The crystal structures of a dihydrofurylsilane and a dihydrofurylgermane are reported. Hirshfeld surface analyses were performed to investigate the intermolecular interactions.
Keywords: crystal structure, dihydrofuranyl groups (DHF), Hirshfeld surface analysis, dihydrofurylsilan, dihydrofurylgermane
Abstract
The title compounds Si(C4H5O)4 (1) and Ge(C4H5O)4 (2) are dihydrofuryl compounds of silicon and germanium and are useful building blocks for the functionalization of these elements. Both structures crystallize in space group P21/n in the monoclinic crystal system with two molecules in the asymmetric unit: the Si and Ge atoms adopt slightly distorted tetrahedral geometries, while the C4H5O moieties exhibit shallow envelope conformations. Through a Hirshfeld surface analysis of the structures, interactions within the crystal packing could be elucidated: compound 1 features a polymeric chain in the (101) plane via C—H⋯O hydrogen bonds whereas in 2 C—H⋯O hydrogen bonds create a polymeric chain in the (010) plane.
1. Chemical context
The first dihydrofurylsilanes (DHF) were prepared by Lukevits and co-workers in the 1980s (Gevorgyan et al., 1989 ▸). The dihydrofuryl substituent is a good leaving group for nucleophilic substitution on silicon and has therefore been further investigated since then (Lukevits et al., 1993 ▸). Primarily carbon nucleophiles (e.g. lithium alkyls) can be used for substitution of the DHF groups (Lukevits et al., 1993 ▸). Nitrogen and oxygen nucleophiles (e.g. LiNEt2 or t-butanol) also serve to cleave the Si–C(DHF) bond: this is an alternative way of introducing an Si—N bond into a compound compared to conventional synthesis methods using, for example, methoxysilanes (Bauer & Strohmann, 2014 ▸). With an oxygen nucleophile such as pyrocatechol, pentavalent silicates can be synthesized (Tacke et al., 1991 ▸, 1993 ▸). Hydrides, likewise, are useful for substitutions (e.g. LiAlH4) (Gevorgyan et al., 1990 ▸, 1992 ▸). Thus, silanes can be synthesised in a very precise way (Lukevics et al., 1985 ▸, 1997 ▸). Analogous to DHF silanes, it is also possible to form germanes with dihydrofuryl substituents. The substitution of the DHF groups on germanium is possible via lithium alkyls or hydrides, similar to silanes (Lukevics et al., 1985 ▸). The crystal structures of various dihydrofurylsilanes such as bis(4,5-dihydrofuran-2-yl)(dimethyl)silane and (4,5-dihydrofuran-2-yl)(methyl)diphenylsilane (Schmidt et al., 2022 ▸) or tris(4,5-dihydrofuran-2-yl)(methyl)silane and tris(4,5-dihydrofuran-2-yl)(phenyl)silane (Krupp et al., 2020 ▸) are already known. Here, we report the crystal structures of tetrakis(4,5-dihydrofuran-2-yl)silane, Si(C4H5O)4 (1) and tetrakis(4,5-dihydrofuran-2-yl)germane, Ge(C4H5O)4 (2) and their extended structures, which were investigated using a Hirshfeld surface analysis. The two compounds are already known in the literature (Lukevics et al., 1984 ▸; Ertschak et al., 1982 ▸).

2. Structural commentary
The molecular structure of 1 is shown in Fig. 1 ▸ and selected bond lengths and angles of the solid-state structure are shown in Table 1 ▸. There are two molecules in the asymmetric unit. The listed bond lengths of the Si–C(DHF) links are all in a comparable range. In addition, the bond lengths are consistent with the characteristic Si—C bond length (Allen et al., 1987 ▸). The C—Si—C bond angles deviate from the ideal value of 109.47° and indicate a slightly distorted tetrahedron. This has already been described in related compounds by Strohmann and co-workers (Krupp et al., 2020 ▸; Schmidt et al., 2022 ▸). This slight distortion is possibly due to the packing in the solid state. The bond lengths of the C=C bonds within the dihydrofuranyl substituent show agreement with bond lengths known in the literature. The C—C single bond between the two sp 3 carbon atoms shows a clear deviation from the median known in literature, and is nearly in the lower quartile. This was also previously described by Strohmann and co-workers (Krupp et al., 2020 ▸; Schmidt et al., 2022 ▸). The DHF rings of the structure do not show complete planarity and have the r.m.s deviations shown in Table 2 ▸. The largest deviation of an atom from the planar position is shown by the sp 3 carbon atom C28, which is located next to the oxygen atom O7. This has also been reported for comparable structures (Schmidt et al., 2022 ▸). In addition, Table 2 ▸ shows the dihedral angles between the normals of two rings.
Figure 1.
The molecular structure of compound 1 with displacement ellipsoids drawn at the 50% probability level.
Table 1. Selected geometric parameters (Å, °) for 1 .
| Si1—C1 | 1.8632 (10) | Si2—C17 | 1.8621 (10) |
| Si1—C5 | 1.8611 (9) | Si2—C21 | 1.8598 (10) |
| Si1—C9 | 1.8604 (10) | Si2—C25 | 1.8615 (10) |
| Si1—C13 | 1.8633 (10) | Si2—C29 | 1.8609 (10) |
| C5—Si1—C1 | 110.75 (4) | C21—Si2—C17 | 106.75 (4) |
| C5—Si1—C13 | 108.60 (4) | C21—Si2—C25 | 109.54 (4) |
| C9—Si1—C1 | 109.39 (4) | C21—Si2—C29 | 112.87 (4) |
| C9—Si1—C5 | 106.46 (4) | C25—Si2—C17 | 110.57 (4) |
| C9—Si1—C13 | 113.04 (4) | C29—Si2—C17 | 108.47 (4) |
| C13—Si1—C1 | 108.60 (4) | C29—Si2—C25 | 108.64 (4) |
Table 2. Conformations (Å, °) of the DHF rings for compound 1 .
| DHF ring | r.m.s. deviation | Largest deviation | Angle between ring normals |
|---|---|---|---|
| C1–C4/O1 | 0.067 | C4 −0.0936 (6) | — |
| C5–C8/O2 | 0.038 | C8 −0.0521 (8) | 82.18 (4) a |
| C9–C12/O3 | 0.034 | C12 −0.0468 (7) | 42.32 (4) a |
| C13–C16/O4 | 0.049 | C16 −0.0679 (7) | 45.01 (4) a |
| C17–C20/O5 | 0.054 | C20 −0.0934 (6) | — |
| C21–C24/O6 | 0.050 | C24 −0.0699 (7) | 48.41 (6) b |
| C25–C28/O7 | 0.093 | C28 0.1298 (9) | 55.30 (6) b |
| C29–C32/O8 | 0.029 | C32 −0.0397 (7) | 81.77 (4) b |
Notes: (a) compared to C1–C4/O1; (b) compared to C17–C20/O5.
The molecular structure of 2 is shown in Fig. 2 ▸. There are two molecules in the asymmetric unit and selected bond lengths and angles are given in Table 3 ▸. The Ge—C bonds are in a comparable range and are consistent with similar bond lengths in the literature (Lazraq et al., 1988 ▸). As already described for structure 1, the germane 2 also shows a slight deviation from the ideal tetrahedral value for the C—Ge—C bond angles, which can also be explained by the packing in the solid state. Likewise, the bond lengths of the C=C groups within the dihydrofuranyl rings show consistency with bond lengths known in the literature, as well as the C—C bond between two sp 3 carbon atoms showing similar peculiarities as previously described (Krupp et al., 2020 ▸; Schmidt et al., 2022 ▸). The DHF rings of the structure do not show complete planarity and have the r.m.s deviations shown in Table 3 ▸. Compared to 1, the deviations in 2 are smaller and, again, the sp 3 carbon atom C28, which is located next to O7, shows the highest deviation. However, this does not apply to the C21–C24/O6 ring as this has a very low r.m.s. deviation and C21 shows the highest deviation. The dihedral angles between the normals of two rings are listed in Table 4 ▸.
Figure 2.
The molecular structure of compound 2 with displacement ellipsoids drawn at the 50% probability level.
Table 3. Selected geometric parameters (Å, °) for 2 .
| Ge1—C1 | 1.9331 (13) | Ge2—C17 | 1.9370 (13) |
| Ge1—C5 | 1.9326 (14) | Ge2—C21 | 1.9290 (13) |
| Ge1—C9 | 1.9299 (14) | Ge2—C25 | 1.9329 (13) |
| Ge1—C13 | 1.9351 (14) | Ge2—C29 | 1.9353 (14) |
| C1—Ge1—C13 | 109.37 (6) | C21—Ge2—C17 | 108.03 (6) |
| C5—Ge1—C1 | 109.74 (6) | C21—Ge2—C25 | 108.58 (5) |
| C5—Ge1—C13 | 108.78 (6) | C21—Ge2—C29 | 112.88 (6) |
| C9—Ge1—C1 | 107.67 (6) | C25—Ge2—C17 | 107.92 (5) |
| C9—Ge1—C5 | 108.46 (6) | C25—Ge2—C29 | 106.91 (6) |
| C9—Ge1—C13 | 112.79 (6) | C29—Ge2—C17 | 112.36 (6) |
Table 4. Conformations (Å, °) of the DHF rings for compound 2 .
| DHF ring | r.m.s. deviation | Largest deviation | Angle between ring normals |
|---|---|---|---|
| C1–C4/O1 | 0.015 | C3 −0.0196 (12) | — |
| C5–C8/O2 | 0.038 | C8 −0.0526 (9) | 81.75 (6) a |
| C9–C12/O3 | 0.017 | C12 0.0240 (13) | 62.38 (7) a |
| C13–C16/O4 | 0.029 | C16 0.0399 (11) | 82.47 (7) a |
| C17–C20/O5 | 0.032 | C20 0.0442 (11) | — |
| C21–C24/O6 | 0.007 | C21 0.0094 (10) | 87.22 (9) b |
| C25–C28/O7 | 0.068 | C28 −0.0941 (9) | 45.36 (6) b |
| C29–C32/O8 | 0.033 | C32 0.0462 (9) | 80.68 (7) b |
Notes: (a) compared to C1–C4/O1; (b) compared to C17–C20/O5.
3. Supramolecular features
In order to quantify the intermolecular interactions in the crystal structure, a Hirshfeld surface analysis was carried out, generated by CrystalExplorer21 (Spackman et al., 2021 ▸). The Hirshfeld surface of 1 is shown in Fig. 3 ▸, where the red areas represent closer interactions between adjacent atoms. The Hirshfeld surface is mapped over d
norm, in the range −0.11 to 1.37 a.u. The distribution of the different interactions is illustrated by the two-dimensional fingerprint plots (Fig. 4 ▸). Interactions identified by the Hirshfeld surface are mostly H⋯H interactions, which contribute 69.9% to the crystal packing. The close interaction H23A⋯H23A
i [symmetry code: (i) −x, −y, −z] with a distance of 2.22 (4) Å was identified by the red spots on the Hirshfeld surface. However, these red spots show only a small proportion of the interactions indicated in the fingerprint. Furthermore, a C30⋯H24B
ii [symmetry code: (ii)
 − x,
− x,
 + y,
+ y,
 − z] van der Waals interaction with a separation of 2.796 (16) Å was also identified. The contribution of the C⋯H interactions is 10.7%, which is a low contribution to the crystal packing. Besides these interactions, H⋯O interactions could be identified and contribute 19.2% of the structure in the solid state. Hydrogen bonds between C—H⋯O, which are indicated by red spots on the Hirshfeld surface are listed in Table 5 ▸. The C—H⋯O hydrogen bonds C8—H8A⋯O5ii, C27—H27B⋯O3iii and C31—H31A⋯O4 can be described as having a D
1
1(2) graph-set motif. C23—H23B⋯O5i is described by
− z] van der Waals interaction with a separation of 2.796 (16) Å was also identified. The contribution of the C⋯H interactions is 10.7%, which is a low contribution to the crystal packing. Besides these interactions, H⋯O interactions could be identified and contribute 19.2% of the structure in the solid state. Hydrogen bonds between C—H⋯O, which are indicated by red spots on the Hirshfeld surface are listed in Table 5 ▸. The C—H⋯O hydrogen bonds C8—H8A⋯O5ii, C27—H27B⋯O3iii and C31—H31A⋯O4 can be described as having a D
1
1(2) graph-set motif. C23—H23B⋯O5i is described by
 (7) (Etter et al., 1990 ▸). Through the hydrogen bonds C27—H27B⋯O3iii and C31—H31A⋯O4, a part of the crystal packing is defined along the [101] direction (Fig. 5 ▸).
