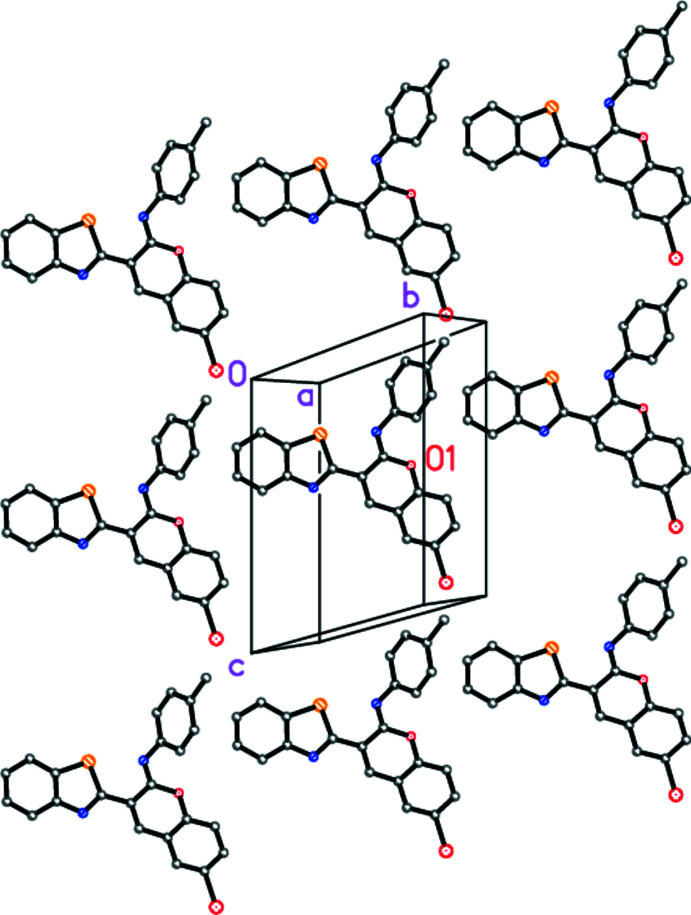
In the crystal structure of the title compound, the C=N—C angle is wide [125.28 (8)°]. The benzothiazole and chromene ring systems are almost coplanar and lie parallel to (1
 0); the toluene ring system is rotated by ca 40° out of the chromene plane.
0); the toluene ring system is rotated by ca 40° out of the chromene plane.
Keywords: crystal structure, benzo[d]thiazole, chromene, imine, π–π-stacking
Abstract
The title compound, C23H15BrN2OS, was the unexpected product in an attempted synthesis of the isomeric 3-(benzo[d]thiazol-2-yl)-6-bromo-1-p-tolylquinolin-2(1H)-one. The Cchromene=N—C angle is wide [125.28 (8)°]. The benzothiazole and chromene ring systems are almost coplanar, with their planes parallel to (1
 0); the toluene ring system is rotated by ca 40° out of the chromene plane. The molecular packing involves layers with π-stacking, borderline ‘weak’ hydrogen bonds and possible C—H⋯π contacts.
0); the toluene ring system is rotated by ca 40° out of the chromene plane. The molecular packing involves layers with π-stacking, borderline ‘weak’ hydrogen bonds and possible C—H⋯π contacts.
1. Chemical context
Benzothiazoles exhibit strong fluorescence and luminescence properties (Wang et al., 2010 ▸). Incorporated benzothiazole moieties are present in many commercially important organofluorescent materials that have attracted significant research interest in the field of organic light-emitting diodes (Lu et al., 2017 ▸; Metwally et al., 2022a
▸,b
▸). Coumarin (IUPAC name 2H-chromen-2-one) is a natural product and flavouring agent. Recently, a series of novel benzothiazolyl-coumarin hybrids have been synthesized as potential biological agents and efficient emitting materials (Azzam et al., 2021 ▸, 2022a
▸,b
▸,c
▸,d
▸; Wu et al., 2011 ▸). We have previously prepared 3-(benzo[d]oxazol, -imidazole, -thiazol-2-yl)-2H-chromen-2-imine and their corresponding coumarin analogues 3-(benzo[d]oxazol-, -imidazol, -thiazol-2-yl)-2H-chromen-2-one, through the reaction of salicylaldehyde with 2-cyanomethyl-benzoxazole, -benzimidazole, and -benzothiazole, respectively (Elgemeie, 1989 ▸). Some derivatives of these ring systems, known commercially as coumarin-6, coumarin-7 and coumarin-30, have been used as laser dyes in medical applications (Das et al., 2021 ▸; Satpati et al., 2009 ▸). Recently, we have synthesized some coumarin derivatives that exhibit fluorescence properties (Elgemeie & Elghandour, 1990 ▸; Elgemeie et al., 2000a
▸,b
▸; Elgemeie et al., 2015 ▸) as part of our research interest in exploiting new coumarin and benzothiazole derivatives for biological and photochemical materials (Azzam et al., 2017a
▸,b
▸, 2020a
▸,b
▸,c
▸,d
▸; Metwally et al., 2021a
▸,b
▸). Here, we describe a one-pot reaction of N-[2-(benzo[d]thiazol-2-yl)acetyl]benzohydrazide (1) with 5-bromo-salicylaldehde (2) and 4-p-toluidine (5) (Fig. 1 ▸). The mass spectrum of the product was, however, inconsistent with the proposed structure, 3-(benzo[d]thiazol-2-yl)-6-bromo-1-p-tolylquinolin-2(1H)-one (6). Therefore, the X-ray crystal structure was determined, showing the exclusive presence of N-[3-(benzo[d]thiazol-2-yl)-6-bromo-2H-chromen-2-ylidene]-4-methylbenzenamine (7), an isomer of 6, as the sole product in the solid state; this was unexpected because the C=O moiety of the coumarin framework is usually chemically robust. The formation of 7 presumably involves the initial formation of the adduct 3 followed by elimination of benzohydrazide; the intermediate 4 then reacts with p-toluidine to give the final product 7 by elimination of water.

