Abstract
Background
While nasopharyngeal (NP) swabs are considered the gold standard for severe acute respiratory coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase-polymerase chain reaction (RT-PCR) detection, several studies have shown that saliva is an alternative specimen for COVID-19 diagnosis and screening.
Methods
To analyze the utility of saliva for the diagnosis of COVID-19 during the circulation of the Omicron variant, participants were enrolled in an ongoing cohort designed to assess the natural history of SARS-CoV-2 infection in adults and children. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Cohen’s kappa coefficient were calculated to assess diagnostic performance.
Results
Overall, 818 samples were collected from 365 outpatients from January 3 to February 2, 2022. The median age was 32.8 years (range: 3–94 years). RT-PCR for SARS-CoV-2 was confirmed in 97/121 symptomatic patients (80.2%) and 62/244 (25.4%) asymptomatic patients. Substantial agreement between saliva and combined nasopharyngeal/oropharyngeal samples was observed with a Cohen’s kappa value of 0.74 [95% confidence interval (CI): 0.67–0.81]. Sensitivity was 77% (95% CI: 70.9–82.2), specificity 95% (95% CI: 91.9–97), PPV 89.8% (95% CI: 83.1–94.4), NPV 87.9% (95% CI: 83.6–91.5), and accuracy 88.5% (95% CI: 85.0-91.4). Sensitivity was higher among samples collected from symptomatic children aged three years and older and adolescents [84% (95% CI: 70.5–92)] with a Cohen’s kappa value of 0.63 (95% CI: 0.35–0.91).
Conclusions
Saliva is a reliable fluid for detecting SARS-CoV-2, especially in symptomatic children and adolescents during the circulation of the Omicron variant.
Keywords: COVID-19, SARS-CoV-2, Omicron, Saliva, Accuracy
Background
On November 26, 2021, the World Health Organization (WHO) designated lineage B.1.1.529 a variant of concern (VOC) named Omicron, on the advice of WHO’s Technical Advisory Group on Virus Evolution [1]. At the beginning of January 2022, Rio de Janeiro, Brazil, experienced a considerable increase in cases of COVID-19. Genomic sequencing studies carried out by the COVID-19 Oswaldo Cruz Foundation (Fiocruz) Genomic Surveillance Network and other institutions suggested that the Omicron variant was responsible for 96% of these cases (http://www.genomahcov.fiocruz.br/dashboard-en/) [2].
While nasopharyngeal (NP) swabs are considered the gold standard for SARS-CoV-2 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) detection, several studies have shown that saliva is an alternative specimen for COVID-19 diagnosis and screening [3–6], including for asymptomatic persons and outpatients [7, 8]. Collecting saliva is non-invasive and is better tolerated and accepted than swabs [7]. As a result, there is a reduced infection risk for healthcare workers and decreased personal protective equipment usage because direct interaction between healthcare workers and patients can be avoided [9, 10]. Yee et al. observed that the performance of saliva and NP swabs were comparable for symptomatic and asymptomatic pediatric patients [9]. In addition, a study showed a 100% positive agreement for the Omicron variant in saliva swabs compared to paired mid-turbinate swabs [11].
We report the accuracy of unstimulated whole saliva (UWS) through drooling compared to combined nasopharyngeal and deep oropharyngeal (OP) swabs in outpatients with suspected COVID-19 and their household contacts. We also compared the distribution of Cycle threshold (Ct) values in the UWS and combined NP/OP of asymptomatic and symptomatic patients.
Methods
Patient information and clinical samples were derived from an open, prospective cohort study designed to assess the natural history of SARS-CoV-2 infection in adults and children. Contacts residing in the same domicile as the index case were offered enrolment in the study. Asymptomatic and symptomatic adults, adolescents, and children were recruited at the Evandro Chagas National Institute of Infectious Diseases (INI) and Germano Sinval Faria Health Centre [12], located in the metropolitan region of Rio de Janeiro during the study period. Residents of the community of Manguinhos, a region with less favorable socioeconomic characteristics, were recruited at the Germano Sinval Faria Health Centre. On the other hand, the participants recruited at the outpatient clinic (INI) came from throughout the metropolitan region of Rio de Janeiro, which includes a number of municipalities that are far from the capital and have a different sociodemographic profile.
Regular home visits according to a pre-defined schedule were made to collect samples (serum, saliva, and naso-oral swabs specimens) and complete the study forms. We collected clinical data, including sociodemographic information, date of symptom onset (if any of the following were present: cough, shortness of breath, fever, chills, headache, loss of taste or smell, fatigue, muscle aches, sore throat, xerostomia, nasal congestion or rhinorrhea, arthralgia, prostration, abdominal pain, nausea, vomiting or diarrhea, and skin rashes), and type of sample collected (UWS vs. NP/OP). The present manuscript described the results of a selection of the cohort participants with an available paired specimen collected during their regular study visits or unscheduled study visits in case a participant developed COVID-19 symptoms during the circulation of Omicron in January 2022. Some participants had more than one paired sample collection during the period.
