Abstract
Researchers are constantly searching for drugs to combat the coronavirus pandemic caused by SARS-CoV-2, which has lasted for over two years. Natural compounds such as phenolic acids are being tested against Mpro and AAK1, which are key players in the SARS-CoV-2 life cycle. This research work aims to study the ability of a panel of natural phenolic acids to inhibit the virus's multiplication directly through Mpro and indirectly by affecting the adaptor-associated protein kinase-1 (AAK1). Pharmacophore mapping, molecular docking, and dynamic studies were conducted over 50 ns and 100 ns on a panel of 39 natural phenolic acids. Rosmarinic acid (16) on the Mpro receptor (− 16.33 kcal/mol) and tannic acid (17) on the AAK1 receptor (− 17.15 kcal/mol) exhibited the best docking energy against both receptors. These favourable docking score values were found to be superior to those of the co-crystallized ligands. Preclinical and clinical research is required before using them simultaneously to halt the COVID-19 life cycle in a synergistic manner.
Subject terms: Medicinal chemistry, Theoretical chemistry, Virtual drug screening, Target identification
Introduction
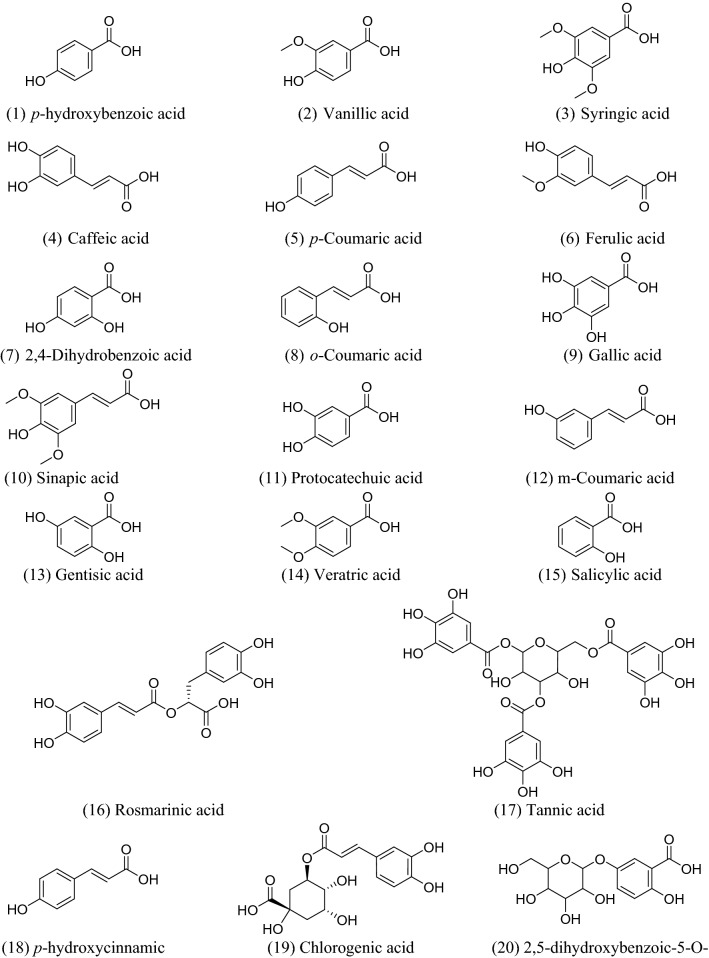
There are numerous potential sources of phenolic acids, such as fruits, vegetables, spices, and herbs1. These substances are naturally occurring by-products or secondary metabolites of plants, and they are found in food on a regular basis. They provide essential health benefits for people, including antioxidant, anti-inflammatory, anticancer, anti-allergic, antihypertensive, and antiviral capabilities2. Many studies have demonstrated the effectiveness of these substances in preventing viruses from causing serious health issues3–5. Natural phenolic acids and their derivatives have shown strong inhibitory effects on a variety of human viruses, including coronavirus (SARS-CoV-2)6–10. Based on the target, there are two groups of potential anti-coronavirus medications: one targets the human immune system or human cells, while the other focuses on the coronavirus itself11,12. SARS-CoV-2 has seven major structural proteins, and several human targets, such as transmembrane serine protease 2 (TMPRSS2), cathepsin L, and adaptor-associated protein kinase 1 (AAK1), are involved in the progression of symptoms linked to SARS-CoV-2 infections14–16. Phenolic acid products have been identified as excellent candidates as anti-SARS-CoV treatments, especially towards Mpro and AAK119,20. Inhibition of AAK1 could be a good strategy to prevent SARS-CoV-2 entry. The carbonyl and hydroxyl groups of phenolic acids play a major role in forming good binding interactions with both Mpro and AAK121–23. These findings have encouraged researchers to investigate natural phenolic acids against these two plausible targets in the SARS-CoV-2 life cycle, in an effort to find new natural and safe treatments for COVID-19. The selected library of natural phenolic acids is represented in Fig. 1.
Figure 1.
Natural phenolic acids that are investigated in this research work.
Methods
In this study, molecular operating environment (MOE, 2019.0102) software was used for pharmacophoric generation, searching and molecular docking24–26, energy minimized structures were gained by applying MMFF94x force field until RMSD gradient of 0.1 kcal mol−1 Å−1 was reached. The protein data bank (PDB) was the source of the X-ray crystallographic structures of SARS CoV-2 main protease enzyme (Mpro) (ID: 7AEH) and adaptor-associated protein kinase 1 (AAK1) (ID: 4WSQ). Triangle matcher as a placement method and London dG as a scoring algorithm were used to determine the pharmacophoric properties of both enzymes and the binding site in each protein file using the co-crystallized ligand. AutoDock Vina 1.1.2. Software was also used for conducting the molecular docking study to confirm the results. SwissADME was used to evaluate the ADME properties of the promising compounds27.
Pharmacophore mapping
SARS CoV-2 main protease enzyme (Mpro)
The binding characteristics for the co-crystallized ligand were chosen, stored, and utilized to search for potential matches from the phenolic acids database using the PDB file (ID: 7AEH) that was obtained from the protein data bank.
Adaptor-associated protein kinase 1 (AAK1)
The PDB file (ID: 4WSQ) was obtained for mapping the pharmacophoric characteristics of AAK1 and examined to determine the most crucial binding properties. For the purpose of choosing the most appropriate phenolic acid structures, the created pharmacophore was preserved and utilized.
Molecular docking
SARS CoV-2 main protease enzyme (Mpro)
The PDB file (ID: 7AEH) was used to identify the required key binding features for the ligand through analysis of the binding interactions of the co-crystallized ligand and to perform the docking procedure.
Adaptor-associated protein kinase 1 (AAK1)
PDB file (ID: 4WSQ) was used for performing molecular docking simulations, the tested compounds were docked at the binding pocket of the enzyme.
Molecular dynamics simulations
The best docking postures were subjected to molecular dynamics (MD) computations to get insights into the stability of the protein–ligand interaction. The CHARMM-GUI solution builder created the input files for the MD calculations using the CHARMM force field parameters for proteins28. The simulation box dimensions of Mpro/Mpro-16 and AAK1/AAK1-16 were 98 × 98 × 98 nm and 132 × 132 × 132 nm, respectively, containing four sodium ions and seven chloride ions. The protein–ligand combination was subjected to the CHARMM36 forcefield. Prior to production simulation, the system's energy consumption was minimized using the steepest descent algorithm (5000 steps). The complex was then subjected to NVT and NPT ensemble, simulating for 125 ps at 300.15 K temperature utilizing 400 kJ mol−1 nm2 and 40 kJ mol−1 nm2 positional restrictions on the backbone and side chains, respectively, to equilibrate the complex for stabilizing its temperature and pressure. The complex is then put through a 100-ns production simulation run in an NPT ensemble at 300.15 K and 1 bar29.