Figure 1.
The synthesis of compound 7.
2. Structural commentary
The molecule of 7 is shown in Fig. 2 ▸. The structure determination makes clear that the unexpected product is a chromene derivative with an exocyclic imino function rather than a quinoline with an exocyclic oxo function. Bond lengths and angles may be regarded as normal, except that the C9=N9—C17 angle is very wide at 125.28 (8)°; selected values are given in Table 1 ▸. The benzothiazole and chromene ring systems are almost coplanar, with an interplanar angle of 7.59 (2)°; associated with this is a short intramolecular contact S1⋯N9 2.7570 (8) Å. The toluene ring system is appreciably rotated out of the chromene plane, with an interplanar angle of 40.38 (2)°.
Figure 2.
The molecule of compound 7 in the crystal. Ellipsoids represent 50% probability levels.
Table 1. Selected geometric parameters (Å, °).
| S1—C7A | 1.7340 (9) | C9—O1 | 1.3819 (11) |
| S1—C2 | 1.7512 (9) | O1—C10 | 1.3751 (12) |
| C2—N3 | 1.3094 (12) | N9—C17 | 1.4127 (12) |
| C2—C8 | 1.4705 (12) | C13—Br1 | 1.8969 (10) |
| C9—N9 | 1.2708 (12) | ||
| C7A—S1—C2 | 88.85 (4) | C3A—C7A—S1 | 109.69 (7) |
| N3—C2—S1 | 115.73 (7) | C10—O1—C9 | 121.82 (7) |
| C2—N3—C3A | 110.78 (8) | C9—N9—C17 | 125.28 (8) |
| N3—C3A—C7A | 114.92 (8) |
3. Supramolecular features
There are few short contacts between molecules; two borderline ‘weak’ hydrogen bonds are listed in Table 2 ▸. A tenable packing analysis attributes a central role to the ring systems; individual rings are denoted here as A (thiazole), B (benzo ring of benzothiazole), C (pyran ring of chromene), D (benzo ring of chromene) and E (tolyl). The molecules lie with rings A–D almost parallel to (1
 0) (Fig. 3 ▸), and there are weak stacking effects A⋯D [intercentroid distance 3.5910 (5) Å, offset 1.12 Å; operator 1 − x, 1 − y, 1 − z], C⋯C [3.6184 (5) Å, 1.35 Å; 2 − x, 1 − y, 1 − z] and C⋯D [3.6308 (5) Å, 1.27 Å; 2 − x, 1 − y, 1 − z] (Fig. 4 ▸). Two possible C—H⋯π interactions are represented by the contacts H21⋯Cg(B) [Cg = centroid; H⋯Cg 2.89 Å, C—H⋯Cg 122°; −1 + x, −1 + y, z] and H6⋯Cg(E) [H⋯Cg 2.87 Å, C—H⋯Cg 124°; x, 1 + y, z]; the angles are narrow, but the interactions do not necessarily involve the ring centroids. The contacts H7⋯Br1 and H6⋯Cg(E) lie within the parent layer; H22⋯N3 is formed to a neighbouring layer and H21⋯Cg(B) to the next layer but one.
0) (Fig. 3 ▸), and there are weak stacking effects A⋯D [intercentroid distance 3.5910 (5) Å, offset 1.12 Å; operator 1 − x, 1 − y, 1 − z], C⋯C [3.6184 (5) Å, 1.35 Å; 2 − x, 1 − y, 1 − z] and C⋯D [3.6308 (5) Å, 1.27 Å; 2 − x, 1 − y, 1 − z] (Fig. 4 ▸). Two possible C—H⋯π interactions are represented by the contacts H21⋯Cg(B) [Cg = centroid; H⋯Cg 2.89 Å, C—H⋯Cg 122°; −1 + x, −1 + y, z] and H6⋯Cg(E) [H⋯Cg 2.87 Å, C—H⋯Cg 124°; x, 1 + y, z]; the angles are narrow, but the interactions do not necessarily involve the ring centroids. The contacts H7⋯Br1 and H6⋯Cg(E) lie within the parent layer; H22⋯N3 is formed to a neighbouring layer and H21⋯Cg(B) to the next layer but one.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C7—H7⋯Br1i | 0.95 | 3.11 | 3.7721 (10) | 128 |
| C22—H22⋯N3ii | 0.95 | 2.63 | 3.5716 (13) | 169 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 3.
Layer structure of compound 7 (without hydrogen atoms) showing the asymmetric unit (indicated by the label O1) and further translation-related molecules viewed perpendicular to the plane (1
 0). A second layer is related to the first by inversion.
0). A second layer is related to the first by inversion.
