Abstract
Objective:
To examine the relationship between Vitamin D status and pain intensity and disability in individuals with and without knee pain, and to examine the role of epigenetics in this relationship.
Design:
Cross-sectional analysis of data from the UPLOAD-2 study (Understanding Pain and Limitations in OsteoArthritic Disease-2).
Participants:
189 individuals aged 45–65 years and older.
Measurements:
Serum Vitamin D levels, pain related interference and characteristic pain intensity measures, and the epigenetic clock GrimAge derived from blood analyses.
Results:
Lower Vitamin D was associated with advanced epigenetic aging (AgeAccelGrim), greater pain and disability and that (AgeAccelGrim) mediated the relationship between Vitamin D status and self-reported pain (ab = −0.0799; CI [−0.1492, −0.0237]) and disability (ab = −0.0669; CI [−0.1365, −0.0149]) outcomes.
Conclusion:
These data support the notion that lifestyle factors such as nutrition status play a key role in aging process, as well as the development and maintenance of age-related diseases such as pain. Modifying nutrition status could help promote healthy aging and reduce pain.
Keywords: Vitamin D, epigenetic aging, pain
Introduction
Knee osteoarthritis (KOA) has quickly become one of the leading causes of pain and disability in aging populations 1. There is large interindividual variability in pain and disability outcomes of those diagnosed with KOA. Often, radiographic imaging findings do not correlate with reported pain sensitivity and pain-related disability or interference 2. As such, there is a great need to understand the mechanisms involved in pain and disability within KOA populations and to identify targets that may mitigate negative outcomes.
Recently, the effect of nutrition status on pain outcomes has become of great interest due to the high potential of modification of one’s diet as well as its low side effect profile. We 3–5 and others 6–8 have previously reported the effects of nutrition status on outcomes across various pain states. Additionally, we have shown that a dietary intervention significantly improved pain sensitivity and disability in individuals with KOA 9. One nutrient that has been historically associated with pain and disability outcomes is 25-dihydroxyvitamin D, commonly referred to as calciferol or simply Vitamin D 10. Vitamin D has also been implicated in KOA, given its mechanisms of action regarding bone health 11. Vitamin D is a fat-soluble vitamin naturally present in some foods, and fortified in others. However, the primary source of Vitamin D in humans is the conversion of cholesterol to the inactive form of Vitamin D by UV light (sunlight), which is then converted to the active form (1,25-hydroxyvitamin D) in the liver and kidneys to be used throughout the body 12. While Vitamin D has previously been associated with pain and disability, as well as KOA, its mechanism of action promoting either negative or positive outcomes has yet to be elucidated.
One of the most important discoveries related to Vitamin D is that of the discovery of the transcription factor Vitamin D receptor (VDR), a member of the steroid nuclear receptor family 13. The effect of liganded VDR depends on the epigenetic landscape of the targeted gene, and it is evident that there is a role of Vitamin D in regulating gene expression 14. There is also evidence to suggest that Vitamin D can alter DNA methylation 15 – a phenomenon necessary for the regulation of biological processes including cell differentiation and genomic imprinting. Additionally, there have been changes within epigenetic clocks, noted with aging and in those with chronic pain conditions such as KOA 16. These epigenetic clocks are based on weighted linear combinations of DNA methylation level at a certain number of CpG sites, and have been shown to be relevant in predicting future outcomes including all-cause mortality17. Thus, it is worth investigating if the Vitamin D association with pain and disability outcomes is mediated by changes in an individual’s epigenetic landscape, as estimated by epigenetic clocks. In this manuscript, we assessed the potential relationship of Vitamin D’s effects on pain intensity and disability through associations in epigenetic aging in individuals with and without KOA. We hypothesized that associations between Vitamin D levels with pain intensity and interference in persons with KOA would be significantly mediated by epigenetic aging.
Methods
Participants
Participants were adults between the ages of 45–85 with and without symptomatic knee osteoarthritis (KOA) recruited from the University of Florida (UF; Gainesville, Florida, USA) and the University of Alabama at Birmingham (UAB; Birmingham, Alabama, USA). Individuals who self-identified as non-Hispanic and “African American/Black” (NHB) or non-Hispanic and “White/Caucasian/European” (NHW) and English speaking, were eligible for inclusion. Individuals were excluded if they reported 1) significant surgery to the index (i.e., most painful) knee (e.g., total knee replacement surgery); 2) cardiovascular disease or history of acute myocardial infarction; 3) uncontrolled hypertension (blood pressure > 150/95 mmHg); 4) systemic rheumatic diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia); 5) neuropathy; 6) chronic opioid use; 7) serious psychiatric illness; 8) neurological disease (e.g., Parkinson’s, multiple sclerosis, stroke with loss of sensory or motor function, or uncontrolled seizures); 9) pregnant; 10) significantly greater pain in a body site other than the knee. All participants provided written informed consent and the study was IRB approved and conducted in accordance with the Declaration of Helsinki. Participants were recruited as part of a parent study designed to examine ethnic and race group differences in physical symptoms, psychosocial functioning, and pain-related central nervous system structure and functioning in KOA. The present study is an ancillary investigation that aimed to determine brain and epigenetic aging patterns in KOA, thus, only measures relevant to the study hypotheses are included and presented below.