(7) (Etter et al., 1990 ▸). Through the hydrogen bonds C27—H27B⋯O3iii and C31—H31A⋯O4, a part of the crystal packing is defined along the [101] direction (Fig. 5 ▸).
Figure 3.
Hirshfeld surface analysis of 1 showing close contacts in the crystal. (a) The hydrogen bonds between C8—H8A⋯O5ii, C23i—H23B
i⋯O5, C27iii—H27B
iii⋯O3 and C31—H31A⋯O4 are labelled [symmetry codes: (i) −
 + x,
+ x,
 + y,
+ y,
 − z; (ii)
− z; (ii)
 + x,
+ x,
 − y,
− y,
 + z; (iii)
+ z; (iii)
 + x,
+ x,
 − y,
− y,
 + z]. (b) The close hydrogen–hydrogen interaction H23A⋯H23A
i and close carbon–hydrogen interaction C30ii⋯H24B are labelled [symmetry codes: (i) −x + 1, −y + 1, −z + 2; (ii)
+ z]. (b) The close hydrogen–hydrogen interaction H23A⋯H23A
i and close carbon–hydrogen interaction C30ii⋯H24B are labelled [symmetry codes: (i) −x + 1, −y + 1, −z + 2; (ii)
 − x, −
− x, −
 + y,
+ y,
 − z].
− z].
Figure 4.
Two-dimensional fingerprint plots for compound 1, showing (a) all contributions, and (b)–(d) delineated into the contributions of atoms within specific interacting pairs (blue areas).
Table 5. Hydrogen-bond geometry (Å, °) for 1 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C23—H23B⋯O5i | 0.974 (18) | 2.531 (18) | 3.3484 (14) | 141.5 (14) |
| C8—H8A⋯O5ii | 0.95 (2) | 2.61 (2) | 3.3800 (15) | 137.9 (15) |
| C27—H27B⋯O3iii | 0.92 (2) | 2.61 (2) | 3.4200 (16) | 147.1 (18) |
| C31—H31A⋯O4 | 0.986 (18) | 2.561 (18) | 3.5358 (14) | 169.6 (15) |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 .
.
Figure 5.
A part of the crystal packing of compound 1
via hydrogen bonds C27–H27B⋯O3iii and C31—H31A⋯O4 in the (101) plane. C—H⋯O hydrogen bonds are shown as dashed blue lines. [Symmetry codes: (iii) −
 + x,
+ x,
 − y,
− y,
 + z].
+ z].
For the Hirshfeld surface analysis of 2, a surface mapped over d
norm in the range −0.15 to 1.33 a.u. was used (Fig. 6 ▸). The distribution of the various interactions is illustrated by the two-dimensional fingerprint plots (Fig. 7 ▸). The distribution of the interactions is very similar and minimally larger for H⋯H (71.6%) than for 1. The interactions between H31B⋯H31B
i at 2.17 (4) Å and H7B⋯H7B
i [symmetry code: (i) −x, −y, −z] at 2.20 (4) Å are visible as red spots and could be identified as close interactions by the Hirshfeld surface. The contribution of the van der Waals interactions is slightly lower at 10.0%. The interaction C32⋯H4A
ii [symmetry code: (ii)
 − x,
− x,
 + y,
+ y,
 − z] at 2.79 (2) Å could also be detected on the Hirshfeld surface. As in the case of 1, interactions of the form H⋯O could be determined, which contribute 18.3% to the crystal packing. These hydrogen bonds were detected by red spots on the Hirshfeld surface and are shown in Table 6 ▸. C4—H4A⋯O7i and C23–H23A⋯O4ii can be described by the graph-set motif D
1
1(2). In contrast, the hydrogen bond C31—H31A⋯O7iii is described by the graph-set motif
− z] at 2.79 (2) Å could also be detected on the Hirshfeld surface. As in the case of 1, interactions of the form H⋯O could be determined, which contribute 18.3% to the crystal packing. These hydrogen bonds were detected by red spots on the Hirshfeld surface and are shown in Table 6 ▸. C4—H4A⋯O7i and C23–H23A⋯O4ii can be described by the graph-set motif D
1
1(2). In contrast, the hydrogen bond C31—H31A⋯O7iii is described by the graph-set motif
 (7) (Etter et al., 1990 ▸). With C4—H4A⋯O7iii and C31—H31A⋯O7i, a part of the crystal packing, which forms a plane in the [010] direction, can be seen in Fig. 8 ▸.
(7) (Etter et al., 1990 ▸). With C4—H4A⋯O7iii and C31—H31A⋯O7i, a part of the crystal packing, which forms a plane in the [010] direction, can be seen in Fig. 8 ▸.
Figure 6.
Hirshfeld surface analysis of 2 showing close contacts in the crystal. (a) The hydrogen bonds between C4iii—H4A
iii⋯O7, C23—H23A⋯O4ii and C31i—H31A
i⋯O7 are labelled [symmetry codes: (i)
 + x, −
+ x, −
 + y,
+ y,
 − z; (ii) −
− z; (ii) −
 + x,
+ x,
 − y,
− y,
 + z; (iii) x, y, 1 + z]. (b) The close hydrogen–hydrogen interactions H31B⋯H31B
i and H7B⋯H7B
i [symmetry code: (i) −x, −y, −z] and the close carbon–hydrogen interaction C32⋯H4A
ii are labelled [symmetry code: (ii)
+ z; (iii) x, y, 1 + z]. (b) The close hydrogen–hydrogen interactions H31B⋯H31B
i and H7B⋯H7B
i [symmetry code: (i) −x, −y, −z] and the close carbon–hydrogen interaction C32⋯H4A
ii are labelled [symmetry code: (ii)
 − x,
− x,
 + y,
+ y,
 − z].
− z].
Figure 7.
Two-dimensional fingerprint plots for compound 2, showing (a) all contributions, and (b)–(d) delineated into the contributions of atoms within specific interacting pairs (blue areas).
Table 6. Hydrogen-bond geometry (Å, °) for 2 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C31—H31A⋯O7i | 0.964 (19) | 2.60 (2) | 3.3279 (18) | 132.1 (14) |
| C23—H23A⋯O4ii | 0.97 (2) | 2.57 (2) | 3.530 (2) | 168.0 (17) |
| C4—H4A⋯O7iii | 0.93 (2) | 2.63 (2) | 3.398 (2) | 140.8 (17) |
Symmetry codes: (i)
 ; (ii)
; (ii)
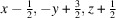 ; (iii)
; (iii)
 .
.
Figure 8.
A part of the crystal packing of compound 2
via hydrogen bonds C4—H4A⋯O7i and C23—H23A⋯O4ii in the (010) plane. C—H⋯O hydrogen bonds are shown as dashed blue lines [symmetry codes: (i)
 − x, −
− x, −
 + y, 3/2 − z; (ii) −
+ y, 3/2 − z; (ii) −
 + x,
+ x,
 − y,
− y,
 + z].
+ z].
4. Database survey
A search of the Cambridge Crystallographic Database (Groom et al., 2016 ▸; WebCSD, accessed January 2023) for the term 2-(4,5-dihydrofuryl)silanes gave bis(4,5-dihydrofuran-2-yl)(dimethyl)silane and (4,5-dihydrofuran-2-yl)(methyl)diphenylsilane (CSD refcodes GAVJUM and GAVKAT; Schmidt et al., 2022 ▸) as well as tris(4,5-dihydrofuran-2-yl)(methyl)silane and tris(4,5-dihydrofuran-2-yl)(phenyl)silane (YUYCED and YUYCON; Krupp et al., 2020 ▸), previously published by our group. These compounds show comparable Si—C(DHF), C—C and C=C bond lengths to those of 1 and 2. They also display similar (DHF)C—Si—C(DHF) bond angles and also a slightly distorted tetrahedron. In addition, a deviation in the planarity of the dihydrofuryl rings was found there. An extended search for 3-(4,5-dihydrofuryl)silanes revealed the compounds [4-(4-fluorophenyl)-5-(4-nitrophenyl)-4,5-dihydrofuran-3-yl](trimethyl)silane (JIVLIM; Li & Zhang, 2018 ▸), (1′S,2R)-5-methyl-4-(t-butyldiphenylsilyl)-2,3-dihydro-furan-2-carboxylic acid (1′-phenylethyl)amide (PUXCAM; Evans et al., 2001 ▸) and 2,2-dichloro-5-phenyl-4-(trimethylsilyl)-3(2H)-furanone (YIHDOI; Murakami et al., 1994 ▸), which have little resemblance to the structure of 1. Tetrakis(2-furanyl)silane was also found in the database when searching for (2-furanyl)silane (XAMZOA; Neugebauer et al., 2000 ▸).
A search for 2-(4,5-dihydrofuryl)germane and an extended search for 3-(4,5-dihydrofuryl)germane found no hits.
5. Synthesis and crystallization
Compound 1 and 2 have already been described by Lukevits and Ertschak (Lukevics et al., 1984 ▸; Ertschak et al., 1982 ▸). For the synthesis of tetrakis(4,5-dihydrofuran-2-yl)silane (1), tert-butyllithium (31.0 ml, 1.90 M in pentane, 58.90 mmol, 4.00 eq.) was added at 228 K to a solution of 2,3-dihydrofuran (4.14 g, 59.10 mmol, 4.00 eq.) in diethyl ether (approx. 100 ml). The reaction solution was stirred for 1 h at room temperature. Then, tetrachlorosilane (2.50 g, 14.70 mmol, 1.00 eq.) was added at 243 K and the reaction solution was stirred for 1 h. The resulting solid was separated by inert filtration. The obtained solution was concentrated in vacuo and crystallized at 243 K. The solvent was removed and the solid was washed with cold diethyl ether. The product tetrakis(4,5-dihydrofuran-2-yl)silane (1) (3.05 g, 10.0 mmol, 68%) was obtained as colourless blocks.
1H NMR: (600.29 MHz, C6D6): δ = 2.25 [dt, 3 J HH = 2.57 Hz, 3 J HH = 9.72 Hz, 8H; Si(CCHCH 2)4], 4.06 [t, 3 J HH = 9.72 Hz, 8H; Si(COCH 2)4], 5.88 [t, 3 J HH = 2.57 Hz, 4H; Si(CCH)4] ppm. {1H}13C NMR: (150.94 MHz, C6D6): δ = 31.4 [4C; Si(CCHCH2)4], 71.0 [4C; Si(COCH2)4], 117.8 [4C; Si(CCH)4], 155.1 [4C; (Si(CO)4] ppm. {1H}29Si NMR: (119.26 MHz, C6D6): δ = −51.40 [s, 1Si; Si(DHF)4] ppm.
For the synthesis of tetrakis(4,5-dihydrofuran-2-yl)germane (2), tert-butyllithium (19.60 ml, 1.90 M in pentane, 37.30 mmol, 4.00 eq.) was added at 228 K to a solution of 2,3-dihydrofuran (2.60 g, 37.30 mmol, 4.00 eq.) in diethyl ether (approx. 100 ml). The reaction solution was stirred for 1 h at rt. Tetrachlorogermane (2.00 g, 9.33 mmol, 1.00 eq.) was added at 213 K and the reaction solution was stirred for 1 h. The resulting solid was separated by inert filtration. The obtained solution was concentrated in vacuo and crystallized at 243 K. The solvent was removed, and the solid was washed with cold diethyl ether. The product tetrakis(4,5-dihydrofuran-2-yl)germane (2) (2.94 g, 8.44 mmol, 91%) was obtained as colourless blocks.
