Abstract
Background
The latent monkeypox outbreak has become the most emergent public health challenge globally. This study was conducted to assess the acceptability, and willingness to take and pay for a hypothetical Monkeypox vaccine among the Vietnamese general public as well as investigate preference for individual vaccine attributes.
Methods
An online cross-sectional study was conducted using snowball sampling among 842 respondents in Vietnam in 2022. A Discrete choice experiment (DCE) on preference for six major attributes of vaccine: effectiveness, immunity duration, side effects, mortality rate, restriction, and the cost was applied.
Results
Fear of the impact of monkeypox on public health and the economy, vaccine service satisfaction and responsibility to the community were the most weighted factors in the decision to take a hypothetical monkeypox vaccine. Two-thirds of participants were willing to take the vaccine, while insufficient information on monkeypox and the vaccine were the main reasons for vaccine hesitancy. For vaccine attributes, the mortality rate after seven days of vaccination was the most weighted while cost was the least influential attribute. Factors associated with willingness to take and to pay for the monkeypox vaccine included knowledge of transmission, geographical location, service satisfaction, and risk of infection, while financial burden and fear of vaccine were major drivers of hesitancy.
Conclusion
Our findings underline an urgent need for effective information dissemination through social media and counseling. The implementation of nationwide monkeypox vaccination requires prioritization and support for high-risk groups as well as consideration for the country’s financial resources.
In July 2022, the World Health Organization declared monkeypox a global health emergency [1-3]. Mass vaccination is a prioritized response to the latent monkeypox pandemic, given that over 70% of the world’s population has lost immunity against smallpox and, consequently monkeypox [2]. However, unlike COVID-19, monkeypox is not an entirely novel disease, and a preventive vaccine is available for the general population. Controversies about the COVID-19 vaccine have largely impacted the acceptance of the current monkeypox vaccine, specifically in heightened wariness about long-term side effects and immunity duration. Common concerns include the risk of autoinoculation, disseminated infection, and post-vaccination encephalitis [1]. Therefore, understanding public preference for the monkeypox vaccine is central to the development and implementation of vaccination programs.
The monkeypox epidemic that emerged during the COVID-19 pandemic resurged in many settings due to insufficient vaccination and boosting coverage [4]. This global experience emphasizes the importance of introducing a new monkeypox vaccine, which must be not only effective and accepted but also timely. From a public health perspective, it is critical to ensure the necessary infrastructure for service delivery and encourage access to and effective use of vaccines by promoting awareness, acceptance, readiness, and willingness to take the vaccine among the public. Given the novelty of the monkeypox outbreak to date, there is little evidence of the willingness to receive and pay for a new monkeypox vaccine and suggestions of monkeypox vaccination hesitancy in the context of the COVID-19 pandemic. Few studies demonstrated that the younger group, having knowledge and perception of disease severity, and receiving health warnings were substantially correlated with lower monkeypox vaccine hesitancy [5,6].
Besides empirical evidence on social and structural barriers to vaccination, frameworks for understanding vaccination motivation are needed to assess willingness to receive a monkeypox vaccine. For instance, the integrated model of the Health Belief Model and Theory of Reasoned Action identified behavioral determinants such as benefits, limitations, attitude, and subjective norms to be correlated with vaccine acceptance [7,8]. Meanwhile, a more detailed and pandemic-specific model, the World Health Organization increasing vaccination model, defines perception, social conformism, confidence in the vaccine, and convenience in accessing a vaccine as some of the most significant determinants of vaccination willingness [9,10]. Although existing frameworks have been updated extensively to adapt to new health emergencies, the economic aspects of these frameworks are lacking which is, however, very important for policy implications in resource-scare settings. In addition, one’s decision to take a monkeypox vaccine is more likely to be influenced by personal preference for known vaccine attributes rather than the mere perception of severity, benefits, and limitations as suggested by conventional frameworks. This change in vaccine perception means that simply evaluating attributes independently is not adequate to conclude public preference for a vaccine; instead, several attributes should be evaluated simultaneously under different scenarios to understand the complex decision process by specific beneficial groups. Discrete choice experiments (DCEs) are a useful technique to assess individual preference by choosing several hypothetical alternatives with different attributes. DCEs have their theoretical underpinning in the Random Utility Model, which models the choices of individuals among discrete sets of alternatives and describes the preferences by a utility function. Personal choice, or preference, is the alternative with the highest perceived utility [11]. The application of DCEs is evident in health services and policy research as well as studies of vaccination preferences but is underused in resource-scare settings, given their complexity in the study design and implementation [12]. This study aimed to assess the knowledge of the general Vietnamese population on monkeypox and evaluate their willingness to take and pay for a hypothetical monkeypox vaccine.
