Abstract
The common marmoset (Callithrix jacchus), a New World NHP, has emerged as important animal model in multiple areas of translational biomedical research. The quality of translational research in marmosets depends on early diagnosis, treatment, and prevention of their spontaneous diseases. Here, we characterize an outbreak of infectious cholangiohepatitis that affected 7 adult common marmosets in a single building over a 10-mo period. Marmosets presented for acute onset of lethargy, dull mentation, weight loss, dehydration, hyporexia, and hypothermia. Blood chemistries at presentation revealed markedly elevated hepatic and biliary enzymes, but mild neutrophilia was detected in only 1 of the 7. Affected marmosets were unresponsive to rigorous treatment and died or were euthanized within 48 h of presentation. Gross and histopathologic examinations revealed severe, necrosuppurative cholangiohepatitis and proliferative cholecystitis with bacterial colonies and an absence of gallstones. Perimortem and postmortem cultures revealed single or dual isolates of Escherichia coli and Pseudomonas aeruginosa. Other postmortem findings included bile duct hyperplasia, periportal hepatitis, bile peritonitis, ulcerative gastroenteritis, and typhlitis. Environmental contamination of water supply equipment with Pseudomonas spp. was identified as the source of infection, but pathogenesis remains unclear. This type of severe, infectious cholangiohepatitis with proliferative cholecystitis with Pseudomonas spp. had not been reported previously in marmosets, and we identified and here describe several contributing factors in addition to contaminated drinking water.
Introduction
The common marmoset (Callithrix jacchus) colony at our institution comprises more than 200 animals, ranging in age from infant to geriatric. At the time of this outbreak, animals were housed either individually, in pairs, or in family groups in a single building. Approximately 2 y prior to the outbreak, the colony was genetically diversified by introducing 57 marmosets from 2 German institutions into the breeding colony. The colony is regularly screened for known pathogens of laboratory marmosets including Giardia spp., Klebsiella pneumoniae, Campylobacter spp., Salmonella spp., and Shigella spp.7,23 Weight loss and diarrhea due to Giardia spp., Klebsiella pneumoniae, or Campylobacter spp. infection are diagnosed and treated frequently within the colony, whereas infection with Salmonella or Shigella spp. is rare. Noninfectious metabolic and degenerative conditions managed in the colony include obesity, dental disease, gastrointestinal disease, and bone disease, including fibrous osteodystrophy.7,29
Liver disease occasionally is diagnosed in marmosets in our colony, including in obese marmosets with liver enzyme elevations secondary to hepatic lipidosis (steatosis), and has been reported in other colonies.13,18 Liver amyloidosis as a sequalae of chronic inflammatory pathologies can occur but typically presents in marmosets with reduced body weight. Marmosets are highly susceptible to viral hepatitis, but our colony has had no known cases associated with these agents.9,25 However, from November 2019 through August 2020, 7 adult common marmosets sporadically presented with a similar constellation of clinical findings: acute onset severe lethargy, dull mentation, anorexia with or without dehydration, and marked elevation of liver enzymes in serum. This case series describes an outbreak of acute bacterial cholecystitis, cholangiohepatitis, and typhlitis caused by Pseudomonas spp. that was traced to the water supply system and equipment. To our knowledge, this report is the first to demonstrate infectious cholecystitis due to environmental contamination in a marmoset colony.
Case Series
Clinical presentations.
The index case was an experimentally naïve, 9-y-old male marmoset imported from a German institution 2 y prior to presentation. The family group of this marmoset was reported for weight loss. Subsequent fecal testing was positive for both Giardia spp. and Campylobacter coli. After treatment of the group with tinidazole (62.5 mg, single oral gavage) and azithromycin (40 mg/kg PO once followed by 20 mg/kg PO daily for 4 d), this single adult male remained PCR positive for C. coli. A new treatment plan was started for this male, but the marmoset refused oral medication. The next day, this marmoset was acutely lethargic with a dull mentation. No other neurologic deficits were noted, but he remained minimally responsive to the environment unless directly stimulated. On physical exam, he was severely dehydrated and later that day became frankly icteric. Radiographs showed moderate distention of the stomach and bowel and decreased serosal detail in the abdominal cavity. Consistent with these findings, hypoechogenic free fluid was present in the abdomen on ultrasonography. Exploratory laparotomy revealed severe peritonitis with yellow, viscous abdominal effusion consistent with bile. This marmoset was euthanized intraoperatively due to poor prognosis. Intraoperative cultures were submitted, and a full necropsy was performed.
Over the next 10 mo, 6 additional adult common marmosets presented similarly, with clinical signs of acute lethargy, obtundation, anorexia, hypothermia, and dehydration. Abdominal palpation was unremarkable, and abdominal ultrasonography was performed in a subset of animals. On ultrasound examination, some marmosets had no remarkable findings whereas others showed a hyperechoic gallbladder wall with branching hyperechogenicity throughout the liver, indicative of biliary tract inflammation. Aggressive supportive care was initiated immediately in all cases; this included intravenous and subcutaneous fluid administration, intravenous antibiotics, gastrointestinal support, and heat or oxygen support as indicated. Despite treatment, all marmosets showed progressive clinical signs and were euthanized or died within 48 h of initial presentation.
Materials and Methods
Animals.