Figure 4.
Stacking of ring systems in the structure of 7 (without hydrogen atoms). The view direction is parallel to the c axis. The label O1 indicates the molecule of the chosen asymmetric unit.
4. Database survey
The searches employed the routine ConQuest (Bruno et al., 2002 ▸), part of Version 2022.3.0 of the CSD (Groom et al., 2016 ▸).
We recently reported the structure of the mixed coumarin/benzo[d]thiazole derivative 3-(benzo[d]thiazol-2-yl)-2H-chromen-2-one [3-(1,3-benzothiazol-2-yl)-2H-1-benzopyran-2-one] (Abdallah et al., 2022 ▸). The structure of the 4-oxo isomer had already been published by Lohar et al. (2018 ▸). Two more related structures were published by others at the same time (Singh et al., 2022 ▸). The current structure, however, bears an imine (=NAr) rather than an oxo substituent at atom C2 of the chromene (and thus is strictly not a coumarin). Only one other such structure was found in the database; its substituent at the imine nitrogen atom is pyridin-2-ethyl (refcode ITEVAF; Ahamed & Ghosh, 2011 ▸) and its C=N—C angle is much narrower than in 7 at 118.5 (7)°. A further search was therefore performed for structures with an =NAr group at the 2-position of a chromene ring system. This gave 18 hits with a considerable spread of C=N—C angles, namely 120.5–127.9°, mean value 123.4 (24)°. Nine of these structures appeared in the same publication (Shishkina et al., 2019 ▸), and, like 7, none of them had an interplanar angle close to the calculated gas-phase optimum of 0°.
5. Synthesis and crystallization
5-Bromo-salicylaldehyde 2 (2.01 g, 0.01 mol), p-toluidine 5 (1.07 g, 0.01 mol) and solid ammonium acetate (0.77 g, 0.01 mol) were added to a solution of N-[2-(benzo[d]thiazol-2-yl)acetyl]benzohydrazide 1 (3.11 g, 0.01 mol) in ethanol (25 mL). The reaction mixture was refluxed for 3 h, and the solid thus formed was collected by filtration and recrystallized from ethanol.
Yellow crystals (seen under the microscope to be orange/yellow dichroic); yield: 94% (4.21 g); m.p. 501–503 K; IR (KBr, cm−1): ν 3052, (CH-aromatic), 2918, 2852 (CH3), 1554 (C=N), 1591, 1476 (C=C). 1H NMR (400 MHz, DMSO-d 6) δ: 2.51 (s, 3H, CH3), 7.16–8.28 (m, 11H, 2 C6H4, C6H3), 8.73 (s, 1H, CH-pyran). 13C NMR (100 MHz, DMSO-d6 ) δ: 21.2 (CH3), 116.5, 118.0, 121.7, 122.5 (2), 123.1, 123.9, 125.8, 127.0, 129.9 (2), 132.0, 134.1, 134.5, 135.1, 137.6, 141.7, 145.6, 152.0, 152.1 (aromatic carbons, pyran ring), 160.5 (C=N). Analysis: calculated for C23H15BrN2OS (447.35): C 61.75, H 3.38, N 6.26, S 7.17%. Found: C 61.86, H 3.50, N 6.06, S 6.99%.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The methyl group was included as an idealized rigid group allowed to rotate but not tip (C—H 0.98 Å, H—C—H 109.5°). Other hydrogen atoms were included using a riding model starting from calculated positions, with C—H 0.95 Å. The U(H) values were fixed at 1.5 × U eq of the parent carbon atoms for methyl H atoms and 1.2 × U eq for other hydrogen atoms.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | C23H15BrN2OS |
| M r | 447.34 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 100 |
| a, b, c (Å) | 7.34138 (10), 10.6720 (2), 12.9247 (2) |
| α, β, γ (°) | 104.5034 (16), 90.2462 (12), 103.9961 (14) |
| V (Å3) | 948.97 (3) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.29 |
| Crystal size (mm) | 0.20 × 0.15 × 0.12 |
| Data collection | |
| Diffractometer | XtaLAB Synergy |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2022 ▸) |
| T min, T max | 0.902, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 126966, 12477, 11717 |
| R int | 0.030 |
| (sin θ/λ)max (Å−1) | 0.928 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.044, 0.086, 1.27 |
| No. of reflections | 12477 |
| No. of parameters | 254 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.00, −0.64 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989023002979/yz2032sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023002979/yz2032Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989023002979/yz2032Isup3.cml
CCDC reference: 2252955
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge support by the Open Access Publication Funds of the Technical University of Braunschweig.
