/FVC between groups, but data were not included in any of the three published abstracts.
11. Mucus wet or dry weight
Medium term
McIlwaine 1991 did not publish data on this outcome, but in one abstract reported that in comparison to CCPT, 12 participants produced more sputum with AD and three produced more with PEP. In another abstract they reported that AD "showed a statistically significant (P < 0.01) greater ability to mobilise sputum" compared to CCPT. In the third abstract authors reported "the net weight of sputum obtained was significantly greater with AD than with PEP or CCPT (P < 0.01)." It is unclear how they collected, analysed or interpreted these data.
Long term
Participants kept daily diaries recording, amongst other things, sputum production; however, McIlwaine 2010 provided no data or narrative findings.
Additional outcomes that have arisen from the review
Clinical score
Medium term
Only one of the three abstracts related to McIlwaine 1991 mentioned clinical scores, noting that there was no difference between groups, but they did not name the score or provide data.
Long term
On entering the study and at three‐monthly intervals, each participant had a full clinical assessment, including Shwachman and Huang scores (McIlwaine 2010). There was no difference in Shwachman score between groups in the mean change from baseline (7.53 (SD 9.01) with CCPT versus 3.12 (SD 6.24) with AD); whereas the Huang score was improved in the AD group as compared to the CCPT group (−0.88 (SD 4.86) with CCPT versus 2.32 (SD 4.31) with AD; P = 0.04).
Sputum culture
Long term
McIlwaine 2010 asked participants at each clinic visit to provide a sputum specimen for bacteriological culture, but they provided no data or narrative findings.
Comparison 6: conventional chest physiotherapy versus exercise
There were no medium‐term or long‐term studies comparing CCPT with exercise in this review. There was one short‐term RCT with 17 participants, conducted over a two‐week period of hospitalisation, which compared CCPT alone (three sessions day) with exercise combined with CCPT (two exercise sessions and one CCPT session per day) (Cerny 1989). See Table 6.
Primary outcomes
1. Pulmonary function tests
The publication resulting from this study reported that while both groups improved during the hospital admission, there were no differences between groups (Cerny 1989), indicating that the extent of the changes in all tests was the same for both groups. There were also no differences in pulmonary function score between the CCPT and exercise groups at admission or discharge. Cerny 1989 presented data graphically. However, our analysis of the original data provided by the study author showed more improvement in respiratory function in the CCPT group than the exercise group for all pulmonary function outcomes analysed.
The inconsistency in findings between the publication and our meta‐analysis should be interpreted with acknowledgement of differences in baseline respiratory function values between groups in this study, with those in the CCPT group having lower admission values for FEV1 and FEF25–75 values (P < 0.05). There was no attempt to match participants for disease severity in the study and this may have influenced the magnitude of improvement during a two‐week admission for acute exacerbation.
a. Forced expiratory volume in one second
Short term
When we analysed the original data provided by the study authors there was a higher FEV1 % predicted in the CCPT group compared to the exercise group (MD 7.05, 95% CI 3.15 to 10.95; P < 0.001; very low‐certainty evidence; Analysis 7.1). This is in contrast to findings in the publication, which reported no differences between groups in FEV1 % predicted.
7.1. Analysis.

Comparison 7: Conventional chest physiotherapy (CCPT) versus exercise, Outcome 1: Forced expiratory volume in 1 second (FEV 1) % predicted
b. Forced vital capacity
Short term
When we analysed the original data provided by the study authors there was a higher FVC % predicted in the CCPT group (MD 7.83, 95% CI 2.48 to 13.18; P = 0.004; very low‐certainty evidence; Analysis 7.2). This is in contrast to findings in the publication, which reported no differences between groups in FVC % predicted.
7.2. Analysis.

Comparison 7: Conventional chest physiotherapy (CCPT) versus exercise, Outcome 2: Forced vital capacity (FVC) % predicted
c. Average forced expiratory flow between 25% and 75% of forced vital capacity
Short term
When we analysed the original data provided by the study authors there was a higher FEF25–75 % predicted in the CCPT group (MD 4.74, 95% CI 1.94 to 7.54; P = 0.0009; very low‐certainty evidence; Analysis 7.3). This is in contrast to findings in the publication, which reported no differences between groups in FEF25–75 % predicted.
7.3. Analysis.

Comparison 7: Conventional chest physiotherapy (CCPT) versus exercise, Outcome 3: Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted
2. Number of respiratory exacerbations
Respiratory exacerbations were defined by time to first respiratory exacerbation or any single or combined reports of number of days in hospital, admissions to hospital, IV antibiotic courses or IV antibiotic days per year.
a. Number of days in hospital for respiratory exacerbations
Short term
The short‐term study comparing CCPT with exercise reported length of hospital stay during a single admission for acute exacerbation, and the publication concluded that this was similar between groups (Cerny 1989). The mean (SD) duration of the hospitalisation was 13 (2.6) days for the nine people in the CCPT group and 13 (3) days for the eight people in exercise group; when analysed in RevMan this showed no difference between groups (MD 0.00, 95% CI −2.68 to 2.68; P = 1.0; very low‐certainty evidence; Analysis 7.4).
7.4. Analysis.

Comparison 7: Conventional chest physiotherapy (CCPT) versus exercise, Outcome 4: Number of days in hospital
Secondary outcomes
2. Adherence to therapy, satisfaction and individual preference
Short term
While the study investigators did not report specifically on this outcome, they did note that all CCPT treatments were completed as required for the study, and 96% of the scheduled exercise therapy sessions were completed (Cerny 1989).
4. Objective change in exercise capacity
Short term
Participants performed the exercise test (protocol name not specified) on a cycle ergometer. The initial load of 0.3 W/kg was increased by 0.3 W/kg every two minutes and exercise stopped when participants could no longer continue despite encouragement or when SaO2 had decreased by more than 15% or to less than 75% of the baseline level. Investigators monitored SaO2, electrocardiographic activity and heart rate continuously. There were no changes in peak load and peak heart rate from admission to discharge between groups (Cerny 1989).
5. Additional lung function tests
Short term
Participants performed spirometry each day immediately before the morning treatment, 15 minutes after the morning treatment and every hour for five hours after the morning treatment. There may have been a slight improvement in pulmonary functions 15 minutes post‐treatment, which lasted over the five‐hour follow‐up period, after 85% of the treatment sessions (Cerny 1989).
Data on additional lung function tests could not be extracted from the publication and study authors did not provide original data. There were no differences in pulmonary function scores between the exercise and CCPT groups at admission or discharge.
7. Blood oxygen levels
Short term
Investigators estimated SaO2 % with an ear oximeter, but provided no data (Cerny 1989).
11. Mucus wet or dry weight
Short term
Cerny 1989 determined daily sputum volume expectorated by measuring and summing the amounts of sputum accumulated during the following time intervals: from the time of awakening (7 a.m. to 8 a.m.) to the beginning of treatment, during and for one‐half hours following treatment, from 9:30 a.m. to 11:30 a.m., from 11:30 a.m. to 2:30 p.m., from 2:30 p.m. to 8:30 p.m. and from 8:30 p.m. to wake‐up. They recorded sputum volume, wet weight and dry weight (after four days of drying in an oven) expressed in units per hour.
There were no differences in 24‐hour sputum volume and dry weight; the greatest volume was collected after the morning treatment, with no differences between the CCPT and exercise groups; they provided no data.
Additional outcomes that have arisen from the review
Both treatments induced a productive cough and an equal number of coughs (Cerny 1989).
Discussion
This review set out to determine if there was any advantage of CCPT (a technique used for more than six decades to clear pulmonary secretions) over alternative ACTs (developed to encourage independence in self‐care). Outcomes included pulmonary function, number of respiratory exacerbations, QoL, adherence, changes in exercise capacity, ventilation scanning, blood oxygen levels, nutritional status, mortality, mucus transport rate, adverse outcomes and cost–benefit analyses. We excluded studies of less than seven days' duration (including single‐treatment studies) because CF is a chronic disorder in which such studies are inadequate for describing efficacy, safety or long‐term acceptability of any interventions for this population.
Summary of main results
We included 21 studies (778 participants), but found insufficient evidence to confirm or exclude any differences between alternative ACTs and CCPT in terms of their outcome measures. Furthermore, data from the included studies did not demonstrate that any of the alternative ACTs were better than CCPT in people with CF. This may reflect insufficient data for meta‐analysis rather than equivalence between techniques, but may also suggest that CCPT is as effective as other more novel ACTs. The heterogeneity in methodologies and the outcome measures chosen by investigators and how they collected data makes it difficult to perform meta‐analyses of data from the different studies.
Overall, we found limited evidence that participants were more adherent to, preferred or were more satisfied with alternative ACTs compared to CCPT; however, the outcome measures used were predominantly self‐reported and, therefore, subjective and open to bias. In the 14 studies that included a report of individual preference, without exception these showed that individuals preferred self‐administered ACTs that facilitated independence. However, as the studies measured preference in different ways, we could not combine the individual study results in an analysis.
Studies undertaken during acute exacerbations demonstrated relatively large gains in respiratory function irrespective of ACT using standard lung function techniques. Longer‐term studies demonstrated smaller improvements or deterioration over time.
Comparison 1: conventional chest physiotherapy versus positive expiratory pressure
The most common comparison reported was CCPT versus PEP (9 studies, 191 participants, 171 analysed) of which six were medium‐term studies (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), and three were long‐term studies (Costantini 2001; Gaskin 1998; McIlwaine 1997).
All evidence was graded as very low‐certainty. There was no difference in lung function (FEV1, FVC or FEF25–75 % predicted) in the medium‐to‐long term. No study reported the number of respiratory exacerbations per year, but one long‐term study (40 participants) reported no difference in the number of hospital admissions for respiratory exacerbations per year (McIlwaine 1997). A further single study (66 participants) reported no difference between groups in QoL after two years (Gaskin 1998). Six medium‐term studies reported that participants preferred PEP as it could be performed independently and with less discomfort than CCPT (Dadparvar 1995; McIlwaine 1991; Steen 1991; Tonnesen 1982; Tyrrell 1986; van Asperen 1987), but one study found that participants reverted to CCPT during an exacerbation (van Asperen 1987). One long‐term study reported that participants preferred PEP (Costantini 2001), and a further study reported slightly better adherence to PEP than CCPT (McIlwaine 1997). Three studies reported no difference in mucus weight between groups (Steen 1991; Tyrrell 1986; van Asperen 1987), and two reported greater sputum production with PEP (McIlwaine 1991; Tonnesen 1982). Although three medium‐term studies and two long‐term studies measured the change in exercise capacity, they did not report any results (Gaskin 1998; McIlwaine 1997; Steen 1991; Tyrrell 1986; van Asperen 1987). There was limited evidence for measures of lung scanning and blood oxygen levels, with no clear differences between interventions. Three studies reported results for nutritional status and again found no difference between intervention groups at any time point (Costantini 2001; Steen 1991; Tyrrell 1986). One study reported a single death during the course of the study (Steen 1991). Two studies reported adverse events, which were rare and evenly distributed between intervention groups (Costantini 2001; McIlwaine 1997). Limited narrative reports also showed no difference between CCPT and PEP in terms of clinical score and changes in bacteriological cultures.
Comparison 2: conventional chest physiotherapy versus extrapulmonary mechanical percussion
Three studies compared CCPT with therapies involving extrapulmonary mechanical percussion (274 participants, 252 analysed); this was HFCC in two studies (Arens 1994; Sontag 2010), and MP in one study (Bauer 1994). Two studies reported short‐term results (Arens 1994; Bauer 1994), no study reported in the medium term and one study reported long‐term results (Sontag 2010).
All evidence was graded as very low certainty. The two short‐term studies reported no difference in FEV1 and FVC % predicted (Arens 1994; Bauer 1994). No lung function data were available for the long‐term study, so the results were reported narratively; there were no differences in rate of decline in FEV1 or FVC % predicted between the two groups, but the annual rate of decline in FEF25–75 was greater in those using HFCC than those using CCPT (Sontag 2010). The two short‐term studies reported fewer days in hospital due to respiratory exacerbations in the extrapulmonary MP group (Arens 1994; Bauer 1994), but there was no difference in time to first IV antibiotics between the groups in the long‐term study (Sontag 2010).
Only the long‐term study investigated the impact on QoL during the study and found no difference between the groups for any of the 12 HRQoL domain scores (Sontag 2010). There was no difference in adherence rate between the groups in any study (Arens 1994; Bauer 1994; Sontag 2010), but the long‐term study reported significantly lower treatment satisfaction scores in the CCPT group compared to the HFCC group (Sontag 2010). Only one short‐term study performed additional lung function tests and found no differences after 14 days (Arens 1994). Both short‐term studies reported SpO2, but only one provided any data and found no differences between groups after 14 days (Arens 1994). Both short‐term studies reported improvements in weight gain in the HFCC group compared to the CCPT group, but with a high degree of heterogeneity (Arens 1994; Bauer 1994). The long‐term study investigated an association of BMI percentile and age with time to exacerbation, but the study authors did not report any specific results (Sontag 2010). One short‐term study reported mucus weight and results showed that sputum production was similar between groups after 24 hours (Arens 1994). Only this short‐term study reported adverse events including haemoptysis following treatments resolved after 24 hours (one participant in the HFCC group compared to two participants in the CCPT group); and some participants in the HFCC group reported chest pain and nausea (Arens 1994). One short‐term study measured sputum cultures at baseline (Arens 1994), and one long‐term study only used FEV1 % predicted decline model as a covariate (differences were not significant) (Sontag 2010).
Comparison 3: conventional chest physiotherapy versus active cycle of breathing techniques
There were no eligible studies comparing CCPT with ACBT directly, but there were three studies that compared CCPT with specific components of both ACBT and CCPT, namely FET (Reisman 1988; Steen 1991), and directed huffing and coughing (Bain 1988). One study reported in the short‐term (Bain 1988), one in the medium term (Steen 1991), and one in the long term (Reisman 1988).
All evidence was graded as very low certainty. No study reported any difference in rate of decline between groups in FEV1, and FVC % predicted at any time point (Bain 1988; Reisman 1988; Steen 1991). One short‐term study concluded that directed coughing was as effective as CCPT measured by FEF25–75 without providing any data (Bain 1988), while the long‐term study found the annual decline in FEF25–75 was less in the CCPT with FET group compared to FET alone (Reisman 1988). Only the long‐term study reported respiratory exacerbations and found no difference between groups in the number of hospital admissions for a respiratory exacerbation or for the number of days in hospital for respiratory exacerbations (Reisman 1988). The short‐term study did not specifically report adherence to therapy; however, one participant was withdrawn from the study due to lack of compliance and a further participant due to a parent's request (Bain 1988). The measures of adherence and preference were ad hoc and inconsistent in the other studies; the long‐term study (67 participants) did report that 64 participants were consistently compliant with their therapy (Reisman 1988).
No study reported QoL. Impact on exercise capacity was reported using daily diaries; the medium‐term study did not provide any data (Steen 1991) and the long‐term study reported no differences in the level of activity as a risk factor for decline in FEV1 between groups (Reisman 1988). There were no differences in additional lung function measures in either the short‐term study (Bain 1988) or the medium‐term study (Steen 1991); the long‐term study did not report this outcome (Reisman 1988). Only the medium‐term study used lung imaging as an outcome measure and reported no difference between groups (Steen 1991). Blood oxygen levels were measured only in the short‐term study and showed no difference between groups on day 15 (Bain 1988). Nutritional status was only used to describe participant characteristics in all studies. There were no data for an analysis of mucus weight; both the short‐term and medium‐term studies narratively reported no difference between groups at the end of the study (Bain 1988; Steen 1991). Two studies used participant diaries to record adherence and change in symptoms (Reisman 1988; Steen 1991). The medium‐term study narratively reported no difference in clinical scores (Steen 1991). When we analysed the data from the long‐term study, we found the Shwachman score was higher with CCPT combined with FET than with FET alone; however, the original paper reported no difference between groups (Reisman 1988). No studies used mortality as a comparator between groups, but one study reported a death as a reason for loss to follow‐up (Steen 1991).
Comparison 4: conventional chest physiotherapy versus oscillating positive expiratory pressure devices
Six studies (259 participants, 237 analysed) compared CCPT with O‐PEP devices (such as Acapella, Aerobika, Flutter device, RC‐cornet and IPV). Of these, four studies included the Flutter device as a comparator (225 participants, 207 analysed) (Giles 1996; Gondor 1999; Homnick 1998; Sontag 2010), and two studies compared CCPT to IPV (34 participants, 30 analysed) (Hare 2002; Homnick 1995). There were three short‐term studies (Gondor 1999; Hare 2002; Homnick 1998), two medium‐term studies (Giles 1996; Homnick 1995), and one long‐term study (Sontag 2010).
All evidence was graded as very low certainty. Three short‐term studies reported lung function outcomes, but only one provided data for analysis (Homnick 1998). This study compared CCPT to the Flutter device and found no differences between groups in FEV1 % predicted, FVC % predicted and FEF25–75 (Homnick 1998). A further study compared CCPT to the Flutter device and narratively reported a greater improvement in FEV1 % predicted, FVC % predicted and FEF25–75 in the Flutter device group (Gondor 1999). The third short‐term study compared CCPT with IPV and narratively reported no difference between groups in mean change from baseline in FEV1 % predicted, FVC % predicted or FEF25–75 (Hare 2002). One medium‐term study comparing CCPT with IPV, provided data for analysis that showed no difference in rate of decline in FEV1 % predicted, FVC % predicted and FEF25–75 (Homnick 1995). The second medium‐term study narratively concluded that CCPT was as effective as the Flutter device (Giles 1996); the long‐term study reported similar conclusions (Sontag 2010). No studies reported number of exacerbations per year. All three short‐term studies reported no difference in the length of hospital stay for a respiratory exacerbation, with two providing data (45 participants, 42 analysed) (Gondor 1999; Homnick 1998), and one reporting narratively (Hare 2002). One medium‐term study reported no difference between groups in number of hospitalisations, length of hospital stay or days of IV antibiotics (Homnick 1995). The long‐term study reported no difference between groups in the time to the first course of IV antibiotics (Sontag 2010).
Only the long‐term study narratively reported QoL score (using the CFQ) and found no difference across domains between CCPT and the Flutter device (Sontag 2010). Studies used daily adherence logs, diaries or telephone diaries to measure adherence to therapy and individual preference; it should also be noted that the methods used in the studies to collect adherence information was varied and mostly subjective. One short‐term study noted that participants in the IPV group were "generally satisfied with the device," but it is unclear how this was assessed or analysed (Hare 2002). Participant satisfaction was reported to be lower in the CCPT group compared to the Flutter device group or IPV group in the medium term (Giles 1996; Homnick 1995). Investigators in the long‐term study stated that satisfaction with therapy was an independent predictor of withdrawing; participants withdrew at higher rates than expected, and the rate was significantly higher in the CCPT group compared to the Flutter device group (Sontag 2010). Only the long‐term study reported results for adherence to treatment and found no difference between intervention groups (Sontag 2010). Only one short‐term study undertook a cost–benefit analysis of the intervention reporting that CCPT therapy was labour‐intensive and entailed significant costs in a hospital setting (Homnick 1998). Only one short‐term study reported an objective change in exercise capacity (6MWT distance) and found no difference between groups (Gondor 1999). Two short‐term studies undertook additional measures of lung function; neither identified any differences between groups in any measure (Hare 2002; Homnick 1998). Only one short‐term study reported the change from baseline in blood oxygen levels and while levels in both groups improved, there were no differences between groups (Gondor 1999). One medium‐term study reported no changes in weight and BMI in either group at the end of the study (Homnick 1995). BMI was tested as a covariate in the FEV1 and FVC % predicted decline models in the long‐term study, in which study authors described an association of BMI percentile and age with time to exacerbation but did not report any specific results (Sontag 2010). Only one medium‐term study measured sputum weight and found no difference between groups, either wet or dry weight (Giles 1996). Two short‐term studies reporting on adverse events each stated no complications occurred with either therapy (Hare 2002; Homnick 1998). In one medium‐term study, one participant experienced haemoptysis, but this occurred in conjunction with the onset of an acute infection (Homnick 1995). Two short‐term studies reported clinical score with no difference between groups at end of study (Hare 2002; Homnick 1998). No study used mortality as a comparator between groups.
Comparison 5: conventional chest physiotherapy versus autogenic drainage
Two cross‐over RCTs (54 participants, 47 analysed) compared CCPT with AD (McIlwaine 1991; McIlwaine 2010). One study reported in the medium term (McIlwaine 1991), and one study provided data for the long term (McIlwaine 2010).
All evidence was graded as very low certainty. Neither study found any difference in FEV1 % predicted, FVC % predicted or FEF25–75 between groups at the end of the studies (McIlwaine 1991; McIlwaine 2010). Neither study reported number of respiratory exacerbations per year and only the long‐term study reported number of hospitalisations and number of hospital days (McIlwaine 2010). In contrast to the published paper, our analysis found fewer admissions in the AD group than the CCPT group (MD 0.24, 95% CI 0.06 to 0.42; P = 0.008; Analysis 6.4); we found no difference in the proportion of admissions per number of participants in each group (McIlwaine 2010). Investigators indicated that duration of hospitalisations were recorded during the study, but these data were not reported in the paper (McIlwaine 2010).
Neither study reported QoL (McIlwaine 1991; McIlwaine 2010). Both studies reported that participants preferred AD over CCPT; reasons for this included comfort, convenience, independence, ease of use, more control and flexibility over treatment times, and less interruption to daily living (McIlwaine 1991; McIlwaine 2010). We only reported the first phase of the long‐term study as there were a high proportion of participants allocated to AD for the first phase who either refused to switch to CCPT for the second phase or incorporated AD breathing techniques into their CCPT, which led to a cross‐over effect (McIlwaine 2010). This study also reported that participants kept daily diaries recording activity levels, but provided no data or narrative findings in the publication (McIlwaine 2010). The medium‐term study reported no differences in FEV1/FVC between groups, but did not provide any data (McIlwaine 1991). This study also narratively reported that the AD group produced more sputum than the CCPT group, but without details of how these data were collected, analysed or interpreted (McIlwaine 1991). Furthermore, this study noted that there was no difference in clinical scores between groups, but did not name the score or provide data (McIlwaine 1991). The long‐term study reported no difference between groups in change from baseline in Shwachman score, but a greater improvement in Huang score in the AD group compared to the CCPT group (McIlwaine 2010). In the long‐term study, participants were asked at each clinic visit to provide a sputum specimen for bacteriological culture, but reported no data or narrative findings. Neither study reported adverse events or mortality (McIlwaine 1991; McIlwaine 2010).
