Abstract
Because fluoroquinolones have an immunomodulatory effect on cytokine production by lipopolysaccharide (LPS)-treated human monocytes, we examined the effect of fluoroquinolones on the survival of mice injected with a lethal dose of LPS. Trovafloxacin (100 mg/kg), ciprofloxacin (250 mg/kg), and tosufloxacin (100 mg/kg) protected 75% (P = 0.0001), 25% (P = 0.002), and 50% (P = 0.002), respectively, of mice against death. The fluoroquinolones significantly reduced serum levels of interleukin-6 and tumor necrosis factor alpha in LPS-treated mice. The protective effects of fluoroquinolones in LPS-induced shock in mice may also occur in humans.
Septic shock that results from gram-negative bacterial infections is a major cause of death among patients in intensive care units (17). The presence of bacterial lipopolysaccharide (LPS) in the bloodstream causes fever, hypotension, multiple organ failure and, in severe cases, septic shock and death (16). Tumor necrosis factor (TNF) and interleukin-1 (IL-1) have been shown to be important mediators of shock; both induce changes that are similar to those induced by endotoxin or live gram-negative bacteria (7, 18). Although concentrations of these cytokines fluctuate during sepsis (21), TNF is elevated in the sera of patients in septic shock, and most studies have shown an association between TNF levels, severity of shock, and fatality. The serum levels of IL-1, IL-6, and IL-10 are also frequently elevated in these patients. High peak levels or persisting elevation of IL-6 levels has been stated to be a predictor of adverse outcome (21).
TNF, IL-1, and platelet-activating factor have been identified as important mediators in development of inflammation induced by sepsis (2, 4, 11). However, clinical trials in humans with sepsis or septic shock with TNF-α blocking antibodies (1, 8), IL-1 receptor antagonists (12), and platelet-activating factor receptor antagonists (10) have been unsuccessful. Also, attempts to downregulate the inflammatory process by using methylprednisolone (6) or to prevent the initiation of sepsis by using monoclonal antibodies directed against endotoxin (5, 14, 22) have failed to show benefit of these treatment modalities.
We have previously shown that trovafloxacin reduces the in vitro production of TNF-α, IL-1β, and IL-6 by LPS-stimulated human monocytes (15). In the present study, we considered it of interest to determine whether three structurally related fluoroquinolones—trovafloxacin, ciprofloxacin, and tosufloxacin—affect serum concentrations of cytokines and prevent or reduce mortality in mice injected with a lethal dose of LPS.
Antibiotics.
Trovafloxacin (Pfizer, Inc., Groton, Conn.), ciprofloxacin (Bayer Corp., West Haven, Conn.), and tosufloxacin (Abbott Laboratories, Chicago, Ill.) were dissolved as directed by the supplier and administered orally by gavage.
Mice.
Outbred Swiss Webster (SW) and inbred BALB/C female mice weighing approximately 20 g at the beginning of each experiment were purchased from Taconic Farms, Inc. (Germantown, N.Y.) and given water and food ad libitum.
Effect of antibiotics on mortality induced by LPS.
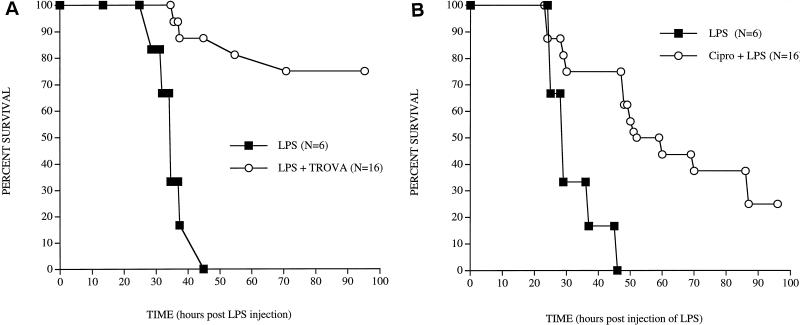
In preliminary experiments, doses of 10 or 100 μg of LPS (Escherichia coli O111:B4; Sigma Chemical Co., St. Louis, Mo.) administered intravenously (i.v.) resulted in 100% survival. Mortality following a dose of 500 μg varied from 50 to 100%; a 1,000-μg dose always resulted in 100% mortality. The effect of pretreatment with antibiotics on mortality was investigated by treating SW mice orally with doses of 100 or 200 mg of trovafloxacin, 100 or 250 mg of ciprofloxacin, or 100 or 200 mg of tosufloxacin per kg at 47, 17, and 1 h prior to the injection of 1,000 μg of LPS. Control mice received antibiotics alone or LPS alone. Mice were observed daily at 8 a.m. and 5 p.m., and mortality was recorded for 1 week after the injection of LPS. The Kaplan-Meier product limited-survival analysis was used to analyze the survival data (StatView, version 4.02; Abacus Concepts, Berkeley, Calif.). A P value of ≤0.05 was considered significant. A series of 11 experiments with 6 to 10 SW mice in each treatment group revealed comparable results. The results of representative experiments are shown in Fig. 1. Treatment with 100 or 200 mg of trovafloxacin per kg resulted in survival of 75% (Fig. 1A) and 67% of the mice, respectively (P = 0.001), whereas treatment with 250 mg of ciprofloxacin per kg resulted in survival of 25% of the mice (P = 0.008) (Fig. 1B). Treatment with a 100-mg/kg dose did not afford significant protection against death. An experiment of the same design in BALB/c mice (10 mice in each group) using 250 mg of ciprofloxacin per kg resulted in 30% survival (P = 0.001). Survival rates following treatment of SW mice with 100 or 200 mg of tosufloxacin per kg were 50% (P = 0.003) and 30% (P = 0.008), respectively (six mice in each group).