A colony of approximately 200 common marmosets (Callitrhix jacchus) is maintained at our institution for breeding and for use in auditory neurophysiology research. Of the 7 marmosets included in this case series, 6 were from a group of 57 obtained from 2 institutions (German Primate Center and University Hospital of Düsseldorf) in 2017; the remaining animal was born at our institution. In addition, 6 of the affected marmosets were male, and all 7 animals were 3 to 11 y old (Table 1). These animals were experimentally naïve and used only for breeding; only one was singly housed during this period. All affected marmosets were considered clinically stable prior to presentation, and routine examination including CBC counts and serum chemistries had revealed no concerning abnormalities (Table 2).
Table 1.
Demographic characteristics of affected marmosets
| Case no. | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age (years) | 9 | 7 | 3 | 5 | 7 | 10 | 11 |
| Sex | Male | Male | Male | Male | Female | Male | Male |
| Baseline weight (g) | 380 | 430 | 390 | 470 | 440 | 430 | 390 |
| Facility space | Room 2 | Room 1B | Room 1A | Room 1B | Room 2a | Room 2 | Room 2a |
| Housing status | Family group | Breeding pair | Singly housed | Breeding pair | Breeding pair | Family group | Breeding pair |
These animals were housed in the same family group.
Table 2.
Blood chemistry prior to clinical presentation and at time of presentation
| Blood chemistry prior to clinical presentationa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case no. | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Reference rangeb | |
| BUN (mg/dL) | 20 | 21 | 28 | 14 | 20 | 31 | 59 | 9.5–52.4 (23.6) |
| ALT (U/L) | 0 | 4 | 0 | 178 | 17 | 60 | 74 | 0.8–45.3 (3.0) |
| AST (U/L) | 145 | 152 | 137 | 197 | 103 | 343 | 185 | 51.2–316.2 (155.7) |
| ALP (U/L) | 111 | 147 | 93 | 65 | 48 | 69 | 69 | 44.0–426.3 (115.5) |
| GGT (U/L) | 8 | 2 | 11 | 12 | 2 | 11 | 8 | 0.2–13.5 (2.8) |
| Total bilirubin (mg/dL) | 0.3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.3 | 0.4 | 0–0.3 (0.0) |
| Blood chemistry at time of presentation | ||||||||
| Case no. | ||||||||
| 2 | 3 | 4 | 5 | 7 | Reference range | |||
| BUN (mg/dL) | 127 | 45 | NA | 40 | 170 | 9.5–52.4 (23.6) | ||
| ALT (U/L) | 41 | 261 | NA | > 600 | > 600 | 0.8–45.3 (3.0) | ||
| AST (U/L) | 364 | 7,665 | 1,100 | 71 | 1,416 | 51.2–316.2 (155.7) | ||
| ALP (U/L) | 5,160 | 2,617 | NA | 252 | THTC | 44.0–426.3 (115.5) | ||
| GGT (U/L) | 230 | 205 | 754 | 9 | 64 | 0.2–13.5 (2.8) | ||
| Total bilirubin (mg/dL) | 0.5 | 5.1 | 10.8 | 0.2 | 0.2 | 0–0.3 (0.0) | ||
At the time of this outbreak, all marmosets in the colony were housed in 2 adjacent rooms in a single animal facility. Marmosets used for breeding were housed in family groups consisting of a breeding pair and as many as 3 generations of their offspring. Marmosets being used for auditory experiments were housed in pairs or singly when necessary for study purposes. Marmosets were provided thermoneutral perching in the form of a nest box or hanging shelter as well as mounted wood branches. These materials were sanitized or replaced on a rotating basis to allow for expression of normal scent-marking behaviors. All colony animals were fed a regulated amount of a gel-based diet (Callitrichid Diet 5LK6, LabDiet, St Louis, MO) to maintain healthy body condition and were provided rotating edible enrichment consisting of cereals, dried fruits, chickpeas, and live wax worms. Marmosets had ad libitum access to autoclaved water bottles that were hand-filled with chlorinated water (target level 1.0 to 1.5 ppm) from a carboy system. The animal rooms were maintained between 74 to 84 °F (23 to 29 °C) and on a 14:10-h light cycle. The colony was not maintained as SPF for any agents. Marmosets were tested and treated for common pathogens as clinically indicated.
All animals in this case study were covered by a breeding protocol approved by the Johns Hopkins University IACUC. This colony was maintained in an AAALAC-accredited facility.
Clinical pathology.
Perimortem CBC counts and serum chemistries were performed using blood collected into 500-µL EDTA and serum separator tubes, respectively. Samples were either analyzed by the Johns Hopkins Phenotyping Core using automated hematology (Procyte, IDEXX, Westbrook, ME) and clinical chemistry (Respons 910 Vet, DiaSys, Waterbury, CT) or shipped to IDEXX Bioanalytics (Columbia, MO) for analysis. Serum from a single case and a healthy conspecific were submitted to IDEXX Bioanalytics for ammonia level measurement. No hemolysis or lipidemia was noted in any of these samples.
Necropsy and histopathology.
Complete diagnostic necropsies were performed after marmosets either died spontaneously or were euthanized. Before euthanasia, marmosets were deeply anesthetized with intramuscular alfaxalone, which was performed using intravenous or intracardiac pentobarbital-based euthanasia solution. Tissues were fixed in 10% neutral buffered formalin, routinely processed into 5 µm sections, and stained with hematoxylin and eosin for microscopic examination. Some sections were also processed for Brown Hopps Gram, Ziel–Neelson acid fast, and Warthin–Starry stains by using standard histology techniques.