Comparison 6: conventional chest physiotherapy versus exercise
One short‐term RCT (17 participants) conducted over a two‐week period of hospitalisation compared CCPT alone (three sessions per day) with exercise combined with CCPT (two exercise sessions and one CCPT session per day) (Cerny 1989).
We did not grade the certainty of the evidence for this comparison. There were inconsistencies in the findings for pulmonary function outcomes between the original publication and the analysis we performed. While the publication stated that both groups improved during hospitalisation with no difference between the groups, our analysis of the original data provided by the study author showed a greater improvement in respiratory function in the CCPT group than the exercise group for all pulmonary function outcomes analysed (FEV1 % predicted, FVC % predicted and FEF25–75). These differences should be interpreted with acknowledgement of the lower admission values for FEV1 % predicted and FEF25–75 in the CCPT group compared to the exercise group and the fact that there was no attempt to match participants for disease severity in the study, which may have influenced the magnitude of change during the admission (Cerny 1989). The study did not report the number of respiratory exacerbations, but did report that the length of hospital stay during a single hospital admission was similar between groups. Adherence to therapy was not specifically reported, but it was noted by the authors that all CCPT sessions and 96% of the exercise sessions were completed by participants. They reported no differences in exercise capacity (measured using peak load and peak heart rate as outcomes from a cycle ergometry test) from admission to discharge between groups. Spirometry was performed on multiple occasions before and after the therapy sessions and there were no differences between groups. There were no differences between groups in 24‐hour sputum volume and dry weight; investigators stated that the greatest volume was collected after the morning treatment with no differences between the CCPT and exercise groups, but provided no data. The study did not measure the additional outcomes of lung imaging, blood oxygen levels, anthropometric measures, cost analysis and mortality.
Overall completeness and applicability of evidence
The studies in this review included mixed populations with regard to age, gender, disease severity and stability so the results have some applicability to individuals with CF. However, it should be noted that there is limited evidence for some comparisons, specifically between CCPT and exercise; the lack of evidence should not lead to the assumption of effectiveness, or lack of, for any therapy. It should also be noted that all studies included in this review were undertaken over 10 years ago, with the most recent in 2010 (McIlwaine 2010; Sontag 2010) and the oldest in 1982 (Tonnesen 1982). The developments in diagnosis and management of individuals with CF have been considerable in recent years, including the introduction of highly effective modulator therapies. Results should therefore be interpreted with caution, as generalisability to current cohorts of people living with CF may be limited.
The measurement of treatment satisfaction and QoL as well as the impact of treatment burden are seen as essential considerations for all individuals with CF. However, only two studies objectively measured the impact of the interventions on QoL (Gaskin 1998; Sontag 2010); and whilst 14/21 studies reported some measure of participant satisfaction or preference, outcomes were collected using a wide variety of methods and often lacked clarity and transparency.
The use of sputum weight or volume as an outcome measure should be interpreted with caution. Seven studies measured sputum weight or volume collected over 24 hours or less following ACT treatment. Sputum collected after a single treatment cannot be considered a reliable surrogate for long‐term airway clearance of any intervention (Giles 1996).
The inclusion of physical activity exercise as a management strategy is now seen as an essential component in the treatment of people with CF. Nine studies included some outcomes related to physical activity or exercise; however, only one short‐term study directly compared the impact of CCPT and exercise on lung function (Cerny 1989). There is a need for robust further research in this area in particular before any clear conclusions can be made.
Quality of the evidence
We assessed the certainty of the evidence for our most important chosen outcomes using GRADE (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). We assessed the certainty of the evidence for each outcome as very low. We downgraded the certainty of the evidence for imprecision (small numbers of studies and participants) and risks of bias and incomplete data reporting. We had concerns over withdrawals being biased against CCPT, as evidenced in the Sontag 2010 and McIlwaine 2010. This means that we are very uncertain about the estimate.
There was substantial heterogeneity in the meta‐analyses of lung function data (FEV1 % predicted, FVC % predicted and FEF25–75). These results may reflect differences between centres in terms of treatments, training or measurement techniques. However, even within individual centres, studies undertaken less than a decade apart, comparing the same interventions, demonstrated results that were not in agreement (McIlwaine 1991; McIlwaine 1997). Another factor that may have contributed to the heterogeneity of these results could be the small numbers per participant group seen across virtually all studies. Only one study recruited more than 70 participants (Sontag 2010), and no single study recruited more than 36 participants to each study group and the mean number was far lower. This then introduces possibility that most studies included in this review were inadequately powered to find differences between treatments if they existed and estimates of effect would be imprecise.
The absence of differences between treatments as demonstrated by the primary outcome measures chosen for this review may indicate that there are indeed no differences between treatment techniques. However, it may also indicate that the outcome measures selected for this review and for the original studies, were too insensitive to detect differences between treatments. Even in measures that appear comparable, such as lung function outcomes, some studies specified that these were undertaken under laboratory conditions according to internationally recognised standards (Arens 1994; Gondor 1999; Homnick 1998; McIlwaine 1997; McIlwaine 2010), whereas others used measures from participants performing simple spirometry at home under poorly standardised conditions (van Asperen 1987). The differences in methodological processes may have impacted on the results. It is possible that future studies may need to describe the methods used in more detail to allow greater standardisation and or consider utilising more sensitive indices of lung function such as LCI or pulmonary scanning. However, measures such as these have not yet been validated in terms of clinical importance or relevance.
It is difficult to assess the extent to which researchers' viewpoints may have influenced individual reporting of preference or satisfaction, especially since current trends tend to favour independence and self‐therapy in terms of ACTs. No studies used standardised or comparable measures of individual preference, but some studies measured this using informal methods. In the absence of any other clear objective distinctions between treatments, softer parameters such as individual preference seem to escalate in importance. This is particularly pertinent in a lifelong disease such as CF, where it is assumed that compliance with ACTs will be associated with a smaller annual decline in respiratory function. It is also assumed that the more satisfied individuals are with a treatment regimen, the more likely they will be to adhere to treatment.
There was a great sense of frustration when we could not access original data and could not retrieve them from the published manuscripts. At times, a degree of re‐analysis of original data was required, and this presented the possibility that the outcome described in the original paper would not quite be duplicated following re‐analysis for the purpose of meta‐analysis (this did not occur in the data submitted to date).
Potential biases in the review process
For the 2022 update of the review, we replaced the use of the Jadad system of scoring with the RoB 1 tool available within the Review Manager 5 and presented our findings within this framework. Validated scoring systems such as those proposed by Jadad place great value upon randomisation and double blinding (Jadad 1996). While randomisation and complete follow‐up remain important criteria in physiotherapy studies, many physiotherapy studies cannot easily incorporate blinding and are inevitably disadvantaged when measured using such scores. These studies become vulnerable to exclusion from review and the potential to recognise valuable clinical information may be missed.
Systematic reviews of physiotherapy studies face the challenge of problematic quality selection systems used for scoring individual studies. The Cochrane RoB 1 tool provides a more comprehensive method of assessing bias. However, many of the studies included in this review did not report adequate information to allow for the risk of bias to be determined resulting in over half of the studies being deemed at unclear risk of bias. Additionally, a number of studies did not report outcome measures sufficiently and were deemed to have a high risk of bias.
As in the previous version of this Cochrane Review of physiotherapy studies for CF, we encountered substantial challenges because of the great number of interventions, outcome measures and study durations. There has been a great deal of professional debate with regard to terminology reflecting the specific interventions. It is thus likely to cause some discomfort that we have chosen to 'lump' for the purpose of meta‐analysis certain interventions that appear to have similarities, for example those involving mechanical percussive devices. It is hoped that in future updates of this review and in the presence of large numbers of studies related to each intervention, there would be no need for such combinations.
Agreements and disagreements with other studies or reviews
This review is in agreement with the other published reviews in this topic area that have compared ACTs for CF (Bradley 2006; Burnham 2021; Heinz 2022; Morrison 2020; Radtke 2022; Wilson 2019; Wilson 2023). An overview of Cochrane Reviews reviewed six ACT Cochrane Reviews (Burnham 2021; Main 2005; McIlwaine 2019; Morrison 2020; Warnock 2015; Wilson 2023); it reported moderate‐certainty evidence that PEP therapy and vibrating (oscillating) devices had a similar effect on lung function as measured by FEV1 after six months of treatment (Wilson 2019).
In addition, Radtke 2022 recently reviewed the evidence of whether physical exercise training improves low aerobic fitness, improves HRQoL and slows the decline in lung function in people with CF and compared the duration of intervention. They reported that in studies with an active training programme of physical activity lasting over six months had a positive effect on exercise capacity when compared to usual care. However, the magnitude of improvement in exercise capacity was interpreted as small. In addition, they reported that physical activity interventions may have no effect on FEV1 % predicted.
In summary, this current review is in agreement with the other available reviews to support the efficacy of one ACT over another.
Authors' conclusions
Implications for practice.
There remains considerable debate regarding the dosage and frequency of airway clearance techniques (ACTs) as well as the ongoing question whether ACT can be replaced by other treatment modalities such as physical activity and exercise. This review cannot move this debate further forward per se. However, it does provide some evidence that ACTs may have the potential to slow the progression of diseases in the longer term as measured by lung function measures and this should be taken into account when considering the potential removal of an ACT from a treatment regimen.
There appeared to be no advantage of alternative ACTs over conventional chest physiotherapy (CCPT) in terms of effect on respiratory function. It is important to note that this result may simply reflect the paucity of evidence rather than a definitive conclusion that CCPT is no better than any of the alternatives. There appeared to be a tendency for individuals to prefer self‐administered ACTs. These seemed to offer more choice, independence and convenience in performing this daily routine. Clinicians may consider this when providing advice on which ACT is most appropriate for individuals old enough to be capable of self‐treatment. However, it should be noted that from this review there was no evidence to confirm or exclude any differences between CCPT and alternative ACTs, so CCPT should not be ruled out as an option if deemed appropriate and acceptable to the individual.
The introduction of highly effective cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies in 2021 has rapidly changed the clinical care landscape for people living with cystic fibrosis (CF), with some people reporting that respiratory secretions are less problematic after starting these new treatments. People living with CF and healthcare teams have begun to question whether some treatments that have historically been integral to CF care, including ACTs, are still needed. However, despite these advances, many individuals may continue to benefit from ACTs, including those with respiratory exacerbations, those who have established CF lung disease and bronchiectasis, those who do not have access to highly effective modulator therapies, those who have not had a good treatment response, or those who decline to take them. In addition, there are large populations of people living with other chronic respiratory disorders involving excess mucus production or difficulties clearing secretions, who may benefit from effective ACTs.
Examination of raw data submitted by study authors showed that although between‐group differences may not have been significant, it was clear that specific participants responded positively or negatively to individual treatments. Results from the only study comparing CCPT and exercise showed that there were greater improvements in participants with lower baseline lung function. This further highlights that individualisation and choice of ACT strategy may depend on individual needs at different points in time and an individual's responses to any ACT may also therefore be variable: for example, during periods of exacerbation compared with clinical stability. In terms of managing individuals, 'statistical' recommendations may not always be appropriate, and some experimentation may be required to provide the perfect solution for a particular individual's needs. As further studies are conducted, it may become apparent that some techniques may better suit certain ages and stages of the disease and this should be taken into account in future research to facilitate this. Other factors such as pulmonary hyperreactivity may, for example, preclude certain techniques. There was no evidence from meta‐analysis of available studies that any technique offered an advantage during acute pulmonary exacerbation compared to stable disease.
Recent research findings from breath‐by‐breath longitudinal monitoring of ACTs undertaken at home suggest that ACTs can have clear clinical benefit in terms of improving lung function, but only if they are performed as recommended (Raywood 2022a; Raywood 2022b). Results also suggested that the majority of children and young people with CF were not using ACTs as recommended and not deriving any benefit. Variability of quality as well as quantity of routinely performed ACTs may have been implicated in some of the studies included in this review, potentially masking clinically important changes in participants who were performing ACTs correctly. There is a need to find ways of ensuring improvements in the quality of ACTs performed at home. If this can be achieved, individuals who need to use ACTs will have clear guidance on techniques that will have clinical benefit. This will substantially reduce burden of care by decreasing time wasted on treatments that do not work, and streamlining efforts on techniques that do.
In conclusion this review cannot yet recommend any single treatment above others. Physiotherapists and people with CF might feel more comfortable trying different ACTs until a method is found that suits the individual best.
Implications for research.
More than half the publications potentially relevant to this review were excluded because they involved single treatments only, or a study duration of less than seven days. Only six studies out of the 21 identified for inclusion were undertaken over a period of one year or more. The chronic nature of CF necessitates daily treatment during the individual's lifetime. It is thus unlikely that safety or efficacy can be demonstrated over very short study intervals. More adequately powered long‐term and multicentre randomised controlled trials comparing alternative ACTs with CCPT need to be included in this review before clinically valuable information can be gained with regard to treatment efficacy and safety. Importantly, longitudinal adherence in terms of both quality and quantity of these complex ACT interventions in individual participants will need to be measured in order to properly assess efficacy.
Highly effective CFTR modulator therapies also provide new research opportunities for people living with CF, many of whom have found the burden of treatment for their condition difficult to bear. Future research appears focussed on expanding the availability of highly effective treatments, but also on the judicious and evidence‐based withdrawal of historic treatments, especially those that have proved burdensome. ACTs have been acknowledged as the most burdensome of all CF treatments, but also as one of the most important to date. It is possible that future studies should begin to explore the impact of withdrawing ACTs in specific individuals who are very well on modulator therapies.
These profound improvements in care have meant that many children and young adults now maintain lung function in the normal range with a smaller decline in lung function and better overall prognosis. However, these treatments are not a cure, and there is a need to monitor disease progression and response to therapies, and thus a call to develop new measures that adequately detect mild disease and which are sensitive to changes in health. Well‐established standard measures of respiratory function, for example FEV1, are proving to be insensitive outcomes measures in CF research, and other outcomes, for example composite health measures (Filipow 2021) or inert gas washout techniques, may improve the ability to differentiate between treatments, especially during childhood.
Included studies were in general deemed to have an unclear or high risk of bias scored poorly on quality assessment, and it is likely that no single study was adequately powered to identify clinical differences. More long‐term, well‐designed studies with adequate participant numbers are needed to resolve this issue. We recommend studies of sufficient participant numbers and of sufficient duration to determine if there is a difference in important outcome measures such as rate of decline in respiratory function, quality of life and independence. Other outstanding areas of research include identifying sensitive selection criteria (including age and severity of disease) for different ACTs.
The wide variability in response to physiotherapy treatments by individuals continues to challenge healthcare professionals, but suggests that treatment selection may depend on factors that have not yet been identified. Future studies need to focus on the relationship between specific clinical or physiological features that may predict positive responses to specific ACTs at specific points in time. It may also be useful for studies to systematically monitor and record possible treatment‐related adverse effects.
One of the key results from this review and something that should be taken into consideration in future research were the challenges in the many studies to recruit and retain participants for the duration of the study. In some studies there were a disproportionate number of dropouts which has the potential to have impacted the results of the study. Intention‐to‐treat analysis of participants who have withdrawn early can dilute or mask any real effects of interventions in remaining participants (Sontag 2010). In addition, the exclusion of participants with early hospital discharge from any analysis of the data, may result in changing the conclusions of a study (Gondor 1999).
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2023 | New search has been performed |
Included studies We included seven new studies (14 references) (Bain 1988; Giles 1996; Gondor 1999; Hare 2002; Sontag 2010; Steen 1991; Tonnesen 1982). The previously identified study Davidson 1992 (abstract) has now been published in full and this publication is now included as the primary reference for this study(McIlwaine 2010). A more recent abstract related to the Dadparvar study was published in 1995 with a larger data set, so this is now included as the primary reference for this study (Dadparvar 1995). Excluded studies We excluded 32 new studies (54 references) (Baran 1977; Bilton 1992; Cantin 2006; Elkins 2005; Fitzgerald 2005; Hofmeyr 1986; Holsclaw 1977; Keller 2001; Klig 1989; Lorin 1971; Lyons 1992; McCarren 2006; McDonnell 1986; Phillips 1998; Placidi 2006; Regelmann 1990; Reix 2012; Roos 1987; Salh 1989; Sanchez Riera 1999; Skopnik 1986; Steven 1992; Sutton 1985; Tannenbaum 2007; van der Schans 1991; van Hengstum 1988; Varekojis 2003; Verboon 1986; Warwick 1991; White 1997; Williams 2001; Wong 2000). One study formerly listed as Kraig 1995 has been excluded (Kirkpatrick 1995). On careful review, it was clear that this study did not meet inclusion criteria, although it had been included in the 2005 review. It was also clear that an error in the primary author's name in the printed copy had resulted in this abstract being included with his forename (Kraig) rather than his surname Kirkpatrick (Kirkpatrick 1995). In this review, Kraig 1995 has been amended to Kirkpatrick 1995 and excluded. |
| 5 May 2023 | New citation required but conclusions have not changed | A new co‐author (SR) has joined the review team and two authors have stepped down (CvdS and AP). The conclusions have not changed, however some additional observations have been reported. This new review does provide some evidence that airway clearance techniques (ACTs) may have the potential to slow the progression of diseases in the longer term as measured by lung function measures and this should be taken into consideration when considering the potential removal of an ACT from a treatment regimen. In addition, an important issue that arose from examination of raw data submitted by study authors was that, although group differences may not have indicated significant changes between treatments, it was clear that some participants responded extremely positively or negatively to individual treatments and choice of ACT strategy may depend on the individual's needs at different points of time, e.g. during an exacerbation compared to during periods of stability and an individual's responses to the ACT may also therefore be variable. As further studies are conducted, it may become apparent that some techniques may better suit certain ages and stages of the disease and this should be taken into account in future research to facilitate this. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 18 February 2009 | Amended | Converted to new review format. The Synopsis has been replaced by a new Plain Language Summary. Amendments in light of comments from the Group's Statistician have been made throughout the review. |
| 18 February 2009 | New search has been performed | Following on from as search of the Group's Cystic Fibrosis Trials Register four trials have been added to excluded studies (Chatham 2004; Grasso 2000; Stites 2006; Warwick 2004). An additional reference to an already excluded trial was also added (Braggion 1995). |
| 15 November 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
Risk of bias information from the 2005 review
The original 2005 review incorporated the use of the Jadad system of scoring publication quality and, as no studies were double‐blinded, this immediately limited the total score to 3 points out of a maximum of 5 (Jadad 1996). Whilst the Jadad scoring system has largely been superseded by a more comprehensive risk of bias assessment in Cochrane Reviews, for consistency, the new additions to this review have also had a Jadad score evaluation added to the Characteristics of included studies table. In this updated review, the maximal Jadad score remained at 3/5 points, since blinding is largely not feasible in this type of research. In this review, two studies scored 0 points because the method of randomisation was alternate allocation (Hare 2002; Homnick 1998). Four studies scored only 1/5 points, because although the study was described as randomised, the method of randomisation was not described and withdrawals and dropouts were not reported (Dadparvar 1995; Giles 1996; Homnick 1995; McIlwaine 1991). In general, those receiving scores of 0 or 1 were considered to be poor in quality with a high potential risk of bias.
There were 10 studies the scored 2/5 on the Jadad scale, and amongst these there was a mix of those considered at high potential risk of bias (Cerny 1989; Costantini 2001; Gaskin 1998; Steen 1991; Tyrrell 1986; van Asperen 1987), and those considered at moderate risk (Arens 1994; Bauer 1994; Gondor 1999; Reisman 1988). The remaining four studies received a Jadad score of 3/5, and amongst these there was a mix of those considered at moderate risk of potential bias (McIlwaine 2010; Sontag 2010), and those considered at low risk of potential bias (Bain 1988; McIlwaine 1997).
Although a methodological score of 2/5 is very low, these studies remained the current best available evidence within the field and it was considered reasonable to include them. It is not feasible in many cases for physiotherapists to incorporate blinding into study design, since participants are perfectly aware of the treatment they are receiving. The Jadad system and similar validated scoring systems can be a disadvantage to studies of this nature, and low scores are inevitable, since 2/5 points are allocated for blinding (Jadad 1996).
Acknowledgements
Enormous gratitude is extended to authors who gave valuable time to locate and send original data in order to facilitate this review. Data were gratefully received from the authors of the following studies: Cerny 1989; Dadparvar 1995; Homnick 1995; Homnick 1998; van Asperen 1987.
