Abstract
Purpose
Hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is a serious medical condition with a high short-term mortality rate, making accurate prognostic assessment essential for informed clinical decision-making. In this study, we aimed to develop a simple and effective prognostic model for predicting short-term mortality in patients with HBV-ACLF.
Patients and Methods
To achieve our objective, we enrolled both a cross-sectional cohort (n = 291) and a retrospective cohort (n = 185) in this study. We collected laboratory and clinical data from these cohorts and performed univariate and multivariate logistic regression analyses to identify independent predictors of short-term mortality. Subsequently, we developed a novel prognostic score for HBV-ACLF, which was validated and assessed using receiver operating characteristic (ROC) curve analysis to determine its performance.
Results
Our analysis revealed that the admission prealbumin (PAB) level was a robust independent predictor of 30-day mortality, with an area under the receiver operating characteristic (AUROC) of 0.760. Moreover, we developed the HIAPP score, a prognostic-score model based on PAB. The HIAPP score was significantly lower in survivors compared to non-survivors (−2.80±0.21 vs 0.97±0.41, P < 0.001). The HIAPP score’s AUROC value was 0.899, which was found to be superior to the MELD score (AUROC = 0.795) and the CLIF-C ACLF score (AUC =0.781) and comparable to the COSSH-ACLF II score (AUC =0.825) for predicting 30-day mortality. These findings were also validated in a separate cohort, further supporting the utility of the HIAPP score as a prognostic tool for HBV-ACLF patients.
Conclusion
Our study identifies the admission PAB level as a simple and valuable predictive index for 30-day mortality in HBV-ACLF patients. Furthermore, the HIAPP score, which incorporates PAB, PLT, INR, HE, and age, is an easy-to-use and pragmatic prognostic score in predicting short-term mortality.
Keywords: hepatitis B virus related acute on chronic liver failure, short term prognosis, prealbumin, prognostic model
Graphical Abstract
Introduction
Acute-on-chronic liver failure (ACLF) is a severe clinical syndrome characterized by acute liver decompensation, organ failures, and a high rate of short-term mortality triggered by an acute insult.1–3 In China, HBV-ACLF accounts for more than 70% of ACLF cases due to the high prevalence of HBV infection.4,5 While liver transplantation (LT) is the definitive therapeutic option for liver failure,6 the shortage of LT resources in China leads to an extremely poor prognosis for HBV-ACLF patients, with a high short-term mortality rate ranging from 29.7% to 40%.7,8 Thus, accurate assessment of the clinical condition and prognosis is critical for effective HBV-ACLF management and allocation of LT resources.
In recent years, several prognostic models have been developed for HBV-ACLF, including the COSSH-ACLF II score, the FT3 correlation formula score, and the P5 score based on plasminogen.9–11 These models have demonstrated high prognostic value for predicting poor outcomes in patients with HBV-ACLF. However, they also have some limitations. The COSSH-ACLF II score requires an accurate score for hepatic encephalopathy and involves complex calculations. The FT3 correlation formula score requires further validation in a larger group of patients with HBV-ACLF. Additionally, the detection of plasminogen is not widely used in clinics, limiting the practical application of the P5 score based on plasminogen. Currently, the model of end-stage liver disease (MELD) is a frequently used prognostic score for predicting short-term outcomes in patients with HBV-ACLF. However, its predictive accuracy is limited, and thus, there is a need for more accurate and easily applicable prognostic indicators and models for HBV-ACLF.
Prealbumin (PAB), also referred to as transthyretin, is mainly synthesized by the liver and is minimally influenced by venous replenishment.12 Besides serving as a sensitive nutritional protein marker, PAB is also a sensitive biomarker for assessing the severity of liver diseases.13 In patients who have undergone hepatectomy or LT, serum prealbumin has been demonstrated to be a useful prognostic indicator for predicting postoperative complications and survival.14,15 However, the potential utility of PAB in prognosticating HBV-ACLF has not been comprehensively investigated.
In this study, we investigated the potential prognostic value of PAB in HBV-ACLF patients and found it to be a significant predictor of short-term mortality. Subsequently, we developed and validated a prognostic score based on PAB to accurately predict the short-term mortality in HBV-ACLF patients.
Materials and Methods
Study Design and Patients
This study included two cohorts. The first cohort (cohort 1, n=291) was a cross-sectional study that included 185 HBV-ACLF, 27 HBV-DCC, 28 HBV-LC, 26 CHB, and 25 HCs. The aim of this cohort was to investigate the association between PAB level and 30-day mortality in HBV-ACLF patients. The second cohort (cohort 2) was a retrospective cohort consisting of 185 HBV-ACLF inpatients, which was further divided into a deriving cohort (65%) and a validation cohort (35%). The deriving cohort was used to identify independent and powerful prognostic factors for short-term mortality and to develop a new prognostic score, while the validation cohort was used to validate the new score’s prognostic ability. Patients with HBV-ACLF were recruited based on the APASL criteria, which required positive HBsAg for at least 6 months, serum total bilirubin (TBIL) levels of 5 mg/dL or higher, INR levels of 1.5 or higher or prothrombin activity levels below 40%, and the development of ascites and/or encephalopathy within 4 weeks.16 Patients with other types of hepatitis viruses, alcoholic liver disease, autoimmune liver disease, or liver cancer were excluded. All subjects were recruited or enrolled at the Second Affiliated Hospital of Anhui Medical University between May 1, 2012, and November 1, 2021. All HBV-ACLF patients were followed up for 30 days or until death, whichever occurred first, and the 30-day mortality rate was determined. The study was approved by the ethical committee of the Second Affiliated Hospital of Anhui Medical University and was conducted in accordance with the 1975 declaration of Helsinki. Written informed consent was obtained from all participants for the use of their clinical data in this study.
