Abstract
Modern conformal radiation therapy using techniques such as modulation, image guidance and motion management have changed the face of radiotherapy today offering superior conformity, efficiency, and reproducibility to clinics worldwide. This review assesses the impact of these advanced radiotherapy techniques on patient toxicity and survival rates reported from January 2017 to September 2020. The main aims are to establish if dosimetric and efficiency gains correlate with improved survival and reduced toxicities and to answer the question ‘What is the clinical evidence for the most effective implementation of VMAT?’. Compared with 3DCRT, improvements have been reported with VMAT in prostate, locally advanced cervical carcinoma and various head and neck applications, leading to the shift in technology to VMAT. Other sites such as thoracic neoplasms and nasopharyngeal carcinomas have observed some improvement with VMAT although not in line with improved dosimetric measures, and the burden of toxicity and the incidence of cancer related deaths remain high, signaling the need to further mitigate toxicity and increase survival. As technological advancement continues, large randomised long-term clinical trials are required to determine the way-forward and offer site-specific recommendations. These studies are usually expensive and time consuming, therefore utilising pooled real-world data in a prospective nature can be an alternative solution to comprehensively assess the efficacy of modern radiotherapy techniques.
Introduction
Cancer is the second leading cause of death globally 1,2 and a major public health concern. For over a century, radiotherapy used alone or in combination with other treatment modalities such as chemotherapy or surgery, has been proven effective for the treatment and management of cancer. 3 Owing to the critical role of radiotherapy in the treatment of cancer, advances in radiotherapy techniques are likely to have major clinical impact and necessitate review of optimum evidence-based practice.
Modern radiation therapy techniques employ modulated photon (Intensity Modulated Radiation Therapy—IMRT, Volumetric Modulated Arc Therapy—VMAT) or particle (Intensity Modulated Proton Therapy—IMPT) beams and the dosimetric gain over 3D conformal radiotherapy (3DCRT) has been widely studied. 4 Published surveys 5 and reviews suggest a shift in usage from 3DCRT to VMAT 6 specifically, combined with varying dose fractionation schemes [hypofractionation, Stereotactic Body Radiation Therapy (SBRT) and Simultaneous Integrated Boost (SIB)]. Additionally, the effect of image guidance (Image Guided Radiation Therapy, IGRT) and motion management systems on dose delivery, target positioning accuracy and reproducibility, warrants the assessment of collective clinical impact of these practices with modulated therapies.
Unlike fixed-field IMRT, VMAT allows simultaneous motion of gantry, MLC and dose rate using dynamic modulated arcs, resulting in increased conformality and enhanced sparing of the critical structures near the target. 7 Techniques of VMAT are diverse and can employ flattening filter free (FFF) beams, standard, tangential (t-VMAT) or restricted angles (R-VMAT). The application of IMRT clinical trial outcomes 8 to VMAT is proof of increased VMAT implementation.
Some authors consider VMAT as a type of IMRT, and since its introduction 4 the initial divide in the literature’s nomenclature has blurred considerably in recent years, particularly with the advent of comparative proton studies.
IMPT utilises proton pencil beams which produce distinct dose distributions when compared to photons due to the characteristic Bragg peak, resulting in maximum dose deposition at a finite tissue depth followed by a sharp dose fall-off with no exit dose. 9 Dosimetric studies suggest there may be advantages in the use of protons over photons with findings of normal tissue sparing and improved target conformity. 9–13
Previously, there have been two review papers on the clinical use of VMAT and its outcomes, one assessing VMAT at the start of its implementation (2000–2010) 14 and the other looking at the clinical outcomes of its implementation (2009–2016). 4 Both noted increases in the global usage and clinical implementation of VMAT with many publications tailored to planning and feasibility studies; however, clinical outcome studies were emerging but scarce and reporting only acute toxicities.
This paper seeks to review the impact of modern radiotherapy techniques and treatment schemes on patient clinical outcomes for seven clinical sites during 2017–2021 and to establish if improved survival and reduced toxicities relate to dosimetric and efficiency gains. In analyzing the available literature on reported clinical outcomes where VMAT has been employed this review seeks to answer the question ‘What is the clinical evidence for the most effective implementation of VMAT?’
Methods
This analysis strictly followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 15 The search engines used were the National Library of Medicine (PubMed/Medline) and The British Journal of Radiology (BJR) database. These identified articles from January 2017 to October 2021 which recorded clinical outcomes post radiotherapy using the keywords “radiotherapy, intensity-modulated” OR “VMAT” OR “arc radiotherapy” AND “treatment outcomes” OR “clinical trials” OR “evidence-based” OR “clinical outcomes.”.
Inclusion and exclusion criteria
Publications were selected for inclusion if they were published within the above timeframe, English language only, full text articles which reported clinical outcomes (survival and toxicities) after modern radiotherapy schemes.
Exclusion criteria included any case reports, comment abstracts, dosimetric only studies, wrong technique (chemotherapy, tomotherapy, carbon ion therapy etc.) and whose main aim does not assess the treatment outcomes of VMAT.
Results
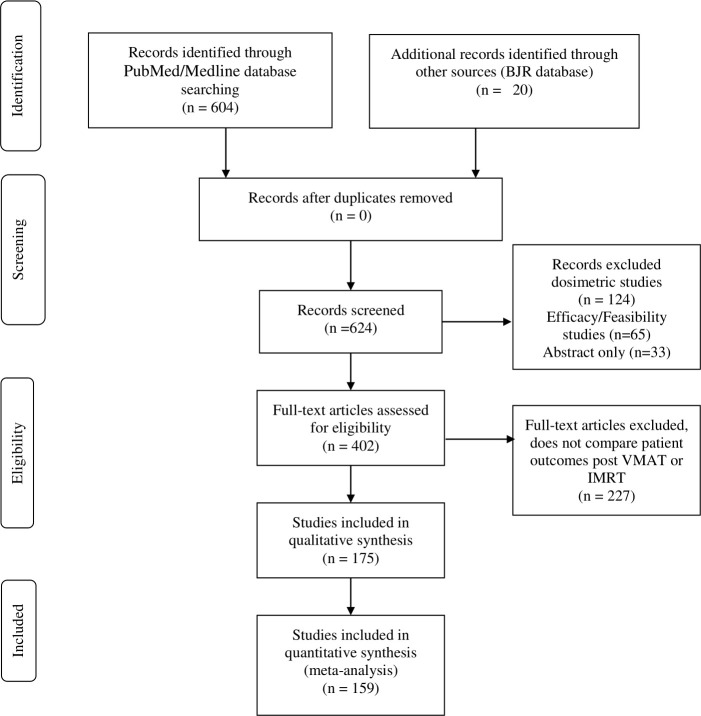
The PubMed search yielded 604 articles while searches through the BJR database identified 20 articles. After title and abstract examination for relevance and removal of publications which are present in the exclusion criteria, 175 publications remained and are included in this review (Figure 1). These assessed papers involved; retrospective studies; prospective studies and review papers.
Figure 1.
PRISMA flow diagram. IMRT, intensity modulated radiation therapy; VMAT, volumetric modulated arc therapy.
Prostate
The management of prostate cancer can utilise radiotherapy (photons, protons, or brachytherapy) surgery, or active surveillance. 16 Advances in photon therapy resulted in extensive publications on dosimetric efficacy for prostate cancer and even led to the establishment of radiotherapy guidelines. The European Association of Urology (EAU) now recommends either dose escalated IMRT or VMAT as standard therapy for prostate carcinoma, due to reduced toxicity compared to 3DCRT. 16 VMAT has been widely accepted and may be considered as first choice for radiotherapy prostate treatments due to significant reduction of rectal volume doses and improved efficiency due to reduction of MUs for some models of treatment units. 17
Clinical implementation and recommendations 16 are currently present without substantial empirical data from well-designed perspective clinical benefit studies. 17 Nonetheless, recently published clinical outcome studies assessed the impact of IMRT and VMAT along with fractionation schemes and escalated doses (SIB, hypofractionation, SBRT) and image guidance techniques (IGRT) on toxicity and survival.
Hypofractionated schemes
Treatment fractionation has several known benefits: repair of sublethal damage to normal tissue, reoxygenation of hypoxic tumour cells, and redistribution of tumour cells to radiosensitive phases of the cell cycle. 18 Hypofractionated radiotherapy delivers larger than 2Gy-Fraction daily doses resulting in fewer total fractions during radiotherapy. 19 For cases of prostate cancer, hypofractionation regimens are guided by the staging. Current recommendations propose ultrahypofractionation for low to intermediate risk and moderate hypofractionation regimens for high risk. 20 The American Society of Radiation Oncology (ASTRO) defines SBRT as “an external beam radiation therapy (EBRT) method used to precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions” 21 and a few studies investigate its use in prostate cancer therapy. 22,23
A recent study 24 comparing hypofractionation (70 Gy in 28 F) vs conventional fractionation (80 Gy in 40 F) utilising VMAT as the treatment technique reported no significant difference in biochemical relapse-free survival (BRFS) between the groups (94.6% vs 95% respectively (p = 0.704)), and therefore support the use of hypofractionated regimes for localised prostate cancer therapy. Another study by Vassis et al 25 also assessed hypofractionation (60 Gy in 20 F) against conventional fractionation (78 Gy in 39 F) utilising VMAT and reported hypofractionated radiotherapy schemes produce no significant difference in freedom from biochemical failure (FFBF) and late toxicities; however, significant reduction in proctitis and urinary frequency was observed. A third study 26 of 206 males treated with step-and shoot IMRT concluded a hypofractionated regimen of 72 Gy in 2.4 Gy fractions increased the biologically effective radiation dose to the prostate, providing better control than conventionally fractionated of 75.6 Gy in 1.8 Gy fractions. Additionally, hypofractionated schemes reduced treatment duration (8.4–6 weeks) and did not correlate to increased late urinary toxicity incidence. 26 A non-significant increase in rectal bleeding was observed, however, all cases resolved with treatment 26 thus concluding that this regime is safe and effective. 27 In fact, in the United Kingdom, hypofractionated radiation therapy (60 Gy in 20 Fractions) has been recommended as the new standard of care for localised prostate cases 8 and these results guide fractionation in VMAT.
In 2017, a publication by Haque et al 28 highlighted the lack of completed Phase III randomised trials comparing outcomes of prostate cancer patients treated with conventional fractionation to SBRT. Though consensus in prescription has not been concluded, studies reported good clinical outcomes for low-risk disease for a SBRT scheme of 35–36.25 Gy in 5 daily fractions 29–32 however, its effectiveness in high or intermediate risk disease is less clear. A study in Philadelphia assessing 263 localised prostate cancer patients found no difference in 5-year FFBF between matched SBRT and conventionally fractionated IMRT groups and no significant difference in toxicity, concluding SBRT can be a suitable alternative treatment for patients with prostate cancer. 22
Dosimetric comparison of proton- and photon-based hypofractionated SBRT by Goddard et al 23 concluded when Hounsfield unit (HU) uncertainties were not addressed, IMPT and VMAT treatment plans were comparable for target coverage, conformity and OAR sparing, with proton-based plans reducing OAR dose more than VMAT. However, when HU uncertainty is considered, VMAT surpasses IMPT in terms of target conformity, and OAR sparing 23 Additionally, a recent clinical outcome study comparing IMRT, and proton beam therapy concluded that no significant difference in biochemical failure, local failure, regional failure and distant failure was seen with these techniques. 33 Therefore, VMAT still has a role in the future of prostate radiotherapy and further prospective studies are required to recommend another treatment modality.