We are also extremely grateful for the support of Nikki Jahnke (Managing Editor of the Cochrane Cystic Fibrosis and Genetic Disorders (CFGD) Review Group), Sherie Smith (who helped compile the summary of findings tables), Tracey Remmington (previous Managing Editor of the CFGD Review Group) and Ashley Jones (formerly medical statistician at the CFGD Review Group). We are very grateful to Dr Connor Brenna, Department of Anesthesiology & Pain Medicine in the University of Toronto, Toronto, ON, Canada for translational assistance with the Danish paper (Tonnesen 1982).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms | Date of most recent search |
| AMED EBSCO (Allied and Complementary Medicine; 1985 to 2002) | SEARCH 1: Physical Therapy or Physiotherapy AND Cystic Fibrosis SEARCH 2: Physical Therapy Techniques or PhysiotherapyTechniques AND Cystic Fibrosis |
2002 (day and month unknown) |
| CINAHL EBSCO (Allied and Complementary Medicine; 1985 to 2002) | SEARCH 1: Physical Therapy or Physiotherapy AND Cystic Fibrosis SEARCH 2: Physical Therapy Techniques or Physiotherapy Techniques AND Cystic Fibrosis |
2002 (day and month unknown) |
| ClinicalTrials.gov (clinicaltrials.gov) | ADVANCED SEARCH Condition or disease: cystic fibrosis OR mucoviscidosis OR mucoviscidoses Other terms: postural drainage OR vibration OR vibratory OR vibration OR percussion OR cup OR cupping OR huff OR huffing OR directed cough OR directed coughing OR shake OR shaking OR ((conventional OR traditional OR chest) AND physiotherapy) Study type: Interventional Studies (Clinical Trials) |
29 June 2022 |
| WHO International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/) |
BASIC SEARCH (cystic fibrosis OR mucoviscidosis OR mucoviscidoses) AND (postural drainage OR vibration OR vibratory OR vibration OR percussion OR cup OR cupping OR huff OR huffing OR directed cough OR directed coughing OR shake OR shaking OR ((conventional OR traditional OR chest) AND physiotherapy)) |
29 June 2022 |
Data and analyses
Comparison 1. Conventional chest physiotherapy (CCPT) versus specific other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Forced expiratory volume in 1 second (FEV 1) (% predicted) | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 CCPT versus PEP | 6 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.39, 2.29] | |
| 1.1.2 CCPT versus HFCC/MP | 2 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐5.49, 1.29] | |
| 1.1.3 CCPT versus ACBT/FET | 1 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐0.39, 5.99] | |
| 1.1.4 CCPT versus O‐PEP devices | 2 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐5.79, 7.39] | |
| 1.1.5 CCPT versus AD | 2 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐2.52, 6.14] | |
| 1.1.6 CCPT versus exercise | 1 | Mean Difference (IV, Random, 95% CI) | 7.05 [3.15, 10.95] | |
| 1.2 Forced vital capacity (FVC) (% predicted) | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 CCPT versus PEP | 6 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.71, 3.51] | |
| 1.2.2 CCPT versus HFCC/MP | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐8.05, 0.33] | |
| 1.2.3 CCPT versus ACBT/FET | 1 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.83, 4.43] | |
| 1.2.4 CCPT versus O‐PEP devices | 2 | Mean Difference (IV, Random, 95% CI) | 6.13 [‐2.92, 15.19] | |
| 1.2.5 CCPT versus AD | 2 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐3.62, 4.40] | |
| 1.2.6 CCPT versus exercise | 1 | Mean Difference (IV, Random, 95% CI) | 7.83 [2.48, 13.18] | |
| 1.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) (% predicted) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 CCPT versus PEP | 4 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.86, 3.12] | |
| 1.3.2 CCPT versus HFCC/MP | 2 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐2.53, 3.52] | |
| 1.3.3 CCPT versus ACBT/FET | 1 | Mean Difference (IV, Random, 95% CI) | 6.00 [0.55, 11.45] | |
| 1.3.4 CCPT versus O‐PEP devices | 2 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐7.56, 10.09] | |
| 1.3.5 CCPT versus AD | 2 | Mean Difference (IV, Random, 95% CI) | 2.23 [‐8.96, 13.42] | |
| 1.3.6 CCPT versus exercise | 1 | Mean Difference (IV, Random, 95% CI) | 4.74 [1.94, 7.54] | |
| 1.4 Number of hospital admissions | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 CCPT versus PEP | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 1.4.2 CCPT versus ACBT/FET | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.62] |
| 1.5 Number of days in hospital | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 CCPT versus O‐PEP devices | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Schwachman score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 CPT versus ACBT/FET | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Conventional chest physiotherapy (CCPT) versus positive expiratory pressure (PEP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 6 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.39, 2.29] | |
| 2.1.1 Medium term | 4 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐2.04, 3.11] | |
| 2.1.2 Long term | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.08 [‐11.69, 5.54] | |
| 2.2 Forced vital capacity (FVC) % predicted | 6 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.71, 3.51] | |
| 2.2.1 Medium term | 4 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐3.48, 5.61] | |
| 2.2.2 Long term | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐13.05, 7.03] | |
| 2.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 4 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.86, 3.12] | |
| 2.3.1 Medium term | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐5.37, 4.36] | |
| 2.3.2 Long term | 1 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐13.30, 6.18] | |
| 2.4 Number of hospital admissions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Long term | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
Comparison 3. Conventional chest physiotherapy (CCPT) versus high‐frequency chest compression (HFCC)/mechanical percussive (MP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Short term | 2 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐5.49, 1.29] | |
| 3.2 Forced vital capacity (FVC) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Short term | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐8.05, 0.33] | |
| 3.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 Short term | 2 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐2.53, 3.52] | |
| 3.4 Number of days in hospital for respiratory exacerbations | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Short term | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.69, 1.10] |
| 3.5 Blood oxygen levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Short term | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.29, 0.49] |
| 3.6 Weight (change from baseline) (kg) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.6.1 Short term | 2 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.87, ‐0.07] |
Comparison 4. Conventional chest physiotherapy (CCPT) versus active cycle of breathing therapy (ACBT)/forced expiratory technique (FET).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Long term | 1 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐0.39, 5.99] | |
| 4.2 Forced vital capacity (FVC) % predicted | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Long term | 1 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.83, 4.43] | |
| 4.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Long term | 1 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [0.55, 11.45] | |
| 4.4 Number of participants per group requiring ≥ 1 hospital admission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.4.1 Long term | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.62] |
| 4.5 Blood oxygen levels (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 Short term | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.49, 1.09] |
| 4.6 Shwachman score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.6.1 Long term | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 3.90 [1.52, 6.28] |
Comparison 5. Conventional chest physiotherapy (CCPT) versus oscillating positive expiratory pressure (O‐PEP) devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Short term | 1 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐6.85, 8.25] | |
| 5.1.2 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | 1.12 [‐12.40, 14.64] | |
| 5.2 Forced vital capacity (FVC) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 Short term | 1 | Mean Difference (IV, Random, 95% CI) | 11.30 [‐1.54, 24.14] | |
| 5.2.2 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐9.31, 13.31] | |
| 5.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 Short term | 1 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐7.23, 13.63] | |
| 5.3.2 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | ‐3.62 [‐20.18, 12.94] | |
| 5.4 Number of days in hospital | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.4.1 Short term | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.81, 1.36] |
| 5.4.2 Medium term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.55, 6.95] |
| 5.5 Number of days of intravenous antibiotics for respiratory exacerbations | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.5.1 Medium term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐16.36, 13.16] |
Comparison 6. Conventional chest physiotherapy (CCPT) versus autogenic drainage (AD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐2.52, 6.14] | |
| 6.1.1 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | 1.29 [‐4.07, 6.65] | |
| 6.1.2 Long term | 1 | Mean Difference (IV, Random, 95% CI) | 2.79 [‐4.54, 10.12] | |
| 6.2 Forced vital capacity (FVC) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐3.62, 4.40] | |
| 6.2.1 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐4.00, 6.72] | |
| 6.2.2 Long term | 1 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐6.88, 5.20] | |
| 6.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 2 | Mean Difference (IV, Random, 95% CI) | 2.23 [‐8.96, 13.42] | |
| 6.3.1 Medium term | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐7.49, 3.23] | |
| 6.3.2 Long term | 1 | Mean Difference (IV, Random, 95% CI) | 9.71 [‐3.35, 22.77] | |
| 6.4 Number hospital admissions | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.4.1 Long term | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.06, 0.42] |
| 6.5 Proportion of admissions per number of participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.5.1 Long term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.71] |
Comparison 7. Conventional chest physiotherapy (CCPT) versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Forced expiratory volume in 1 second (FEV 1) % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Short term | 1 | Mean Difference (IV, Fixed, 95% CI) | 7.05 [3.15, 10.95] | |
| 7.2 Forced vital capacity (FVC) % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 Short term | 1 | Mean Difference (IV, Fixed, 95% CI) | 7.83 [2.48, 13.18] | |
| 7.3 Average forced expiratory flow between 25% and 75% expired FVC (FEF 25–75) % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 Short term | 1 | Mean Difference (IV, Fixed, 95% CI) | 4.74 [1.94, 7.54] | |
| 7.4 Number of days in hospital | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.4.1 Short term | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐2.68, 2.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arens 1994.
| Study characteristics | ||
| Methods | Single centre (Los Angeles, USA), parallel design RCT Participants randomly allocated to receive either HFCC or CCPT 3 times a day for 14 days during admission to hospital for acute pulmonary exacerbation |
|
| Participants | 54 recruited but 4 dropped out and excluded from analysis Analysis on 50 people with CF diagnosed by sweat chloride levels and clinical findings: 25 in each group Mean age 20.5 years; range 16–24 years CCPT: mean age: 18 (SD 1.3) years HFCC: mean age: 22.9 (SD 2.0) years 32 male, 18 female |
|
| Interventions | CCPT vs HFCC (ThAIRaphy bronchial drainage system). All participants also received IV antibiotics, inhaled bronchodilators and nutritional support during their admission. CCPT: 30‐min treatments, 3 times daily of conventional methods of percussion and PD. Aerosolised bronchodilators (albuterol 0.5 mL diluted in 5 mL normal saline) were delivered performed 10–15 min before CCPT administration. PD performed in 6 positions (4 lying and 2 sitting), and percussion administered for 4 min in every position. HFCC: 30‐min treatments, 3 times daily, while receiving aerosolised bronchodilator (albuterol 0.5 mL diluted in 5 mL of normal saline), seated upright during entire session. The HFCC treatment consisted of 6 frequency periods (4–5 min each), range 6–19 Hz. Between each frequency period, the device was stopped. The participant was instructed to perform a maximal inspiratory manoeuvre and then the pulsating device was reactivated at 25 Hz for the duration of an expiratory manoeuvre. The participant was instructed to actively cough between each of these expiratory manoeuvres, which were repeated 3 times. |
|
| Outcomes | Weight, respiratory rate, and SpO2 recorded regularly throughout hospitalisation. In addition, haemoglobin, WBC count, ANC, serum protein and albumin concentrations drawn on admission and at days 7 and 14 of hospitalisation. Pulmonary function tests performed on admission and immediately before physiotherapy treatment at weekly intervals (days 1, 8 and 14). Sputum collected for 24 h after beginning HFCC or CCPT. The first hour and 24‐h wet sputum collection samples measured. The dry sputum weight measured after slow desiccation in a commercial microwave for 10–60 min. Sputum weight (wet and dry), VC, FEV1, FEF25–75, SpO2, RV, RV/TLC. Participant satisfaction in the HFCC group recorded. | |
| Notes | Study reported in 1 abstract (Arens 1993) and 1 full‐text manuscript (Arens 1994), which is the primary reference for this study although some details were extracted from the abstract. Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote): "Patients were randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although 50 participants included and analysed, study authors suggested 4 additional participants were excluded. Quote: "3 additional patients originally assigned to receive HFCC, and one patient assigned to the CPT [chest physiotherapy] regimen, did not comply to the chest physiotherapy schedules and were excluded from the study." |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Unclear risk | Participants satisfaction only reported in HFCC group. |
Bain 1988.
| Study characteristics | ||
| Methods | Single‐centre (Melbourne, Australia), parallel design RCT Participants randomly allocated to receive either directed coughing or CCPT 3 times per day for 14 days during admission to hospital for acute pulmonary exacerbation |
|
| Participants | People with CF, aged > 7 years, able to expectorate sputum and perform lung function tests 46 recruited (23 in each group) but 8 failed to complete the study and were excluded from analysis. 4 participants from each arm: 4 due to febrile illness, 1 due to lack of compliance, 1 due to a large haemoptysis, 1 due to participant request and 1 was discharged before the study completed Analysis on 38 people with CF who were hospitalised with a pulmonary exacerbation Mean age 13 years; range 9–18 years 29 male and 17 female Stratified into 2 groups at baseline: CCPT (n = 19): median age 12 (range 9–18) years, mean FEV1% predicted 59.1 (SD 27.5) Directed coughing (n = 19): median age 13 (range 9–16) years, mean FEV1% predicted 55.6 (SD 19.7) |
|
| Interventions | Participants in both groups had physiotherapy treatment 3 times per day for 14 days. Nebulised salbutamol was administered prior to each physiotherapy treatment. Week‐day morning and afternoon treatments were done by the staff physiotherapist allocated to that particular participant for the 14 days of the study. The evening treatment was done by the ward nursing staff. On the weekends, participants were seen by the physiotherapist rostered on duty in the mornings and by the ward nursing staff in the afternoon and evening. All participants received 1‐to‐1 treatments in uninterrupted sessions. None exercised prior to physiotherapy treatments or before the pulmonary function tests. Standard PD positions were used. 4 lung segments drained at each treatment based on the latest chest radiograph. If this showed generalised changes or a clear chest the segments drained were anterior segments of left and right upper lobes, lateral segment of right lower lobe, lateral segment of left lower lobe and posterior basal segments of both lower lobes. For each drainage position the following treatment was done. CCPT: 2‐min percussion (clapping) over the segment, followed by 6–8 vibrations over the segment as the participant gently and completely exhaled, followed by the coughing sequence. In the sitting position, participant did 5 quick, shallow huffs or pants, followed by 3 forced expiratory huffs and then coughed effectively until all the loosened sputum was cleared from the larger airways. The coughing sequence was then repeated. The participant resumed the same drainage position and the percussion, vibrations and coughing sequence were repeated once more. This regimen was continued until all 4 segments were drained (a minimum of 16 coughs per treatment required). There was no time limit for this treatment, but it was kept as close to 35 minutes as possible. Directed coughing: conducted over 35 minutes. The participant remained seated throughout the treatment and every 5 min was directed to perform the huffing and coughing sequence twice. The participant did 5 quick, shallow huffs or pants, followed by 3 forced expiratory huffs and then coughed effectively until all loosened sputum was cleared from the larger airways. The sequence was then repeated. |
|
| Outcomes | Pulmonary function and sputum characteristics. On admission all participants had a chest radiograph. They performed pulmonary function tests and a sputum specimen was obtained for culture. Their height and weight were measured. On the results of the chest radiograph, FEV1 and the volume of sputum cleared, the participants were graded according to their pulmonary status (grade 2 = mild‐to‐moderate lung disease, grade 3 = severe lung disease). VC, FEV1 and FEF25–75. TLC and RV were calculated from measurements of TGV in an integrated flow, pressure compensated total body plethysmograph. Maximum expiratory flow–volume curves were performed in the body plethysmograph and expiratory flow was measured at 70%, 60% and 40% of TLC and corrected for lung volume by dividing by TLC and expressed as TLC/s. Maximum static inspiratory and expiratory pressures at the mouth were measured. Ear oximetry was measured with a Hewlett Packard ear oximeter. Sputum was also collected after both physiotherapy sessions. The sputum was weighed and the volume measured. In addition, the colour and consistency was noted and graded on a scale of 1–5. Colour 1 = light yellow, 5 = dark green/brown. Consistency 1 = thin, 5 = thick and tenacious. | |
| Notes | Study reported in 1 full‐text manuscript (Bain 1988), which is the primary reference for this study. Jadad score: 3/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation was used after participants were graded according to pulmonary status (grade 2: mild‐to‐moderate lung disease, grade 3: severe lung disease), based on chest radiograph, FEV1 and the volume of sputum cleared. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants (4 from each group) withdrew from the study (0 lost to follow‐up). 4 due to febrile illness, 1 due to lack of compliance, 1 due to a large haemoptysis, 1 due to participant request and 1 was discharged before the study was completed. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Unclear risk | Treatments were supervised by either a physiotherapist or nurse, depending on time of day or day of week. |
Bauer 1994.
| Study characteristics | ||
| Methods | Single‐centre (Alabama, USA), parallel design RCT for 54 participants (3 dropped out) Participants randomly allocated to receive either manual or mechanical chest percussion 3 times per day for approximately 12 days during admission to hospital for acute pulmonary exacerbation. 27 of the original participants were admitted a 2nd time during the study and agreed to participate in the opposite arm of the study (cross‐over analysis) (5 dropped out, 22 analysed). |
|
| Participants | 54 participants aged > 6 years agreed to participate and could perform adequate spirometry and signed consent forms. 3 dropped out: 1 'dismissed' after 2 days of hospitalisation, 1 not compliant with the protocol, and 1 complained that manual percussion was painful. Therefore, 51 participants participated fully in the study, providing the data for analysis. 27 participants were admitted a 2nd time during the study and agreed to participate in the opposite arm of the study (cross‐over analysis). Of these 27 participants, 5 were excluded: 2 were dismissed early, 1 was non‐compliant and 2, assigned to mechanical percussion, requested to return to manual percussion because of personal preference. Therefore, 22 participants participated fully in both arms of the study (cross‐over group). Manual percussion: (n = 36, including cross‐over): mean age 17 (SEM 1.4) years; 18 males; mean days in hospital 12.5 (SEM 0.5) days Mechanical percussion: (n = 37, including cross‐over): mean age 15.9 years (SEM 1.4); 19 males; mean days in hospital 11.4 (SEM 0.5) days |
|
| Interventions | Participants were randomly assigned to receive either manual or mechanical percussion for the entire hospitalisation. Participants admitted to the hospital twice during the course of the study were allowed to participate a 2nd time but were non‐randomly assigned to receive the form of percussion not received during the first participation. No participant was allowed to participate more than twice. Percussion was performed 3 times per day by respiratory care practitioners who recorded each session in the hospital chart, noting time, positions, cough and secretion assessments, oximetry readings and complications. Each percussion session was performed in 2 upright positions (upper portion of the back and upper anterior portion of the chest), 4 flat positions (mid‐portion of the chest, anterior portion of the back, left and right sides), and 4 Trendelenburg positions (lower portion of the chest, anterior portion of the back, left and right sides). Manual percussion: 30 min with cupped hands Mechanical percussion: 30 min with Vibracare percussor or a model 9000 percussor 25–35 Hz, with (quote) "sufficient pressure to change the voice." |
|
| Outcomes | Hospital duration, participant preference, FVC, FEV1, FEF25–75, weight. | |
| Notes | Study reported in 1 abstract (Bauer 1990) and 1 full‐text manuscript (Bauer 1994), which is the primary reference for this study. No details were extracted from the abstract. Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote) "Patients were randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/54 participants dropped out of the first phase (51 analysed) and 5/27 participants dropped out in the second phase (22 analysed). 1 early discharge, 1 non‐compliant, 1 complained manual percussion painful. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | High risk | Final analysis combined and included data from 22 participants on repeated admissions. Participants who were less well and required more‐frequent admissions may thus have been over‐represented in this population sample. Although unpaired t‐test used on combined analysis, data were paired for 22 participants within the analysis. |
Cerny 1989.
| Study characteristics | ||
| Methods | Single‐centre (Buffalo, USA), parallel design RCT Participants randomly allocated to receive either 2 cycle ergometer exercise sessions plus 1 bronchial hygiene treatment session per day or 3 bronchial hygiene treatment sessions per day for approximately 13 days during admission to hospital for acute pulmonary exacerbation. |
|
| Participants | 17 participants admitted to hospital for acute pulmonary exacerbation. All 17 completed the study. Participants not matched for disease severity. Mean pulmonary function score in both groups indicated severe disease (CCPT group slightly worse). Admission based on symptoms of increased shortness of breath, coughing and sputum production, and decreased lung function. Participant gender not reported CCPT (n = 8): mean age 15.9 (SD 4.9) years, mean length of stay 13.0 (SD 2.6) days Cycle ergometer exercise plus CCPT (n = 9): mean age 15.4 (SD 4.9) years, mean length of stay 13.0 (SD 3.0) days |
|
| Interventions | Participants were randomly assigned to either 2 cycle ergometer exercise sessions and 2 bronchial hygiene treatment sessions per day or 3 bronchial hygiene treatment sessions per day. All participants were treated similarly with IV antibiotics, inhaled bronchodilators, pancreatic enzymes, and water‐soluble vitamins A and E and multivitamin preparations. CCPT: PD was preceded by inhaled β2‐receptor agonist bronchodilators. PD in 6 positions, with chest percussion, vibration and forced expiration was done for 20–40 minutes 3 times daily, except for 1 participant who received 2 afternoon treatments for 4 treatments per day. Physiotherapy given between 8 a.m. and 9:30 a.m., 3 p.m. and 4:30 p.m., and 7 p.m. and 9 p.m. Cycle ergometer exercise plus CCPT: the group exercised during the first 2 sessions and received PD during the 3rd session. Coughing was encouraged during and after all therapy sessions. Coughs were counted for 15 min following each therapy session. Workloads in the first 2 days were set to an HR of 25–40% of the HRR. This was tolerated for 5–10 minutes, except in 2 participants who received supplemental oxygen at a level to maintain SaO2 > 90% on days 1 and 2. After 3–4 days, all exercised at an HR ≥ 40% of their peak HRR. From day 4 to discharge, exercise time was increased to a target duration of 15–20 minutes and was individually adjusted based on the participant's HR and SaO2 response to the admission exercise test. By the end of the hospitalisation, all were worked at 45–65% of HRR. |
|
| Outcomes | Pulmonary function and response to a progressive, incremental cycle ergometer exercise test were measured on admission and before discharge. Participants were discharged after resolution of pulmonary function test results, chest radiograph, shortness of breath and elimination of fever. FVC, ERV, IC, FEV1, and FEF25–75 were measured by spirometry; FRC and Raw were measured by body plethysmography. RV = FRC – ERV, TLC = FRC + IC, and SGAW = 1/Raw × FRC − 1 were calculated. SaO2 was estimated with an ear oximeter. The exercise test was performed on a cycle ergometer. The initial load of 0.3 W/kg was increased by 0.3 W/kg every 2 minutes. Exercise was stopped when the participants could no longer continue despite encouragement or when SaO2 had decreased by > 15% or to < 75% of the baseline level. Daily sputum volume expectorated was measured and summed during different time intervals in the day and during and after treatment. Sputum volume, wet weight and dry weight were recorded and expressed in units per hour. |
|
| Notes | Study reported in 1 full‐text manuscript (Cerny 1989), which is the primary reference for this study. Study author provided summary data (% predicted and SEM) on admission and discharge for FVC, FEV1 and FEF25–75. Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote) "Patients were randomly assigned," "No attempt was made to match patients for disease severity." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 participants recruited and analysed. All PD sessions were completed, 96% of scheduled exercise sessions completed. |
| Selective reporting (reporting bias) | High risk | Numerical data were not provided for any outcome in the manuscript except age, height, weight and pulmonary function score. Results were either presented in graphical form only (for FRC, FVC, FEV1, FEF25–75) with change from baseline superimposed on the graph, or summarised in text without numerical data for the remaining outcome measures (ERV, IC, Raw, RV, TLC, SGAW). "No significant differences" between treatments in any outcome measures reported but no data provided. Limited additional summary data provided by study authors. |
| Other bias | High risk | Differences in baseline FEV1, FEF25–75 between groups, with CCPT group significantly lower. Sample size small. |
Costantini 2001.
| Study characteristics | ||
| Methods | Single‐centre (Milan, Italy) parallel design RCT Participants (newborn infants with CF) were randomly assigned to PEP mask or CCPT (PD with percussion and vibration) and after a 14‐day training period, they were followed up as outpatients for 1 year. |
|
| Participants | In 2001 abstracts: 26 infants (14 boys, 12 girls), diagnosed by newborn screening: IRT and genotype, and within the first 4 months of age on enrolment. Numbers allocated to each group not reported. In 1998 abstract: 12 newborn infants (5 boys), diagnosed with CF via newborn screening and positive sweat test within 2nd month of life. CCPT (n = 5) versus PEP (n = 7). |
|
| Interventions | 2001 abstract: no details of either intervention are provided. Randomly assigned then "trained for a 2 week period" in conventional 'PD and percussion' or PEP mask. Both treatments consisted of 30‐min sessions twice daily. Chest wall vibration was mentioned as part of technique in 1998 but not 2001. 1998 abstract: no details of either intervention in terms of frequency or duration were provided. |
|
| Outcomes | 2001 abstract: growth, CXR, SaO2, physical examination, sputum culture were assessed initially (baseline), and after 6 and 12 months of treatment. Courses of antibiotic therapy were recorded and the onset of possible adverse effects related to the techniques was monitored. 1998 abstract: growth, CXR, SaO2, clinical examination, courses of antibiotic treatment, and onset of adverse effects (quote: "assessed every 6 months"). |
|
| Notes | Study reported in 3 abstracts (Costantini 1998; 2001; 2001). The 2001 abstracts (same data set) are the primary references for this study. Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: 2001: participants were "randomly assigned." 1998: participants were "assigned" in "prospective randomized trial." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2001 abstracts: 5 participants in PEP and 4 in CCPT group developed GOR. 3/4 CCPT participants dropped out of the study due to the "severity of their symptoms." They were "not evaluated." The numbers of participants in each group at randomisation were not reported, so the significance of these dropouts could not be assessed. 1998 abstract: no attrition reported. |
| Selective reporting (reporting bias) | High risk |
|
| Other bias | High risk |
|
Dadparvar 1995.