NP/OP and UWS were collected on the same visit. Nurses collected NP/OP swabs. UWS was collected, under nurses’ supervision, by asking the participant to accumulate saliva (at least 1–2 mL) in the mouth for one minute and then drooling into a sterile container without coughing or clearing their throats. UWS was collected only for children who drool saliva (aged three years and older). Combined NP/OP swabs were placed in a Falcon tube with 3 mL of Viral Transport Medium (VTM). Samples were transferred on the same day in a refrigerated bag to the Laboratory of Respiratory Viruses and Measles, a national reference laboratory for SARS-CoV-2 RT-PCR testing.
On receipt at the national reference laboratory, a suspension was prepared consisting of UWS and VTM that had a final volume of 2 mL. Before preparing aliquots for extractions, all samples were vortex-homogenized for 30 s. The viral RNA was extracted automatedly using 300 µl of the sample and Perkin-Elmer Chemagic machine/chemistry. SARS-CoV-2 positive cases were confirmed by real-time RT–PCR assays using the SARS-CoV-2 Molecular E/RP Kit (Biomanguinhos, Rio de Janeiro, Brazil) [13] based on the protocol previously designed by Corman et al. [14]. Amplifications were conducted in the ABI7500 platform using the following conditions: reverse transcription (50 °C, 15 min), reverse transcriptase inactivation and DNA polymerase activation (95 °C, 2 min), followed by 45 cycles of DNA denaturation (95 °C, 20 s) and annealing–extension (58 °C, 30 s). All samples with sigmoid curves crossing the threshold line up to cycle 40 were considered positive. Negative and positive controls were included in each extraction and real-time RT–PCR batch.
Cycle threshold (Ct) values of RT-PCR targeting the E gene were recorded for all positive results. Frequencies and percentages were reported for categorical variables. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with a 95% confidence interval (CI), were calculated to assess diagnostic performance [15]. The Cohen’s kappa coefficient was used to estimate the agreement between the UWS and combined NP/OP swabs results as a reference gold standard. We used Wilcoxon’s signed-rank test to compare the Ct values of UWS and combined NP/OP samples.
Furthermore, we compared Ct values between asymptomatic and symptomatic RT-PCR confirmed patients and between concordant and discordant UWS and NP/OP groups with the Kruskal-Wallis test followed by a Dunn post-hoc test [16]. Also, the Chi-square test or Fisher’s exact test for selected categorical variables was conducted to assess differences in discordant results. All statistical analyses were performed using the software R, version 4.1.0 (R Core Team, 2021). Statistical significance was set at a p-value ≤ 0.05.
Results
From January 3, 2022 to February 2, 2022, 365 outpatients were enrolled. The median age was 32.8 years, ranging from 3 to 94 years, with 111 (30.4%) patients under 18 years old. There were 221 (60.5%) female patients. A total of 119 (32.6%) patients had symptomatic, and 246 (67.4%) had an asymptomatic infection during the first sample collection. Selected baseline characteristics at enrollment are summarized in Table 1. None of the patients were hospitalized during the study period, and those who developed symptoms had mild or moderate disease. The median time between the onset of symptoms and the first sample collection was three days. Two participants developed symptoms in the interval between the two sample collections. RT-PCR for SARS-CoV-2 was confirmed in 97 out of 121 symptomatic patients (80.2%) either by detectable SARS-CoV-2 in NP/OP swab or UWS samples; most were adults (n = 71, 73.2%). Detectable RT-PCR for SARS-CoV-2 was observed in 62 out of 244 (25.4%) asymptomatic patients, and the majority also corresponded to adult patients (n = 44, 71.0%).
Table 1.