Microbiologic analysis.
Depending on the appearance of the organs during necropsy, samples for postmortem culture were collected from the gallbladder, liver, or abdominal cavity by using culturettes (Culture Swab Amies without charcoal, BBL, Becton Dickinson, Franklin Lakes, NJ) and were submitted to IDEXX Reference Laboratories (North Grafton, MA) for processing. A single abdominal culture was obtained aseptically during exploratory laparotomy (case 1), whereas the remaining samples were selected based on organ pathology and minimal likelihood of contamination. In addition to samples for culture, swabs from case 7 were submitted to IDEXX Bioanalytics for PCR testing for C. coli, Campylobacter jejuni, Klebsiella pneumoniae, Salmonella spp., and Shigella spp. Fresh liver samples from cases 2 and 7 were frozen at −20 °C and submitted to IDEXX Bioanalytics for PCR testing of the following pathogens: adeno-associated virus; Corynebacterium bovis; Corynebacterium spp. (HAC2); Epstein–Barr virus; human adenovirus, Hantaan orthohantavirus; human cytomegalovirus; hepatitis viruses A, B, and C; human herpesviruses 6 and 8; HIV 1 and 2, human papillomaviruses 16 and 18; herpes simplex viruses 1 and 2; human T-lymphotropic viruses 1 and 2; lymphocytic choriomeningitis virus; Mycoplasma spp., Seoul orthohantavirus, Sin Nombre orthohantavirus, Treponema pallidum, and vesicular stomatitis virus.
Results
Clinical pathology.
Serum chemistry analyses were performed in 5 of the 7 cases. Results included marked increases in ALT, AST, ALP, and GGT, consistent with severe acute liver failure and cholestasis (Table 2). In addition, 2 cases showed hyperbilirubinemia, and 2 others had hypoalbuminemia. Consistent with the severe dehydration noted on physical exam, BUN and creatinine values were severely elevated, suggesting prerenal azotemia.
Serum ammonia levels were measured in case 7 and in a clinically healthy German-origin marmoset. Levels were over 599 µmol/L for case 7 and 209 µmol/L in the healthy control. Published reference ranges for serum ammonia levels are not currently available for marmosets.
CBC counts results were relatively unremarkable (Table 3). Both RBC and total WBC parameters were within normal limits for all cases. Mild neutrophilia (6.53 × 103 cells/µL) was present in only one animal; otherwise leukocyte subtypes were normal (less than 3 × 103 cells/µL).
Table 3.
CBC count at presentation
| Case no. | Reference rangea | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | ||
| WBC (×103/µL) | 4.15 | 8.7 | 3.4 | 2.89 | 3.17 | 12.0 ± 10.8 |
| Neutrophils (×103/µL) | 2.30 (55.4%) | 6.53 (75.0%) | 2.76 (81.2%) | 2.00 (69.2%) | 2.39 (75.5%) | 3.2 ± 1.5 |
| Lymphocytes (×103/µL) | 1.68 (40.5%) | 1.65 (19.0%) | 0.47 (13.8%) | 0.79 (27.3%) | 0.54 (17.0%) | 3.0 ± 1.6 |
| Monocytes (×103/µL) | 0.16 (3.9%) | 0.44 (5.0%) | 0.11 (3.2%) | 0.10 (3.5%) | 0.03 (0.9%) | 0.25 ± 0.18 |
| Eosinophils (×103/µL) | 0 (0.0%) | 0.09 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.03 (0.9%) | 0.23 ± 0.14 |
| Basophils (×103/µL) | 0.01 (0.2%) | 0 (0.0%) | 0.06 (1.8%) | 0 (0.0%) | 0.18 (5.7%) | 0.16 ± 0.15 |
Necropsy and histopathology.
Gross appearance of the gallbladders ranged from dark green to tan to white; all had thickened walls (up to 2 mm) with bile contents that ranged from yellow to hemorrhagic to purulent. None of the gallbladders could be expressed (Figure 1 A). No obvious stones were observed. All livers had multiple pale-to-tan nodules randomly dispersed throughout the parenchyma (Figure 1 B), and 3 of the 7 cases had peritonitis with fibrinous adhesions. In 5 of the 7 cases, the cecal mucosal surface had raised, multifocal to coalescing, yellow to white plaques, often with peripheral hemorrhage (Figure 1 C). Histologically, all 7 cases showed necrotizing typhlitis.
Figure 1.