| Study characteristics | ||
| Methods | Single‐centre (Philadelphia, USA) prospective randomised 2‐group cross‐over design Participants (clinically stable people with CF) received 3 months of each therapy in random order. No washout period reported. Radionuclide assessment of whole lung and peripheral lung clearance was performed at baseline and end of each 3‐month treatment period. |
|
| Participants | 1995 abstract: 20 clinically stable participants with CF (age and gender not reported) 1990 abstract: 13 clinically stable participants with CF, age range: 18–34 years, mean age: 25.7 (SD 5) years; 7 males, 6 females were 'selected' |
|
| Interventions |
CCPT PEP No indication of frequency or duration of prescription for each. |
|
| Outcomes | Clinical scoring, chest radiography, TC99m‐DTPA radionuclide scanning (dynamic acquisition done in supine position for 30 min) and pulmonary function (FVC, FEV1, FEF, TLC) including lung volumes were recorded at baseline and 3 and 6 months following completion of CCPT and PEP phases. Data analysed to obtain T1/2 clearance in min of each whole lung and periphery. Preference determined by "standardised written questionnaire." | |
| Notes | Study reported in 2 abstracts (Darbee 1990, Dadparvar 1995). The 1995 abstract includes analysis of a larger population and is the primary reference for this study. Original data on 20 participants in 1995 abstract obtained from study author. Jadad score: 1/5 (cross‐over study) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of cross‐over intervention randomised, but no details provided. 13 participants were 'selected' – unclear on what basis or whether purposive. 1995 abstract provided no details on 20 participants. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding withdrawals or dropouts in either abstract. Participant data obtained on 20 participants from study authors. |
| Selective reporting (reporting bias) | High risk | Clinical scores, chest radiography and pulmonary function including lung volumes were measured but not reported in 1990. |
| Other bias | High risk | No washout period between each period of intervention could cause a potential carry‐over risk. Preference for PEP reported – but no details on how this was analysed, or what proportion preferred PEP. Age, gender or numbers allocated to each group were not reported. Conclusion in 1990 abstract that both modalities equally effective, while 1995 abstract concludes PEP better for lung clearance while CCPT better for regional lung ventilation. |
Gaskin 1998.
| Study characteristics | ||
| Methods | Single‐centre (Toronto, Canada) prospective parallel design RCT 66 participants (with CF attending adult and paediatric clinics) were "randomly assigned" to standard PD and percussion (CCPT) or PEP mask therapy (Astra Meditec) for 2 years. |
|
| Participants | 66 participants, 61 participants completed. Mean age 21.6 (SD 8.3) at enrolment; age range 11–45 years 34 males, 32 females CCPT: mean age 21.9 (SD 8.7) years PEP: mean age 21.3 (SD 8.0) years |
|
| Interventions |
CCPT PEP No indication of frequency or duration of, or adherence with each prescription. |
|
| Outcomes | Followed up every 3 months for 2 years: FVC, FEV1, QoL (Quality of Well Being score), Brasfield CXR score, exercise testing (cycle ergometry). Compliance and exercise diaries kept by participants. | |
| Notes | Study reported in 1 abstract from 12th NACF conference (Gaskin 1998). Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote) "patients were randomly assigned". Numbers assigned to each group not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants (gender not reported) withdrew from study (0 lost to follow‐up): 4 withdrew from CCPT group immediately after randomisation; 1 from PEP group moved away. |
| Selective reporting (reporting bias) | High risk | Only baseline FEV1 and rate of decline trajectory provided. Secondary analysis of FEV1 data included only those aged < 19 years (unclear how many) and those compliant with treatments (unclear how many). No other outcome data reported – FVC, CXR score, QoL and exercise test results were absent. Compliance data not reported. |
| Other bias | High risk | Analysis with or without the withdrawn and non‐compliant participants in secondary analysis did not change the conclusions of the study. Numbers of non‐compliant participants were not reported. Conclusions that PEP was "valid alternative" to CPT not supported by results reported. |
Giles 1996.
| Study characteristics | ||
| Methods | Single‐centre (Denver, USA) prospective randomised cross‐over study. 14 participants had 12 weeks of participation, comprising 2 weeks of usual treatment, followed by 4 weeks of either Flutter device or CCPT treatment at home, then a 2‐week washout period then 4 weeks of the alternative treatment. | |
| Participants | 14 people with CF. Unclear how they were selected. Age and gender of participants not reported |
|
| Interventions |
Flutter device treatment: (twice daily treatments for 15 min) Standard PD and clapping: (twice daily treatments) |
|
| Outcomes | Pulmonary function tests (FVC and FEV1), wet and dry sputum weights, participant treatment preference questionnaire. | |
| Notes | Study reported in 1 abstract from 10th NACF conference (Giles 1996). Jadad score: 1/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote) "patients were randomly treated". Numbers assigned to each group not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding withdrawals or dropouts in abstract. |
| Selective reporting (reporting bias) | High risk | Only FVC and FEV1 reported from pulmonary function data. Data presented as combined mean from both arms for each therapy, so first‐arm effects not discrete. No numerical data for questionnaire findings. Outcome measure data presented but statistical analyses not reported. |
| Other bias | High risk | The time prescribed for Flutter device treatments was limited to 15 min, while CCPT treatments did not appear to have a time limit. This may impede comparison. Sputum collected after a single treatment at the end of each arm unlikely to represent clearance during each 4‐week period adequately. Treatment prescribed or undertaken during the 2‐week washout period was not reported. Flutter device was apparently "preferred overall," but no data or analysis provided to support this conclusion. |
Gondor 1999.
| Study characteristics | ||
| Methods | Single‐centre (Pittsburgh, USA) prospective parallel design RCT 23 participants were "randomly assigned" to standard PD, percussion and vibration (CCPT) or Flutter device therapy 4 times daily during a 14‐day hospital admission for an acute exacerbation. |
|
| Participants | 23 people with CF recruited. 3/23 (all from the CCPT group) were discharged prior to 14 days, and, therefore, data from only 20 (11 male, 9 female: 8 CCPT, 12 Flutter device) were analysed. Mean age 13 years; range 5–21 years CF diagnosis by elevated sweat chloride concentration (> 60 mEq/L) and typical pulmonary and gastrointestinal findings. Children were eligible to participate if they aged ≥ 5 years and could perform spirometry reliably. Participants were excluded if they were unable to ambulate or had experienced an episode of haemoptysis within 3 months of hospitalisation. Participants already using the Flutter device were not excluded. The participants had moderate‐to‐severe lung disease, with only 2 participants in each group having an FEV1 > 80% of predicted. All but 1 participant in the CCPT group and 2 participants in the Flutter device group had small airway function (FEF25–75) < 50% of predicted. |
|
| Interventions | Chest physiotherapy (n = 11) vs Flutter device (n = 12) 4 times daily CCPT: (PD, percussion and vibration) done immediately after participants received prescribed bronchodilator therapy. Participants received manual clapping over the chest wall with the participant positioned so that gravity could aid mucus drainage from the lobes or segment being percussed. PD with percussion and vibration was performed for 2 min in each of 8 standard positions. Following percussion and vibration in each position, participants took deep breaths, coughed and expectorated. Session duration: 15–20 min. Flutter device: treatments, supervised by a respiratory therapist 4 times daily, done immediately after prescribed bronchodilator therapy. The participant was seated with his/her head raised so that the stem of the device was parallel to the floor, placing the cone at a slight tilt away from the participant. After inhaling and holding their breath for 2–3 seconds, participants would slowly exhale through the Flutter device valve, causing oscillations of the steel ball inside the cone of the Flutter device. Participants performed repeated exhalations through the Flutter device valve. Routinely, 3 sets of 15 exhalations were performed over 12–20 min. After each series of exhalations, participants were instructed to huff and cough, thereby aiding expectoration. |
|
| Outcomes | Pulmonary function testing and the 6MWT (on admission, and days 7 and 14 of hospitalisation). Pulmonary function (FVC, FEV1, FEF25–75) was measured in laboratory using standard spirometry, as outlined in the ATS guidelines about 3.0–3.5 hours after bronchodilator therapy. Results were expressed in absolute values, as well as % predicted values based on gender, race, height, weight and age, as described by Schoenberg. 6MWT performed in a hospital corridor, along a 40‐m course marked at 2‐m intervals. Participants were instructed to cover as much distance as possible in 6 min, walking at their own pace. Participants were permitted to rest during the test if necessary. No verbal encouragement was provided by the testing staff. |
|
| Notes | Study reported in 1 abstract (Gondor 1996) and 1 full‐text manuscript (Gondor 1999), which is the primary reference for this study. Jadad score: 2/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: (quote) "patients were randomly assigned" (11 CCPT and 12 Flutter device recruited, 8 CCPT and 12 Flutter device analysed). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pulmonary function and exercise technicians were blinded as to which treatment the participants were receiving. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants from CCPT group were excluded from analysis due to early discharge. There were no data provided for these participants. Including them in analysis changed the conclusions of the study. 2 participants did not do 6MWT, unclear from which group. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | High risk | 3/23 participants (all in CCPT group) were discharged prior to the 14 days of inpatient therapy, and their data excluded from analysis of the 2‐week intervention. The study concluded that those using Flutter device had better pulmonary function after 1 week of therapy (higher FVC and FEV1) and similar improvement in pulmonary function and exercise tolerance compared to CCPT after 2 weeks of therapy, suggesting that Flutter device therapy is an acceptable alternative to standard chest physiotherapy during in‐hospital care of people with CF. However, when data from the 3 excluded participants were included in analysis, the 7‐day findings were no longer statistically significant. Sponsored by Scandipharm Pharmaceuticals (who provided the Flutter devices). |
Hare 2002.
| Study characteristics | ||
| Methods | Single‐centre (Michigan, USA) pilot 'open‐label' comparative trial with 'alternate assignment' 14 participants with CF and an acute exacerbation were admitted to hospital for IV course of antibiotics (duration of admission not reported) and were alternately assigned to receive Percussivetech HF device or standard manual CCPT 4 times daily during hospitalisation. |
|
| Participants | 14 participants Age range 8–28 years 10 male, 4 female |
|
| Interventions |
PercussiveTech HF: 4 times per day during hospitalisation CCPT: standard manual CCPT, 4 times per day during hospitalisation |
|
| Outcomes | "Complete" PFTs (FVC, FEV1, FEF25–75 and RV) and clinical scores measured at baseline and at discharge. Participants were monitored for complications (haemoptysis, hypoxaemia, pneumothorax). | |
| Notes | Study reported in 1 abstract from 16th NACF conference (Hare 2002). Jadad score: 0/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random "alternate assignment," and final numbers in each group not reported, or how the first participant was allocated. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed as "alternate assignment" to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding: not feasible (participants), open‐label (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded: open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding withdrawals or dropouts in abstract. |
| Selective reporting (reporting bias) | High risk | No data or statistical analysis provided. Outcomes were discussed in narrative form only. |
| Other bias | High risk | No data included in abstract, results presented descriptively. Numbers allocated to each group not reported, magnitude of change in clinical outcomes not reported. Nature of statistical analysis not reported. Length of admission not reported, with risk that this differed between groups. Study supported by Vortran Medical Tech (Percussivetech HF manufacturer). |
Homnick 1995.
| Study characteristics | ||
| Methods | Single‐centre (Michigan, USA), parallel design RCT 20 participants stratified by Shwachman score and randomised to either IPV with intrained aerosol or standard manual chest physiotherapy and aerosol treatment for a total study period of 180 days. Participants were instructed to do ≥ 2 treatments each day and were followed up every 30 days. |
|
| Participants | 20 participants with CF who attended the CF centre and who were using standard CPT and aerosol therapy at home were randomised. They were clinically stable, ambulatory, with no history of pneumothorax or haemoptysis, and able to tolerate ≥ 2 IPV or standard CCPT sessions per day. Age range aged 5–24 years CCPT (n = 8): 5 males, 3 females, mean age 10 years; range 5–18 years IPV (n = 8): 5 males, 3 females, mean age 12 years; range 5–24 years |
|
| Interventions |
CCPT: aerosol and CCPT ≥ 2 times a day recorded daily on a log sheet. This consisted of manual percussion for about 2 min in each of 10 PD positions. Standard aerosol therapy with 2 mL saline or odium cromoglicate with an appropriate amount of albuterol given by compressor and updraft nebuliser. IPV with intrained aerosol: participants received instructions on the use of the IPV (10–30 cmH2O) machine and were given saline and albuterol for use in the device. Instructed to use IPV ≥ 2 times a day and record the frequency daily on a log sheet. The IPV group also asked to complete a satisfaction index after each 30‐day period of using IPV (comfort, time spent in therapy and compliance). Dosing for albuterol in both the standard aerosol and IPV groups was 1.25–2.5 mg per treatment. Pressures and frequencies were individually determined by the respiratory therapist. All participants continued their assigned treatments while hospitalised, usually at a frequency of 4 times per day. |
|
| Outcomes | All participants were followed up at 30‐day intervals, measures included participant logs, anthropometric measures (BMI), spirometry (FVC, FEV1, FEF25–75), adverse effects, satisfaction, inpatient and outpatient antibiotic use, number of hospitalisations and adverse events. An adverse reaction was reported in 1 participant who experienced minor haemoptysis in the 4th week of using the IPV device. |
|
| Notes | Study reported in 1 abstract (Homnick 1994) and 1 full‐text manuscript (Homnick 1995), which is the primary reference for this study. Jadad score: 1/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported. Participants were randomised after being stratified by Shwachman score. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants in each group completed study, but no details given on 4 withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported, but each participant participated in 5 sequential 30‐day periods, with outcomes measured on each 30 visit. Only first and last visits were analysed and reported. |
| Other bias | High risk | Only IPV group asked to report on satisfaction. Only 8 participants completed study in each group. Details of, or reasons for, 4 dropouts not reported. Baseline mean BMI was significantly higher in the IPV group (15.2 (SD 1.7)) compared to the CCPT group (7.9 (SD 2.0)) and mean FEV1 (although non‐significant) was higher in the IPV group (70 (SD 12) % predicted) compared to the CCPT group (59 (SD 12) % predicted). |
Homnick 1998.
| Study characteristics | ||
| Methods | Single‐centre (Michigan, USA), preliminary, open‐label, alternate assignment comparative trial 22 adults and children with CF confirmed by sweat test or genetic testing, or both, were alternately allocated to either Flutter device or CCPT during hospitalisation for an acute exacerbation. 7/22 participants were admitted more than once during the study period (data collected during 33 hospital admissions). Duration: maximum 11 days per admission (mean 8.9 days) during an acute exacerbation |
|
| Participants | Participants diagnosed with CF by sweat or genetic testing. 22 participants contributed data during 33 admissions to hospital for pulmonary exacerbations, 15 participants admitted once, 7 participants admitted ≥ 2 times Age range 7–44 years Exclusion criteria included history of severe reaction to aerosolised bronchodilator (significant tachycardia or wheezing), or to CCPT, (increased wheezing, chest pain, or discomfort); history of tobacco use; inability to undertake pulmonary function testing; history of significant haemoptysis (> 240 mL/day on any occasion or > 100 mL/day for several days) or pneumothorax within 1 year of study entry. CCPT (n = 17): mean age 12 years; range 7–21 years Flutter (n = 16): mean age 16.1 years; range 8–44 years |
|
| Interventions | Participants received supervised Flutter therapy or standard, manual CCPT 4 times per day during hospitalisation; all participants received rhDNase 2.5 mg once daily. Respiratory therapists were registered and trained and the same in both groups. Flutter device: 15 min 4 times per day (preceded by albuterol nebuliser 2.5–5 mg in either 2 mL normal saline solution or 20 mg (2 mL) sodium cromoglycate); Flutter device was directly supervised by respiratory therapists using manufacturer's recommendations. CCPT: (standard manual) 30 min 4 times per day (preceded by albuterol nebuliser 2.5–5 mg in either 2 mL normal saline solution or 20 mg (2 mL) sodium cromoglycate); CCPT was administered by respiratory therapists using CF Foundation guidelines incorporated into a hospital protocol. |
|
| Outcomes | Complete pulmonary function tests (FEV1, FVC, FEF25–75, FEV1/FVC, RV, RV/TLC) performed in lung function laboratory using ATS standards at baseline (admission), weekly, and upon discharge from the hospital. Clinical score determined at the time of hospital admission and at discharge. Participants monitored for complications, including haemoptysis, hypoxaemia and pneumothorax. Shwachman score, a CF severity scoring system, a clinical score (modified Case Western) were all performed at baseline. Mean length of hospitalisation, mean number of respiratory treatments during the hospitalisation and adverse events were measured. | |
| Notes | Study reported in 1 abstract (Homnick 1996, n = 14) and 1 full‐text manuscript (Homnick 1998, n = 22), which is the primary reference for this study. Jadad score: 0/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported: initial participant "randomised," then alternate allocation. |
| Allocation concealment (selection bias) | High risk | Alternate allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding: not feasible (participants), open‐label (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded: open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysis of 33 hospitalisations in 22 participants. Multiple enrolment to increase sample size. Although authors stated no participants withdrew from the study, it was unclear if any declined further participation in hospitalisations subsequent to their first admission. |
| Selective reporting (reporting bias) | Unclear risk | Pulmonary function tests after 1st week were measured but not reported. |
| Other bias | High risk | Difference in baseline ages between groups (16 years with Flutter device vs 12 years with CCPT), and almost significant improvement in clinical score in favour of CCPT group not discussed. CCPT treatments were twice as long as Flutter device treatments (30 min with CCPT vs 15 min with Flutter), with uncertain consequences. The 22 participants were measured during 33 admissions, with repeated measures on 7/22 participants, some of whom were admitted more than twice (and who may, therefore, have been over‐represented). Apparently, subgroup analysis of first 22 admissions produced similar results although data not shown. Unclear how subsequent admissions were dealt with in terms of group allocation. |
McIlwaine 1991.
| Study characteristics | ||
| Methods | Single‐centre (Vancouver, Canada), randomised cross‐over study with 3 arms 18 participants with CF randomly allocated into 3 groups (CCPT, PEP or AD) at start of trial, although participants were ultimately to perform each treatment at home for 2 months. There was a 4‐week washout period between each 2‐month treatment cycle, consisting of CCPT. |
|
| Participants | 18 participants with CF (diagnostic criteria not described) Age and gender not reported in publication Unpublished data stated age range 11–27 years; mean age 17.3 years. Pulmonary severity ranged from mild to severe (FVC range, 38–117% of predicted value and Shwachman score, 50–94). |
|
| Interventions |
CCPT: standard PD with percussion PEP: applied with a mask combined with huffing, in a seated position, which utilised collateral ventilation AD: utilising airflow and breathing control to move secretions, in which respiratory rate, amplitude and effort were modulated to mobilise and clear secretions No details of prescription reported (duration, frequency); no details of dropouts or withdrawals. |
|
| Outcomes | Outcomes not explicitly declared. Apparent from results that sputum "net weight" was measured, although it is unclear when this was done or how it was measured (or whether single or repeated treatments). Pulmonary function tests (FVC, FEV1, FEF25–75, FEV1/FVC) performed at baseline and at the beginning and end of each 2‐month intervention period. All 3 abstracts report on "more independence, less discomfort, more 'sense of control'" and "less interruption of daily life", although how and when this was measured was not reported. | |
| Notes | Study reported in 3 abstracts (Davidson 1988, McIlwaine 1988, McIlwaine 1991), the most recent of which is the primary reference for this study, from the 17th European CF conference, although some details were extracted from the other abstracts. An unpublished draft of the full manuscript for this study was provided to the Cochrane Cystic Fibrosis and Genetic Disorders Editorial Base, which provided material additional details. Jadad score: 1/5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Quote: "Using a random cross‐over design, patients were divided into groups." Numbers in each group and order of treatments not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No withdrawals or dropouts reported in any of the abstracts. In unpublished draft of full manuscript, it is apparent 4 participants did not complete the study and were excluded from the analysis: 1 hospitalised during first treatment period (PD) due to an exacerbation (ABPA); 1 withdrew at the end of the 1st treatment period after requiring prednisone to control an allergic reaction to an antibiotic; 2 participants refused to cross over after the 1st treatment period (AD group) due to perceived greater effectiveness at mobilising sputum. |
| Selective reporting (reporting bias) | High risk | No numeric data provided for any outcome in the study, and outcomes for pulmonary function simply described as non‐significant. 2/3 abstracts indicated that AD produced "significantly more sputum" than CCPT, but no data on actual values or magnitude of difference provided. |
| Other bias | High risk | Each participant used each technique for 2 months, and had 1 month of washout between techniques. The washout was CCPT, which was 1 of the 3 techniques. Unclear what impact this may have had. Unclear how multiple repeated pulmonary function values were performed or analysed. Unclear how "independence, discomfort, sense of control or interruption to daily life" were measured, although these were reported in all 3 abstracts. Supported in part by grants from the BC Lung Association, BC Health Care Research Foundation and the Vancouver Foundation, Canadian Pacific Airlines & Astra Meditech (Manufacturer of PEP masks). |
McIlwaine 1997.