Baseline characteristics of the participants enrolled in the study
| Characteristics | Children and adolescents (< 18 years old) n = 111 | Adults (18 years and older) n = 254 |
|---|---|---|
| Sex | ||
| Male | 53 (47.7) | 91 (35.8) |
| Female | 58 (52.3) | 163 (64.2) |
| Age | ||
| 3–12 years | 74/365 (20.3) | - |
| ≥12–18 years | 37/365 (10.1) | - |
| ≥18 years | - | 254/365 (69.3) |
| Mean (SD) | 10.2 (3.7) | 43.7 (16.2) |
| Median (IQR) | 9.7 (7.2, 13.4) | 40.8 (31.7, 56.8) |
| [Minimum, Maximum] | [3.4, 17.5] | [18.3, 94.1] |
| Highest educational attainment | ||
| Primary or lower | 103 (92.8) | 42 (16.5) |
| Secondary | 8 (7.8) | 102 (40.2) |
| University or postgraduate | - | 101 (39.8) |
| Missing | - | 9 (3.5) |
| Main Comorbidities | ||
| Allergic rhinitis, yes | 16 (14.4) | 39 (15.4) |
| Asthma, yes | 14 (12.6) | 11 (4.3) |
| Hypertension, yes | - | 66 (26.0) |
| Obesity, yes | - | 41 (16.1) |
| Diabetes Mellitus, yes | - | 18 (7.1) |
| Thyroid dysfunction, yes | - | 18 (7.1) |
| Cardiovascular disease, yes | - | 12 (4.7) |
| Cancer, yes | - | 5 (2.0) |
| Kidney chronic disease, yes | - | 3 (1.2) |
| HIV Infection, yes | - | 2 (0.8) |
| Symptomatic infection, yes | 33 (27.7) | 86 (72.3) |
IQR interquartile range, SD standard deviation
Overall, 818 samples were collected. Analysis of concordance was conducted between UWS and combined NP/OP samples showing an overall Cohen’s kappa value of 0.74 (95% CI: 0.67–0.81), indicating substantial agreement between the two sampling methods. Sensitivity was 77% (95% CI: 70.9–82.2), specificity 95% (95% CI: 91.9–97), PPV 89.8% (95% CI: 83.1–94.4), NPV 87.9% (95% CI: 83.6–91.5), and accuracy 88.5% (95% CI 85.0- 91.4) (Table 2).
Table 2.
Overall results from combined nasopharyngeal/oropharyngeal swabs and unstimulated whole saliva samples in same-day matched pairs (n = 409)
| Combined nasopharyngeal/oropharyngeal swabs | |||
|---|---|---|---|
| Positive | Negative | Total | |
| Unstimulated Whole Saliva |
N (%) | N (%) | N (%) |
| Positive | 114 (89.8) | 13 (10.2) | 127 (100) |
| Negative | 34 (12.1) | 248 (87.9) | 282 (100) |
| Total | 148 (36.2) | 261 (63.8) | 409 (100) |
The comparison of performance between UWS and combined NP/OP samples, based on the RT-PCR detection of SARS-CoV-2 stratified by age groups and symptoms, is shown in Table 3.
Table 3.
Summary of results obtained from parallel testing of combined nasopharyngeal/oropharyngeal swabs and unstimulated whole saliva stratified by age groups and symptoms (all samples)
| Combined nasopharyngeal/oropharyngeal swabs | ||||||
|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | |||||
| Unstimulated | Positive | Negative | Total | Positive | Negative | Total |
| Whole Saliva | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| Children and adolescents (< 18 years old) | ||||||
| Positive | 10 (83.3) | 2 (16.7) | 12 (100) | 21 (95.5) | 1 (4.5) | 22 (100) |
| Negative | 6 (8.1) | 68 (91.9) | 74 (100) | 4 (36.4) | 7 (63.6) | 11 (100) |
| Adults (18 years and older) | ||||||
| Positive | 30 (81.1) | 7 (18.9) | 37 (100) | 53 (94.6) | 3 (5.4) | 56 (100) |
| Negative | 7 (4.3) | 156 (95.7) | 163 (100) | 17 (50.0) | 17 (50.0) | 34 (100) |
Numbers in brackets stand for the proportion of the total of Unstimulated Whole Saliva for asymptomatic and symptomatic patients in each age group.
Sensitivity was higher among samples collected from symptomatic children and adolescent participants, and specificity was higher among asymptomatic patients regardless of age. The agreement between UWS and paired NP/OP swabs samples was substantial for asymptomatic and symptomatic children and adolescents, asymptomatic adults, and moderate for symptomatic adults (Table 4).
Table 4.