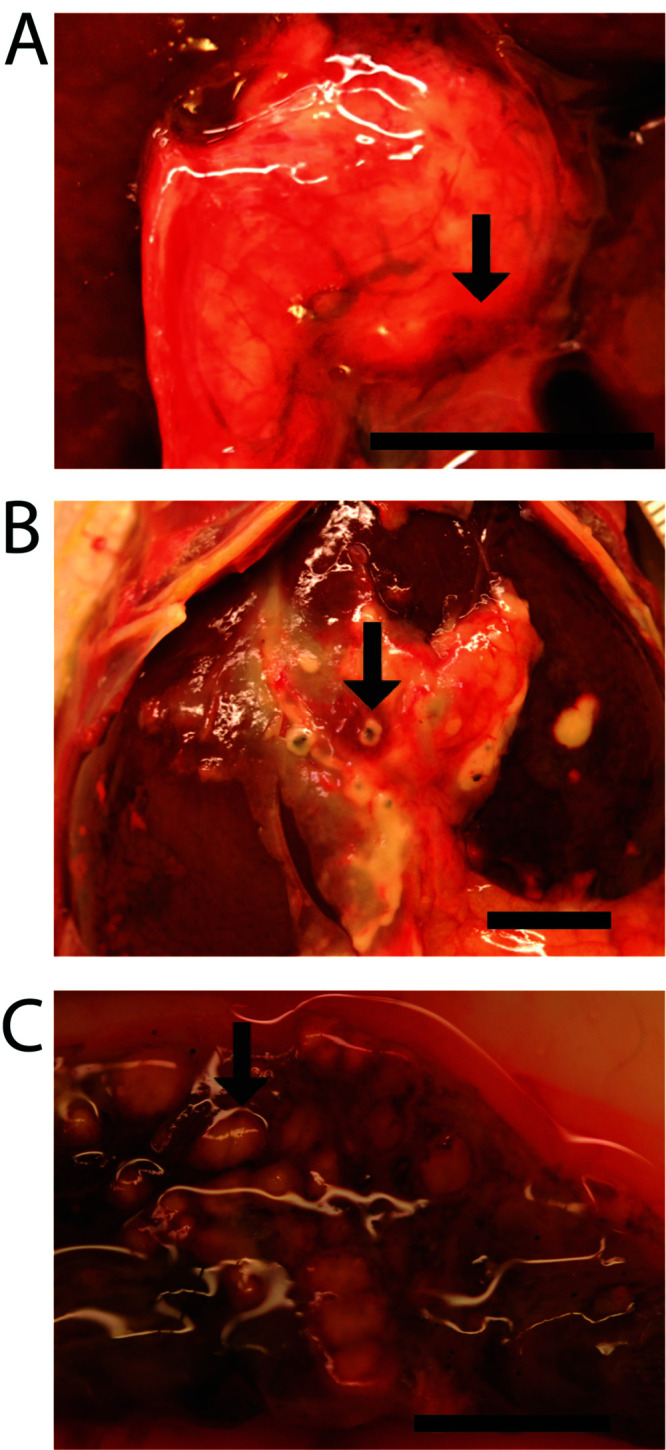
Necrotizing, suppurative inflammation involving multiple organs. (A) The gallbladder is diffusely white to yellow and has a prominent hemorrhagic serosal surface (arrow). (B) Omental fat is loosely adhered to liver capsule and contains 5 to 10, white to yellow, umbilicated nodules that are 2 to 3 mm in diameter (arrow). The liver has dozens of white to yellow umbilicated nodules ranging from 1 to 5 mm in diameter. (C) The mucosal surface of the cecal apex has multifocal to coalescing, 2- to 3-mm, yellow nodules (arrow). Scale bar, 1 cm.
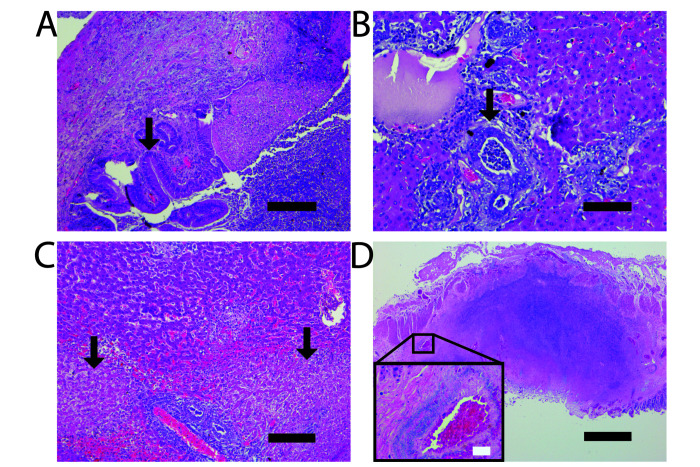
Histopathologic examination of the liver and gallbladder revealed severe, necrotizing, and neutrophilic cholangiohepatitis with proliferative and neutrophilic cholecystitis (Figure 2 A through C) and intralesional bacterial colonies. Gallstones were not detected in any of the cases. Vascular infiltration of bacteria was evident in some examined sections of cecum with necrosis (Figure 2 D). In addition, intrahepatic bile duct hyperplasia, necrotizing periportal hepatitis, and necroulcerative typhlitis were identified in all 7 cases (Figure 2 C and D). Three cases showed gastric involvement, and one showed suppurative nephritis. Other common histopathologic findings included chronic lymphoplasmacytic enteritis (3 of 7 marmosets) and a spectrum of progressive glomerulonephropathy (3 of 7 marmosets). Glomerulonephropathy is a commonly-observed immune-mediated condition of aging marmosets without clinical signs,14,27,36 and is considered to be of minimal clinical significance the current cases.
Figure 2.
Necrotizing, suppurative inflammation involving multiple organs. (A) Transmural neutrophilic infiltration within the gallbladder with epithelial hyperplasia (arrow). (B) Intrahepatic biliary duct hyperplasia (arrow). Occasional bile ducts are filled with neutrophils. (C) Periportal hepatic necrosis and suppurative inflammation. D) Transmural necrotizing neutrophilic infiltration within cecum with peritonitis. Insert: Bacterial rods (hematoxylin positive area) infiltrating a large vessel wall. Hematoxylin and eosin stain; scale bars, 200 µm (A and C), 100 µm (B), 500 µm (D); white scale bar, 30 µm.