Clinical Data
Demographic and clinical data were extracted retrospectively from medical records, and laboratory variables were measured in all subjects within the first 24 hours after admission. Serum levels of prealbumin, albumin, total protein (TP), globulin, total bilirubin (TBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine were quantified using the Beckman Coulter AU5800 automatic biochemical analyzer (USA). Platelet (PLT), neutrophil, and white blood cell (WBC) counts were measured using the Sysmex XT-4000i Hematology Analyzer (Japan). The international normalized ratio (INR) was determined using the Sysmex CS 5100 automatic coagulation analyzer (Japan). Serum Hepatitis B viral DNA (HBV DNA) levels were measured using the Agilent Technologies Mx3000P System (USA).
Calculation of Scores
The scores were calculated using previously reported formulas.9 The Model of End-Stage Liver Disease (MELD) score was calculated as follows: MELD = 3.78 × ln [TBIL (mg/dL)] + 11.2 × ln (INR) + 9.57 × ln [serum creatinine (mg/dL)] + 6.43. The Chronic Liver Failure-ACLF (COSSH-ACLF) IIs score was calculated as follows: COSSH-ACLF IIs = 1.649 × ln(INR) + 0.457 × HE score (HE grade: 0/1, 1–2/2 and 3–4/3) + 0.425 × ln(neutrophil) (109/L) + 0.396 × ln(TB) (umol/L) + 0.576 × ln(serum urea) (mmol/L) + 0.033 × age. The Chronic Liver Failure-Consortium ACLF (CLIF-C ACLF) score was calculated as follows: CLIF-C ACLF = 10 × [0.33 × CLIF- organ failure score (OFs) + 0.04 × age + 0.63 × ln (white blood cell [WBC]) −2].
Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 and MEDCALC software. Quantitative variables were presented as means ± SD or medians (P25-P75), while categorical variables were presented as numbers and percentages. The Student’s t-test or chi-square test was used for group comparisons. The Spearman correlation coefficients were used to assess the correlation between PAB and other laboratory markers. Multivariate logistic regression was used to identify independent prognostic factors for 30-day mortality in HBV-ACLF. A two-tailed P value of ≤0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were used to evaluate the predictive value of various prognostic indices for HBV-ACLF patients.
Results
Clinical Characteristics
Table 1 presents the general characteristics of HBV-ACLF, HBV-DCC, HBV-LC, CHB, and HCs. There were no significant differences in age, gender ratio, or laboratory parameters between the deriving and validation groups of HBV-ACLF patients. However, there were significant gender and age differences among patients with different stages of HBV-related liver diseases. Patients with HBV-ACLF had significantly increased levels of ALT, AST, TBIL, creatinine, neutrophil count, PT, and PT-INR (p < 0.05) and lower levels of albumin and platelet counts compared to other groups. During the 30-day follow-up period, 128 HBV-ACLF patients survived, while 57 patients died.
Table 1.
Clinical Characteristic of the Subjects
| HC (n =25) | CHB (n =26) | HBV-LC (n =28) | HBV-DCC (n = 27) | HBV‐ACLF | ||||
|---|---|---|---|---|---|---|---|---|
| Total (n=185) | Deriving Cohort (n=120) | Validation Cohort (n=65) | P* | |||||
| Age (y) | 45.40±11.95 | 38.15±10.76 | 47.75±12.46 | 54.85±10.75 | 48.39±12.69bd | 48.68±13.61 | 47.86±10.86 | NS |
| Gender (female/male) | 8/17 | 7/19 | 7/21 | 9/18 | 27/158ad | 16/104 | 11/54 | NS |
| WBC (×109/L) | 6.63±1.48 | 5.51±1.68 | 4.13±1.86 | 2.86±1.52 | 7.11±4.56bcd | 7.11±4.08 | 6.56±3.25 | NS |
| Neutrophil (×109/L) | 3.64±1.08 | 2.86±1.09 | 2.19±1.04 | 2.53±4.67 | 4.95±3.38abcd | 5.11±3.66 | 4.64±2.81 | NS |
| Platelet (×109/L) | 207.00(190.00 −253.00) | 178.00(156.50–211.00) | 88.50(61.50–125.25) | 50.00(31.00–84.00) | 87.00(60.00–120.50)abd | 86.50(63.25–125.00) | 92.00 (56.00–115.00) | NS |
| Albumin (g/L) | 46.20±2.67 | 41.17±3.22 | 35.24± 5.66 | 28.40±5.87 | 29.74±4.84abc | 29.62±4.77 | 29.97±5.00 | NS |
| ALT (U/L) | 19.00(15.50–35.50) | 29.00(19.50–77.25) | 51.50(27.25–169.50) | 26.00(18.00–43.00) | 401.00(119.50–751.50)abcd | 403.50(130.25–768.25) | 355.00(112.00–715.00) | NS |
| AST (U/L) | 24.00(22.50–29.50) | 26.00(19.00–47.25) | 52.50(32.50–100.50) | 46.00(33.90–66.10) | 262.00(145.50–599.00)abcd | 263.50(157.00–611.50) | 228.00 (116.00–584.00) | NS |
| Serum bilirubin (mg/dL) | 0.83±0.21 | 0.94±0.76 | 1.57± 1.24 | 2.15±1.51 | 19.42±8.21abcd | 19.52±8.23 | 19.23±8.22 | NS |
| BUN (mmol/L) | 4.88(4.73–5.68) | 4.59(3.84–5.62) | 4.36(3.40–5.60) | 4.92(3.56–7.26) | 4.33(3.07–5.96) | 4.26(3.05–5.65) | 4.44 (3.32–6.16) | NS |
| Creatinine (mg/dL) | 0.78(0.62–0.94) | 0.76(0.64–0.88) | 0.67(0.51–0.76) | 0.86(0.53–1.02) | 0.84(0.70–1.02) | 0.84(0.70–1.02) | 0.83 (0.70–0.95) | NS |
| INR | 0.91(0.87–0.95) | 1.00(0.92–1.06) | 1.18(1.04–1.36) | 1.45(1.35–1.53) | 2.08(1.72–2.63)abcd | 2.17(1.78–2.64) | 2.01 (1.65–2.60) | NS |
| MELD | ND | ND | 5.01±3.70 | 10.61±5.05 | 24.90±6.80abcd | 25.49±6.87 | 24.51±6.49 | NS |
Notes: P* values correspond to the comparison of the deriving and validation groups of HBV-ACLF patients. ND not determined, aP < 0.05 HBV-ACLF vs HCs, bP < 0.05 HBV-ACLF vs CHB, cP < 0.05 HBV-ACLF vs LC, dP < 0.05 HBV-ACLF vs DCC.