ADMET study
SwissADME (http://www.swissadme.ch/index.php) web server was used the study the ADME properties of the promising compounds, it can evaluate the pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. The smiles of the selected library were generated and then the ADME properties were calculated. Lazar toxicity predictor web server (https://lazar.in-silico.ch/predict) was used to estimate the toxicity profile of (16) rosmarinic acid against Mpro and (17) tannic acid against AAK1.
Results and discussions
Pharmacophore mapping and molecular docking on SARS CoV-2 main protease (Mpro)
Pharmacophore generation
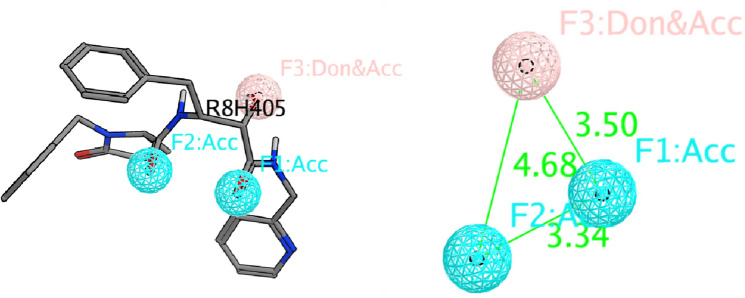
3D Pharmacophores represent the ensembles of the chemically defined interactions of the bioactive conformation of the ligand, accordingly, pharmacophore generation represents an elegant way to decode the chemically encoded ligand information and so becomes a valuable tool in drug design30,31. The selected PDB file for Mpro enzyme with its co-crystallized ligand; R8H, (2 ~ {R})-5-oxidanylidene- ~ {N}-[(2 ~ {R},3 ~ {S})-3-oxidanyl-4-oxidanylidene-1-phenyl-4-(pyridin-2-ylmethylamino)butan-2-yl]-1-(phenylmethyl)pyrrolidine-2-carboxamide [C28 H30 N4 O4] was used to identify the essential pharmacophoric features for better binding with the enzyme. The key amino acids in Mpro active site were HIS-41 and CYS-145 in the catalytic dyad32,33 in addition to GLY-143 that interacts with the oxygen atoms of the co-crystallized ligand. The pharmacophoric features were selected as shown in Fig. 2.
Figure 2.
Pharmacophoric features for SARS CoV-2 Mpro obtained from the co-crystallized ligand (PDB: 7AEH) (Acc: Acceptor/Don: Donor).
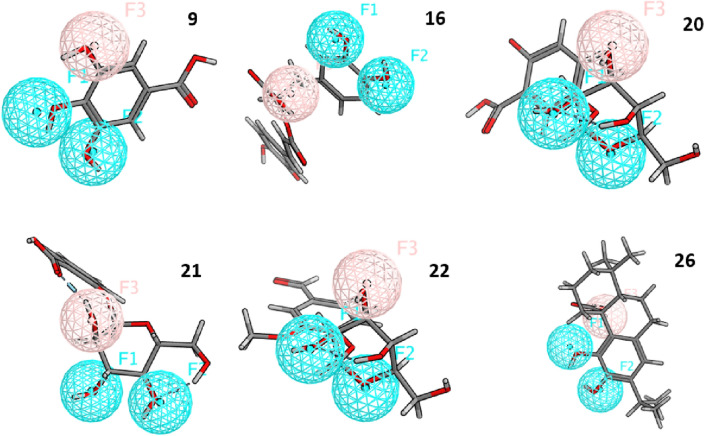
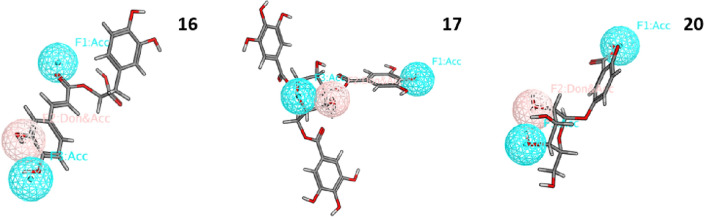
Virtual screening of the selected database against these pharmacophoric features revealed that six hit molecules, (9) gallic acid, (16) rosmarinic acid, (20) 2,5-dihydroxybenzoic-5-O-glucoside, (21) salicylic acid glucoside, (22) glucovanillic acid and (26) carnosic acid, they have showed RMSD value of 0.53, 0.56, 0.29, 0.18, 0.29 and 0.54 respectively, Fig. 3 is showing that these six hits are fitting the generated pharmacophoric features in Mpro.
Figure 3.
Fitting of the best six hits on the generated Mpro pharmacophore.
Molecular docking on SARS Cov-2 Mpro
Molecular docking study was carried out for the hit molecules, their binding energy scores are summarized in Table 1, compared with the co-crystallized ligand (− 10.52 kcal./mol.).
Table 1.
Binding energy scores for the tested acids (Mpro enzyme).
| Compound no | Energy score using MOE (kcal/mol) | Energy score using Autodock Vina (kcal/mol) |
|---|---|---|
| 9 | − 9.78 | − 9.20 |
| 16 | − 16.33 | − 14.53 |
| 20 | − 12.28 | − 12.15 |
| 21 | − 9.81 | − 9.65 |
| 22 | − 9.53 | − 8.43 |
| 26 | − 11.08 | − 11.02 |
| Co-Cry. ligand | − 10.52 | − 10.92 |
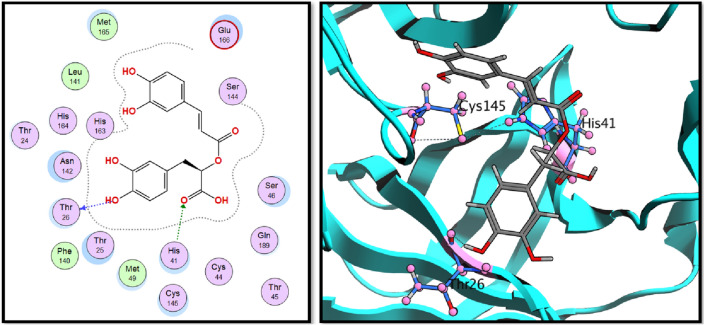
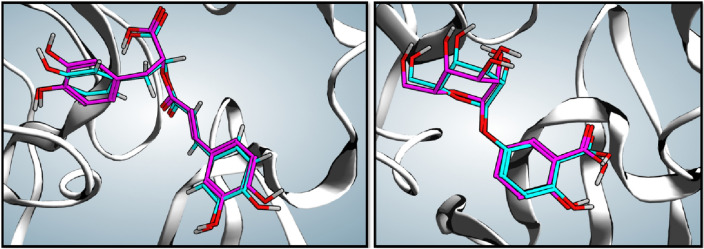
According to the binding energy scores in Table 1, both compounds 16 (rosmarinic acid) and 20 (2,3-dihydroxybenzoic-5-O-glucoside) showed the highest scores, − 16.33 and − 12.28 kcal./mol. Both binding interactions are well demonstrated in Figs. 4 and 5, rosmarinic acid interacts with the catalytic dyad through HIS-41 with its carboxylic acid carbonyl oxygen, while compound 20 (2,5-dihydroxybenzoic-5-O-glucoside) showed the same binding interaction revealing its expected good inhibitory activity on Mpro enzyme. To validate the docking results, the best pose of the tested compounds (16) and (20) using MOE was aligned with the poses obtained from AutoDock Vina (Fig. 6).