METHODS
Study design
The Preference for Vaccine Evaluation & Trail (The PREVENT Study) is a Vietnam nationwide assessment using a patient-centered design to inform health technology development and acceleration. Designed using the Qualtrics system (www.qualtrics.com), the PREVENT study included an online interactive questionnaire to target subjects across different regions of Vietnam from April to August 2022. Eligibility requirements included being Vietnamese living in Vietnam, aged 16 and above, being referred, and agreeing to complete the survey. The snowball sampling method [13] was used for disseminating the survey, involving 20 seeders who were first chosen respondents in all three regions: Northern, Central, and Southern. These seeders were asked to participate in the survey and then refer it to their peers. Participants took approximately 30 minutes to complete the questionnaire and then were encouraged to introduce more acquaintances and colleagues to participate in the survey. Participants were informed of the benefits and risks of participating and gave informed consent by ticking the box at the top of the questionnaire. Records were monitored and tracked using IP address by Qualtrics system to avoid duplicates and ensure the validity of the data set, then extracted, analyzed, and stored safely and confidently and used merely for research purposes.
The PREVENT Study included five topics of interest, namely: 1) monkeypox, 2) COVID-19 vaccine booster for adults, 3) COVID-19 vaccine for children, 4) HIV vaccine, and 5) a hypothetical pandemic in the future. After answering general social demographic questions, each respondent was randomly assigned to one of these five topics, generating five separate data sets. In total, 5700 respondents were included in the PREVENT study. The sub-study of the monkeypox vaccine included 842 complete records, and the response rate was 80.6%.
Measurement and instrument
We applied a standard procedure for generating the research instrument. Initially, a literature review was conducted to identify gaps in knowledge and important facets that have emerged from previous studies. Second, we constructed the questionnaire, covering the breadth of measurement of interest. A group of experts in public health, infectious diseases, health services, econometrics, linguistics, representatives of target groups, and research assistants jointly deliberated throughout the process of translating, rephrasing, piloting, and shorting the questionnaire. Finally, the tool included five major sessions: 1) socio-demographics, 2) history of COVID-19 infection and vaccination, 3) knowledge and perception of monkeypox (15 items in three domains), 4) willingness to take a hypothetical new monkeypox vaccine, and 5) willingness to pay for it. The details questionnaire, construction, and items are presented in Section 1 in the Online Supplementary Document.
Discrete choice experiment (DCE)
Six major drivers of individual choices on vaccination were derived from a thorough review of the literature, namely 1) effectiveness of the vaccine, 2) immunity duration, 3) side effects, 4) mortality rate within seven days after vaccination, 5) restriction if not vaccinated, and 6) costs. These attributes were then assigned two-five levels for choosing, contributing to a total of 31 × 41 × 23 × 51 = 480 possible alternatives (Table 1). Each participant was asked to respond to seven different scenarios based on generated combinations. The sample size was estimated to be 357 per group (Section 2 in the Online Supplementary Document).
Table 1.
Vaccine attributes in Discrete choice experiment
| Attributes | Options | ||||
|---|---|---|---|---|---|
| Effectiveness |
<60% |
60%-90% |
>90% |
|
|
| Immunity duration |
3-6 months |
6-12 months |
1-3 year |
Lifetime |
|
| Side effects |
Light, normal activities |
Severe, fatigue, immobility |
|
|
|
| Mortality rate after vaccinated |
1 / 100 000 |
10 / 100 000 |
|
|
|
| Restriction if not vaccinated |
No restrictions |
Cannot go to crowded places |
|
|
|
| Cost* | 100 000 VND | 200 000 VND | 500 000 VND | 1 000 000 VND | 2 000 000 VND |
VND – Vietnamese đồng
*1 US dollar (US$) = 23 000 VND.
Statistical analysis
Statistical analysis was performed using Qualtrics and STATA software (Stata 15. version 15. StataCorp; 2017). With missing data, we used the Listwise Deletion method to clean data before analyzing it. The Listwise Deletion method handles missing values and removes empty observations of all variables.
The internal consistency reliability was assessed using Cronbach’s alpha. The Cronbach’s alpha value of 0.7 or above was considered acceptable. We also assessed domain-domain correlation, item-item correlation, item-total correlation, and Cronbach’s alpha of the domain if the item was deleted.
The Exploratory Factor Analysis (EFA) using principal component analysis (PCA) was performed to evaluate the optimal structural model of the instrument according to the observed data. The number of factors was determined based on the Scree plot, and parallel analysis, along with eigenvalues and the proportion of variance explained. Items with a loading value ≥0.4 were included in the relevant component. Hence, in terms of the knowledge and perception of monkeypox, there were three domains, including general knowledge about monkeypox (three items), knowledge of transmission routes and ways to prevent monkeypox (nine items), and incorrect knowledge about the route of transmission, and treatment drugs of monkeypox (three items).