supplementary crystallographic information
Crystal data
| C23H15BrN2OS | Z = 2 |
| Mr = 447.34 | F(000) = 452 |
| Triclinic, P1 | Dx = 1.566 Mg m−3 |
| a = 7.34138 (10) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.6720 (2) Å | Cell parameters from 55045 reflections |
| c = 12.9247 (2) Å | θ = 2.3–41.0° |
| α = 104.5034 (16)° | µ = 2.29 mm−1 |
| β = 90.2462 (12)° | T = 100 K |
| γ = 103.9961 (14)° | Block, yellow-orange dichroic |
| V = 948.97 (3) Å3 | 0.20 × 0.15 × 0.12 mm |
Data collection
| XtaLAB Synergy diffractometer | 12477 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Mo) X-ray Source | 11717 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.030 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 41.3°, θmin = 2.0° |
| ω scans | h = −13→13 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2022) | k = −19→19 |
| Tmin = 0.902, Tmax = 1.000 | l = −23→23 |
| 126966 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.044 | H-atom parameters constrained |
| wR(F2) = 0.086 | w = 1/[σ2(Fo2) + (0.0329P)2 + 0.3775P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.27 | (Δ/σ)max = 0.003 |
| 12477 reflections | Δρmax = 1.00 e Å−3 |
| 254 parameters | Δρmin = −0.64 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. Short intramolecular contact: 2.7570 (0.0008) S1 - N9 Least-squares planes (x,y,z in crystal coordinates) and deviations from them (* indicates atom used to define plane) 6.9114 (0.0010) x + 0.6551 (0.0043) y + 0.9221 (0.0052) z = 5.0880 (0.0023) * 0.0005 (0.0007) C17 * -0.0080 (0.0007) C18 * 0.0078 (0.0007) C19 * -0.0001 (0.0007) C20 * -0.0074 (0.0007) C21 * 0.0071 (0.0007) C22 -0.1433 (0.0014) N9 0.0078 (0.0018) C23 Rms deviation of fitted atoms = 0.0062 6.7905 (0.0005) x - 4.3737 (0.0026) y - 3.5046 (0.0028) z = 1.1567 (0.0016) Angle to previous plane (with approximate esd) = 40.378 ( 0.018 ) * -0.0112 (0.0007) C8 * -0.0356 (0.0007) C9 * 0.0189 (0.0007) O1 * 0.0164 (0.0008) C10 * 0.0078 (0.0008) C11 * -0.0097 (0.0008) C12 * -0.0229 (0.0008) C13 * -0.0003 (0.0008) C14 * 0.0198 (0.0008) C15 * 0.0168 (0.0007) C16 -0.1250 (0.0009) Br1 -0.0945 (0.0011) N9 Rms deviation of fitted atoms = 0.0184 6.6853 (0.0007) x - 5.5698 (0.0018) y - 2.4072 (0.0037) z = 1.1618 (0.0013) Angle to previous plane (with approximate esd) = 7.587 ( 0.024 ) * -0.0246 (0.0005) S1 * -0.0299 (0.0006) C2 * 0.0114 (0.0007) N3 * 0.0329 (0.0008) C3A * 0.0037 (0.0008) C4 * -0.0325 (0.0008) C5 * -0.0172 (0.0009) C6 * 0.0192 (0.0008) C7 * 0.0370 (0.0008) C7A Rms deviation of fitted atoms = 0.0254 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.45005 (3) | 0.22816 (2) | 0.24952 (2) | 0.01102 (4) | |
| C2 | 0.53265 (12) | 0.27006 (9) | 0.38418 (7) | 0.00988 (12) | |
| N3 | 0.47875 (11) | 0.17627 (8) | 0.43436 (6) | 0.01092 (11) | |
| C3A | 0.36335 (12) | 0.06293 (9) | 0.36717 (7) | 0.01059 (12) | |
| C4 | 0.27384 (14) | −0.05271 (10) | 0.39829 (8) | 0.01396 (14) | |
| H4 | 0.294443 | −0.059470 | 0.469083 | 0.017* | |
| C5 | 0.15488 (14) | −0.15658 (10) | 0.32326 (9) | 0.01606 (16) | |
| H5 | 0.091595 | −0.234805 | 0.343368 | 0.019* | |
| C6 | 0.12611 (14) | −0.14821 (10) | 0.21763 (9) | 0.01639 (16) | |
| H6 | 0.044937 | −0.221294 | 0.167457 | 0.020* | |