Figure 4.
2D and 3D interactions of rosmarinic acid (16) in the binding pocket of Mpro enzyme.
Figure 5.
2D and 3D interactions of 2,5-dihydroxybenzoic-5-O-glucoside (20) in the binding pocket of Mpro enzyme.
Figure 6.
Alignment of rosmarinic acid (16) and 2,5-dihydroxybenzoic-5-O-glucoside (20) docking poses obtained from MOE (cyan) with that obtained from Autodock Vina (purple).
Pharmacophore mapping and molecular docking on adaptor-associated protein kinase 1 (AAK1)
Pharmacophore generation
4WSQ PDB file was used to identify the key pharmacophoric features of AAK1 enzyme with its co-crystallized ligand (KSA, K-252A [C27H21N3O5]). The pharmacophoric features were selected (Fig. 7).
Figure 7.
Pharmacophoric features for AAK1 enzyme obtained from the co-crystallized ligand (PDB: 4WSQ).
Virtual screening was performed on the generated pharmacophore model, it has showed three hit molecules: (16) rosmarinic acid, (17) tannic acid and (20) 2,3-dihydroxybenzoic-5-O-glucoside with RMSD values of 0.45, 0.20, 0.28, respectively (Fig. 8).
Figure 8.
Fitting of the three hit compounds to the generated AAK1 pharmacophoric features.
Molecular docking against AAK1
Molecular docking study was carried out for the hit molecules, their binding energy scores are summarized in Table 2, compared to the co-crystallized ligand (− 13.28 kcal./mol.).
Table 2.
Binding energy scores for the tested acids (AAK1 enzyme).
| Compound no. | Energy score (kcal/mol) | Energy score using Autodock Vina (kcal/mol) |
|---|---|---|
| 16 | − 10.68 | − 10.52 |
| 17 | − 17.15 | − 16.21 |
| 20 | − 9.40 | − 9.33 |
| Co-crystallized ligand | − 13.28 | − 13.15 |
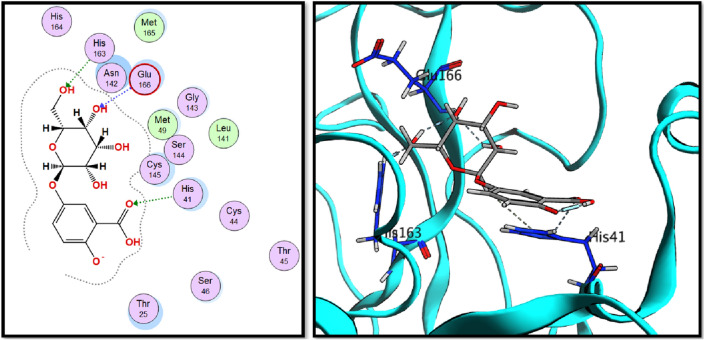
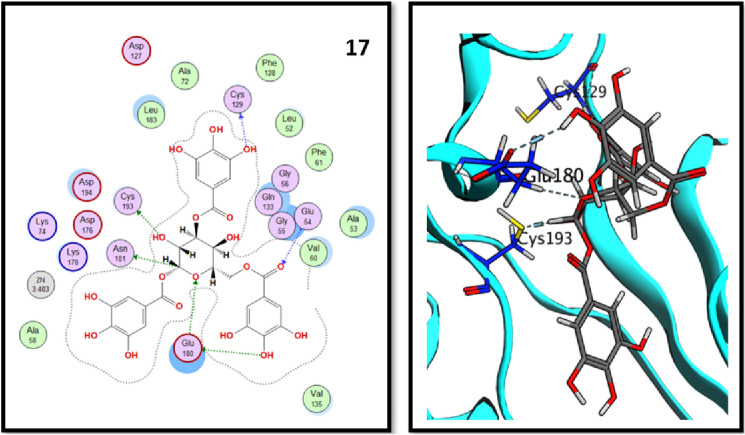
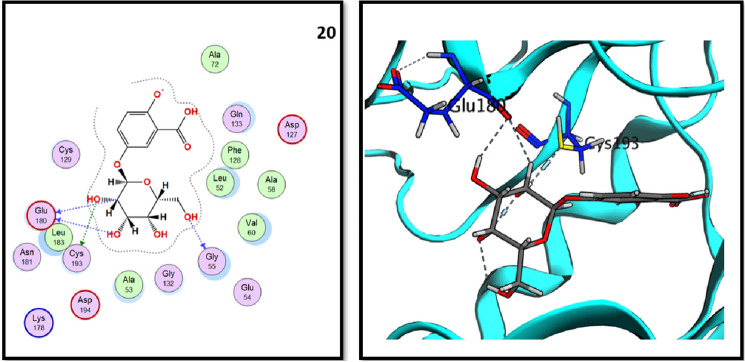
Analysis of molecular docking results revealed that all three tested compounds have the main binding interaction with CYS-19334. Compound (17), tannic acid showed the highest binding energy score (− 17.15 kcal./mol.) with strong H-bond interactions through its hydroxyl groups to CYS-129, GLU-180 and CYS-193 amino acid residues. On the other hand, compound (16), rosmarinic acid showed H-bond interaction through its carboxylic acid OH group to CYS-193 in addition to binding of phenolic OH group to ASP-127 in the binding vicinity. Finally, compound (20), 2,3-dihydroxybenzoic-5-O-glucoside showed the H-bond interactions with GLU-180 and CYS-193 residues through its hydroxyl groups. All obtained results are illustrated in Figs. 9, 10 and 11.
Figure 9.
2D and 3D interactions of rosmarinic acid (16) in the AAK1 active site.
Figure 10.
2D and 3D interactions of tannic acid (17) in the AAK1 active site.
Figure 11.
2D and 3D interactions of 2,5-dihydroxybenzoic-5-O-glucoside (20) in the AAK1 active site.
Molecular dynamics (MD) simulation
The GROMACS software was used to run an MD simulation in order to compare the behaviour of compound (16) rosmarinic acid with MPRO and compound (17) tannic acid with AAK1 across simulation times of 50 and 100 ns, respectively35–37.
Simulation time over 50 ns
Analysis of the root mean square deviation (RMSD)
The root mean square deviation (RMSD) was investigated quantitatively to assess the degree of divergence of each complex protein structure with each ligand from its baseline behaviour. The system's stability is evaluated during the simulation with the help of the RMSD. In two different MD simulations, a control system (a ligand-free structure) and a complex were put up for this38. The stability and convergence of compound 16 in the target Mpro and compound 17 in the target AAK1 were investigated using a 50-ns molecular dynamics (MD) simulation, where the backbone atoms' RMSD value was obtained, as shown in Fig. 12. The findings indicated that the complex-maintained equilibrium for the course of the experiment. The RMSD values of the apoprotein and the compound 17-bound complex were 0.14–0.36 nm. Furthermore, the RMSD values of the apoprotein and the compound 16-bound complex ranged from 0.12 to 0.23 nm. Compounds 16 and 17 behaved consistently inside their pockets throughout the simulation and went farther toward the binding pocket. This could explain why both 16 and 17 have potent inhibitory effects against Mpro and AAK1, respectively.