Microbiology results.
PCR testing of tissues from cases 2 and 7 were negative for all tested pathogens, including Klebsiella spp.; Salmonella spp.; Epstein–Barr virus; hepatitis viruses A, B, and C; lymphocytic choriomeningitis virus, and Mycoplasma spp.
For 6 of the 7 animals, postmortem cultures of the gallbladder, liver, or abdominal cavity returned Pseudomonas spp. as single isolates or in combination with other bacteria (Table 4). In addition, 4 of the 7 cases yielded only Pseudomonas aeruginosa. After the fourth case presented, concern regarding a possible environmental source of infection prompted culture sampling of the feed, food preparation supplies, and the water supply system. Multiple components of the water supply system returned single isolates of Pseudomonas spp. Further investigation of the water supply system revealed malfunctioning of the automated chlorination system, resulting in chlorine levels that were below those required for disinfection (measured chlorine, 0.0 ppm; target level, 1.0 to 1.5 ppm). The entire water system subsequently was replaced with a reverse-osmosis system, and water quality is now monitored through annual sterility testing, filtration system inspection, and filter replacement. No further cases have been identified since this change.
Table 4.
Results of postmortem cultures
| Gallbladder | Liver | Abdominal cavity | |
|---|---|---|---|
| Case 1 | Not sampled | E. coli | E. coli |
| Case 2 | P. aeruginosa | Not sampled | Not sampled |
| Case 3 | Not sampled | P. aeruginosa, E. coli | Not sampled |
| Case 4 | P. aeruginosa | P. aeruginosa | Not sampled |
| Case 5 | P. putida, E. coli | Not sampled | Not sampled |
| Case 6 | P. aeruginosa, E. coli | Not sampled | Not sampled |
| Case 7 | P. aeruginosa | Not sampled | Pseudomonas spp. |
Discussion
Here, we describe a condition that has not been previously reported in a marmoset colony. The top differential diagnosis for the index case was K. pneumonia infection due to the sudden onset of lethargy or anorexia, or sudden death without premonitory signs.26 A minimal hematologic inflammatory response is also consistent with K. pneumonia infections, as seen in a recent published outbreak.26 Furthermore, several of the marmosets in our colony are chronic carriers of K. pneumoniae, in that they are positive via fecal PCR analysis but are otherwise healthy and asymptomatic. However, in this first case, diagnostic abdominal ultrasonography and laparotomy demonstrated gallbladder and liver involvement and necropsies of subsequent animals revealed a clinical problem that we had not diagnosed previously in colony marmosets. Despite the profound neutrophilic infiltration of the gallbladder and liver and evidence of bacterial colonization, none of the affected marmosets showed an inflammatory leukogram, thus presenting a paradoxical clinical picture.
These 7 cases also shared many gross and histologic features. In 5 of the 7 animals, gross pathology examination revealed a cecal mucosal surface with raised, multifocal to coalescing, yellow to white plaques. Histologically, all 7 cases had necrotizing and neutrophilic typhlitis with bacterial colonies (plaques), and one case had evidence of bacterial vascular invasion. The correlation between cecal bacterial infection and infection of the biliary tract and liver is not understood. We suspect that bacterial inoculation via the oral route (i.e., drinking water) resulted in colonization of the upper gastrointestinal tract followed by either ascending infection (e.g., from the duodenum to the biliary tract and, ultimately, the liver) or septicemia after translocation into the portal blood supply.3
The vulnerability of the gallbladder to bacterial colonization usually depends on cholelithiasis. However, because none of these animals had gallstones, other host or bacterial virulence factors must have predisposed individuals the observed bacterial cholecystitis. Gallbladder disease has been reported in baboons, macaques, marmosets, and tamarins, and in general is commonly a consequence of cholelithiasis, experimentally induced immunodeficiency, or parasitic coinfection.17,30,34,35 A search of the literature did not reveal reports of severe infectious cholecystitis or cholangiohepatitis—especially with such acute, severe clinical presentation—in marmosets. Multiple reviews of spontaneous or background pathology in common marmoset colonies report low percentages of hepatitis and cholecystitis, but these review do not include the initial clinical signs.11,16,33 One review article reports infection with P. aeruginosa in 3 colitis cases and 3 hepatitis cases, but the pathogenesis and cause of death were unclear.11
Other common clinical features in our cases were dull mentation and lethargy at presentation. Due to the severely elevated serum liver enzymes indicative of liver failure, hepatic encephalopathy was considered. In this regard, serum ammonia was greatly elevated in case 7 (the only animal tested). Sampling and analyzing ammonia blood levels can be complicated due to its inherent instability and frequent artificial elevation.12 Because reference values are not available for serum ammonia in marmosets, we also submitted a serum sample from a normal marmoset. Although both results are likely artificially elevated due to the shipping time to the testing facility, the scale of difference between the healthy and affected marmosets is consistent with a genuine increase in the affected animal. The CNS signs of obtunded or depressed mentation and ataxia can occur with hyperammonemia, but presence of hyperammonemia among the other 6 marmosets was not verified.