Potential covariates for full models of the decision to take and willingness to pay for the monkeypox vaccine are socioeconomic status, COVID-19 characteristics, related information regarding the COVID-19 vaccine, factors affecting intention to vaccinate for diseases prevention, interpersonal factors, and monkeypox's knowledge and perception. We used Ordered Logistic Regression to identify factors related to the decision to take and willingness to pay for the monkeypox vaccine. The number of observations was 705 with the Pseudo R2 was 0.0790 and 0.0684. The P-value P < 0.05 was considered statistically significant.
In DCE data analysis, individual-based utility models were yielded using Hierarchical Bayes estimation that uses Bayesian methods to probabilistically derive the relative value of each tested variable.
RESULTS
The majority of our sample were females and aged from 16 to 24 years old. The most common education level is bachelor, followed by a mean average monthly income per household of 3-10 million Vietnamese đồng (VND) (Table 2). 62.7% had been infected with COVID-19, mostly in the recent 3-6 months. The majority of our sample also received the mandatory and booster doses of the COVID-19 vaccine. In terms of factors affecting vaccination to prevent disease, the mean score of “concerns about the impact and composition of the vaccine”, “fear of vaccine”, and “responsibility to the community” was 7.0 (standard deviation (SD) = 2.0), 5.9, (SD = 2.4) and 7.1 (SD = 2.6). Regarding interpersonal factors, the average score of “risks of infected diseases”, “fear of the impact of the disease on health and economy”, and “service satisfaction” were 6.8 (SD = 2.0), 7.8 (SD = 1.9) and 7.3 (SD = 1.7), respectively (Table 3). The willingness to receive the monkeypox vaccine was positive in the majority of our sample. Among the 1.5% who were unwilling to get vaccinated for monkeypox, the main concerns were insufficient information about monkeypox (41.2%) and the vaccine’s side effects (32.6%). Vaccine cost was also an economic burden for 63.4% of participants (Table 4).
Table 2.
Demographic characteristics of participants
| Characteristics | n | % |
|---|---|---|
| Gender |
|
|
|
Male
|
239 |
28.6 |
|
Female
|
595 |
71.4 |
| Age group |
|
|
|
16-19
|
298 |
35.4 |
|
20-24
|
345 |
41.0 |
|
>25
|
198 |
23.5 |
| Educational attainment |
|
|
|
Not graduated from high school
|
92 |
11.0 |
|
Graduated from high school
|
65 |
7.7 |
|
College / university / postgraduate
|
683 |
81.3 |
| Marital status |
|
|
|
Single / divorced / widowed
|
691 |
82.3 |
|
Married
|
149 |
17.7 |
| Occupation |
|
|
|
Healthcare worker / medical students
|
357 |
42.5 |
|
Other students
|
219 |
26.1 |
|
Other occupation
|
264 |
31.4 |
| Children status |
|
|
|
No children yet
|
700 |
83.6 |
|
Pregnant / have children
|
137 |
16.4 |
| Monthly household income per capita |
|
|
|
Under 1 million VND*
|
233 |
28.9 |
|
1-3 million VND*
|
154 |
19.1 |
|
3-10 million VND*
|
315 |
39.1 |
|
>10 million VND*
|
104 |
12.9 |
| Area |
|
|
|
Hanoi – Capital city
|
335 |
42.0 |
|
Northern Provinces / cities
|
108 |
13.5 |
|
Southern Provinces / cities
|
114 |
14.3 |
|
Central and Central Highlands
|
174 |
21.8 |
|
Other provinces
|
67 |
8.4 |
| Total | 842 |
VND – Vietnamese đồng
*1 US dollar (US$) = 23 000 VND.
Table 3.
Health characteristics regarding COVID-19, factors affecting vaccination to prevent diseases in general and Interpersonal factors
| Characteristics | n | % |
|---|---|---|
| Personal and family history of COVID-19 |
|
|
|
Nobody has ever had COVID-19
|
117 |
13.9 |
|
You had COVID-19
|
528 |
62.7 |
|
Have adults at home with COVID-19
|
456 |
54.2 |
|
Children <18 years old at home with COVID-19
|
227 |
27.0 |
| Time since the last COVID-19 infection |
|
|
|
Not yet infected
|
264 |
31.7 |
|
1-3 months
|
184 |
22.1 |
|
3-6 months
|
333 |
40.0 |
|
>6 months
|
51 |
6.1 |
| Health status |
|
|
|
Completely healthy (100%)
|
342 |
40.6 |
|
Relatively healthy (80 -<100%)
|
388 |
46.1 |
|
Compromised / with illness
|
112 |
13.3 |
| History of COVID-19 vaccination of individual and family |
|
|
|
Two injections
|
225 |
26.7 |
|
More than three injections / boosters
|
559 |
66.4 |
|
At least one injection for children below 12 years old
|
57 |
6.8 |
|
At least one injection for children from 12-17 years old
|
92 |
10.9 |
|
At least two injections for adults
|
401 |
47.6 |
|
|
Mean
|
SD
|
| Factors affecting vaccination to prevent disease |
|
|
|
Concerns about the impact and composition of the vaccine (range 1-10)
|
7.0 |
2.0 |
|
Fear of vaccine (range 1-10)
|
5.9 |
2.4 |
|
Responsibility to the community (range 1-10)
|
7.1 |
2.6 |
| Interpersonal factors |
|
|
|
Risks of infected diseases (range 1-10)
|
6.8 |
2.0 |
|
Fear of the impact of the disease on health and economy (range 1-10)
|
7.8 |
1.9 |
| Service satisfaction (range 1-10) | 7.3 | 1.7 |
Table 4.