Figure 12.
A depiction of the root mean square deviation (RMSD) from the molecular dynamics simulation trajectories of the Mpro (blue) and Mpro-16 complex (green) in addition to AAK1 (black) and AAk1-17 complex (red) during a 50-ns MD simulation.
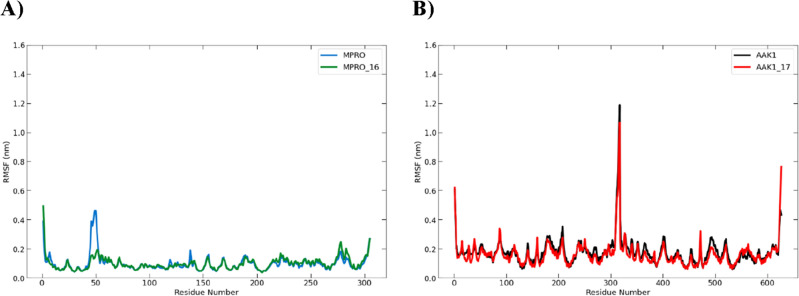
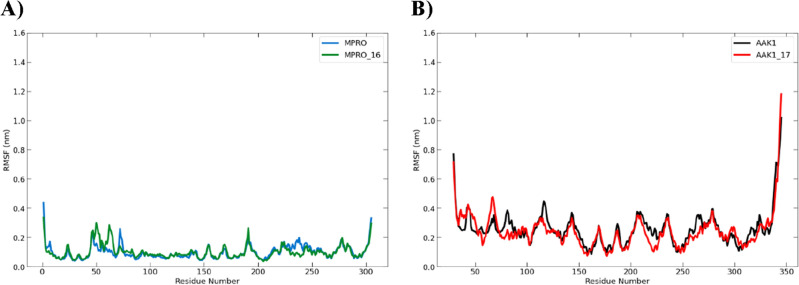
Analysis of the root mean square fluctuation (RMSF)
The local changes in the protein structure brought on by the presence of the suggested inhibitor were examined using the root mean square fluctuation (RMSF)39. Throughout the simulation period, it showed how flexible the protein was. The ranges of 0.03–0.48 nm and 0.15–1.2 nm showed the highest variation. The similar residues in the compounds 16 or 17-bound complexes were less flexible than the native, unbound targets in general. These low-fluctuating residues contributed to the stability of the docked molecules at the binding site (Fig. 13).
Figure 13.
Plot of the root mean square fluctuation (RMSF) for (A) 300-ns molecular dynamics simulation of the Mpro protein (blue) and Mpro-16 complex (green) and (B) 600-ns molecular dynamics simulation of the AAK1 protein (black) and AAK1-17 complex (red).
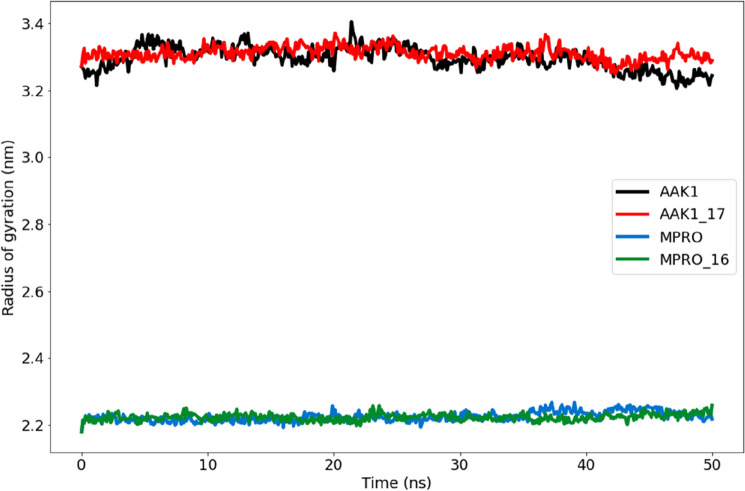
Analysis for the radius of gyration (Rg)
The radius of gyration provides information regarding the size and compactness of protein molecules (Rg). The Rg can be used to track the folding and unfolding of protein structures when ligands are bound40. Rg values for the drug-bound complexes were typically closer to the native unbound Mpro and AAK1 values (Fig. 14). Compound 16 and Mpro had average Rg values of 2.18–2.26 nm, whereas compound 17 and AAK1 had values of 3.22–3.40 nm. A less compact or more unfolded protein–ligand interaction is indicated by a greater Rg. A protein is considered securely folded, though, if its Rg value holds steady during the MD simulation. It is viewed as unfolded if the value of Rg changes with time. Figure 14 illustrates how, as compared to the unbound protein, each complex had very identical properties in terms of compactness and almost constant values of Rg.
Figure 14.
A plot illustrated the radius of gyration (Rg) for the Mpro protein (blue) and compound 16 complex (green) in addition to the AAK1 protein (black) and compound 17 complex (red) during the 50-ns molecular dynamics simulation.
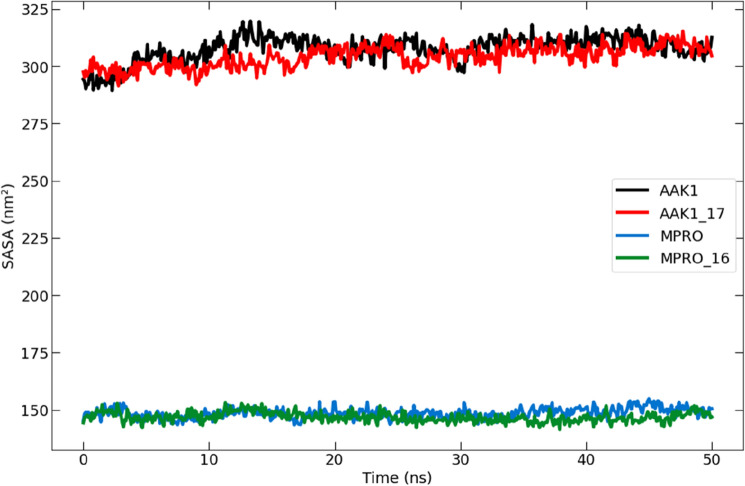
Analysis of solvent-accessible surface area (SASA)
Both with and without ligands, the protein's solvent-accessible surface area (SASA) was studied. The protein–ligand complex's SASA computation is used to forecast the number of conformational changes that the aqueous solvent can access41. As a result, the SASA was used to evaluate interactions between the complex and the solvent throughout the 50-ns MD simulation. For the unbound protein and protein–ligand complexes, Fig. 15 shows the SASA vs. simulation time curve. In addition to compound 17 and AAK1, the SASA averages for compound 16 and Mpro ranged from 141 to 155 nm2 and 290 to 320 nm2, respectively. Each compound binding caused the SASA to slightly increase due to the expanded surface generated by a portion of the bound ligand surface poking out from the protein surface.
Figure 15.
A graph showing the solvent-accessible surface area (SASA) for the Mpro protein (blue) and the complex of compound 16 (green) in addition to the AAK1 protein (black) and the complex of compound 17 (red) during the 50-ns molecular dynamics simulation.