FIG. 1.
Survival in SW mice treated orally with 100 mg of trovafloxacin per kg and injected i.v. with 1,000 μg of LPS 1 h later (A) or treated orally with 250 mg of ciprofloxacin per kg and injected with LPS 1 h later (B). The P values, as determined by Kaplan-Meier product limited-survival analysis, were 0.001 for trovafloxacin and 0.008 for ciprofloxacin.
In separate experiments to determine whether a single dose of antibiotics administered 1 h before 1,000 μg of LPS would also be protective, mice were treated with 100 or 200 mg of trovafloxacin per kg 1 h before injection of LPS. The results of a representative experiment of the three performed revealed that 17% (P = 0.010) of the mice treated at 100 mg/kg and 33% (P = 0.0006) of the mice treated at 200 mg/kg survived longer than the controls injected with LPS alone (10 mice in each group).
In five experiments performed to determine whether administration of the antibiotics after the injection of LPS also would be protective, groups of 5 to 10 SW mice each were injected i.v. or intraperitoneally with 500 or 1,000 μg of LPS and, 1 h or 10 min later, were treated orally with 100 mg of trovafloxacin or 250 mg of ciprofloxacin per kg. The results varied from an earlier death in the LPS-antibiotic treated mice than in controls that received only LPS to 25 and 30% survival rates in mice treated with trovafloxacin or ciprofloxacin, respectively. The reasons for this variation are unclear. Mice appeared ill 1 h following LPS injection, and by 3 h they were huddled together and lethargic. Attempts to administer a second dose of the antibiotics at 6 h after the first dose were not successful.
Effect of antibiotics on serum levels of cytokines.
Determination of serum levels of cytokines was conducted using commercially available enzyme-linked immunosorbent assay reagents (PharMingen, San Diego, Calif., and Endogen, Woburn, Mass.). Quantification was based on a standard curve derived by linear dilution of the cytokine standards included in the respective kits. The detection limits were as follows: 4 pg/ml for IL-2; 8 pg/ml for IL-1β, IL-4, IL-6, IL-10, and TNF-α; and 20 pg/ml for IL-12 (p40) and gamma interferon (IFN-γ). Each assay was performed in duplicate wells. Statistical analysis of the differences in cytokine levels was determined using Welch's modified t test. A P value of ≤0.05 was considered significant.
Initial experiments were performed to determine the effects of 100 mg of either trovafloxacin alone, ciprofloxacin alone, or LPS alone per kg. Controls were as described in Table 1. Mice were bled by cardiac puncture under anesthesia at 1 and 3 h after treatment. The serum levels of each of the cytokines were significantly elevated at 1 and 3 h following injection of LPS compared with sham-treated controls. The combined results of two separate experiments, with 10 mice in each group, are shown in Table 1. Treatment with trovafloxacin resulted in significant increases in the levels of IL-2, IL-4, IL-10, and TNF-α at 1 and 3 h, of IL-6 and IL-12 at 1 h, and of TNF-α at 3 h (Table 1). Treatment with ciprofloxacin resulted in significant increase in levels of IL-2, IL-4, and TNF-α at 1 and 3 h, of IL-12 at 1 h, and of IL-10 and TNF-α at 3 h. Although the increases in levels of cytokines were significantly higher in mice treated with antibiotics than in sham-treated controls (injected with diluent), they were not significantly higher than those noted in mice injected with LPS alone (Table 1).
TABLE 1.