1H NMR: (400.25 MHz, C6D6): δ = 2.26 [dt, 3 J HH = 2.57 Hz, 3 J HH = 9.66 Hz, 8H; Ge(CCHCH 2)4], 4.05 [t, 3 J HH = 9.66 Hz, 8H; Ge(COCH 2)4], 5.62 [t, 3 J HH = 2.57 Hz, 4H; Ge(CCH)4] ppm. {1H}13C NMR: (100.64 MHz, C6D6): δ = 30.8 [4C; Ge(CCHCH2)4], 71.0 [4C; Ge(COCH2)4], 113.8 [4C; Si(CCH)4], 155.7 [4C; (Ge(CO)4] ppm.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 7 ▸. Hydrogen atoms H8A,B, H23A,B, H24A,B, H27A,B and H31A,B for compound 1 and all H atoms for compound 2 were refined independently. Other H atoms were positioned geometrically (C—H = 0.95–1.00 Å) and were refined using a riding model, with U iso(H) = 1.2U eq(C) for CH2 and CH hydrogen.
Table 7. Experimental details.
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C16H20O4Si | C16H20GeO4 |
| M r | 304.41 | 348.91 |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 14.2044 (7), 14.2458 (7), 15.4851 (8) | 14.3828 (5), 14.2069 (5), 15.3594 (6) |
| β (°) | 102.605 (2) | 101.159 (1) |
| V (Å3) | 3057.9 (3) | 3079.13 (19) |
| Z | 8 | 8 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.17 | 2.00 |
| Crystal size (mm) | 0.68 × 0.54 × 0.48 | 0.19 × 0.16 × 0.08 |
| Data collection | ||
| Diffractometer | Bruker D8 VENTURE | Bruker D8 VENTURE |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.532, 0.570 | 0.496, 0.568 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 371220, 9331, 8779 | 71639, 13541, 10143 |
| R int | 0.036 | 0.046 |
| (sin θ/λ)max (Å−1) | 0.714 | 0.807 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.037, 0.103, 1.03 | 0.032, 0.077, 1.02 |
| No. of reflections | 9331 | 13541 |
| No. of parameters | 419 | 539 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.89, −0.38 | 0.67, −0.56 |
Supplementary Material
Crystal structure: contains datablock(s) 1. DOI: 10.1107/S2056989023003158/hb8058sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989023003158/hb80581sup2.hkl
Supporting information file. DOI: 10.1107/S2056989023003158/hb80581sup4.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989023003158/hb80582sup3.hkl
Supporting information file. DOI: 10.1107/S2056989023003158/hb80582sup5.cml
CCDC reference: 2254180
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Crystal data
| C16H20O4Si | F(000) = 1296 |
| Mr = 304.41 | Dx = 1.322 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 14.2044 (7) Å | Cell parameters from 9905 reflections |
| b = 14.2458 (7) Å | θ = 2.6–17.2° |
| c = 15.4851 (8) Å | µ = 0.17 mm−1 |
| β = 102.605 (2)° | T = 100 K |
| V = 3057.9 (3) Å3 | Block, white |
| Z = 8 | 0.68 × 0.54 × 0.48 mm |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Data collection
| Bruker D8 VENTURE diffractometer | 9331 independent reflections |
| Radiation source: microfocus sealed X-ray tube, Incoatec Iµs | 8779 reflections with I > 2σ(I) |
| HELIOS mirror optics monochromator | Rint = 0.036 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 30.5°, θmin = 2.1° |
| ω and φ scans | h = −20→20 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −20→20 |
| Tmin = 0.532, Tmax = 0.570 | l = −22→22 |
| 371220 measured reflections |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.037 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.103 | w = 1/[σ2(Fo2) + (0.0578P)2 + 1.2587P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 9331 reflections | Δρmax = 0.89 e Å−3 |
| 419 parameters | Δρmin = −0.38 e Å−3 |
| 0 restraints |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Si1 | 0.74622 (2) | 0.28841 (2) | 0.75077 (2) | 0.01509 (6) | |
| O1 | 0.93162 (5) | 0.25935 (5) | 0.85344 (5) | 0.02192 (14) | |
| O2 | 0.66166 (7) | 0.30739 (6) | 0.89526 (5) | 0.02813 (17) | |
| O3 | 0.72770 (6) | 0.45724 (6) | 0.65291 (5) | 0.02471 (15) | |
| O4 | 0.68036 (6) | 0.15675 (7) | 0.61688 (6) | 0.03055 (19) | |
| C1 | 0.86791 (7) | 0.32761 (7) | 0.81134 (6) | 0.01724 (16) | |
| C2 | 0.90373 (7) | 0.41431 (7) | 0.82338 (7) | 0.02177 (18) | |
| H2 | 0.871274 | 0.469549 | 0.798507 | 0.026* | |
| C3 | 1.00352 (8) | 0.41171 (8) | 0.88241 (7) | 0.0249 (2) | |
| H3A | 1.003889 | 0.437693 | 0.941784 | 0.030* | |
| H3B | 1.050636 | 0.446387 | 0.855916 | 0.030* | |
| C4 | 1.02411 (7) | 0.30597 (8) | 0.88647 (7) | 0.02419 (19) | |
| H4A | 1.070235 | 0.289812 | 0.849205 | 0.029* | |
| H4B | 1.052002 | 0.286370 | 0.948076 | 0.029* | |
| C5 | 0.67360 (7) | 0.24575 (7) | 0.82938 (6) | 0.01708 (16) | |
| C6 | 0.63131 (8) | 0.16312 (7) | 0.83393 (7) | 0.02348 (19) | |
| H6 | 0.630973 | 0.112660 | 0.793750 | 0.028* | |
| C7 | 0.58398 (9) | 0.16119 (7) | 0.91211 (8) | 0.0264 (2) | |
| H7A | 0.616413 | 0.116278 | 0.957809 | 0.032* | |
| H7B | 0.514695 | 0.144991 | 0.893973 | 0.032* | |
| C8 | 0.59827 (10) | 0.26230 (8) | 0.94469 (8) | 0.0310 (2) | |
| C9 | 0.67957 (7) | 0.39005 (7) | 0.69126 (6) | 0.01753 (16) | |
| C10 | 0.58506 (7) | 0.40639 (8) | 0.68000 (7) | 0.02276 (18) | |
| H10 | 0.540808 | 0.367188 | 0.701030 | 0.027* | |
| C11 | 0.55907 (8) | 0.49611 (8) | 0.62906 (8) | 0.0270 (2) | |
| H11A | 0.537713 | 0.544989 | 0.666015 | 0.032* | |
| H11B | 0.507913 | 0.485739 | 0.575246 | 0.032* | |
| C12 | 0.65508 (8) | 0.52240 (8) | 0.60570 (7) | 0.0257 (2) | |
| H12A | 0.649902 | 0.517179 | 0.541082 | 0.031* | |
| H12B | 0.672832 | 0.587780 | 0.623997 | 0.031* | |
| C13 | 0.76134 (7) | 0.19008 (7) | 0.67562 (6) | 0.01761 (16) | |
| C14 | 0.84148 (7) | 0.14317 (7) | 0.67128 (6) | 0.02059 (17) | |
| H14 | 0.903761 | 0.156174 | 0.706378 | 0.025* | |
| C15 | 0.81904 (8) | 0.06663 (8) | 0.60272 (7) | 0.02422 (19) | |
| H15A | 0.827125 | 0.003588 | 0.630249 | 0.029* | |
| H15B | 0.860033 | 0.071638 | 0.558782 | 0.029* | |
| C16 | 0.71307 (7) | 0.08715 (8) | 0.56085 (7) | 0.02435 (19) | |
| H16A | 0.706698 | 0.111915 | 0.500114 | 0.029* | |
| H16B | 0.674129 | 0.029137 | 0.557871 | 0.029* | |
| Si2 | 0.27704 (2) | 0.32421 (2) | 0.76377 (2) | 0.01562 (6) | |
| O5 | 0.09592 (6) | 0.28846 (5) | 0.65743 (5) | 0.02374 (15) | |
| O6 | 0.27220 (6) | 0.47644 (6) | 0.87285 (5) | 0.02643 (16) | |
| O7 | 0.21828 (7) | 0.14802 (6) | 0.81068 (5) | 0.03273 (19) | |
| O8 | 0.34396 (7) | 0.33545 (6) | 0.60778 (5) | 0.02971 (18) | |
| C17 | 0.15457 (7) | 0.35882 (6) | 0.70150 (6) | 0.01701 (16) | |
| C18 | 0.11405 (8) | 0.44363 (7) | 0.69027 (7) | 0.02251 (18) | |
| H18 | 0.143122 | 0.499966 | 0.716098 | 0.027* | |
| C19 | 0.01501 (8) | 0.43670 (7) | 0.63035 (7) | 0.02359 (19) | |
| H19A | 0.013165 | 0.466606 | 0.572297 | 0.028* | |
| H19B | −0.034782 | 0.465527 | 0.657760 | 0.028* | |
| C20 | 0.00210 (7) | 0.33032 (7) | 0.62152 (7) | 0.02269 (19) | |
| H20A | −0.045780 | 0.308461 | 0.654906 | 0.027* | |
| H20B | −0.020752 | 0.312609 | 0.558609 | 0.027* | |
| C21 | 0.33102 (7) | 0.42965 (7) | 0.82634 (6) | 0.01817 (16) | |
| C22 | 0.41603 (8) | 0.47116 (8) | 0.83225 (8) | 0.0262 (2) | |
| H22 | 0.465781 | 0.449003 | 0.805262 | 0.031* | |
| C23 | 0.42186 (8) | 0.55853 (8) | 0.88805 (9) | 0.0290 (2) | |
| C24 | 0.31965 (8) | 0.56474 (7) | 0.90322 (7) | 0.02153 (18) | |
| C25 | 0.26895 (7) | 0.22739 (7) | 0.84255 (6) | 0.01758 (16) | |
| C26 | 0.30693 (9) | 0.22394 (8) | 0.92899 (7) | 0.0288 (2) | |
| H26 | 0.343000 | 0.272690 | 0.962848 | 0.035* | |
| C27 | 0.28359 (12) | 0.13035 (9) | 0.96465 (8) | 0.0375 (3) | |
| C28 | 0.20576 (9) | 0.09462 (8) | 0.88849 (8) | 0.0285 (2) | |
| H28A | 0.213710 | 0.026545 | 0.879398 | 0.034* | |
| H28B | 0.140908 | 0.105698 | 0.900266 | 0.034* | |