Procedures
Demographic information including age, ethnicity/race, and sex were self-reported during initial phone screening. Eligible individuals were scheduled for a Health Assessment Session (HAS), at which informed consent was obtained prior to study procedures. A health history and pain history, and physical exam were conducted during the HAS. Approximately one week later, participants attended a quantitative sensory testing (QST) session. Clinical pain measures and blood samples were collected at the QST session.
Measures
Graded Chronic Pain Scale (GCPS)
The GCPS is a robust, validated 18 self-reported questionnaire that measures two dimensions of chronic pain severity: pain intensity and pain-related disability. The questionnaire consists of seven items, with six scored on an 11-point Likert scale asking participants to report their current, average and worst pain over the last six months (i.e., 0 = “no pain” to 10 = “pain as bad as it can be”), and how much pain has interfered with daily activities, recreation/social/family activities, and ability to work (i.e., 0 = “no interference” to 10 = “unable to carry out activities”). Scores are then calculated for the two subscales: characteristic pain intensity is calculated as the mean intensity ratings for the current, worst and average pain multiplied by 10; and the pain-related disability score, which is calculated as the mean rating for difficult performing daily, social and work-related activities multiplied by 10, with each score ranging from 0–100. One open-ended question asks participants to report “how many days in the last six months have you been kept from your usual activities because of pain”. Higher scores indicate greater pain and pain-related disability.
Pain Group Classification
Consistent with the Task Force for the Classification of Chronic Pain consensus for the 11th version of the International Classification of Diseases (ICD-11) of the World Health Organization (WHO) recommendations 19, incorporating both pain disability and its duration 20, individuals were categorized based on how limiting their pain in their daily life using the Graded Chronic Pain Scale (GCPS) 21. Scores from the GCPS characteristic pain intensity scale and Disability points were then used to categorize participants according to a pain grade: Grade 0 = no reported pain intensity; Grade 1 = low disability (i.e., <3 disability points) and low pain intensity (i.e., <50); Grade 2 = low disability-high intensity pain (i.e., ≥50); Grade 3 = high disability-moderately limiting (i.e., 3–4 Disability Points), regardless of pain intensity; Grade 4 = high disability-severely limiting (i.e., 5–6 Disability Points), regardless of pain intensity.21 Pain Groups were defined based on pain grade as follows: No chronic pain (i.e., Grade 0), Low impact pain (i.e., Grades 1–2), High impact pain (i.e., Grade 3–4).
Blood Collection and Processing
Blood samples were collected from the forearm or hand vein at the onset of the quantitative sensory testing session and included collection of a 10ml K2 EDTA tube and a 7ml Corvac Serum Separator tube that were subsequently used for DNA methylation and Vitamin D analyses, respectively.
DNA Extraction and Methylation Analysis
The EDTA tube was centrifuged at 3000rpm for 10 minutes and the buffy coat was carefully extracted and transferred to a cryovial for −80-degree storage. To isolate genomic DNA, the frozen buffy coat samples were thawed at 37°C to dissolve homogeneously. ~200 ul (or 150–200 ul) of sample was lysed in R.B.C lysis buffer and centrifuged at 6000 rpm for 5 minutes at room temperature. The supernatant was discarded and sodium EDTA solution was added to the pellet and vortex gently to remove RBC clumps. Homogenate was incubated at 50–55°C with Proteinase K and SDS solution. Following incubation, equal volume of phenol was added, mixed, and centrifuged at 10,000 rpm for 10 minutes. Supernatant was transferred in a fresh tube and equal volume of phenol-chloroform-isoamyl alcohol was added, mixed and centrifuged at the same rpm. Again, supernatant was transferred in a fresh tube and equal volume of chloroform-isoamyl alcohol was added followed by centrifugation at same rpm conditions. Supernatant was transferred in a fresh tube and 1/10th volume of 3M sodium acetate along with 2 volumes of absolute alcohol was added. The precipitated DNA was washed with 70% ethanol by centrifugation at 10,000 rpm for 5 minutes. The pellet was air dried and dissolved in Tris-EDTA buffer. The dissolved DNA was qubit quantified and visualized on agarose gel for quality assessment. Sodium Bisulfite conversion and EPIC methylation array was performed by Moffitt Cancer Center, Molecular Genomics Core located at 3011 Holly Dr., Tampa, FL 33612.
DNA Methylation Age Calculation
The raw data generated by Illumina EPIC array (.idat files) underwent quality control and normalization prior to the calculation of DNAmGrimAge via an online calculator (https://dnamage.genetics.ucla.edu/home). The normalized beta values were obtained using ChAMP (Chip Analysis Methylation Pipeline for Illumina HumanMethylation EPIC) protocol 22[Cruz-Almeida et al., 2021, under review]. These normalized beta values were subset to sites required for the calculation of DNA Methylation Age and uploaded with a sample annotation file as per the protocol document that accompanies the online calculator. The age-adjusted AgeAccelGrim variable was calculated as the difference between chronological age and DNAmGrimAge.