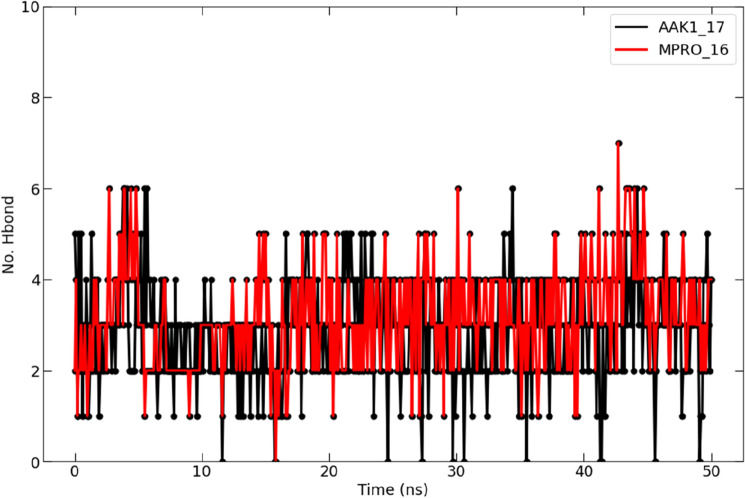
Analysis of hydrogen bond
The protein–ligand complex is stabilized by hydrogen bonds that form between the receptor and ligand. It also impacts the design of medications and their specificity, metabolization, and adsorption42. Therefore, the hydrogen bonds in each ligand–protein combination were studied. Figure 16 displays the total number of hydrogen bonds discovered in the complex after a 50-ns simulation. Each complex had between one and six hydrogen bonds, with two of them remaining constant over the course of the simulation. Additionally, each compound showed a similar hydrogen-bonding pattern throughout the simulation, as seen in Fig. 16.
Figure 16.
A graph presenting the numbers of hydrogen bonds that were seen in the complex formed by compound 16 (with Mpro in red color) and compound 17 (with AAK1 in black color) during the 50-ns molecular dynamics simulation.
Simulation time over 100 ns
Analysis of the root mean square deviation (RMSD)
When the simulation period was extended to 100 nm, we found that the complex still maintained equilibrium for the course of the experiment (Fig. 17). The RMSD values of the apoprotein and the compound 17-bound complex reached 0.58 nm. Furthermore, the RMSD values of the apoprotein and the compound 16-bound complex reached 0.29 nm. So, compounds 16 and 17 still behaved consistently inside their pockets throughout the simulation and went farther toward the binding pocket.
Figure 17.
Plot of the root mean square deviation (RMSD) for (A) 100-ns molecular dynamics simulation of the Mpro protein and Mpro-16 complex and (B) 100-ns molecular dynamics simulation of the AAK1 protein and AAK1-17 complex.
Analysis of the root mean square fluctuation (RMSF)
Throughout the extended simulation period to 100 ns, it still showed how flexible the proteins were. The similar residues in the compounds 16- and 17-bound complexes were less flexible than the native, unbound targets. These low-fluctuating residues contributed to the stability of the docked molecules at the binding site (Fig. 18).
Figure 18.
Plot of the root mean square fluctuation (RMSF) for (A) 300-ns molecular dynamics simulation of the Mpro protein (blue) and Mpro-16 complex (green) and (B) 350-ns molecular dynamics simulation of the AAK1 protein (black) and AAK1-17 complex (red).
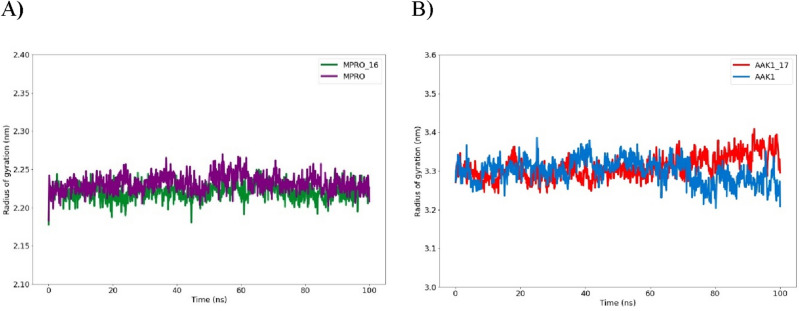
Analysis for the radius of gyration (Rg)
Rg values for the drug-bound complexes were still typically closer to the native unbound Mpro and AAK1 values. Figure 19 illustrates how, as compared to the unbound protein, each complex had very identical properties in terms of compactness and almost constant values of Rg.
Figure 19.
A plot illustrated the radius of gyration (Rg) for (A) the Mpro protein and compound 16 complex in addition to the AAK1 protein and (B) compound 17 complex during the 100-ns molecular dynamics simulation.
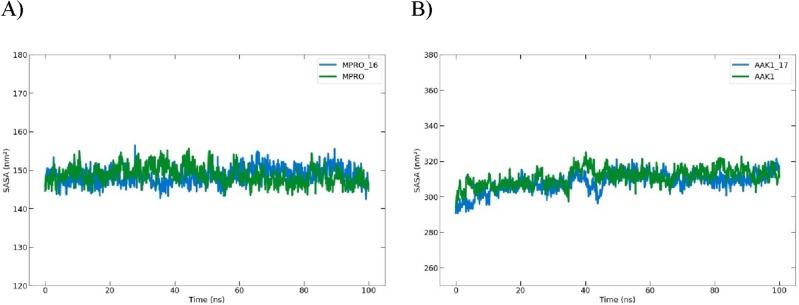
Analysis of solvent-accessible surface area (SASA)
Each compound binding still caused SASA to increase slightly due to the expanded surface resulting from a portion of the binding surface bound from the protein surface (Fig. 20).
Figure 20.
A graph showing the solvent-accessible surface area (SASA) for (A) the Mpro protein and the complex of compound 16, and (B) the AAK1 protein and the complex of compound 17 during the 100-ns molecular dynamics simulation.
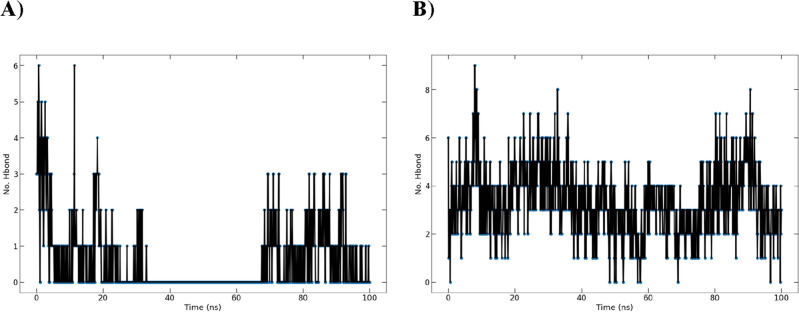
Analysis of hydrogen bond
The complex formed by compound 17 with AAK1 (in black color) still had between one and six hydrogen bonds, with two of them remaining constant over the course of the simulation (100 ns). But the complex formed by compound 16 with Mpro (in red color) had between one and three hydrogen bonds, without making any hydrogen bonding around 30–70 ns period (Fig. 21).
Figure 21.
A graph presenting the numbers of hydrogen bonds that were seen in the complex formed by (A) compound 16 (with Mpro in red color) and (B) compound 17 (with AAK1 in black color) during the 100-ns molecular dynamics simulation.