| C29 | 0.34752 (7) | 0.28210 (7) | 0.68322 (6) | 0.01786 (16) | |
| C30 | 0.39575 (7) | 0.20199 (7) | 0.68338 (7) | 0.02305 (19) | |
| H30 | 0.406776 | 0.157557 | 0.730256 | 0.028* | |
| C31 | 0.43036 (8) | 0.19189 (8) | 0.59856 (8) | 0.0278 (2) | |
| C32 | 0.38774 (9) | 0.28001 (8) | 0.54776 (7) | 0.0271 (2) | |
| H32A | 0.439067 | 0.316510 | 0.528906 | 0.033* | |
| H32B | 0.338577 | 0.262374 | 0.494472 | 0.033* | |
| H23A | 0.4712 (13) | 0.5512 (12) | 0.9465 (11) | 0.041 (5)* | |
| H23B | 0.4341 (13) | 0.6133 (13) | 0.8545 (11) | 0.041 (4)* | |
| H8A | 0.6292 (14) | 0.2656 (14) | 1.0055 (13) | 0.052 (5)* | |
| H8B | 0.5353 (15) | 0.2977 (14) | 0.9324 (13) | 0.053 (5)* | |
| H27A | 0.3448 (15) | 0.0826 (15) | 0.9746 (13) | 0.059 (6)* | |
| H27B | 0.2664 (15) | 0.1338 (15) | 1.0187 (14) | 0.061 (6)* | |
| H31A | 0.5013 (13) | 0.1894 (13) | 0.6088 (11) | 0.040 (4)* | |
| H31B | 0.4046 (14) | 0.1348 (14) | 0.5651 (13) | 0.050 (5)* | |
| H24A | 0.3158 (10) | 0.5717 (11) | 0.9629 (10) | 0.024 (3)* | |
| H24B | 0.2840 (11) | 0.6143 (11) | 0.8672 (10) | 0.030 (4)* |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Si1 | 0.01493 (11) | 0.01596 (12) | 0.01465 (11) | −0.00030 (8) | 0.00382 (8) | −0.00069 (8) |
| O1 | 0.0174 (3) | 0.0222 (3) | 0.0238 (3) | −0.0004 (3) | −0.0006 (3) | 0.0016 (3) |
| O2 | 0.0447 (5) | 0.0198 (3) | 0.0264 (4) | −0.0128 (3) | 0.0220 (3) | −0.0079 (3) |
| O3 | 0.0213 (3) | 0.0232 (3) | 0.0288 (4) | 0.0003 (3) | 0.0036 (3) | 0.0100 (3) |
| O4 | 0.0163 (3) | 0.0400 (5) | 0.0336 (4) | 0.0013 (3) | 0.0016 (3) | −0.0198 (4) |
| C1 | 0.0162 (4) | 0.0208 (4) | 0.0150 (4) | −0.0006 (3) | 0.0039 (3) | 0.0003 (3) |
| C2 | 0.0225 (4) | 0.0212 (4) | 0.0208 (4) | −0.0030 (3) | 0.0030 (3) | −0.0010 (3) |
| C3 | 0.0218 (4) | 0.0290 (5) | 0.0230 (4) | −0.0070 (4) | 0.0026 (4) | −0.0047 (4) |
| C4 | 0.0167 (4) | 0.0316 (5) | 0.0227 (4) | −0.0027 (4) | 0.0008 (3) | 0.0011 (4) |
| C5 | 0.0181 (4) | 0.0170 (4) | 0.0168 (4) | −0.0005 (3) | 0.0054 (3) | −0.0019 (3) |
| C6 | 0.0285 (5) | 0.0179 (4) | 0.0278 (5) | −0.0048 (4) | 0.0145 (4) | −0.0059 (4) |
| C7 | 0.0351 (5) | 0.0192 (4) | 0.0295 (5) | −0.0081 (4) | 0.0171 (4) | −0.0036 (4) |
| C8 | 0.0477 (7) | 0.0216 (5) | 0.0322 (6) | −0.0104 (5) | 0.0271 (5) | −0.0062 (4) |
| C9 | 0.0191 (4) | 0.0174 (4) | 0.0159 (4) | −0.0001 (3) | 0.0033 (3) | −0.0011 (3) |
| C10 | 0.0194 (4) | 0.0243 (5) | 0.0241 (4) | 0.0021 (3) | 0.0037 (3) | 0.0000 (4) |
| C11 | 0.0244 (5) | 0.0257 (5) | 0.0277 (5) | 0.0067 (4) | −0.0016 (4) | 0.0003 (4) |
| C12 | 0.0290 (5) | 0.0210 (4) | 0.0246 (5) | 0.0032 (4) | 0.0000 (4) | 0.0048 (4) |
| C13 | 0.0171 (4) | 0.0190 (4) | 0.0168 (4) | −0.0010 (3) | 0.0037 (3) | −0.0020 (3) |
| C14 | 0.0198 (4) | 0.0205 (4) | 0.0201 (4) | 0.0029 (3) | 0.0012 (3) | −0.0025 (3) |
| C15 | 0.0240 (4) | 0.0221 (4) | 0.0252 (5) | 0.0048 (4) | 0.0025 (4) | −0.0058 (4) |
| C16 | 0.0217 (4) | 0.0270 (5) | 0.0240 (4) | −0.0009 (4) | 0.0043 (3) | −0.0096 (4) |
| Si2 | 0.01722 (12) | 0.01467 (12) | 0.01523 (11) | −0.00029 (8) | 0.00411 (9) | −0.00052 (8) |
| O5 | 0.0218 (3) | 0.0164 (3) | 0.0287 (4) | 0.0020 (2) | −0.0038 (3) | −0.0029 (3) |
| O6 | 0.0260 (4) | 0.0261 (4) | 0.0300 (4) | −0.0089 (3) | 0.0122 (3) | −0.0140 (3) |
| O7 | 0.0474 (5) | 0.0228 (4) | 0.0234 (4) | −0.0128 (3) | −0.0022 (3) | 0.0023 (3) |
| O8 | 0.0418 (5) | 0.0291 (4) | 0.0225 (4) | 0.0143 (3) | 0.0164 (3) | 0.0068 (3) |
| C17 | 0.0193 (4) | 0.0165 (4) | 0.0158 (4) | 0.0002 (3) | 0.0051 (3) | −0.0006 (3) |
| C18 | 0.0257 (5) | 0.0171 (4) | 0.0238 (4) | 0.0023 (3) | 0.0035 (4) | −0.0006 (3) |
| C19 | 0.0244 (4) | 0.0227 (4) | 0.0234 (4) | 0.0074 (4) | 0.0046 (4) | 0.0032 (4) |
| C20 | 0.0178 (4) | 0.0252 (5) | 0.0245 (4) | 0.0024 (3) | 0.0033 (3) | −0.0003 (4) |
| C21 | 0.0218 (4) | 0.0156 (4) | 0.0174 (4) | −0.0007 (3) | 0.0048 (3) | −0.0001 (3) |
| C22 | 0.0233 (5) | 0.0203 (4) | 0.0365 (5) | −0.0024 (4) | 0.0100 (4) | −0.0042 (4) |
| C23 | 0.0243 (5) | 0.0195 (4) | 0.0425 (6) | −0.0054 (4) | 0.0060 (4) | −0.0061 (4) |
| C24 | 0.0268 (5) | 0.0170 (4) | 0.0202 (4) | −0.0022 (3) | 0.0037 (3) | −0.0028 (3) |
| C25 | 0.0187 (4) | 0.0159 (4) | 0.0183 (4) | 0.0007 (3) | 0.0044 (3) | 0.0002 (3) |
| C26 | 0.0353 (6) | 0.0249 (5) | 0.0216 (5) | −0.0109 (4) | −0.0037 (4) | 0.0042 (4) |
| C27 | 0.0539 (8) | 0.0304 (6) | 0.0227 (5) | −0.0157 (5) | −0.0039 (5) | 0.0089 (4) |
| C28 | 0.0301 (5) | 0.0211 (5) | 0.0324 (5) | −0.0068 (4) | 0.0025 (4) | 0.0044 (4) |
| C29 | 0.0181 (4) | 0.0186 (4) | 0.0174 (4) | −0.0007 (3) | 0.0049 (3) | −0.0005 (3) |
| C30 | 0.0219 (4) | 0.0208 (4) | 0.0283 (5) | 0.0028 (3) | 0.0095 (4) | 0.0027 (4) |
| C31 | 0.0267 (5) | 0.0268 (5) | 0.0330 (5) | 0.0044 (4) | 0.0133 (4) | −0.0041 (4) |
| C32 | 0.0300 (5) | 0.0330 (5) | 0.0210 (4) | 0.0036 (4) | 0.0111 (4) | −0.0027 (4) |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Geometric parameters (Å, º)
| Si1—C1 | 1.8632 (10) | Si2—C17 | 1.8621 (10) |
| Si1—C5 | 1.8611 (9) | Si2—C21 | 1.8598 (10) |
| Si1—C9 | 1.8604 (10) | Si2—C25 | 1.8615 (10) |
| Si1—C13 | 1.8633 (10) | Si2—C29 | 1.8609 (10) |
| O1—C1 | 1.3901 (11) | O5—C17 | 1.3843 (11) |
| O1—C4 | 1.4613 (12) | O5—C20 | 1.4552 (12) |
| O2—C5 | 1.3842 (11) | O6—C21 | 1.3869 (12) |
| O2—C8 | 1.4525 (13) | O6—C24 | 1.4559 (12) |
| O3—C9 | 1.3834 (12) | O7—C25 | 1.3729 (12) |
| O3—C12 | 1.4598 (12) | O7—C28 | 1.4683 (14) |
| O4—C13 | 1.3853 (11) | O8—C29 | 1.3853 (12) |
| O4—C16 | 1.4587 (12) | O8—C32 | 1.4583 (13) |
| C1—C2 | 1.3334 (13) | C17—C18 | 1.3332 (13) |
| C2—H2 | 0.9500 | C18—H18 | 0.9500 |
| C2—C3 | 1.5102 (14) | C18—C19 | 1.5099 (15) |
| C3—H3A | 0.9900 | C19—H19A | 0.9900 |
| C3—H3B | 0.9900 | C19—H19B | 0.9900 |
| C3—C4 | 1.5332 (16) | C19—C20 | 1.5291 (15) |
| C4—H4A | 0.9900 | C20—H20A | 0.9900 |
| C4—H4B | 0.9900 | C20—H20B | 0.9900 |
| C5—C6 | 1.3304 (13) | C21—C22 | 1.3297 (14) |
| C6—H6 | 0.9500 | C22—H22 | 0.9500 |
| C6—C7 | 1.5078 (14) | C22—C23 | 1.5071 (15) |
| C7—H7A | 0.9900 | C23—C24 | 1.5230 (15) |
| C7—H7B | 0.9900 | C23—H23A | 1.022 (18) |
| C7—C8 | 1.5251 (15) | C23—H23B | 0.974 (18) |
| C8—H8A | 0.95 (2) | C24—H24A | 0.942 (14) |
| C8—H8B | 1.01 (2) | C24—H24B | 0.971 (16) |
| C9—C10 | 1.3358 (13) | C25—C26 | 1.3303 (14) |
| C10—H10 | 0.9500 | C26—H26 | 0.9500 |
| C10—C11 | 1.5049 (15) | C26—C27 | 1.5073 (16) |
| C11—H11A | 0.9900 | C27—C28 | 1.5175 (17) |
| C11—H11B | 0.9900 | C27—H27A | 1.09 (2) |
| C11—C12 | 1.5317 (16) | C27—H27B | 0.92 (2) |
| C12—H12A | 0.9900 | C28—H28A | 0.9900 |
| C12—H12B | 0.9900 | C28—H28B | 0.9900 |
| C13—C14 | 1.3343 (13) | C29—C30 | 1.3307 (13) |
| C14—H14 | 0.9500 | C30—H30 | 0.9500 |
| C14—C15 | 1.5065 (14) | C30—C31 | 1.5065 (15) |
| C15—H15A | 0.9900 | C31—C32 | 1.5341 (17) |
| C15—H15B | 0.9900 | C31—H31A | 0.986 (18) |
| C15—C16 | 1.5315 (15) | C31—H31B | 0.99 (2) |
| C16—H16A | 0.9900 | C32—H32A | 0.9900 |
| C16—H16B | 0.9900 | C32—H32B | 0.9900 |
| C5—Si1—C1 | 110.75 (4) | C21—Si2—C17 | 106.75 (4) |
| C5—Si1—C13 | 108.60 (4) | C21—Si2—C25 | 109.54 (4) |
| C9—Si1—C1 | 109.39 (4) | C21—Si2—C29 | 112.87 (4) |
| C9—Si1—C5 | 106.46 (4) | C25—Si2—C17 | 110.57 (4) |
| C9—Si1—C13 | 113.04 (4) | C29—Si2—C17 | 108.47 (4) |
| C13—Si1—C1 | 108.60 (4) | C29—Si2—C25 | 108.64 (4) |
| C1—O1—C4 | 106.85 (8) | C17—O5—C20 | 107.10 (7) |
| C5—O2—C8 | 107.31 (8) | C21—O6—C24 | 107.11 (8) |
| C9—O3—C12 | 107.07 (8) | C25—O7—C28 | 106.23 (8) |
