The title compound crystallizes with a disordered nitro group in twinned crystals. In the crystal, the N—H group donates a hydrogen bond to a nitro oxygen atom, generating chains propagating in the [101] direction. The amide carbonyl oxygen atom is not involved in the hydrogen bonding.
Keywords: N-alkoxyacetanilides, phenacetin congeners, nitro products, crystal structure, hydrogen bonding
Abstract
The title compound, C9H10N2O4, crystallizes with a disordered nitro group in twinned crystals. Both the methoxy group and the acetamide groups are nearly coplanar with the phenyl ring, and the C—N—C—O torsion angle [0.2 (4)°] is also insignificantly different from zero. Overall, the 12-atom methoxyphenylacetamide group is nearly planar, with an r.m.s. deviation of 0.042 Å. The nitro group is twisted out of this plane by about 30°, disordered into two orientations with opposite senses of twist. In the crystal, the N—H group donates a hydrogen bond to a nitro oxygen atom, generating chains propagating in the [101] direction. The amide carbonyl oxygen atom is not involved in the hydrogen bonding.
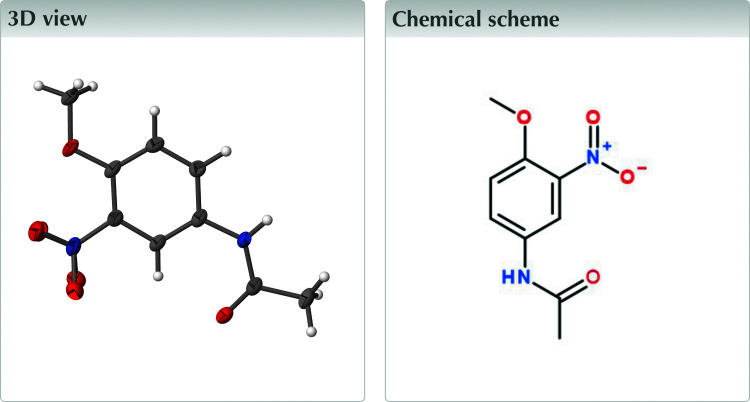
Structure description
Belonging to the class of 4-alkoxyacetanilides (4-AAs), phenacetin [N-(4-ethoxyphenyl)acetamide] was the first synthetic fever reducer and non-opioid analgesic to go on the market worldwide as early as the 1890s. It is generally believed that the analgesic effects of 4-AAs are due to their actions on the sensory tracts of the spinal cord, while the antipyretic actions arise from their actions on the brain where the temperature set point is lowered (Dalmann et al., 2015 ▸; Flower & Vane, 1972 ▸). In vivo, 4-AAs mostly undergo oxidative O-dealkylation to give N-(4-hydroxphenyl)acetamide (Brodie & Axelrod, 1948 ▸; Kapetanović & Mieyal, 1979 ▸), the clinically relevant analgesic, while small portions may undergo deacylation, producing carcinogenic, kidney-damaging 4-alkoxyanilines and/or their N-oxidation products, namely, N-(4-alkoxyphenyl)hydroxylamine and 1-alkoxy-4-nitrosobenzene (Prescott, 1980 ▸).
There has been extensive information on phase I and phase II biotransformation of 4-AAs (Estus & Mieyal, 1983 ▸; Hinson, 1983 ▸; Kapetanović & Mieyal, 1979 ▸; Taxak et al., 2013 ▸), but little is known about their biotransformation by non-enzymatic mechanisms, including those mediated by nitric oxide-derived free radical and non-free radical oxidants (viz., nitrogen dioxide, carbonate radical, and peroxynitrous acid). Studies from our laboratory have shown, for instance, that N-(4-hydroxyphenyl)acetamide forms nitrated products along with varying amounts of dimers when reacted with the said nitric oxide-derived oxidants under physiologically relevant conditions (Deere et al., 2023 ▸; Uppu & Martin, 2004 ▸). We reason that similar products (or their positional isomers) may be formed in the reactions of 4-AAs with nitric oxide-derived oxidants or other cellular oxidants like the hypochlorite/hypochlorous acid conjugate acid/base system (pH ≃ 7.53).