Vitamin D Assay
Blood was collected into a Corvac tube and wrapped in foil to protect from light. After 30 minutes, samples were centrifuged at 1800G for 10 minutes and then transferred to a 0.5mL serum aliquot into an amber cryovial and stored at −80°C until processed for assays. Vitamin D was measured on a TOSOH Bioscience AIA-900 (South San Francisco, CA) using immunofluorescence.
Statistical analyses
All analyses were completed using SPSS v27.0 (Armonk, NY: IBM Corp). Prior to running analyses, the data were cleaned so that only those with data for all variables of interest were included in the analyses. Analyses of variance (ANOVA) were employed to observe if there were differences in Vitamin D levels across pain groups, age, sex, race and study site. Next, regression-based mediation analyses23 were performed to determine whether AgeAccelGrim mediated the relationship between Vitamin D and pain and disability, with Vitamin D as the independent variable (X), AgeAccelGrim as the mediator (M), and the continuous GCPS derived characteristic pain intensity or pain-related disability as the dependent/outcome variables (Y) (Figures 1 and 2). Age, race, sex and study site were included as covariates in all analyses due to their known association with the variables of interest (i.e., Vitamin D levels, age-related biological changes (i.e., AgeAccelGrim), pain intensity and disability).
Figure 1.
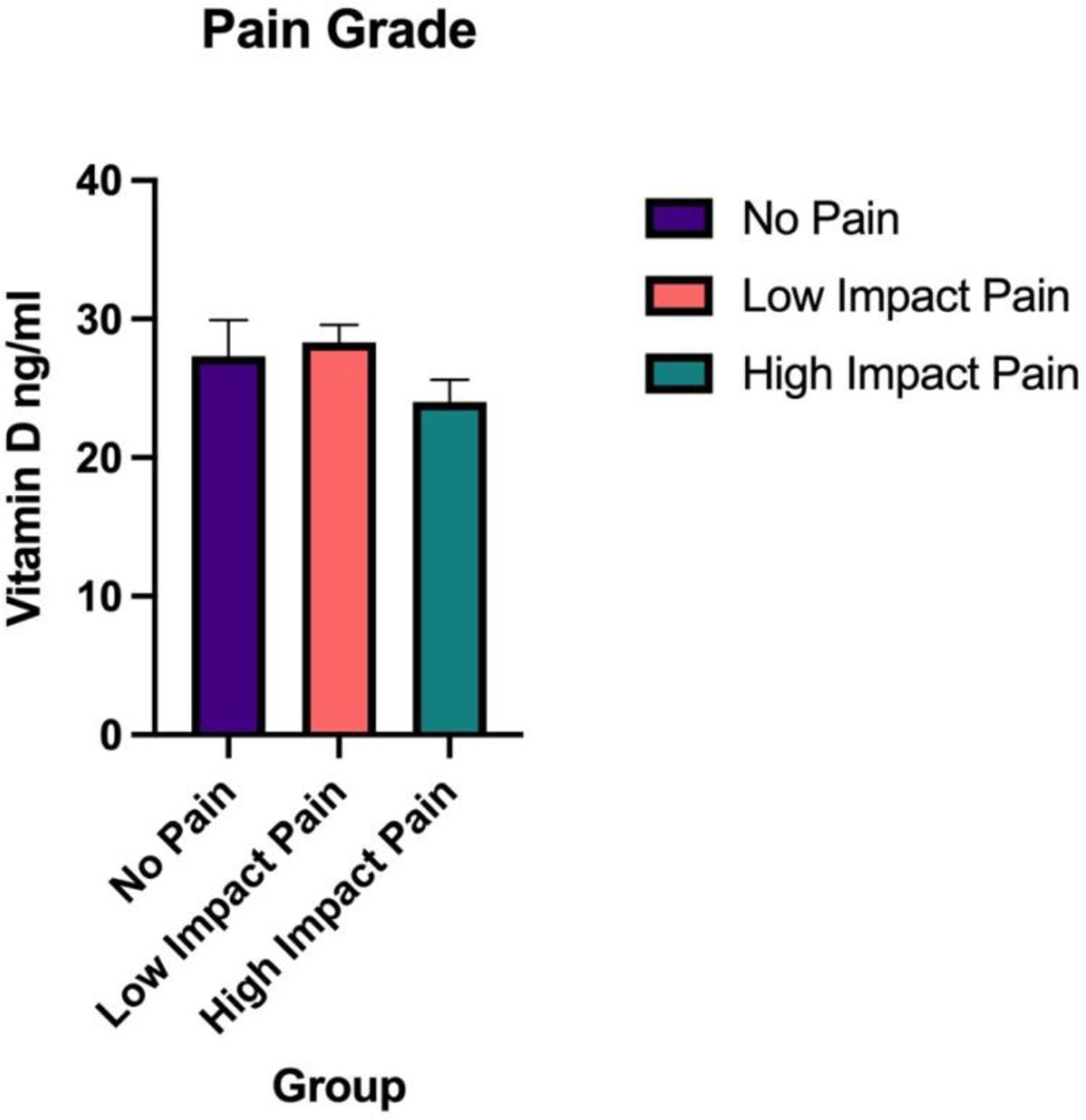
Vitamin D levels (ng/mL) by pain grade groups (No Pain, Low Impact Pain and High Impact Pain). Individuals with High Impact Pain had significantly lower levels of Vitamin D compared to Low Impact and No Pain groups.