Principle component analysis (PCA)
The conformational distribution during the simulation time was investigated using the PCA approach, as well as the large-scale collective motions of the protein in protein–ligand complexes on the simulation-generated trajectories. It was assumed that the complex that takes up less phase space with a stable cluster is more stable than the complex that takes up more space with a nonstable cluster43. During simulations of compound 16 bound to Mpro and compound 17 bound to AAK1 proteins, the first two principal components (PC1 and PC2) were chosen to investigate their projection of trajectories in phase space. The results clearly showed that Mpro-16 complex occupied smaller regions of phase space than AAK1-17 complex (Fig. 22).
Figure 22.
First two eigenvectors describe the protein motion in phase space for (A) Mpro-16 and (B) AAK1-17 complexes.
Binding energy estimation by MM/PBSA method
The Molecular Mechanics/Poisson Boltzmann Surface Area (MM/PBSA) method throughout the 100 ns simulations was selected for rescoring complexes because it is the fastest force field-based method that computes the free energy of binding, as compared to the other computational free energy methods, such as free energy perturbation (FEP) or thermodynamic integration (TI) methods. The MM/PBSA calculation was performed using g-mmpbsa software. The calculated binding free energies are shown in Table 3. The average overall binding free energy of the Mpro-16 and AAK1-17 complexes are − 39.776 (± 41.068) kJ/mol and − 171.265 (± 53.984) kJ/mol, respectively.
Table 3.
Calculated binding free energies of tested compounds 16 and 17 [kJ/mol].
| Complex | (kJ/mol) | van der Waal energy | Electrostatic energy | Polar solvation energy | SASA energy |
|---|---|---|---|---|---|
| Mpro-16 | − 39.776 (± 41.068) | − 58.763 (± 51.562) | − 12.993 (± 21.540) | 39.232 (± 68.765) | − 7.252 (± 6.553) |
| AAK1-17 | − 171.265 (± 53.984) | − 203.855 (± 25.396) | − 150.308 (± 69.756) | 209.561 (± 29.494) | − 26.663 (± 0.712) |
ADME study
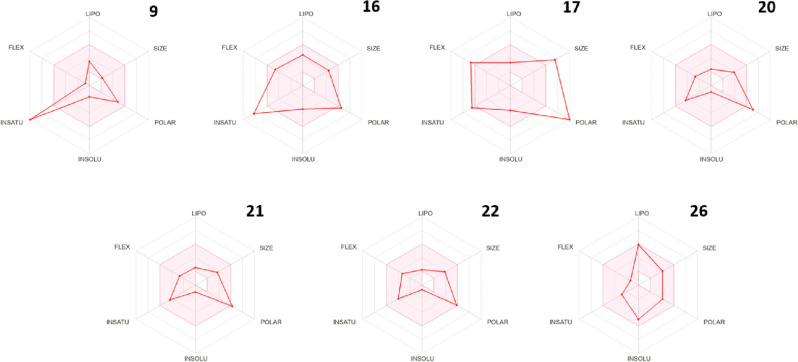
Selected compounds that showed good binding activity to both tested enzymes (9, 16, 17, 20, 21, 22 and 26) were studied and tested through the SwissADME web tool. First, we studied the bioavailability through a radar chart that tests six parameters, Lipophilicity (LIPO), Size, Polarity (POLAR), Insolubility (INSOL), Unsaturation (UNSAT) and Flexibility (FLEX). Compounds 22 and 26 showed no violation, compounds 9, 20 and 21 showed only one violation in the chart, while compounds 16 and 17 showed more than one violation due to their high hydrophilic characters of both compounds revealing their expected oral bioavailability (Fig. 23).
Figure 23.
The bioavailability radar chart for the tested compounds (the colored zone is the suitable physicochemical space for oral bioavailability).
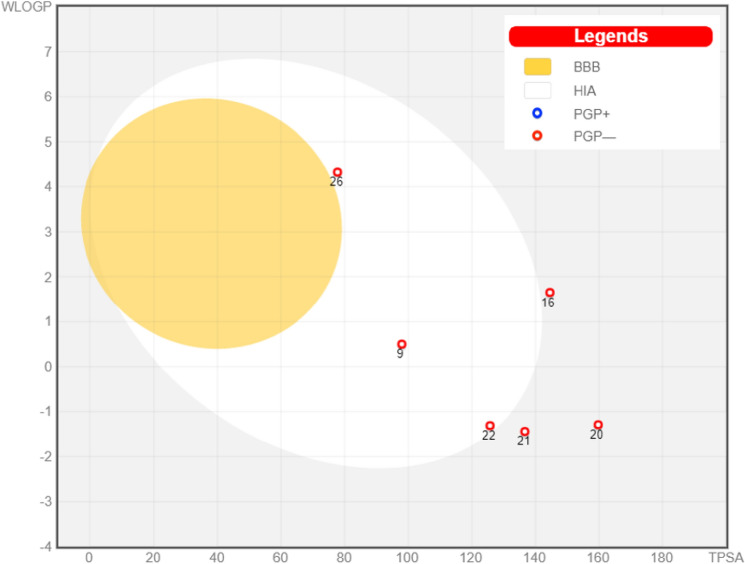
The SwissADME server also provides a BOILED EGG chart to indicate the human intestinal absorption (white part), blood–brain barrier penetration (the yellow part)44, and the probability of the tested compound acting as a substrate for permeability glycoprotein (PGP) which is an efflux pump for many drugs (blue color if it’s a possible substrate or red color if it’s not)45. Only three of the tested compounds showed good gastrointestinal absorption (compounds 9, 22 and 26), which exhibited a good balance between lipophilic and hydrophilic characters. Other tested phenolic acid derivatives exhibited poor gastrointestinal absorption due to their high hydrophilic characters, especially for tannic acid (compound 17), which did not appear in the chart due to extreme hydrophilic properties. All the seven tested compounds are not substrates for PGP, so they will not be susceptible to cell efflux (Fig. 24).
Figure 24.
BOILED-EGG chart for the seven tested compounds (Yellow area is blood–brain barrier; BBB, white area is human intestinal absorption; HIA, red circles mean that these compounds are non-substrate for PGP).
Eventually, applying Lipinski’s rule of five46 to predict the probability of the tested compounds being orally active, all showed no violations in the four parameters (log P, molecular weight, number of H-bond donor groups and number of H-bond acceptor groups) except for compound 17 (three violations) and compound 20 (one violation), this was because compound 17 (tannic acid) is extremely hydrophilic with high molecular weight (Table 4).
Table 4.