Towards better understanding of these possibilities and to shed light on molecular targets, we have synthesized the title compound, C9H10N2O4 [N-(4-methoxy-3-nitrophenyl)acetamide]: crystals grown in water were analyzed by X-ray diffraction. Combined with the recent revelations of mechanisms of action of N-(4-hydroxyphenyl)acetamide through indirect activation of CB1 receptors by 4-aminophenol [hydrolysis product of N-(4-hydroxyphenyl)acetamide] and endocannabinoid reuptake inhibitor AM404 (Bertolini et al., 2006 ▸; Zygmunt et al., 2000 ▸), the information presented here may provide useful insights into molecular targets for 4-AAs and their nitrated metabolites.
The title compound, shown in Fig. 1 ▸, crystallizes with a disordered nitro group in twinned crystals. Both the methoxy group and the acetamide groups are nearly coplanar with the phenyl ring, with respective torsion angles 0.0 (4)° for C9—O2—C4—C5 and 4.9 (4)° for C7—N1—C1—C2. The C1—N1—C7—O1 torsion angle is also insignificantly different from zero, 0.2 (4)°. Overall, the atoms of the 12-atom methoxyphenylacetamide group are almost coplanar with an r.m.s. deviation of 0.042 Å. The nitro group is twisted out of this plane by 23.5 (2) and 35.6 (2)°, disordered into two orientations with opposite senses of twist. The dihedral angle between the two disordered C—NO2 planes is 59.2 (2)°. The N—H group donates intermolecular hydrogen bonds to the nitro oxygen atom at x −
 ,
,
 − y, z −
− y, z −
 , with an N1⋯O3A distance of 3.122 (4) Å (Table 1 ▸), thereby forming chains propagating in the [101] direction, as shown in Fig. 2 ▸. The unit cell is shown in Fig. 3 ▸. Interestingly, the amide carbonyl oxygen atom is not involved in the hydrogen bonding.
, with an N1⋯O3A distance of 3.122 (4) Å (Table 1 ▸), thereby forming chains propagating in the [101] direction, as shown in Fig. 2 ▸. The unit cell is shown in Fig. 3 ▸. Interestingly, the amide carbonyl oxygen atom is not involved in the hydrogen bonding.
Figure 1.

The title molecule with displacement ellipsoids drawn at the 50% probability level.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O3i | 0.87 (3) | 2.59 (3) | 3.410 (5) | 157 (2) |
| N1—H1N⋯O3A i | 0.87 (3) | 2.37 (3) | 3.122 (4) | 145 (3) |
Symmetry code: (i)
 .
.
Figure 2.
The hydrogen-bonding scheme. Only one orientation of the disordered NO2 group is shown.
Figure 3.
The unit-cell packing. Only one orientation of the disordered NO2 group is shown.