Figure 2.
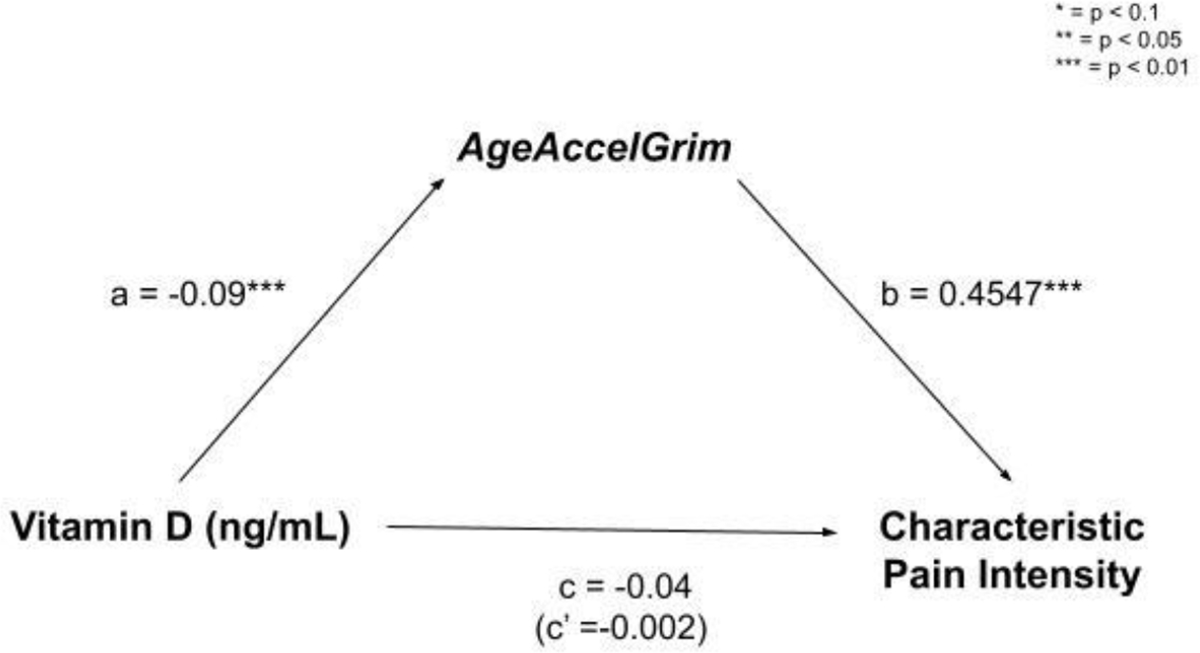
Schematic of mediation model including Vitamin D status (X), AgeAccelGrim (M), and characteristic pain intensity (Y).
Results
Sample Characteristics
Of the 245 individuals who participated in the study, only 189 individuals had complete pain, epigenetics, Vitamin D and all covariate data comprising the present study sample. Participants in the entire sample were mostly female (63.7%), 44.6% non-Hispanic Black (NHB), and had a mean age of 57.9 years (± 8.1 years). As a whole, the sample had a mean Vitamin D serum level of 26.7 ng/mL (± 12.8 ng/mL). The mean AgeAccelGrim was 2.4 years (± 5.6 years). There were no significant differences in Vitamin D levels between sex, race and study site categories (p>0.05). Sample demographic characteristics across the three pain groups are presented in Table 1.
Table 1.
Sample demographic characteristics of present study sample (alpha level=0.95; p<0.05*).
| Mean (SD) or No. | (%) | P* | ||
|---|---|---|---|---|
| No pain | Low impact pain | High impact pain | ||
| N | 26 | 106 | 57 | |
| Chronological age (years) | 59.6 (9.5) | 58.6 (8.03) | 56.3 (7.2) | 0.122 |
| DNAmGrimAge (years) | 59.4 (6.6) | 60.04 (7.6) | 61.5 (8.3) | 0.427 |
| AgeAccelGrim (years) | 0.23 (3.35) | 1.31 (5.46) | 5.00 (5.61) | <0.001* |
| Sex | ||||
| Male | 9 (34.6) | 37 (34.9) | 22 (38.6) | 0.887 |
| Female | 17 (65.4) | 69 (65.1) | 35 (61.4) | |
| Race | ||||
| Non-Hispanic black | 10 (38.5) | 41 (38.7) | 33 (57.9) | 0.057 |
| Non-Hispanic white | 16 (61.5) | 65 (61.3) | 24 (42.1) | |
| Study site | ||||
| University of Florida | 17 (65.4) | 66 (62.3) | 34 (59.6) | 0.879 |
| University of Alabama at Birmingham | 9 (34.6) | 40 (37.7) | 23 (40.4) | |
Vitamin D Levels Association with Pain and Epigenetic Aging
There was a significant difference in Vitamin D levels (F(2,118)=4.060, p=0.046) between the pain groups (No Pain, Low-Impact Pain and High Impact Pain), with individuals in the High Impact Pain group showing significantly lower mean levels of Vitamin D (24.01 ng/mL) compared to the Low Impact Pain (28.30 ng/mL) and No Pain (27.30 ng/mL) groups (Figure 1).