| C13—O4—C16 | 107.27 (8) | C29—O8—C32 | 107.34 (8) |
| O1—C1—Si1 | 117.64 (7) | O5—C17—Si2 | 117.21 (7) |
| C2—C1—Si1 | 129.18 (8) | C18—C17—Si2 | 129.46 (8) |
| C2—C1—O1 | 113.14 (8) | C18—C17—O5 | 113.31 (9) |
| C1—C2—H2 | 125.0 | C17—C18—H18 | 125.1 |
| C1—C2—C3 | 109.94 (9) | C17—C18—C19 | 109.80 (9) |
| C3—C2—H2 | 125.0 | C19—C18—H18 | 125.1 |
| C2—C3—H3A | 111.5 | C18—C19—H19A | 111.5 |
| C2—C3—H3B | 111.5 | C18—C19—H19B | 111.5 |
| C2—C3—C4 | 101.21 (8) | C18—C19—C20 | 101.38 (8) |
| H3A—C3—H3B | 109.3 | H19A—C19—H19B | 109.3 |
| C4—C3—H3A | 111.5 | C20—C19—H19A | 111.5 |
| C4—C3—H3B | 111.5 | C20—C19—H19B | 111.5 |
| O1—C4—C3 | 106.46 (8) | O5—C20—C19 | 106.85 (8) |
| O1—C4—H4A | 110.4 | O5—C20—H20A | 110.4 |
| O1—C4—H4B | 110.4 | O5—C20—H20B | 110.4 |
| C3—C4—H4A | 110.4 | C19—C20—H20A | 110.4 |
| C3—C4—H4B | 110.4 | C19—C20—H20B | 110.4 |
| H4A—C4—H4B | 108.6 | H20A—C20—H20B | 108.6 |
| O2—C5—Si1 | 116.71 (7) | O6—C21—Si2 | 115.61 (7) |
| C6—C5—Si1 | 130.14 (7) | C22—C21—Si2 | 131.30 (8) |
| C6—C5—O2 | 113.14 (8) | C22—C21—O6 | 113.05 (9) |
| C5—C6—H6 | 124.9 | C21—C22—H22 | 125.0 |
| C5—C6—C7 | 110.13 (9) | C21—C22—C23 | 110.01 (9) |
| C7—C6—H6 | 124.9 | C23—C22—H22 | 125.0 |
| C6—C7—H7A | 111.5 | C22—C23—C24 | 101.63 (8) |
| C6—C7—H7B | 111.5 | C22—C23—H23A | 111.4 (10) |
| C6—C7—C8 | 101.42 (8) | C22—C23—H23B | 110.5 (10) |
| H7A—C7—H7B | 109.3 | C24—C23—H23A | 111.2 (10) |
| C8—C7—H7A | 111.5 | C24—C23—H23B | 108.8 (10) |
| C8—C7—H7B | 111.5 | H23A—C23—H23B | 112.7 (14) |
| O2—C8—C7 | 107.24 (8) | O6—C24—C23 | 106.83 (8) |
| O2—C8—H8A | 107.2 (12) | O6—C24—H24A | 106.6 (9) |
| O2—C8—H8B | 107.8 (11) | O6—C24—H24B | 107.2 (9) |
| C7—C8—H8A | 112.0 (12) | C23—C24—H24A | 114.7 (9) |
| C7—C8—H8B | 111.2 (12) | C23—C24—H24B | 110.5 (9) |
| H8A—C8—H8B | 111.2 (16) | H24A—C24—H24B | 110.6 (13) |
| O3—C9—Si1 | 120.36 (7) | O7—C25—Si2 | 118.49 (7) |
| C10—C9—Si1 | 126.06 (8) | C26—C25—Si2 | 128.12 (8) |
| C10—C9—O3 | 113.58 (9) | C26—C25—O7 | 113.39 (9) |
| C9—C10—H10 | 125.0 | C25—C26—H26 | 125.4 |
| C9—C10—C11 | 109.94 (9) | C25—C26—C27 | 109.14 (10) |
| C11—C10—H10 | 125.0 | C27—C26—H26 | 125.4 |
| C10—C11—H11A | 111.4 | C26—C27—C28 | 101.14 (9) |
| C10—C11—H11B | 111.4 | C26—C27—H27A | 111.9 (11) |
| C10—C11—C12 | 101.67 (8) | C26—C27—H27B | 114.0 (14) |
| H11A—C11—H11B | 109.3 | C28—C27—H27A | 108.9 (11) |
| C12—C11—H11A | 111.4 | C28—C27—H27B | 115.5 (14) |
| C12—C11—H11B | 111.4 | H27A—C27—H27B | 105.5 (17) |
| O3—C12—C11 | 107.13 (8) | O7—C28—C27 | 105.41 (9) |
| O3—C12—H12A | 110.3 | O7—C28—H28A | 110.7 |
| O3—C12—H12B | 110.3 | O7—C28—H28B | 110.7 |
| C11—C12—H12A | 110.3 | C27—C28—H28A | 110.7 |
| C11—C12—H12B | 110.3 | C27—C28—H28B | 110.7 |
| H12A—C12—H12B | 108.5 | H28A—C28—H28B | 108.8 |
| O4—C13—Si1 | 118.38 (7) | O8—C29—Si2 | 117.54 (7) |
| C14—C13—Si1 | 128.60 (7) | C30—C29—Si2 | 128.85 (8) |
| C14—C13—O4 | 112.99 (8) | C30—C29—O8 | 113.34 (9) |
| C13—C14—H14 | 124.9 | C29—C30—H30 | 124.9 |
| C13—C14—C15 | 110.26 (9) | C29—C30—C31 | 110.25 (9) |
| C15—C14—H14 | 124.9 | C31—C30—H30 | 124.9 |
| C14—C15—H15A | 111.5 | C30—C31—C32 | 101.63 (8) |
| C14—C15—H15B | 111.5 | C30—C31—H31A | 112.3 (10) |
| C14—C15—C16 | 101.39 (8) | C30—C31—H31B | 112.4 (11) |
| H15A—C15—H15B | 109.3 | C32—C31—H31A | 112.8 (11) |
| C16—C15—H15A | 111.5 | C32—C31—H31B | 110.1 (11) |
| C16—C15—H15B | 111.5 | H31A—C31—H31B | 107.6 (15) |
| O4—C16—C15 | 106.81 (8) | O8—C32—C31 | 106.97 (8) |
| O4—C16—H16A | 110.4 | O8—C32—H32A | 110.3 |
| O4—C16—H16B | 110.4 | O8—C32—H32B | 110.3 |
| C15—C16—H16A | 110.4 | C31—C32—H32A | 110.3 |
| C15—C16—H16B | 110.4 | C31—C32—H32B | 110.3 |
| H16A—C16—H16B | 108.6 | H32A—C32—H32B | 108.6 |
| Si1—C1—C2—C3 | 176.08 (7) | Si2—C17—C18—C19 | 177.05 (7) |
| Si1—C5—C6—C7 | 177.67 (8) | Si2—C21—C22—C23 | 176.66 (8) |
| Si1—C9—C10—C11 | 179.51 (7) | Si2—C25—C26—C27 | −178.46 (9) |
| Si1—C13—C14—C15 | 177.84 (8) | Si2—C29—C30—C31 | 172.10 (8) |
| O1—C1—C2—C3 | −1.60 (12) | O5—C17—C18—C19 | −0.92 (12) |
| O2—C5—C6—C7 | −1.12 (13) | O6—C21—C22—C23 | −0.58 (14) |
| O3—C9—C10—C11 | −1.00 (12) | O7—C25—C26—C27 | 1.49 (15) |
| O4—C13—C14—C15 | −0.17 (13) | O8—C29—C30—C31 | −1.61 (13) |
| C1—Si1—C5—O2 | 55.68 (9) | C17—Si2—C21—O6 | 47.49 (8) |
| C1—Si1—C5—C6 | −123.08 (10) | C17—Si2—C21—C22 | −129.70 (11) |
| C1—Si1—C9—O3 | 36.98 (9) | C17—Si2—C25—O7 | 53.20 (9) |
| C1—Si1—C9—C10 | −143.56 (9) | C17—Si2—C25—C26 | −126.85 (11) |
| C1—Si1—C13—O4 | −173.06 (8) | C17—Si2—C29—O8 | 45.79 (9) |
| C1—Si1—C13—C14 | 9.04 (11) | C17—Si2—C29—C30 | −127.70 (10) |
| C1—O1—C4—C3 | 14.79 (10) | C17—O5—C20—C19 | 12.05 (11) |
| C1—C2—C3—C4 | 10.34 (11) | C17—C18—C19—C20 | 8.05 (11) |
| C2—C3—C4—O1 | −14.89 (10) | C18—C19—C20—O5 | −11.93 (10) |
| C4—O1—C1—Si1 | 173.47 (7) | C20—O5—C17—Si2 | 174.56 (6) |
| C4—O1—C1—C2 | −8.56 (11) | C20—O5—C17—C18 | −7.20 (11) |
| C5—Si1—C1—O1 | 68.57 (8) | C21—Si2—C17—O5 | −172.97 (7) |
| C5—Si1—C1—C2 | −109.02 (10) | C21—Si2—C17—C18 | 9.12 (11) |
| C5—Si1—C9—O3 | 156.69 (7) | C21—Si2—C25—O7 | 170.57 (8) |
| C5—Si1—C9—C10 | −23.85 (10) | C21—Si2—C25—C26 | −9.48 (12) |
| C5—Si1—C13—O4 | 66.43 (9) | C21—Si2—C29—O8 | −72.29 (9) |
| C5—Si1—C13—C14 | −111.48 (10) | C21—Si2—C29—C30 | 114.22 (10) |
| C5—O2—C8—C7 | 8.28 (14) | C21—O6—C24—C23 | −11.72 (11) |
| C5—C6—C7—C8 | 5.96 (13) | C21—C22—C23—C24 | −6.51 (13) |
| C6—C7—C8—O2 | −8.43 (13) | C22—C23—C24—O6 | 10.84 (11) |
| C8—O2—C5—Si1 | 176.40 (8) | C24—O6—C21—Si2 | −169.79 (7) |
| C8—O2—C5—C6 | −4.63 (13) | C24—O6—C21—C22 | 7.92 (12) |
| C9—Si1—C1—O1 | −174.39 (7) | C25—Si2—C17—O5 | −53.90 (8) |
| C9—Si1—C1—C2 | 8.02 (11) | C25—Si2—C17—C18 | 128.20 (9) |
| C9—Si1—C5—O2 | −63.14 (8) | C25—Si2—C21—O6 | −72.25 (8) |
| C9—Si1—C5—C6 | 118.10 (10) | C25—Si2—C21—C22 | 110.57 (11) |
| C9—Si1—C13—O4 | −51.47 (9) | C25—Si2—C29—O8 | 166.02 (8) |
| C9—Si1—C13—C14 | 130.62 (9) | C25—Si2—C29—C30 | −7.47 (11) |
| C9—O3—C12—C11 | 7.40 (11) | C25—O7—C28—C27 | −20.72 (13) |
| C9—C10—C11—C12 | 5.34 (12) | C25—C26—C27—C28 | −13.98 (15) |
| C10—C11—C12—O3 | −7.57 (11) | C26—C27—C28—O7 | 20.51 (14) |
| C12—O3—C9—Si1 | 175.37 (7) | C28—O7—C25—Si2 | −167.63 (7) |
| C12—O3—C9—C10 | −4.16 (12) | C28—O7—C25—C26 | 12.41 (13) |
| C13—Si1—C1—O1 | −50.60 (8) | C29—Si2—C17—O5 | 65.13 (8) |
| C13—Si1—C1—C2 | 131.81 (9) | C29—Si2—C17—C18 | −112.78 (10) |
| C13—Si1—C5—O2 | 174.85 (7) | C29—Si2—C21—O6 | 166.57 (7) |
| C13—Si1—C5—C6 | −3.91 (12) | C29—Si2—C21—C22 | −10.62 (12) |
| C13—Si1—C9—O3 | −84.16 (8) | C29—Si2—C25—O7 | −65.72 (9) |
| C13—Si1—C9—C10 | 95.30 (9) | C29—Si2—C25—C26 | 114.22 (11) |
| C13—O4—C16—C15 | 11.15 (12) | C29—O8—C32—C31 | −7.05 (12) |
| C13—C14—C15—C16 | 6.83 (12) | C29—C30—C31—C32 | −2.78 (12) |
| C14—C15—C16—O4 | −10.65 (11) | C30—C31—C32—O8 | 5.86 (12) |
| C16—O4—C13—Si1 | 174.68 (7) | C32—O8—C29—Si2 | −168.91 (7) |
| C16—O4—C13—C14 | −7.10 (12) | C32—O8—C29—C30 | 5.58 (13) |
Tetrakis(4,5-dihydrofuran-2-yl)silane (1). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C23—H23B···O5i | 0.974 (18) | 2.531 (18) | 3.3484 (14) | 141.5 (14) |
| C8—H8A···O5ii | 0.95 (2) | 2.61 (2) | 3.3800 (15) | 137.9 (15) |
| C27—H27B···O3iii | 0.92 (2) | 2.61 (2) | 3.4200 (16) | 147.1 (18) |
| C31—H31A···O4 | 0.986 (18) | 2.561 (18) | 3.5358 (14) | 169.6 (15) |
Symmetry codes: (i) −x+1/2, y+1/2, −z+3/2; (ii) x+1/2, −y+1/2, z+1/2; (iii) x−1/2, −y+1/2, z+1/2.