Abbreviations: WBC, White blood cell count; ALT, Alanine transaminase; AST, Aspartate transaminase; BUN, blood urea nitrogen; INR, International normalized ratio; MELD, Model for end-stage liver disease.
Decreased Levels of PAB Were Closely Associated with Disease Severity, and HBV-ACLF Patients Had the Lowest PAB Level
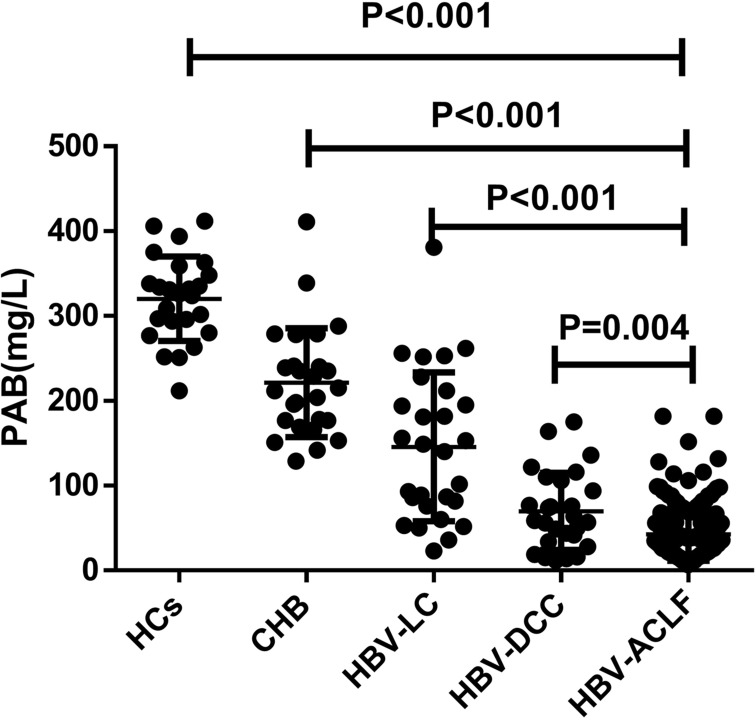
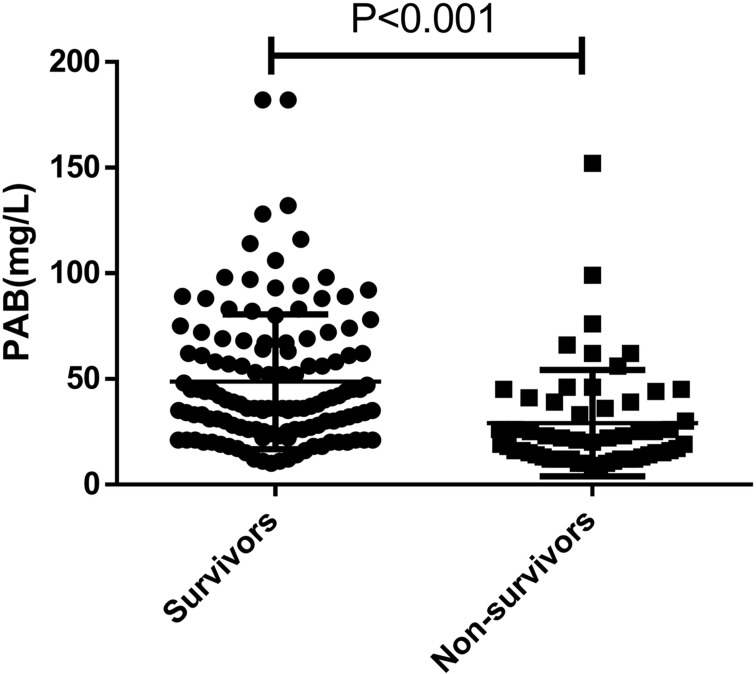
As we know, prealbumin can reflect liver function, but its level changes in different stages of HBV-related liver disease have not been thoroughly studied. In this study, we aimed to investigate the relationship between prealbumin (PAB) levels and different stages of HBV-related liver disease. Using cohort 1 (Table 1), we conducted a cross-sectional study and found that HBV-ACLF patients had significantly lower PAB levels (42.66±31.27 mg/L) on admission compared to HCs (320.40±49.81 mg/L), CHB (221.54±64.27 mg/L), HBV-LC (145.82±87.64 mg/L), and HBV-DCC (69.78±45.39 mg/L) patients (all P < 0.05; Figure 1). We also observed a decrease in serum PAB levels with the progression of HBV-related liver disease. Moreover, our findings showed that serum PAB levels in non-survivors (29.16±3.33, n=57) were significantly lower than in survivors (48.67±2.82, n=128) among patients with HBV-ACLF (P<0.001, Figure 2).
Figure 1.
The comparison of PAB levels in HBV-ACLF, HBV-DCC, HBV-LC, CHB, and HCs patients. Values are presented as mean± SD. The level of PAB in patients with HBV-ACLF was significantly lower than that in patients with HBV-DCC, HBV-LC, CHB, and HCs.
Figure 2.
The serum PAB levels in non-survivor (n = 57) and survivor (n = 128) patients with HBV-ACLF.