Epigenetic Aging Mediates Association between Vitamin D Levels with Pain
With both characteristic pain intensity and pain-related disability as the dependent variables, complete mediations of Vitamin D by AgeAccelGrim were observed. Indirect effects were calculated by multiplying the a and b pathways constituting the effect. Even after controlling for age, race, and sex, and study site there was a significant indirect effect of Vitamin D status on characteristic pain intensity through AgeAccelGrim ab = −0.0409; CI [−0.1492, −0.0237] (Figure 2). The mediator AgeAccelGrim accounted for a large portion of the total effect, PM = 0.95. Additionally, there was also a significant indirect effect of Vitamin D status on pain-related disability through AgeAccelGrim ab = −0.0483; CI [−0.1365, −0.0149] (Figure 3).
Figure 3.
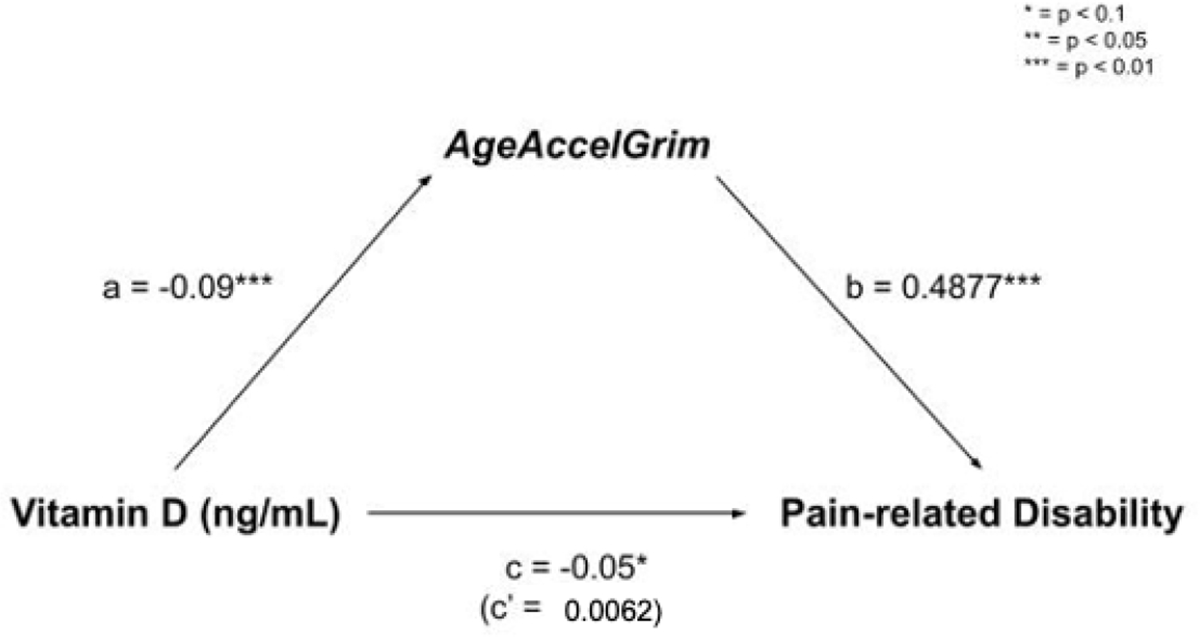
Schematic of mediation model including Vitamin D status (X), AgeAccelGrim (M), and Pain-related Disability (Y).
Discussion
Vitamin D status has been associated with pain outcomes in a variety of conditions, however, the mechanisms linking Vitamin D to pain and disability remain poorly understood. Here, we sought to understand whether the previously reported associations between Vitamin D and self-reported pain sensitivity and disability were attenuated or mediated by the GrimAge epigenetic clock in individuals with or at risk for KOA. Between groups, individuals within the High Impact Pain group had lower serum Vitamin D levels compared to Low Impact Pain and No Pain groups. Additionally, those with High Impact Pain had significantly higher AgeAccelGrim, indicating possible accelerated epigenetic aging. Upon employing a regression-based mediation model, there were significant indirect effects of Vitamin D on pain intensity and pain-related disability mediated by AgeAccelGrim. Thus, the data from this sample suggest that Vitamin D may be associated with pain and disability outcomes through interactions with an epigenetic biomarker previously reported to predict all-cause mortality.