| C7 | 0.21439 (14) | −0.03497 (10) | 0.18570 (8) | 0.01469 (15) | |
| H7 | 0.195092 | −0.029543 | 0.114386 | 0.018* | |
| C7A | 0.33289 (12) | 0.07121 (9) | 0.26171 (7) | 0.01109 (13) | |
| C8 | 0.65444 (12) | 0.40075 (9) | 0.44104 (7) | 0.01000 (12) | |
| C9 | 0.69589 (12) | 0.51144 (9) | 0.39019 (7) | 0.01044 (12) | |
| O1 | 0.81113 (10) | 0.63197 (7) | 0.44747 (6) | 0.01236 (11) | |
| N9 | 0.62892 (12) | 0.49654 (8) | 0.29581 (7) | 0.01224 (12) | |
| C10 | 0.87706 (12) | 0.65155 (9) | 0.55151 (7) | 0.01079 (12) | |
| C11 | 0.98434 (13) | 0.77861 (9) | 0.60325 (8) | 0.01284 (14) | |
| H11 | 1.010923 | 0.847551 | 0.567020 | 0.015* | |
| C12 | 1.05226 (13) | 0.80324 (10) | 0.70912 (8) | 0.01385 (14) | |
| H12 | 1.126245 | 0.889327 | 0.745864 | 0.017* | |
| C13 | 1.01084 (13) | 0.70054 (10) | 0.76081 (8) | 0.01306 (14) | |
| Br1 | 1.09469 (2) | 0.73707 (2) | 0.90683 (2) | 0.01723 (3) | |
| C14 | 0.90564 (13) | 0.57339 (9) | 0.70921 (8) | 0.01269 (13) | |
| H14 | 0.880313 | 0.504533 | 0.745531 | 0.015* | |
| C15 | 0.83692 (12) | 0.54769 (9) | 0.60237 (7) | 0.01077 (12) | |
| C16 | 0.72340 (12) | 0.41962 (9) | 0.54312 (7) | 0.01106 (13) | |
| H16 | 0.696241 | 0.347267 | 0.575708 | 0.013* | |
| C17 | 0.64703 (13) | 0.59939 (9) | 0.24293 (7) | 0.01134 (13) | |
| C18 | 0.66395 (14) | 0.56395 (10) | 0.13211 (8) | 0.01423 (14) | |
| H18 | 0.674436 | 0.476506 | 0.097566 | 0.017* | |
| C19 | 0.66550 (15) | 0.65591 (10) | 0.07226 (8) | 0.01490 (15) | |
| H19 | 0.680212 | 0.631073 | −0.002518 | 0.018* | |
| C20 | 0.64577 (13) | 0.78423 (10) | 0.12042 (8) | 0.01351 (14) | |
| C21 | 0.62680 (14) | 0.81793 (10) | 0.23071 (8) | 0.01380 (14) | |
| H21 | 0.611872 | 0.904390 | 0.264656 | 0.017* | |
| C22 | 0.62919 (13) | 0.72820 (9) | 0.29233 (8) | 0.01265 (13) | |
| H22 | 0.618755 | 0.754253 | 0.367493 | 0.015* | |
| C23 | 0.64635 (18) | 0.88259 (12) | 0.05472 (10) | 0.02078 (19) | |
| H23A | 0.613539 | 0.961990 | 0.098977 | 0.031* | |
| H23B | 0.554014 | 0.841125 | −0.006738 | 0.031* | |
| H23C | 0.771864 | 0.908668 | 0.029148 | 0.031* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01261 (8) | 0.01125 (8) | 0.00922 (8) | 0.00140 (6) | 0.00060 (6) | 0.00424 (6) |
| C2 | 0.0102 (3) | 0.0100 (3) | 0.0097 (3) | 0.0025 (2) | 0.0010 (2) | 0.0031 (2) |
| N3 | 0.0119 (3) | 0.0103 (3) | 0.0107 (3) | 0.0012 (2) | 0.0001 (2) | 0.0044 (2) |
| C3A | 0.0103 (3) | 0.0104 (3) | 0.0113 (3) | 0.0019 (2) | 0.0004 (2) | 0.0040 (2) |
| C4 | 0.0142 (3) | 0.0127 (3) | 0.0152 (4) | 0.0007 (3) | −0.0004 (3) | 0.0067 (3) |
| C5 | 0.0144 (3) | 0.0132 (3) | 0.0200 (4) | −0.0004 (3) | −0.0013 (3) | 0.0070 (3) |
| C6 | 0.0151 (4) | 0.0131 (3) | 0.0185 (4) | −0.0005 (3) | −0.0031 (3) | 0.0036 (3) |
| C7 | 0.0155 (3) | 0.0136 (3) | 0.0131 (4) | 0.0007 (3) | −0.0021 (3) | 0.0029 (3) |
| C7A | 0.0113 (3) | 0.0111 (3) | 0.0107 (3) | 0.0021 (2) | 0.0004 (2) | 0.0034 (2) |
| C8 | 0.0101 (3) | 0.0096 (3) | 0.0107 (3) | 0.0023 (2) | 0.0011 (2) | 0.0036 (2) |
| C9 | 0.0104 (3) | 0.0096 (3) | 0.0116 (3) | 0.0018 (2) | 0.0014 (2) | 0.0038 (2) |
| O1 | 0.0136 (3) | 0.0105 (2) | 0.0122 (3) | 0.0001 (2) | −0.0009 (2) | 0.0043 (2) |
| N9 | 0.0145 (3) | 0.0111 (3) | 0.0116 (3) | 0.0022 (2) | 0.0009 (2) | 0.0049 (2) |