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Crystal data
| C16H20GeO4 | F(000) = 1440 |
| Mr = 348.91 | Dx = 1.505 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 14.3828 (5) Å | Cell parameters from 8295 reflections |
| b = 14.2069 (5) Å | θ = 2.6–23.2° |
| c = 15.3594 (6) Å | µ = 2.00 mm−1 |
| β = 101.159 (1)° | T = 100 K |
| V = 3079.13 (19) Å3 | Block, colourless |
| Z = 8 | 0.19 × 0.16 × 0.08 mm |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Data collection
| Bruker D8 VENTURE diffractometer | 13541 independent reflections |
| Radiation source: microfocus sealed X-ray tube, Incoatec Iµs | 10143 reflections with I > 2σ(I) |
| HELIOS mirror optics monochromator | Rint = 0.046 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 35.0°, θmin = 2.2° |
| ω and φ scans | h = −23→23 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −22→22 |
| Tmin = 0.496, Tmax = 0.568 | l = −24→24 |
| 71639 measured reflections |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | All H-atom parameters refined |
| wR(F2) = 0.077 | w = 1/[σ2(Fo2) + (0.0339P)2 + 0.639P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max = 0.002 |
| 13541 reflections | Δρmax = 0.67 e Å−3 |
| 539 parameters | Δρmin = −0.56 e Å−3 |
| 0 restraints |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ge1 | 0.76646 (2) | 0.79656 (2) | 0.25601 (2) | 0.01725 (4) | |
| O1 | 0.85960 (11) | 0.80852 (8) | 0.11071 (9) | 0.0405 (3) | |
| O2 | 0.57907 (7) | 0.77199 (8) | 0.15770 (7) | 0.0234 (2) | |
| O3 | 0.78696 (8) | 0.96600 (8) | 0.35784 (8) | 0.0284 (2) | |
| O4 | 0.82066 (8) | 0.67821 (10) | 0.40676 (8) | 0.0360 (3) | |
| C1 | 0.83902 (10) | 0.74624 (10) | 0.17308 (9) | 0.0198 (2) | |
| C2 | 0.87060 (12) | 0.66001 (11) | 0.16430 (11) | 0.0266 (3) | |
| H2 | 0.8597 (14) | 0.6081 (15) | 0.2001 (13) | 0.042 (6)* | |
| C3 | 0.91833 (13) | 0.65498 (12) | 0.08575 (12) | 0.0284 (3) | |
| H3A | 0.8873 (15) | 0.6142 (16) | 0.0419 (14) | 0.046 (6)* | |
| H3B | 0.9832 (15) | 0.6336 (15) | 0.1007 (13) | 0.040 (6)* | |
| C4 | 0.91262 (16) | 0.75656 (12) | 0.05442 (12) | 0.0337 (4) | |
| C5 | 0.64477 (10) | 0.84006 (10) | 0.19256 (9) | 0.0192 (2) | |
| C6 | 0.61438 (11) | 0.92783 (11) | 0.17540 (10) | 0.0240 (3) | |
| H6 | 0.6487 (14) | 0.9854 (14) | 0.1928 (12) | 0.032 (5)* | |
| C7 | 0.51501 (11) | 0.92654 (13) | 0.12080 (11) | 0.0289 (3) | |
| C8 | 0.49159 (10) | 0.82158 (12) | 0.11877 (10) | 0.0250 (3) | |
| H8A | 0.4679 (15) | 0.7944 (14) | 0.0582 (14) | 0.038 (6)* | |
| H8B | 0.4508 (13) | 0.8053 (12) | 0.1537 (12) | 0.021 (4)* | |
| C9 | 0.83515 (10) | 0.90293 (10) | 0.31455 (9) | 0.0200 (2) | |
| C10 | 0.92565 (11) | 0.92402 (13) | 0.31889 (11) | 0.0288 (3) | |
| H10 | 0.9719 (16) | 0.8885 (15) | 0.2931 (14) | 0.049 (6)* | |
| C11 | 0.94986 (12) | 1.01363 (13) | 0.37105 (12) | 0.0319 (4) | |
| H11A | 0.9997 (14) | 1.0013 (14) | 0.4282 (13) | 0.039 (6)* | |
| H11B | 0.9740 (13) | 1.0595 (14) | 0.3353 (12) | 0.034 (5)* | |
| C12 | 0.85364 (12) | 1.04082 (12) | 0.39196 (11) | 0.0280 (3) | |
| H12A | 0.8284 (13) | 1.0973 (14) | 0.3656 (13) | 0.034 (5)* | |
| H12B | 0.8548 (13) | 1.0425 (13) | 0.4541 (13) | 0.032 (5)* | |
| C13 | 0.74617 (9) | 0.69873 (10) | 0.33799 (9) | 0.0188 (2) | |
| C14 | 0.66946 (10) | 0.64706 (11) | 0.33906 (10) | 0.0221 (3) | |
| H14 | 0.6147 (14) | 0.6514 (14) | 0.2963 (13) | 0.035 (5)* | |
| C15 | 0.68669 (11) | 0.58031 (12) | 0.41642 (11) | 0.0267 (3) | |
| H15A | 0.6842 (14) | 0.5155 (14) | 0.3967 (13) | 0.033 (5)* | |
| H15B | 0.6374 (14) | 0.5901 (14) | 0.4575 (13) | 0.036 (5)* | |
| C16 | 0.78647 (11) | 0.60903 (12) | 0.46348 (10) | 0.0259 (3) | |
| H16A | 0.8283 (14) | 0.5559 (15) | 0.4694 (13) | 0.039 (6)* | |
| H16B | 0.7876 (12) | 0.6372 (13) | 0.5198 (12) | 0.024 (5)* | |
| Ge2 | 0.72156 (2) | 0.67613 (2) | 0.73024 (2) | 0.01539 (3) | |
| O5 | 0.66868 (7) | 0.79050 (9) | 0.57513 (7) | 0.0292 (2) | |
| O6 | 0.65130 (9) | 0.67107 (9) | 0.89028 (8) | 0.0319 (3) | |
| O7 | 0.90223 (7) | 0.71570 (7) | 0.83475 (7) | 0.02075 (19) | |
| O8 | 0.73350 (7) | 0.50898 (8) | 0.63530 (7) | 0.0237 (2) | |
| C17 | 0.73712 (9) | 0.77827 (10) | 0.65076 (9) | 0.0172 (2) | |
| C18 | 0.80834 (11) | 0.83944 (11) | 0.65802 (10) | 0.0241 (3) | |
| H18 | 0.8624 (14) | 0.8432 (14) | 0.7071 (13) | 0.032 (5)* | |
| C19 | 0.79407 (11) | 0.90342 (12) | 0.57842 (11) | 0.0266 (3) | |
| H19A | 0.7938 (14) | 0.9697 (14) | 0.5976 (13) | 0.035 (5)* | |
| H19B | 0.8440 (14) | 0.8900 (14) | 0.5411 (13) | 0.037 (6)* | |
| C20 | 0.69603 (11) | 0.87337 (12) | 0.52929 (11) | 0.0265 (3) | |
| H20A | 0.6984 (13) | 0.8555 (13) | 0.4700 (12) | 0.027 (5)* | |
| H20B | 0.6490 (14) | 0.9218 (15) | 0.5329 (13) | 0.037 (5)* | |
| C21 | 0.64670 (9) | 0.72187 (10) | 0.81286 (9) | 0.0182 (2) | |
| C22 | 0.59783 (10) | 0.80127 (11) | 0.81080 (10) | 0.0235 (3) | |
| H22 | 0.5888 (14) | 0.8425 (14) | 0.7631 (13) | 0.038 (6)* | |
| C23 | 0.56360 (13) | 0.81459 (12) | 0.89642 (12) | 0.0294 (3) | |
| C24 | 0.60078 (15) | 0.72545 (13) | 0.94731 (11) | 0.0324 (4) | |
| H24A | 0.5493 (16) | 0.6861 (15) | 0.9582 (14) | 0.045 (6)* | |
| H24B | 0.6453 (16) | 0.7397 (16) | 1.0018 (15) | 0.046 (6)* | |
| C25 | 0.84566 (9) | 0.64211 (9) | 0.79573 (8) | 0.0166 (2) | |
| C26 | 0.88659 (10) | 0.55803 (11) | 0.81029 (10) | 0.0215 (3) | |
| H26 | 0.8613 (14) | 0.4975 (15) | 0.7893 (13) | 0.040 (6)* | |
| C27 | 0.98385 (10) | 0.56899 (11) | 0.86794 (10) | 0.0216 (3) | |
| H27A | 1.0330 (14) | 0.5386 (14) | 0.8448 (13) | 0.034 (5)* | |
| H27B | 0.9826 (14) | 0.5428 (14) | 0.9284 (13) | 0.033 (5)* | |
| C28 | 0.99617 (10) | 0.67601 (11) | 0.86798 (9) | 0.0203 (2) | |
| H28A | 1.0206 (14) | 0.7041 (13) | 0.9236 (13) | 0.027 (5)* | |
| H28B | 1.0331 (13) | 0.6967 (12) | 0.8283 (12) | 0.022 (5)* | |
| C29 | 0.66849 (9) | 0.56460 (10) | 0.66718 (9) | 0.0185 (2) | |
| C30 | 0.58084 (10) | 0.53090 (11) | 0.64952 (10) | 0.0236 (3) | |
| H30 | 0.5260 (13) | 0.5604 (14) | 0.6667 (12) | 0.032 (5)* | |
| C31 | 0.57860 (10) | 0.43934 (11) | 0.59975 (11) | 0.0250 (3) | |
| H31A | 0.5534 (13) | 0.3918 (14) | 0.6335 (12) | 0.033 (5)* | |
| H31B | 0.5415 (15) | 0.4461 (15) | 0.5397 (14) | 0.047 (6)* | |
| C32 | 0.68365 (11) | 0.42402 (10) | 0.59885 (10) | 0.0227 (3) | |
| H23A | 0.4953 (16) | 0.8203 (15) | 0.8901 (14) | 0.043 (6)* | |
| H23B | 0.5908 (16) | 0.8691 (16) | 0.9306 (15) | 0.050 (7)* | |
| H4A | 0.8817 (15) | 0.7617 (15) | −0.0042 (14) | 0.040 (6)* | |
| H4B | 0.9781 (17) | 0.7862 (16) | 0.0636 (15) | 0.052 (7)* | |
| H32A | 0.6975 (14) | 0.4156 (15) | 0.5409 (14) | 0.041 (6)* | |
| H32B | 0.7082 (13) | 0.3748 (13) | 0.6351 (12) | 0.027 (5)* | |
| H7A | 0.4702 (16) | 0.9630 (15) | 0.1448 (14) | 0.048 (6)* | |
| H7B | 0.5180 (15) | 0.9528 (15) | 0.0576 (14) | 0.045 (6)* |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ge1 | 0.01688 (6) | 0.01865 (7) | 0.01668 (6) | 0.00177 (5) | 0.00433 (5) | 0.00107 (5) |
| O1 | 0.0733 (10) | 0.0227 (6) | 0.0364 (6) | 0.0195 (6) | 0.0374 (7) | 0.0121 (5) |
| O2 | 0.0211 (4) | 0.0209 (5) | 0.0254 (5) | 0.0006 (4) | −0.0023 (4) | 0.0009 (4) |
| O3 | 0.0227 (5) | 0.0265 (6) | 0.0361 (6) | −0.0032 (4) | 0.0055 (4) | −0.0116 (5) |
| O4 | 0.0179 (4) | 0.0551 (8) | 0.0320 (6) | −0.0060 (5) | −0.0026 (4) | 0.0239 (6) |
| C1 | 0.0215 (6) | 0.0198 (6) | 0.0192 (6) | 0.0030 (5) | 0.0070 (5) | 0.0028 (5) |