Drug-likeness of the tested compounds (Lipinski’s rule of five).
| Compound no. | Properties | Comment |
|---|---|---|
| 9 |
Log P = 0.21 (< 5) Molecular weight = 170.12 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 4 (≤ 5) No of H-bond acceptor atoms (Os + Ns) = 5 (≤ 10) |
No violation |
| 16 |
Log P = 1.52 (< 5) Molecular weight = 360.31 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 5 (≤ 5) No of H-bond acceptor atoms (Os + Ns) = 8 (≤ 10) |
No violation |
| 17 |
Log P = -0.48 (< 5) Molecular weight = 636.47 g/mol (> 500) No of H-bond donor groups (OHs + NHs) = 11 (> 5) No of H-bond acceptor atoms (Os + Ns) = 18 (> 10) |
Three violations |
| 20 |
Log P = − 1.07 (< 5) Molecular weight = 315.25 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 6 (> 5) No of H-bond acceptor atoms (Os + Ns) = 9 (≤ 10) |
One violation |
| 21 |
Log P = − 0.91 (< 5) Molecular weight = 300.26 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 5 (≤ 5) No of H-bond acceptor atoms (Os + Ns) = 8 (≤ 10) |
No violation |
| 22 |
Log P = − 0.81 (< 5) Molecular weight = 314.29 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 4 (≤ 5) No of H-bond acceptor atoms (Os + Ns) = 8 (≤ 10) |
No violation |
| 26 |
Log P = 3.82 (< 5) Molecular weight = 332.43 g/mol (< 500) No of H-bond donor groups (OHs + NHs) = 3 (≤ 5) No of H-bond acceptor atoms (Os + Ns) = 4 (≤ 10) |
No violation |
The overall data shows that compounds 16 (rosmarinic acid) and 17 (tannic acid) require structural modifications to improve their pharmacokinetic properties and to be suitable for oral administration. Toxicity profile of our most promising natural acids 16 and 17 against Mpro and was investigated through lazar toxicity predictor, the obtained results revealed that both (16) rosmarinic acid and (17) tannic acid are non-carcinogenic in mice, rats and rodents model, additionally, AAK1 showed to be non-mutagenic in Salmonella typhimurium model. With Maximum Recommended Daily Dose in Human equal 4.35 (mg/kg_bw/day) and 137.0 (mg/kg_bw/day) respectively.
Conclusion
The objective of this study was to find natural phenolic acids that can target SARS-CoV-2's main protease (Mpro) and adaptor-associated protein kinase 1 (AAK1) enzymes. The researchers employed several molecular modelling techniques such as pharmacophore mapping, molecular docking, molecular dynamics, and ADME studies. Rosmarinic acid (16) and 2,5-dihydroxybenzoic-5-O-glucoside (20) demonstrated remarkable binding affinities for both target enzymes and, as such, could act as dual inhibitors. Tannic acid (17) showed good potential in fitting all the required pharmacophoric features in AAK1 with the lowest docking score. These results were confirmed through pharmacophore mapping, molecular docking, and dynamic studies over 50 ns and extended to 100 ns. In terms of the ADME study, Rosmarinic acid has low bioavailability due to its high polarity and thus requires structural modification to improve its bioavailability as a promising dual target for COVID-19 prevention via main protease inhibition and endocytosis inhibition.
Acknowledgements
The researchers would like to acknowledge Deanship of scientific research, Taif University for funding this work.
Author contributions
H.I.G.: validation, revision and writing. A.B.: conceptualization, methods, writing. M.K.E.-A.: wrote the draft of the manuscript text, methods and review. H.O.T.: prepared figures, methods and writing. R.I.A.: methods, figures check and writing. A.J.O.: methodology, validation, editing. A.A.E.-M.: data curation, writing and revision. D.A.: methods, data analysis and revision. All authors reviewed the manuscript.
Funding
Deanship of scientific research, Taif University, Saudi Arabia has supported this research work.
Data availability
All data generated or analyzed during this study are included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prabhu S, Molath A, Choksi H, Kumar S, Mehra R. Classifications of polyphenols and their potential application in human health and diseases. Int. J. Physiol. Nutr. Phys. Educ. 2021;6:293–301. doi: 10.22271/journalofsport.2021.v6.i1e.2236. [DOI] [Google Scholar]
- 2.El-Nashar HA, Mostafa NM, Abd El-Ghffar EA, Eldahshan OA, Singab ANB. The genus Schinus (Anacardiaceae): A review on phytochemicals and biological aspects. Nat. Prod. Res. 2022;36:4839–4857. doi: 10.1080/14786419.2021.2012772. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24:1–10. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehrawat R, Rathee P, Akkol EK, Khatkar S, Lather A, Redhu N, Khatkar A. Phenolic acids-versatile natural moiety with numerous biological applications. Curr. Top. Med. Chem. 2022;22:1472–1484. doi: 10.2174/1568026622666220623114450. [DOI] [PubMed] [Google Scholar]
- 5.Montenegro-Landívar MF, Tapia-Quirós P, Vecino X, Reig M, Valderrama C, Granados M, Cortina JL, Saurina J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021;801:1–14. doi: 10.1016/j.scitotenv.2021.149719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y-H, Zhang B-Y, Qiu L-P, Guan R-F, Ye Z-H, Yu X-P. Structure properties and mechanisms of action of naturally originated phenolic acids and their derivatives against human viral infections. Curr. Med. Chem. 2017;24:4279–4302. doi: 10.2174/0929867324666170815102917. [DOI] [PubMed] [Google Scholar]
- 7.Kowalczyk M, Golonko A, Świsłocka R, Kalinowska M, Parcheta M, Swiergiel A, Lewandowski W. Drug design strategies for the treatment of viral disease. Plant phenolic compounds and their derivatives. Front. Pharmacol. 2021;1:1–21. doi: 10.3389/fphar.2021.709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabet R, Sisakht M, Emami L, Sabahi Z. Comparison of COVID-19 virus main protease inhibition activities of phenolic acids by molecular docking. Trends Pharmaceut. Sci. 2021;7:117–126. [Google Scholar]
- 9.Sohail, M.I., Siddiqui, A., Erum, N., Kamran, M. Phytomedicine and the COVID-19 pandemic. in: Phytomedicine, Elsevier, 2021, pp. 693–708.