Synthesis and crystallization
N-(4-Methoxy-3-nitrophenyl)acetamide was synthesized by the acetylation of 4-methoxy-3-nitroaniline using acetic anhydride. Typically, 20 mmol (3.36 g) of 4-methoxy-3-nitroaniline in 30 ml of glacial acetic acid was refluxed for 2 h with 20% molar excess (24 mmol; 2.46 g) of acetic anhydride. The reaction mixture was stirred continuously during the reaction. In the end, the mixture was dried under vacuum, and the residue was purified by recrystallization twice from deionized water. Single crystals of the title compound were grown from an aqueous solution by slow cooling of a hot and nearly saturated solution.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The crystal chosen for data collection was found to be a three-component nonmerohedral twin with approximate fractions of 0.962: 0.024: 0.014. Refinement was against a twin4.hkl file prepared by TWINABS and the twin fractions were not refined.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C9H10N2O4 |
| M r | 210.19 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 90 |
| a, b, c (Å) | 10.8740 (8), 7.0136 (6), 12.2891 (12) |
| β (°) | 92.313 (5) |
| V (Å3) | 936.48 (14) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 1.02 |
| Crystal size (mm) | 0.32 × 0.09 × 0.04 |
| Data collection | |
| Diffractometer | Bruker Kappa APEXII DUO CCD |
| Absorption correction | Multi-scan (TWINABS; Bruker, 2016 ▸) |
| T min, T max | 0.742, 0.961 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 2937, 1675, 1360 |
| R int | 0.047 |
| (sin θ/λ)max (Å−1) | 0.607 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.059, 0.176, 1.08 |
| No. of reflections | 1675 |
| No. of parameters | 160 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.28, −0.35 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314623002985/hb4429sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623002985/hb4429Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623002985/hb4429Isup3.cml
CCDC reference: 2253001
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| C9H10N2O4 | F(000) = 440 |
| Mr = 210.19 | Dx = 1.491 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 10.8740 (8) Å | Cell parameters from 3893 reflections |
| b = 7.0136 (6) Å | θ = 5.3–69.2° |
| c = 12.2891 (12) Å | µ = 1.02 mm−1 |
| β = 92.313 (5)° | T = 90 K |
| V = 936.48 (14) Å3 | Needle, yellow |
| Z = 4 | 0.32 × 0.09 × 0.04 mm |
Data collection
| Bruker Kappa APEXII DUO CCD diffractometer | 1675 independent reflections |
| Radiation source: IµS microfocus | 1360 reflections with I > 2σ(I) |
| QUAZAR multilayer optics monochromator | Rint = 0.047 |
| φ and ω scans | θmax = 69.4°, θmin = 5.3° |
| Absorption correction: multi-scan (TWINABS; Bruker, 2016) | h = −13→13 |
| Tmin = 0.742, Tmax = 0.961 | k = −8→8 |