Willing to inject and pay for vaccine characteristics of respondents
| Characteristics | n | % |
|---|---|---|
| Willingness to injection |
|
|
|
Will not vaccinate
|
13 |
1.5 |
|
Not decided yet, waiting for more information
|
111 |
13.2 |
|
Will vaccine once vaccine becomes officially approved
|
551 |
65.4 |
|
Ready to vaccinate in the vaccine trial
|
167 |
19.8 |
| Reasons for vaccine hesitancy |
|
|
|
Insufficient information about monkeypox
|
296 |
41.2 |
|
Not feeling well
|
61 |
8.5 |
|
Having underlying diseases / comorbidities
|
27 |
3.8 |
|
Allergic
|
42 |
5.9 |
|
Insufficient information about vaccine’s side effects
|
234 |
32.6 |
|
Not decided, waiting for more information
|
58 |
8.1 |
|
Cannot access health institutions to vaccinate
|
22 |
3.1 |
|
Still suffering from side effects of last vaccination
|
30 |
4.2 |
|
Feeling vaccination is unnecessary
|
26 |
3.6 |
| Willing to pay |
|
|
|
Unwilling to pay
|
162 |
19.2 |
|
20% of cost
|
124 |
14.7 |
|
50% of cost
|
258 |
30.6 |
|
80% of cost
|
122 |
14.5 |
|
Full cost
|
176 |
20.9 |
| Economic burden |
|
|
|
No
|
302 |
36.6 |
| Yes | 522 | 63.4 |
The EFA results about monkeypox's knowledge and perception included three factors. 1) General knowledge about monkeypox: mean (m) = 6.82 (SD = 4.18), Cronbach’s alpha = 0.90; 2) knowledge of transmission routes and ways to prevent monkeypox: m = 4.34 (SD = 3.31), Cronbach’s alpha = 0.86; 3) misconception on monkeypox: m = 1.30 (SD = 2.7) and Cronbach’s alpha = 0.75 (Table 5).
Table 5.
Exploratory factor analysis of knowledge and perception of monkeypox among participants
| Items | n (%) | General knowledge about monkeypox | Knowledge of transmission routes and ways to prevent monkeypox | Misconception on monkeypox |
|---|---|---|---|---|
| Monkeypox only exists in Western and Middle Africa |
814 (79.6) |
0.5973 |
|
|
| Vietnam is at risk of developing a monkeypox outbreak |
648 (63.4) |
|
0.4256 |
|
| Monkeypox is spreading fast globally |
626 (61.2) |
|
0.6014 |
|
| It is difficult to spread monkeypox between humans |
208 (20.3) |
|
|
0.7706 |
| Monkeypox is spread through sexual means |
651 (63.7) |
0.9087 |
|
|
| Monkeypox is spread through skin contact and droplets |
465 (45.5) |
|
0.6168 |
|
| Monkeypox has the same manifestation as smallpox |
383 (37.5) |
|
0.5136 |
|
| Having multiple pustules is a symptom of monkeypox |
346 (33.9) |
|
0.5822 |
|
| Fever, headache, swollen lymph nodes are symptoms of monkeypox |
460 (45.0) |
|
0.6254 |
|
| Monkeypox can result in fatigue and death |
374 (36.6) |
|
0.623 |
|
| Treatment for monkeypox is available |
70 (6.8) |
|
|
0.4476 |
| Not eating wild animals is a prevention means |
318 (31.1) |
|
0.5922 |
|
| Reducing interactions with wild animals is a prevention means |
378 (37.0) |
|
0.6495 |
|
| Monkeypox is not transmitted through handshakes, hugs, and kisses |
121 (11.8) |
|
|
0.7863 |
| Strengthening personal hygiene and nutritional plan are prevention means |
625 (61.1) |
0.9104 |
|
|
| Reliability (Cronbach’s alpha) |
|
0.89 |
0.86 |
0.75 |
| Score (range 0-10), mean (SD) | 6.14 (1.40) | 4.54 (3.30) | 1.40 (2.80) |
SD – standard deviation
Participants who were female and married had a higher willingness to take the monkeypox vaccine. Participants who resided in the South had a lower willingness to take than those from Hanoi, while residents in Central and Central Highlands had higher vaccine acceptance and willingness to pay. Participants who had higher risks of infected diseases and service satisfaction scores had a higher level of willingness to inject the vaccine. A higher score of knowledge of transmission routes and ways to prevent monkeypox and satisfaction with service were positive factors associated with willingness to pay for the monkeypox vaccine. By contrast, a higher score of fear of the vaccine and the burden of medical expenses were likely to be associated with a lower level of willingness to pay for monkeypox vaccine (Table 6).