- 10.Liu H, Zhu W, Wu Y, Jiang C, Huo L, Belal A. COVID-19 pandemic between severity facts and prophylaxis. Nat. Prod. Commun. 2021;16:1270. [Google Scholar]
- 11.Gil C, Ginex T, Maestro I, Nozal V, Barrado-Gil L, Cuesta-Geijo MÁ, Urquiza J, Ramírez D, Alonso C, Campillo NE. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020;63:12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 12.Eldeeb E, Belal A. Two promising herbs that may help in delaying corona virus progression. Int. J. Trend Sci. Res. Dev. 2020;4:764–766. [Google Scholar]
- 13.Raj R. Analysis of non-structural proteins, NSPs of SARS CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 2021;25:1–10. doi: 10.1016/j.bbrep.2020.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qayed WS, Ferreira RS, Silva JRA. In silico study towards repositioning of FDA-approved drug candidates for anticoronaviral therapy: Molecular docking, molecular dynamics and binding free energy calculations. Molecules. 2022;27:1–22. doi: 10.3390/molecules27185988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillaiyar T, Laufer S. Kinases as potential therapeutic targets for anti-coronaviral therapy. J. Med. Chem. 2021;65:955–982. doi: 10.1021/acs.jmedchem.1c00335. [DOI] [PubMed] [Google Scholar]
- 16.Ayele AG, Enyew EF, Kifle ZD. Roles of existing drug and drug targets for COVID-19 management. Metabolism Open. 2021;11:1–10. doi: 10.1016/j.metop.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam MT, Sarkar C, El-Kersh DM, Jamaddar S, Uddin SJ, Shilpi JA, Mubarak MS. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020;34:2471–2492. doi: 10.1002/ptr.6700. [DOI] [PubMed] [Google Scholar]
- 20.Sayed AM, Khattab AR, AboulMagd AM, Hassan HM, Rateb ME, Zaid H, Abdelmohsen UR. Nature as a treasure trove of potential anti-SARS-CoV drug leads: A structural/mechanistic rationale. RSC Adv. 2020;10:19790–19802. doi: 10.1039/D0RA04199H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souid I, Korchef A, Souid S. In silico evaluation of Vitis amurensis Rupr. polyphenol compounds for their inhibition potency against CoVID-19 main enzymes Mpro and RdRp. Saudi Pharm. J. 2022;30:1–15. doi: 10.1016/j.jsps.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gligorijevic N, Radomirovic M, Nedic O, Stojadinovic M, Khulal U, Stanic-Vucinic D, Cirkovic Velickovic T. Molecular mechanisms of possible action of phenolic compounds in COVID-19 protection and prevention. Int. J. Mol. Sci. 2021;22:1–29. doi: 10.3390/ijms222212385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belal A, Elsayed A, Gharib AF, Ali Alqarni MA, Soliman AM, Mehany AB, Elanany MA. Toward the discovery of SARS CoV-2 main protease inhibitors: exploring therapeutic potentials of evodiamine and its derivatives, virtual screening, molecular docking, and molecular dynamic studies. Nat. Prod. Commun. 2022;17:3082. [Google Scholar]
- 24.Vilar S, Cozza G, Moro S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008;8:1555–1572. doi: 10.2174/156802608786786624. [DOI] [PubMed] [Google Scholar]
- 25.Mehany A, Belal A, Santali EY, Shaaban S, Abourehab MA, El-Feky OA, Diab M, Abou Galala F, Elkaeed EB, Abdelhamid G. Biological effect of quercetin in repairing brain damage and cerebral changes in rats: Molecular docking and in vivo studies. BioMed Res. Int. 2022;1:1. doi: 10.1155/2022/8962149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehany AB, Belal A, Mohamed AF, Shaaban S, Abdelhamid G. Apoptotic and anti-angiogenic effects of propolis against human bladder cancer: Molecular docking and in vitro screening. Biomarkers. 2022;27:138–150. doi: 10.1080/1354750X.2021.2020903. [DOI] [PubMed] [Google Scholar]
- 27.Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawfik HO, Belal A, Abourehab MA, Angeli A, Bonardi A, Supuran CT, El-Hamamsy MH. Dependence on linkers’ flexibility designed for benzenesulfonamides targeting discovery of novel hCA IX inhibitors as potent anticancer agents. J. Enzyme Inhib. Med. Chem. 2022;37:2765–2785. doi: 10.1080/14756366.2022.2130285. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Farouk F, Elmaaty AA, Elkamhawy A, Tawfik HO, Alnajjar R, Abourehab MAS, Saleh MA, Eldehna WM, Al-Karmalawy AA. Investigating the potential anticancer activities of antibiotics as topoisomerase II inhibitors and DNA intercalators: In vitro, molecular docking, molecular dynamics, and SAR studies. J. Enzyme Inhib. Med. Chem. 2023;38:2171029. doi: 10.1080/14756366.2023.2171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Šribar D, Noonan T, Deng L, Nguyen TN, Pach S, Machalz D, Bermudez M, Wolber G, Schaller D. Next generation 3D pharmacophore modeling. WIREs Comput. Mol. Sci. 2020;10:1–20. [Google Scholar]
- 31.Belal A, Elanany MA, Raafat M, Hamza HT, Mehany AB. Calendula officinalis phytochemicals for the treatment of wounds through matrix metalloproteinases-8 and 9 (MMP-8 and MMP-9): in silico approach. Nat. Prod. Commun. 2022;17:848. [Google Scholar]
- 32.Lockbaum GJ, Henes M, Lee JM, Timm J, Nalivaika EA, Thompson PR, Kurt Yilmaz N, Schiffer CA. Pan-3C protease inhibitor rupintrivir binds SARS CoV-2 main protease in a unique binding mode. Biochemistry. 2021;60:2925–2931. doi: 10.1021/acs.biochem.1c00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Refaey RH, El-Ashrey MK, Nissan YM. Repurposing of renin inhibitors as SARS COV-2 main protease inhibitors: A computational study. Virology. 2021;554:48–54. doi: 10.1016/j.virol.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrell FJ, Szklarz M, Azeez KRA, Elkins JM, Knapp S. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24:401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousef RG, Ibrahim A, Khalifa MM, Eldehna WM, Gobaara IM, Mehany AB, Elkaeed EB, Alsfouk AA, Metwaly AM, Eissa IH. Discovery of new nicotinamides as apoptotic VEGFR-2 inhibitors: Virtual screening, synthesis, anti-proliferative, immunomodulatory, ADMET, toxicity, and molecular dynamic simulation studies. J. Enzyme Inhib. Med. Chem. 2022;37:1389–1403. doi: 10.1080/14756366.2022.2070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elrazaz EZ, Serya RA, Ismail NS, Albohy A, Abou El Ella DA, Abouzid KA. Discovery of potent thieno [2, 3-d] pyrimidine VEGFR-2 inhibitors: Design, synthesis and enzyme inhibitory evaluation supported by molecular dynamics simulations. Bioorg. Chem. 2021;113:1–16. doi: 10.1016/j.bioorg.2021.105019. [DOI] [PubMed] [Google Scholar]
- 37.Belhassan A, Zaki H, Chtita S, Alaqarbeh M, Alsakhen N, Benlyas M, Lakhlifi T, Bouachrine M. Camphor, Artemisinin and Sumac Phytochemicals as inhibitors against COVID-19: Computational approach. Comput. Biol. Med. 2021;136:1–17. doi: 10.1016/j.compbiomed.2021.104758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guterres H, Im W. Improving protein-ligand docking results with high-throughput molecular dynamics simulations. J. Chem. Inf. Model. 2020;60:2189–2198. doi: 10.1021/acs.jcim.0c00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jena AB, Kanungo N, Nayak V, Chainy G, Dandapat J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: Insights from computational studies. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-81462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali A, Ali A, Warsi MH, Rahman MA, Ahsan MJ, Azam F. Toward the discovery of a novel class of leads for high altitude disorders by virtual screening and molecular dynamics approaches targeting carbonic anhydrase. Int. J. Mol. Sci. 2022;23:1–20. doi: 10.3390/ijms23095054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa RAD, Rocha JAD, Pinheiro AS, Costa ADSD, Rocha ECD, Silva RC, Gonçalves ADS, Santos CB, Brasil DDS. A computational approach applied to the study of potential allosteric inhibitors protease NS2B/NS3 from Dengue virus. Molecules. 2022;27:1–20. doi: 10.3390/molecules27134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Wong AR, Yang AWH, Hung A. Interaction of compounds derived from the Chinese medicinal formula Huangqi Guizhi Wuwu Tang with stroke-related numbness and weakness targets: An in-silico docking and molecular dynamics study. Comput. Biol. Med. 2022;146:1–9. doi: 10.1016/j.compbiomed.2022.105568. [DOI] [PubMed] [Google Scholar]
- 43.Elmaaty AA, Darwish KM, Khattab M, Elhady SS, Salah M, Hamed MI, Al-Karmalawy AA, Saleh MM. In a search for potential drug candidates for combating COVID-19: Computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J. Biomol. Struct. Dyn. 2022;40:8866–8893. doi: 10.1080/07391102.2021.1918256. [DOI] [PubMed] [Google Scholar]
- 44.Daina A, Zoete V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharom F. The P-glycoprotein efflux pump: How does it transport drugs? J. Membr. Biol. 1997;160:161–175. doi: 10.1007/s002329900305. [DOI] [PubMed] [Google Scholar]
- 46.Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.