All 7 marmosets in this case series were used for breeding. During the same period in which the clinical cases occurred, the mortality rate was higher than expected in their offspring. Between March 2019 and July 2020, 86% (6 of 7) of the affected adult breeding marmosets had multiple offspring die within 48 h of birth, totaling 23 offspring. Ten of those 23 infants (43%) had histologic hepatobiliary disease that varied in severity. Liver lesions included necrosis, thrombosis, hemorrhage, bile stasis, and biliary duct hyperplasia, and in one case, hepatobiliary disease that resembled extrahepatic biliary atresia, with inflammation and bile plugging. By comparison, in healthy adult breeding pairs during the same timeframe only 1 of 38 offspring (2.6%) that died or were euthanized within 48 h had hepatobiliary disease. Due to their small size and lack of postmortem indication, cultures were not obtained from any of the infant marmosets. Although a cause for the hepatic lesions was not readily apparent in these cases, the findings in the infants likely are related to the parents’ chronic infection with Pseudomonas spp. Since we eliminated Pseudomonas spp. from the water supply, none of the infant marmosets that have died or been euthanized have had hepatobiliary lesions identified on necropsy.
Several animals used for neurologic studies in our facility have chronic cranial implants that are monitored via culture of implant margins for implant-associated infections, including Staphylococcus aureus, Streptococcus spp., and Pseudomonas spp.5 When infection is detected during screening, these animals are treated if clinical signs are present. None of these chronically implanted animals were included in the case series we described here.
Pseudomonas spp. are ubiquitous in the environment and an opportunistic pathogen in humans and many other species.37 They are difficult to eliminate due to their ability to form a biofilm. As is clear from this case series, P. aeruginosa infections can be life-threatening, progress rapidly, rarely affect the leukogram, and can be difficult to treat. Treatment is often hindered by the organism’s ability to form biofilms, which is a self-generated matrix of extracellular polysaccharides, proteins, and lipids. The biofilm protects bacterial colonies from environmental stressors, temperature changes, and phagocytosis by the immune system.10,37
In clinical settings, P. aeruginosa is intrinsically resistant to many antibiotics, limiting the range of antibiotics for which sensitivity testing is indicated.37 In our cases, Pseudomonas isolates were susceptible to the antibiotics ceftazidime, imipenem, amikacin, gentamicin, ciprofloxacin, enrofloxacin, and marbofloxacin. A single gallbladder isolate was resistant to enrofloxacin.
We reviewed these cases for a common predisposing feature for susceptibility to this infection. In humans, P. aeruginosa is opportunistic and infections occur in immunocompromised individuals.37 To investigate the possibility of an underlying primary infection causing immunosuppression, postmortem liver samples from 2 of the 7 cases were tested for common gastrointestinal and hepatic parasites, viruses, and bacteria. Although none of the cases had a recent clinical history of diarrhea or systemic illness, other individual marmosets in our colony are fecal positive for Giardia, and a previous outbreak in Salmonella in 2015 resulted in ulcerative colitis. In addition, in humans, Giardia has been reported as a cause of acute cholecystitis.3,4 We also considered coinfection with Helicobacter spp., given that our animals’ hepatobiliary inflammation and chronic enteritis are consistent with reports in other marmoset colonies and other species of NHP.31,33 Other potential infectious agents included Epstein–Barr virus; hepatitis viruses A, B, and C; lymphocytic choriomeningitis virus, and Mycoplasma spp. Both liver samples were PCR negative for all agents. Although less likely, another potential pathogen was Callitrichine herpesvirus 3, which causes a lymphoproliferative disease and B cell lymphoma of the gastrointestinal tract and associated lymph nodes that often results in intraabdominal masses. In cases of infection with Callitrichine herpesvirus 3, hematology often reveals neutrophilia with left shift and elevated liver enzymes.28 Although 3 of our 7 marmosets had chronic lymphoplasmacytic enteritis, none had enlarged lymph nodes or evidence of lymphoproliferative disease or B cell lymphoma, thus ruling out Callitrichine herpesvirus 3. Historically, callitrichid hepatitis can be caused by lymphocytic choriomeningitis virus, an arenavirus that results in rapidly progressive illness or sudden death,8 as was seen in this case series. When affected animals are discovered perimortem, clinical chemistry analyses likely will reveal elevated serum AST and total bilirubin in conjunction with lymphocytosis.8 Mus musculus serve as a natural reservoir, and although outbreaks of callitrichid hepatitis are most commonly reported in zoos and animal parks, an outbreak in a primate research facility is possible if feral rodents are present.32
After we cultured Pseudomonas at necropsy, we began to search for the source of an infection. Immediately after the failed chlorination system was discovered, it was replaced with a reverse-osmosis filtration system. Currently, autoclaved bottles are filled directly from the filtration system and changed 3 times each week. Since this revised sanitation policy was implemented, no additional cases have occurred. With the source of infection identified and removed, the remaining question is why only a small subset of the population succumbed to severe infection while the majority remained unaffected despite a common water supply. Only 3% of the colony was affected, but no obvious pattern of infection was determined, and disease occurred in both animal rooms. Two affected animals were cohoused, but their cases occurred 2 mo apart. Signalment of affected animals showed a strong predisposition toward older male marmosets. The average age of cases was 7.5 years, and all but one of the affected animals was male. In addition, 86% (6 of 7 animals) of cases occurred in a cohort of animals obtained from 2 institutions in Germany during 2017. This population of marmosets represents approximately 25% of the colony, but the genetic relatedness of the animals in this cohort is unknown.