IMRT vs VMAT
A study in 2017 of patients treated with SIB-IMRT and SIB-VMAT to the whole pelvis recorded no significant difference in the rate of acute genitourinary (GU) or gastrointestinal (GI) toxicities and no reported late Grade III toxicity of GI and GU except for rectal toxicity between the two groups. 34 The recommendation was therefore that dose escalation using SIB-IMRT or VMAT with daily CBCT will reduce radiation toxicity to the bladder and rectum. 34 A year later, Tondel et al studied the use of daily CBCT vs weekly orthogonal images on a 250 patient cohort receiving 3DCRT using field-in-field technique. 17 Though Tøndel observed daily CBCT verification significantly reduced rectal irradiation, this gain was not translated into a reduction of acute side-effects. 17 A similar study as Tøndel using VMAT may produce more promising clinical outcomes.
Randomised clinical outcome studies are emerging 35,36 for locally advanced high-risk carcinoma of the prostate and conclude prostate and pelvic lymph node IMRT is safe. Although higher Grade II toxicities are observed when compared with prostate only studies 36 low levels of GI and GU toxicity scores from physician and patient reported assessments were achieved 24 months after treatment. 35 Whilst regional nodal irradiation provides a survival advantage to patients with localised high-risk breast cancer; studies have not concluded whether the same effect is seen in prostate cancer. 35
Owing to the time and financial implications of randomised clinical outcome studies, currently the literature has described IMRT findings more than VMAT. However, VMAT has been used to assess fractionation 25,37,38 and image guidance 34,39 implying that it has been widely adopted as the gold-standard for prostate radiotherapy.
Anorectal
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide 40 and chemoradiotherapy is the current standard of practice 41 owing to works of Ajani et al. 42 Although historically the radiation prescription with 3DCRT was conservative, toxicity incidence was high and often required extended mid-treatment breaks resulting in reduced oncological outcomes and substantial late pelvic radiation morbidity. 43
Current studies advocate for the use of IMRT and VMAT over 3DCRT as there are several benefits: anal sphincter sparing 44 ; increased conformity and homogeneity 45 ; toxicity reduction and improved clinical outcomes (Table 1). 50 In 2017, Muirhead et al assessed the implementation of IMRT (step-and-shoot IMRT, VMAT and tomotherapy) in the UK for anal cancer and concluded that although not universal, these techniques were gaining frequency in the UK. 43 This national audit also observed a small improvement in Grade III/IV toxicity, though statistically insignificant due to the small cohort of patients, incidence of GI (specifically diarrhoea) and haematological Grade III/IV toxicity differed between IMRT and 3DCRT with sequential boost. 43
Table 1.
Toxicity and survival rates for anorectal cancer patients from five retrospective studies
| [ref] | Period of study | N | Prescription | RT technique | Median follow-up | Outcomes- toxicity | Outcomes- Survival |
|---|---|---|---|---|---|---|---|
| 46 | 09/2014– 08/2016 | 11 |
cT2N0 Prescription: 50.4 Gy/28 to PTV 42 Gy/28 to the elective nodal PTV cT3–T4/N0–N3 59.4 Gy/33F- 61.2 Gy/34F to PTV Clinical nodes 50.4 Gy/30F if ≤3 cm or 54 Gy/30F if >3 cm Elective nodal PTV45 Gy/30F |
No comparison- all cases treated with VMAT-two coplanar arcs of 360° and received concurrent chemotherapy MMC (12 mg/m2) |
12 months (6–37 months) |
Skin
G3 (27.3%) G2 45.5% GI G3 18.2% G2 45.5% G3 18.2% (required hospital admission) GU G2 27.3% Haematologic G2 anaemia (18.2%), neutropaenia (27.3%), thrombocytopaenia (27.3%) |
one-year OS 89% 3 year OS 71%; one- and 3 year PFS were both 75%; 2 year CFS was 68% |
| 47 | 02/2011–04/2016 | 21 | Phase I 39.6 Gy, 1.8 Gy/fraction Phase II 14.4 Gy up to a total dose of 54 Gy (n = 4) 19.8 up to 59.4 Gy (n = 15) |
No comparison- all patients were treated with 2 full arc 6 MV VMAT plans | 35.5 months (3–71 months) |
Skin acute G3 (one pt) G2(47.6%); G1(38%); G0 (two pts) GU toxicity G1(47.6%) G0 (52.4%) GI toxicity Acute G3 (one pt) G2 (9.5%) G1(47.6%) G0(42.8%) |
2 year OS 91% 2 year DFS 73% 2 year LC 81% |
| 48 | 09/2007 −03/2015 |
155 | Phase I 36 Gy (1.8 Gy/ Fraction) Phase II 23.4 Gy (1.8 Gy/Fraction) |
IMRT (n = 39) or VMAT (n = 15), or HT (n = 97) Phase II IMRT (n = 16, until 2011), VMAT (n = 17), HT (n = 61) or 3D-conformal EBRT (CRT, n = 61) |
38 months (12–51 months) |
Skin
Acute G3 (22%) No late Grade III cutaneous toxicity GI Toxicity late G3 (3/137 patients (anal incontinence) |
4 year LC 82%; 4 years OS 82%; 4 year CSS was 90% |
| 49 | 05/2006 −01/2015. | 172 | Whole pelvis at a dose of 45 Gy/ 25F |
Image guided IMRT (n = 45) 3DCRT (n = 99) |
53 months (range, 18–95 months) 3DCRT group 43 months (range, 17–69 months) IG-IMRT group. |
Overall acute G3 or G4 toxicity IG-IMRT (8.9%) vs 3DCRT (20.2%) p = 0.042 GI G0-2 IG-IMRT(93.3%) 3DCRT (84.8%) G3/4 IG-IMRT (9.7%) vs 3DCRT (14.1%) p = 0.039 |
4 year OS 81.6% IMRT and 67.9% 3DCRT (p = 0.12 4 year DFS 53.8% IMRT and 51.8% 3DCRT(p = 0.51, 4-yer LFFS 88% IMRT and 75.1% 3DCRT(p = 0.031 4-year- DFFS 64.5% IMRT and 62% 3DCRT (p = 0.61) |
| 50 | 11/2011–11/2013 | 15 | Phase I SIB: 37.5 Gy/25F to PTV1 and 45 Gy/25F to PTV2 T2 disease 54 Gy/5F PTV boost T3–T4 disease 59 Gy/7 F Involved nodes < 5 cm 54 Gy nodes > 5 cm 59 Gy. |
Sliding window IMRT (n = 7) VMAT (n = 8) | 26 months (13–42 months) |
Skin
Acute G3 -Radiation Dermatitis (27%) GI Nausea (1.3%) Diarrhoea (13.3%) Haematologic Neutropaenia G3 (6.6%) |
Three-year CFS and DFS rates were both 86% 3 year OS rate was 88% |
CGS, cancer free survival; CSS, cancer-specific survival; 3DCRT, 3D conformal radiotherapy; DFS, disease free survival.
Terms: ; 3DCRT, 3D conformal radiotherapy; CFS, cancer free survival; CSS, cancer-specific survival; DFS, disease free survival; DFFS, distant failure-free survival; EBRT, external beam radiation therapy; GU, genitourinary; IMRT, intensity modulated radiation therapy; LFFS, local failure–free survival; OS, overall survival; PTV, planning target volume; RT, radiation therapy; VMAT, volumetric modulated arc therapy.
Additional studies reported similar findings to Muirhead et al reporting IMRT 41 and VMAT alongside IGRT 46–49 reduce acute GI and haematological toxicity and increase overall and 5-year DFS. Of note, statistically significant findings reported patients treated with fixed-gantry IMRT delivered with a sliding window technique presented a significantly higher risk of acute Grade III (or more) toxicity compared to those treated with VMAT or helical tomotherapy (38.5% vs 15.3%, p = 0.049). 48 Toxicity and survival rates for anorectal cancer patients from five retrospective trials 46–50 are tabulated in Table 1. These report 1- to 3-year overall survival of approximately 90% is achievable with low G3 toxicity levels. These publications confirmed the safety and efficacy of photon modulated therapies and recommend the adoption of VMAT alongside IGRT as the standard of care for anorectal cancer.
Physician-weighted and patient reported outcomes (PROs) represent a critical aspect of toxicity evaluation. An extension of the UK nationwide study conducted by Gilbert et al reported high overall 1-year oncological outcomes for overall, disease-free and colostomy-free survival consistent with the reported prospective and randomised studies of IMRT in anal cancer. 51 The 1-year PRO toxicity data are consistent with centre reported data and suggest IMRT techniques (VMAT, tomotherapy and static IMRT) reduce bowel toxicity and male sexual dysfunction. Despite the improvement with IMRT techniques acute GI and hematological toxicity 52 should be further reduced to minimise unplanned treatment breaks and hospitalisation. 41 There is a clear need for further optimisation and development of planning techniques to reduce OAR dose 52 combined with randomised prospective studies with extended follow-up to further validate the observations published and determine the durability of the findings. The usefulness of VMAT-SBRT for lymph-node recurrent cases of CRC was also studied by Franzese et al and the efficacy for local disease control confirmed. 53
Planning studies have shown that proton therapy could significantly reduce the dose to the OARs especially pelvic bone marrow; 54 however to date, no published clinical trial is present in the literature, so inferences can only be made from dosimetric outcomes. An ongoing clinical trial will provide answers to the usefulness of proton therapy with CRC in the next 5 years. 12
Gynaecological
Cervical cancer has the second highest incidence among females and is the third leading cause of cancer-related death among females worldwide. 1 Owing to the well-established nature of VMAT and IMRT, current literature uses these techniques to assess fractionation schemes (SIB and hypofractionation) or compare with other techniques such as brachytherapy if not feasible 55 and long-term studies compare IMRT and VMAT to 3DCRT. 56,57
Cases of locally advanced cervical cancer (LACC) respond favourably to VMAT with low haematologic toxicity incidence and promising survival rates (Table 2). 58 Authors even suggest that SIB-VMAT can be an effective treatment technique for irradiation of LACC where brachytherapy cannot be facilitated. 55,62 The combined use of image guidance and VMAT (IG-VMAT) in patients with LACC reported low haematologic toxicity and promising survival rates. 58 Current literature recommends the adoption of hypofractionated schemes, image guidance protocols and simultaneous integrated boost (SIB-VMAT) towards the enhanced management of LACC. 63
Table 2.