| Study characteristics | ||
| Methods | Single‐centre (Vancouver, Canada), parallel design RCT with 2 arms 40 participants with CF stratified by FEV1, gender and age and matched pairs were randomly allocated into either twice daily CCPT or PEP for 12 months |
|
| Participants | 40 children and adolescents with confirmed CF diagnosis (Gibson Cooke method) stable disease and Shwachman scores 52–93 and judged to be "competent and compliant in daily PD & Perc" and in performing pulmonary function tests. Participants were excluded if within 1 month of IV antibiotics, hospital admission or other intensive therapies. No DNase or inhaled antimicrobials. Age range 6–17 years 36 participants completed. 2 from each group dropped out because of non‐compliance with treatment (performance of physiotherapy < 85% of the time) or non‐attendance at clinic. Participants with equivalent FEV1, age and sex were stratified. CCPT (n = 20): mean age 9.8 years; range 6–14 years; 12 males, 8 females PEP (n = 20): mean age 10.4 years; range 6–17 years; 10 males, 10 females < 85% compliance with performance of twice a day treatment was considered an endpoint for non‐compliance and participants were removed from the study. |
|
| Interventions |
CCPT: participant assumed each of 5 or 6 PD positions. In each position, the chest wall was percussed for 3–5 min, followed by deep breathing exercises combined with vibration on expiration, forced expirations and vigorous coughing, i.e. 5–7 min in each position (30 min in total) twice daily. PEP: a manometer was used to create a steady PEP of 10–20 cmH2O during the middle part of expiration. Treatment in a sitting position; the participant breathed in and out through the mask 15 times (approximately 2 min). Tidal volume inspirations and expiration was slightly active against the mask. Participants then removed the mask and performed 2 or 3 forced expirations, followed by a cough to clear secretions that had been mobilised to the central airways. This was followed by a 1‐ to 2‐min period of relaxed controlled breathing. The sequence was repeated 6 times (approximately 20 min) twice daily. No study participant received DNase or inhaled antimicrobials during study period. |
|
| Outcomes | FEV1 was primary outcome measure. FVC, FEV1 and FEF25–75, and clinical status (including Shwachman and Huang scores, sputum samples) were measured at baseline and 3‐month intervals. CXR at baseline and 12 months (Brasfield score). Compliance with physiotherapy "monitored closely" throughout study via daily record of treatment adherence and a monthly questionnaire (physical activity level, how the participant was feeling, amount of cough, sputum productivity, participants impression of the technique, adverse reactions, compliance). Secondary outcomes included number of hospitalisations, CXR and participant's own evaluation of the technique. | |
| Notes | Study reported in 2 abstracts (McIlwaine 1995, McIlwaine 1996) and 1 full‐text manuscript (McIlwaine 1997), which is the primary reference for this study, from the Journal of Pediatrics. Details in abstracts corresponded with those in the paper. Jadad score: 3/5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified by FEV1, gender and age and matched pairs were "randomly assigned by computer." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not blinded (physiotherapist). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians, pulmonary function technicians and radiologist blinded to the method of physiotherapy treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 from each group dropped out because of non‐compliance with treatment (performance of physiotherapy < 85% of the time) or non‐attendance at clinic. |
| Selective reporting (reporting bias) | High risk | Numerical data not reported for Shwachman score, Huang score, CXR, microbiology and preference. Lung function outcomes only reported graphically as change from baseline. |
| Other bias | Unclear risk | Only participants "competent and compliant in daily PD & Perc" were recruited and retained in the study. Effect of non‐compliance unclear. |
McIlwaine 2010.
| Study characteristics | ||
| Methods | Single‐centre (Vancouver, Canada), cross‐over RCT 36 participants were "matched as pairs" by FEV1, Shwachman scores, age and gender, and then members of each pair were randomised by computer into either PD with percussion or AD for 2 years, with cross‐over at 12 months (only 1st year reported). Participants were instructed to perform ≥ 2 treatments each day. Note: only results from the first year of the study were reported, as 10/17 participants who had completed performing AD for the first year refused to change back to PD for the 2nd year. |
|
| Participants | 36 participants aged 12–18 years with confirmed diagnosis of CF who were compliant in performing daily physiotherapy using the PD technique for ≥ 1 year prior to the study were enrolled and matched as pairs, using FEV1 (within 15%) as the primary match, and Shwachman scores (within 15 points), age (within 3 years) and same sex as the secondary match. CCPT (n = 18): mean age 13.7 years, 9 males AD (n = 18): mean age 14.0 years, 9 males Of 3 dropouts, 2 were from PD group (1 pregnancy, 1 ABPA) and 1 from AD group (non‐compliance). 1 female, the other 2 not reported. |
|
| Interventions |
CCPT: PD performed in each of 5 or 6 PD positions. In each position, the chest wall was percussed by a 2nd person for 3–5 min followed by deep breathing exercises combined with vibrations on expiration. This was followed by 2–3 huffs. The participant was then encouraged to cough and expectorate any mucus produced. This was followed by a short period of relaxed controlled breathing. The cycle was repeated for each PD position. PD was performed twice daily with 6 positions drained in the morning and 5 in the evening. Treatment took approximately 30 min to complete. AD: performed in a sitting position twice daily. The participant began by inhaling a tidal volume breath followed by a 3‐second pause at the end of inspiration. Expiration was performed through the mouth as a sigh, with the participant actively exhaling down to RV. This cycle of tidal volume breathing was repeated, commencing at a low lung volume level until the mucus located in the small airways was felt to have moved proximal to the middle airways. The participant then adjusted their level of breathing to a medium lung volume and then to a high lung volume, gradually mobilising the mucus up the airway towards the mouth. After the mucus reached the mouth and was expelled, the above procedure was repeated until the participant felt that all the mucus was evacuated from the chest. The length of time to complete this technique varied with each participant, but on average required 30 min. |
|
| Outcomes | The primary outcome measured was the rate of decline in FEV1 % predicted. Shwachman and Huang scores measured at baseline. Pulmonary function tests (FVC, FEV1, FEF25–75) were measured at 3‐monthly intervals. Competency of technique was performed at each clinical visit and a sputum sample was obtained. Adherence was monitored by daily diary (number and duration of treatments, activity level and sputum production) and monthly telephone call from physiotherapist (to check adherence, health status and answer any concerns). The number and duration of hospitalisations, sputum bacteriology, antibiotics and participant evaluation of the technique were recorded. Statistical analysis clearly explained. | |
| Notes | Study was reported in 2 abstracts (Davidson 1992, McIlwaine 1992) and 1 full‐text manuscript (McIlwaine 2010), which is the primary reference for this study, from Pediatric Pulmonology, although some details were extracted from the abstracts. Jadad score: 3/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants matched as pairs, using FEV1; within 15% as the primary match, and Shwachman scores (within 15 points), age (within 3 years), and same sex as the secondary match. Each member of pair then randomly assigned to either group by computer. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not blinded (personnel: participants were asked to demonstrate technique at each clinic visit). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians and pulmonary function technicians blinded to the method of physiotherapy treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant attrition at 12‐month cross‐over. 10/17 participants who completed 1st year preferred AD and refused to change back to PD. During 1st year, 2 dropouts from PD group (1 pregnant, 1 grew aspergillosis) and 1 dropout from AD group (non‐compliance). |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes clearly reported, but no numerical data provided on other outcomes measured, e.g. preference, number of treatments performed, length of treatments, activity level, sputum production, sputum bacteriology. |
| Other bias | High risk | This cross‐over study was supposed to incorporate 2 arms (1 year for each arm). However, a significant proportion of people using AD during the 1st year refused to return to CCPT and of the 7/17 who agreed to return to performing PD, 5 used AD to augment their PD (strong cross‐over effect in 2nd year). The numbers in the AD arm refusing to cross over differed slightly from both abstracts (8/18 refusing) to the primary paper (10/17 refusing). The Shwachman score at enrolment of 36 participants also differed slightly between the Davidson abstract (45–98) and the McIlwaine abstract and primary paper (65–98). Only year 1 data used and no formal cross‐over analysis performed. |
Reisman 1988.
| Study characteristics | ||
| Methods | Single‐centre (Toronto, Canada), prospective parallel design RCT 67 participants with established CF diagnosis and mild or moderate disease were stratified by age, sex and pulmonary impairment before being randomised within each stratum into either CCPT plus FET or FET only for 3 years (although mean follow‐up in both groups was 2.4 years). Participants were instructed to have ≥ 2 treatments each day. |
|
| Participants | 67 participants aged 7–21 years diagnosed with CF (elevated sweat chloride levels, characteristic clinical findings or positive family history) and mild‐to‐moderate disease (FEV1 > 40% predicted) were enrolled. Participants were stratified for age (7–11 years, 12–16 years and 17–21 years), sex and pulmonary impairment (mild: FEV1 > 80% predicted; moderate: FEV1 40–80% predicted), then randomly assigned within each stratum to 1 of 2 groups. 63/67 participants completed study (2 sisters in the CCPT group moved to another city 6 months after the study began, and 2 participants in the FET group withdrew after 5 and 6 months because of family anxiety associated with discontinuation of CCPT. Neither of these had demonstrated any physical decline). CCPT with FET (n = 30): mean age 12.6 (SD 4.2) years; 18 males, 12 females FET alone (n = 33): mean age 11.8 (SD 3.0) years; 20 males, 13 females |
|
| Interventions | Both interventions were prescribed twice daily (although 8 participants in FET group and 5 participants in CCPT with FET group reported completing all therapy in a single session). In addition to verbal instructions, all participants received a written outline of their specific protocol, and they were asked to keep a diary reporting adherence to their physiotherapy regimen. CCPT: performed according to any previous routine: PD accompanied by percussion. Participants were instructed in the routine PD positions to be performed each day, with 8 min of percussion per position. Percussion was performed manually or with the aid of a mechanical percussor. Several brands of percussor were used, but all had similar stroke force and frequency. This group were also instructed to perform FET as below. FET: participants were told to discontinue PD percussion. They were taught deep breathing with maximal expiratory effort and coughing throughout inhalational therapy, which consisted of β2‐bronchodilator inhalation delivered by mask and compressor. The technique of FET consisted of 2 maximal inspirations, each followed by a prolonged, controlled, forced expiration, and then 3 normal quiet inspirations, each followed by a prolonged, controlled, forced expiration. A minimum of 3 coughs performed, or coughs performed until there was no more sputum to expectorate. All participants advised to perform as much physical activity and sport as they could, which was monitored by questionnaire. The mean time per day spent on physiotherapy before the study period was the same in both groups (around 36 min). |
|
| Outcomes | Posteroanterior and lateral chest radiographs (Brasfield CXR score) and FVC, FEV1, FEF25–75, were obtained twice every year in conjunction with scheduled clinic visits Shwachman scores, hospital days and 24‐hour sputum were also measured every 6 months. A 'Jones Stage 1' exercise test was conducted at baseline and after ≥ 1 year (work capacity, respiratory rate and HR). Exacerbations were treated with IV antibiotics and "vigorous physiotherapy." Physical activity was monitored using an annual questionnaire and adherence to the physiotherapy regimen was evaluated annually and scored with the scoring system of Passero and colleagues (Passero 1981). |
|
| Notes | Study reported in 1 full‐text manuscript (Reisman 1988), which is the primary reference for this study. Jadad score: 2/5. Trial over 2.4 years, data given as decline per year. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported. Participants were stratified by age, sex and FEV1 and then (quote) "randomly assigned within each stratum to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not reported (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants from each group withdrew: 2 sisters from the PD plus FET group as they moved away after 6 months; and 2 from the FET group after 5 and 6 months due to anxiety over stopping PD. None of these 4 demonstrated any physical decline. Only 25 participants completed exercise testing (younger participants unwilling to complete). Sputum microbiology only reported rather than volume or weight in 24 hours from each technique. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Sputum microbiology only reported rather than volume or weight in 24 hours from each technique. |
| Other bias | Unclear risk | The study was designed as a 3‐year prospective trial. The reason for early termination at 2.4 years was not reported but may have related to the greater rate of decline in the FET only group, and numbers of exacerbations in the FET group. On face value, the number of hospitalisations and hospital days (due to exacerbations) during study period appeared substantially greater in FET group (even when outlier removed), although this was apparently non‐significant. |
Sontag 2010.
| Study characteristics | ||
| Methods | Multicentre (20 centres, study based in Denver, Colorado, USA), prospective parallel design RCT 166 participants with CF were stratified by age and randomly assigned to CCPT, FD or HFCWO. |
|
| Participants | 166 participants with established CF diagnosis (sweat chloride or genotype with 2 documented CFTR mutations), and FEV1 > 45% were randomly assigned to CCPT, FD or HFCWO, using electronic randomisation, stratified by age (children: 7–11 years, adolescents: 12–17 years, adults: ≥ 18 years). Age range of adults not reported. Children: n = 86, 61 completed study, 25 withdrew (14 from CCPT, 9 from FD and 2 from HFCWO). Adolescents: n = 44, 30 completed study, 14 withdrew (12 from CCPT, 1 from FD and 1 from HFCWO). Adults: n = 36, 19 completed study, 17 withdrew (9 from CCPT, 6 from FD and 2 from HFCWO). Excluded if hospitalised for a pulmonary exacerbation or had gross haemoptysis (> 249 mL) within 60 days prior to screening, or a pneumothorax in the 6 months preceding screening. |
|
| Interventions | Participants were trained to perform the treatment twice daily for 20–40 min/session. CCPT: administered by an available carer using a wedge provided to assist with appropriate positioning. Positioning, percussion (vibration) and FET with coughing between each of 6 positions. After each position, participants were instructed to perform 3 FET and cough. Flutter device: self‐administered utilising the Flutter device (Scandipharm, Birmingham, Alabama), incorporating Flutter device airway vibration, and FET with coughing. Flutter device treatment was divided into 3 stages: 1. loosening and mobilisation breaths, followed by 2. mucus mobilisation and 3. expectoration. HFCWO: self‐administered utilising the Vest (Hill‐Rom, Minneapolis, Minnesota) incorporating HFCWO, deep breathing and FET with coughing between each frequency. Each frequency was instructed to be performed for 5 min with deep breathing to TLC every 2 min, and each cycle was followed by 3 FET. |
|
| Outcomes | Clinical status (rate of (FEV1) decline, time to need for IV antibiotics to treat pulmonary exacerbations, use of other pulmonary therapies), anthropometrics, and spirometry (FEV1, FVC, FEF25–75) were assessed every 3 months for 3 years, following a screening visit within 7 days of randomisation. At 'select visits,' a validated Treatment Satisfaction Survey and CF‐specific HRQoL instrument, the Cystic Fibrosis Questionnaire were administered, and adherence was measured using the daily telephone diary. | |
| Notes | Study reported in 3 abstracts (Accurso 2004; Modi 2006; Quittner 2004) and 2 full‐text manuscripts (Sontag 2010; Modi 2010). Sontag 2010 is the primary reference for this study, from Pediatric Pulmonology. Jadad score: 3/5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by age (children: 7–11 years, adolescents: 12–17 years, adults: ≥ 18 years) and randomly assigned using electronic randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not reported (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size of 180 participants not met (with power = 85%) and 3‐year RCT terminated early because of disproportionate dropouts in 3 groups. 56 (33.7%) of participants withdrew from the trial. 15 participants withdrew within 60 days of randomisation and were not included in analyses. 2 were children (1 CCPT, 1 Flutter device), 7 adolescents (6 CCPT, 1 Flutter device) and 6 adults (4 CCPT, 2 Flutter device). 11 of these 15 withdrew on day of randomisation. A further 41 (24 from CCPT group) withdrew after first 60 days and were included in ITT analysis. 23 were children (13 CCPT, 8 Flutter device, 2 HFCWO), 7 adolescents (6 CCPT, 1 HFCWO), 11 adults (5 CCPT, 4 Flutter device, 2 HFCWO). Reasons for dropouts after 60 days (n = 41) were moved or lost to follow‐up (n = 13); lack of time (n = 7); preferred another therapy (n = 4); decrease in health (n = 4); compliance (n = 4); wanted to participate in another study (n = 3); family stress (n = 2); and lack of interest (n = 2); no reasons given for 3 participants. Participants randomised to the HFCC group who withdrew from the study had significantly lower FEV1 % predicted and FVC % predicted (P < 0.03 and P < 0.01) at baseline than those who continued in the study, adjusted for age group. Subgroup analysis showed that within the adolescents, baseline FVC % predicted was significantly higher (P < 0.02) in the HFCC group (94.9%) compared to the CCPT group (84.9%). 110 participants completed the entire study: 61 children (17 CCPT, 20 Flutter device, 24 HFCWO), 30 adolescents (3 CCPT, 11 Flutter device, 16 HFCWO), 19 adults (3 CCPT, 4 Flutter device, 12 HFCWO). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on those who finished the study. |
| Other bias | High risk |
|
Steen 1991.
| Study characteristics | ||
| Methods | Single‐centre (Belfast, Northern Ireland), cross‐over RCT 28 participants with CF were randomly allocated to 1 of 4 treatment groups, each to be carried out for 4 weeks with no washout period, comprising: CCPT and FET, 5 min PEP plus CCPT and FET, PEP alone, and PEP and FET alone. All participants were fully assessed after each 4‐week intervention before being randomly allocated to 1 of the remaining treatment groups. |
|
| Participants | 28 participants were recruited aged 8–21 years (5 severe, 14 moderate, 9 mild disease) with confirmed CF diagnosis (elevated sweat sodium > 70 mmol on 2 occasions) who were able to co‐operate with pulmonary function testing and "highly motivated" to complete the trial. Gender split of population not described. 24 completed the trial, 4 withdrawals: 1 severely affected girl of 18 years died; 1 girl of 8 years became non‐compliant and 2 girls asked to be withdrawn (1 after developing a pneumothorax while on CCPT plus PEP and 1, who was a copious sputum producer, withdrew after 2 days on CCPT plus PEP "as PEP alone was failing to clear secretions" – she had previously carried out PEP alone, which she considered was as effective as CCPT but no better). |
|
| Interventions | Bronchodilator response was assessed by recording PEFR using a Wrights Peak Flow Meter, before and 30 min after salbutamol nebuliser (dose 2.5 mg). PEFR improved by ≥ 10% in 4 participants who had inhaled bronchodilators prior to each physiotherapy treatment throughout the study. CCPT: a combination of PD (the drainage positions as described by Kendig and Clarwick (Kendig 1977)), percussion, breathing exercises and FET consisting of 1 or 2 forced expirations with an open glottis from mid‐lung volume to low‐lung volume followed by a period of relaxed controlled diaphragmatic breathing. CCPT plus PEP: 5 min PEP followed by CCPT as above. PEP delivered using an Astra or Vitapep mask. The participant sat with elbows resting on a table, applied the face mask firmly and, using a slightly active expiration, the outflow valve was adjusted to give an expiratory resistance of 10–15 cmH2O. The participant breathed through the mask 10–15 times followed by forced expiration and cough, if required. The cycle was then repeated. PEP alone: in the sitting position as above. PEP and FET: PEP and FET as described above in the sitting position. FET alone: 5 older participants with "good FET" agreed to carry out FET alone as a separate treatment arm. The treatments were carried out consecutively with no wash‐out period. |
|
| Outcomes | Participants initially underwent clinical assessment and CXR and were given Shwachman and Chrispin‐Norman scores. Daily diaries were kept noting changes in cough, sputum characteristics, wheeze, appetite, exercise tolerance and shortness of breath. Sputum was sent for culture each month and during acute exacerbations. Participants also noted their general impression about each treatment. Data presented on Shwachman and Chrispin‐Norman scores, weight of sputum and pulmonary function (FEV1, FVC, PEFR). Although not explicitly described, presumably the data collection procedure described at baseline was reproduced at each visit: a physiotherapy treatment was carried out with pulmonary function tests being recorded before and at 20 min and 120 min after the completion of treatment. PEFR was measured and FVC and FEV1 using a Vitalograph spirometer. Sputum was collected and weighed over this 2‐hour period. | |
| Notes | Study is reported in 1 abstract (Steen 1985, n = 25) and 1 full‐text manuscript (Steen 1991, n = 28), which is the primary reference for this study. Jadad score: 2/5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported: participants were "randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not reported (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrawn (1 death, 1 non‐compliant, 1 pneumothorax and 1 considered treatment was ineffective). |
| Selective reporting (reporting bias) | High risk | Only 1 pulmonary function figure reported for each intervention. Unclear whether this was from the test recorded before or 20 min or 120 min after the completion of treatments. No numerical data provided for between‐group changes: reported as "no significant alteration" in growth, Shwachman and Chrispin‐Norman scores neither was there any significant change in pulmonary function tests or expectorated sputum weight (between any of the 5 treatments). Data from daily diaries not reported (changes in cough, sputum characteristics, wheeze, appetite, exercise tolerance and shortness of breath). |
| Other bias | High risk |
Astra Medical and Vitalograph provided PEP masks for the study. |
Tonnesen 1982.
| Study characteristics | ||
| Methods | Single‐centre (The National Hospital of Copenhagen, Denmark), cross‐over RCT. 15 participants with CF randomly allocated to either CCPT or PEP, each to be carried out for 4 weeks. Unclear whether washout period between |
|
| Participants | 15 participants with CF. Means of CF diagnosis not specified. 12 had chronic Pseudomonas aeruginosa infection Age range 12–29 years 6 male, 9 female |
|
| Interventions |
PEP breathing mask with a rectifier valve and tracheal tube connection: applied in sitting position, with an expiratory resistance "chosen according to the patient's tolerance" (mean initial resistance 3.9 (SD 0.3) decreased to 3.6 (SD 0.3) at 14 days). PEP delivered via a mask, at home, for 15 min (with 2‐ to 3‐min breaks), 3 times daily for 1 month CCPT: 1–3 times per day given by "the usual therapist" (a mix of participant's mother, father and physiotherapist), with 20–30 min "consisting of vibrations, tapotement, expansion exercises, and standing drainage on a specialized, tilt‐able bed." |
|
| Outcomes | Peak flow, FVC, FEV1, RV, FRC, TLC were obtained at baseline and after 1 month of either PEP or CCPT. Sputum samples taken (unclear at which time points – 24 samples from 14 participants). 12/14 participants had CXR at baseline and 1 month after treatment. The other 2 had "completely normal chest radiographs and were not checked after the treatments." A multifaceted disease severity scoring system was applied where points were assigned on the basis of weight and height, physical activity, presence of clubbed fingers, CXR and CF complications: 1 being very severe and 10 being unaffected. The cohort mean was 5.9. | |
| Notes | Data extraction with assistance from Dr Connor Brenna, Department of Anesthesiology & Pain Medicine in the University of Toronto, Toronto, ON, Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not specified, but the paper stated that 15 participants were assigned to therapy on the basis of a randomised cross‐over design. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not described (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The radiologist assessing paired CXRs for each participant was blind to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 participants recruited, 1 withdrew (and was not included in the analysis). The paper described a participant (14F) who withdrew 2 days into PEP treatment "due to a feeling of secretion stagnation in the lungs," when she and her mother became sceptical about the PEP treatment and felt that CCPT was necessary. The paper noted that the participant "later began mask treatment and postural drainage with apparently good effect." |
| Selective reporting (reporting bias) | Unclear risk | Paper reported that every 14 days, the outpatient check‐ups included lung function examination, sputum culture for bacteria, clinical examination and instructions regarding how to use the mask. However, only data at beginning and end of each month were reported. |
| Other bias | Unclear risk | Potentially the fact that CCPT and PEP were carried out for different durations (15 min with PEP vs 20–30 min with CCPT) and at different frequencies (3 times per day with PEP vs 1–3 times per day with CCPT). Authors described a clinical score where "1 point represents a patient in extremely poor condition and 10 points a completely unaffected patient." Yet they suggest the range in the participant group was 0–9, with mean score of 5.9. |
Tyrrell 1986.