| C2 | 0.0319 (7) | 0.0198 (7) | 0.0325 (8) | 0.0051 (6) | 0.0171 (6) | 0.0063 (6) |
| C3 | 0.0369 (8) | 0.0197 (7) | 0.0323 (8) | 0.0067 (6) | 0.0161 (7) | 0.0023 (6) |
| C4 | 0.0546 (11) | 0.0231 (8) | 0.0308 (8) | 0.0133 (8) | 0.0265 (8) | 0.0067 (6) |
| C5 | 0.0195 (5) | 0.0217 (7) | 0.0169 (5) | 0.0028 (5) | 0.0047 (4) | 0.0005 (5) |
| C6 | 0.0259 (6) | 0.0211 (7) | 0.0245 (7) | 0.0041 (6) | 0.0039 (5) | 0.0028 (5) |
| C7 | 0.0270 (7) | 0.0315 (9) | 0.0276 (7) | 0.0090 (7) | 0.0034 (6) | 0.0087 (6) |
| C8 | 0.0192 (6) | 0.0334 (8) | 0.0217 (6) | 0.0038 (6) | 0.0021 (5) | 0.0010 (6) |
| C9 | 0.0202 (6) | 0.0205 (6) | 0.0190 (6) | −0.0008 (5) | 0.0029 (5) | 0.0006 (5) |
| C10 | 0.0201 (6) | 0.0361 (9) | 0.0300 (7) | −0.0024 (6) | 0.0041 (5) | 0.0017 (7) |
| C11 | 0.0253 (7) | 0.0341 (9) | 0.0322 (8) | −0.0095 (7) | −0.0045 (6) | 0.0069 (7) |
| C12 | 0.0331 (8) | 0.0209 (7) | 0.0273 (7) | −0.0070 (6) | −0.0007 (6) | −0.0003 (6) |
| C13 | 0.0177 (5) | 0.0215 (6) | 0.0172 (5) | 0.0033 (5) | 0.0035 (4) | 0.0016 (5) |
| C14 | 0.0223 (6) | 0.0218 (7) | 0.0208 (6) | −0.0018 (5) | 0.0006 (5) | 0.0023 (5) |
| C15 | 0.0231 (6) | 0.0291 (8) | 0.0271 (7) | −0.0021 (6) | 0.0028 (5) | 0.0074 (6) |
| C16 | 0.0218 (6) | 0.0314 (8) | 0.0240 (7) | 0.0004 (6) | 0.0030 (5) | 0.0088 (6) |
| Ge2 | 0.01513 (6) | 0.01417 (6) | 0.01657 (6) | −0.00089 (5) | 0.00231 (4) | −0.00060 (5) |
| O5 | 0.0213 (5) | 0.0340 (6) | 0.0278 (5) | −0.0084 (5) | −0.0062 (4) | 0.0122 (5) |
| O6 | 0.0454 (7) | 0.0298 (6) | 0.0246 (5) | 0.0172 (5) | 0.0168 (5) | 0.0083 (5) |
| O7 | 0.0192 (4) | 0.0148 (5) | 0.0254 (5) | 0.0014 (4) | −0.0029 (4) | −0.0022 (4) |
| O8 | 0.0208 (4) | 0.0193 (5) | 0.0304 (5) | −0.0021 (4) | 0.0036 (4) | −0.0091 (4) |
| C17 | 0.0168 (5) | 0.0165 (6) | 0.0174 (5) | 0.0014 (5) | 0.0014 (4) | 0.0010 (4) |
| C18 | 0.0243 (6) | 0.0230 (7) | 0.0226 (6) | −0.0061 (5) | −0.0016 (5) | 0.0029 (5) |
| C19 | 0.0234 (6) | 0.0243 (7) | 0.0299 (7) | −0.0048 (6) | −0.0001 (6) | 0.0077 (6) |
| C20 | 0.0231 (6) | 0.0293 (8) | 0.0254 (7) | −0.0026 (6) | 0.0005 (5) | 0.0105 (6) |
| C21 | 0.0173 (5) | 0.0186 (6) | 0.0189 (6) | −0.0007 (5) | 0.0041 (4) | 0.0012 (5) |
| C22 | 0.0229 (6) | 0.0215 (7) | 0.0275 (7) | 0.0037 (5) | 0.0088 (5) | 0.0046 (6) |
| C23 | 0.0310 (7) | 0.0268 (8) | 0.0338 (8) | 0.0086 (7) | 0.0149 (6) | 0.0009 (6) |
| C24 | 0.0447 (10) | 0.0310 (9) | 0.0261 (7) | 0.0095 (8) | 0.0180 (7) | 0.0020 (6) |
| C25 | 0.0168 (5) | 0.0156 (6) | 0.0171 (5) | −0.0003 (5) | 0.0025 (4) | −0.0008 (4) |
| C26 | 0.0219 (6) | 0.0165 (6) | 0.0242 (6) | 0.0014 (5) | −0.0001 (5) | 0.0000 (5) |
| C27 | 0.0210 (6) | 0.0203 (7) | 0.0225 (6) | 0.0054 (5) | 0.0018 (5) | 0.0011 (5) |
| C28 | 0.0172 (5) | 0.0227 (7) | 0.0200 (6) | 0.0023 (5) | 0.0015 (4) | 0.0000 (5) |
| C29 | 0.0210 (5) | 0.0158 (6) | 0.0180 (5) | −0.0014 (5) | 0.0023 (4) | −0.0008 (5) |
| C30 | 0.0205 (6) | 0.0193 (7) | 0.0293 (7) | −0.0024 (5) | 0.0007 (5) | −0.0024 (5) |
| C31 | 0.0222 (6) | 0.0163 (6) | 0.0326 (8) | −0.0011 (5) | −0.0047 (6) | 0.0009 (6) |
| C32 | 0.0264 (6) | 0.0162 (6) | 0.0238 (6) | −0.0027 (5) | 0.0008 (5) | −0.0022 (5) |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Geometric parameters (Å, º)
| Ge1—C1 | 1.9331 (13) | Ge2—C17 | 1.9370 (13) |
| Ge1—C5 | 1.9326 (14) | Ge2—C21 | 1.9290 (13) |
| Ge1—C9 | 1.9299 (14) | Ge2—C25 | 1.9329 (13) |
| Ge1—C13 | 1.9351 (14) | Ge2—C29 | 1.9353 (14) |
| O1—C1 | 1.3776 (17) | O5—C17 | 1.3806 (16) |
| O1—C4 | 1.4592 (19) | O5—C20 | 1.4641 (19) |
| O2—C5 | 1.3852 (17) | O6—C21 | 1.3816 (17) |
| O2—C8 | 1.4649 (17) | O6—C24 | 1.4624 (19) |
| O3—C9 | 1.3797 (17) | O7—C25 | 1.3877 (16) |
| O3—C12 | 1.4595 (18) | O7—C28 | 1.4619 (16) |
| O4—C13 | 1.3822 (16) | O8—C29 | 1.3840 (17) |
| O4—C16 | 1.4603 (19) | O8—C32 | 1.4592 (17) |
| C1—C2 | 1.323 (2) | C17—C18 | 1.3314 (19) |
| C2—H2 | 0.95 (2) | C18—H18 | 0.97 (2) |
| C2—C3 | 1.501 (2) | C18—C19 | 1.505 (2) |
| C3—H3A | 0.93 (2) | C19—H19A | 0.99 (2) |
| C3—H3B | 0.97 (2) | C19—H19B | 1.02 (2) |
| C3—C4 | 1.518 (2) | C19—C20 | 1.526 (2) |
| C4—H4A | 0.93 (2) | C20—H20A | 0.952 (18) |
| C4—H4B | 1.02 (2) | C20—H20B | 0.97 (2) |
| C5—C6 | 1.331 (2) | C21—C22 | 1.326 (2) |
| C6—H6 | 0.97 (2) | C22—H22 | 0.93 (2) |
| C6—C7 | 1.511 (2) | C22—C23 | 1.503 (2) |
| C7—C8 | 1.528 (2) | C23—C24 | 1.529 (2) |
| C7—H7A | 0.96 (2) | C23—H23A | 0.97 (2) |
| C7—H7B | 1.05 (2) | C23—H23B | 0.97 (2) |
| C8—H8A | 1.00 (2) | C24—H24A | 0.97 (2) |
| C8—H8B | 0.899 (18) | C24—H24B | 0.97 (2) |
| C9—C10 | 1.325 (2) | C25—C26 | 1.3313 (19) |
| C10—H10 | 0.98 (2) | C26—H26 | 0.96 (2) |
| C10—C11 | 1.509 (3) | C26—C27 | 1.5119 (19) |
| C11—H11A | 1.04 (2) | C27—H27A | 0.95 (2) |
| C11—H11B | 0.960 (19) | C27—H27B | 1.00 (2) |
| C11—C12 | 1.530 (3) | C27—C28 | 1.531 (2) |
| C12—H12A | 0.94 (2) | C28—H28A | 0.946 (19) |
| C12—H12B | 0.951 (19) | C28—H28B | 0.930 (18) |
| C13—C14 | 1.328 (2) | C29—C30 | 1.3266 (19) |
| C14—H14 | 0.92 (2) | C30—H30 | 0.974 (19) |
| C14—C15 | 1.503 (2) | C30—C31 | 1.506 (2) |
| C15—H15A | 0.97 (2) | C31—H31A | 0.964 (19) |
| C15—H15B | 1.046 (19) | C31—H31B | 0.98 (2) |
| C15—C16 | 1.532 (2) | C31—C32 | 1.529 (2) |
| C16—H16A | 0.96 (2) | C32—H32A | 0.96 (2) |
| C16—H16B | 0.950 (18) | C32—H32B | 0.920 (19) |
| C1—Ge1—C13 | 109.37 (6) | C21—Ge2—C17 | 108.03 (6) |
| C5—Ge1—C1 | 109.74 (6) | C21—Ge2—C25 | 108.58 (5) |
| C5—Ge1—C13 | 108.78 (6) | C21—Ge2—C29 | 112.88 (6) |
| C9—Ge1—C1 | 107.67 (6) | C25—Ge2—C17 | 107.92 (5) |
| C9—Ge1—C5 | 108.46 (6) | C25—Ge2—C29 | 106.91 (6) |
| C9—Ge1—C13 | 112.79 (6) | C29—Ge2—C17 | 112.36 (6) |
| C1—O1—C4 | 107.12 (11) | C17—O5—C20 | 106.95 (11) |
| C5—O2—C8 | 106.84 (11) | C21—O6—C24 | 107.04 (12) |
| C9—O3—C12 | 106.83 (12) | C25—O7—C28 | 106.57 (10) |
| C13—O4—C16 | 107.29 (11) | C29—O8—C32 | 107.01 (11) |
| O1—C1—Ge1 | 115.90 (10) | O5—C17—Ge2 | 118.30 (9) |
| C2—C1—Ge1 | 130.51 (11) | C18—C17—Ge2 | 128.10 (11) |
| C2—C1—O1 | 113.56 (13) | C18—C17—O5 | 113.59 (12) |
| C1—C2—H2 | 124.1 (12) | C17—C18—H18 | 125.9 (12) |
| C1—C2—C3 | 110.22 (13) | C17—C18—C19 | 110.14 (13) |
| C3—C2—H2 | 125.5 (12) | C19—C18—H18 | 123.9 (12) |
| C2—C3—H3A | 112.3 (13) | C18—C19—H19A | 110.0 (11) |
| C2—C3—H3B | 113.2 (12) | C18—C19—H19B | 110.1 (11) |
| C2—C3—C4 | 101.79 (13) | C18—C19—C20 | 101.56 (12) |
| H3A—C3—H3B | 106.4 (17) | H19A—C19—H19B | 113.0 (16) |
| C4—C3—H3A | 111.9 (14) | C20—C19—H19A | 111.0 (12) |
| C4—C3—H3B | 111.4 (13) | C20—C19—H19B | 110.7 (11) |
| O1—C4—C3 | 107.18 (12) | O5—C20—C19 | 107.20 (12) |
| O1—C4—H4A | 109.2 (13) | O5—C20—H20A | 107.9 (11) |
| O1—C4—H4B | 106.5 (13) | O5—C20—H20B | 106.7 (12) |
| C3—C4—H4A | 111.6 (13) | C19—C20—H20A | 110.3 (11) |
| C3—C4—H4B | 111.0 (13) | C19—C20—H20B | 111.1 (12) |
| H4A—C4—H4B | 111.1 (18) | H20A—C20—H20B | 113.3 (16) |
| O2—C5—Ge1 | 117.07 (10) | O6—C21—Ge2 | 116.77 (10) |
| C6—C5—Ge1 | 129.06 (12) | C22—C21—Ge2 | 129.17 (11) |
| C6—C5—O2 | 113.85 (13) | C22—C21—O6 | 113.68 (13) |
| C5—C6—H6 | 127.5 (12) | C21—C22—H22 | 123.3 (13) |
| C5—C6—C7 | 109.74 (14) | C21—C22—C23 | 110.38 (13) |
| C7—C6—H6 | 122.8 (12) | C23—C22—H22 | 126.3 (12) |
| C6—C7—C8 | 101.79 (12) | C22—C23—C24 | 101.63 (12) |
| C6—C7—H7A | 114.6 (13) | C22—C23—H23A | 114.8 (13) |
| C6—C7—H7B | 107.8 (12) | C22—C23—H23B | 114.0 (13) |