Toxicity and survival rates for cervical cancer patients for retrospective and prospective study designs
| [ref] | Study design | Period of study | N | Prescription | RT technique | Median follow-up | Outcomes- Toxicity | Outcomes- Survival |
|---|---|---|---|---|---|---|---|---|
| 58 | Retrospective | 01/2013–12/2014 | 18 | PTV 50.4 Gy in 28 F | VMAT:2 6 MV coplanar mono-isocentric arcs | 30.5 months (IQR: 13–36.25 months). |
Acute haematologic toxicity Haemoglobin G0: 23.5% G1: 17.7% G2: 58.8% TLC G0: 47% G1: 35.5% G2: 17.7% Platelets G0: 88.2% G1: 5.9% G2: 5.9% |
OS 72.2% (95%CI: 62.1–80.5%) DFS 2 year DFS was 63.3% (95% CI: 52.8–72.4%) |
| 59 | Retrospective | 12/2010–05/2017 IMRT: 12/2010–09/2012 VMAT: 05/2013–05/2017 |
398 | Phase I: whole pelvis VMAT or IMRT 45/25F or 50 Gy/28F 25–28 Phase II: Lymph node boost up to 60–70 Gy |
IMRT (n = 67) VMAT (n = 331) |
IMRT 35.07 (range, 4.80–90.37) months VMAT 25.47 (range, 0.93–58.93) months |
VMAT group, the incidence of Grade 3 and four acute anaemia/erythropenia were 3.6 and 0.9%. acute Grade 3 and 4 leukopenia were 8.5 and 0.6% |
IMRT
3 year OS: 76.2% 3 year DFS: 76.4% 3 year LC: 83.1% 3 year DMFS: 86.1% VMAT 3 year OS 80.5% 3 year DFS 65.4% 3 year LC 88.7% 3 year DMFS 78.1%. CCRT vs non-CCRT 3 year OS in the CCRT 84.8vs 65.4% non-CCRT (p = 0.005) |
| 57 | Multicentre randomised control trial | 11/2012–08/2015 | 278 | Phase I 45 Gy/25F or 50.4 Gy/28F |
IMRT- (inverse planning approaches: IMRT, VMAT, and tomotherapy) (n = 129) 3DCRT (Four field box technique) (n = 149) |
Acute effects assessed during treatment |
Physician Reported Outcomes
No Grade 5 AE Grade 3 and 4 AEs: 16.4%(IMRT);11.0% (3DCRT) (p = 0.28) GI AEs Acute Grade 2 GI AEs (IMRT- 26.2% v 3DCRT- 22.1%; p = 0.43). Patient reported outcomes Mean decrease- EPIC urinary summary score (wk3 and 5 compared with baseline) 3 week results IMRT (26.0 [SD, 14.5] v 22.5 [SD, 11.3]; p = 0.04 5 week results IMRT −210.4 [SD, 17.5] v 3DCRT 25.6 [SD, 15.3]; p = 0.03 EPIC urinary score at 5 weeks favoured the IMRT arm (estimate, 24.59; SD, 2.19; p = 0.04) |
Not analyzed |
| 60 | Prospective study | 09/2011–04/2015 | 30 | Macroscopic disease 66 Gy/30F; Pelvis 54 Gy/30F |
SIB-VMAT technique two 3600_ arcs with 6 MV | 32 months (range: 8–50 months) |
Acute GI toxicity
G0- 30%; G1-23%; G2-43% Late GI toxicity G0-70%; G1- 30% Acute Urinary G0-23%; G1-40%; G2-37% Late urinary G0-90%; G1-10% Acute Vaginal G0-7%; G1-63%; G2-23% Late vaginal toxicity G0-77%; G1-23% Acute Rectal toxicity G0-47%; G1-30%; G2-23% Late Rectal toxicity G0-73%; G1-20% Acute Hematologic G0-70%; G1-30% Late Hematologic G0-87%; G1-13% |
3 year OS- 93% 3 year LC 80%, Clinical outcomes by stage (II vs III) 3 year OS Stage II- 100% 3 year OS Stage III- 85% 3 year LC (Stg II) 91% 3 year LC (Stg III) 67% |
| 61 | Retrospective | 01/2007–12/2016 | 123 | 45 Gy/25F or 50.4 Gy/28F | 3DCRT IMRT VMAT Tomotherapy |
32.2 months | Not assessed |
Survival rates < 70 years vs >70 years
3 year OS 63.9vs 50.6% 5 year OS 60.4vs 39.1 % 3 year CSS 68.2vs 70.9% 5 year CSS 78.2vs 82.1 % 3 year LRRFS 82.8vs 82.1% 5 year LRRFS 78.2vs 82.1% 3 year LRFS 85.3vs 82.1% 5 year LRFS 82.6vs 82.1% |
Terms: 3DCRT, 3D conformal radiotherapy; AE, adverse events; CSS, cancer-specific survival; DFS, disease free survival; DMFS, distant metastasis-free survival; EPIC, expanded prostate cancer index composite; IMRT, intensity modulated radiation therapy; LRRFS, locoregional recurrence-free survival; OS, overall survival; PRO-CTCAE, the Patient-Reported Outcomes–Common Terminology Criteria for Adverse Events; TLC, total leukocyte count; VMAT, volumetric modulated arc therapy
A comparative study of IMRT and VMAT by Lin et al 59 observed no significant difference in 3-year survival rates (OS, DFS, LC and DMFS) however, VMAT was superior to IMRT for certain toxicity incidences, including acute anaemia, chronic enterocolitis and higher cystitis, and early-stage (IA-IIA) overall survival rates. Kloop et al 57 compared physician and patient reported outcomes for two cohorts: 3DCRT and IMRT. Klopp defined IMRT as any inverse planning technique and therefore included IMRT, VMAT and tomotherapy in the second group. Findings from this randomised study cannot be conclusive about VMAT since both tomotherapy and IMRT were also used, 57 however, it revealed pelvic IMRT decreased impact on bowel and urinary function and quality of life (QOL) metrics. Therefore, a reduction in the decline of physical function is observed with IMRT, VMAT and tomotherapy compared with standard pelvic radiotherapy.
Though long-term follow-up is still suggested to determine the impact on late toxicity and survival rates, the literature is consistent that VMAT has been well tolerated, safe and effective and its use alongside hypofractionation and image guidance has resulted in tangible clinical evidence of reduced GI and GU toxicity and enhanced QOL.
Breast
3DCRT has been the gold-standard for the treatment of breast carcinoma, however, results from dosimetric and efficiency studies have steered the shift to modern techniques in many centres. Due to the involuntary motion of the lungs during radiotherapy, motion management strategies namely deep inspiration breath-hold (DIBH) is encouraged alongside photon modulated techniques to minimise heart and lung doses. 64 Owing to the increase in 10-year survival rates, it is critical to understand and minimise long-term toxicities and late cardiac events. 65–67 For carcinoma of the breast, this means the investigation of both modulated techniques and motion management systems.
Jagsi et al 68 compared patients treated with 3DCRT on free-breathing scans vs step-and-shoot IMRT on DIBH scans and concluded that IMRT with DIBH has potential benefit to preserve cardiac ejection fraction, among patients with left-sided disease with internal mammary nodal involvement. Another study 69 reported a reduction in mean heart doses and expected years of life lost with DIBH compared to free breathing (FB). A prospective, randomised study by Choi et al 70 compared 3DCRT (50.4 Gy in 28 F followed by 9 Gy in 5 F boost) to SIB-IMRT (50.4 Gy in 28 F to the breast and 57.4 Gy in 28 F to the tumour bed) reported no significant difference in 3 year LRRFS, DMFS, RFS and OS. However, the IMRT cohort experienced a reduction in Grade II or higher radiation dermatitis (27.8% IMRT vs 37.1% 3DCRT) and lower dose to the ipsilateral lung and heart (for LBreast), therefore there is promising data for the use of IMRT for early-stage breast cancer. 70
Literature on the use of VMAT for breast radiotherapy has reported enhanced tumour coverage, increased dose homogeneity and conformity, 71 with one main drawback, the generation of low-dose baths specifically to the contralateral breast, lung and heart which exceed that of 3DCRT. 72 In a study by Ma et al, 73 VMAT was associated with an increase in mean heart dose and low-dose volume to the lung, compared with 3D-CRT, possibly explaining the higher use of tangential IMRT over VMAT.
An estimation of excess absolute risk (EAR) in terms of developing a secondary cancer in three organs [contralateral breast (CB), contralateral lung (CL), ipsilateral lung (IL)] after exposure to radiation was determined by Haciislamoglu et al. 74 They observed a significantly lower EAR risk with field in field (FiF) technique and a statistically lower secondary cancer risk compared with IMRT and VMAT. 74 Additionally, the volume of low dose (3 Gy and 5 Gy) to normal tissue was significantly higher with IMRT and VMAT than FiF. 74 Whole breast hypofractionated VMAT has been studied and implemented in some centres and reports of safety, efficiency and tolerated patient experience has been noted. 75 New techniques such as tangential VMAT (t-VMAT) and tangential IMRT (t-IMRT) has been compared and found while t-VMAT produced higher target homogeneity and conformity, t-IMRT for left-sided breast carcinoma resulted in significant reduction in heart and lung doses and a greater than 40% reduction in heart and lung EAR. 71
The optimal radiation technique to treat breast cancer can vary with patient anatomy and laterality of the breast cancer and size of the treated field. Long-term clinical trials and theoretical estimation studies are required to determine the effects of the low-dose baths seen with VMAT on the healthy tissue and secondary cancer induction. Though some dosimetric promise has been reported with proton therapy (mean heart dose < 1 Gy), 11 the clinical impact on late cardiac toxicity of this and other modern techniques is currently unknown and a minimum of 10 years of follow-up would be required to determine the effect of modern techniques on toxicity and survival. 76
Thoracic neoplasms
Stage I non-small cell lung cancer (NSCLC) can be optimally treated with surgery providing good local control and survival outcome. 77 However, for a significant number of patients, this option is not viable due to comorbidities and as a result receive concurrent chemoradiation therapy (CCRT). Historically, CCRT corresponded to relatively poor outcomes: long-term survival 15–30%; local control 40–50% 77 ; median survival time of 28.7 months. 78 Radiation-induced toxicity, specifically radiation pneumonitis (RP) incidence impacts survival and QOL, 79 and thus is a factor of interest when comparing techniques and treatment efficacy.
In recent years, the use of modern conformal techniques (IMRT, VMAT, IGRT, SBRT) have dramatically changed the treatment capability and expected clinical outcomes for NSCLC (Table 3). 86 Though large variations in treatment plans are seen with planners of different experience levels, 87 studies have reported increased 5-year OS rates and favourable toxicity profile with SBRT using IMRT or VMAT. 77,88 Chi et al 88 assessed the clinical outcomes of following particle beam therapy and SBRT and observed for both techniques incidence of severe toxicity (Grade III-V), chest wall toxicity and RP were low, and no significant difference was seen between techniques in incidence of Grade IV-V toxicity. Additionally, Liao et al 2018 78 reported comparable incidence of RP between IMRT and passive scatter proton therapy (PSPT) and IMRT reported a slightly better, but not significant, overall survival rates (p = 0.297) may be produced with PSPT for NSCLC. Another study observing the effect of IMPT and concurrent chemotherapy on thoracic tumours also observed low toxicity for advanced inoperable cases; however, due to the short follow-up and non-randomised study design concluded that further randomised prospective trials are required to validate and accurately quantify the effect of IMPT use. 82 Although it cannot be concluded that PSPT or SBRT is better for the treatment of NSCLC, we can say with confidence that these are viable options and offer improved outcomes than those seen historically.
Table 3.