Table 6.
Factors associated with willingness to take and willingness to pay for monkeypox vaccine among participants
| Characteristics | Willingness to take |
Willingness to pay |
||
|---|---|---|---|---|
|
(1 “not inject” – 4 “willing to participate in trials”)
|
(1 “no pay” – 5 “100% fee”)
|
|||
|
|
OR
|
95% CI
|
OR
|
95% CI
|
| Socio-economic |
|
|
|
|
| Gender (female vs. male – Ref.) |
0.50* |
0.34-0.73 |
|
|
| Marital status (married vs. single / divorced / widowed – Ref.) |
0.57† |
0.36-0.92 |
|
|
| Monthly household income per capita (<1 million VND – Ref.) |
|
|
|
|
|
1-3 million VND
|
1.22 |
0.77-1.92 |
1.79* |
1.21-2.64 |
|
3-10 million VND
|
0.71‡ |
0.48-1.07 |
1.43† |
1.03-2.00 |
|
>10 million VND or above
|
0.69 |
0.39-1.22 |
4.57* |
2.77-7.54 |
| Location (vs. Hanoi – Ref.) |
|
|
|
|
|
Northern provinces
|
1.41 |
0.84-2.35 |
1.34 |
0.88-2.04 |
|
Southern provinces
|
0.60† |
0.37-0.96 |
1.36 |
0.91-2.03 |
|
Central and Central Highlands
|
1.57† |
1.02-2.41 |
1.85* |
1.29-2.66 |
|
Other provinces
|
0.88 |
0.46-1.68 |
0.79 |
0.47-1.34 |
| Health status (vs. Completely healthy 100% – Ref) |
|
|
|
|
|
Relatively healthy (80 -<100%)
|
0.64† |
0.45-0.91 |
0.76‡ |
0.56-1.04 |
|
Compromised/with illness
|
0.47* |
0.28-0.79 |
0.61† |
0.40-0.95 |
| History of COVID-19 vaccination of self and family |
|
|
|
|
|
Had mandatory two injections
|
|
|
1.36† |
1.00-1.85 |
|
At least two injections for adults
|
0.71† |
0.51-0.98 |
|
|
| Knowledge and perception of monkeypox |
|
|
|
|
|
Knowledge of transmission routes and ways to prevent monkeypox (unit: score)
|
|
|
1.04† |
1.00-1.09 |
| Factors affecting vaccination to prevent disease |
|
|
|
|
|
Fear of vaccine (unit: score)
|
|
|
0.95* |
0.93-0.97 |
| Interpersonal factors |
|
|
|
|
|
Risks of infected diseases (unit: score)
|
1.06* |
1.02-1.09 |
|
|
|
Fear of the impact of the disease on health and economy (unit: score)
|
0.93* |
0.90-0.97 |
|
|
|
Service Satisfaction (unit: score)
|
1.04† |
1.00-1.09 |
1.06* |
1.03-1.09 |
| Economic burden (yes vs. no – Ref) | 0.76 | 0.54-1.07 | 0.46* | 0.34-0.61 |
OR – odds ratio, CI – confidence interval
*P < 0.01, †P < 0.05, ‡P < 0.1.
Our DCE result suggested that mortality rate had the most influence on the decision-making process, followed by immunity and vaccine effectiveness. The cost was the least weighted attribute at 7.8 points (Figure 1). The best scenario for respondents included above 90% effectiveness with life-long immunity, minor side effects, low mortality rate, and no limitation. For this scenario, the mean willingness to pay among participants was 200 000 VND. The actual vaccine package based on the current smallpox vaccine included: 60%-90% effectiveness, life-long immunity, potential adverse side effects such as fatigue, fever or blister, insignificant mortality rate, and few limitations if not vaccinated. For this package, the generated willingness to pay was 187 000 VND (Table 7 and Table 8).
Figure 1.