| C10 | 0.0100 (3) | 0.0104 (3) | 0.0120 (3) | 0.0023 (2) | 0.0009 (2) | 0.0032 (2) |
| C11 | 0.0122 (3) | 0.0100 (3) | 0.0154 (4) | 0.0011 (2) | 0.0009 (3) | 0.0033 (3) |
| C12 | 0.0127 (3) | 0.0117 (3) | 0.0155 (4) | 0.0020 (3) | 0.0002 (3) | 0.0015 (3) |
| C13 | 0.0126 (3) | 0.0138 (3) | 0.0116 (3) | 0.0029 (3) | −0.0006 (3) | 0.0016 (3) |
| Br1 | 0.01926 (5) | 0.01788 (5) | 0.01143 (4) | 0.00218 (3) | −0.00160 (3) | 0.00057 (3) |
| C14 | 0.0132 (3) | 0.0125 (3) | 0.0119 (3) | 0.0027 (3) | −0.0004 (3) | 0.0028 (3) |
| C15 | 0.0101 (3) | 0.0103 (3) | 0.0118 (3) | 0.0023 (2) | 0.0004 (2) | 0.0029 (2) |
| C16 | 0.0113 (3) | 0.0104 (3) | 0.0116 (3) | 0.0022 (2) | 0.0004 (2) | 0.0036 (2) |
| C17 | 0.0124 (3) | 0.0111 (3) | 0.0106 (3) | 0.0015 (2) | 0.0002 (2) | 0.0043 (2) |
| C18 | 0.0184 (4) | 0.0125 (3) | 0.0108 (3) | 0.0021 (3) | 0.0001 (3) | 0.0029 (3) |
| C19 | 0.0184 (4) | 0.0156 (4) | 0.0099 (3) | 0.0017 (3) | 0.0000 (3) | 0.0043 (3) |
| C20 | 0.0142 (3) | 0.0147 (3) | 0.0127 (4) | 0.0020 (3) | 0.0002 (3) | 0.0068 (3) |
| C21 | 0.0161 (3) | 0.0131 (3) | 0.0140 (4) | 0.0046 (3) | 0.0025 (3) | 0.0060 (3) |
| C22 | 0.0153 (3) | 0.0127 (3) | 0.0112 (3) | 0.0040 (3) | 0.0025 (3) | 0.0048 (3) |
| C23 | 0.0267 (5) | 0.0210 (4) | 0.0186 (5) | 0.0057 (4) | 0.0018 (4) | 0.0125 (4) |
Geometric parameters (Å, º)
| S1—C7A | 1.7340 (9) | C15—C16 | 1.4382 (13) |
| S1—C2 | 1.7512 (9) | C17—C18 | 1.4010 (13) |
| C2—N3 | 1.3094 (12) | C17—C22 | 1.4010 (13) |
| C2—C8 | 1.4705 (12) | C18—C19 | 1.3915 (14) |
| N3—C3A | 1.3809 (12) | C19—C20 | 1.3977 (15) |
| C3A—C4 | 1.4055 (13) | C20—C21 | 1.3964 (14) |
| C3A—C7A | 1.4082 (13) | C20—C23 | 1.5057 (14) |
| C4—C5 | 1.3849 (14) | C21—C22 | 1.3934 (13) |
| C5—C6 | 1.4085 (15) | C4—H4 | 0.9500 |
| C6—C7 | 1.3872 (14) | C5—H5 | 0.9500 |
| C7—C7A | 1.4015 (13) | C6—H6 | 0.9500 |
| C8—C16 | 1.3600 (13) | C7—H7 | 0.9500 |
| C8—C9 | 1.4616 (12) | C11—H11 | 0.9500 |
| C9—N9 | 1.2708 (12) | C12—H12 | 0.9500 |
| C9—O1 | 1.3819 (11) | C14—H14 | 0.9500 |
| O1—C10 | 1.3751 (12) | C16—H16 | 0.9500 |
| N9—C17 | 1.4127 (12) | C18—H18 | 0.9500 |
| C10—C11 | 1.3896 (13) | C19—H19 | 0.9500 |
| C10—C15 | 1.3985 (13) | C21—H21 | 0.9500 |
| C11—C12 | 1.3931 (14) | C22—H22 | 0.9500 |
| C12—C13 | 1.3954 (14) | C23—H23A | 0.9800 |
| C13—C14 | 1.3849 (13) | C23—H23B | 0.9800 |
| C13—Br1 | 1.8969 (10) | C23—H23C | 0.9800 |
| C14—C15 | 1.4049 (13) | ||
| C7A—S1—C2 | 88.85 (4) | C22—C17—N9 | 123.92 (8) |
| N3—C2—C8 | 120.14 (8) | C19—C18—C17 | 120.48 (9) |
| N3—C2—S1 | 115.73 (7) | C18—C19—C20 | 121.05 (9) |
| C8—C2—S1 | 124.12 (6) | C21—C20—C19 | 117.95 (9) |
| C2—N3—C3A | 110.78 (8) | C21—C20—C23 | 121.46 (9) |
| N3—C3A—C4 | 124.80 (8) | C19—C20—C23 | 120.58 (9) |
| N3—C3A—C7A | 114.92 (8) | C22—C21—C20 | 121.80 (9) |
| C4—C3A—C7A | 120.23 (8) | C21—C22—C17 | 119.69 (9) |
| C5—C4—C3A | 118.40 (9) | C5—C4—H4 | 120.8 |
| C4—C5—C6 | 121.12 (9) | C3A—C4—H4 | 120.8 |
| C7—C6—C5 | 121.06 (9) | C4—C5—H5 | 119.4 |
| C6—C7—C7A | 118.06 (9) | C6—C5—H5 | 119.4 |
| C7—C7A—C3A | 121.11 (8) | C7—C6—H6 | 119.5 |
| C7—C7A—S1 | 129.11 (7) | C5—C6—H6 | 119.5 |
| C3A—C7A—S1 | 109.69 (7) | C6—C7—H7 | 121.0 |
| C16—C8—C9 | 119.94 (8) | C7A—C7—H7 | 121.0 |