Toxicity and survival rates for thoracic neoplasms and NSCLC for retrospective and prospective study designs
| [ref] | Study design | Period of study | N | Prescription- diagnosis and staging | RT technique | Median follow-up | Outcomes- toxicity | Outcomes- survival |
|---|---|---|---|---|---|---|---|---|
| 80 | Retrospective | 09/2015–10/2018 | 27 | 60 Gy/8 F Patient cohort: Central thoracic oligometastases |
SBRT- IMRT or SBRT-VMAT |
11.6 months (IQR 6.5–19.4 months) | No Grade > 3 toxicities Acute Grade II toxicity-3 (11.1%) Grade II dysphagia-one case: 1 month post-SBRT resolved by 3 month Grade II radiation pneumonitis - 2 cases resolved with steroid treatment. Grade 2 late Toxicity - fatigue, One (3.7%) |
1 year IFC 95.2% (95% confidence interval [CI] 86.6–100.0%) 2 year IFC 85.7% (95% CI 68.3–100.0%) 1 year PFS 42.8% (95% CI 26.1–70.1%) 2 year PFS 23.4% (95% CI 9.8–55.9%) 1 year OS 82.7% (95% CI 68.6–99.7%) 2 year OS 69.5% (95% CI 51.0–94.7%) |
| 81 | Retrospective | 03/2011–09/2016 | 134 | 52 Gy/26F or 64 Gy/32F Diagnosis: Stage I-IV NSCLC |
VMAT- 6 MV single arc or two-arc | 18.6 months (range, 2–45 months) |
Radiation pneumonitis
Grade 0–2 (N = 120) Grade 3–4 (N = 14) Fibrosis Grade 0–2 (N = 122) Grade 3–4 (=12) Further toxicity classification seen in Table 2 |
2 year PFS 18.2% median PFS 7.6 months 2 year OS 38.4%, median survival time of 18.6 months |
| 77 | Retrospective | 11/2007-06/2016 | 300 | 48 Gy in 12 Gy × 4 fractions. Diagnosis: Stage I NSCLC T1a: 59% (N = 111) T1b: 30% (N = 57) T2: 11% (N = 21) All patients were N0 and M0. |
2007–2010, Patients treated with (n > 7) coplanar/ non-coplanar beams After 2010, all patients were treated (VMAT) two half arcs |
18 months (IQR: 9–33 months) |
No Grade IV or V toxicity observed Fatigue (41%; N = 77) Chest wall pain (10%; N = 19) Dyspnea (7%; N = 14) Radiation pneumonitis (4%; N = 8, including 2% of Grade 3), Dermatitis(4%;N = 7) Cough (3%;N = 6) Rib fractures (2%; N = 3) Esophagitis (1%; N = 1) |
1 year OS 83% [95% CI; 78–89%] (N = 128) 2 year OS 65% [95% CI: 57–73%](N = 78) 4 years, the OS 37% [95% CI; 29–47%] (N = 53) Median survival 37 months. 1 year DFS 75% [95% CI: 68–81%] (N = 114) 2 year DFS 49% [95% CI: 42–58%] (N = 60) 4 year DFS 31% [95% CI: 24–41%] (N = 41) |
| 6 | Retrospective | 01/2009-03/2017 | 3872 | Diagnosis: Stage III NSCLC | 3DCRT - N = 1178 (30.4%) IMRT N = 1847 (47.7%) VMAT N = 847 (21.9%) |
Not stated | Not assessed | 1 year OS 3CRT Group 74.4% (71.8–76.8); 1 year OS IMRT Group 74.4% (71.8–76.8) 1 year OS VMAT Group 77.5% (74.6–80.2) 5 years OS 3DCRT 22.4% (95% CI, 20.0–25.0) 5 year OS IMRT 23.5% (95% CI, 21.3–25.7) 5 year OS VMAT 23.9% (95% CI, 20.0–28.0) |
| 82 | Non-randomised trial | 2012–2016 | 51 | Not specified newly diagnosed or recurrent Stage II or III NSCLC |
Multifield optimised IMPT plans three or four beams | 23.0 months (range 0.9–60.1 months) | Pneumonitis G1 16% G2 14% Cardiac toxicity G1 6% G2 8% Oesophagitis G1 37% G2 43% G3 6% Radiation dermatitis G1 33% G2 31% G3 6% Pain G1 37% G2 29% Oesophageal stricture G3 2% Fatigue G1 63% G2 27% G3 2% Further analysis refer to Table 4 |
Median OS 33.9 months Median DFS 12.6 months 3 year LC 78.3% 3 year DFFS 51% Further analysis refer to Table 2 |
Terms: 3DCRT, 3D conformal radiotherapy; DFFS, distant failure-free survival; DFS, disease free survival; IFC, in field control; IMRT, intensity modulated radiation therapy; LC, Local control; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RT, radiation therapy; SBRT, stereotactic body radiation therapy; VMAT, volumetric modulated arc therapy.
Retrospective studies 6,77,80,89 record improved survival and reduced RP incidence and severe toxicity with VMAT compared with 3DCRT and even IMRT and report a significant increase in use of VMAT after 2010. 6,77 Radiotherapy management of locally advanced NSCLC has therefore improved with modern photon therapies; however, the survival rates and toxicity incidence can possibly be improved by standardising treatment planning, implementation of new techniques, increased use of motion management systems and assessment of fractionation schemes. As an example, VMAT techniques with FFF beams are potentially advantageous as they employ higher dose rates and jaw-tracking technology which may further increase conformity and OAR sparing. 81 At the very least, it can be concluded that the use of VMAT in NSCLC and other thoracic neoplasms offers no detriment to survival rates historically seen.
Head and neck
Radiotherapy has and continues to play a critical role in the management and treatment regime for head and neck (HNC) cancers. 9 Historically, photon-based techniques dominated HNC treatment; however, there is a growing interest in modulated proton therapy for clinical use. One major drawback of IMPT is its high sensitivity to anatomical changes which is critical in HNC due to inter- and intrafractional motion and target volume changes with high weight loss. 9
| Toxicity profiles for patients with locally advanced head and neck cancer (LAHNC) have improved but remain significant 90 with frequent, though reduced, reports of xerostomia. 91 Sequential (SEQ) boost vs SIB for LAHNC reported comparable survival rates; however, acute toxicity incidence benefited with SEQ-IMRT. 90 LAHNC are susceptible to locoregional recurrence and as such reirradiation was explored. 92 Bahl et al recommended a prescription >46 Gy for inoperable recurrent tumours as < 45 Gy showed a higher incidence of progressive disease ( =0.01). 93 Additionally, QUANTEC guidelines were released 10 years ago with much of the evidence based on conventional 3D conformal therapy. There is therefore a need for validation and prospective studies to guide the optimisation of the treatment plans generated in this era. 94 |
Nasopharyngeal cancer
For nasopharyngeal cancers (NPCs), the advent of IMRT facilitated reduction in dose to OARs and improved target homogeneity. 95 A few randomised controlled trials have assessed the clinical benefit of IMRT compared to 3DCRT. 96–98 Studies comparing IMRT and VMAT in NPC have shown the variation in plan conformity, coverage and homogeneity are marginal. 84 Additionally, clinical outcomes of tumour control, survival, and changes in QOL are comparative between IMRT or VMAT (Table 4). 83–85
Table 4.
Toxicity and survival rates for nasopharyngeal carcinoma for retrospective and prospective study designs
| [ref] | Study design | Period of study | N | Prescription- diagnosis and staging | RT technique | Median follow-up | Outcomes- toxicity | Outcomes- Survival |
|---|---|---|---|---|---|---|---|---|
| 83 | Retrospective | 10/2010-05/2014 | 20 | SIB-VMAT three target volumes: 70 Gy/35F, 63 Gy/35F and 56 Gy/35F SEQ-VMAT two targets: 46 Gy/23F boost field 24 Gy/12F Diagnosis: nasopharyngeal cancer; clinical stage of II, III, IVA, or IVB |
SIB- VMAT And SEQ-VMAT |
All patients:
47 months (range 8–70) f Survivors: 51 months (range 26–70) |
Haematological toxicities
Grade III or higher toxicities Anaemia 4 (20%) Leukocytopaenia 12 (60%) Thrombocytopaenia1 (5%) Elevation of creatinine 1 (5%) Hyponatraemia 9 (45%) Non-haematological toxicities, Grade III or higher toxicities Mucositis 11 (55%) Dysphagia 10 (50%) Dermatitis 8 (40%) |
3 year OS 85% [95% CI 69–100] 3 year PFS rates 65% (95% CI 44–86) 3 year LCR, 78% (95% CI 59–97), 3 year RCR 88% (95% CI 73–100) 3 year DMFR were 79% (95% CI 61–97) |
| 84 | Prospective | 06/2013-08/2015 | 80 | The prescribed doses were as follows: 68–72 Gy to the PGTVnx, 64–68 Gy to the PGTVnd, 60 Gy to the PTV1, and 54–56 Gy to the PTV2, in 30–33 fractions Staging: (i) newly diagnosed cases of primary NPC with pathological confirmation Stage I-IVB |
Single or two-arc VMAT (N = 40) 7–9 field IMRT (N = 40) |
29 months (range,6–48 months) |
Not assessed | 2 year estimated LRFS 100% VMAT and IMRT 2 year RRFS and 2 year LRRFS 97.4% VMAT 100% IMRT 2 year DMFS and DFS 90% VMAT and 95% IMRT 2 year OS rates were similar between two groups, with the 92.4% VMAT and 97.5% IMRT Local failure did not occur in both groups until the end of follow-up |
| 85 | Prospective | 03/2013-12/2015 | 140 | The prescribed dose and fractionation for PTV-H, PTV-M, and PTV-L were 69.96 Gy, 59.40 Gy, and 52.80 Gy in 33 fractions, respectively New diagnosis of non-distant metastasis NPC |
7-field SIB-IMRT (n = 74) Dual arc SIB-VMAT (n = 66) |
IMRT group46 months (range, 2 - 59 months) VMAT group 38 months (range, 12–58 months) |
Not assessed |
Survival rates VMAT vs IMRT
The 3 year LRRFS 96.6% VMAT group and 91.4% IMRT 3 year DMFS 89.4% VMAT; 90.0% IMRT 3 year FFS 86.1% VMAT; 79.8% IMRT 3 year OS 87.4% VMAT 91.3% IMRT (p value > 0.05 Survival rates: N0 vs N2-3 disease 3 year LRRFS DMFS, FFS, and OS rates for N0– one were 96.9, 94.0, 88.8, and 95.7% 82.5%, 82.2%, 76.3%, and 80.5% for N2–3 (all p values < 0.05) Also, larger GTV was observed to be predictive of poorer LRRFS. |
Terms: 3DCRT, 3D conformal radiotherapy; DFFS, distant failure-free survival; DFS, disease free survival; DMFR, distant metastasis free rates; DMFS, distant metastasis-free survival; FFS, failure-free survival; IMRT, intensity modulated radiation therapy; LC, Local control; LCR, local control rates; LRRFS, locoregional relapse-free survival; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RT, radiation therapy; SBRT, stereotactic body radiation therapy; VMAT, volumetric modulated arc therapy.
It has been shown that particle therapy (IMPT) can offer OAR sparing and target conformity with promising initial findings. 99 However, evidence for the translation of these benefits to efficacy and toxicity are limited. It is therefore safe to say despite the enhancement in NPC management in the last 30 years, the burden of long-term toxicities, which impair QOL, is still present. Adaptive radiotherapy, IGRT and the comparison of clinical outcomes (more than 5 years) between particle and photon therapies fueled by PROs is the next step for research. Additionally, specific research geared towards the decrease in cognitive and hearing impairment is necessary to improve QOL of the survivors.
Oropharyngeal cancer
The average 5-year overall survival for oropharyngeal cancers is 65% with traditional radiotherapy techniques. 9 The use of VMAT for oropharyngeal carcinoma has been published and viewed as safe and effective with increasing rates of survival and disease control. 100 Prospective proton therapy studies 101 have shown clinical benefits for oropharyngeal cancer with reduced rates of PEG-tube replacement, acute hospitalisation and narcotic requirements compared to VMAT. Although longer follow- up is needed to determine long-term effects, initial findings show a reduction in acute toxicity and hence improved quality of life. Owing to the sensitivity of proton beams to radiological density changes, the use of on-board imaging to confirm setup and anatomical reproducibility is mandatory 9 alongside long-term prospective studies that can clearly quantify the clinical benefit of IMPT over IMRT and establish recommendations for safe and effective treatment.
Multiple brain metastases
Whole brain radiotherapy (WBRT) was the initial practice of care for multiple brain metastases 102 and single large brain metastases with the latter also employing the use of surgical resection. 91 Currently, there has been a shift from WBRT to SRS where possible as a 12-fold reduction in 1-year local failure is gained with the use of SRS 103 along with increased OAR sparing, improved outcomes, and increased life expectancy. 102
Additionally, WBRT is associated with reduced QOL from decreased neurocognitive function and increased memory loss. A Phase II RTOG 0933 clinical trial 104 proposed employing strategies to avoid the hippocampus during WBRT (HA-WBRT) as a possible approach to mitigate these events. Some practical strategies have been published, 105 which have been employed for cases of diffused metastases where SRS was not permissible. 106
The commercial solutions for delivering SRS are wide ranging employing Cobalt-60 sources (Gamma Knife (GK) unit), photon-based deliveries (CyberKnife (CK)) or a gantry-based linear accelerator system with stereotactic capabilities. 107 Historically, the use of GK platform for radiosurgery was preferred; however, treating more than five metastases with GK burdens staff resources as treatment is associated with long times (1–3 h) especially as the cobalt-60 source decayed. 108 The use of Linac-based SRS employing FFF beams have now been extensively used as its high dose rate is more time efficient (approximately 20 min). 108 VMAT SRS when compared to GK also improved target conformity with no significant difference between 6 MV FFF and 10 MV FFF. However, gradient index may be a more relevant parameter to study and one downfall is the increased low-dose baths compared to GK for which the clinical significance is currently unknown 108
Glioblastoma multiforme (GBM)
Published literature has illustrated the dosimetric and efficiency advantages of VMAT to IMRT for GBM management in treatment time reduction, dose reduction to the brain stem, hippocampi, optic chiasm and cochleae and improved target coverage and conformity, 109–111 therefore explaining the increased implementation of VMAT over IMRT without clinical outcome data. Sheu et al was the first to assess the clinical benefit of VMAT for GBM and deduce if dosimetric advantages translated to clinical outcomes. 108 Toxicity was assessed and recorded weekly and an MRI with contrast taken 1-month post-RT.