Cytokine levels in blood of mice at 1 and 3 h after administration of LPS, ciprofloxacin, or trovafloxacin
| Groupa | Time (h) | Serum cytokine level (ng/ml)b of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-1α | IL-2 | IL-4 | IL-6 | IL-10 | IL-12 | IFN-γ | TNF-α | ||
| Control | 1 | 0.16 ± 0.8 | 0.57 ± 0.2 | 2.32 ± 0.9 | 0.58 ± 0.1 | 0.47 ± 0.03 | 1.79 ± 0.08 | 0.12 ± 0.01 | 1.43 ± 0.5 |
| 3 | 0.26 ± 0.07 | 0.48 ± 0.1 | 0.56 ± 0.04 | 0.53 ± 0.06 | 0.62 ± 0.1 | 2.2 ± 0.1 | 0.31 ± 0.08 | 0.64 ± 0.1 | |
| LPS | 1 | 0.3 ± 0.1* | 1.2 ± 0.5* | 6.4 ± 1.3* | 20.4 ± 10.5 | 0.9 ± 0.4 | 5.8 ± 2.1* | 1.0 ± 0.1* | 4.6 ± 1.6* |
| 3 | 1.2 ± 0.5* | 3.6 ± 0.9* | 2.1 ± 0.2* | 31.2 ± 1.5* | 1.9 ± 0.3* | 42.0 ± 12.3* | 1.3 ± 0.08* | 10.0 ± 3.2* | |
| Cipro | 1 | 0.23 ± 0.1 | 1.6 ± 0.3* | 9.9 ± 2.0* | 1.0 ± 0.3 | 0.7 ± 0.1 | 9.7 ± 3.5* | 0.9 ± 0.1* | 1.0 ± 0.4 |
| 3 | 0.38 ± 0.1 | 2.2 ± 0.9* | 2.5 ± 0.3* | 0.5 ± 0.2 | 1.7 ± 0.4* | 2.6 ± 1.5 | 1.4 ± 0.1* | 5.5 ± 1.7* | |
| Trova | 1 | 0.24 ± 0.08 | 0.9 ± 0.2* | 8.1 ± 2.5* | 2.3 ± 0.9* | 1.3 ± 0.4* | 5.1 ± 1.1* | 1.1 ± 0.2* | 1.4 ± 0.6 |
| 3 | 0.18 ± 0.1 | 1.9 ± 0.5* | 1.8 ± 0.1* | 0.2 ± 0.05 | 1.9 ± 0.5* | 1.8 ± 1.2 | 1.3 ± 0.1* | 8.9 ± 1.0* | |
Combined results using 10 mice per group. Control, mice treated with diluent of the antibiotics perorally (p.o.) and diluent of LPS i.v.; LPS, mice treated with diluent of the antibiotics p.o. and 1,000 μg of LPS i.v.; Cipro, mice treated with 100 mg of ciprofloxacin p.o. per kg and diluent of LPS i.v.; Trova, mice treated with 100 mg of trovafloxacin p.o. per kg and diluent of LPS i.v.
∗, P value of ≤0.05 compared to the control group (Welch's modified t test).
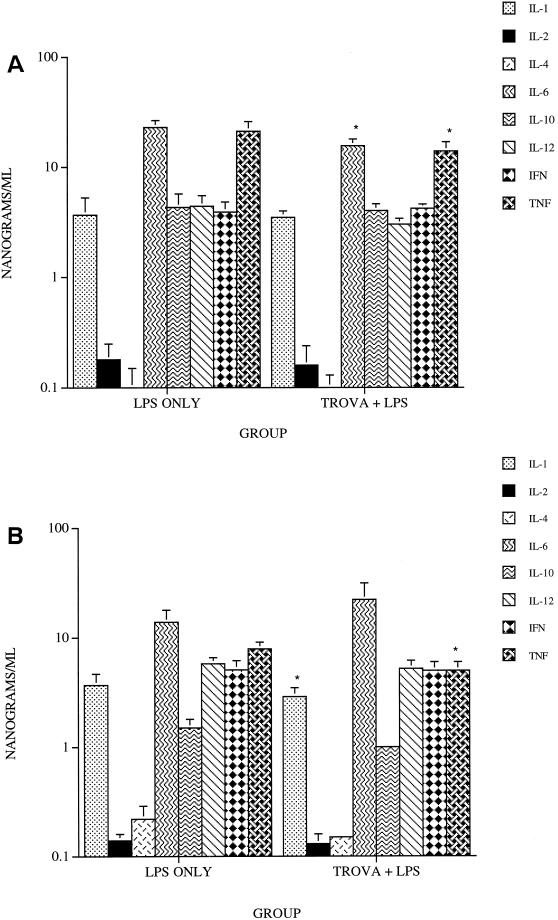
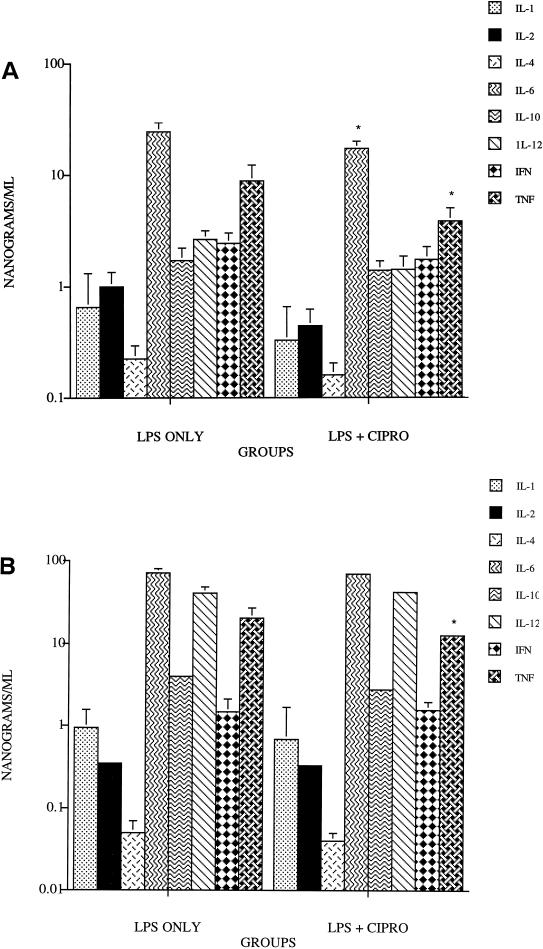
The effect of prior treatment with trovafloxacin, ciprofloxacin, or tosufloxacin on serum cytokine levels was examined 1 and 4 h after injection of LPS. Treatment with trovafloxacin resulted in a significant reduction in the levels of IL-6 and TNF-α (P = 0.009 and P = 0.01, respectively) at 1 h after LPS injection (Fig. 2A) and of IL-1 and TNF-α at 4 h (P = 0.04 and P = 0.003, respectively) (Fig. 2B). Treatment with ciprofloxacin resulted in significant reduction in levels of IL-6 and TNF-α (P = 0.02 and P = 0.05, respectively) 1 h after LPS injection (Fig. 3A); only the levels of TNF-α were significantly reduced (P = 0.05) at 4 h (Fig. 3B). Results with tosufloxacin were similar to those noted with ciprofloxacin (data not shown). The serum levels of each of the other cytokines were not significantly different from those of the controls.
