Abstract
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in the United States. Although certain genetic predispositions may contribute to one's risk for developing CRC, dietary and lifestyle factors may play an important role as well. In a recent study in Nature, Dmitrieva-Posocco et al, reveal a potential protective role of the ketogenic diet in colorectal cancer growth and progression. Administration of a ketogenic diet to CRC-bearing mice demonstrated a tumor-suppressive effect. Specifically, the ketone body β-hydroxybutyrate (BHB) exhibited the ability to suppress epithelial cell proliferation and inhibit tumor growth. BHB acts on cancer cells through regulation of homeodomain-only protein Hopx, known regulator of CRC. Furthermore, BHB requires a surface receptor Hcar to induce Hopx expression and suppress proliferation of intestinal epithelial cells. Taken together, these results describe a new therapeutic approach of using dietary intervention for the prevention and treatment of colorectal cancer.
Keywords: ketogenic diet, BHB, colorectal cancer, metabolites
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer and the second leading cause of cancer-related death in the United States (Siegel et al, 2022). Risk factors for developing CRC include genetic mutations, diet, and lifestyle factors (Islami et al, 2018). Screening by colonoscopy remains the most important and cost-effective strategy in reducing the incidence and mortality of this disease, as treatment typically includes local excision by surgery or partial colectomy, removal of nearby lymph nodes, chemotherapy, and radiation therapy—all of which have significant side effects.
Although the risk for CRC is largely controlled by environmental and lifestyle factors (Islami et al, 2018), the individual risk for developing CRC can also be increased by certain genetic mutations. This is particularly apparent in autosomal dominant genetic disorders, such as Lynch syndrome (hereditary nonpolyposis colon cancer) and familial adenomatous polyposis. Patients with Lynch syndrome, an inherited disorder characterized by mutations in the DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2 or by a deletion of EPCAM (Boland et al, 2022; Idos and Valle, 1993), are predisposed to the development of cancer, including an increased risk for CRC (Sehgal et al, 2014). Despite the genetic predisposition to CRC in patients with Lynch syndrome, the age of onset and rate of progression is highly variable among patients, indicating a critical role for modifiable environmental factors in disease manifestation even in the case of defined genetic mutations.
Optimizing Lifestyle for CRC Prevention
Although common, there are several ways to decrease the rate of incidence, as well as the mortality rate of CRC. Cancer screenings have been an effective method in the early detection of CRC. Stool tests are a noninvasive method that can be used to detect blood or altered DNA in the stool (Abbaszadegan et al, 2007). Colonoscopies and sigmoidoscopies are also common procedures for CRC screening. According to data from the SEER program at the NIH, between 2010 and 2019, the death rates associated with CRC have decreased by 2.0% on average each year (SEER Cancer Stat Facts: Colorectal Cancer, 2022), likely due to the increased availability and use of these screening methods.
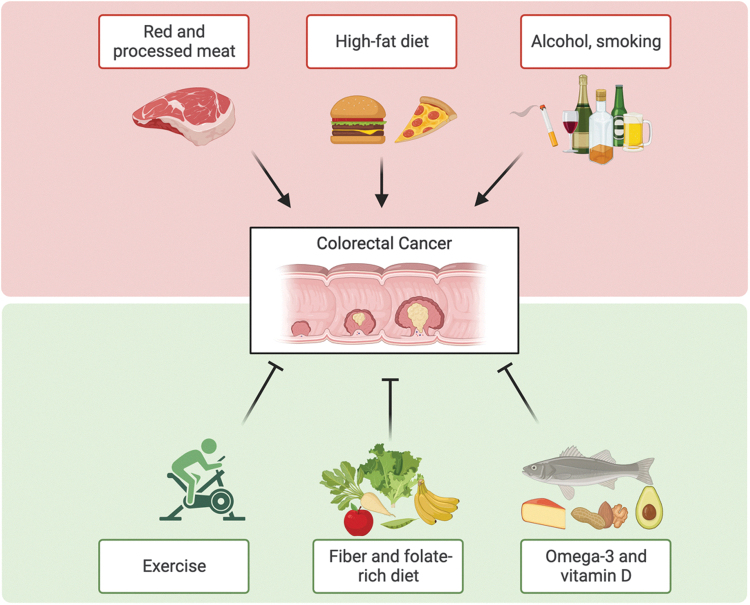
In addition to early detection, clinicians and scientists are increasingly recognizing the value that lies in optimizing a patient's lifestyle, and more specifically their diets, in cancer prevention (Fig. 1). Since only a small number of CRC cases are related to a known genetic predisposition, there is a critical role for adjustable behavioral practices and lifestyle habits (Kirkegaard et al, 2010; MacFarlane and Stover, 2007). Some lifestyle factors, such as obesity and sedentary lifestyles, have been shown to increase the risk for developing CRC (Baena and Salinas, 2015). Moreover, seminal epidemiological studies have linked CRC incidence to diet, and dietary patterns characteristic of the modern human lifestyle have been associated with an increase in CRC prevalence (Brenner et al, 2014).