| 2937 measured reflections | l = −14→14 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.059 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.176 | w = 1/[σ2(Fo2) + (0.0926P)2 + 0.7816P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max < 0.001 |
| 1675 reflections | Δρmax = 0.28 e Å−3 |
| 160 parameters | Δρmin = −0.35 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Twinned crystal. Refinement was vs. an HKLF4 file prepared by TWINABS. All H atoms were located in difference maps and those on C were thereafter treated as riding in geometrically idealized positions with C—H distances of 0.95 Å for phenyl and 0.98 Å for methyl. The coordinates of the amide H atom were refined. Uiso(H) values were constrained to be 1.2Ueq for the attached atom (1.5 for methyl). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.29551 (15) | 0.2148 (3) | 0.64386 (16) | 0.0309 (5) | |
| O2 | 0.84160 (14) | 0.4030 (3) | 0.47776 (15) | 0.0291 (5) | |
| O3 | 0.6667 (4) | 0.4337 (10) | 0.7443 (4) | 0.0382 (13) | 0.500 (6) |
| O4 | 0.8325 (3) | 0.3096 (8) | 0.6813 (4) | 0.0450 (16) | 0.500 (6) |
| O3A | 0.6722 (4) | 0.3056 (10) | 0.7606 (3) | 0.0377 (13) | 0.500 (6) |
| O4A | 0.8073 (3) | 0.4813 (6) | 0.6859 (3) | 0.0366 (13) | 0.500 (6) |
| N1 | 0.34765 (17) | 0.1800 (3) | 0.46794 (18) | 0.0246 (5) | |
| H1N | 0.323 (3) | 0.145 (4) | 0.403 (3) | 0.030* | |
| N2 | 0.72034 (18) | 0.3692 (3) | 0.67532 (19) | 0.0314 (6) | |
| C1 | 0.4726 (2) | 0.2346 (3) | 0.4747 (2) | 0.0248 (6) | |
| C2 | 0.5355 (2) | 0.2771 (3) | 0.5717 (2) | 0.0248 (6) | |
| H2 | 0.495195 | 0.269873 | 0.638738 | 0.030* | |
| C3 | 0.6593 (2) | 0.3309 (4) | 0.5696 (2) | 0.0249 (6) | |
| C4 | 0.7224 (2) | 0.3447 (3) | 0.4729 (2) | 0.0252 (6) | |
| C5 | 0.6570 (2) | 0.2991 (4) | 0.3768 (2) | 0.0278 (6) | |
| H5 | 0.696789 | 0.305718 | 0.309488 | 0.033* | |
| C6 | 0.5349 (2) | 0.2441 (3) | 0.3779 (2) | 0.0255 (6) | |
| H6 | 0.492590 | 0.212081 | 0.311263 | 0.031* | |
| C7 | 0.2676 (2) | 0.1729 (3) | 0.5507 (2) | 0.0260 (6) | |
| C8 | 0.1392 (2) | 0.1083 (4) | 0.5162 (2) | 0.0316 (6) | |
| H8A | 0.102811 | 0.039286 | 0.576348 | 0.047* | |
| H8B | 0.143220 | 0.024068 | 0.452865 | 0.047* | |
| H8C | 0.088432 | 0.219750 | 0.497165 | 0.047* | |
| C9 | 0.9015 (2) | 0.4168 (4) | 0.3751 (2) | 0.0308 (6) | |
| H9A | 0.906509 | 0.289913 | 0.342208 | 0.046* | |
| H9B | 0.984699 | 0.468291 | 0.387608 | 0.046* | |
| H9C | 0.854022 | 0.501614 | 0.325949 | 0.046* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0196 (9) | 0.0424 (11) | 0.0312 (12) | −0.0022 (7) | 0.0055 (7) | 0.0013 (8) |
| O2 | 0.0149 (8) | 0.0369 (11) | 0.0359 (11) | −0.0033 (6) | 0.0060 (7) | 0.0021 (8) |
| O3 | 0.036 (2) | 0.052 (4) | 0.027 (2) | −0.003 (2) | 0.0027 (17) | −0.009 (2) |
| O4 | 0.0188 (19) | 0.078 (4) | 0.037 (3) | −0.0051 (18) | −0.0031 (15) | 0.010 (2) |
| O3A | 0.033 (2) | 0.052 (4) | 0.027 (2) | −0.0082 (19) | −0.0026 (16) | 0.002 (2) |
| O4A | 0.0236 (19) | 0.048 (3) | 0.039 (2) | −0.0111 (16) | 0.0014 (15) | −0.0081 (18) |