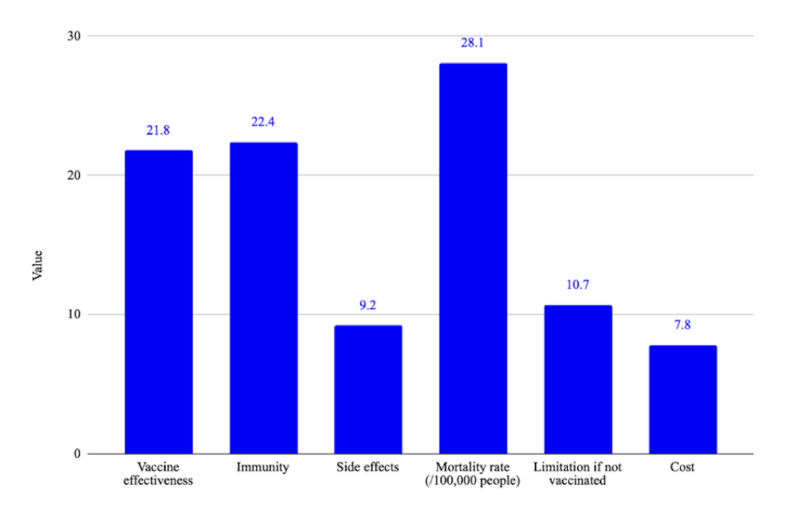
Feature importance of vaccine attributes during the decision-making process.
Table 7.
The optimal monkeypox vaccine attributes package
|
Vaccine effectiveness
|
More than 90% |
|
Immunity
|
Life-long |
|
Side effects
|
Minor, do not affect daily activities |
|
Mortality rate ( / 100 000 people)
|
One death |
|
Limitation if not vaccinated
|
No |
| Cost* | 200 000 VND |
VND – Vietnamese đồng
*1 US dollar (US$) = 23 000 VND.
Table 8.
The actual vaccine package based on the current smallpox vaccine
|
Vaccine effectiveness
|
60%-90% |
|
Immunity
|
Life-long |
|
Side effects
|
Severe, fatigue and immobility |
|
Mortality rate ( / 100 000 people)
|
One death |
|
Limitation if not vaccinated
|
No |
| Cost* | 187 000 VND |
VND – Vietnamese đồng
*1 US dollar (US$) = 23 000 VND.
DISCUSSION
Our findings indicate a high willingness to take monkeypox vaccines and a moderate willingness to pay, provided that sufficient information about the pandemic and vaccine characteristics are available; this preference was the same among the general population and health professionals / medical students. While most participants had a basic understanding of monkeypox, more information should be given on monkeypox symptoms and transmission. Drivers of vaccine decisions were fear of the impact on community health and economy, satisfaction with health and vaccination services, sense of responsibility to the community, and concerns about the composition of the vaccine. Willingness to take and to pay for the vaccines vary not only with socio-economic characteristics and vaccine attributes but also with the history of personal experience with COVID-19.
Overall, while the basic knowledge of smallpox, such as the current situation and basic symptoms, was reported as above average, our participants demonstrated a poor comprehension of monkeypox; this is consistent with recent studies on monkeypox. In fact, Indonesian general health practitioners recorded only 9% with a thorough understanding of the disease, while 23.1% mistook antibiotics as a required monkeypox treatment [5,14]. Critical public misperception can be attributed to the ineffective dissemination of monkeypox information. Indeed, more than 20% of our sample were unaware of human-to-human monkeypox transmission, nor believed that monkeypox could transmit through intimacy. In addition, among all predictors of vaccine hesitancy identified in this study and available literature, concerns about the composition of the monkeypox vaccine were consistently ranked as the most important [5,15]. Compared to COVID-19, monkeypox information was significantly less effectively communicated to the public. Although the current state of monkeypox is comparable to that of COVID-19 in January 2020, monkeypox's basic information was significantly less effectively communicated to the public than COVID-19's, which was correctly perceived by up to 86% of the population and broadcasted intensively worldwide at that time [16,17]. Informing the public about the severity and basic symptoms of monkeypox is critically important even when clinical information is not yet official, as delays in early communication had resulted in a failure to control the disease in other recent outbreaks [18].
Although the monkeypox vaccine was widely accepted, just 20% of respondents were willing to pay for it, even when people had knowledge about the disease and the related risks. Since the modern monkeypox vaccine is not yet available, the willingness to pay was determined based on smallpox vaccine attributes, which included a level of effectiveness below 90%, a life-long immunity, potential adverse side effects such as fatigue, fever or blister, and insignificant mortality rate [19,20]. For this vaccine package, participants were willing to pay 187 000 VND (8US$), which amounted to only 3.7% of the national monthly household income per capita of approximately 5 million VND (US$212). Furthermore, Vietnamese’ willingness to pay was only one-fifth of the willing to pay (WTP) recorded in Indonesia, a middle-income peer. The WTP calculated in a cross-sectional study among Indonesian frontline physicians was US$37 on average, equivalent to 19.2% of their national monthly household income per capita [21,22]. Compared to COVID-19, the WTP recorded in this study is also one-half of the WTP recorded for the COVID-19 vaccine in Vietnam in 2021 [23].