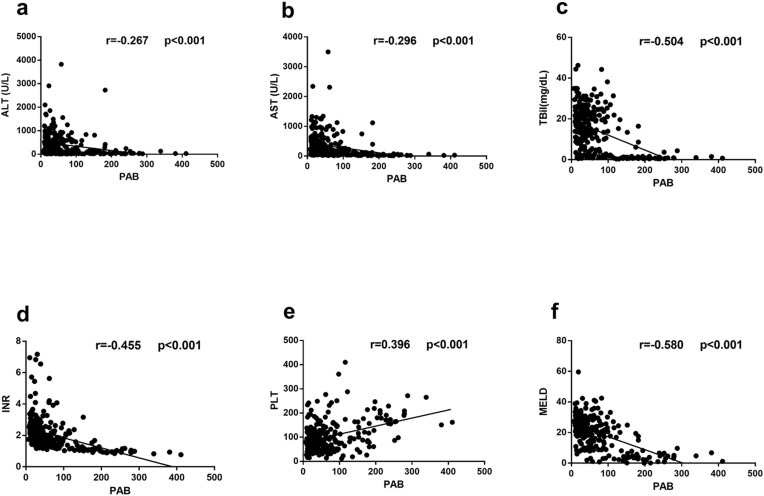
Next, we investigated the relationship between serum PAB levels and various biomarkers of liver injury (Figure 3). Our analysis revealed that serum PAB levels were positively correlated with PLT (r = 0.396, P < 0.001) and negatively correlated with ALT (r = −0.267, P < 0.001), AST (r = −0.296, P < 0.001), TBIL (r = −0.504, P < 0.001), and INR (r = −0.455, P < 0.001). Moreover, we observed a significant inverse relationship between serum PAB levels and the Model for End-Stage Liver Disease (MELD) score, which is commonly used to assess the severity of the disease in HBV-ACLF patients (r = −0.580, P < 0.001).
Figure 3.
Correlations between serum PAB levels and liver injury parameters in patients with HBV-related liver diseases. Correlations between PAB and ALT (a), AST (b), TBIL (c), INR (d), PLT (e), MELD (f).
PAB for Prognostic Prediction for HBV‐ACLF Patients in the Deriving Cohort
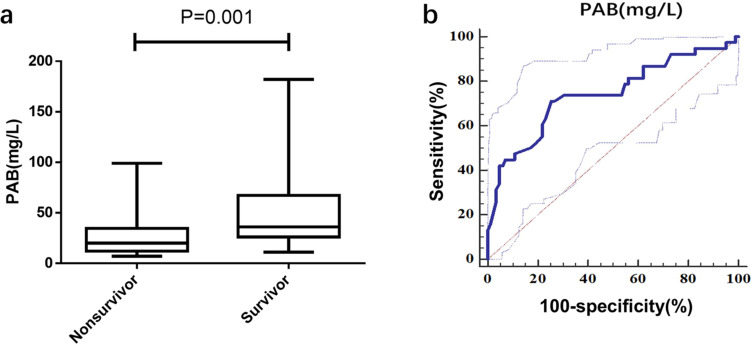
Based on the aforementioned findings, we aimed to investigate whether reduced serum PAB levels could serve as a prognostic indicator for mortality in HBV-ACLF patients. We enrolled a derivation cohort (n = 120) in this study, and Table 2 outlines the cohort’s characteristics. Upon admission, non-survivors had a significantly lower PAB level than survivors (P = 0.001) (Figure 4a). Logistic regression analysis revealed that PAB was a significant and independent predictor of short-term mortality in HBV‐ACLF. The PAB achieved an AUC of 0.760 (sensitivity = 72.97%, specificity = 74.39%) for 30-day mortality of HBV-ACLF (Figure 4b). Based on the median PAB level in ACLF patients, we classified them into high-PAB (>34mg/L) or low-PAB (≤34mg/L) groups. The low-PAB group had a significantly higher mortality rate than the high-PAB group (P < 0.001; Table 3).
Table 2.
Baseline Characteristics of the Deriving Cohort Enrolled in This Study on Admission
| Variables | Total Population (n=120) | Nonsurvivor (n = 38) | Survivor (n =82) | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | 0R (95% CI) | P | ||||
| Clinical characteristics | |||||||
| Age (y) | 48.68±13.61 | 54.34±12.78 | 46.05±13.25 | 1.049(1.017–1.082) | 0.003 | 1.068(1.021–1.118) | 0.005 |
| Gender (female/male) | 16/104 | 5/33 | 11/71 | 0.978(0.314–3.042) | 0.969 | ||
| SBP | 38(31.67%) | 14 (36.8%) | 24 (29.3%) | 1.410(0.625–3.178) | 0.408 | ||
| HE (mild/serve) | 5/12 | 4/10 | 1/2 | 15.361(4.071–57.965) | <0.001 | 12.025(1.719–84.105) | 0.009 |
| Laboratory parameters | |||||||
| WBC (×109/L) | 7.11±4.08 | 7.86±4.03 | 6.77±4.08 | 1.065(0.970–1.169) | 0.186 | – | – |
| Neutrophil (×109/L) | 5.11±3.66 | 6.05±3.59 | 4.69±3.63 | 1.102(0.992–1.226) | 0.071 | ||
| Platelet (×109/L) | 86.50(63.25–125.00) | 79.00(50.75–103.00) | 95.00(67.25–139.50) | 0.991(0.983–0.999) | 0.033 | 0.985(0.971–0.999) | 0.039 |
| Total protein (g/L) | 57.82±8.07 | 55.91±7.88 | 58.71±8.04 | 0.957(0.910–1.005) | 0.081 | – | – |
| Albumin (g/L) | 29.62±4.77 | 29.40±3.99 | 29.72±5.11 | 0.986(0.909–1.069) | 0.729 | ||
| Globulin (g/L) | 28.20±7.81 | 26.51±7.01 | 28.99±8.07 | 0.958(0.909–1.009) | 0.108 | ||
| A/G | 1.04(0.84–1.40) | 1.05(0.92–1.43) | 1.01(0.81–1.40) | 1.330(0.619–2.854) | 0.465 | – | – |
| ALT (U/L) | 403.50(130.25–768.25) | 574.00(281.50–940.25) | 349.50(99.50–654.50) | 1.001(1.000–1.002) | 0.027 | – | – |