FIG. 1.
Schematic overview of the role of lifestyle and dietary supplements in colorectal cancer development. Figure was prepared using BioRender.
For example, a Western diet has previously been shown to enhance tumorigenicity of intestinal progenitor cells and to suppress antitumor immunity (Beyaz et al, 2016; Fu et al, 2019; Ringel et al, 2020). Furthermore, multiple epidemiological studies have suggested that excessive intake of animal protein, especially red meat, is associated with increased risk of developing CRC (Aggarwal and Shishodia, 2006). In contrast, little is known about diets that are associated with effective prevention or treatment of intestinal tumorigenesis and mechanistic studies of nutrition and nutritional supplements in CRC are critically needed.
Other modifiable lifestyle factors, including cigarette smoking and alcohol consumption, have been linked to CRC risk in prospective studies of Western populations (Amitay et al, 2020). Mechanistically, acetaldehyde, a metabolite of ethanol/alcohol, can affect DNA synthesis, repair, alteration of structure and function of glutathione, and increase in colonic mucosal proliferation (Win et al, 2017).
Diet has recently emerged as an effective and noninvasive lifestyle intervention against neoplastic diseases (Kanarek et al, 2020). However, concrete recommendations for how diet can be used to prevent and treat cancer remain sparse. High-fiber diets have been shown to protect against CRC (Kunzmann et al, 2015), although there is still varying evidence (Romaneiro and Parekh, 2012). It has been suggested that fiber exerts its protective role against CRC by blocking the conversions of primary bile acids into more toxic secondary bile acids, such as deoxycholic acid.
Bacterial fermentation of fiber can also produce short chain fatty acids (SCFAs), mainly acetic acid, butyric acid, and propionic acid, which may also confer protection against CRC (Baena and Salinas, 2015). Modulation of tumor suppressors, such as p21, through the inhibition of histone deacetylases (HDACs), is thought to contribute to the anticancer effects of SCFAs. Upregulation of tumor suppressor p21, for example, can lead to a reduction in epithelial inflammation and an increase in the apoptosis of cancer cells (Wang et al, 2019).
Studies have also indicated a protective role of folic acid in reducing the risk of CRC. Supplementation of folic acid has been shown to decrease one's risk for developing CRC by about 20–25% and may also decrease the risk of mortality in patients with CRC (Sanjoaquin et al, 2005). Mechanisms by which folic acid may exert its protective effects include its role in maintaining DNA stability and integrity (Kim, 2006). Vitamin B6, a cofactor in folate metabolism (MTHFR), has also been implicated to be protective against CRC (Theodoratou et al, 2008). However, further studies are needed to confirm these potentially protective effects of folic acid and vitamin B6 intake.