No significant difference was observed in median OS (18.4 months IMRT vs 22 months VMAT: p = 0.33) and dermatological toxicities (81% alopecia; 58% erythema); however, fatigue (57%) and headaches (20%) were reported by both groups with no difference in toxicity incidence as well. Sheu et al concluded that care should be used in correlating dosimetric gain to clinical effects and centres should understand new techniques before adoption. 108
Another study assessed the impact of chemoradiation using VMAT on survival and disease progression or tumour failure at the contralateral hippocampus (cHC) for 82 patients with GBM over 4 years (2014–2018). 112 The median follow-up for survivors was 11.7 months (range, 3.6–39.1) with a median OS of 23.5 months (95% CI: 18.4–28.7 months) and median PFS of 9.7 months (95% CI, 7.9–11.5 months). 6- and 12-month cHC failure-free rates were high at 98.7 and 97.2% respectively and overall tumour-failure at the cHC was low with 7.3% observed at the cHC and 9.8% failure observed at a 1-cm margin to the cHC. 83 Wee et al therefore concluded that chemoradiation using HA-VMAT produced low incidence of cHC- and cHC + 1 cm-failure, and therefore can be safe in newly diagnosed cases of GBM once this technique does not impair target coverage. 112
Although there are several dosimetric studies of the potential impact of proton therapy for GBM, a recent publication 113 observed that although the radiation exposure to normal tissue responsible for cognitive function was significantly less with proton therapy this did not translate to improved cognitive outcomes.
Oesophageal
The use of radiotherapy for both resectable and unresectable oesophageal cancer is well understood and is effective and essential to its management. 114 Numerous planning studies highlighted the superiority of IMRT over 3DCRT; however, the question of association between dosimetric gain and clinical effects remain. Xu et al reported a significant reduction in survival for patients treated with 3DCRT (p = 0.007) when compared to IMRT; however, the two techniques produced similar incidence of radiation pneumonitis and radiation oesophagitis. 114 Another study highlighted by Gwynne et al 115 assessed long-term clinical outcomes of patients treated with 3D-CRT (n = 413) and IMRT (n = 263) and reported a statistically significant increase in the risk of dying and of locoregional recurrence with 3DCRT compared to IMRT (72.6% vs 52.9%, p < 0.0001; p = 0.0038 respectively).
Cone beam CT (CBCT) has been proven to be reliable in pre-treatment target verification and greater setup reproducibility in the treatment of oesophageal and gastrooesophageal cancers 115,116 and has since been mandated in the UK’s NeoSCOPE/SCOPE two trial for IGRT usage. Initially, due to concerns of the effect of the low-dose baths from IMRT and VMAT NeoScope did not allow these techniques in their earlier trials, however, owing to the benefits seen with these techniques they have been mandated to be used in the current SCOPE two trial. 117,118
Zhao et al 13,119 reported both the favourability of reduced toxicity (less than Grade IV) with modern techniques and concern of high LR failure. Dosimetric studies point to proton therapy for clinical improvement, 120 however, multicentre, long-term (greater than 5 years) prospective randomised trials aimed at technique standardisation, effective comparison of the various techniques and ultimately recommendations are needed. 114
Radiotherapy and pregnancy
Malignant tumours occur in 1:1000 pregnancies 121 with breast cancer followed by gynaecological malignancies and lymphomas being the most diagnosed tumours in pregnant females. 122 Radiotherapy during pregnancy, though not impossible, require careful considerations based on tumour location and gestational age as the foetal effects are vast: abortion, foetal death, microcephaly, and foetal malformations. 123 Modern photon modulated techniques require specific considerations and multidisciplinary input owing to the creation of low dose-baths by IMRT or VMAT approaches and use of kV-generated images for IGRT. 122
Breast radiotherapy in pregnancy
Intraoperative radiotherapy (IORT) 124 has been identified as a satisfactory boost to the tumour bed in carcinoma of the breast. However, treatment of breast cancer with radiation poses a challenge due to the proximity of the foetus to the tumour bed. Hence, international consensus supports a gestation stage-based treatment approach for breast cancer and recommends possible post-ponement of near-term patients more than 37 weeks of gestation where the treatment can be post-poned until post-partum in near-term patients at more than 37 weeks of gestation. 122
In 2011, the Italian European Institute of Oncology treated the first pregnant patient with electron beam intraoperative radiotherapy (ELIOT) at week 15 of gestation and an estimated dose to the foetus was 0.84 mGy. These results suggest that ELIOT can therefore be considered as a treatment option for anticipated boost therapy during the first and second trimester of pregnancy and whole breast radiotherapy post-poned until after childbirth. 122 The inclusion of a multidisciplinary team goes without saying and as much as possible treatment should follow established guidelines for non-pregnant patients. 125
Pelvic radiotherapy in pregnancy
Spontaneous abortion has been observed within 3–6 weeks of pelvic RT. 122 Cases of cervical cancer diagnosed after the 20th week of gestation; a treatment delay can be considered in the interest of the foetus without a significant effect on the prognosis. 122 No international consensus or recommendations have been published for pelvic RT during pregnancy as the risk of foetal defects and abortion with radiotherapy is significant. 122
Lymphoma and pregnancy
Evens et al 126 investigated the effects of chemotherapy, RT or a combination of both in Hodgkin’s lymphoma and non-Hodgkin’s lymphoma in a series of 90 pregnant females. Four cases (4.4%) utilised radiotherapy with Stage I and IIA diagnoses and dose prescription of 25–30 Gy. No spontaneous abortions, neonatal intensive care unit admission or malformations were reported therefore radiotherapy in pregnant patients with lymphoma may be feasible and modern radiotherapy techniques can be explored.
Oral cancer and pregnancy
The incidence of oral cancer during pregnancy is less than 2% and the treatments are as follows: surgery (56.4%), chemotherapy (12.8%), radiotherapy (28.2%), no treatment during pregnancy (23.1%). 120 Like other clinical sites, no clear guidelines exist for the treatment of oral cancer during pregnancy 120,127 Treatment strategies should involve careful investigation of patients staging and social history. 128
Takahashi et al 120 studied a 36-year old tongue cancer patient treated during pregnancy using FFF-VMAT technique. Dosimetric comparison between tomotherapy, single arc VMAT and FFF-VMAT showed significant out of field doses due to scatter from the flattening filter. FFF-VMAT attained the lowest simulated foetal dose with phantom study and was therefore selected with the following prescription: involved nodes - 66 Gy in 33 F, tumour bed and ipsilateral neck - 60 Gy in 33 F; contralateral neck - 54 Gy in 33 F. The actual foetal dose was measured using in vivo dosimetry and calculated to be 30 mGy and a baby was born healthy at 37 weeks.
Brainstem gliomas
Brainstem gliomas though rare (approximately 2% of adult gliomas) occur more in younger adults, 128 and hence must be studied when evaluating the suitability of radiotherapy during pregnancy. Brainstem gliomas are associated with high maternal mortality, 128 therefore treatment with surgery or radiotherapy should not be delayed. Despite the high mortality, Rosen et al observed 128 some favourable pregnancy outcomes and, concluded that the most optimum treatment plan can be determined through in vivo monitoring and phantom estimation studies.
Oropharyngeal cancer in pregnancy
Pineda et al observed the effectiveness of 6 MV IMRT on a patient with oropharyngeal cancer treated during pregnancy and concluded that in this case radiotherapy alone provided good local control to the patient and did not result in any foetal abnormalities at birth or 18 month post-delivery. 129 This is a promising outcome; however, care must be taken for radiotherapy delivery during pregnancy.
Conclusion
VMAT has been widely adopted throughout many centres and many clinical sites. Proof of this adoption can be seen in its use to evaluate the effectiveness of other techniques (chemotherapy regimens, SIB, FFF, SBRT, IGRT and hypofractionation) 37–39,130–133 and the application of outcomes in dose fractionation gained through static IMRT studies seamlessly applied to VMAT.
VMAT has surpassed 3DCRT in most sites and has been proven to be more efficient while providing increased OAR sparing, reduced toxicity, and improved survival rates. As such, authors as well as some multicentre clinical trials 117,118 recommend the adoption of VMAT for treatment of prostate and gynaecological carcinomas as well as thoracic neoplasms—specifically NSCLC—and head and neck applications (SRS, HA-WBRT, nasopharyngeal, oropharyngeal, oesophageal and gastroesophageal carcinomas). Long survival sites such as breast have not ruled out 3DCRT through field-in-field techniques and advocate for selection of radiation technique based on patient anatomy and laterality of the breast cancer and size of the treated field. The concern of the low-dose baths seen in VMAT on healthy tissue and secondary cancer induction is especially important for breast radiotherapy and when addressed may steer the shift to VMAT from the mainstay of 3DCRT.
Although authors have and continue to report many improvements with VMAT, most study designs are retrospective in nature and assess small patient cohorts. Additionally, toxicity and survival has not fallen as theoretically expected in some cases namely thoracic neoplasms and nasopharyngeal carcinomas, therefore requiring focus into standardising treatment planning and further assessment of fractionation schemes. There is a need for more prospective, multicentre, long-term, randomised clinical trials with large patient cohorts to accurately answer our research question.
Prospective randomised clinical trials are expensive, lengthy and may have small study groups, however, the use of pooled real-world data in a prospective nature can be an alternative solution to comprehensively assess the efficacy of modern radiotherapy techniques. 91 Collaborations between the European Organisation of Research and Treatment of Cancer and the European Society for Radiotherapy and Oncology) has produced the - E²-RADIatE (EORTC 1811 study) platform designed to collect real-world data through prospective data registries in radiotherapy. 134 This and other initiatives like this may be the direction for definitive evaluation of techniques efficacy and their impact on toxicity and survival.
Footnotes
Acknowledgment: PhD research partially funded by IAEA Doctoral CRP E24022
Contributor Information
Sherisse Ornella Hunte, Email: sherisse.hunte@outlook.com.
Catharine H Clark, Email: catharine.clark@nhs.net.
Nikolay Zyuzikov, Email: nikolay.zyuzikov@sta.uwi.edu.