| C16—C8—C2 | 119.48 (8) | C10—C11—H11 | 120.5 |
| C9—C8—C2 | 120.55 (8) | C12—C11—H11 | 120.5 |
| N9—C9—O1 | 121.20 (8) | C11—C12—H12 | 120.2 |
| N9—C9—C8 | 120.80 (8) | C13—C12—H12 | 120.2 |
| O1—C9—C8 | 118.00 (8) | C13—C14—H14 | 120.5 |
| C10—O1—C9 | 121.82 (7) | C15—C14—H14 | 120.5 |
| C9—N9—C17 | 125.28 (8) | C8—C16—H16 | 119.6 |
| O1—C10—C11 | 117.00 (8) | C15—C16—H16 | 119.6 |
| O1—C10—C15 | 121.11 (8) | C19—C18—H18 | 119.8 |
| C11—C10—C15 | 121.89 (9) | C17—C18—H18 | 119.8 |
| C10—C11—C12 | 118.90 (9) | C18—C19—H19 | 119.5 |
| C11—C12—C13 | 119.52 (9) | C20—C19—H19 | 119.5 |
| C14—C13—C12 | 121.77 (9) | C22—C21—H21 | 119.1 |
| C14—C13—Br1 | 119.00 (7) | C20—C21—H21 | 119.1 |
| C12—C13—Br1 | 119.21 (7) | C21—C22—H22 | 120.2 |
| C13—C14—C15 | 119.05 (9) | C17—C22—H22 | 120.2 |
| C10—C15—C14 | 118.86 (8) | C20—C23—H23A | 109.5 |
| C10—C15—C16 | 118.19 (8) | C20—C23—H23B | 109.5 |
| C14—C15—C16 | 122.95 (8) | H23A—C23—H23B | 109.5 |
| C8—C16—C15 | 120.85 (8) | C20—C23—H23C | 109.5 |
| C18—C17—C22 | 119.00 (8) | H23A—C23—H23C | 109.5 |
| C18—C17—N9 | 116.72 (8) | H23B—C23—H23C | 109.5 |
| C7A—S1—C2—N3 | 0.05 (7) | C9—O1—C10—C11 | −176.93 (8) |
| C7A—S1—C2—C8 | −179.77 (8) | C9—O1—C10—C15 | 2.96 (13) |
| C8—C2—N3—C3A | 179.03 (8) | O1—C10—C11—C12 | 179.31 (8) |
| S1—C2—N3—C3A | −0.79 (10) | C15—C10—C11—C12 | −0.57 (14) |
| C2—N3—C3A—C4 | −176.34 (9) | C10—C11—C12—C13 | −0.22 (14) |
| C2—N3—C3A—C7A | 1.36 (11) | C11—C12—C13—C14 | 0.92 (15) |
| N3—C3A—C4—C5 | 177.03 (9) | C11—C12—C13—Br1 | −177.29 (7) |
| C7A—C3A—C4—C5 | −0.56 (14) | C12—C13—C14—C15 | −0.80 (14) |
| C3A—C4—C5—C6 | 1.09 (16) | Br1—C13—C14—C15 | 177.41 (7) |
| C4—C5—C6—C7 | −0.75 (17) | O1—C10—C15—C14 | −179.19 (8) |
| C5—C6—C7—C7A | −0.15 (16) | C11—C10—C15—C14 | 0.68 (13) |
| C6—C7—C7A—C3A | 0.68 (15) | O1—C10—C15—C16 | −0.42 (13) |
| C6—C7—C7A—S1 | −175.47 (8) | C11—C10—C15—C16 | 179.46 (8) |
| N3—C3A—C7A—C7 | −178.14 (9) | C13—C14—C15—C10 | 0.00 (13) |
| C4—C3A—C7A—C7 | −0.33 (14) | C13—C14—C15—C16 | −178.71 (9) |
| N3—C3A—C7A—S1 | −1.32 (10) | C9—C8—C16—C15 | 0.14 (13) |
| C4—C3A—C7A—S1 | 176.50 (7) | C2—C8—C16—C15 | −177.59 (8) |
| C2—S1—C7A—C7 | 177.19 (10) | C10—C15—C16—C8 | −1.08 (13) |
| C2—S1—C7A—C3A | 0.69 (7) | C14—C15—C16—C8 | 177.64 (9) |
| N3—C2—C8—C16 | 5.52 (13) | C9—N9—C17—C18 | 145.41 (10) |
| S1—C2—C8—C16 | −174.66 (7) | C9—N9—C17—C22 | −41.55 (14) |
| N3—C2—C8—C9 | −172.19 (8) | C22—C17—C18—C19 | 0.84 (14) |
| S1—C2—C8—C9 | 7.62 (12) | N9—C17—C18—C19 | 174.24 (9) |
| C16—C8—C9—N9 | −178.29 (9) | C17—C18—C19—C20 | −1.56 (15) |
| C2—C8—C9—N9 | −0.59 (13) | C18—C19—C20—C21 | 0.79 (15) |
| C16—C8—C9—O1 | 2.26 (13) | C18—C19—C20—C23 | −179.58 (10) |
| C2—C8—C9—O1 | 179.97 (8) | C19—C20—C21—C22 | 0.68 (15) |
| N9—C9—O1—C10 | 176.74 (8) | C23—C20—C21—C22 | −178.95 (10) |
| C8—C9—O1—C10 | −3.82 (12) | C20—C21—C22—C17 | −1.37 (15) |
| O1—C9—N9—C17 | −6.20 (14) | C18—C17—C22—C21 | 0.59 (14) |
| C8—C9—N9—C17 | 174.37 (8) | N9—C17—C22—C21 | −172.29 (9) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H7···Br1i | 0.95 | 3.11 | 3.7721 (10) | 128 |
| C22—H22···N3ii | 0.95 | 2.63 | 3.5716 (13) | 169 |
Symmetry codes: (i) x−1, y−1, z−1; (ii) −x+1, −y+1, −z+1.