Dietary fish intake has also been shown to have a protective effect on CRC (Wu et al, 2012). Fish is typically high in vitamin D and omega-3 fatty acids, such as alpha-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid. Studies have also shown that vitamin D is inversely associated with CRC. Supplementation with calcium and vitamin D have shown a protective effect against CRC, likely due to their ability to decrease epithelial cell proliferation and promote differentiation (Peters et al, 2001; Ryan-Harshman and Aldoori, 2007). However, calcium and vitamin D may be dependent on each other to achieve their full effects (Grau et al, 2003). Magnesium may also offer protection against CRC but may be dependent on the relative levels of calcium as well (Chen et al, 2012; Dai et al, 2012).
Omega-3 fatty acids are substrates for the cyclooxygenase 2 (COX-2) enzyme, which converts these fatty acids into prostaglandin E3 (PGE3). PGE3, unlike PGE2, is considered to be anti-inflammatory in nature and could possibly inhibit the proliferation of tumor cells (Yang et al, 2014). In addition, given the ability of non-steroidal anti-inflammatory drugs (NSAIDs) to inhibit cyclooxygenase enzymes and, therefore, prevent the release of tumor-promoting PGE2, they are also associated with a decreased CRC risk (Garcia-Albeniz and Chan, 2011). Several epidemiological studies have indeed shown the anti-CRC effects of NSAIDs, particularly aspirin; however, these effects may be dependent on levels of COX-2 expression in the tumors of CRC patients (Chan et al, 2007; Rothwell et al, 2010). Selective inhibition of COX-1 by low-dose aspirin has also shown a significant reduction in colonic mucosal prostaglandins, suggesting a COX-1-dependent role in CRC protection as well (Patrono, 2001; Ruffin et al, 1997).
Although some studies suggest that CRC can be prevented in the general population through dietary and lifestyle interventions, addressing this gap in our knowledge may result in the design of a dietary intervention for prevention purposes based on a detailed mechanistic understanding of diet-derived molecules in modulating physiological and neoplastic events.
The Role of Ketogenic Diet and Beta-Hydroxybutyrate in CRC
Mechanistic studies of nutrition in human diseases are in their infancy, and in the majority of cases we lack the scientific understanding needed to improve therapeutic outcomes by optimizing dietary composition.
In a recent study, we probed the effect of diet on intestinal tumorigenesis (Dmitrieva-Posocco et al, 2022). We systematically studied several macro- and micronutrient-defined diets for their impact on CRC in mice. To identify dietary interventions that can impact intestinal tumor growth, we fed mice six different diets and induced CRC using several genetic and nongenetic models. We started with the azoxymethane (AOM)/dextran sodium sulfate (DSS) model, using injection of the carcinogen AOM, followed by three cycles of DSS in the drinking water. We found that high-fat low-carbohydrate ketogenic diet (KD) exerts a profound tumor-suppressive effect across different treatment protocols. Similar to the AOM/DSS model, KD effectively suppressed tumor development in a genetic model of CRC, in which both alleles of the tumor suppressor gene Apc are deleted from a subset of colonic epithelial cells (Dmitrieva-Posocco et al, 2019; Hinoi et al, 2007), resulting in a sporadic transformation pattern (CDX2CreERT-Apcfl/fl).
The KD is a high-fat adequate protein low-carbohydrate diet designed to produce ketosis through mimicking the metabolic changes of starvation (Fedorovich et al, 2018). Although used primarily for treating seizures in children, KD has also been shown to be useful in treating intractable seizures in adults, and possibly for treating other nonepileptic conditions, such as brain tumors, Alzheimer's disease, and diabetes (McDonald and Cervenka, 2018).
KD feeding induces a state of ketosis, which is a survival mechanism that provides an alternative source of energy during prolonged starvation or lack of carbohydrate ingestion. Fat metabolism and its by-product ketone bodies are essential mechanisms for survival in animals and humans. The two main ketone bodies, β-hydroxybutyrate (BHB) and acetoacetate (AcAc), serve as a circulating alternative energy source for tissues during nutrient deprivation (Newman and Verdin, 2013). We identified that one of the main ketone bodies, BHB, but not AcAc, suppresses intestinal proliferation and overall inhibits tumor growth.