Andrew Nisbet, Email: andrew.nisbet@ucl.ac.uk.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. doi: 10.3322/canjclin.55.2.74 [DOI] [PubMed] [Google Scholar]
- 3. Gianfaldoni S, Gianfaldoni R, Wollina U, Lotti J, Tchernev G, Lotti T. An overview on radiotherapy: from its history to its current applications in dermatology. Open Access Maced J Med Sci 2017; 5: 521–25. doi: 10.3889/oamjms.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macchia G, Deodato F, Cilla S, Cammelli S, Guido A, Ferioli M, et al. Volumetric modulated arc therapy for treatment of solid tumors: current insights. Onco Targets Ther 2017; 10: 3755–72. doi: 10.2147/OTT.S113119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber J, Vial P, White P, Menzies N, Deshpande S, Bromley R, et al. A survey of modulated radiotherapy use in australia & new zealand in 2015. Australas Phys Eng Sci Med 2017; 40: 811–22. doi: 10.1007/s13246-017-0590-y [DOI] [PubMed] [Google Scholar]
- 6. Peng J, Pond G, Donovan E, Ellis PM, Swaminath A. A comparison of radiation techniques in patients treated with concurrent chemoradiation for stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2020; 106: 985–92: S0360-3016(19)34545-6. 10.1016/j.ijrobp.2019.12.027 [DOI] [PubMed] [Google Scholar]
- 7. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008; 35: 310–17. doi: 10.1118/1.2818738 [DOI] [PubMed] [Google Scholar]
- 8. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 chhip trial. Lancet Oncol 2016; 17: 1047–60. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreno AC, Frank SJ, Garden AS, Rosenthal DI, Fuller CD, Gunn GB, et al. Intensity modulated proton therapy (IMPT) - the future of IMRT for head and neck cancer. Oral Oncol 2019; 88: 66–74. doi: 10.1016/j.oraloncology.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H, Zeng J, Bowen SR, Rengan R. Proton therapy for malignant pleural mesothelioma: A three case series describing the clinical and dosimetric advantages of proton-based therapy. Cureus 2017; 9: e1705. doi: 10.7759/cureus.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley JA, Dagan R, Ho MW, Rutenberg M, Morris CG, Li Z, et al. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast cancer compared with photons. Int J Radiat Oncol Biol Phys 2016; 95: 411–21. doi: 10.1016/j.ijrobp.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 12. Jordan K. Proton Therapy in Reducing Toxicity in Anal Cancer. Available from: https://clinical trials.gov/ct2/show/NCT03018418
- 13. Xi M, Lin SH. Recent advances in intensity modulated radiotherapy and proton therapy for esophageal cancer. Expert Rev Anticancer Ther 2017; 17: 635–46. doi: 10.1080/14737140.2017.1331130 [DOI] [PubMed] [Google Scholar]
- 14. Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 2011; 84: 967–96. doi: 10.1259/bjr/22373346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–29. doi: 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 17. Tøndel H, Lund J-Å, Lydersen S, Wanderås AD, Aksnessæther B, Jensen CA, et al. Radiotherapy for prostate cancer – does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? results from a randomized controlled trial. Radiotherapy and Oncology 2018; 126: 229–35. doi: 10.1016/j.radonc.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 18. Avkshtol V, Dong Y, Hayes SB, Hallman MA, Price RA, Sobczak ML, et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Res Rep Urol 2016; 8: 145–58. doi: 10.2147/RRU.S58262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Houtte PV. IASLC Thoracic Oncology. 2nd edn. Elsevier Inc; 2018. [Google Scholar]
- 20. Fransson P, Nilsson P, Gunnlaugsson A, Beckman L, Tavelin B, Norman D, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol 2021; 22: 235–45. doi: 10.1016/S1470-2045(20)30581-7 [DOI] [PubMed] [Google Scholar]
- 21. Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, et al. American society for therapeutic radiology and oncology (ASTRO) and american college of radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: 326–32. doi: 10.1016/j.ijrobp.2009.09.042 [DOI] [PubMed] [Google Scholar]
- 22. Oliai C, Bernetich M, Brady L, Yang J, Hanlon A, Lamond J, et al. Propensity score matched comparison of SBRT versus IMRT for the treatment of localized prostate cancer. J Radiat Oncol 2016; 5: 187–95. 10.1007/s13566-015-0237-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goddard LC, Brodin NP, Bodner WR, Garg MK, Tomé WA. Comparing photon and proton-based hypofractioned sbrt for prostate cancer accounting for robustness and realistic treatment deliverability. Br J Radiol 2018; 91(1085): 20180010. 10.1259/bjr.20180010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong Q-Z, Xia X, Gao H, Xu Y-G, Zhao T, Wu Q-H, et al. Hypofractionated versus conventionally fractionated image-guided volumetric-modulated arc radiotherapy for localized prostate cancer: a phase II randomized trial from china. Aging (Albany NY) 2021; 13: 6936–44. doi: 10.18632/aging.202551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vassis S, Nöldeke B, Christiansen H, von Klot CA, Merten R. Moderately HRT vs. CRT for localized prostate cancer using image-guided VMAT with SIB: evaluation of acute and late toxicities. Strahlenther Onkol 2020; 196: 598–607. doi: 10.1007/s00066-020-01589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffman KE, Voong KR, Levy LB, Allen PK, Choi S, Schlembach PJ, et al. Randomized trial OF hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol 2018; 36: 2943–49. doi: 10.1200/JCO.2018.77.9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faria S, Ruo R, Perna M, Cury F, Duclos M, Sarshoghi A, et al. Long-term results of moderate hypofractionation to prostate and pelvic nodes plus androgen suppression in high-risk prostate cancer. Pract Radiat Oncol 2020; 10: e514-20. doi: 10.1016/j.prro.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 28. Haque W, Butler EB, Teh BS. Stereotactic body radiation therapy for prostate cancer-a review. Chin Clin Oncol 2017; 6: S10. doi: 10.21037/cco.2017.06.05 [DOI] [PubMed] [Google Scholar]
- 29. Rucinska M, Kieszkowska-Grudny A, Nawrocki S. SHARP hypofractionated stereotactic radiotherapy is well tolerated in prostate cancer : toxicity and quality of life assessment. Strahlenther Onkol 2016; 192: 449–57. doi: 10.1007/s00066-016-0971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hannan R, Tumati V, Xie X-J, Cho LC, Kavanagh BD, Brindle J, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-results from a multi-institutional clinical trial. Eur J Cancer 2016; 59: 142–51. doi: 10.1016/j.ejca.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 31. Kim HJ, Phak JH, Kim WC. Hypofractionated stereotactic body radiotherapy in low- and intermediate-risk prostate carcinoma. Radiat Oncol J 2016; 34: 260–64. doi: 10.3857/roj.2015.01571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera FG, Valerio M, Berthold D, Tawadros T, Meuwly J-Y, Vallet V, et al. 50-gy stereotactic body radiation therapy to the dominant intraprostatic nodule: results from a phase 1a/b trial. International Journal of Radiation Oncology*Biology*Physics 2019; 103: 320–34. 10.1016/j.ijrobp.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 33. Barsky AR, Carmona R, Verma V, Santos PMG, Both S, Bekelman JE, et al. Comparative analysis of 5-year clinical outcomes and patterns of failure of proton beam therapy versus intensity modulated radiation therapy for prostate cancer in the postoperative setting. Pract Radiat Oncol 2021; 11: e195-202. doi: 10.1016/j.prro.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 34. Daoud MA, Aboelnaga EM, Alashry MS, Fathy S, Aletreby MA. Clinical outcome and toxicity evaluation of simultaneous integrated boost pelvic IMRT/VMAT at different dose levels combined with androgen deprivation therapy in prostate cancer patients. Onco Targets Ther 2017; 10: 4981–88. doi: 10.2147/OTT.S141224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reis Ferreira M, Khan A, Thomas K, Truelove L, McNair H, Gao A, et al. Phase 1/2 dose-escalation study of the use of intensity modulated radiation therapy to treat the prostate and pelvic nodes in patients with prostate cancer. Int J Radiat Oncol Biol Phys 2017; 99: 1234–42. doi: 10.1016/j.ijrobp.2017.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dearnaley D, Griffin CL, Lewis R, Mayles P, Mayles H, Naismith OF, et al. Toxicity and patient-reported outcomes of a phase 2 randomized trial of prostate and pelvic lymph node versus prostate only radiotherapy in advanced localised prostate cancer (PIVOTAL). Int J Radiat Oncol Biol Phys 2019; 103: 605–17. doi: 10.1016/j.ijrobp.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruner DW, Pugh SL, Lee WR, Hall WA, Dignam JJ, Low D, et al. Quality of life in patients with low-risk prostate cancer treated with hypofractionated vs conventional radiotherapy: a phase 3 randomized clinical trial. JAMA Oncol 2019; 5: 664–70. doi: 10.1001/jamaoncol.2018.6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lieng H, Pintilie M, Bayley A, Berlin A, Bristow R, Chung P, et al. Long-term outcomes of a phase II trial of moderate hypofractionated image-guided intensity modulated radiotherapy (IG-IMRT) for localized prostate cancer. Radiother Oncol 2017; 122: 93–98. doi: 10.1016/j.radonc.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 39. Nakamura K, Mizowaki T, Inokuchi H, Ikeda I, Inoue T, Kamba T, et al. Decreased acute toxicities of intensity-modulated radiation therapy for localized prostate cancer with prostate-based versus bone-based image guidance. Int J Clin Oncol 2018; 23: 158–64. doi: 10.1007/s10147-017-1174-2 [DOI] [PubMed] [Google Scholar]
- 40. Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020; 70: 313. 10.3322/caac.21609 [DOI] [PubMed] [Google Scholar]
- 41. Joseph K, Warkentin H, Mulder K, Doll C. Minimizing hematological toxicity in the management of anal cancer patients. Expert Rev Qual Life Cancer Care 2018; 3: 27–33. doi: 10.1080/23809000.2018.1438845 [DOI] [Google Scholar]
- 42. Ajani JA, Winter KA, Gunderson LL. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal. JAMA 2008; 299: 1914. 10.1001/jama.299.16.1914 [DOI] [PubMed] [Google Scholar]
- 43. Muirhead R, Drinkwater K, O’Cathail SM, Adams R, Glynne-Jones R, Harrison M, et al. Initial results from the royal college of radiologists’ UK national audit of anal cancer radiotherapy 2015. Clinical Oncology 2017; 29: 188–97. doi: 10.1016/j.clon.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dapper H, Rodríguez I, Münch S, Peeken JC, Borm K, Combs SE, et al. Impact of VMAT-IMRT compared to 3D conformal radiotherapy on anal sphincter dose distribution in neoadjuvant chemoradiation of rectal cancer. Radiat Oncol 2018; 13: 237. doi: 10.1186/s13014-018-1187-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin J-C, Tsai J-T, Chen L-J, Li M-H, Liu W-H. Compared planning dosimetry of TOMO, VMAT and IMRT in rectal cancer with different simulated positions. Oncotarget 2017; 8: 42020–29. 10.18632/oncotarget.14923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aboelnaga EM, Fathy S, Daoud MA. Simultaneous integrated boost by volumetric modulated arc therapy (VMAT) with concurrent mitomycin and capecitabine in anal canal carcinoma. Original Article Middle East Journal of Cancer 2019; 10: 183–93. [Google Scholar]
- 47. Yordanov K, Cima S, Richetti A, Pesce G, Martucci F, Azinwi NC, et al. Concurrent chemoradiation with volumetric modulated arc therapy of patients treated for anal cancer-acute toxicity and treatment outcome. J Gastrointest Oncol 2017; 8: 361–67. doi: 10.21037/jgo.2017.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Bari B, Lestrade L, Franzetti-Pellanda A, Jumeau R, Biggiogero M, Kountouri M, et al. Modern intensity-modulated radiotherapy with image guidance allows low toxicity rates and good local control in chemoradiotherapy for anal cancer patients. J Cancer Res Clin Oncol 2018; 144: 781–89. doi: 10.1007/s00432-018-2608-6 [DOI] [PubMed] [Google Scholar]