| AST (U/L) | 263.50(157.00–611.50) | 491.50(239.00–859.50) | 214.00(121.75–424.75) | 1.001(1.000–1.002) | 0.046 | – | – |
| Serum bilirubin (mg/dL) | 19.52±8.23 | 20.47±6.81 | 19.08±8.82 | 1.021(0.974–1.069) | 0.389 | ||
| BUN (mmol/L) | 4.26(3.05–5.65) | 4.35(3.08–6.94) | 4.19(3.02–5.33) | 1.048(0.957–1.147) | 0.311 | ||
| Creatinine (mg/dL) | 0.84(0.70–1.02) | 0.86(0.71–1.15) | 0.81(0.70–1.00) | 2.314(0.888–6.030) | 0.086 | – | |
| INR | 2.17(1.78–2.64) | 2.59(2.06–4.13) | 2.04(1.66–2.39) | 3.117(1.772–5.483) | <0.001 | 2.197(1.005–4.805) | 0.049 |
| Lg (DNA) | 4.93±1.75 | 5.12±1.54 | 4.83±1.84 | 1.120(0.897–1.397) | 0.317 | – | |
| Prealbumin | 34.00(20.00–61.00) | 20.00(12.00–34.50) | 36.00(26.00–67.25) | 0.959(0.937–0.982) | 0.001 | 0.963(0.934–0.994) | 0.018 |
| HIAPP score | −1.63±2.74 | 0.97±0.41 | −2.80±0.21 | <0.001 | |||
| MELD | 25.49±6.78 | 29.78±7.73 | 23.50±5.25 | <0.001 | |||
Note: Data presented as means ± SD or medians (P25‐P75).
Abbreviations: SBP, spontaneous bacterial peritonitis; HE, Hepatic encephalopathy; A/G, albumin/globulin.
Figure 4.
Using PAB on admission for predicting the prognosis of HBV‐ACLF. (a) the concentrations of PAB in Non-survivor (n = 38) and Survivor (n =82) patients in the deriving cohort. (b) ROC analysis shows the performance of PAB in distinguishing Non-survivor patients from Survivor patients in the deriving cohort.
Table 3.
Mortality According to PAB Levels
| Mortality | P value | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| With low levels of PAB (n = 59) | 28 (47.5%) | – | – | – | – |
| With high levels of PAB (n = 61) | 10(16.4%) | <0.001 | 0.217 | 0.093 | 0.507 |
Note: P values indicate statistical significance.
Abbreviation: PAB, Prealbumin.
Development and Evaluation of a PAB -Based Prognostic Model for HBV‐ACLF Patients in the Deriving Cohort
We investigated the performance of PAB in combination with other indicators at admission to predict HBV-ACLF outcomes. We used univariate and multivariate logistic regression analysis on the deriving cohort (cohort 2) and found that PAB, PLT, INR, HE, and age were all independent prognostic predictors of short-term mortality (all P<0.05, as summarized in Table 2). Based on these findings, we developed a prognostic model (HIAPP) using the following equation: HIAPP = 2.981*HE + 0.787*INR + 0.066*age - 0.038*PAB - 0.015*PLT - 4.119, where HE = 0 for patients without HE, and HE = 1 for patients with HE. This model combines multiple indicators to provide a more accurate prediction of short-term mortality in HBV-ACLF patients.
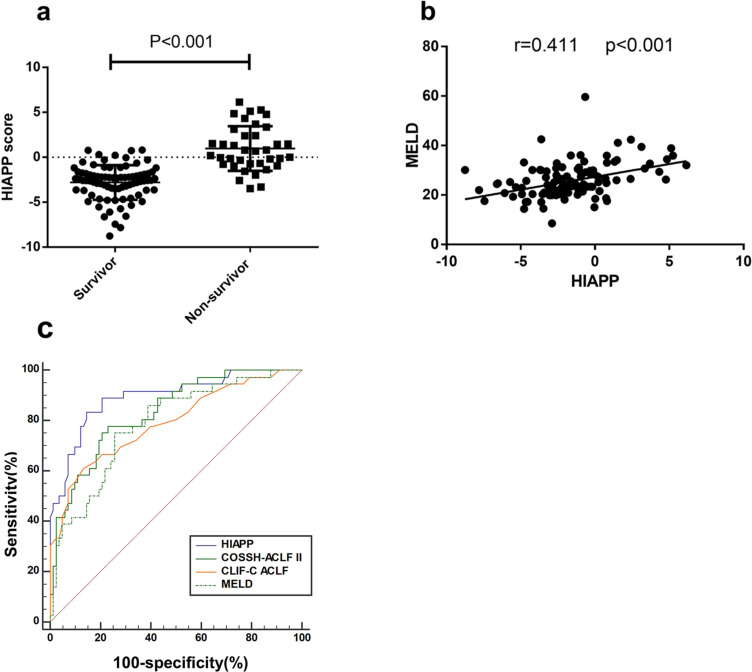
In the non-survivor group, the HIAPP score was significantly higher (0.97±0.41) compared to the survivor group (−2.80±0.21) (P < 0.001; Figure 5a). Additionally, there was a positive correlation observed between the HIAPP score and MELD score (r = 0.411, P < 0.001) (Figure 5b). The HIAPP score achieved an AUC of 0.899 (sensitivity = 83.8%, specificity = 85.7%, cut‐off = −1.09) for 30-day mortality, which demonstrated greater predictive ability than the MELD (AUC = 0.795, sensitivity = 73.7%, specificity = 74.4%, p < 0.05) and CLIF-C ACLF (AUC = 0.781, sensitivity = 59.46%, specificity = 86.59%, p < 0.05) scores. The HIAPP score showed comparable predictive ability to the COSSH-ACLF II score (AUC = 0.825, sensitivity = 75.68%, specificity = 76.83%, p > 0.05) (Figure 5c).