It was shown that BHB regulates class I HDACs, which are known as a family of proteins that suppresses and enhances transcription and as promising agents against cancer (Gregoretti et al, 2004). In addition, BHB serves as an inhibitor of NLRP3-mediated mouse inflammatory diseases through suppression of caspase-1 activity and IL-1β secretion (Youm et al, 2015). Another group of researchers showed that KD and BHB controls gut microbiota followed by intestinal Th17 cells regulation, particularly BHB directly suppresses Bifidobacterium growth in a dose-dependent manner in vitro (Ang et al, 2020).
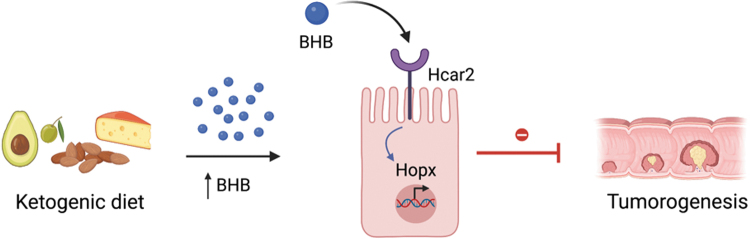
Although these mechanisms do not contribute to the ability of BHB to inhibit intestinal tumor growth, we found that BHB suppresses epithelial cell proliferation and tumor growth through regulation of homeodomain-only protein Hopx, a known regulator of CRC (Yamashita et al, 2013). Furthermore, we identified that BHB requires a surface receptor Hcar2 (Gpr109a) to induce Hopx expression, which inhibits proliferation of IEC (Fig. 2). Finally, we found evidence that elevated levels of BHB and HOPX are negatively correlated with human cell cycle progression. Taken together, these results describe a new therapeutic approach for cancer prevention and treatment.
FIG. 2.
Ketogenic diet and its metabolite BHB act on the cell surface receptor Hcar2 to induce Hopx protein expression, which suppresses proliferation of cancer cells and CRC progression. Figure was prepared using BioRender. BHB, β-hydroxybutyrate; CRC, colorectal cancer.
Given the data presented, KD, and especially BHB, appear to be beneficial dietary elements for cancer prevention and therapy. High levels of serum BHB were associated with less proliferation capability of human epithelial cells from the large intestine, suggesting an association between ketone synthesis and epithelial growth (Dmitrieva-Posocco et al, 2022). These findings call for the systematic evaluation of KD and BHB as new tools in the clinical management of CRC.
Conclusion
We summarized in this study several links between dietary factors and CRC development. In our recent study, we identified new mechanisms by which KDs, and in particular the ketone body BHB, suppress growth and progression of CRC. Our findings support the idea that adjusting one's diet and nutrient uptake can be beneficial in the prevention of CRC development and potentially other types of cancer. Therefore, dietary intervention may be used as a promising new therapeutic strategy together with existing therapies for cancer treatment. However, further investigations are needed to test this hypothesis.
Acknowledgments
We thank the members of the Levy laboratory for discussions and input.
Authors' Contributions
S.K. and G.R. performed literature research and wrote the article. M.L. guided literature research and edited the article. All authors contributed to the article and approved the submitted version.
Disclosure Statement
No competing financial interests exist.
Funding Information
M.L. is supported by the NIH Director's New Innovator Award (DP2-AG-067511), an American Cancer Society Scholar Award, the Pew Scholar Program, the Searle Scholar program, the Edward Mallinckrodt Jr Foundation, and grants from the Abramson Cancer Center, Penn Institute for Immunology, Penn Center for Molecular Studies in Digestive and Liver Diseases, Penn Center for Precision Medicine, Penn Institute on Aging, Penn Center of Excellence in Environmental Toxicology (P30-ES-013508), and the Borrelli Family Pilot Grant in Lynch Syndrome. Figures were created with BioRender.com.