FIG. 2.
Levels of serum cytokines in mice treated orally with 100 mg of trovafloxacin per kg and injected i.v. with 1,000 μg of LPS at 1 h (A) and 4 h (B) after injection. ∗, P values of ≤0.05 compared with LPS alone.
FIG. 3.
Levels of serum cytokines in mice treated orally with 100 mg of ciprofloxacin per kg and injected i.v. with 1,000 μg of LPS at 1 h (A) and 4 h (B) after injection. ∗, P values of ≤0.05 compared with LPS alone.
The in vitro immunomodulatory activity of fluoroquinolones has been described by a number of investigators using both mouse and human immune cells (3, 15, 19). The results described above reveal that oral treatment with trovafloxacin, ciprofloxacin, or tosufloxacin in mice prior to the induction of a lethal LPS-induced septic shock-like state significantly protects against death. Significant protection was conferred even when a single dose of trovafloxacin was administered 1 h before LPS.
The mechanism(s) by which these antibiotics protect LPS-injected mice against death is not clear. Following treatment with the fluoroquinolones alone, each of the cytokines tested except for IL-1β was upregulated in mice. This, along with the downregulation of IL-6 and TNF-α observed after administration of LPS to the antibiotic treated mice, may, at least partially, have been responsible for their protective activity. Although cytokine responses vary considerably in patients with sepsis, the proinflammatory cytokine network and lack of appropriately modulated anti-inflammatory mediators are considered central to the pathophysiology of the sepsis syndrome (9, 13, 20).
The doses of trovafloxacin used in the present studies were higher than and those of ciprofloxacin were comparable to those used in humans when corrected for the per-square-meter surface area of mice and humans. Blood and tissue levels in mice given these antibiotics by gavage were not performed and were beyond the scope of this study. Our results reveal that each of the three fluoroquinolones significantly reduced LPS-induced mortality in mice and modulated mediator cytokines in vivo. The protective effects of fluoroquinolones in LPS-induced shock in mice may also occur in humans.