| Study characteristics | ||
| Methods | Single‐centre (Nottingham, UK), cross‐over RCT 19 participants with mild‐to‐moderate CF underwent 1 month of PEP treatment and 1 month of CCPT in random order. |
|
| Participants | 19 participants aged 10–18 years with mild‐to‐moderate CF (Shwachman scores: 47–85 (mean 62), and Chrispin‐Norman CXR scores: 3–20 (mean 12.6)) were enrolled, but 3 excluded from analysis due to non‐compliance (not defined, or when these participants were excluded). 16 participants completed: mean age 13 years; range 10–18 years; 7 male, 9 female Children who showed airway reversibility with salbutamol were asked to use it before treatment throughout the study. How many children did this was not reported. |
|
| Interventions |
CCPT: 2 sessions of PD, percussion and coughing daily PEP: using a manometer, a resistor was attached to the outflow valve such that the participant breathed against a pressure of 10–15 cmH2O: 10 breaths through the mask followed by forced expiratory coughing in the sitting position. Repeated for 20 min twice daily. No washout period described. |
|
| Outcomes | Outcomes recorded at the beginning and end of each month: sputum weight, PEFR, FEV0.75, FVC (using a Vitalograph), before and 20 and 90 min after a single treatment under supervision (either CCPT performed by a parent or treatment with the PEP mask). Diary cards were used to record symptoms (sleep, cough, wheeze, activity, and sputum production, with a maximum score of 13). | |
| Notes | Study reported in 1 abstract (Tyrrell 1985, n = 15) and 1 full‐text manuscript (Tyrrell 1986, n = 19), which is the primary reference for this study. Jadad score: 2/5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments undertaken "in random order," but no details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not reported (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants excluded from analysis due to non‐compliance, but unclear which treatment arm they were in, or what constituted non‐compliance. Only 15 participants shown in Figure 2 of publication, instead of 16. Data on lung function and sputum only presented for a single supervised physiotherapy session, but unclear when in the study this was done or whether the change was calculated from measures at 20 or 90 min after the single treatment. |
| Selective reporting (reporting bias) | High risk | No numerical data related to change after 1 month of treatment were reported. Although authors reported "no significant difference in FEV0.75, FVC, or PEFR at the end of the two treatment periods was shown," it was unclear how the analysis was done or whether this related to within‐ or between‐group changes. Antibiotic consumption "was the same for each month." Mean scores from diary cards reported, but no statistical analysis or granular detail on changes in symptoms of sleep, cough, wheeze, activity or sputum production. Only anecdotal impressions related to preference provided. |
| Other bias | High risk | No washout period between interventions, so risk of carry‐over effects. In Figure 2 of the publication, % change from baseline values in 1 month appeared to be presented, in which all FEV0.75 values from participants in the first PEP arm appeared to have deteriorated. Unclear how the analysis was done. The results apparently averaged for each participant over the intervention period, potentially masking significant variation in clinical status. Data on lung function and sputum were only presented for a single supervised physiotherapy session, but it is unclear when in the study this was done or how it was calculated. |
van Asperen 1987.
| Study characteristics | ||
| Methods | Single‐centre (New South Wales, Australia), cross‐over RCT 13 participants with CF (stable disease and sputum producers) undertook in random order either 4 weeks of PEP mask plus forced expiration and coughing or PD and percussion plus forced expiration and coughing. |
|
| Participants | 13 participants with CF (daily sputum production and no change in treatment in the 2 months prior to the study), 10 completed (2 withdrew due to acute exacerbations and 1 withdrew electively). Age range 7–18 years Gender not reported |
|
| Interventions |
CCPT: standard PD with manual percussion to all areas followed by forced expiration and coughing on a twice daily basis, lasting ≥ 20 min per session. PEP: PEP mask for 10–15 breaths, achieving an expiratory pressure of 10–15 cmH2O, followed by forced expiration and coughing conducted for 20 min on a twice daily basis. No washout period described. |
|
| Outcomes | Baseline evaluation by physiotherapist of sputum volume in the hour from the commencement of the regimen; and spirometry using a vitalograph or a Collins water‐sealed spirometer with recording of FEV1, FVC and FEF25–75. Participants then completed a diary card for 4 weeks (cough score (0–3) day and night; activity score (0–3); sputum volume in the hour after each treatment and PEFR using a Wright mini‐peak flowmeter 1 hour after treatment). | |
| Notes | Study reported in 1 short full‐text manuscript (Van Asperen 1987). Jadad score: 2/5. Published data in litres/second, but % predicted obtained from study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated "randomly in a crossover fashion" to the treatment regimens, but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible (participants), not reported (personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants did not complete: 1 withdrew electively, 2 withdrawn due to acute infective exacerbations. Not reported which treatment arm they were in, or when they withdrew. Sputum only collected from 6 participants on both occasions (quote) "Unfortunately, sputum volumes measured by the physiotherapist were too variable and also insufficient in number to draw any definite conclusions from." PEFR data only reported from 8 participants, recorded "satisfactorily" in 8/10 participants, although criteria for 'satisfactory' not provided. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but only anecdotal impressions related to preference provided. Pretreatment pulmonary function presented as litre/second and % predicted, while post‐treatment pulmonary function only presented as litres/second. |
| Other bias | Unclear risk | The results were averaged for each participant over the 4‐week study period, potentially masking significant variation in clinical status. All participants were "fully active" during the treatment periods and so activity score was not used for comparison. Astra Pharmaceuticals supplied PEP masks, PEFR meters and volume measurers. |
6MWT: 6‐minute walk test; ABPA: allergic bronchopulmonary aspergillosis; AD: autogenic drainage; ANC: absolute neutrophil count; ATS: American Thoracic Society; BMI: body mass index; CCPT: conventional chest physiotherapy; CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator; CXR: chest X‐ray; ERV: expiratory reserve volume; FET: forced expiratory technique; FEV1: forced expiratory volume in one second; FEF25–75: average forced expiratory flow between 25% and 75% of FVC; FRC: functional residual capacity; FVC: forced vital capacity; GOR: gastro‐oesophageal reflux; h: hour; HFCC: high‐frequency chest compression; HFCWO: high‐frequency chest wall oscillation; HR: heart rate; HRR: heart rate reserve; HIC: inspiratory capacity; HRQoL: health‐related quality of life; IPV: intrapulmonary percussive ventilation; IRT: immunoreactive trypsinogen; ITT: intention‐to‐treat; IV: intravenous; min: minute; n: number; NACF: North American Cystic Fibrosis; PD: postural drainage; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; PEP: positive expiratory pressure; QoL: quality of life; Raw: airway resistance; RCT: randomised controlled trial; RV: residual volume; SaO2: oxygen saturation of arterial blood; SD: standard deviation; SGAW: specific airways conductance; SEM: standard error of the mean; SpO2: saturation of haemoglobin with oxygen using pulse oximetry; TGV: thoracic gas volume; TLC: total lung capacity; VC: vital capacity; WBC: white blood cell.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baldwin 1994 | Study < 7 days in duration. Cross‐over: 1 treatment each of 2 regimens over 2 non‐consecutive days. |
| Baran 1977 | Study < 7 days in duration: no valid comparison. |
| Bilton 1992 | Study < 7 days in duration. |
| Braggion 1995 | Study < 7 days in duration: excluded because 2 treatments for 2 days for each of 4 interventions: CCPT vs PEP versus HFCC versus control. |
| Button 2003 | CCPT with and without head‐down tip. Excluded because no valid comparison group. The study focus was on "effect of tip" not effect of CCPT. |
| Cantin 2006 | Study < 7 days in duration. Single treatments and no valid comparison group. |
| Chatham 2004 | Study < 7 days in duration: single treatment cross‐over. "Standard" physiotherapy included ACBT and, therefore, not strictly CCPT as defined in this review. |
| Corten 2020 | Assisted AD vs "standard" airway clearance technique, but the "standard" technique group included a mix of CCPT, Flutter device, ACBT and active play, not possible to extricate CCPT results. |
| de Boeck 1984 | Study < 7 days in duration: 1 treatment on 2 consecutive mornings. Vigorous coughing vs CCPT. |
| Desmond 1983 | CCPT vs no physiotherapy (no active control). |
| Elkins 2005 | Study < 7 days in duration. Single treatments and no valid comparison group. |
| Falk 1984 | Study < 7 days in duration, cross‐over design with 1 treatment session each for 4 regimens over 2 days. |
| Fitzgerald 2005 | No CCPT group or valid comparison group. |
| Ghasempour 2019 | No detail on length of treatment, so potentially < 7 days. Insufficient detail on what 'CCPT' entailed. |
| Giles 1995 | Study < 7 days in duration: randomised cross‐over design (CCPT vs AD) with 2 treatment regimens over 2 days. |
| Grasso 2000 | Excluded because no valid comparison group. Determined effects of different music therapy used as adjunct to CCPT. |
| Hartsell 1978 | Study < 7 days in duration: CCPT vs mechanical percussor, single treatment only. Included non‐CF participants. |
| Hofmeyr 1986 | Study < 7 days in duration and no valid comparison. |
| Holsclaw 1977 | Study < 7 days in duration: no valid comparison. |
| Hristara‐Papadopoulou 2005 | Study < 7 days in duration, 2 single treatments (ACBT and CCPT) administered by physiotherapist at clinic visits approximately 3 months apart. |
| Keller 2001 | Excluded because no valid comparison group. Comparator not a recognised technique for airway clearance (30‐min harmonica playing). Also unclear that the primary intervention was CCPT (described as "individually suited" physiotherapy). |
| Kerrebijn 1982 | Study < 7 days in duration: single treatments comparing CCPT with aerosol treatment. |
| Kirkpatrick 1995 | Study < 7 days in duration: randomised cross‐over design with 2 regimens (manual chest percussion vs acoustic percussion) each performed once over 2 days. 5 participants (ages not reported). |
| Klig 1989 | Study design not randomised or quasi‐randomised. |
| Kluft 1996 | Study < 7 days in duration: randomised cross‐over design, 2 days each of CCPT and HFCWO over a 4‐day period. |
| Konstan 1994 | Study < 7 days in duration: randomised cross‐over design with 3 regimens (CCPT vs Flutter device vs cough) each performed twice over 2 weeks. |
| Lannefors 1992 | Study < 7 days in duration: randomised cross‐over design with 1 treatment of each of 3 regimens over 3 separate days. |
| Lorin 1971 | Study < 7 days in duration: no valid comparison groups. |
| Lyons 1992 | Study < 7 days in duration: single treatments over 4 days. |
| Maayan 1989 | Study < 7 days in duration; randomised cross‐over design with 1 treatment each of 4 regimens (CCPT vs 2 aerosols vs CCPT plus aerosol). |
| Majaesic 1996 | Study < 7 days in duration: randomised cross‐over design comparing CCPT vs HFCC. |
| Marks 2004 | Study < 7 days in duration, randomised cross‐over design of single treatment of IPV vs CCPT. |
| Martinez Rodriguez 2017 | Insufficient detail on chest physiotherapy to be clear it was CCPT – pulmonary rehabilitation programme vs "chest physiotherapy." |
| Maxwell 1979 | Study < 7 days in duration: randomised cross‐over design of single treatment of mechanical vs manual percussion. |
| McCarren 2006 | Study < 7 days in duration: single treatments and no valid comparison groups. |
| McDonnell 1986 | Study < 7 days in duration: no valid comparisons. |
| Morris 1982 | Study < 7 days in duration: randomised cross‐over design of single treatments of CCPT vs mechanical percussor. |
| Natale 1994 | Study < 7 days in duration: randomised cross‐over comparison of 3 regimens over 5 days, 1 treatment each of IPV, IPV plus CCPT, CCPT alone. |
| Newhouse 1998 | Study < 7 days in duration: randomised cross‐over design with single interventions of 3 regimens (CCPT vs IPV vs Flutter device). |
| Oberwaldner 1986 | Excluded because of ABC 'withdrawal' study design (A = 10 months of high‐pressure PEP, then B = 2 months reverting to prestudy treatment, then C = 6 months PEP). |
| Orlik 2000 | Study of each intervention < 7 days in duration and non‐randomised study design. |
| Orlik 2001 | Study design not randomised or quasi‐randomised. |
| Phillips 1998 | Study < 7 days in duration. |
| Placidi 2006 | Study < 7 days in duration. |
| Pryor 1979 | Study < 7 days in duration: randomised cross‐over with only 2 treatments (CCPT vs FET). |
| Regelmann 1990 | Excluded because no valid comparison group. Conventional CPT vs 'other.' |
| Reix 2012 | Study < 7 days in duration. |
| Roos 1987 | CCPT group included AD and PEP. |
| Rossman 1982 | Study < 7 days in duration: randomised cross‐over design with 1 treatment each of 5 regimens over 5 days. |
| Salh 1989 | Study < 7 days in duration in the exercise vs CCPT arms. |
| Samuelson 1994 | Study < 7 days in duration, cross‐over single treatment RCT of CCPT vs therabed. |
| Sanchez Riera 1999 | Study < 7 days in duration. |
| Scherer 1998 | Study < 7 days in duration, randomised cross‐over comparison of 1 treatment each of 5 regimens (2 forms of high‐frequency oral airway and 2 forms of chest wall oscillation vs CCPT). |
| Skopnik 1986 | Study < 7 days in duration. |
| Steven 1992 | Study < 7 days in duration. |
| Stites 2006 | Study < 7 days in duration, single treatment cross‐over comparison of CCPT or HFCWO. Cross‐over interval variable between 72 hours and 10 days. |
| Sutton 1985 | Study < 7 days in duration. |
| Tannenbaum 2007 | No CCPT group. |
| Tonnesen 1984 | Cross‐over non‐randomised study, second arm prospective (first arm retrospective); CCPT vs PEP. |
| van der Schans 1991 | Study < 7 days in duration: single treatments and no CCPT group. |
| van Hengstum 1988 | Study not randomised or quasi‐randomised and < 7 days in duration. |
| Varekojis 2003 | Study < 7 days in duration: 2 days for each treatment. |
| Verboon 1986 | Study < 7 days in duration and no valid comparison. |
| Warwick 1990 | Study < 7 days in duration; randomised cross‐over design with 1 treatment each of 4 regimens. |
| Warwick 1991 | Non‐randomised study with prospectively collected pulmonary function data on 16 people with CF compared with their retrospectively retrieved pulmonary function data. |
| Warwick 2004 | Study < 7 days in duration, 4 single treatment sessions of CCPT or HFCC over 4 days. |
| White 1997 | Study < 7 days in duration: single treatments and no CCPT group. |
| Williams 2001 | Study < 7 days in duration. 48 hours between single treatments. |
| Wong 2000 | Study < 7 days in duration. |
ACBT: active cycle of breathing therapy; AD: autogenic drainage; CCPT: conventional chest physiotherapy; CF: cystic fibrosis; FET: forced expiratory technique; HFCC: high‐frequency chest compression; HFCWO: high‐frequency chest wall oscillation; IPV: intrapulmonary percussive ventilation; PEP: positive expiratory pressure.
Differences between protocol and review
At the 2022 update, we added the secondary outcome of 'Mucus wet or dry weight' as this outcome has been used frequently in airway clearance studies in recent years and is commonly perceived to be a measure of expectorated mucus volume. Also at this update, we added summary of findings tables in line with current Cochrane guidance.
Contributions of authors
Up to 2016
Eleanor Main, Ammani Prasad and the Cochrane Cystic Fibrosis and Genetic Disorders Group conducted searches for relevant studies.
Eleanor Main, Ammani Prasad and Cees van der Schans wrote the protocol for the review and independently evaluated which studies should be included.
Eleanor Main and Ammani Prasad extracted data from included studies, liaised with the study authors to obtain additional data, performed data entry, data analysis, interpretation of the results and wrote the review.
Eleanor Main acted as guarantor for this review.
From 2016
Eleanor Main, Sarah Rand and the Cochrane Cystic Fibrosis and Genetic Disorders Group conducted searches for relevant studies.
Eleanor Main wrote the protocol for the review and Eleanor Main and Sarah Rand independently evaluated which studies should be included.
Eleanor Main and Sarah Rand extracted data from included studies, liaised with the study authors to obtain additional data, performed data entry, data analysis, interpretation of the results and wrote the review.
Eleanor Main acts as guarantor for this review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health and Care Research, UK
This systematic review was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
EM: none.