Our results also demonstrated cost as the least influential factor when making vaccine decisions compared to other clinical attributes, where all factors were weighted at least twice as much cost. Also, there was a strong correlation between satisfaction with vaccination services during COVID-19 and willingness to take and pay for the monkeypox vaccine. In terms of geographical difference, despite having a lower average income, participants in the Central and Central Highlands were more willing to take and pay for the vaccine than those in Hanoi and other major provinces. A possible explanation is that residents in rural areas were generally more physically and financially impacted by the COVID-19 pandemic, which resulted in heightened wariness to the prevalent pandemic. As rural communities also have smaller populations, it is more convenient to encourage adherence to government-led social measures, such as taking a monkeypox vaccine [24,25].
The most important implication of our findings for government intervention design emphasizes on effective communication campaigns and enhanced service accessibility. Transparent communication is needed about monkeypox presentation, risks, recommendations of clinicians, and the active role of the population in prevention and management. Given the increasing popularity of online platforms, information should be dispensed through social media as well. In the interest of early communication, public figures and influential individuals should be recruited or encouraged to raise awareness about the disease, especially in the domains where monkeypox substantially differs from COVID-19, such as transmission mode and symptoms. Social listening insights on public perception and misinformation should be more widely utilized to inform risk communication and identify areas that need addressing, such as the potential stigmatization of LGBTQ+ groups associated with the misconception that monkeypox is transmitted through homosexual contact [26]. At the same time, conventional means such as television and public infographics should still maintain the role of an official and trusted standard-setting platform to avoid misinformation. Second, financial support schemes, such as discounts during the first three months of vaccination launch, can be provided to help citizens transition from free-of-charge COVID-19 vaccines to compulsory and with charge monkeypox, as well as to promote early vaccination among the public.
The main strength of our study is the complexity of the DCE scenarios that we provided to respondents and the geographical variation. However, a limitation was that as monkeypox is a recently emerged health issue after a long period of COVID-19, many participants may be influenced by their previous perception of COVID-19 when answering specific questions, such as willingness to pay for vaccines. Lastly, data collected through self-reporting may be subject to bias, and we did not survey a representative sample of the general population. For instance, our sample was made up of a majority of women, people with higher education, and students. This trend is attributed to the fact that our survey was distributed online and that participation was voluntary, meaning people who are more conscious of their health or engage in online content more frequently would be more likely to respond to our survey. Nonetheless, this study is among the first and most significant efforts to examine national knowledge of monkeypox and provides early insights on how monkeypox vaccination and management should proceed in the coming stage. Future research direction can be on the optimal price and discount level, cost distribution strategy across different socioeconomic and geographical groups, social factors and other factors related to the COVID-19 isolation period that influence the decision to vaccinate.
CONCLUSION
Our study presented lower-than-expected knowledge of monkeypox among Vietnamese respondents. Although the monkeypox vaccine was widely accepted, the willingness to pay was critically low. As lack of information and misinformation were the primary predictors of vaccine reluctance and unwillingness to pay, urgent and effective information dissemination should be implemented, particularly through social media and television. Regarding vaccination, authorities should prioritize high-risk groups and consider the population's willingness to be vaccinated to determine the optimal vaccine price without compromising the country's economic status and limited health resources.
Additional material
Acknowledgments
The authors would like to thank ActionAid Vietnam and the research collaborators.
Ethics statement: The protocol was approved by ethical review committees designated by the Vietnam Ministry of Health, decision number 164/GCN-HDDDNCYSH-DHYHN and 13/HDDD-DHDT.
Footnotes
Funding: This work was funded by Vingroup Joint Stock Company (Vingroup JSC), Vingroup and supported by Vingroup Innovation Foundation (VINIF) under project code VINIF.2020.COVID-19.DA03, and ActionAid Vietnam.
Authorship contributions: Conceptualization, methodology, design, and measures development: BXT, LAD, CL; Data collection: BXT, HPT, THTD; Data analysis: BXT, MNLV, THTD; Supervision: BXT, LB, PA, GF; Writing: All authors.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
REFERENCES
- 1.World Health Organization. Monkeypox WHO Fact Sheet. 2018.
- 2.Simpson K, Heymann D, Brown CS, Edmunds WJ, Elsgaard J, Fine P, et al. Human monkeypox – After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-81. 10.1016/j.vaccine.2020.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO declares monkeypox a global health emergency as infections soar (press release). 2022.