Figure 5.
The correlations between HIAPP and other scores on admission in the deriving cohorts (a) HIAPP score distribution of non-survivor and survivor HBV-ACLF patients in the deriving cohorts (b) The correlations between HIAPP and MELD (c) ROC analysis shows the performance of the HIAPP, COSSH-ACLF II, CLIF-C ACLF, and MELD scores.
HIAPP Performance in the Validation Cohort
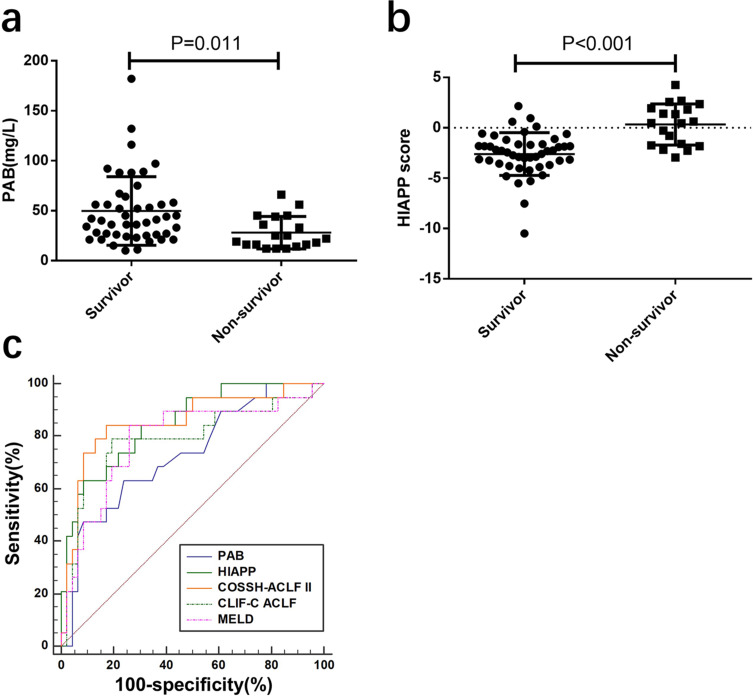
We conducted a retrospective validation study on a cohort of HBV-ACLF patients (n=65) to evaluate the performance of PAB and HIAPP in predicting mortality. In this cohort, non-survivors had significantly lower PAB levels compared to survivors (28.00±3.74 vs 49.74±5.07 mg/L, P=0.011; Figure 6a). The AUC of PAB levels for predicting 30-day mortality was 0.729, with a sensitivity of 63.2% and specificity of 76.1%. The HIAPP score of non-survivors (0.33 ± 0.47) was significantly higher than that of survivors (−2.60 ± 0.31) (P < 0.001; Figure 6b). The HIAPP score model showed an AUROC of 0.850 for predicting 30-day mortality, which was higher than the MELD score (AUROC=0.786) and CLIF-C ACLF score (AUROC=0.787), and comparable to the COSSH-ACLF II score (AUROC=0.854) (Figure 6c).
Figure 6.
The performance of PAB, HIAPP, COSSH-ACLF II, CLIF-C ACLF and MELD for predicting the 30‐day mortality in the validation cohort (a) PAB level of non-survivor and survivor of HBV‐ACLF patients in the validation cohort. (b) HIAPP score of non-survivors and survivors in the validation cohort. (c) ROC analysis shows the performance of PAB, HIAPP, COSSH-ACLF II, CLIF-C ACLF, and MELD scores in the validation cohort.
Discussion
Hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) is recognized as the primary cause of ACLF in China, and it has been linked to a high rate of short-term mortality, imposing a substantial burden on both patients and their families, as well as society as a whole.17,18 Therefore, there is a pressing need for a simple and effective prognostic scoring system to enable early patient classification and determine the appropriate treatment strategy. To this end, previous studies have utilized various clinical indicators that reflect liver function, organ failure, infection, and inflammation, based on the pathological course of ACLF, to develop prognostic models.19–21
As previously mentioned, our study identified INR, HE, age, and platelet count as powerful and independent predictors of 30-day mortality.11,22 Thrombocytopenia, a common coagulation disorder in chronic liver disease, has been included in multiple prognostic scores due to its close relationship with disease progression.23 In our study, we found that platelet count decreased with the progression of HBV-related liver disease, with the lowest counts seen in patients with HBV-ACLF. This is likely due to reduced thrombopoietin secretion and splenic sequestration of platelets in the presence of liver failure.24 Our findings indicate that low platelet counts are associated with impaired liver function and a poor prognosis, which is consistent with the results of a retrospective observational cohort study.25
The half-life of prealbumin is relatively short, at only 1.9 days, making it a sensitive and specific indicator of recent nutritional status and liver function. It is also an ideal candidate indicator for predicting patient prognosis.26 A lower serum PAB level suggests decreased liver synthesis capacity and a poor prognosis. Studies have reported a significant reduction in PAB levels in patients with liver cirrhosis, which is closely related to impaired liver function.27 In patients with decompensated cirrhosis, serum prealbumin is significantly correlated with the Model for End-Stage Liver Disease (MELD) score and serves as a prognostic indicator.28 Additionally, serum prealbumin is an acute-phase protein, which is closely related to inflammation and infection. Various cytokines, such as IL-6, are synthesized in response to inflammation, resulting in decreased synthesis and low serum concentrations of these proteins, including PAB.29 Liver failure is known to cause severe liver function damage and a stronger inflammatory response than other liver diseases, such as cirrhosis. Previous studies have shown that uncontrolled inflammatory responses and immunological dysfunction play important roles in the pathogenesis of acute-on-chronic liver failure, which also affects patient prognosis.30 Based on the above, we hypothesize that PAB may be a sensitive and specific short-term prognostic factor for acute-on-chronic liver failure. Our data showed that serum PAB levels were significantly correlated with important laboratory parameters of liver injury, such as platelet count, alanine aminotransferase, aspartate aminotransferase, and international normalized ratio, as well as the MELD score. This suggests that serum PAB levels may reflect the severity of HBV-related acute-on-chronic liver failure. Furthermore, HBV-related acute-on-chronic liver failure patients had significantly lower PAB levels, and the receiver operating characteristic curve analysis demonstrated that the serum PAB level at admission could accurately predict the 30-day mortality of HBV-related acute-on-chronic liver failure. Notably, PAB showed potential as a predictor of HBV-related acute-on-chronic liver failure prognosis, but it has not yet been incorporated into any prognostic scoring system.