- 49. Huang C-M, Huang M-Y, Tsai H-L, Huang C-W, Ma C-J, Lin C-H, et al. A retrospective comparison of outcome and toxicity of preoperative image-guided intensity-modulated radiotherapy versus conventional pelvic radiotherapy for locally advanced rectal carcinoma. J Radiat Res 2017; 58: 247–59. doi: 10.1093/jrr/rrw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yucel S, Kadioglu H, Gural Z, Akgun Z, Saglam EK. Outcomes of patients with anal cancer treated with volumetric-modulated arc therapy or intensity-modulated radiotherapy and concurrent chemotherapy. J Cancer Res Ther 2021; 17: 51–55. doi: 10.4103/jcrt.JCRT_774_16 [DOI] [PubMed] [Google Scholar]
- 51. Gilbert A, Drinkwater K, McParland L, Adams R, Glynne-Jones R, Harrison M, et al. UK national cohort of anal cancer treated with intensity-modulated radiotherapy: one-year oncological and patient-reported outcomes. Eur J Cancer 2020; 128: 7–16. doi: 10.1016/j.ejca.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 52. Kronborg C, Serup-Hansen E, Lefevre A, Wilken EE, Petersen JB, Hansen J, et al. Prospective evaluation of acute toxicity and patient reported outcomes in anal cancer and plan optimization. Radiother Oncol 2018; 128: 375–79. doi: 10.1016/j.radonc.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 53. Franzese C, Fogliata A, Clerici E, Franceschini D, Villa E, D’Agostino G, et al. Toxicity profile and early clinical outcome for advanced head and neck cancer patients treated with simultaneous integrated boost and volumetric modulated arc therapy. Radiat Oncol 2015; 10: 224. doi: 10.1186/s13014-015-0535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ojerholm E, Kirk ML, Thompson RF, Zhai H, Metz JM, Both S, et al. Pencil-beam scanning proton therapy for anal cancer: a dosimetric comparison with intensity-modulated radiotherapy. Acta Oncol 2015; 54: 1209–17. doi: 10.3109/0284186X.2014.1002570 [DOI] [PubMed] [Google Scholar]
- 55. Sukhikh ES, Sukhikh LG, Lushnikova PA, Tatarchenko MA, Abdelrahman AR. Dosimetric and radiobiological comparison of simultaneous integrated boost and sequential boost of locally advanced cervical cancer. Phys Med 2020; 73: 83–88. doi: 10.1016/j.ejmp.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 56. Du X, Tao J, Sheng X, Lu C, Yu H, Wang C, et al. Intensity-modulated radiation therapy for advanced cervical cancer: A comparison of dosimetric and clinical outcomes with conventional radiotherapy. Gynecol Oncol 2012; 125: 151–57. 10.1016/j.ygyno.2011.12.432 [DOI] [PubMed] [Google Scholar]
- 57. Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG ONCOLOGY-RTOG 1203. J Clin Oncol 2018; 36: 2538–44. doi: 10.1200/JCO.2017.77.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahmad I, Chufal KS, Bashir I, Bhatt CP, Bajpai R, Sharma L, et al. Early clinical outcomes, patterns of failure, and acute haematologic toxicity of image-guided volumetric modulated arc therapy (IG-VMAT) in the definitive treatment of locally advanced carcinoma cervix. Clin Med Insights Oncol 2018; 12: 1179554918783990. doi: 10.1177/1179554918783990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin Y, Ouyang Y, Chen K, Lu Z, Liu Y, Cao X. Clinical outcomes of volumetric modulated arc therapy following intracavitary/interstitial brachytherapy in cervical cancer: A single institution retrospective experience. Front Oncol 2019; 9: 760. doi: 10.3389/fonc.2019.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mazzola R, Ricchetti F, Fiorentino A, Levra NG, Fersino S, Di Paola G, et al. Weekly cisplatin and volumetric-modulated arc therapy with simultaneous integrated boost for radical treatment of advanced cervical cancer in elderly patients: feasibility and clinical preliminary results. Technol Cancer Res Treat 2017; 16: 310–15. doi: 10.1177/1533034616655055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hou P, Hsieh C, Wei M, Hsiao S, Shueng P. Differences in treatment outcomes and prognosis between elderly and younger patients receiving definitive radiotherapy for cervical cancer. IJERPH 2020; 17: 4510. doi: 10.3390/ijerph17124510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho B. Intensity-modulated radiation therapy: A review with A physics perspective. Radiat Oncol J 2018; 36: 1–10. doi: 10.3857/roj.2018.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chino J, Annunziata CM, Beriwal S, Bradfield L, Erickson BA, Fields EC, et al. Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol 2020; 10: 220–34. doi: 10.1016/j.prro.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol 2018; 8. doi: 10.3389/fonc.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Janssen S, Rades D, Meyer A, Fahlbusch FB, Wildfang I, Meier A, et al. Local recurrence of breast cancer: conventionally fractionated partial external beam re-irradiation with curative intention. Strahlenther Onkol 2018; 194: 806–14. doi: 10.1007/s00066-018-1315-1 [DOI] [PubMed] [Google Scholar]
- 66. Janssen S, Käsmann L, Fahlbusch FB, Rades D, Vordermark D. Side effects of radiotherapy in breast cancer patients. Strahlenther Onkol 2017; 194: 136–42. 10.1007/s00066-017-1197-7 [DOI] [PubMed] [Google Scholar]
- 67. Duma M-N, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R, et al. Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the german society of radiation oncology (degro). Strahlenther Onkol 2019; 195: 861–71. doi: 10.1007/s00066-019-01495-w [DOI] [PubMed] [Google Scholar]
- 68. Jagsi R, Griffith KA, Moran JM, Ficaro E, Marsh R, Dess RT, et al. A randomized comparison of radiation therapy techniques in the management of node-positive breast cancer: primary outcomes analysis. Int J Radiat Oncol Biol Phys 2018; 101: 1149–58. doi: 10.1016/j.ijrobp.2018.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simonetto C, Eidemüller M, Gaasch A, Pazos M, Schönecker S, Reitz D, et al. Does deep inspiration breath-hold prolong life? individual risk estimates of ischaemic heart disease after breast cancer radiotherapy. Radiother Oncol 2019; 131: 202–7. doi: 10.1016/j.radonc.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 70. Choi KH, Ahn SJ, Jeong JU, Yu M, Kim JH, Jeong BK, et al. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: A randomized clinical trial of KROG 15-03. Radiother Oncol 2021; 154: 179–86. doi: 10.1016/j.radonc.2020.09.043 [DOI] [PubMed] [Google Scholar]
- 71. Karpf D, Sakka M, Metzger M, Grabenbauer GG. Left breast irradiation with tangential intensity modulated radiotherapy (t-IMRT) versus tangential volumetric modulated arc therapy (t-VMAT): trade-offs between secondary cancer induction risk and optimal target coverage. Radiat Oncol 2019; 14: 156. doi: 10.1186/s13014-019-1363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Byrne M, Archibald-Heeren B, Hu Y, Fong A, Chong L, Teh A. Comparison of semiautomated tangential VMAT with 3DCRT for breast or chest wall and regional nodes. J Appl Clin Med Phys 2018; 19: 684–93. doi: 10.1002/acm2.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ma C, Zhang W, Lu J, Wu L, Wu F, Huang B, et al. Dosimetric comparison and evaluation of three radiotherapy techniques for use after modified radical mastectomy for locally advanced left-sided breast cancer. Sci Rep 2015; 5: 12274. 10.1038/srep12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haciislamoglu E, Cinar Y, Gurcan F, Canyilmaz E, Gungor G, Yoney A. Secondary cancer risk after whole-breast radiation therapy: field-in-field versus intensity modulated radiation therapy versus volumetric modulated arc therapy. BJR 2019; 92: 20190317. 10.1259/bjr.20190317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fiorentino A, Gregucci F, Mazzola R, Figlia V, Ricchetti F, Sicignano G, et al. Intensity-modulated radiotherapy and hypofractionated volumetric modulated arc therapy for elderly patients with breast cancer: comparison of acute and late toxicities. Radiol Med 2019; 124: 309–14. doi: 10.1007/s11547-018-0976-2 [DOI] [PubMed] [Google Scholar]
- 76. Bradley JA, Mendenhall NP. Annual Review of Medicine Novel Radiotherapy Techniques for Breast Cancer. 2017. Available from: 10.1146/annurev-med-042716 [DOI] [PubMed]
- 77. Dubaere E, Goffaux M, Wanet M, Bihin B, Gheldof C, Demoulin A-S, et al. Long term outcome after 48 gy stereotactic ablative body radiotherapy for peripheral stage i non-small cell lung cancer. BMC Cancer 2019; 19(1): 639. doi: 10.1186/s12885-019-5863-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liao Z, Lee JJ, Komaki R, Gomez DR, O’reilly MS, v FF, et al. Advanced non-small-cell lung cancer. J Clin Oncol 2018; 36: 1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Farr KP, Khalil AA, Grau C. Patient-reported lung symptoms and quality of life before and after radiation therapy for non-small cell lung cancer: correlation with radiation pneumonitis and functional imaging. Acta Oncol 2019; 58: 1523–27. doi: 10.1080/0284186X.2019.1634835 [DOI] [PubMed] [Google Scholar]
- 80. Cooke R, Camilleri P, Chu K-Y, O’Cathail SM, Robinson M, Van Den Heuvel F, et al. Stereotactic body radiotherapy for moderately central and ultra-central oligometastatic disease: initial outcomes. Tech Innov Patient Support Radiat Oncol 2020; 13: 24–30. doi: 10.1016/j.tipsro.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pokhrel D, Halfman M, Sanford L. FFF-VMAT for SBRT of lung lesions: improves dose coverage at tumor-lung interface compared to flattened beams. J Appl Clin Med Phys 2020; 21: 26–35. doi: 10.1002/acm2.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elhammali A, Blanchard P, Yoder A, Liao Z, Zhang X, Ronald Zhu X, et al. Clinical outcomes after intensity-modulated proton therapy with concurrent chemotherapy for inoperable non-small cell lung cancer. Radiother Oncol 2019; 136: 136–42. doi: 10.1016/j.radonc.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 83. Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016; 38 Suppl 1: E1886-95. 10.1002/hed.24341 [DOI] [PubMed] [Google Scholar]
- 84. Huang T-L, Tsai M-H, Chuang H-C, Chien C-Y, Lin Y-T, Tsai W-L, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma treated by volumetric-modulated arc therapy versus intensity-modulated radiotherapy. Radiat Oncol 2020; 15: 84. doi: 10.1186/s13014-020-01532-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Imano N, Murakami Y, Nakashima T, Takeuchi Y, Takahashi I, Doi Y, et al. Clinical outcomes of concurrent chemoradiotherapy with volumetric modulated arc therapy in patients with locally advanced nasopharyngeal carcinoma. Jpn J Radiol 2017; 35: 673–80. doi: 10.1007/s11604-017-0680-5 [DOI] [PubMed] [Google Scholar]
- 86. Tini P, Nardone V, Pastina P, Pirtoli L, Correale P, Giordano A. The effects of radiotherapy on the survival of patients with unresectable non-small cell lung cancer. Expert Rev Anticancer Ther 2018; 18: 593–602. doi: 10.1080/14737140.2018.1458615 [DOI] [PubMed] [Google Scholar]
- 87. Tambe NS, Pires IM, Moore C, Cawthorne C, Beavis AW. Validation of in-house knowledge-based planning model for advance-stage lung cancer patients treated using vmat radiotherapy. Br J Radiol 2020; 93(1106): 20190535. doi: 10.1259/bjr.20190535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chi A, Chen H, Wen S, Yan H, Liao Z. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017; 123: 346–54. doi: 10.1016/j.radonc.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jin X, Lin B, Chen D, Li L, Han C, Zhou Y, et al. Safety and outcomes of volumetric modulated arc therapy in the treatment of patients with inoperable lung cancer. J Cancer 2019; 10: 2868–73. doi: 10.7150/jca.31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vlacich G, Stavas MJ, Pendyala P, Chen SC, Shyr Y, Cmelak AJ. A comparative analysis between sequential boost and integrated boost intensity-modulated radiation therapy with concurrent chemotherapy for locally-advanced head and neck cancer. Radiat Oncol 2017; 12: 13. doi: 10.1186/s13014-016-0756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. De Felice F, Bonomo P, Sanguineti G, Orlandi E. Moderately accelerated intensity-modulated radiation therapy using simultaneous integrated boost: practical reasons or evidence-based choice? A critical appraisal of literature. Head Neck 2020; 42: 3405–14. doi: 10.1002/hed.26400 [DOI] [PubMed] [Google Scholar]