SR: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arens 1994 {published data only}
- Arens R, Gozal D, Omlin KJ, Vega J, Boyd KP, Keens TG, et al. Comparative efficacy of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 1993;Suppl 9:239. [CFGD REGISTER: PE24a] [DOI] [PubMed] [Google Scholar]
- Arens R, Gozal D, Omlin KJ, Vega J, Boyd KP, Keens TG, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1994;150(4):1154-7. [CFGD REGISTER: PE24b] [DOI] [PubMed] [Google Scholar]
Bain 1988 {published data only}
- Bain J, Bishop J, Olinsky A. Evaluation of directed coughing in cystic fibrosis. British Journal of Diseases of the Chest 1988;82(2):138-48. [CFGD REGISTER: PE11] [DOI] [PubMed] [Google Scholar]
Bauer 1994 {published data only}
- Bauer M, Schoumacher R. Comparison of efficacy of manual and mechanical percussion in cystic fibrosis. Pediatric Pulmonology 1990;Suppl 5:249. [CFGD REGISTER: PE21a] [Google Scholar]
- Bauer ML, McDougal J, Schoumacher RA. Comparison of manual and mechanical chest percussion in hospitalized patients with cystic fibrosis. Journal of Pediatrics 1994;124(2):250-4. [CFGD REGISTER: PE21b] [DOI] [PubMed] [Google Scholar]
Cerny 1989 {published data only}
- Cerny FJ. Relative effects of bronchial drainage and exercise for in-hospital care of patients with cystic fibrosis. Physical Therapy 1989;69(8):633-9. [CFGD REGISTER: PE13] [DOI] [PubMed] [Google Scholar]
Costantini 2001 {published data only}
- Costantini D, Brivio A, Brusa D, Delfino R, Fredella C, Russo M, et al. PEP mask versus postural drainage in CF infants a long-term comparative trial. Pediatric Pulmonology 2001;Suppl 22:308. [CFGD REGISTER: PE94c] [Google Scholar]
- Costantini D, Brivio A, Delfino R, Fredella C, Russo MC, Sguera A. PEP mask versus postural drainage in CF infants a long-term comparative trial. In: Proceedings of the 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P100. [CFGD REGISTER: PE94b]
- Costantini D, Brivio A, Delfino R, Sguera A, Brusa D, Padoan R, et al. PEP mask versus postural drainage in CF infants. Pediatric Pulmonology 1998;Suppl 17:342. [CFGD REGISTER: PE94a] [Google Scholar]
Dadparvar 1995 {published data only}
- Dadparvar S, Darbee J, Jehan A, Bensel K, Slizofski WJ, Holsclaw D. Tc-DIPA aerosol ventilation evaluates the effectiveness of PEP mask in the treatment of cystic fibrosis. European Respiratory Journal 1995;8(Suppl 19):177s. [CFGD REGISTER: PE42b] [Google Scholar]
- Darbee J, Dadparvar S, Bensel K, Jehan A, Watkins M, Holsclaw D. Radionuclide assessment of the comparative effects of chest physical therapy and positive expiratory pressure mask in Cystic Fibrosis. Pediatric Pulmonology 1990;Suppl 5:251. [CFGD REGISTER: PE42a] [Google Scholar]
Gaskin 1998 {published data only}
- Gaskin L, Corey M, Shin J, Reisman JJ, Thomas J, Tullis DE. Long term trial of conventional postural drainage and percussion vs. positive expiratory pressure. Pediatric Pulmonology 1998;Suppl 17:345. [CFGD REGISTER: PE96] [Google Scholar]
Giles 1996 {published data only}
- Giles D, Sontag M, Wagener J, Accurso F. Effect of one month of treatment with flutter valve or postural drainage and clapping on pulmonary function and sputum recovery in cystic fibrosis. Pediatric Pulmonology 1996;Suppl 13:307. [CFGD REGISTER: PE70] [Google Scholar]
Gondor 1999 {published data only}
- Gondor M, Nixon PA, Mutich R, Rebovich PJ, Orenstein DM. Comparison of flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1999;28(4):255-60. [CFGD REGISTER: PE71b] [DOI] [PubMed] [Google Scholar]
- Gondor M, Nixon PA, Rebovich PJ, Orenstein DM. A comparison of the flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1996;Suppl 13:307. [CFGD REGISTER: PE71a] [DOI] [PubMed] [Google Scholar]
Hare 2002 {published data only}
- Hare KL, Homnick DN, Cucos D, Marks JH. The PercussiveTech HF device compared to standard chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 2002;Suppl 24:316. [CFGD REGISTER: PE139] [Google Scholar]
Homnick 1995 {published data only}
- Homnick D, Spillers C, White F. The intrapulmonary percussive ventilator compared to standard aerosol therapy and chest physiotherapy in the treatment of patients with cystic fibrosis. Pediatric Pulmonology 1994;Suppl 10:213. [CFGD REGISTER: PE27a] [Google Scholar]
- Homnick DN, White F, Castro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatric Pulmonology 1995;20(1):50-5. [CFGD REGISTER: PE27b] [DOI] [PubMed] [Google Scholar]
Homnick 1998 {published data only}
- Homnick DN, Anderson K, Marks JH. Comparison of the flutter device to standard chest physiotherapy in hospitalized patients with cystic fibrosis: a pilot study. Chest 1998;114(4):993-7. [CFGD REGISTER: PE72b] [DOI] [PubMed] [Google Scholar]
- Homnick DN, Marks JH. Comparison of the flutter device to standard chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 1996;Suppl 13:308. [CFGD REGISTER: PE72a] [DOI] [PubMed] [Google Scholar]
McIlwaine 1991 {published and unpublished data}
- Davidson AG, McIlwaine PM, Wong TK, Nakielna EM, Pirie GE. Physiotherapy in cystic fibrosis: a comparative trial of positive expiratory pressure, autogenic drainage and conventional percussion and drainage techniques. Pediatric Pulmonology 1988;Suppl 2:137. [CFGD REGISTER: PE44a] [Google Scholar]
- McIlwaine PM, Davidson AG, Wong LT, Pirie GE, Nakielna EM. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of cystic fibrosis. Excerpta Medica 1988;74:R(d)3. [CFGD REGISTER: PE44b] [Google Scholar]
- McIlwaine PM, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of cystic fibrosis. In: 17th European Cystic Fibrosis Conference; 1991 Jun 18-21; Copenhagen, Denmark. 1991:S8.4. [CFGD REGISTER: PE44c]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage therapy in the treatment of cystic fibrosis (as supplied 21 July 2003). Data on file 2003. [CFGD REGISTER: PE44f]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage therapy in the treatment of cystic fibrosis. Unpublished article 2014. [CFGD REGISTER: PE44d]
McIlwaine 1997 {published data only}
- Button B, Herbert R, Maher C. Positive expiratory pressure therapy better maintains pulmonary function than postural drainage and percussion in patients with cystic fibrosis. Australian Journal of Physiotherapy 1998;44(4):285-6. [CFGD REGISTER: PE54d] [Google Scholar]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Israel Journal of Medical Sciences 1996;32(Suppl):S193. [CFGD REGISTER: PE54b] [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Journal of Pediatrics 1997;131(4):570-4. [CFGD REGISTER: PE54c] [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Pediatric Pulmonology 1995;Suppl 12:268. [CFGD REGISTER: PE54a] [DOI] [PubMed] [Google Scholar]
McIlwaine 2010 {published data only}
- Davidson AG, Wong LT, Pirie GE, McIlwaine PM. Long-term comparative trial of conventional percussion and drainage physiotherapy versus autogenic drainage in cystic fibrosis. Pediatric Pulmonology 1992;14(S8):235. [CFGD REGISTER: PE47a] [Google Scholar]
- McIlwaine M, Wong LT, Chilvers M, Davidson GF. Long-term comparative trial of two different physiotherapy techniques; postural drainage with percussion and autogenic drainage, in the treatment of cystic fibrosis. Pediatric Pulmonology 2010;45(11):1064-9. [CFGD REGISTER: PE47c] [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Long-term comparative trial of conventional percussion and drainage physiotherapy versus autogenic drainage in cystic fibrosis. In: 11th International Cystic Fibrosis Congress; 1992 Aug 22-27; Dublin, Ireland. 1992:AHP32. [CFGD REGISTER: PE47b]
Reisman 1988 {published data only}
- Reisman JJ, Rivington-Law B, Corey M, Marcotte J, Wannamaker E, Harcourt D, et al. Role of conventional physiotherapy in cystic fibrosis. Journal of Pediatrics 1988;113(4):632-6. [CFGD REGISTER: PE12] [DOI] [PubMed] [Google Scholar]
Sontag 2010 {published data only}
- Accurso FJ, Sontag MK, Koenig JM, Quittner AL. Multi-center airway secretion clearance study in cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314. [CFGD REGISTER: PE152a] [Google Scholar]
- Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology 2010;35(9):1028-37. [CFGD REGISTER: PE152e] [DOI] [PubMed] [Google Scholar]
- Modi AC, Sontag MK, Koenig JM, Accurso FJ, Quittner AL, Investigators and Coordinators of the Airway Secretion Clearance Study. Adherence to airway clearance therapies in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl:S97. [CFGD REGISTER: PE152c] [Google Scholar]
- NCT00839644. Airway secretion clearance in cystic fibrosis. clinicaltrials.gov/show/NCT00839644 (first received 9 February 2009). [CFGD REGISTER: PE152g]
- Quittner AL, Modi AC, Accurso FJ, Koenig JM, Sontag MK, Oermann C, et al. Treatment satisfaction, health-related quality of life and airway clearance therapies in patients with cystic fibrosis. Pediatric Pulmonology 2004;8(Suppl 27):314. [CFGD REGISTER: PE152b] [Google Scholar]
- Sontag MK, Quittner AL, Modi AC, Koenig JM, Giles D, Oermann CM, et al. Lessons learned from a randomized trial of airway secretion clearance techniques in cystic fibrosis. Pediatric Pulmonology 2010;45(3):291-300. [CFGD REGISTER: PE152d] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steen 1991 {published data only}
- Steen HJ, Redmond AO, O'Neill D, Beattie F. Evaluation of the PEP mask in cystic fibrosis. Acta Paediatrica Scandinavia 1991;80(1):51-6. [CFGD REGISTER: PE14b] [DOI] [PubMed] [Google Scholar]
- Steen HJ, Redmond AO, O'Neill D, Beattie F. Has the PEP mask a role in the management of teenage patients? In: 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3-8; Jerusalem, Israel. 1985:94. [CFGD REGISTER: PE14a]
Tonnesen 1982 {published data only}
- Tonnesen P, Kelstrup M. Self-administered positive end expiratory pressure (PEEP) using a face mask as an alternative to conventional lung physiotherapy. Ugeskrift for Laeger 1982;144(21):1532-6. [CFGD REGISTER: PE5] [PubMed] [Google Scholar]
Tyrrell 1986 {published data only}
- Tyrrell JC, Hiller EJ, Martin J. Face mask physiotherapy in cystic fibrosis. Archives of Disease in Childhood 1986;61(6):598-611. [CFGD REGISTER: PE31a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell JC, Martin J, Hiller EJ. 'PEP' mask physiotherapy in cystic fibrosis. In: 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3-8; Jerusalem, Israel. 1985:23. [CFGD REGISTER: PE31b]
van Asperen 1987 {published data only}
- Asperen PP, Jackson L, Hennessy P, Brown J. Comparison of a positive expiratory pressure (PEP) mask with postural drainage in patients with cystic fibrosis. Australian Paediatric Journal 1987;23(5):283-4. [CFGD REGISTER: PE10] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baldwin 1994 {published data only}
- Baldwin DR, Hill AL, Peckham DG, Knox AJ. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respiratory Medicine 1994;88(1):49-53. [CFGD REGISTER: PE19] [DOI] [PubMed] [Google Scholar]
Baran 1977 {published data only}
- Baran D, Penalosa A, Degre S. Physical working capacity before and after chest physiotherapy in cystic fibrosis. Cystic Fibrosis 1977;-:239-44. [CFGD REGISTER: PE67] [Google Scholar]
Bilton 1992 {published data only}
- Bilton D, Dodd M, Webb AK. The benefits of exercise combined with physiotherapy in cystic fibrosis. Pediatric Pulmonology 1990;Suppl 5:253. [CFGD REGISTER: PE41a] [Google Scholar]
- Bilton D, Dodd ME, Abbot JV, Webb AK. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respiratory Medicine 1992;86(6):507-11. [CFGD REGISTER: PE41b] [DOI] [PubMed] [Google Scholar]
Braggion 1995 {published data only}
- Braggion C, Cappelletti LM, Cornacchia M, Zanolla L, Mastella G. Short-term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross- over randomized study. Pediatric Pulmonology 1995;19(1):16-22. [CFGD REGISTER: PE26c] [DOI] [PubMed] [Google Scholar]
- Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Mastella G. Short-term effects of 3 physiotherapy (CPT) regimens in cystic fibrosis (CF) patients hospitalized for a pulmonary exacerbation: a cross-over randomized trial. In: 18th European Cystic Fibrosis Conference; 1993 May 21-26; Madrid, Spain. 1993:W9.3. [CFGD REGISTER: PE26a] [DOI] [PubMed]
- Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Matella G. Short-term effects of three chest physiotherapy regimens on patients with cystic fibrosis hospitalized for pulmonary exacerbation: a crossover randomized study. Excerpta Medica International Congree Series 1993;1034:239-46. [CFGD REGISTER: PE26c] [Google Scholar]
Button 2003 {published data only}PE88
- Button BM, Catto-Smith AG, Olinsky A, Phelan PD, Story I. Newborn screening in cystic fibrosis: the physiotherapist's dilemma in safe and effective treatment – to tip or not to tip? American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A130. [CENTRAL: 404023] [CFGD REGISTER: PE88e] [Google Scholar]
- Button BM, Heine R, Catto-Smith A, Olinsky A, Phelan PD, Story I. A twelve month comparison of standard versus modified chest physiotherapy in twenty infants with cystic fibrosis. Pediatric Pulmonology 1997;Suppl 14:299. [CENTRAL: 208199] [CFGD REGISTER: PE88a] [Google Scholar]
- Button BM, Heine RG, Catto Smith AG, Phelan PD, Olinsky A. Postural drainage and gastro-oesophageal reflux in infants with cystic fibrosis. Archives of Disease in Childhood 1997;76(2):148-50. [CENTRAL: 137610] [CFGD REGISTER: PE88g] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button BM, Heine RG, Catto-Smith A, Olinsky A, Phelan PD, Ditchfield M, et al. The five year follow-up of two groups of newly diagnosed infants with CF randomized to receive standard (with tip) or modified (without tip) physiotherapy during infancy. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P111. [CENTRAL: 354395] [CFGD REGISTER: PE88d]
- Button BM, Heine RG, Catto-Smith A, Olinsky A, Phelan PD, Story I. Chest physiotherapy for children with CF-birth to two years: issues to consider. Netherlands Journal of Medicine 1999;54(Suppl):S18-9. [CENTRAL: 291231] [CFGD REGISTER: PE88b] [Google Scholar]
- Button BM, Heine RG, Catto-Smith AG, Olinsky A, Phelan PD, Ditchfield MR, et al. Chest physiotherapy in infants with cystic fibrosis: to tip or not? A five-year study. Pediatric Pulmonology 2003;35(3):208-13. [CENTRAL: 431301] [CFGD REGISTER: PE88f] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Button BM, Heine RG, Catto-Smith AG, Phelan PD, Olinsky A. Chest physiotherapy, gastro-oesophageal reflux, and arousal in infants with cystic fibrosis. Archives of Disease in Childhood 2004;89(5):435-9. [CENTRAL: 997712] [CFGD REGISTER: PE88i] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button BM, Olinsky A, Catto-Smith A, Story I. The effects of standard and modified physiotherapy positions and states of arousal including non-nutritive sucking, crying and sleep on gastroesophageal reflux in young infants with CF. Pediatric Pulmonology 1999;Suppl 19:289. [CENTRAL: 291232] [CFGD REGISTER: PE88h] [Google Scholar]
- Button BM, Phelan P, Olinsky AD, Catto-Smith AG, Heine RG, Ditchfield M, et al. The five year follow-up of two groups of newly diagnosed infants with CF randomized to receive standard or modified physiotherapy during infancy. Pediatric Pulmonology 2000;Suppl 20:301-2. [CENTRAL: 315354] [CFGD REGISTER: PE88c] [Google Scholar]
Cantin 2006 {published data only}
- Cantin AM, Bacon M, Berthiaume Y. Mechanical airway clearance using the Frequencer electro-acoustical transducer in cystic fibrosis. Clinical and Investigative Medicine 2006;29(3):159-65. [CFGD REGISTER: PE159b] [PubMed] [Google Scholar]
- Cantin AM, Berthiaume Y. Clearance of airway secretions with the Frequencer in patients with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):322. [CFGD REGISTER: PE159] [Google Scholar]
Chatham 2004 {published data only}
- Chatham K, Ionescu AA, Nixon LS, Shale DJ. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. European Respiratory Journal 2004;23(3):435-9. [CFGD REGISTER: PE149] [DOI] [PubMed] [Google Scholar]
Corten 2020 {published data only}
- Corten L, Morrow BM. The use of assisted autogenic drainage in children with cystic fibrosis; a pilot randomized controlled study. Physiotherapy Practice & Research 2020;41(1):79-89. [CFGD REGISTER: PE297b] [Google Scholar]
- PACTR201501001016415. Assisted autogenic drainage (chest physiotherapy) in South African children with cystic fibrosis. trialsearch.who.int/Trial2.aspx?TrialID=PACTR201501001016415 (first received 26 January 2015). [CFGD REGISTER: PE297a]
de Boeck 1984 {published data only}
- Boeck C, Zinman R. Cough versus chest physiotherapy. A comparison of the acute effects on pulmonary function in patients with cystic fibrosis. American Review of Respiratory Disease 1984;129(1):182-4. [CFGD REGISTER: PE59] [DOI] [PubMed] [Google Scholar]
Desmond 1983 {published data only}
- Desmond KJ, Schwenk WF, Thomas E, Beaudry PH, Coates AL. Immediate and long-term effects of chest physiotherapy in patients with cystic fibrosis. Journal of Pediatrics 1983;103(4):538-42. [CFGD REGISTER: PE77] [DOI] [PubMed] [Google Scholar]
Elkins 2005 {published data only}
- Elkins MR, Eberl S, Constable C, White J, Robinson M, Daviskas E, et al. The effect of manual chest physiotherapy, positive expiratory pressure (PEP), and oscillating PEP on mucociliary clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):321. [CFGD REGISTER: PE158] [Google Scholar]
Falk 1984 {published data only}
- Falk M, Kelstrup M, Andersen JB, Kinoshita T, Falk P, Stovring S, et al. Improving the ketchup bottle method with positive expiratory pressure, PEP, in cystic fibrosis. European Journal of Respiratory Diseases 1984;65(6):423-32. [CFGD REGISTER: PE7] [PubMed] [Google Scholar]
Fitzgerald 2005 {published data only}
- Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics 2005;116(4):e549-54. [CFGD REGISTER: BD102b] [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Hilton J, Smith L, Jepson B. Is dornase alfa (Pulmozyme) more effective before or after physiotherapy? A cross-over, randomised, placebo-controlled trial. Pediatric Pulmonology 2001;Suppl 22:309-10. [CFGD REGISTER: BD102a] [Google Scholar]
Ghasempour 2019 {published data only}
- Ghasempour M, Bilan N, Rezazadehsaatlou M. Positive expiratory pressure (PEP) versus conventional chest physiotherapy in pediatric patients with acute exacerbation of cystic fibrosis. International Journal of Pediatrics 2019;7(1):8881-8. [CFGD REGISTER: PE276] [Google Scholar]
Giles 1995 {published data only}
- Giles DR, Wagener JS, Accurso FJ, Butler Simon N. Short-term effects of postural drainage with clapping vs autogenic drainage on oxygen saturation and sputum recovery in patients with cystic fibrosis. Chest 1995;108(4):952-4. [CFGD REGISTER: PE66] [DOI] [PubMed] [Google Scholar]
Grasso 2000 {published data only}
- Grasso MC, Button BM, Allison DJ, Sawyer SM. Benefits of music therapy as an adjunct to chest physiotherapy in infants and toddlers with cystic fibrosis. Pediatric Pulmonology 2000;29(5):371-81. [CFGD REGISTER: PE99b] [DOI] [PubMed] [Google Scholar]
- Grasso MC, Button BM, Sawyer SM, Allison DJ. Music: meeting the challenge of adherence to chest physiotherapy for infants and toddlers with cystic fibrosis. Pediatric Pulmonology 1998;Suppl 17:397. [CFGD REGISTER: PE99a] [DOI] [PubMed] [Google Scholar]
Hartsell 1978 {published data only}
- Hartsell M, Traver G, Taussig LM. Comparison of manual percussion and vibration (P &V) vs mechanical vibration (MV) alone on maximal expiratory flows. In: 19th Annual Meeting Cystic Fibrosis Club Abstracts. 1978:49. [CFGD REGISTER: PE30]
Hofmeyr 1986 {published data only}
- Hofmeyr JL, Webber BA, Hodson ME. Evaluation of positive expiratory pressure as an adjunct to chest physiotherapy in the treatment of cystic fibrosis. Thorax 1986;41(12):951-4. [CFGD REGISTER: PE9b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber BA, Hofmeyr JL, Hodson ME, Batten JC. Evaluation of positive expiratory pressure as an adjunct to postural drainage. In: 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3-8; Jerusalem, Israel. 1985:95. [CFGD REGISTER: PE9a]
Holsclaw 1977 {published data only}
- Holsclaw DS, Tecklin JS. The effectiveness of bronchial drainage and aerosol inhalation in cystic fibrosis. Cystic Fibrosis 1977;-:230-8. [CFGD REGISTER: PE2b] [Google Scholar]
- Tecklin JS, Holsclaw DS Jr. Bronchial drainage with aerosol medications in cystic fibrosis. Physical Therapy 1976;56(9):999-1003. [CFGD REGISTER: PE2a] [DOI] [PubMed] [Google Scholar]
Hristara‐Papadopoulou 2005 {published data only}
- Hristara-Papadopoulou A, Tsanakas J. Results of active cycle of breathing techniques and conventional physiotherapy in mucociliary clearance in children with cystic fibrosis. Hippokratia 2007;11(4):202-4. [CFGD REGISTER: PE240a] [PMC free article] [PubMed] [Google Scholar]
- Hristara-Papadopoulou A, Varsamis P, Diomou G. Physiotherapy in cystic fibrosis: an empirical comparison of two methods. Zeitschrift fur physiotherapeuten 2005;57(3):536-42. [CFGD REGISTER: PE240b] [Google Scholar]
Keller 2001 {published data only}
- Keller H, Liniger W, Fopp A, Hoch M, Knopfli B. Effects of daily harmonica play during in-hospital care of patients with cystic fibrosis. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P339. [CFGD REGISTER: PE126]
Kerrebijn 1982 {published data only}
- Kerrebijn KF, Veentjer R, Bonzet VD, Water E. The immediate effect of physiotherapy and aerosol treatment on pulmonary function in children with cystic fibrosis. European Journal of Respiratory Disease 1982;63(1):35-42. [CFGD REGISTER: PE76] [PubMed] [Google Scholar]
Kirkpatrick 1995 {published data only}
- Kirkpatrick KR, Howard D, Ter-Pogossian M, Kollef MH. A direct comparison of manual chest percussion with acoustic percussion, an experimental treatment for cystic fibrosis. American Journal of Respiratory and Critical Care Medicine Supplements 1995;151(4):A738. [CFGD REGISTER: PE37] [Google Scholar]
Klig 1989 {published data only}
- Klig S, Denning C, Jacoby J, Xia F, Gaerlan P, Bisberg D, et al. A biopsychosocial examination of two methods of pulmonary therapy. Pediatric Pulmonology 1989;Suppl 4:128. [CFGD REGISTER: PE39] [Google Scholar]
Kluft 1996 {published data only}
- Kluft J, Beker L, Castagnino M, Gaiser J, Chaney H, Fink RJ. A comparison of bronchial drainage treatments in cystic fibrosis. Pediatric Pulmonology 1996;22(4):271-4. [CFGD REGISTER: PE84] [DOI] [PubMed] [Google Scholar]
Konstan 1994 {published data only}
- Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. Journal of Pediatrics 1994;124(5 Pt 1):689-93. [CFGD REGISTER: PE22] [DOI] [PubMed] [Google Scholar]
Lannefors 1992 {published data only}
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis – a comparison between postural drainage, PEP-mask and physical exercise. In: 11th International Cystic Fibrosis Congress; 1992 Aug 22-27; Dublin, Ireland. 1992. [CFGD REGISTER: PE46a] [PubMed]
- Lannefors L, Wollmer P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748-53. [CFGD REGISTER: PE46b] [PubMed] [Google Scholar]
Lorin 1971 {published data only}
- Lorin MI, Denning CR. Evaluation of postural drainage by measurement of sputum volume and consistency. American Journal of Physical Medicine 1971;50(5):215-9. [CFGD REGISTER: PE80] [MEDLINE: ] [PubMed] [Google Scholar]
Lyons 1992 {published data only}
- Lyons E, Chatham K, Campbell IA, Prescott RJ. Evaluation of the Flutter VRP1 device in young adults with cystic fibrosis. Thorax 1992;47(3):237P. [CFGD REGISTER: PE60b] [Google Scholar]
- Lyons E, Chatham K, Campbell IA, Prescott RJ. Evaluation of the flutter VRP1 device in young adults with cystic fibrosis. In: 11th International Cystic Fibrosis Congress; 1992 Aug 22-27; Dublin, Ireland. 1992:AHP30. [CFGD REGISTER: PE60a]
Maayan 1989 {published data only}
- Maayan C, Bar-Yishay E, Yaacobi T, Marcus Y, Katznelson D, Yahav Y, et al. Immediate effect of various treatments on lung function in infants with cystic fibrosis. Respiration 1989;55(3):144-51. [CFGD REGISTER: IB56] [DOI] [PubMed] [Google Scholar]