Rates of sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and Cohen’s kappa values of unstimulated whole saliva compared to combined nasopharyngeal/oropharyngeal swabs stratified by age group and symptoms (all samples)
| Asymptomatic | Symptomatic | |
|---|---|---|
| Children and adolescents (< 18 years old) | ||
| Sensitivity Estimate % (95% CI) | 62.5 (42.6–78.9) | 84.0 (70.5–92.0) |
| Specificity Estimate % (95% CI) | 97.1 (90.5–99.2) | 87.5 (49.5–98.0) |
| Positive Predictive Value Estimate % (95% CI) | 83.3 (51.6–97.9) | 95.5 (77.2–99.9) |
| Negative Predictive Value Estimate % (95% CI) | 91.9 (83.2–97.0) | 63.6 (30.8–89.1) |
| Accuracy Estimate % (95% CI) | 90.7 (82.5–95.9) | 84.9 (68.1–94.9) |
| Cohen’s kappaa Estimate % (95% CI) | 0.66 (0.44–0.88) | 0.63 (0.35–0.91) |
| Adults (18 years and older) | ||
| Sensitivity Estimate % (95% CI) | 81.1 (67.1–90.0) | 75.7 (68.9–81.4) |
| Specificity Estimate % (95% CI) | 95.7 (92.0-97.8) | 85.0 (64.2–94.7) |
| Positive Predictive Value Estimate % (95% CI) | 81.1 (64.8–92.0) | 94.6 (85.1–98.9) |
| Negative Predictive Value Estimate % (95% CI) | 95.7 (91.4–98.3) | 50.0 (32.4–67.6) |
| Accuracy Estimate % (95% CI) | 93.0 (88.5–96.1) | 77.8 (67.8–85.9) |
| Cohen’s kappaa Estimate % (95% CI) | 0.77 (0.65–0.88) | 0.49 (0.30–0.67) |
CI Confidence Interval, a Cohen’s kappa values: <0: No agreement; 0-0.20: Slight agreement; 0.21–0.40: Fair agreement; 0.41–0.60: Moderate agreement; 0.61–0.80: Substantial agreement; 0.81-1.0: Almost perfect agreement
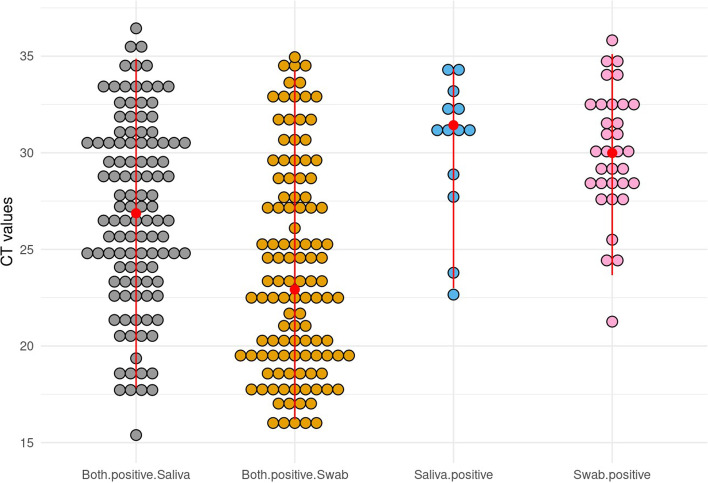
Overall, the distribution of Ct values of UWS (median, 27.7; IQR, 23.8–31.1) and paired NP/OP swabs (median, 25.2; IQR, 20.1–29.9) were different (p < 0.05). In addition, the distribution of Ct values from concordant results for UWS (median 26.9; IQR, 23.5–30.5) and paired NP/OP swabs (median 22.9; IQR, 19.5–27.6) was different (p < 0.05) (Fig. 1). However, there was no difference when the paired samples were discordant and positive only by UWS (median 31.4; IQR, 28.9–32.4) or by NP/OP swabs (median 30.0; IQR, 28.4–32.2), p = 0.99 (Fig. 1).
Fig. 1.
Cycle threshold values for E gene for unstimulated whole saliva and combined nasopharyngeal/oropharyngeal swabs. Distribution of Cycle threshold (Ct) values by testing concordance. Each “dot” represents a positive SARS-CoV-2 RT-PCR result. Red dots represent the median Ct values for both UWS and combined NP/OP swabs and positive for either UWS or combined NP/OP swabs. Data only include results of positive RT-PCR for SARS-CoV-2.
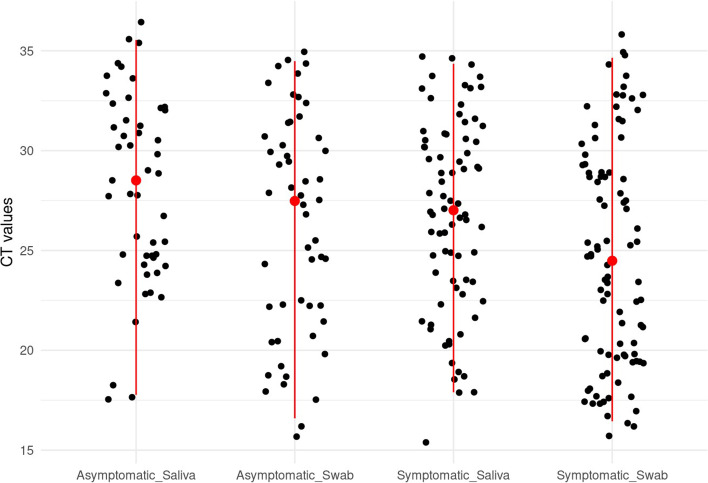
Moreover, no differences were observed between the UWS from asymptomatic patients (median 28.5; IQR, 24.6–32.0) and paired NP/OP (median 27.5; IQR, 22.2–30.6), p = 0.70. Marginal statistical significance was observed among paired samples from symptomatic patients (median 27.0; IQR, 23.4–30.5 for UWS and median 24.5; IQR, 19.8–28.9 for NP/OP), p = 0.08 (Fig. 2).
Fig. 2.