In the present study, we used the epigenetic clock DNAmGrimAge developed and validated by Horvath and colleagues. DNAmGrimAge strongly predicts life-span and health-span17. In our sample, the High Impact Pain group had significantly greater AgeAccelGrim, with their epigenetic age being ~5 years older compared to their true chronological age. Other epigenetic clocks, such as Horvath’s pan tissue clock and Hannum’s clock have shown accelerated epigenetic aging is related to deficient Vitamin D status24. However, we have previously shown that out of all of the epigenetic clocks, only DNAmGrimAge was associated with the multidimensional experience of pain [Cruz-Almeida et al., 2021, under review]. Furthermore, the DNA surrogates used to create the DNAmGrimAge clock (beta-2 microglobulin, growth differentiation factor-15 (GDF-15), plasminogen activator inhibitor-1 (PAI-1), TIMP metallopeptidase inhibitor-1, leptin, cystatin C and adrenomedullin (ADM)) and their related plasma proteins (ADM, C-reactive protein (CRP), PAI-1 and GDF-15) and smoking have previously been associated with pain outcomes 17, making it a good option for exploration in this study. For example, CRP is a protein made by the liver that increases when there is inflammation present in the body, and it the relationship between inflammation and pain has been well established25. Leptin, an appetite-regulating hormone and pro-inflammatory adipokine has been shown to predict body pain and may be a driver of musculoskeletal pain states26. GDF-15 has also been previously shown to be associated with analgesia by inhibiting Nav1.8 sodium channel activity in the primary sensory neurons27. Another study found that higher expression of PAI-1 was associated with increased dysmenorrhea in women with endometriosis 28. Thus, there is ample reason to suggest that the DNAmGrimAge clock would be appropriate for these analyses.
Vitamin D is a fat soluble secosteroid, that is often referred to as a hormone due to its ability to participate in chemical messaging throughout the body 29. Though Vitamin D is present naturally in some foods such as fish, as well as fortified in a variety of other foodstuffs, approximately 90% of human Vitamin D synthesis comes from exposure to UV rays from sunlight 15. In our sample, not only is it possible that there are deficiencies in the diet of Vitamin D rich foods, but exacerbated pain and disability can also make it extremely difficult for the High Impact Pain group to physically get outside and spend time exposed to sunlight. It is also worth noting that even though race was controlled for in these analyses, NHBs frequently show deficient Vitamin D levels30 and were more likely to be in the High Impact Pain group; this relationship between race and Vitamin D should be explored as to explain possible racial differences seen in chronic pain.
It has been shown that Vitamin D has important roles in homeostasis 31, disease pathogenesis 32, and human development and function 33. At the genomic level, Vitamin D primarily exerts its effects through interactions with the Vitamin D receptor (VDR). The binding of Vitamin D to VDR results in the heterodimerization with the retinoid X receptor (RXR). The RXR and VDR heterodimer then has the ability to bind to Vitamin D response elements, which primarily are found in the promotor regions of Vitamin D responsive genes34. This binding results in subsequent upregulation or suppression of transcription of the genes involved. There is also evidence to suggest that Vitamin D can affect DNA methylation, one of the other ways the epigenome influences gene expression. DNA methylation involves the transfer of a methyl group to cytosine residues. It can regulate gene transcription and expression by the recruitment of proteins involved in gene repression, or inhibiting the binding of transcription factors due to alterations in the conformational state of DNA 35, 36. In general, increased DNA methylation is associated with the compaction of chromatin filaments, which leads to decreased transcription factor access and subsequent repression of the gene in that region 37. The mechanism by which Vitamin D participates in DNA methylation is currently being understood38–40. In mice supplemented with cholecalciferol, BHMT-1 expression was increased in the CD4+ T cells of mice, likely occurring through a VDR dependent pathway. BHMT-1 codes for the enzyme that catalyzes the reaction of methionine to S-adenosylmethionine – a methyl donor used in DNA methylation41. Additionally, DNA methyltransferase expression was found to be downregulated in CD4+ T cells of cholecalciferol supplemented mice42. There is also evidence suggesting the effect of Vitamin D on other enzymes involved in DNA methylation, as VDR binding sites have been located upstream to DNMT1, DMNT3, TET1 and TET3 regions34. The specific loci that Vitamin and VDR may participate in gene expression and to what extent is currently being elucidated.
We acknowledge that there were some limitations to our study, the first being the small sample size with a slightly unequal distribution of the sexes and races within our sample due to the elimination of participants during the cleaning of the data. Future studies are needed that have an equal representation of males and females and inclusion of various ethnic and racial groups. Second, we acknowledge the variability that comes with using self-reported measures. However, given the subjectivity of the pain experience, self-reported measures are the most useful way to gain insight on an individual’s daily experience over time. Future studies should aim to examine Vitamin D levels, epigenetics and other pain measures, such as Quantitative Sensory Testing physical performance measures, in order to gain more quantitative data about the pain experience. Additionally, future studies should aim to include a variety of nutrients that are also known to interact with the epigenome (e.g. Vitamins A and E) in order to truly understand to what extent Vitamin D alone or in combination is having an impact on gene expression and subsequent pain and disability outcomes 43, 44.
While novel, the data from this study highlight the important role that Vitamin D plays within the genomic environment, as well as in relation to health outcomes including pain intensity and disability. These data also add to the growing body of evidence of the importance of a nutrient-dense, high-quality diet in the potential development and maintenance of painful conditions. In addition to the lack of negative side effects of dietary interventions, they do not have the potential of addition that many of the current therapies for chronic pain have, such as opioid medications. In this case, it would also be imperative to improve on occupational health protocols to ensure adequate sunlight exposure, as well as possibly explore Vitamin D supplementation for those experiencing pain. Additionally, the incorporation opportunities for sunlight exposure to daily work and home activities as well as possible Vitamin D supplementation in geographically northern regions is strongly suggested for the prevention of pain. Continuing to understand the mechanisms of specific nutrients as well as the potential benefits of high-quality diet patterns is imperative to allow individuals with pain to live longer, healthier, and enjoyable lives.