Majaesic 1996 {published data only}
- Majaesic CM, Montgomery M, Jones R, King M. Reduction in sputum viscosity using high frequency chest compressions (HFCC) compared to conventional chest physiotherapy (CCP). Pediatric Pulmonology 1996;Suppl 13:308. [Google Scholar]
Marks 2004 {unpublished data only}
- Marks J, Hare K, Homnick D. The PercussiveTech HF Device compared to standard chest physiotherapy in patients with cystic fibrosis. In: 13th International Cystic Fibrosis Congress; 2000 Jun 4-8; Stockholm, Sweden. 2000. [CFGD REGISTER: PE109b]
- Marks JH, Hare KL, Homnick DN. Pulmonary function and sputum production in patients with cystic fibrosis: a pilot study comparing the percussive tech HF device and standard chest physiotherapy. Pediatric Pulmonology 1999;Suppl 19:290. [CFGD REGISTER: PE109a] [DOI] [PubMed] [Google Scholar]
- Marks JH, Hare KL, Saunders RA, Homnick DN. Pulmonary function and sputum production in patients with cystic fibrosis: a pilot study comparing the PercussiveTech HF device and standard chest physiotherapy. Chest 2004;125(4):1507-11. [CFGD REGISTER: PE109c] [DOI] [PubMed] [Google Scholar]
Martinez Rodriguez 2017 {published data only}
- Martinez Rodriguez ME, Suarez Cortina L, Maiz Carro L, Ruiz-De-Valbuena M, Jimenez Cosmes L. A pulmonary rehabilitation program to increase adherence to airway clearance techniques for children and adults with cystic fibrosis. Journal of Cystic Fibrosis 2017;16 Suppl 1:S58. [CFGD REGISTER: PE248] [Google Scholar]
Maxwell 1979 {published data only}
- Maxwell M, Redmond A. Comparative trial of manual and mechanical percussion technique with gravity-assisted bronchial drainage in patients with cystic fibrosis. Archives of Disease in Childhood 1979;54(7):542-4. [CFGD REGISTER: PE3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarren 2006 {published data only}
- McCarren B, Alison JA. Comparison of vibration to other physiotherapy interventions for secretion clearance. European Respiratory Journal 2005;Suppl 49:497s. [CFGD REGISTER: PE160a] [Google Scholar]
- McCarren B, Alison JA. Physiological effects of vibration in subjects with cystic fibrosis. European Respiratory Journal 2006;27(6):1204-9. [CFGD REGISTER: PE160b] [DOI] [PubMed] [Google Scholar]
McDonnell 1986 {published data only}
- McDonnell T, McNicholas WT, FitzGerald MX. Hypoxaemia during chest physiotherapy in patients with cystic fibrosis. Irish Journal of Medical Science 1986;155:345-8. [CFGD REGISTER: OV9] [DOI] [PubMed] [Google Scholar]
Morris 1982 {published data only}
- Morris D, Barbero G, Konig P, Woodruff C, Kline J, Martinez R. The effect of mechanical and manual percussion on pulmonary function in cystic fibrosis patients. In: 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington DC. 1982:135. [CFGD REGISTER: PE83]
Natale 1994 {published data only}
- Natale JE, Pfeifle J, Homnick DN. Comparison of intrapulmonary percussive ventilation and chest physiotherapy. A pilot study in patients with cystic fibrosis. Chest 1994;105(6):1789-93. [CFGD REGISTER: PE23] [DOI] [PubMed] [Google Scholar]
Newhouse 1998 {published data only}
- Newhouse P, White F, Marks J, Hamnick D. Pulmonary function testing and sputum production in patients with cystic fibrosis: a pilot study comparing the flutter device, intrapulmonary percussion ventilator and standard chest physiotherapy. Pediatric Pulmonology 1995;Suppl 12:269. [CFGD REGISTER: PE56a] [Google Scholar]
- Newhouse PA, White F, Marks JH, Homnick DN. The intrapulmonary percussive ventilator and flutter device compared to standard chest physiotherapy in patients with cystic fibrosis. Clinical Pediatrics 1998;37(7):427-32. [CFGD REGISTER: PE56b] [DOI] [PubMed] [Google Scholar]
Oberwaldner 1986 {published data only}
- Oberwaldner B, Evans JC, Zach MS. Forced expirations against a variable resistance: a new chest physiotherapy method in cystic fibrosis. Pediatric Pulmonology 1986;2(6):358-67. [DOI] [PubMed] [Google Scholar]
Orlik 2000 {published data only}
- Orlik T. Evaluation of the efficiency of selected thoracic physiotherapy methods used in the treatment of patients with cystic fibrosis [Ocena efektywnosci wybranych metod fizjoterapii klatki piersiowej stosowanej w leczeniu chorych na mukowiscydoze]. Medycyna Wieku Rozwojowego 2000;4(3):233-46. [CFGD REGISTER: PE140] [PubMed] [Google Scholar]
Orlik 2001 {published data only}
- Orlik T, Sands D. Long-term evaluation of effectiveness for selected chest physiotherapy methods used in the treatment of cystic fibrosis [Dlugofalowa ocena skutecznosci wybranych metod fizjoterapii klatki piersiowej stoswanych w leczeniu chorych na mukowiscydoze]. Medycyna Wieku Rozwojowego 2001;5(3):245-57. [CFGD REGISTER: PE125b] [PubMed] [Google Scholar]
- Orlik T, Sands D. Long-term study of efficiencies of select physiotherapy methods used in the treatment of cystic fibrosis. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P113. [CFGD REGISTER: PE125a]
Phillips 1998 {published data only}
- Phillips GE, Pike SE, Rosenthal M, Bush A. Holding the baby: head downwards positioning for physiotherapy does not cause gastro-oesophageal reflux. European Respiratory Journal 1998;12(4):954-7. [CFGD REGISTER: PE101] [DOI] [PubMed] [Google Scholar]
Placidi 2006 {published data only}
- Placidi G, Cornacchia M, Cappelletti LM, Mastella G, Assael BM, Braggion C. Short-term effects of positive airway pressure on sputum clearance by directed coughing: a cross-over, randomized study. Pediatric Pulmonology 2001;Suppl 22:313-4. [CFGD REGISTER: PE128a] [Google Scholar]
- Placidi G, Cornacchia M, Polese G, Zanolla L, Assael BM, Braggion C. Chest physiotherapy with positive airway pressure: a pilot study of short-term effects on sputum clearance in patients with cystic fibrosis and severe airway obstruction. Respiratory Care 2006;51(10):1145-53. [CFGD REGISTER: PE128b] [PubMed] [Google Scholar]
Pryor 1979 {published data only}
- Hodson ME, Batten JC, Pryor JA, Webber BA. Evaluation of the forced expiration technique as an adjunct to postural drainage in the treatment of cystic fibrosis. In: 9th Meeting European Working Group for Cystic Fibrosis; 1979 Jun 12-13; Noordwijkerhout, The Netherlands. 1979:57. [CFGD REGISTER: PE78b]
- Pryor JA, Webber BA, Hodson ME, Batten JC. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. British Medical Journal 1979;2(6187):417-8. [CFGD REGISTER: PE78a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor JA, Webber BA. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979;65(10):304-7. [CFGD REGISTER: PE78c] [PubMed] [Google Scholar]
Regelmann 1990 {published data only}
- Regelmann WE, Elliott GR, Clawson CC, Warwick WJ. Reduction of sputum Ps. Aeruginosa density by antibiotics improves lung function in CF more than bronchodilators and chest physiotherapy alone. Pediatric Pulmonology 1988;Suppl 2:97. [CFGD REGISTER: PI65a] [DOI] [PubMed] [Google Scholar]
- Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. American Review of Respiratory Disease 1990;4(Pt 1):914-21. [CFGD REGISTER: PI65b] [DOI] [PubMed] [Google Scholar]
Reix 2012 {published data only}
- NCT01509235. Self drainage in pediatric cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT01509235 (first received 12 January 2012). [CFGD REGISTER: PE183c]
- Reix P, Aubert F, Kassai B, Bige V, Bellon G. Better satisfaction of cystic fibrosis paediatric patients with autogenic drainage associated to exercise compared to conventional chest physiotherapy. Journal of Cystic Fibrosis 2009;8 Suppl 2:S73. [ABSTRACT NO: 293] [CFGD REGISTER: PE183a] [Google Scholar]
- Reix P, Aubert F, Werck-Gallois MC, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2012;58(4):241-7. [CENTRAL: 841658] [CFGD REGISTER: PE183b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roos 1987 {published data only}
- Roos S, Birrer P, Rüdeberg A, Kraemer R. First experience with intrapulmonary percussive ventilation (IPV) in the treatment of patients with cystic fibrosis. In: 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987. [CFGD REGISTER: PE34]
Rossman 1982 {published data only}
- Rossman C, Waldes R, Sampson D, Newhouse M. Does chest physiotherapy improve mucus removal in patients with cystic fibrosis? In: 8th International Cystic Fibrosis Congress; 1980. 1980:32a. [CFGD REGISTER: PE6a]
- Rossman CM, Waldes R, Sampson D, Newhouse MT. Effect of chest physiotherapy on the removal of mucus in patients with cystic fibrosis. American Review of Respiratory Disease 1982;126(1):131-5. [CFGD REGISTER: PE6b] [DOI] [PubMed] [Google Scholar]
Salh 1989 {published data only}
- Salh W, Bilton D, Dodd M, Webb AK. Effect of exercise and physiotherapy in aiding sputum expectoration in adults with cystic fibrosis. Thorax 1989;44(12):1006-8. [CFGD REGISTER: PE63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuelson 1994 {published data only}
- Samuelson W, Woodward V, Lowe V. Utility of a dynamic air therapy bed vs. conventional chest physiotherapy in adult CF patients. Pediatric Pulmonology 1994;Suppl 10:313. [CFGD REGISTER: PE51] [Google Scholar]
Sanchez Riera 1999 {published data only}
- Sanchez Riera H, Dapena Fernandez FJ, Gomez Dominguez F, Ortega Ruiz F, Elias Hernandez T, Montemayor Rubio T, et al. Comparative study of the efficacy of 2 respiratory physiotherapy protocols for patients with cystic fibrosis [Estudio comparativo de la eficacia de dos protocolos de fisioterapia respiratoria en pacientes con fibrosis quistica]. Archivos de Bronconeumologia 1999;35(6):275-9. [CFGD REGISTER: PE106] [DOI] [PubMed] [Google Scholar]
Scherer 1998 {published data only}
- Scherer TA, Barandun J, Martinez E, Wanner A, Rubin EM. Effect of high-frequency oral airway and chest wall oscillation and conventional chest physical therapy on expectoration in patients with stable cystic fibrosis. Chest 1998;113(4):1019-27. [CFGD REGISTER: PE91] [DOI] [PubMed] [Google Scholar]
Skopnik 1986 {published data only}
- Skopnik H, Waters W, Horwitz AE, Roth B, Genz-Bohm G. Evaluation of knock and vibration therapy and autogenic drainage in cystic fibrosis using a ventilation zintigraphical method [Beurteilung der Klopf- und Vibrations-Therpie und autogene Drainage bei cystischer Fibrose mittels eines ventilationsszintigraphischen Verfahrens]. Monatsschrift fur Kinderheilkunde 1986;134:583. [CFGD REGISTER: PE131] [Google Scholar]
Steven 1992 {published data only}
- Steven MH, Pryor JA, Webber BA, Hodson MR. Physiotherapy versus cough alone in the treatment of cystic fibrosis. New Zealand Journal of Physiotherapy 1992;20:31-7. [CFGD REGISTER: PE65] [Google Scholar]
Stites 2006 {published data only}
- Stites SW, Perry GV, Peddicord T, Cox G, McMillan C, Becker B. Effect of high-frequency chest wall oscillation on the central and peripheral distribution of aerosolized diethylene triamine penta-acetic acid as compared to standard chest physiotherapy in cystic fibrosis. Chest 2006;129(3):712-7. [CFGD REGISTER: PE162] [DOI] [PubMed] [Google Scholar]
Sutton 1985 {published data only}
- Sutton PP, Lopez Vidriero MT, Pavia D, Newman SP, Clay MM, Webber B, et al. Assessment of percussion, vibratory-shaking and breathing exercises in chest physiotherapy. European Journal of Respiratory Diseases 1985;66(2):147-52. [CFGD REGISTER: PE81] [MEDLINE: ] [PubMed] [Google Scholar]
- Sutton PP, Parker RA, Webber BA, Newman SP, Garland N, Lopez-Vidriero MT, et al. Assessment of the forced expiration technique, postural drainage and directed coughing in chest physiotherapy. European Journal Respiratory Diseases 1983;64(1):62-8. [CFGD REGISTER: PE32b] [PubMed] [Google Scholar]
Tannenbaum 2007 {published data only}
- Tannenbaum E, Prasad SA, Dinwiddie R, Main E. Chest physiotherapy during anaesthesia for children with cystic fibrosis: effects on respiratory function. Pediatric Pulmonology 2007;42(12):1152-8. [CFGD REGISTER: PE129b] [DOI] [PubMed] [Google Scholar]
- Tannenbaum E, Prasad SA, Main E. The effect of chest physiotherapy on cystic fibrosis patients undergoing general anaesthesia for an elective surgical procedure. Pediatric Pulmonology 2001;Suppl 22:315. [CFGD REGISTER: PE129a] [Google Scholar]
Tonnesen 1984 {published data only}
- Tonnesen P, Stovring S. Positive expiratory pressure (PEP) as lung physiotherapy in cystic fibrosis: a pilot study. European Journal of Respiratory Diseases 1984;65(6):419-22. [PubMed] [Google Scholar]
van der Schans 1991 {published data only}
- Schans CP, Mark TW, Vries G, Piers DA, Beekhuis H, Dankert-Roelse JE, et al. Effect of positive expiratory pressure breathing in patients with cystic fibrosis. Thorax 1991;46(4):252-6. [CFGD REGISTER: PE142] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hengstum 1988 {published data only}
- Hengstum M, Festen J, Beurskens C, Hankel M, Beekman F, Corstens F. Conventional physiotherapy and forced expiration manoeuvres have similar effects on tracheobronchial clearance. European Respiratory Journal 1988;1(8):758-61. [CFGD REGISTER: PE64] [PubMed] [Google Scholar]
Varekojis 2003 {published data only}
- Castile R, Tice J, Flucke R, Filbrun D, Varekojis S, McCoy K. Comparison of three sputum clearance methods in in-patients with cystic fibrosis. Pediatric Pulmonology 1998;Suppl 17:329. [CFGD REGISTER: PE93a] [Google Scholar]
- Varekojis SM, Douce FH, Flucke RL, Filbrun DA, Tice JS, McCoy KS, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respiratory Care 2003;48(1):24-8. [CFGD REGISTER: PE93b] [PubMed] [Google Scholar]
Verboon 1986 {published data only}
- Verboon JM, Bakker W, Dijkman JH. Effect of the forced expiration technique and postural drainage in adults with cystic fibrosis. In: 9th International Cystic Fibrosis Congress; 1984 Jun 9-15; Brighton, England. 1984:2.17. [CFGD REGISTER: PE8a]
- Verboon JM, Bakker W, Sterk PJ. De waarde van de 'forced expiration technique' (FET). Ned Tijdschr Fhysiotherapie 1987;97:62-4. [CFGD REGISTER: PE8c] [Google Scholar]
- Verboon JM, Bakker W, Sterk PJ. The value of the forced expiration technique with and without postural drainage in adults with cystic fibrosis. European Journal of Respiratory Diseases 1986;69(3):169-74. [CFGD REGISTER: PE8b] [PubMed] [Google Scholar]
Warwick 1990 {published data only}
- Warwick WJ, Wielnski CI. Matched pair comparison of manual chest physical therapy (CPT) and the thairapy bronchial drainage vest (ThBVD) system. Pediatric Pulmonology 1990;Suppl 5:177. [CFGD REGISTER: PE43] [Google Scholar]
Warwick 1991 {published data only}
- Warwick WJ, Hansen LG. The long-term effect of high-frequency chest compression therapy on pulmonary complications of cystic fibrosis. Pediatric Pulmonology 1991;11(3):265-71. [DOI] [PubMed] [Google Scholar]
Warwick 2004 {published data only}
- Warwick WJ, Wielinski CL, Hansen LG. Comparison of expectorated sputum after manual chest physical therapy and high-frequency chest compression. Biomedical Instrumentation Technology 2004;38(6):470-5. [CFGD REGISTER: PE153] [DOI] [PubMed] [Google Scholar]
White 1997 {published data only}
- White D, Stiller K, Willson K. The role of thoracic expansion exercises during the active cycle of breathing techniques. Physiotherapy Theory & Practice 1997;13(2):155-62. [CFGD REGISTER: PE61b] [Google Scholar]
- White D, Stiller K. Are thoracic expansion exercises necessary during the active cycle of breathing techniques for adult cystic fibrosis patients? Israel Journal of Medical Sciences 1996;32(Suppl):S275. [CFGD REGISTER: PE61a] [Google Scholar]
Williams 2001 {published data only}
- Parsons DW, Williams MT, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Chest physiotherapy: Improvements in lung function and ventilation are associated with physiotherapy-assisted treatment. Pediatric Pulmonology 1995;Suppl 12:271. [CFGD REGISTER: PE57a] [Google Scholar]
- Williams M, Parsons D, Martin A, Ellis E, Giles S, Staugas RE, et al. Chest physiotherapy (CPT) and cystic fibrosis: physiotherapist-assisted treatment is more energy efficient. Australian and New Zealand Journal of Medicine 1995;25:441. [CFGD REGISTER: PE57c] [Google Scholar]
- Williams M, Parsons D, Martin A, Giles S, Staugas RE. Energy expenditure during chest physiotherapy in normal and cystic fibrosis (CF) subjects. Australian and New Zealand Journal of Medicine 1994;24:445. [CFGD REGISTER: PE57b] [Google Scholar]
- Williams MT, Parsons DW, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Acute respiratory infection in patients with cystic fibrosis with mild pulmonary impairment: comparison of two physiotherapy regimens. Australian Journal of Physiotherapy 2001;47(4):227-36. [CFGD REGISTER: PE57d] [DOI] [PubMed] [Google Scholar]
- Williams MT, Parsons DW, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Energy expenditure during physiotherapist-assisted and self-treatment in cystic fibrosis. Physiotherapy and Practice 2000;16(2):57-67. [CFGD REGISTER: PE57e] [Google Scholar]
Wong 2000 {published data only}
- Wong LT, McIlwaine PM, Davidson AG, Lillquist YP. Gastroesophageal reflux during chest physiotherapy: a comparison of positive expiratory pressure and postural drainage with percussion. Pediatric Pulmonology 1999;Suppl 19:288. [CFGD REGISTER: PE108a] [Google Scholar]
- Wong LT, McIlwaine PM, Davidson AG. Gastroesophageal reflux during chest physiotherapy: a comparison of positive expiratory pressure and postural drainage with percussion. In: 13th International Cystic Fibrosis Congress; 2000 Jun 4-8; Stockholm, Sweden. 2000:130. [CFGD REGISTER: PE108b]
Additional references
Bell 2020
- Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respiratory Medicine 2020;8(1):65-124. [DOI: 10.1016/S2213-2600(19)30337-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2006
- Bradley JM, Moran FM, Elborn JS. Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: an overview of five Cochrane systematic reviews. Respiratory Medicine 2006;100(2):191-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brewin 1989
- Brewin CR, Bradley C. Patient preferences and randomised clinical trials. BMJ 1989;299(6694):313-5. [DOI: 10.1136/bmj.299.6694.313] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnham 2021
- Burnham P, Stanford G, Stewart R. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD009595. [DOI: 10.1002/14651858.CD009595.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Boeck 2020
- De Boeck K. Cystic fibrosis in the year 2020: a disease with a new face. Acta Paediatrica 2020;109(5):893-9. [DOI: 10.1111/apa.15155] [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Ewart 1901
- Ewart WE. The treatment of bronchiectasis and of chronic bronchial affections by posture and by respiratory exercises. Lancet 1901;158(4063):70–2. [DOI: 10.1016/S0140-6736(01)85059-5] [DOI] [Google Scholar]
Filipow 2021
- Filipow N, Davies G, Main E, Sebire NJ, Wallis C, Ratjen F, et al. Unsupervised phenotypic clustering for determining clinical status in children with cystic fibrosis. European Respiratory Journal 2021;58(2):2002881. [DOI: 10.1183/13993003.02881-2020] [DOI] [PubMed] [Google Scholar]
Godfrey 1971
- Godfrey S, Mearns M. Pulmonary function and response to exercise in cystic fibrosis. Archives of Disease in Childhood 1971;46(246):144-51. [DOI: 10.1136/adc.46.246] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinz 2022
- Heinz KD, Walsh A, Southern KW, Johnstone Z, Regan KH. Exercise versus airway clearance techniques for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2022, Issue 6. Art. No: CD013285. [DOI: 10.1002/14651858.CD013285.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Kendig 1977
- Kendig EL, Clarwick V, Waring W. Chapter title unavailable. In: Waring W, editors(s). Diagnostic and Therapeutic Procedures in Disorders of the Respiratory Tract in Children. 3rd edition. Philadelphia: Saunders, 1977:114-9. [Google Scholar]
McIlwaine 2019
- McIlwaine M, Button B, Nevitt SJ. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD003147. [DOI: 10.1002/14651858.CD003147.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2008
- McPherson K. Do patients' preferences matter? BMJ 2008;337:a2034. [DOI: 10.1136/bmj.a2034] [DOI] [PubMed] [Google Scholar]
Morrison 2020
- Morrison L, Milroy S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD006842. [DOI: 10.1002/14651858.CD006842.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2009
- Myers LB. An exploratory study investigating factors associated with adherence to chest physiotherapy and exercise in adults with cystic fibrosis. Journal of Cystic Fibrosis 2009;8(6):425-7. [DOI: 10.1016/j.jcf.2009.08.005] [DOI] [PubMed] [Google Scholar]
O'Donohoe 2014
- O'Donohoe R, Fullen BM. Adherence of subjects with cystic fibrosis to their home program: a systematic review. Respiratory Care 2014;59(11):1731-46. [DOI: 10.4187/respcare.02990] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Passero 1981
- Passero MA, Remor B, Salomon J. Patient-reported compliance with cystic fibrosis therapy. Clinical Pediatrics 1981;20:264-8. [DOI] [PubMed] [Google Scholar]
Prasad 1998
- Prasad SA, Main E. Finding evidence to support airway clearance techniques in cystic fibrosis. Disability and Rehabilitation 1998;20(6-7):235-46. [DOI] [PubMed] [Google Scholar]
Preference Collaborative Review Group 2008
- Preference Collaborative Review Group. Patients' preferences within randomised trials: systematic review and patient level meta-analysis. BMJ 2008;337:a1864. [DOI: 10.1136/bmj.a1864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire – Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2022
- Radtke T, Smith S, Nevitt SJ, Hebestreit H, Kriemler S. Physical activity and exercise training in cystic fibrosis. Cochrane Database of Systematic Reviews 2022, Issue 8. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raywood 2022a
- Raywood E, Shannon H, Filipow N, Tanriver G, Stanojevic S, Douglas H, et al. Quantity and quality of airway clearance in children and young people with cystic fibrosis. Journal of Cystic Fibrosis 2022;S1569-1993(22):00686-5. [DOI: 10.1016/j.jcf.2022.09.008] [DOI] [PubMed] [Google Scholar]
Raywood 2022b
- Raywood E, Filipow N, Stanojevic S, Shannon H, Douglas H, Tanriver G, et al. Effects of quantity and quality of daily airway clearance treatments on lung function in children and young people with cystic fibrosis: results from Project Fizzyo. Journal of Cystic Fibrosis 2022;21 (Suppl):S164. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Silverman 1996
- Silverman WA, Altman DG. Patients' preferences and randomised trials. Lancet 1996;347(8995):171-4. [DOI: 10.1016/s0140-6736(96)90347-5] [DOI] [PubMed] [Google Scholar]
Udell 2011
- Udell JA, Redelmeier DA. Patient preferences and the ironic nature of randomized trials. Medical Decision Making 2011;31(2):226-8. [DOI: 10.1177/0272989X11399125] [DOI] [PubMed] [Google Scholar]
UK CF Trust 2022
- UK CF Trust. Cystic fibrosis FAQs. www.cysticfibrosis.org.uk/what-is-cystic-fibrosis/faqs (accessed 9 August 2022).
van der Schans 1996
- Schans CP, Mark TW, Rubin BK, Postma DS, Koëter GH. Chest physical therapy: mucus mobilizing techniques. In: JR Bach, editors(s). Pulmonary Rehabilitation. Philadelphia, USA: Hanley & Belfus, 1996:229-46. [Google Scholar]
Warnock 2015
- Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD001401. [DOI: 10.1002/14651858.CD001401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2019
- Wilson LM, Morrison L, Robinson KA. Airway clearance techniques for cystic fibrosis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD011231. [DOI: 10.1002/14651858.CD011231.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2023
- Wilson LM, Saldanha IJ, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2023, Issue 2. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Main 2003
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD002011. [DOI: 10.1002/14651858.CD002011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Main 2005
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD002011. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