Comparison of Cycle threshold values of SARS-CoV-2 RT-PCR obtained from unstimulated whole saliva and combined nasopharyngeal/oropharyngeal swabs specimens for asymptomatic and symptomatic patients. Note. Cycle threshold (Ct) values are distributed by testing concordance between asymptomatic and symptomatic patients. Each “dot” represents a positive SARS-CoV-2 RT-PCR result. Red dots represent the median Ct values for unstimulated whole saliva and combined nasopharyngeal/oropharyngeal swabs. Data only include results of positive RT-PCR for SARS-CoV-2.
Selected sociodemographic and clinical characteristics of the participants with discordant results is shown in Table 5. No differences were observed between the groups.
Table 5.
Sociodemographic and clinical characteristics of the participants with discordant results
| Characteristics | Concordant results | Discordant results | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Sex | |||
| Male | 128 (40.3) | 16 (34.0) | 0.416 |
| Female | 190 (59.7) | 31 (66.0) | |
| Age | |||
| <18 years | 98 (30.8) | 13 (27.7) | 0.660 |
| ≥18 years | 220 (69.2) | 34 (72.3) | |
| Highest educational attainment | |||
| Primary or lower | 123 (39.7) | 22 (47.8) | 0.294 |
| Secondary or higher | 187(60.3) | 24 (52.2) | |
| Main Comorbidities | |||
| Allergic rhinitis (< 18 years) | |||
| No | 83 (84.7) | 12 (92.3) | 0.688* |
| Yes | 15 (15.3) | 1 (7.7) | |
| Asthma (< 18 years) | |||
| No | 85 (86.7) | 12 (92.3) | 1.000* |
| Yes | 13 (13.3) | 1 (7.7) | |
| Allergic rhinitis (≥ 18 years) | |||
| No | 185 (84.1) | 30 (88.2) | 0.533 |
| Yes | 35 (15.9) | 4 (11.8) | |
| Asthma (≥ 18 years) | |||
| No | 210 (95.5) | 33 (97.1) | 1.000* |
| Yes | 10 (4.5) | 1 (2.9) | |
| Hypertension (≥ 18 years) | |||
| No | 164 (74.5) | 24 (70.6) | 0.624 |
| Yes | 56 (25.5) | 10 (29.4) | |
| Obesity (≥ 18 years) | |||
| No | 188 (85.5) | 25 (73.5) | 0.079 |
| Yes | 32 (14.5) | 9 (26.5) | |
| Diabetes Mellitus (≥ 18 years) | |||
| No | 203 (92.3) | 33 (97.1) | 0.482* |
| Yes | 17 (7.7) | 1 (2.9) | |
| Thyroid dysfunction (≥ 18 years) | |||
| No | 203 (92.3) | 33 (97.1) | 0.482* |
| Yes | 17 (7.7) | 1 (2.9) |
Highest educational attainment (missing = 9); *Fisher’s exact test
Discussion
In this study, saliva samples had high sensitivity (84%) for the detection of SARS-CoV-2, with substantial agreement (Cohen’s kappa = 0.63) with combined NP/OP in symptomatic children and adolescents. In addition, saliva detected SARS-CoV-2 in 13 patients with negative combined NP/OP results.
Published studies comparing saliva with nasopharyngeal or oropharyngeal secretions to diagnose SARS-CoV-2 infection have shown conflicting results [7, 8, 17–26]. Banerjee et al. demonstrated high sensitivity (93%) and specificity (96.2%) of saliva specimens compared to paired NP swabs for SARS-CoV-2 detection in children (age range 5–18 years) [27]. Al Suwaidi et al., in a prospective study including 476 children (age range 3–18 years), suggested saliva as an alternative to upper respiratory tract samples for the diagnosis of SARS-CoV-2 [28].
We found that sensitivity was inferior for saliva in asymptomatic [62.5% (95% CI: 42.6–78.9)] compared with symptomatic [(84.0% (95% CI: 70.5–92.0)] children and adolescents. In contrast, Yee et al. reported that for symptomatic and asymptomatic pediatric patients not previously diagnosed with COVID-19, the performances of saliva and NP swabs were comparable (positive percent agreement: 82.4% versus 85.3%) [9].
Our results suggest that, according to the sensitivity estimates, saliva-based RT-PCR should be used with caution for asymptomatic SARS-CoV-2 infection screening during the circulation of the Omicron variant.
In asymptomatic and symptomatic adults, the sensitivity of saliva RT-PCR was 81.1% (95% CI: 67.1–90.0) and 75.7% (95% CI: 68.9–81.4), respectively. Similar results were reported by Pasomsub et al., who studied 200 sample pairs of nasopharyngeal and throat swabs and saliva samples from 200 individuals suspected to have COVID-19 [20]. In a prospective study with symptomatic outpatients, Landry et al. showed an overall sensitivity for SARS-CoV-2 detection of 85.7% for pure saliva compared to simultaneously-collected NP swabs [29]. Also, the median Ct value was significantly lower for NP swabs than for saliva.