In humans, Pseudomonas infections are associated with water, similar to this case series. Pseudomonas infection outbreaks in humans are prevalent in hospitals, and strategies for its elimination have been reviewed.22 Devices, instrumentation, water tubing, and electronic faucets are particularly important factors in transmission. In addition, Pseudomonas has been transmitted to the lung during bronchoscopy36 and to the biliary system after endoscopic procedures. The human biliary tract is particularly susceptible to Pseudomonas infections.15 Ten patients who underwent endoscopic retrograde cholangiopancreatography (ERCP) with a single contaminated endoscope grew P. aeruginosa serotype 10 from their bile samples, and 5 of the 10 patients had clinical manifestations including cholecystitis and abscesses. P. aeruginosa strains from the bile and elevator endoscope channel were cultured to show the cross-contamination between patients when endoscopes were not cleaned and disinfected appropriately.1 Bacterial infection is considered a main complication of ERCP, especially with bacteria like Pseudomonas that can form biofilms. Recently, new standards for disinfection highlight the worldwide problem of bacterial contamination during endoscopy.6,21
In summary, we describe here a case series in common marmosets with severe acute cholangiohepatitis and cholecystitis associated with Pseudomonas spp. contamination of the water supply. Six of the affected 7 marmosets had been imported into our colony from institutions in Germany, all of the affected marmosets were experimentally naïve, and 6 of the 7 animals were older males. This case series highlights the importance of considering environmental factors in disease outbreaks even when discrete patterns are unclear. This novel disease presentation is of potential importance for all institutions that maintain marmoset colonies.
Acknowledgments
We thank the other JHU laboratory animal medicine and veterinary pathology residents and faculty who were involved in these cases for their insight and service for these cases.
References
- 1.Allen JI, Allen MO, Olson MM, Gerding DN, Shanholtzer CJ, Meier PB, Vennes JA, Silvis SE. 1987. Pseudomonas infection of the biliary system resulting from use of a contaminated endoscope. Gastroenterology 92:759–763. 10.1016/0016-5085(87)90029-1. [DOI] [PubMed] [Google Scholar]
- 2.An Z, Braseth AL, Sahar N. 2021. Acute cholangitis: Causes, diagnosis, and management. Gastroenterol Clin North Am 50:403–414. 10.1016/j.gtc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Araki H, Shimizu S, Hayashi K, Yamada T, Kusakabe A, Kanie H, Mizuno Y, Kojima I, Saitou A, Nagao K, Suzuki Y, Toyohara T, Suzuki T, Uchida E, Uno K, Nakazawa T. 2017. Acute acalculous cholecystitis caused by Giardia lamblia. Intern Med 56:1657–1662. 10.2169/internalmedicine.56.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson NE, Cheney C, Rholl V, Burris D, Hadro N. 2001. Biliary giardiasis in a patient with human immunodeficiency virus. J Clin Gastroenterol 33:167–170. 10.1097/00004836-200108000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Association of Primate Veterinarians. 2021. Association of Primate Veterinarians cranial implant care for nonhuman primates in biomedical research. J Am Assoc Lab Anim Sci 60:496–501. 10.30802/AALAS-JAALAS-21-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azimirad M, Alebouyeh M, Sadeghi A, Khodamoradi E, Aghdaei HA, Mohammad Alizadeh AH, Zali MR. 2019. Bioburden and transmission of pathogenic bacteria through elevator channel during endoscopic retrograde cholangiopancreatography: Application of multiple-locus variable-number tandem-repeat analysis for characterization of clonal strains. Expert Rev Med Devices 16:413–420. 10.1080/17434440.2019.1604215. [DOI] [PubMed] [Google Scholar]
- 7.Baxter VK, Shaw GC, Sotuyo NP, Carlson CS, Olson EJ, Zink MC, Mankowski JL, Adams RJ, Hutchinson EK, Metcalf Pate KA. 2013. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One 8:e82747. 10.1371/journal.pone.0082747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady AG, Carville AAL. 2012. Chapter 12. Digestive system diseases of nonhuman primates, p 589–627. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman primates in biomedical research (2nd edition). Boston (MA): Academic Press. [Google Scholar]
- 9.Carrion R, Jr, Patterson JL. 2012. An animal model that reflects human disease: The common marmoset (Callithrix jacchus). Curr Opin Virol 2:357–362. 10.1016/j.coviro.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlan LM, Cotter PD, Hill C, Alvarez-Ordóñez A. 2016. New weapons to fight old enemies: Novel strategies for the (bio)control of bacterial biofilms in the food industry. Front Microbiol 7:1641. 10.3389/fmicb.2016.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David JM, Dick EJ, Jr, Hubbard GB. 2009. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). J Med Primatol 38:347–359. 10.1111/j.1600-0684.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.eClinPath.com. [Internet]. Ammonia. [Cited DD Month YYYY]. Available at: https://eclinpath.com/chemistry/liver/liver-function-tests/ammonia/.