Several prognostic scoring systems have been developed to aid in the early detection and optimization of ACLF management. However, the commonly used prognostic scores for ACLF, such as CLIF‐SOFA, and CLIF-C ACLF, were developed and validated using data from other pathological types of ACLF in the European population. Therefore, their ability to accurately predict short-term outcomes of patients with HBV‐ACLF in China needs additional research. Moreover, these scores require many parameters and a complex evaluation of organ failure, making them difficult to apply in clinical practice. Although the MELD score, which includes creatinine, TBiL, and INR, is still widely used to predict the prognosis of patients with ACLF, it does not consider the impact of clinical complications and age on the prognosis. Thus, a simple and practical prognostic score for HBV-ACLF patients is urgently needed to facilitate the early classification of patients and determine the appropriate treatment strategy.
Based on the existing scores and the findings of this study, we have proposed a prognostic model for short-term mortality in patients with HBV-ACLF, called HIAPP, which is based on PAB, PLT, INR, HE, and age. Our study has shown that the HIAPP model has an excellent linear relationship with the MELD score, indicating that it has potential as a prognostic model for the short-term prognosis of HBV-ACLF. Furthermore, in predicting the short-term outcome of HBV-ACLF patients, we have demonstrated that the HIAPP score outperforms the MELD score and the CLIF-C ACLF score and is comparable with the recently reported COSSH-ACLF II score, which has a high predictive value for the mortality of HBV-ACLF patients in China. Importantly, we have also revealed that HIAPP requires only routine biochemical and hematological indicators, making it more feasible for clinical application, especially in primary hospitals, and reducing the patient’s medical burden.
Our study has several limitations that should be considered. First, the sample size was relatively small, and future studies with larger sample sizes are needed to confirm our findings. Second, we developed and validated the HIAPP model in a retrospective cohort study, and therefore, its performance should be further evaluated in prospective longitudinal cohorts with complete follow-up data. Additionally, the generalizability of our findings may be limited as our study only included patients with HBV‐ACLF from a single center in China.
To conclude, our study highlights the potential of PAB as a predictive marker and the HIAPP score as an easy-to-use pragmatic tool for short-term prognosis in HBV-ACLF patients. The results demonstrate that the HIAPP score can aid in expanding the clinical applicability of early patient triage and determining appropriate management strategies, particularly in primary hospitals. Despite the limitations of our study, including the small sample size and lack of a prospective longitudinal cohort, the findings provide valuable insights into the development of a simple and practical prognostic model for HBV-ACLF patients.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81902056), the Provincial Natural Science Research Project of Anhui Colleges (KJ2019A0276) and the Research Fund Project of Anhui Medical University (2021xkj050).
Abbreviations
HBV-ACLF, Hepatitis B virus-related acute-on-chronic liver failure; PAB, Prealbumin; PLT, Platelet; INR, International normalized ratio; HE, Hepatic encephalopathy; ACLF, Acute-on-chronic liver failure; MELD, Model for End‐stage Liver Disease; DCC, Decompensated cirrhosis; LC, Liver cirrhosis; CHB, Chronic hepatitis B; TBIL, Total bilirubin; ROC, Receiver operating characteristic.
Data Sharing Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy restrictions but are available from the corresponding author on reasonable request.