References
- Abbaszadegan MR, Tavasoli A, Velayati A, et al. Stool-based DNA testing, a new noninvasive method for colorectal cancer screening, the first report from Iran. World J Gastroenterol 2007;13(10):1528–1533; doi: 10.3748/wjg.v13.i10.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006;71(10):1397–1421; doi: 10.1016/j.bcp.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Amitay EL, Carr PR, Jansen L, et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer 2020;122(11):1604–1610; doi: 10.1038/s41416-020-0803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020;181(6):1263–1275.e16; doi: 10.1016/j.cell.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena R, Salinas P. Diet and colorectal cancer. Maturitas 2015;80(3):258–264; doi: 10.1016/j.maturitas.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Beyaz S, Yilmaz ÖH. Molecular pathways: Dietary regulation of stemness and tumor initiation by the PPAR-δ pathway. Clin Cancer Res 2016;22(23):5636–5641; doi: 10.1158/1078-0432.CCR-16-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland CR, Yurgelun MB, Mraz KA, et al. Managing gastric cancer risk in lynch syndrome: Controversies and recommendations. Familial Cancer 2022;21(1):75–78; doi: 10.1007/s10689-021-00235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383(9927):1490–1502; doi: 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007;356(21):2131–2142; doi: 10.1056/NEJMoa067208 [DOI] [PubMed] [Google Scholar]
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: A meta-analysis of prospective studies. Eur J Clin Nutr 2012;66(11):1182–1186; doi: 10.1038/ejcn.2012.135 [DOI] [PubMed] [Google Scholar]
- Dai Q, Sandler RS, Barry EL, et al. Calcium, magnesium, and colorectal cancer. Epidemiology 2012;23(3):504–505; doi: 10.1097/EDE.0b013e31824deb09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O, Dzutsev A, Posocco DF, et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity 2019;50(1):166–180.e7; doi: 10.1016/j.immuni.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O, Wong AC, Lundgren P, et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 2022;605(7908):160–165; doi: 10.1038/s41586-022-04649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorovich SV, Voronina PP, Waseem TV. Ketogenic diet versus ketoacidosis: What determines the influence of ketone bodies on neurons? Neural Regen Res 2018;13(12):2060–2063; doi: 10.4103/1673-5374.241442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Coulter S, Yoshihara E, et al. FXR regulates intestinal cancer stem cell proliferation. Cell 2019;176(5):1098–1112.e18; doi: 10.1016/j.cell.2019.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Albeniz X, Chan AT. Aspirin for the prevention of colorectal cancer. Best Pract Res Clin Gastroenterol 2011;25(4):461–472; doi: 10.1016/j.bpg.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: Results of a randomized trial. J Natl Cancer Inst 2003;95(23):1765–1771. doi: 10.1093/jnci/djg110 [DOI] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol 2004;338(1):17–31. doi: 10.1016/j.jmb.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Hinoi T, Akyol A, Theisen BK, et al. Mouse model of colonic adenoma-carcinoma progression based on Somatic Apc inactivation. Cancer Res 2007;67(20):9721–9730. doi: 10.1158/0008-5472.CAN-07-2735 [DOI] [PubMed] [Google Scholar]
- Idos G, Valle L. Lynch Syndrome. University of Washington: Seattle, Seattle (WA); 1993. Available from: http://europepmc.org/abstract/MED/20301390 [Last accessed: August 16, 2022]. [PubMed] [Google Scholar]
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68(1):31–54; doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature 2020;579(7800):507–517; doi: 10.1038/s41586-020-2124-0 [DOI] [PubMed] [Google Scholar]