A prospective study in symptomatic outpatients in Australia showed that 84.6% of patients with positive NP swabs had SARS-CoV-2 RNA detected in saliva, and 2% of saliva samples from patients with negative NP swabs were also positive [8]. Furthermore, the median Ct value was significantly lower in NP swabs than in saliva, suggesting a higher viral load in NP swabs [8]. Plantamura et al. also described Ct values as significantly lower in NP swabs than in saliva [30]. Similar Ct results were also observed in our study. On the other hand, Tutuncu et al. showed that mean Ct values of NP and saliva samples in mildly symptomatic and asymptomatic patients were not significantly different [31]. We may not have found a difference between the NP/OP and UWS samples of symptomatic participants due to the sample size of this group since marginal statistical significance was observed between paired NP/OP and UWS samples.
In our study, the overall Ct values in saliva were higher than those observed in the combined NP/OP swabs reflecting lower levels of viral nucleic acid, which may have impacted saliva sensitivity in the detection of SARS-CoV-2. The following factors may partially explain this difference: 1) When collecting saliva, patients accumulate saliva for one minute and then drool it into a sample container. In this way, some patients may not have provided a sufficient sample; 2) Combined collection of nasopharyngeal and oropharyngeal swabs may increase the concentration of viral particles in the sample, thereby increasing the sensitivity of NP/OP to UWS.
No sociodemographic and clinical characteristics evaluated were associated with the discordant combined NP/OP and UWS RT-PCR results.
The greatest strength of our study was a large cohort focusing on symptomatic and asymptomatic outpatients, including adults, children, and adolescents, and the opportunity to identify asymptomatic participants with the Omicron variant during regular cohort study visits and symptomatic participants during unscheduled visits.
Among the limitations of the study were: 1) Inclusion of only outpatients and not hospitalized participants; 2) Children under three were not enrolled; therefore, data cannot be generalized to this age group because saliva was collected by spontaneous production, not by swab collection; 3) Participants were enrolled in the household of an index case; therefore the pretest probability was high; 4) In the present study, there was good agreement between the Ct values of samples obtained via the two different sampling modes. On the other hand, the presence or absence of symptoms did not have a significant effect on Ct values. We may have failed to detect a difference in Ct values between symptomatic and asymptomatic patients due to insufficient statistical power.
Conclusions
In summary, our findings suggest saliva is an adequate fluid for detecting SARS-CoV-2, especially for confirmation in symptomatic children and adolescents but should be used with caution for asymptomatic SARS-CoV-2 infection screening during the circulation of the Omicron variant. The negative predictive value was not high enough to exclude COVID-19, so a negative result should not be considered definitive, and the collection of additional samples is recommended especially in situations of high prevalence of COVID-19.
Acknowledgements
We wish to thank all our patients for their willingness to participate in the study.
Abbreviations
- CI
Confidence interval
- COVID-19
Coronavirus disease, 2019
- Ct
Cycle threshold
- Fiocruz
Oswaldo Cruz Foundation
- NP
Nasopharyngeal
- NPV
Negative predictive value
- OP
Oropharyngeal
- PPV
Positive predictive value
- RT-PCR
Real-time reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- UWS
Unstimulated whole saliva
- VTM
Viral Transport Medium
- WHO
World Health Organization
- VOC
Variant of concern
Authors' contributions
Conceptualization: GC, MO, LG and PB; Data curation: GC and FM-F; Formal analysis: GC and WT; Funding acquisition: GC, JW, CS, MS and PB; Investigation: GC, MO, PR, MB, APC, EM, IM, HS, LD, FM, VN, MS and PB; Methodology: GC, WT and PB; Project administration: LG, JW, CS, MS and PB; Resources: MB, APC, EM, IM, and LD; Software: GC, WT and FM-F; Supervision: GC, LG, APC, IM, LD and PB; Validation: GC, MO, WT, LG, MB, APC, EM, IM, LD, FM, OE, VN, AP, JW, CS, MS and PB; Visualization: GC, MO, LG, PR, TF, SP, MB, APC, EM, HS, LD, FM-F, OE, FM, VN, AP, JW, CS, MS and PB; Writing original draft: GC; Writing - review & editing: MO, WT, LG, PR, TF, SP, MB, APC, EM, IM, HS, LD, FM-F, OE, FM, VN, AP, JW, CS, MS and PB. The author(s) read and approved the final manuscript.