- 4.Our World in Data. Coronavirus (COVID-19) Vaccinations. 2022. [Google Scholar]
- 5.Temsah M-H, Aljamaan F, Alenezi S, Alhasan K, Saddik B, Al-Barag A, et al. Monkeypox caused less worry than COVID-19 among the general population during the first month of the WHO Monkeypox alert:Experience from Saudi Arabia. Travel Med Infect Dis. 2022;49:102426. 10.1016/j.tmaid.2022.102426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner KE, Ssekyanzi H, Sicsic J, Mueller JE, Toomey T, Ulrich AK, et al. What drives willingness to receive a new vaccine that prevents an emerging infectious disease? A discrete choice experiment among university students in Uganda. PLoS One. 2022;17:e0268063. 10.1371/journal.pone.0268063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fall E, Izaute M, Chakroun-Baggioni N.How can the health belief model and self-determination theory predict both influenza vaccination and vaccination intention? A longitudinal study among university students. Psychol Health. 2018;33:746-64. 10.1080/08870446.2017.1401623 [DOI] [PubMed] [Google Scholar]
- 8.Kan T, Zhang J.Factors influencing seasonal influenza vaccination behaviour among elderly people: a systematic review. Public Health. 2018;156:67-78. 10.1016/j.puhe.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald NE.Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161-4. 10.1016/j.vaccine.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 10.Harrison JA, Mullen PD, Green LW.A meta-analysis of studies of the health belief model with adults. Health Educ Res. 1992;7:107-16. 10.1093/her/7.1.107 [DOI] [PubMed] [Google Scholar]
- 11.Mangham LJ, Hanson K, McPake B.How to do (or not to do) ... Designing a discrete choice experiment for application in a low-income country. Health Policy Plan. 2009;24:151-8. 10.1093/heapol/czn047 [DOI] [PubMed] [Google Scholar]
- 12.Mühlbacher A, Johnson FR.Choice Experiments to Quantify Preferences for Health and Healthcare: State of the Practice. Appl Health Econ Health Policy. 2016;14:253-66. 10.1007/s40258-016-0232-7 [DOI] [PubMed] [Google Scholar]
- 13.Goodman LA.Snowball sampling. Ann Math Statist. 1961;32:148-70. 10.1214/aoms/1177705148 [DOI] [Google Scholar]
- 14.Harapan H, Setiawan AM, Yufika A, Anwar S, Wahyuni S, Asrizal FW, et al. Knowledge of human monkeypox viral infection among general practitioners: a cross-sectional study in Indonesia. Pathog Glob Health. 2020;114:68-75. 10.1080/20477724.2020.1743037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najeeb H, Huda Z.Monkeypox virus: A spreading threat for Pakistan? Ann Med Surg (Lond). 2022;79:103977. 10.1016/j.amsu.2022.103977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmons S, Barjaková M, Robertson D, Belton C, Lunn P. Public understanding and perceptions of the COVID-19 test-and-trace system. The Economic and Social Research Institute. 2020. [Google Scholar]
- 17.Chen E, Lerman K, Ferrara E.Tracking Social Media Discourse About the COVID-19 Pandemic: Development of a Public Coronavirus Twitter Data Set. JMIR Public Health Surveill. 2020;6:e19273. 10.2196/19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudd R, Baur C.Health literacy and early insights during a pandemic. J Commun Healthc. 2020;13:13-6. 10.1080/17538068.2020.1760622 [DOI] [Google Scholar]
- 19.Bonilla-Guerrero R, Poland GA.Smallpox vaccines: current and future. J Lab Clin Med. 2003;142:252-7. 10.1016/S0022-2143(03)00143-4 [DOI] [PubMed] [Google Scholar]
- 20.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287-94. 10.1097/00006454-200404000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Harapan H, Wagner AL, Yufika A, Setiawan AM, Anwar S, Wahyuni S, et al. Acceptance and willingness to pay for a hypothetical vaccine against monkeypox viral infection among frontline physicians: A cross-sectional study in Indonesia. Vaccine. 2020;38:6800-6. 10.1016/j.vaccine.2020.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CEIC. Indonesia Monthly Earnings. 2022.
- 23.Nguyen LH, Hoang MT, Nguyen LD, Ninh LT, Nguyen HTT, Nguyen AD, et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Trop Med Int Health. 2021;26:1303-13. 10.1111/tmi.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linh TNQ, Hanh TTT, Shaw R.COVID-19 initial preparedness and response in Vietnam during the first six months of the pandemic and the lessons for Sendai framework implementation. Int J Disaster Resil Built Environ. 2021;12:143-55. 10.1108/IJDRBE-07-2020-0080 [DOI] [Google Scholar]
- 25.Ho HT, Jenkins C, Ta HQ, Bui CL, Van Hoang M, Santin O.Digital support for caregivers of patients with non-communicable diseases during COVID-19: Lessons from a cancer case study in Vietnam. J Glob Health. 2021;11:03095. 10.7189/jogh.11.03095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aquino YSJ, Cabrera N, Salisi J, Yarcia LE.Monkeypox and the legacy of prejudice in targeted public health campaigns. BMJ Glob Health. 2022;7:e010630. 10.1136/bmjgh-2022-010630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