- Kim Y. Folate: A magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006;55(10):1387–1389; doi: 10.1136/gut.2006.095463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: A prospective Danish cohort study. BMJ 2010;341:c5504; doi: 10.1136/bmj.c5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann AT, Coleman HG, Huang WY, et al. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial12. Am J Clin Nutr 2015;102(4):881–890; doi: 10.3945/ajcn.115.113282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane AJ, Stover PJ. Convergence of genetic, nutritional and inflammatory factors in gastrointestinal cancers. Nutr Rev 2007;65(12 Pt 2):S157–S166; doi: 10.1111/j.1753-4887.2007.tb00355.x [DOI] [PubMed] [Google Scholar]
- McDonald TJW, Cervenka MC. The expanding role of ketogenic diets in adult neurological disorders. Brain Sci 2018;8(8):E148; doi: 10.3390/brainsci8080148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014;25(1):42–52; doi: 10.1016/j.tem.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C. Measurement of cyclooxygenase isozyme inhibition in humans: Exploring the clinical relevance of biochemical selectivity. Clin Exp Rheumatol 2001;19(6 Suppl 25):S45–S50. [PubMed] [Google Scholar]
- Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2001;10(12):1267–1274. [PubMed] [Google Scholar]
- Ringel T, Frey N, Ringnalda F, et al. Genome-scale CRISPR screening in human intestinal organoids identifies drivers of TGF-β resistance. Cell Stem Cell 2020;26(3):431–440.e8; doi: 10.1016/j.stem.2020.02.007 [DOI] [PubMed] [Google Scholar]
- Romaneiro S, Parekh N. Dietary fiber intake and colorectal cancer risk: Weighing the evidence from epidemiologic studies. Topics Clin Nutr 2012;27(1):41–47; doi: 10.1097/TIN.0b013e3182461dd4 [DOI] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376(9754):1741–1750; doi: 10.1016/S0140-6736(10)61543-7 [DOI] [PubMed] [Google Scholar]
- Ruffin MT IV, Normolle D, Vaerten MA, et al. Suppression of human colorectal mucosal prostaglandins: Determining the lowest effective aspirin dose. J Natl Cancer Inst 1997;89(15):1152–1160; doi: 10.1093/jnci/89.15.1152 [DOI] [PubMed] [Google Scholar]
- Ryan-Harshman M, Aldoori W. Diet and colorectal cancer. Can Fam Physician 2007;53(11):1913–1920. [PMC free article] [PubMed] [Google Scholar]
- Sanjoaquin MA, Allen N, Couto E, et al. Folate intake and colorectal cancer risk: A meta-analytical approach. Int J Cancer 2005;113(5):825–828; doi: 10.1002/ijc.20648 [DOI] [PubMed] [Google Scholar]
- Sehgal R, Sheahan K, O'Connell PR, et al. Lynch syndrome: An updated review. Genes (Basel) 2014;5(3):497–507; doi: 10.3390/genes5030497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEER Cancer Stat Facts: Colorectal Cancer. National Cancer Institute: Bethesda, MD; 2022. Available from: https://seer.cancer.gov/statfacts/html/colorect.html [Last accessed: July 5, 2022]. [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72(1):7–33; doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- Theodoratou E, Farrington SM, Tenesa A, et al. Dietary vitamin B6 intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2008;17(1):171–182; doi: 10.1158/1055-9965.EPI-07-0621 [DOI] [PubMed] [Google Scholar]
- Wang G, Yu Y, Wang YZ, et al. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J Cell Physiol 2019;234(10):17023–17049; doi: 10.1002/jcp.28436 [DOI] [PubMed] [Google Scholar]
- Win AK, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 2017;26(3):404–412; doi: 10.1158/1055-9965.EPI-16-0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Feng B, Li K, et al. Fish consumption and colorectal cancer risk in humans: A systematic review and meta-analysis. Am J Med 2012;125(6):551–559.e5; doi: 10.1016/j.amjmed.2012.01.022 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Katoh H, Watanabe M. The Homeobox Only Protein Homeobox (HOPX) and colorectal cancer. Int J Mol Sci 2013;14(12):23231–23243; doi: 10.3390/ijms141223231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Jiang Y, Fischer SM. Prostaglandin E3 metabolism and cancer. Cancer Lett 2014;348(0):1–11; doi: 10.1016/j.canlet.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21(3):263–269; doi: 10.1038/nm.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]