References
- Abdallah, A. E. M., Elgemeie, G. H. & Jones, P. G. (2022). IUCrData, 7, x220332. [DOI] [PMC free article] [PubMed]
- Ahamed, B. N. & Ghosh, P. (2011). Dalton Trans. 40, 6411–6419. [DOI] [PubMed]
- Azzam, R. A., Elboshi, H. A. & Elgemeie, G. H. (2020a). ACS Omega, 5, 30023–30036. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elboshi, H. A. & Elgemeie, G. H. (2022d). Antibiotics, 11, 1799. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elgemeie, G. H., Elsayed, R. E., Gad, N. M. & Jones, P. G. (2022b). Acta Cryst. E78, 369–372. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elgemeie, G. H., Elsayed, R. E. & Jones, P. G. (2017a). Acta Cryst. E73, 1820–1822. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elgemeie, G. H., Elsayed, R. E. & Jones, P. G. (2017b). Acta Cryst. E73, 1041–1043. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elgemeie, G. H., Gad, N. M. & Jones, P. G. (2022c). IUCrData, 7, x220412. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elgemeie, G. H. & Osman, R. R. (2020d). J. Mol. Struct. 1201, 127194.
- Azzam, R. A., Elgemeie, G. H., Seif, M. M. & Jones, P. G. (2021). Acta Cryst. E77, 891–894. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Elsayed, R. E. & Elgemeie, G. H. (2020b). ACS Omega, 5, 26182–26194. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Gad, N. M. & Elgemeie, G. H. (2022a). ACS Omega, 7, 35656–35667. [DOI] [PMC free article] [PubMed]
- Azzam, R. A., Osman, R. R. & Elgemeie, G. H. (2020c). ACS Omega, 5, 1640–1655. [DOI] [PMC free article] [PubMed]
- Bruno, I. J., Cole, J. C., Edgington, P. R., Kessler, M., Macrae, C. F., McCabe, P., Pearson, J. & Taylor, R. (2002). Acta Cryst. B58, 389–397. [DOI] [PubMed]
- Das, A., Das, S., Biswas, A. & Chattopadhyay, N. (2021). J. Phys. Chem. B, 125, 13482–13493. [DOI] [PubMed]
- Elgemeie, G. H. (1989). Chem. Ind. 19, 653–654.
- Elgemeie, G. H., Ahmed, K. A., ahmed, E. A., helal, M. H. & Masoud, D. M. (2015). Pigm. Resin Technol. 44, 87–93.
- Elgemeie, G. H. & Elghandour, A. H. (1990). Bull. Chem. Soc. Jpn, 63, 1230–1232.
- Elgemeie, G. H., Shams, H. Z., Elkholy, Y. M. & Abbas, N. S. (2000a). Phosphorus Sulfur Silicon, 165, 265–272.
- Elgemeie, G. H., Shams, Z., Elkholy, M. & Abbas, N. S. (2000b). Heterocycl. Commun. 6, 363–268.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Lohar, S., Dhara, K., Roy, P., Sinha Babu, S. P. & Chattopadhyay, P. (2018). ACS Omega, 3, 10145–10153. [DOI] [PMC free article] [PubMed]
- Lu, F., Hu, R., Wang, S., Guo, X. & Yang, G. (2017). RSC Adv. 7, 4196–4202.
- Metwally, N. H., Elgemeie, G. H. & Fahmy, F. G. (2022b). Egypt. J. Chem. 65, 679–686.
- Metwally, N. H., Elgemeie, G. H. & Jones, P. G. (2021a). Acta Cryst. E77, 615–617. [DOI] [PMC free article] [PubMed]
- Metwally, N. H., Elgemeie, G. H. & Jones, P. G. (2021b). Acta Cryst. E77, 1054–1057. [DOI] [PMC free article] [PubMed]
- Metwally, N. H., Elgemeie, G. H. & Jones, P. G. (2022a). Acta Cryst. E78, 445–448. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Satpati, A. K., Kumbhakar, M., Nath, S. & Pal, H. (2009). Photochem. Photobiol. 85, 119–129. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. C71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. A71, 3–8.
- Shishkina, S. V., Konovalova, I. S., Kovalenko, S. M., Trostianko, P. V., Geleverya, A. O., Nikolayeva, L. L. & Bunyatyan, N. D. (2019). Acta Cryst. B75, 887–902. [DOI] [PubMed]
- Siemens (1994). XP. Siemens Analytical X–Ray Instruments, Madison, Wisconsin, USA.
- Singh, R., Chen, D.-G., Wang, C.-H., Wu, C.-C., Hsu, C.-H., Wu, C.-H., Lai, T.-Y., Chou, P. & Chen, C. (2022). J. Mater. Chem. B, 10, 6228–6236. [DOI] [PubMed]
- Wang, H., Chen, G., Xu, X., Chen, H. & Ji, S. (2010). Dyes Pigments, 86, 238–248.
- Wu, W., Wu, W., Ji, S., Guo, H. & Zhao, J. (2011). Dalton Trans. 40, 5953–5963. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989023002979/yz2032sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023002979/yz2032Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989023002979/yz2032Isup3.cml
CCDC reference: 2252955
Additional supporting information: crystallographic information; 3D view; checkCIF report