| N1 | 0.0163 (10) | 0.0324 (12) | 0.0251 (12) | −0.0046 (7) | 0.0012 (8) | −0.0011 (9) |
| N2 | 0.0188 (10) | 0.0402 (13) | 0.0349 (14) | −0.0045 (9) | −0.0023 (9) | 0.0080 (11) |
| C1 | 0.0164 (11) | 0.0231 (12) | 0.0348 (15) | 0.0006 (8) | 0.0023 (9) | 0.0026 (10) |
| C2 | 0.0172 (11) | 0.0254 (13) | 0.0320 (15) | −0.0010 (8) | 0.0048 (9) | 0.0030 (10) |
| C3 | 0.0196 (12) | 0.0275 (13) | 0.0276 (14) | 0.0002 (9) | 0.0015 (9) | 0.0024 (10) |
| C4 | 0.0156 (11) | 0.0237 (12) | 0.0368 (16) | −0.0005 (8) | 0.0061 (9) | 0.0038 (10) |
| C5 | 0.0229 (12) | 0.0311 (14) | 0.0299 (15) | 0.0003 (9) | 0.0081 (10) | 0.0019 (11) |
| C6 | 0.0223 (12) | 0.0283 (13) | 0.0261 (14) | 0.0009 (9) | 0.0029 (9) | −0.0028 (10) |
| C7 | 0.0177 (12) | 0.0259 (13) | 0.0346 (16) | 0.0002 (9) | 0.0019 (10) | 0.0038 (10) |
| C8 | 0.0177 (12) | 0.0337 (14) | 0.0433 (17) | −0.0034 (9) | 0.0016 (10) | −0.0016 (12) |
| C9 | 0.0192 (12) | 0.0397 (15) | 0.0343 (15) | −0.0022 (10) | 0.0087 (10) | 0.0011 (11) |
Geometric parameters (Å, º)
| O1—C7 | 1.209 (3) | C2—H2 | 0.9500 |
| O2—C4 | 1.358 (3) | C3—C4 | 1.399 (3) |
| O2—C9 | 1.446 (3) | C4—C5 | 1.391 (4) |
| O3—N2 | 1.142 (5) | C5—C6 | 1.383 (3) |
| O4—N2 | 1.288 (5) | C5—H5 | 0.9500 |
| O3A—N2 | 1.271 (5) | C6—H6 | 0.9500 |
| O4A—N2 | 1.233 (4) | C7—C8 | 1.511 (3) |
| N1—C7 | 1.366 (3) | C8—H8A | 0.9800 |
| N1—C1 | 1.411 (3) | C8—H8B | 0.9800 |
| N1—H1N | 0.87 (3) | C8—H8C | 0.9800 |
| N2—C3 | 1.459 (3) | C9—H9A | 0.9800 |
| C1—C2 | 1.383 (4) | C9—H9B | 0.9800 |
| C1—C6 | 1.394 (3) | C9—H9C | 0.9800 |
| C2—C3 | 1.400 (3) | ||
| C4—O2—C9 | 116.4 (2) | C6—C5—C4 | 120.9 (2) |
| C7—N1—C1 | 127.4 (2) | C6—C5—H5 | 119.5 |
| C7—N1—H1N | 119.2 (19) | C4—C5—H5 | 119.5 |
| C1—N1—H1N | 113.4 (19) | C5—C6—C1 | 121.4 (2) |
| O4A—N2—O3A | 118.5 (3) | C5—C6—H6 | 119.3 |
| O3—N2—O4 | 126.7 (4) | C1—C6—H6 | 119.3 |
| O3—N2—C3 | 120.4 (3) | O1—C7—N1 | 123.6 (2) |
| O4A—N2—C3 | 122.0 (3) | O1—C7—C8 | 122.2 (2) |
| O3A—N2—C3 | 118.8 (3) | N1—C7—C8 | 114.3 (2) |
| O4—N2—C3 | 112.7 (3) | C7—C8—H8A | 109.5 |
| C2—C1—C6 | 119.0 (2) | C7—C8—H8B | 109.5 |
| C2—C1—N1 | 123.4 (2) | H8A—C8—H8B | 109.5 |
| C6—C1—N1 | 117.6 (2) | C7—C8—H8C | 109.5 |
| C1—C2—C3 | 119.0 (2) | H8A—C8—H8C | 109.5 |
| C1—C2—H2 | 120.5 | H8B—C8—H8C | 109.5 |
| C3—C2—H2 | 120.5 | O2—C9—H9A | 109.5 |
| C4—C3—C2 | 122.6 (2) | O2—C9—H9B | 109.5 |
| C4—C3—N2 | 121.5 (2) | H9A—C9—H9B | 109.5 |
| C2—C3—N2 | 115.9 (2) | O2—C9—H9C | 109.5 |
| O2—C4—C5 | 124.1 (2) | H9A—C9—H9C | 109.5 |
| O2—C4—C3 | 118.9 (2) | H9B—C9—H9C | 109.5 |
| C5—C4—C3 | 117.0 (2) | ||
| C7—N1—C1—C2 | 4.9 (4) | C9—O2—C4—C5 | 0.0 (4) |
| C7—N1—C1—C6 | −175.6 (2) | C9—O2—C4—C3 | −179.3 (2) |
| C6—C1—C2—C3 | 0.9 (4) | C2—C3—C4—O2 | 178.2 (2) |
| N1—C1—C2—C3 | −179.6 (2) | N2—C3—C4—O2 | −2.8 (4) |
| C1—C2—C3—C4 | 0.4 (4) | C2—C3—C4—C5 | −1.1 (4) |
| C1—C2—C3—N2 | −178.6 (2) | N2—C3—C4—C5 | 177.9 (2) |