- 13.Franco-Mahecha OL, Carrasco SE. 2021. Hepatic steatosis, a lesion reported in captive aged common marmosets. Aging Pathobiol Ther 3:14–16. 10.31491/APT.2021.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han HJ, Powers SJ, Gabrielson KL. 2022. The common marmoset: Biomedical research animal model applications and common spontaneous diseases. Toxicol Pathol 50:628–637. 10.1177/01926233221095449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JS, Moon C, Mun SJ, Lee JE, Lee SO, Lee S, Lee SH. 2021. Antimicrobial susceptibility trends and risk factors for antimicrobial resistance in Pseudomonas aeruginosa bacteremia: 12-year experience in a tertiary hospital in Korea. J Korean Med Sci 36:e273. 10.3346/jkms.2021.36.e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G. 2006. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp Toxicol Pathol 57:405–410. 10.1016/j.etp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Kessler MJ. 1982. Clinical note: Calcium bilirubinate gallstones in an aged rhesus monkey (Macaca mulatta). Am J Primatol 2:291–294. 10.1002/ajp.1350020306. [DOI] [PubMed] [Google Scholar]
- 18.Kramer JA, Grindley J, Crowell AM, Makaron L, Kohli R, Kirby M, Mansfield KG, Wachtman LM. 2015. The common marmoset as a model for the study of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Vet Pathol 52:404–413. 10.1177/0300985814537839. [DOI] [PubMed] [Google Scholar]
- 19.Kramer R, Burns M. 2019. Chapter 6. Normal clinical and biological parameters of the common marmoset (Callithrix jacchus), p 93–107. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Boston (MA): Academic Press. [Google Scholar]
- 20.Kuehnel F, Grohmann J, Buchwald U, Koeller G, Teupser D, Einspanier A. 2012. Parameters of haematology, clinical chemistry, and lipid metabolism in the common marmoset and alterations under stress conditions. J Med Primatol 41:241–250. 10.1111/j.1600-0684.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- 21.Larsen S, Russell RV, Ockert LK, Spanos S, Travis HS, Ehlers LH, Mærkedahl A. 2020. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. EClinicalMedicine 25:100451. 10.1016/j.eclinm.2020.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loveday HP, Wilson JA, Kerr K, Pitchers R, Walker JT, Browne J. 2014. Association between healthcare water systems and Pseudomonas aeruginosa infections: A rapid systematic review. J Hosp Infect 86:7–15. 10.1016/j.jhin.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Ludlage E, Mansfield K. 2003. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med 53:369–382. [PubMed] [Google Scholar]
- 24.Magden ER, Mansfield KG, Simmons JH, Abee CR. 2015. Chapter 17. Nonhuman primates, p 771–930. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine (3rd edition). Boston (MA): Academic Press. [Google Scholar]
- 25.Mansfield K. 2003. Marmoset models commonly used in biomedical research. Comp Med 53:383–392. [PubMed] [Google Scholar]
- 26.Mansfield KG, Fox JG. 2019. Chapter 16. Bacterial diseases, p 265–287. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Boston (MA): Academic Press. [Google Scholar]
- 27.Marini RP. 2019. Chapter 12. Diseases of the urogenital system, p. 195–212. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Boston (MA): Academic Press. [Google Scholar]
- 28.Mätz-Rensing K, Bleyer M. 2019. Chapter 15. Viral diseases of common marmosets, p 251–264. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Boston (MA): Academic Press. [Google Scholar]
- 29.Olson EJ, Shaw GC, Hutchinson EK, Schultz-Darken N, Bolton ID, Parker JB, Morrison JM, Baxter VK, Pate KA, Mankowski JL, Carlson CS. 2015. Bone disease in the common marmoset: Radiographic and histological findings. Vet Pathol 52:883–893. 10.1177/0300985815589354. [DOI] [PubMed] [Google Scholar]
- 30.Sasseville VG, Mansfield KG. 2010. Overview of known nonhuman primate pathogens with potential to affect colonies used for toxicity testing. J Immunotoxicol 7:79–92. 10.3109/15476910903213521. [DOI] [PubMed] [Google Scholar]
- 31.Saunders KE, Shen Z, Dewhirst FE, Paster BJ, Dangler CA, Fox JG. 1999. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J Clin Microbiol 37:146–151. 10.1128/JCM.37.1.146-151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scanga CA, Holmes KV, Montali RJ. 1993. Serologic evidence of infection with lymphocytic choriomeningitis virus, the agent of callitrichid hepatitis, in primates in zoos, primate research centers, and a natural reserve. J Zoo Wildl Med 24:469–474. [Google Scholar]
- 33.Shen Z, Feng Y, Sheh A, Everitt J, Bertram F, Paster BJ, Fox JG. 2015. Isolation and characterization of a novel Helicobacter species, Helicobacter jaachi sp. nov., from common marmosets (Callithrix jaachus). J Med Microbiol 64:1063–1073. 10.1099/jmm.0.000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slingluff JL, Williams JT, Blau L, Blau A, Dick EJ, Jr, Hubbard GB. 2010. Spontaneous gallbladder pathology in baboons. J Med Primatol 39:92–96. 10.1111/j.1600-0684.2009.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KM, Calle P, Raphael BL, James S, Moore R, McAloose D, Baitchman E. 2006. Cholelithiasis in 4 callitrichid species (Leontopithecus, callithrix). J Zoo Wildl Med 37:44–48. 10.1638/05-032.1. [DOI] [PubMed] [Google Scholar]
- 36.Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. 2011. The marmoset as a model of aging and age-related diseases. ILAR J 52:54–65. 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thi MTT, Wibowo D, Rehm BHA. 2020. Pseudomonas aeruginosa biofilms. Int J Mol Sci 21:8671. 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhou H, Jiang Q, Wang Q, Li S, Huang Y. 2020. Bronchoscope-related Pseudomonas aeruginosa pseudo-outbreak attributed to contaminated rinse water. Am J Infect Control 48:26–32. 10.1016/j.ajic.2019.06.013. [DOI] [PubMed] [Google Scholar]