Funding
This work was funded by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) [FAPERJ/E26/201.379/2021 JCNE (G.C), [FAPERJ/E-26/210.146/2020 COVID (P.B); FAPERJ/E-26/200.935/2022 CNE (P.B), FAPERJ/E26/210.196/2020 (M.S)]; Department of Science and Technology (DECIT) of the Brazilian Ministry of Health (25000.072811/2016-19) (P.B), The National Council for Scientific and Technological Development (CNPq), Ministry of Science, Technology and Innovation (MCT) [402457/2020-0] (M.S); INOVA Oswaldo Cruz Foundation (Fiocruz) [VPPCB-005-FIO-20-2] (M.S); UK Medical Research Council [MR/V033530/1] (J.W and C.S). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Data underlying the study cannot be made publicly available due to ethical concerns, as data contain several personally identifiable information. Data are available from Oswaldo Cruz Foundation for researchers who meet the criteria for access to confidential data. Contact information: Institutional Ethics and Research Committee of the Evandro Chagas National Institute of Infectious Diseases, email: cep@ini.fiocruz.br or Guilherme Amaral Calvet; email: guilherme.calvet@ini.fiocruz.br.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by The Brazilian National Research Ethics Commission and the London School of Tropical Medicine and Hygiene. Written informed consent was obtained from all subjects involved in the study. Children and adolescents were enrolled following written informed assent and by consent by the parent or legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. [cited Feb 06 2022]. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 2.Ministério da Saúde. Fundação Oswaldo Cruz. Dashboard Rede Genômica. Vigilância Genômica do SARS-CoV-2 no Brasil. Dados gerados pela rede genômica Fiocruz e/ou depositados na plataforma Gisaid por outras instituições a partir de amostras brasileiras. [cited Feb 06 2022]. http://www.genomahcov.fiocruz.br/dashboard/.
- 3.Ibrahimi N, Delaunay-Moisan A, Hill C, Le Teuff G, Rupprecht JF, Thuret JY, Chaltiel D, Potier MC. Screening for SARS-CoV-2 by RT-PCR: saliva or nasopharyngeal swab? Rapid review and meta-analysis. PLoS ONE. 2021;16(6):e0253007. doi: 10.1371/journal.pone.0253007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ota K, Yanagihara K, Sasaki D, Kaku N, Uno N, Sakamoto K, Kosai K, Miyazaki T, Hasegawa H, Fujita A, et al. Detection of SARS-CoV-2 using qRT-PCR in saliva obtained from asymptomatic or mild COVID-19 patients, comparative analysis with matched nasopharyngeal samples. PLoS ONE. 2021;16(6):e0252964. doi: 10.1371/journal.pone.0252964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhruddin KS, Haiat A, Ngo HC, Panduwawala C, Chang JWW, Samaranayake LP. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) viral positivity and their burden in saliva of asymptomatic carriers - a systematic review and meta-analysis. Acta Odontol Scand. 2022;80(3):182–90. doi: 10.1080/00016357.2021.1977385. [DOI] [PubMed] [Google Scholar]
- 6.Bulfoni M, Sozio E, Marcon B, De Martino M, Cesselli D, De Carlo C, Martinella R, Migotti A, Vania E, Zanus-Fortes A et al. Validation of a Saliva-Based Test for the Molecular Diagnosis of SARS-CoV-2 Infection. Dis Markers. 2022;2022:6478434. [DOI] [PMC free article] [PubMed]
- 7.Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Warren JL, Geng B, Muenker MC, Moore AJ, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(13):1283–6. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a Noninvasive Specimen for Detection of SARS-CoV-2. J Clin Microbiol. 2020;58(8):e00776-20. [DOI] [PMC free article] [PubMed]
- 9.Yee R, Truong TT, Pannaraj PS, Eubanks N, Gai E, Jumarang J, Turner L, Peralta A, Lee Y, Dien Bard J. Saliva Is a Promising Alternative Specimen for the Detection of SARS-CoV-2 in Children and Adults. J Clin Microbiol. 2021;59(2):e02686-20. [DOI] [PMC free article] [PubMed]
- 10.Uddin MKM, Shirin T, Hossain ME, Alam AN, Ami JQ, Hasan R, Miah M, Shaly NJ, Ahmed S, Rahman SMM, et al. Diagnostic performance of Self-Collected Saliva Versus nasopharyngeal swab for the Molecular Detection of SARS-CoV-2 in the clinical setting. Microbiol Spectr. 2021;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marais G, Hsiao N-y, Iranzadeh A, Doolabh D, Enoch A, Chu C-y, Williamson C, Brink A, Hardie D. Saliva swabs are the preferred sample for Omicron detection. medRxiv 2021:2021.2012.2022.21268246.
- 12.Brasil P, Damasceno L, Fuller T, Bastos LS, Cruz OG, Medeiros F, Calvet GA, Resende P, Whitworth J, Smith C, et al. Cohort-profile: Household transmission of SARS-CoV-2 in a low-resource community in Rio de Janeiro, Brazil. BMJ Open. 2022;12(12):e067212. doi: 10.1136/bmjopen-2022-067212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministério da Saúde. Instituto de Tecnologia em imunobiológicos—Bio-Manguinhos, FIOCRUZ. KIT MOLECULAR SARS-CoV2 (E)—Bio-Manguinhos. Rio do Janeiro; 2020 [cited Feb 15 2023]. [9 p]. Available from: https://www.bio.fiocruz.br/images/molec-sars-cov2-e-96r-04-05-2020-lotes-11ao18.pdf.