REFERENCES
- 1.Abraham E, Anzueto A, Gutierrez G, Tessler S, Pedro G S, Wunderink R, Nogare A D, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbark M, Light R B, Poole L, Allred R, Constant J, Pennington J, Porter S the Norasept II Study Group. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 2.Anderson B O, Bensard D D, Harken A H. The role of platelet activating factor and its antagonists in shock, gram negative sepsis and multiple organ failure Surg. Gynecol Obstet. 1991;172:415–424. [PubMed] [Google Scholar]
- 3.Bailly S, Fay M, Ferrua B, Gougerot-Pocidalo M A. Ciprofloxacin treatment in vivo increases the ex vivo capacity of lipopolysaccharide-stimulated human monocytes to produce IL-1, IL-6 and tumour necrosis factor-alpha Clin. Exp Immunol. 1991;85:331–334. doi: 10.1111/j.1365-2249.1991.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 5.Bone R C, Balk R A, Fein A M, Perl T M, Wenzel R P, Reines H D, Quenzer R W, Iberti T J, Macintyre N, Schein R M. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, clinical trial. Crit Care Med. 1995;23:994–1006. doi: 10.1097/00003246-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bone R C, Fisher C J, Clemmer T P, Slotman G J, Metz C A, Balk R A the Methylprednisolone Severe Sepsis Study Group. A controlled trial of high-dose methyl-prednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 7.Butler L D, Layman N K, Cain R L, Riedl P E, Mohler K M, Bobbitt J L, Belagajie R, Sharp J, Bendele A M. Interleukin 1-induced pathophysiology: induction of cytokines, development of histopathologic changes, and immunopharmacologic intervention. Clin Immunol Immunopathol. 1989;53:400–421. doi: 10.1016/0090-1229(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Damas P, Canivet J L, DeGroote D, Wrindts Y, Albert A, Franchimont P, Lamy M. Sepsis and serum cytokine concentrations. Crit Care Med. 1997;25:405–412. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Dhainaut J F, Tenaillon A, Le Tulzo Y, Schlemmer B, Solet J P, Wolff M, Holzapfel L, Zeni F, Dreyfuss D, Mira J P, Vathaire F D, Guinot P the BN52021 Sepsis Study Group. Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med. 1994;22:1720–1728. [PubMed] [Google Scholar]
- 11.Dinarello C A. Interleukin-1. Rev Infect Dis. 1984;6:51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Fisher C J, Jr, Dhainaut J F, Opal S M, Pribble J P, Balk R A, Slotman G J, Iberti T J, Rackow E C, Shapiro M J, Greenman R L, Reines H D, Shelly M P, Thompson B W, LaBrecque J F, Catalano M A, Knaus W A, Sadoff J C. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome. J Am Med Assoc. 1994;271:1836–1849. [PubMed] [Google Scholar]
- 13.Gardlund B, Sjolin J, Nilsson A, Roll M, Wickerts C J, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 14.Greenman R I, Schein R M, Martin M A, Russell J A The Xoma Sepsis Study Group. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. J Am Med Assoc. 1991;266:1097–1102. [PubMed] [Google Scholar]
- 15.Khan A A, Slifer T R, Remington J S. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob Agents Chemother. 1998;42:1713–1717. doi: 10.1128/aac.42.7.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayeux P R. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997;51:415–435. doi: 10.1080/00984109708984034. [DOI] [PubMed] [Google Scholar]
- 17.Parillo J E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 18.Remick D G, Kunkel R G, Larrick J W, Kunkel S L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Investig. 1987;56:583–590. [PubMed] [Google Scholar]
- 19.Shalit I. Immunological aspects of new quinolones. Eur J Clin Microbiol Infect Dis. 1991;10:262–266. doi: 10.1007/BF01966999. [DOI] [PubMed] [Google Scholar]
- 20.Van der Poll T, de Waal Malefyt R, Coyle S M, Lowry S F. Anti-inflammatory cytokine responses during sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin-1 receptor type II, IL-10, and IL-13. J Infect Dis. 1977;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- 21.Waage A, Steinshamn S. Cytokines in septic shock. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 581–589. [Google Scholar]
- 22.Ziegler E J, Fisher C J, Sprung C L, Straube R C, Sadoff J C, Foulke G E, Wortel C H, Fink M P, Dellinger R P, Teng N N, Allen E, Berger H J, Knatterud G L, LoBuglio A F, Smith C R the HA-1A Sepsis Study Group. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]