| O3—N2—C3—C4 | 147.7 (4) | O2—C4—C5—C6 | −178.8 (2) |
| O4A—N2—C3—C4 | 29.0 (4) | C3—C4—C5—C6 | 0.5 (4) |
| O3A—N2—C3—C4 | −160.7 (4) | C4—C5—C6—C1 | 0.8 (4) |
| O4—N2—C3—C4 | −37.3 (4) | C2—C1—C6—C5 | −1.5 (4) |
| O3—N2—C3—C2 | −33.3 (5) | N1—C1—C6—C5 | 179.0 (2) |
| O4A—N2—C3—C2 | −152.0 (3) | C1—N1—C7—O1 | 0.2 (4) |
| O3A—N2—C3—C2 | 18.4 (5) | C1—N1—C7—C8 | 179.9 (2) |
| O4—N2—C3—C2 | 141.8 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O3i | 0.87 (3) | 2.59 (3) | 3.410 (5) | 157 (2) |
| N1—H1N···O3Ai | 0.87 (3) | 2.37 (3) | 3.122 (4) | 145 (3) |
Symmetry code: (i) x−1/2, −y+1/2, z−1/2.
Funding Statement
The authors acknowledge the support from the National Institutes of Health (NIH) through the National Institute of General Medical Science (NIGMS) grant No. 5 P2O GM103424–17 and the US Department of Education (US DoE; Title III, HBGI Part B grant No. P031B040030). Its contents are solely the responsibility of authors and do not represent the official views of NIH, NIGMS, or US DoE. The upgrade of the diffractometer was made possible by grant No. LEQSF(2011–12)-ENH-TR-01, administered by the Louisiana Board of Regents.
References
- Bertolini, A., Ferrari, A., Ottani, A., Guerzoni, S., Tacchi, R. & Leone, S. (2006). CNS Drug Rev. 12, 250–275. [DOI] [PMC free article] [PubMed]
- Brodie, B. & Axelrod, J. (1948). J. Pharmacol. Exp. Ther. 94, 29–38. [PubMed]
- Bruker (2016). APEX2, SAINT and TWINABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dalmann, R., Daulhac, L., Antri, M., Eschalier, A. & Mallet, C. (2015). Neuropharamacology 91, 63–70. [DOI] [PubMed]
- Deere, C. J., Agu, O. A., Hines, J. E. III & Uppu, R. M. (2023). Unpublished observations.
- Estus, G. S. & Mieyal, J. J. (1983). Drug Metab. Dispos. 11, 471–476. [PubMed]
- Flower, R. J. & Vane, J. R. (1972). Nature, 240, 410–411. [DOI] [PubMed]
- Hinson, J. A. (1983). Environ. Health Perspect. 49, 71–79. [DOI] [PMC free article] [PubMed]
- Kapetanović, I. M. & Mieyal, J. J. (1979). J. Pharmacol. Exp. Ther. 209, 25–30. [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Prescott, L. P. (1980). Br. J. Clin. Pharmacol. 10, 291S–298S. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Taxak, N., Chaitanya Prasad, K. & Bharatam, P. V. (2013). Comput. Theor. Chem. 1007, 48–56.
- Uppu, R. M. & Martin, R. J. (2004). The Toxicologist (supplement to Toxicol. Sci.) 84, 319–319
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zygmunt, P. M., Chuang, H., Movahed, P., Julius, D. & Högestätt, E. D. (2000). Eur. J. Pharmacol. 396, 39–42. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314623002985/hb4429sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623002985/hb4429Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623002985/hb4429Isup3.cml
CCDC reference: 2253001
Additional supporting information: crystallographic information; 3D view; checkCIF report