Acknowledgments:
UPLOAD2 participants and study team; UAB National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR003096.
Funding:
This work was supported by NIH/NIA Grants R01AG059809, R01AG067757 (YCA); and R37AG033906 (RBF). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and DMR-1644779 and the State of Florida.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. Dec 2020;29–30:100587. doi: 10.1016/j.eclinm.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K, Kim HA, Felson DT, et al. Radiographic Knee Osteoarthritis and Knee Pain: Cross-sectional study from Five Different Racial/Ethnic Populations. Sci Rep. Jan 22 2018;8(1):1364. doi: 10.1038/s41598-018-19470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Totsch SK, Quinn TL, Strath LJ, et al. The impact of the Standard American Diet in rats: Effects on behavior, physiology and recovery from inflammatory injury. Scand J Pain. Oct 2017;17:316–324. doi: 10.1016/j.sjpain.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 4.Strath LJ, Brooks MS, Sorge RE, Judd SE. Relationship between diet and relative risk of pain in a cross-sectional analysis of the REGARDS longitudinal study. Pain Manag. Aug 25 2021;doi: 10.2217/pmt-2021-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushik AS, Strath LJ, Sorge RE. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain Ther. Dec 2020;9(2):487–498. doi: 10.1007/s40122-020-00200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tick H Nutrition and pain. Phys Med Rehabil Clin N Am. May 2015;26(2):309–20. doi: 10.1016/j.pmr.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 7.Sibille KT, King C, Garrett TJ, et al. Omega-6: Omega-3 PUFA Ratio, Pain, Functioning, and Distress in Adults With Knee Pain. Clin J Pain. Feb 2018;34(2):182–189. doi: 10.1097/AJP.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques Miranda C, de Lima Campos M, Leite-Almeida H. Diet, body weight and pain susceptibility - A systematic review of preclinical studies. Neurobiol Pain. Aug-Dec 2021;10:100066. doi: 10.1016/j.ynpai.2021.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strath LJ, Jones CD, Philip George A, et al. The Effect of Low-Carbohydrate and Low-Fat Diets on Pain in Individuals with Knee Osteoarthritis. Pain Med. Jan 1 2020;21(1):150–160. doi: 10.1093/pm/pnz022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helde-Frankling M, Bjorkhem-Bergman L. Vitamin D in Pain Management. Int J Mol Sci. Oct 18 2017;18(10)doi: 10.3390/ijms18102170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garfinkel RJ, Dilisio MF, Agrawal DK. Vitamin D and Its Effects on Articular Cartilage and Osteoarthritis. Orthop J Sports Med. Jun 2017;5(6):2325967117711376. doi: 10.1177/2325967117711376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifopoulos C Vitamin D: An overview. Mediterr J Rheumatol. Dec 2017;28(4):206–209. doi: 10.31138/mjr.28.4.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd DR, MacDonald PN. Vitamin D Receptor. In: Lennarz WJ, Lane MD, eds. Encyclopedia of Biological Chemistry (Second Edition). Academic Press; 2013:540–544. [Google Scholar]
- 14.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum Dis Clin North Am. Feb 2012;38(1):13–27. doi: 10.1016/j.rdc.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong LTC, Booth DR, Parnell GP. Vitamin D and its Effects on DNA Methylation in Development, Aging, and Disease. Mol Nutr Food Res. Oct 20 2020:e2000437. doi: 10.1002/mnfr.202000437 [DOI] [PubMed] [Google Scholar]
- 16.Vidal-Bralo L, Lopez-Golan Y, Mera-Varela A, et al. Specific premature epigenetic aging of cartilage in osteoarthritis. Aging (Albany NY). Sep 28 2016;8(9):2222–2231. doi: 10.18632/aging.101053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). Jan 21 2019;11(2):303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott AM, Smith BH, Smith CW, Chambers AW. Changes in chronic pain severity over time: the Chronic Pain Grade as a valid measure. Pain. Dec 1 2000;88(3):303–308. doi: 10.1016/S0304-3959(00)00337-7 [DOI] [PubMed] [Google Scholar]
- 19.Nugraha B, Gutenbrunner C, Barke A, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain. Jan 2019;160(1):88–94. doi: 10.1097/j.pain.0000000000001433 [DOI] [PubMed] [Google Scholar]
- 20.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and Profile of High-Impact Chronic Pain in the United States. The Journal of Pain. 2019/February/01/2019;20(2):146–160. doi: 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Almeida Y, Sinha P, Rani A, Huo Z, Fillingim RB, Foster T. Epigenetic aging is associated with clinical and experimental pain in community-dwelling older adults. Mol Pain. Jan-Dec 2019;15:1744806919871819. doi: 10.1177/1744806919871819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes AF. Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. Second edition. ed. Methodology in the social sciences. Guilford Press; 2018:xx, 692 pages. [Google Scholar]
