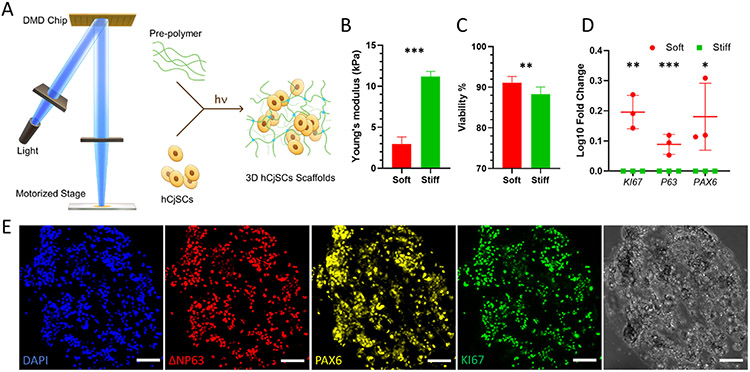
Fig. 2.
DLP-based 3D bioprinting of hydrogel scaffolds supporting the stemness and functionality of the encapsulated hCjSCs. (A) Schematics of the DLP bioprinter setup and the photopolymerization process to fabricate hydrogel scaffolds encapsulating hCjSCs. (B) Compressive modulus of the hCjSCs encapsulated in soft and stiff bioprinted scaffolds (mean ± sd, n = 3). (C) The ratio of PI-negative population measured with flow cytometry representing the percentage of viable cells in soft and stiff bioprinted scaffolds cultured for 5 days (mean ± sd, n = 3). (D) Real time qPCR showing the relative mRNA expression of KI67, P63 and PAX6 of hCjSCs in 2D culture condition (2D control) or 3D hydrogel scaffolds with different stiffness (mean ± sd, n = 3, *: P < 0.05, **: P < 0.01, ***: P < 0.001.). (E) Representative immunofluorescence staining and corresponding bright field images of bioprinted hydrogel scaffolds encapsulating hCjSCs after 2 days of culture expressing ΔNP63, PAX6 and KI67. Scale bars: 100 μm.