Ethical Statement
This study was approved by the ethical committee of the Second Affiliated Hospital of Anhui Medical University and was conducted in accordance with the declaration of Helsinki. All participants provided written informed consent so that their clinical data could be used in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Chen L, Zhang J, Lu T, et al. A nomogram to predict survival in patients with acute-on-chronic hepatitis B liver failure after liver transplantation. Ann Transl Med. 2021;9(7):555. doi: 10.21037/atm-20-6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Z, Zhang Y, Cao Y, et al. A dynamic prediction model for prognosis of acute-on-chronic liver failure based on the trend of clinical indicators. Sci Rep. 2021;11(1):1810. doi: 10.1038/s41598-021-81431-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li TP, Guan SH, Wang Q, Chen LW, Yang K, Zhang H. Soluble mannose receptor as a predictor of prognosis of hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2019;25(37):5667–5675. doi: 10.3748/wjg.v25.i37.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y, Liu QY, Zhang Q, Rong YM, Lu CZ, Li H. Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2020;26(29):4288–4301. doi: 10.3748/wjg.v26.i29.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang K, Pan Y, Jin L, Yu F, Zhang F. Low serum soluble transferrin receptor levels are associated with poor prognosis in patients with hepatitis B virus-related acute-on-chronic liver failure. Biol Trace Elem Res. 2022;201:2757–2764. doi: 10.1007/s12011-022-03385-2 [DOI] [PubMed] [Google Scholar]
- 6.Kim JY, Jun JH, Park SY, Yang SW, Bae SH, Kim GJ. Dynamic regulation of miRNA expression by functionally enhanced placental mesenchymal stem cells promoteshepatic regeneration in a rat model with bile duct ligation. Int J Mol Sci. 2019;20(21):5299. doi: 10.3390/ijms20215299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–2191. doi: 10.1136/gutjnl-2017-314641 [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Lin S, Wang M, Huang J, Zhu Y. The risk of acute kidney injury in hepatitis B virus-related acute on chronic liver failure with tenofovir treatment. Biomed Res Int. 2020;2020:5728359. doi: 10.1155/2020/5728359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Liang X, You S, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75(5):1104–1115. doi: 10.1016/j.jhep.2021.05.026 [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Chen Y, Ding M, Duan Z. Correlation between dynamic changes in free triiodothyronine levels and 90-day prognosis in patients with HBV-related acute-on-chronic liver failure. Eur J Med Res. 2022;27(1):88. doi: 10.1186/s40001-022-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Zhang S, Xie Z, et al. Plasminogen as a prognostic biomarker for HBV-related acute-on-chronic liver failure. J Clin Invest. 2020;130(4):2069–2080. doi: 10.1172/JCI130197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao YY, Teng CL, Peng NF, et al. Serum prealbumin is negatively associated with survival in hepatocellular carcinoma patients after hepatic resection. J Cancer. 2019;10(13):3006–3011. doi: 10.7150/jca.30903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo RR, Liu HT, Deng ZJ, et al. Dose-response between serum prealbumin and all-cause mortality after hepatectomy in patients with hepatocellular carcinoma. Front Oncol. 2021;10:596691. doi: 10.3389/fonc.2020.596691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Y, Cheng JW, Chen FY, et al. A novel preoperative predictive model of 90-day mortality after liver resection for huge hepatocellular carcinoma. Ann Transl Med. 2021;9(9):774. doi: 10.21037/atm-20-7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Liu X, Jiang Y, et al. Low preoperative prealbumin predicts the prevalence of complications following liver transplantation. BMC Gastroenterol. 2021;21(1):233. doi: 10.1186/s12876-021-01818-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao C, Gong J, Zhu S, et al. Nomogram based on blood lipoprotein for estimation of mortality in patients with hepatitis B virus-related acute-on-chronic liver failure. BMC Gastroenterol. 2020;20(1):188. doi: 10.1186/s12876-020-01324-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Chen C, Huang C, Xu W, Hu Q, Chen L. Noninvasive models for predicting poor prognosis of chronic HBV infection patients precipitating acute HEV infection. Sci Rep. 2020;10(1):2753. doi: 10.1038/s41598-020-59670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie Y, Zhang Y, Liu LX, Zhu X. Serum lactate level predicts short-term and long-term mortality of HBV-ACLF patients: a prospective study. Ther Clin Risk Manag. 2020;16:849–860. doi: 10.2147/TCRM.S272463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Zou Z, Shen L, et al. A prediction model for outcome in patients with HBV-ACLF based on predisposition, injury, response and organ failure. Sci Rep. 2020;10(1):20176. doi: 10.1038/s41598-020-77235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue-Meng W, Yang LH, Yang JH, Xu Y, Yang J, Song GB. The effect of plasma exchange on entecavir-treated chronic hepatitis B patients with hepatic de-compensation and acute-on-chronic liver failure. Hepatol Int. 2016;10(3):462–469. doi: 10.1007/s12072-015-9667-4 [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Sun Z, Liu X, et al. HINT: a novel prognostic model for patients with hepatitis B virus-related acute-on-chronic liver failure. Aliment Pharmacol Ther. 2018;48(7):750–760. doi: 10.1111/apt.14927 [DOI] [PubMed] [Google Scholar]
- 23.Gallo P, Terracciani F, Di Pasquale G, Esposito M, Picardi A, Vespasiani-Gentilucci U. Thrombocytopenia in chronic liver disease: physiopathology and new therapeutic strategies before invasive procedures. World J Gastroenterol. 2022;28(30):4061–4074. doi: 10.3748/wjg.v28.i30.4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannini EG, Peck-Radosavljevic M. Platelet dysfunction: status of thrombopoietin in thrombocytopenia associated with chronic liver failure. Semin Thromb Hemost. 2015;41(5):455–461. doi: 10.1055/s-0035-1550432 [DOI] [PubMed] [Google Scholar]
- 25.Xia Q, Dai X, Zhang Y, et al. A modified MELD model for Chinese pre-ACLF and ACLF patients and it reveals poor prognosis in pre-ACLF patients. PLoS One. 2013;8(6):e64379. doi: 10.1371/journal.pone.0064379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Zhang L, Wang J, et al. Prealbumin-to-globulin ratio can predict the chemotherapy outcomes and prognosis of patients with gastric cancer receiving first-line chemotherapy. J Immunol Res. 2020;2020:6813176. doi: 10.1155/2020/6813176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao W, Kong M, Yao J, et al. Changes in prealbumin and body mass index associated with T lymphocyte subsets and nutritional status in chronic hepatitis B and HBV-cirrhosis patients. Clin Lab. 2018;64(11). doi: 10.7754/Clin.Lab.2018.180501 [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Cai LY, Zhong L, et al. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis. J Dig Dis. 2010;11(6):352–357. doi: 10.1111/j.1751-2980.2010.00465.x [DOI] [PubMed] [Google Scholar]
- 29.Myron Johnson A, Merlini G, Sheldon J, Ichihara K; Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419–426. doi: 10.1515/CCLM.2007.051 [DOI] [PubMed] [Google Scholar]
- 30.Martin-Mateos R, Alvarez-Mon M, Albillos A. Dysfunctional immune response in acute-on-chronic liver failure: it takes two to tango. Front Immunol. 2019;10:973. doi: 10.3389/fimmu.2019.00973 [DOI] [PMC free article] [PubMed] [Google Scholar]