- 24.Vetter VM, Spira D, Banszerus VL, Demuth I. Epigenetic Clock and Leukocyte Telomere Length Are Associated with Vitamin D Status but not with Functional Assessments and Frailty in the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci. Oct 15 2020;75(11):2056–2063. doi: 10.1093/gerona/glaa101 [DOI] [PubMed] [Google Scholar]
- 25.Sibille KT, Steingrímsdóttir ÓA, Fillingim RB, et al. Investigating the Burden of Chronic Pain: An Inflammatory and Metabolic Composite. Pain Research and Management. 2016/June/02 2016;2016:7657329. doi: 10.1155/2016/7657329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younger J, Kapphahn K, Brennan K, Sullivan SD, Stefanick ML. Association of Leptin with Body Pain in Women. Journal of women’s health (2002). 2016;25(7):752–760. doi: 10.1089/jwh.2015.5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin W, Zhang W-W, Lyu N, Cao H, Xu W-D, Zhang Y-Q. Growth Differentiation Factor-15 Produces Analgesia by Inhibiting Tetrodotoxin-Resistant Nav1.8 Sodium Channel Activity in Rat Primary Sensory Neurons. Neuroscience Bulletin. 2021/September/01 2021;37(9):1289–1302. doi: 10.1007/s12264-021-00709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alotaibi FT, Peng B, Klausen C, et al. Plasminogen activator inhibitor-1 (PAI-1) expression in endometriosis. PLOS ONE. 2019;14(7):e0219064. doi: 10.1371/journal.pone.0219064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller DN, Kleinewietfeld M, Kvakan H. Vitamin D review. J Renin Angiotensin Aldosterone Syst. Jun 2011;12(2):125–8. doi: 10.1177/1470320311410924 [DOI] [PubMed] [Google Scholar]
- 30.Harris SS. Vitamin D and African Americans. J Nutr. Apr 2006;136(4):1126–9. doi: 10.1093/jn/136.4.1126 [DOI] [PubMed] [Google Scholar]
- 31.Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol. Sep 15 2017;453:36–45. doi: 10.1016/j.mce.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Chen W, Li D, et al. Vitamin D and Chronic Diseases. Aging Dis. May 2017;8(3):346–353. doi: 10.14336/AD.2016.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito S, Leonardi A, Lanciotti L, Cofini M, Muzi G, Penta L. Vitamin D and growth hormone in children: a review of the current scientific knowledge. Journal of Translational Medicine. 2019/March/18 2019;17(1):87. doi: 10.1186/s12967-019-1840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth DR, Ding N, Parnell GP, et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes & Immunity. 2016/June/01 2016;17(4):213–219. doi: 10.1038/gene.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore LD, Le T, Fan G. DNA Methylation and Its Basic Function. Neuropsychopharmacology. 2013/January/01 2013;38(1):23–38. doi: 10.1038/npp.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature Reviews Genetics. 2012/July/01 2012;13(7):484–492. doi: 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- 37.Bell JT, Pai AA, Pickrell JK, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biology. 2011/January/20 2011;12(1):R10. doi: 10.1186/gb-2011-12-1-r10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Review. Frontiers in Physiology. 2014-April-29 2014;5(164)doi: 10.3389/fphys.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckett EL, Duesing K, Martin C, et al. Relationship between methylation status of vitamin D-related genes, vitamin D levels, and methyl-donor biochemistry. Journal of Nutrition & Intermediary Metabolism. 2016/December/01/2016;6:8–15. doi: 10.1016/j.jnim.2016.04.010 [DOI] [Google Scholar]
- 40.Gao X, Zhang Y, Schöttker B, Brenner H. Vitamin D status and epigenetic-based mortality risk score: strong independent and joint prediction of all-cause mortality in a population-based cohort study. Clinical Epigenetics. 2018/June/20 2018;10(1):84. doi: 10.1186/s13148-018-0515-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore JR, Hubler SL, Nelson CD, Nashold FE, Spanier JA, Hayes CE. 1,25-Dihydroxyvitamin D3 increases the methionine cycle, CD4+ T cell DNA methylation and Helios+Foxp3+ T regulatory cells to reverse autoimmune neurodegenerative disease. Journal of Neuroimmunology. 2018/November/15/2018;324:100–114. doi: 10.1016/j.jneuroim.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 42.Zeitelhofer M, Adzemovic MZ, Gomez-Cabrero D, et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. Feb 28 2017;114(9):E1678–e1687. doi: 10.1073/pnas.1615783114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remely M, Ferk F, Sterneder S, et al. Vitamin E Modifies High-Fat Diet-Induced Increase of DNA Strand Breaks, and Changes in Expression and DNA Methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Nutrients. 2017;9(6):607. doi: 10.3390/nu9060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar-El Dadon S, Reifen R. Vitamin A and the epigenome. Crit Rev Food Sci Nutr. Jul 24 2017;57(11):2404–2411. doi: 10.1080/10408398.2015.1060940 [DOI] [PubMed] [Google Scholar]