| C8—C7—H7A | 112.0 (13) | C24—C23—H23A | 111.5 (13) |
| C8—C7—H7B | 112.1 (12) | C24—C23—H23B | 108.5 (14) |
| H7A—C7—H7B | 108.5 (17) | H23A—C23—H23B | 106.3 (17) |
| O2—C8—C7 | 107.01 (12) | O6—C24—C23 | 107.24 (13) |
| O2—C8—H8A | 107.6 (12) | O6—C24—H24A | 106.5 (13) |
| O2—C8—H8B | 103.9 (11) | O6—C24—H24B | 107.8 (13) |
| C7—C8—H8A | 115.4 (12) | C23—C24—H24A | 111.3 (13) |
| C7—C8—H8B | 114.0 (11) | C23—C24—H24B | 112.0 (14) |
| H8A—C8—H8B | 108.1 (16) | H24A—C24—H24B | 111.7 (18) |
| O3—C9—Ge1 | 118.25 (9) | O7—C25—Ge2 | 116.20 (9) |
| C10—C9—Ge1 | 127.58 (12) | C26—C25—Ge2 | 130.20 (11) |
| C10—C9—O3 | 114.16 (14) | C26—C25—O7 | 113.59 (12) |
| C9—C10—H10 | 127.0 (13) | C25—C26—H26 | 128.1 (12) |
| C9—C10—C11 | 109.94 (15) | C25—C26—C27 | 109.57 (13) |
| C11—C10—H10 | 123.1 (13) | C27—C26—H26 | 122.3 (12) |
| C10—C11—H11A | 111.0 (12) | C26—C27—H27A | 113.8 (12) |
| C10—C11—H11B | 109.9 (12) | C26—C27—H27B | 108.9 (11) |
| C10—C11—C12 | 101.51 (13) | C26—C27—C28 | 101.32 (11) |
| H11A—C11—H11B | 109.4 (16) | H27A—C27—H27B | 108.9 (16) |
| C12—C11—H11A | 111.8 (11) | C28—C27—H27A | 110.9 (12) |
| C12—C11—H11B | 113.0 (12) | C28—C27—H27B | 113.0 (11) |
| O3—C12—C11 | 107.40 (13) | O7—C28—C27 | 106.50 (11) |
| O3—C12—H12A | 107.2 (12) | O7—C28—H28A | 108.0 (12) |
| O3—C12—H12B | 105.2 (12) | O7—C28—H28B | 104.8 (11) |
| C11—C12—H12A | 114.5 (12) | C27—C28—H28A | 116.2 (12) |
| C11—C12—H12B | 112.1 (12) | C27—C28—H28B | 113.2 (11) |
| H12A—C12—H12B | 110.0 (16) | H28A—C28—H28B | 107.4 (16) |
| O4—C13—Ge1 | 117.16 (10) | O8—C29—Ge2 | 114.61 (9) |
| C14—C13—Ge1 | 129.45 (11) | C30—C29—Ge2 | 131.68 (11) |
| C14—C13—O4 | 113.39 (13) | C30—C29—O8 | 113.71 (12) |
| C13—C14—H14 | 123.4 (13) | C29—C30—H30 | 125.3 (12) |
| C13—C14—C15 | 110.38 (13) | C29—C30—C31 | 109.97 (13) |
| C15—C14—H14 | 126.2 (13) | C31—C30—H30 | 124.8 (12) |
| C14—C15—H15A | 111.2 (11) | C30—C31—H31A | 107.8 (12) |
| C14—C15—H15B | 111.5 (11) | C30—C31—H31B | 110.4 (13) |
| C14—C15—C16 | 101.60 (12) | C30—C31—C32 | 101.74 (12) |
| H15A—C15—H15B | 109.1 (15) | H31A—C31—H31B | 112.5 (17) |
| C16—C15—H15A | 112.0 (12) | C32—C31—H31A | 112.3 (11) |
| C16—C15—H15B | 111.2 (11) | C32—C31—H31B | 111.5 (13) |
| O4—C16—C15 | 106.89 (12) | O8—C32—C31 | 106.96 (12) |
| O4—C16—H16A | 108.0 (12) | O8—C32—H32A | 106.6 (13) |
| O4—C16—H16B | 108.1 (11) | O8—C32—H32B | 107.1 (12) |
| C15—C16—H16A | 110.4 (12) | C31—C32—H32A | 114.2 (12) |
| C15—C16—H16B | 113.0 (11) | C31—C32—H32B | 111.4 (11) |
| H16A—C16—H16B | 110.2 (16) | H32A—C32—H32B | 110.2 (16) |
| Ge1—C1—C2—C3 | 176.36 (12) | Ge2—C17—C18—C19 | 176.68 (11) |
| Ge1—C5—C6—C7 | 178.01 (11) | Ge2—C21—C22—C23 | −170.77 (12) |
| Ge1—C9—C10—C11 | 179.83 (11) | Ge2—C25—C26—C27 | −178.47 (10) |
| Ge1—C13—C14—C15 | −179.37 (11) | Ge2—C29—C30—C31 | −178.54 (11) |
| O1—C1—C2—C3 | −1.5 (2) | O5—C17—C18—C19 | −1.66 (19) |
| O2—C5—C6—C7 | −0.57 (18) | O6—C21—C22—C23 | 1.84 (19) |
| O3—C9—C10—C11 | −0.27 (19) | O7—C25—C26—C27 | 1.18 (17) |
| O4—C13—C14—C15 | 0.09 (19) | O8—C29—C30—C31 | 0.67 (18) |
| C1—O1—C4—C3 | 2.7 (2) | C17—O5—C20—C19 | 6.77 (18) |
| C1—C2—C3—C4 | 3.0 (2) | C17—C18—C19—C20 | 5.58 (19) |
| C2—C3—C4—O1 | −3.4 (2) | C18—C19—C20—O5 | −7.30 (18) |
| C4—O1—C1—Ge1 | −179.00 (13) | C20—O5—C17—Ge2 | 178.16 (10) |
| C4—O1—C1—C2 | −0.8 (2) | C20—O5—C17—C18 | −3.32 (18) |
| C5—O2—C8—C7 | 8.45 (15) | C21—O6—C24—C23 | 0.6 (2) |
| C5—C6—C7—C8 | 5.62 (17) | C21—C22—C23—C24 | −1.27 (19) |
| C6—C7—C8—O2 | −8.37 (16) | C22—C23—C24—O6 | 0.35 (19) |
| C8—O2—C5—Ge1 | 176.14 (9) | C24—O6—C21—Ge2 | 172.03 (11) |
| C8—O2—C5—C6 | −5.10 (16) | C24—O6—C21—C22 | −1.55 (19) |
| C9—O3—C12—C11 | −4.04 (16) | C25—O7—C28—C27 | −15.01 (14) |
| C9—C10—C11—C12 | −2.19 (18) | C25—C26—C27—C28 | −10.12 (16) |
| C10—C11—C12—O3 | 3.72 (17) | C26—C27—C28—O7 | 14.94 (14) |
| C12—O3—C9—Ge1 | −177.31 (10) | C28—O7—C25—Ge2 | −171.34 (9) |
| C12—O3—C9—C10 | 2.78 (18) | C28—O7—C25—C26 | 8.96 (16) |
| C13—O4—C16—C15 | 6.62 (18) | C29—O8—C32—C31 | 7.81 (15) |
| C13—C14—C15—C16 | 3.90 (18) | C29—C30—C31—C32 | 4.11 (17) |
| C14—C15—C16—O4 | −6.24 (17) | C30—C31—C32—O8 | −7.10 (16) |
| C16—O4—C13—Ge1 | 175.18 (11) | C32—O8—C29—Ge2 | 173.88 (9) |
| C16—O4—C13—C14 | −4.35 (19) | C32—O8—C29—C30 | −5.48 (17) |
Tetrakis(4,5-dihydrofuran-2-yl)germane (2). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C31—H31A···O7i | 0.964 (19) | 2.60 (2) | 3.3279 (18) | 132.1 (14) |
| C23—H23A···O4ii | 0.97 (2) | 2.57 (2) | 3.530 (2) | 168.0 (17) |
| C4—H4A···O7iii | 0.93 (2) | 2.63 (2) | 3.398 (2) | 140.8 (17) |
Symmetry codes: (i) −x+3/2, y−1/2, −z+3/2; (ii) x−1/2, −y+3/2, z+1/2; (iii) x, y, z−1.
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Bauer, J. O. & Strohmann, C. (2014). Angew. Chem. Int. Ed. 53, 720–724. [DOI] [PubMed]
- Bruker (2018). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Ertschak, N., Popelis, Û., Nichler, I. & Lukevics, E. (1982). Zh. Obshch. Khim. 5, 1181–1187.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Evans, D. A., Sweeney, Z. K., Rovis, T. & Tedrow, J. S. (2001). J. Am. Chem. Soc. 123, 12095–12096. [DOI] [PubMed]
- Gevorgyan, V., Borisova, L. & Lukevics, E. (1989). J. Organomet. Chem. 368, 19–21.
- Gevorgyan, V., Borisova, L. & Lukevics, E. (1990). J. Organomet. Chem. 393, 57–67.
- Gevorgyan, V., Borisova, L. & Lukevics, E. (1992). J. Organomet. Chem. 441, 381–387.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Krupp, A., Barth, E. R., Seymen, R. & Strohmann, C. (2020). Acta Cryst. E76, 1514–1519. [DOI] [PMC free article] [PubMed]
- Lazraq, M., Escudié, J., Couret, C., Satgé, J., Dräger, M. & Dammel, R. (1988). Angew. Chem. 100, 885–887.
- Li, T. & Zhang, L. (2018). J. Am. Chem. Soc. 140, 17439–17443. [DOI] [PMC free article] [PubMed]
- Lukevics, E., Gevorgyan, V. & Borisova, L. (1997). Chem. Heterocycl. Compd. 33, 161–163.
- Lukevics, E., Gevorgyan, V., Rosite, S., Gavaps, M. & Mascheika, I. (1984). LZA Vēstis, 1, 109–111.
- Lukevics, E., Gevorgyan, V. N., Goldberg, Y. S. & Shymanska, M. V. (1985). J. Organomet. Chem. 294, 163–171.
- Lukevits, E., Borisova, L. & Gevorgyan, V. (1993). Chem. Heterocycl. Compd. 29, 735–743.
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Murakami, M., Hayashi, M. & Ito, Y. (1994). J. Org. Chem. 59, 7910–7914.
- Neugebauer, P., Klingebiel, U. & Noltemeyer, M. (2000). Z. Naturforsch. B, 55, 913–923.
- Schmidt, A., Krupp, A., Barth, E. R. & Strohmann, C. (2022). Acta Cryst. E78, 23–28. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst. 54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Tacke, R., Lopex–Mras, A., Sperlich, J., Strohmann, C., Kuhs, W. F., Mattern, G. & Sebald, A. (1993). Chem. Ber. 126, 851–861.
- Tacke, R., Sperlich, J., Strohmann, C. & Mattern, G. (1991). Chem. Ber. 124, 1491–1496.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1. DOI: 10.1107/S2056989023003158/hb8058sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989023003158/hb80581sup2.hkl
Supporting information file. DOI: 10.1107/S2056989023003158/hb80581sup4.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989023003158/hb80582sup3.hkl
Supporting information file. DOI: 10.1107/S2056989023003158/hb80582sup5.cml
CCDC reference: 2254180
Additional supporting information: crystallographic information; 3D view; checkCIF report