- 14.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brunink S, Schneider J, Schmidt ML, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed]
- 15.Larner AJ. The 2x2 matrix: contingency, confusion and the metrics of binary classification. London, UK: Springer; 2021. [Google Scholar]
- 16.Altman DG, Bland JM. Parametric v non-parametric methods for data analysis. BMJ. 2009;338:a3167. doi: 10.1136/bmj.a3167. [DOI] [PubMed] [Google Scholar]
- 17.Azzi L, Carcano G, Dalla Gasperina D, Sessa F, Maurino V, Baj A. Two cases of COVID-19 with positive salivary and negative pharyngeal or respiratory swabs at hospital discharge: a rising concern. Oral Dis. 2021;27(Suppl 3):707–9. doi: 10.1111/odi.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A, Fasano M, Sessa F, Tettamanti L, Carinci F, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81(1):e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki S, Fujisawa S, Nakakubo S, Kamada K, Yamashita Y, Fukumoto T, Sato K, Oguri S, Taki K, Senjo H, et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020;81(2):e145–7. doi: 10.1016/j.jinf.2020.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasomsub E, Watcharananan SP, Boonyawat K, Janchompoo P, Wongtabtim G, Suksuwan W, Sungkanuparph S, Phuphuakrat A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2021;27(2):285 e281-285 e284. [DOI] [PMC free article] [PubMed]
- 21.Leung EC, Chow VC, Lee MK, Lai RW. Deep throat saliva as an alternative diagnostic specimen type for the detection of SARS-CoV-2. J Med Virol. 2021;93(1):533–6. doi: 10.1002/jmv.26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal AJ, Mozafarihashjin M, Coomes E, Powis J, Li AX, Paterson A, Anceva-Sami S, Barati S, Crowl G, Faheem A, et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2021;72(6):1064–6. doi: 10.1093/cid/ciaa848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson KE, Barker AP, Hillyard DR, Gilmore N, Barrett JW, Orlandi RR, Shakir SM. Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2. J Clin Microbiol. 2020;58(11):2020.2007.2017.20155754. [DOI] [PMC free article] [PubMed]
- 24.Dogan OA, Kose B, Agaoglu NB, Yildiz J, Alkurt G, Demirkol YK, Irvem A, Doganay GD, Doganay L. Does sampling saliva increase detection of SARS-CoV-2 by RT-PCR? Comparing saliva with oro-nasopharyngeal swabs. J Virol Methods. 2021;290:114049. doi: 10.1016/j.jviromet.2020.114049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne RL, Kay GA, Kontogianni K, Aljayyoussi G, Brown L, Collins AM, Cuevas LE, Ferreira DM, Fraser AJ, Garrod G, et al. Saliva Alternative to Upper Respiratory Swabs for SARS-CoV-2 diagnosis. Emerg Infect Dis. 2020;26(11):2770–1. doi: 10.3201/eid2611.203283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee D, Sasidharan A, Abdulhamid A, Orosco EM, Watts JL, Schuster JE, Myers AL, Weddle G, Selvarangan R. Diagnostic Yield of Saliva for SARS-CoV-2 Molecular Testing in Children. J Pediatr Infect Dis Soc. 2021;10(10):967–9. doi: 10.1093/jpids/piab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Suwaidi H, Senok A, Varghese R, Deesi Z, Khansaheb H, Pokasirakath S, Chacko B, Abufara I, Loney T, Alsheikh-Ali A. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330–5. doi: 10.1016/j.cmi.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landry ML, Criscuolo J, Peaper DR. Challenges in use of saliva for detection of SARS CoV-2 RNA in symptomatic outpatients. J Clin Virol. 2020;130:104567. doi: 10.1016/j.jcv.2020.104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plantamura J, Bousquet A, Otto MP, Bigaillon C, Legland AM, Delacour H, Vest P, Astier H, Valero E, Bylicki O, et al. Performances, feasibility and acceptability of nasopharyngeal swab, saliva and oral-self sampling swab for the detection of severe acute respiratory syndrome coronavirus 2. Eur J Clin Microbiol Infect Dis. 2021;40(10):2191–8. doi: 10.1007/s10096-021-04269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tutuncu EE, Ozgur D, Karamese M. Saliva samples for detection of SARS-CoV-2 in mildly symptomatic and asymptomatic patients. J Med Virol. 2021;93(5):2932–7. doi: 10.1002/jmv.26821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the study cannot be made publicly available due to ethical concerns, as data contain several personally identifiable information. Data are available from Oswaldo Cruz Foundation for researchers who meet the criteria for access to confidential data. Contact information: Institutional Ethics and Research Committee of the Evandro Chagas National Institute of Infectious Diseases, email: cep@ini.fiocruz.br or Guilherme Amaral Calvet; email: guilherme.calvet@ini.fiocruz.br.