- 92. Brodin NP, Tomé WA. Revisiting the dose constraints for head and neck oars in the current era of IMRT. Oral Oncol 2018; 86: 8–18. doi: 10.1016/j.oraloncology.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bahl A, Oinam AS, Kaur S, Verma R, Elangovan A, Bhandari S, et al. Evaluation of acute toxicity and early clinical outcome in head and neck cancers treated with conventional radiotherapy and simultaneous integrated boost arc radiotherapy. World J Oncol 2017; 8: 117–21. doi: 10.14740/wjon1049w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McDowell L, Corry J, Ringash J, Rischin D. Quality of life, toxicity and unmet needs in nasopharyngeal cancer survivors. Front Oncol 2020; 10: 930. doi: 10.3389/fonc.2020.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pow EHN, Kwong DLW, McMillan AS, Wong MCM, Sham JST, Leung LHT, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006; 66: 981–91. 10.1016/j.ijrobp.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 96. Kam MKM, Leung S-F, Zee B, Chau RMC, Suen JJS, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007; 25: 4873–79. doi: 10.1200/JCO.2007.11.5501 [DOI] [PubMed] [Google Scholar]
- 97. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–36. 10.1016/S1470-2045(10)70290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen B-B, Huang S-M, Xiao W-W, Sun W-Z, Liu M-Z, Lu T-X, et al. Prospective matched study on comparison of volumetric-modulated arc therapy and intensity-modulated radiotherapy for nasopharyngeal carcinoma: dosimetry, delivery efficiency and outcomes. J Cancer 2018; 9: 978–86. doi: 10.7150/jca.22843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Franzese C, Fogliata A, Franceschini D, Clerici E, D’Agostino G, Navarria P, et al. Treatment: outcome and toxicity of volumetric modulated arc therapy in oropharyngeal carcinoma. Anticancer Res 2016; 36: 3451–57. [PubMed] [Google Scholar]
- 100. Manzar GS, Lester SC, Routman DM, Harmsen WS, Petersen MM, Sloan JA, et al. Comparative analysis of acute toxicities and patient reported outcomes between intensity-modulated proton therapy (IMPT) and volumetric modulated arc therapy (VMAT) for the treatment of oropharyngeal cancer. Radiother Oncol 2020; 147: 64–74. doi: 10.1016/j.radonc.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 101. Daniela Falco M, Giancaterino S, D’Andrea M, Gimenez De Lorenzo R, Trignani M, Caravatta L, et al. Hippocampal sparing approach in fractionated stereotactic brain VMAT radio therapy: A retrospective feasibility analysis. J Appl Clin Med Phys 2018; 19: 86–93. doi: 10.1002/acm2.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Huff WX, Agrawal N, Shapiro S, Miller J, Kulwin C, Shah M, et al. Efficacy of pre-operative stereotactic radiosurgery followed by surgical resection and correlative radiobiological analysis for patients with 1-4 brain metastases: study protocol for a phase II trial. Radiat Oncol 2018; 13: 252. doi: 10.1186/s13014-018-1178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol 2014; 32: 3810–16. doi: 10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Andreas JJM, Kundapur V. Hippocampus avoidance whole-brain radiation therapy: A practical intensity-modulated radiation therapy planning and delivery approach to RTOG 0933. Journal of Medical Imaging and Radiation Sciences 2015; 46: 78–84. 10.1016/j.jmir.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 105. Zieminski S, Khandekar M, Wang Y. Assessment of multi-criteria optimization (MCO) for volumetric modulated arc therapy (VMAT) in hippocampal avoidance whole brain radiation therapy (HA-WBRT). J Appl Clin Med Phys 2018; 19: 184–90. doi: 10.1002/acm2.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dimitriadis A, Tsang Y, Thomas RAS, Palmer AL, Eaton D, Lee J, et al. Multi-institutional dosimetric delivery assessment of intracranial stereotactic radiosurgery on different treatment platforms. Radiother Oncol 2020; 147: 153–61. doi: 10.1016/j.radonc.2020.05.024 [DOI] [PubMed] [Google Scholar]
- 107. Potrebko PS, Keller A, All S, Sejpal S, Pepe J, Saigal K, et al. GammaKnife versus VMAT radiosurgery plan quality for many brain metastases. J Appl Clin Med Phys 2018; 19: 159–65. doi: 10.1002/acm2.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sheu T, Briere TM, Olanrewaju AM, McAleer MF. Intensity modulated radiation therapy versus volumetric arc radiation therapy in the treatment of glioblastoma-does clinical benefit follow dosimetric advantage? Adv Radiat Oncol 2019; 4: 50–56. doi: 10.1016/j.adro.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Adeberg S, Harrabi SB, Bougatf N, Bernhardt D, Rieber J, Koerber SA, et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma : a dosimetric comparison. Strahlenther Onkol 2016; 192: 770–79. 10.1007/s00066-016-1007-7 [DOI] [PubMed] [Google Scholar]
- 110. Briere TM, McAleer MF, Levy LB, Yang JN. Sparing of normal tissues with volumetric arc radiation therapy for glioblastoma: single institution clinical experience. Radiat Oncol 2017; 12: 79. doi: 10.1186/s13014-017-0810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wee CW, Kim KS, Kim CY, Han JH, Kim YJ, Kim IA. Feasibility of hippocampus-sparing VMAT for newly diagnosed glioblastoma treated by chemoradiation: pattern of failure analysis. Radiat Oncol 2020; 15: 98. doi: 10.1186/s13014-020-01552-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xu D, Li G, Li H, Jia F. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer. Medicine 2017; 96: e7685. 10.1097/MD.0000000000007685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brown PD, Chung C, Liu DD, McAvoy S, Grosshans D, Al Feghali K, et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol 2021; 23: 1337–47. doi: 10.1093/neuonc/noab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gwynne S, Higgins E, Poon King A, Radhakrishna G, Wills L, Mukherjee S, et al. Driving developments in UK oesophageal radiotherapy through the SCOPE trials. Radiat Oncol 2019; 14: 26. doi: 10.1186/s13014-019-1225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hawkins MA, Aitken A, Hansen VN, McNair HA, Tait DM. Set-up errors in radiotherapy for oesophageal cancers--is electronic portal imaging or conebeam more accurate? Radiother Oncol 2011; 98: 249–54. doi: 10.1016/j.radonc.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 116. Hurt CN, Nixon LS, Griffiths GO, Al-Mokhtar R, Gollins S, Staffurth JN, et al. BMC Cancer [Internet]. Available from: http://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-11-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Crosby T, Bridges S, Nixo L. (n.d.). SCOPE2 - A Randomised Phase II/III Trial to Study Radiotherapy Dose Escalation in Patients With Oesophageal Cancer Treated With Definitive Chemo-radiation With an Embedded Phase II Trial for Patients With A Poor Early Response Using Positron Emission Tomography (PET). Clinical Trials.gov; [DOI] [PubMed] [Google Scholar]
- 118. Zhao L, Zhou Y, Mu Y, Chai G, Xiao F, Tan L, et al. Patterns of failure and clinical outcomes of definitive radiotherapy for cervical esophageal cancer. Internet. Available from: www.impactjournals.com/oncotarget [DOI] [PMC free article] [PubMed]
- 119. Ren W, Sun C, Lu N, Xu Y, Han F, Liu YP, et al. Dosimetric comparison of intensity-modulated radiotherapy and volumetric-modulated arc radiotherapy in patients with prostate cancer: A meta-analysis. J Appl Clin Med Phys 2016; 17: 254–62. doi: 10.1120/jacmp.v17i6.6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sato K, Shimamoto H, Mochizuki Y, Hirai H, Tomioka H, Shimizu R, et al. Treatment of oral cancers during pregnancy: A case-based discussion. J Otolaryngol Head Neck Surg 2019; 48: 9. doi: 10.1186/s40463-019-0331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mazzola R, Corradini S, Eidemüeller M, Figlia V, Fiorentino A, Giaj-Levra N, et al. Modern radiotherapy in cancer treatment during pregnancy. Critical Reviews in Oncology/Hematology 2019; 136: 13–19. doi: 10.1016/j.critrevonc.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 122. Stovall M, Blackwell CR, Cundiff J, Novack DH, Palta JR, Wagner LK, et al. Fetal dose from radiotherapy with photon beams: report of AAPM radiation therapy committee task group no. 36. Med Phys 1995; 22: 63–82. 10.1118/1.597525 [DOI] [PubMed] [Google Scholar]
- 123. Sedlmayer F, Reitsamer R, Wenz F, Sperk E, Fussl C, Kaiser J, et al. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol 2017; 12: 23. doi: 10.1186/s13014-016-0749-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Knabben L, Mueller MD. Breast cancer and pregnancy. Horm Mol Biol Clin Investig 2017; 32(1. doi: 10.1515/hmbci-2017-0026 [DOI] [PubMed] [Google Scholar]
- 125. Evens AM, Advani R, Press OW, Lossos IS, Vose JM, Hernandez-Ilizaliturri FJ, et al. Lymphoma occurring during pregnancy: antenatal therapy, complications, and maternal survival in a multicenter analysis. J Clin Oncol 2013; 31: 4132–39. doi: 10.1200/JCO.2013.49.8220 [DOI] [PubMed] [Google Scholar]
- 126. Layton SA, Rintoul M, Avery BS. Oral carcinoma in pregnancy. Br J Oral Maxillofac Surg 1992; 30: 161–64. doi: 10.1016/0266-4356(92)90148-c [DOI] [PubMed] [Google Scholar]
- 127. Takahashi W, Nawa K, Haga A, Yamashita H, Imae T, Ogita M, et al. Acceptable fetal dose using flattening filter-free volumetric arc therapy (FFF VMAT) in postoperative chemoradiotherapy of tongue cancer during pregnancy. Clin Transl Radiat Oncol 2020; 20: 9–12. doi: 10.1016/j.ctro.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rosen A, Anderson V, Bercovic E, Laperriere N, DʼSouza R. Brainstem glioma in pregnancy. Obstetrics & Gynecology 2018. [Google Scholar]
- 129. Martinez Pineda WJ, Calva Espinosa AL, Gonzalez Noguez AG, Osorio Solis C, Vacio Olguin AJ. Tonsil cancer treated with radiotherapy during A pregnancy: A case report. J Radiother Pract 2019; 19: 197–201. doi: 10.1017/S1460396919000608 [DOI] [Google Scholar]
- 130. Rooney KP, Miah AB, Bhide SA, Guerrero-Urbano MT, Sharabiani MT, Newbold KL, et al. Intensity modulated radiotherapy in locally advanced thyroid cancer: outcomes of a sequential phase I dose-escalation study. Radiother Oncol 2018; 127: 43–48. doi: 10.1016/j.radonc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 131. Lertbutsayanukul C, Prayongrat A, Kannarunimit D, Chakkabat C, Netsawang B, Kitpanit S. A randomized phase III study between sequential versus simultaneous integrated boost intensity-modulated radiation therapy in nasopharyngeal carcinoma. Strahlenther Onkol 2018; 194: 375–85. doi: 10.1007/s00066-017-1251-5 [DOI] [PubMed] [Google Scholar]
- 132. Franco P, De Bari B, Arcadipane F, Lepinoy A, Ceccarelli M, Furfaro G, et al. Comparing simultaneous integrated boost vs sequential boost in anal cancer patients: results of a retrospective observational study. Radiat Oncol 2018; 13: 172. doi: 10.1186/s13014-018-1124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW, Bahary J-P, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the nrg oncology rtog 0126 randomized clinical trial. JAMA Oncol 2018; 4: e180039. doi: 10.1001/jamaoncol.2018.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. European Organisation for Research and Treatment of Cancer . E2-RADIatE- THE NEW PLATFORM FOR RADIOTHERAPY.European Organisation FOR Research and Treatment of Cancer. Available from: https://project.eortc.org/e2-radiate/