Abstract
This study aimed to examine the associations between subjective well-being (SWB) and risk of all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VD). We adopted a multidimensional approach to SWB that included the level and breadth of SWB, the latter indicating the extent to which SWB spreads across life domains. Participants (N=171,197; mean age=56.78; SD=8.16 years) were part of the UK Biobank and were followed up to 8.78 years. Domain-general and domain-specific SWB were measured by single items, and the breadth of SWB was indexed with a cumulative score of satisfaction across domains. Dementia incidence was ascertained through hospital and death records. Cox regression was used to examine the association between SWB indicators and risk of all-cause dementia, AD, and VD. General happiness, health and family satisfaction, and satisfaction breadth (satisfaction in multiple domains) were associated with lower risk of all-cause dementia. The associations held after accounting for socio-demographics, health, behavioral, and economic covariates, and depressive symptoms. Health satisfaction and the breadth of satisfaction were also associated with lower risk of AD and VD, with a pattern of slightly stronger associations for VD compared to AD. Some life domains (e.g., health) may be more fruitfully targeted to promote well-being and help protect against dementia, but it is also important to enhance well-being across multiple domains to maximize the protective effects.
Keywords: Subjective well-being, life domains, dementia, Alzheimer’s disease, vascular dementia
1. Introduction
Subjective well-being (SWB) is a multifaceted concept that typically includes both life satisfaction and feelings of happiness, and it can be specific to different domains as well as general (Diener et al., 2018). On the one hand, domain-general SWB reflects overall happiness or life satisfaction without referring to a particular life domain or context. For example, overall happiness reflects the extent to which individuals experience positive feelings in general (Lyubomirsky & Lepper, 1999). On the other hand, domain-specific SWB taps contextualized evaluations and feelings about one or multiple domains (Diener et al., 2018; Sirgy et al., 2020), such as one’s health (Lim et al., 2016), family (Lim et al., 2016), friendship (Doerwald et al., 2021), finances (Doerwald et al., 2021), and work (Kaiser et al., 2020), among others (Sirgy et al., 2020). The theoretical framework of positive health (Seligman, 2008) suggests that greater SWB is predictive of better general health, which has found support in the growing empirical evidence for a protective role of SWB in cognitive health and risk of dementia. For domain-general SWB, positive affect is cross-sectionally associated with better free recall performance (Hill et al., 2005), and a multidimensional measure that encompasses happiness, interest in life, ease of living, and loneliness is longitudinally associated with less cognitive decline over time and lower risk of incident dementia (Rawtaer et al., 2017). For domain-specific SWB, better self-perceived financial well-being (Boo et al., 2021) and health (Stephan et al., 2021) were longitudinally associated with lower risk of dementia. However, limited work on dementia risk has simultaneously examined domain-general and domain-specific SWB within the same cohort. In two studies to our knowledge that have done so, one (N=1,024, Canadian sample) suggested that satisfaction with life overall was longitudinally associated with lower risk of incident dementia, but no associations were found for domain-specific satisfaction (e.g., health, family, etc.; Peitsch et al., 2016), while another (N=8,021, Korean sample) suggested that overall life satisfaction as well as satisfaction with health, economic status, and relationships with spouses and children were longitudinally associated with lower risk of dementia (Zhu et al., 2022). More evidence is needed to understand the roles of domain-general and domain-specific SWB in risk of dementia.
In addition to the level of SWB in general or specific to a particular domain, research also suggests that the extent to which psychosocial and behavioral resources spread across domains and contexts is associated with health outcomes (Drewelies et al., 2019; Lee et al., 2020; Saito et al., 2018). For example, participating in activities across multiple domains (e.g., health, relationships, finance) is associated better cognitive functioning and lower risk of cognitive impairment (Carlson et al., 2012; Lee et al., 2020). Engaging in more compared to fewer types of social relationships (e.g., family and friends) is also associated with lower risk of dementia (Saito et al., 2018). A similar pattern may emerge for SWB: being happy and satisfied with many life domains may be associated with lower risk of dementia. Therefore, drawing on previous research (Saito et al., 2018), we consider breadth as an additional dimension in our approach to SWB, with greater breadth indicating that an individual is satisfied with multiple domains rather than a single domain.
Further, little is known about the associations between SWB with different causes or types of dementia because large samples are required to detect sufficient cases of less common dementia types. It can also be difficult to distinguish between dementia types—the pathologies that lead to Alzheimer’s disease (AD), vascular dementia (VD), and other dementias tend to co-occur (Bennett et al., 2009). In clinical settings, however, differential diagnoses can be made based on more distinct clinical signs and pattern of symptoms (Kester & Scheltens, 2009). AD and VD are the first and second most common causes of dementia (Kester & Scheltens, 2009), but it is not clear whether they may be differently associated with SWB predictors. Higher SWB is consistently related to better cardiovascular health (Kubzansky et al., 2018). Previous studies of other psychological predictors (depression and neuroticism) found stronger associations for VD compared to AD (Barnes et al., 2018; Terracciano et al., 2021). A similar pattern might be expected for the current SWB factors.
Using population-based longitudinal data, we aim to provide a multidimensional account of the association between SWB and risk of dementia. Specifically, we examine the associations between levels of domain-general (general happiness) and domain-specific (health, family, friendship, finances, and work) SWB, as well as the breadth of SWB across domains (described in detail below), with risk of all-cause dementia, AD, and VD. We hypothesize that general happiness, satisfaction with health, family, friendship, finances, and work, as well as the breadth of satisfaction, will be associated with lower risk of all-cause dementia, AD, and VD. We also expect stronger associations for VD compared to AD.
2. Method
Participants and Study Design
Participants are from the UK Biobank (http://www.ukbiobank.ac.uk), an ongoing population-based longitudinal study of human health and common diseases. The UK Biobank enrolled over 500,000 people registered with the UK National Health Service (NHS). Participants completed the first study assessment in 22 assessment centers across the UK between 2006 and 2010, and their health was followed through linked health records from the NHS. SWB measures and covariates were from the first assessment; incident dementia was ascertained from health records. The current sample included 171,197 participants who had data on SWB and were free of prevalent dementia at baseline (Fig.1), and they were followed up for up to 8.78 years (see Results). The UK Biobank obtained ethical approval from the North West Multicenter Research Ethics Committee and informed consent from all participants. This research has been conducted using the UK Biobank Resource (Application Reference Number 57672).
Fig 1.
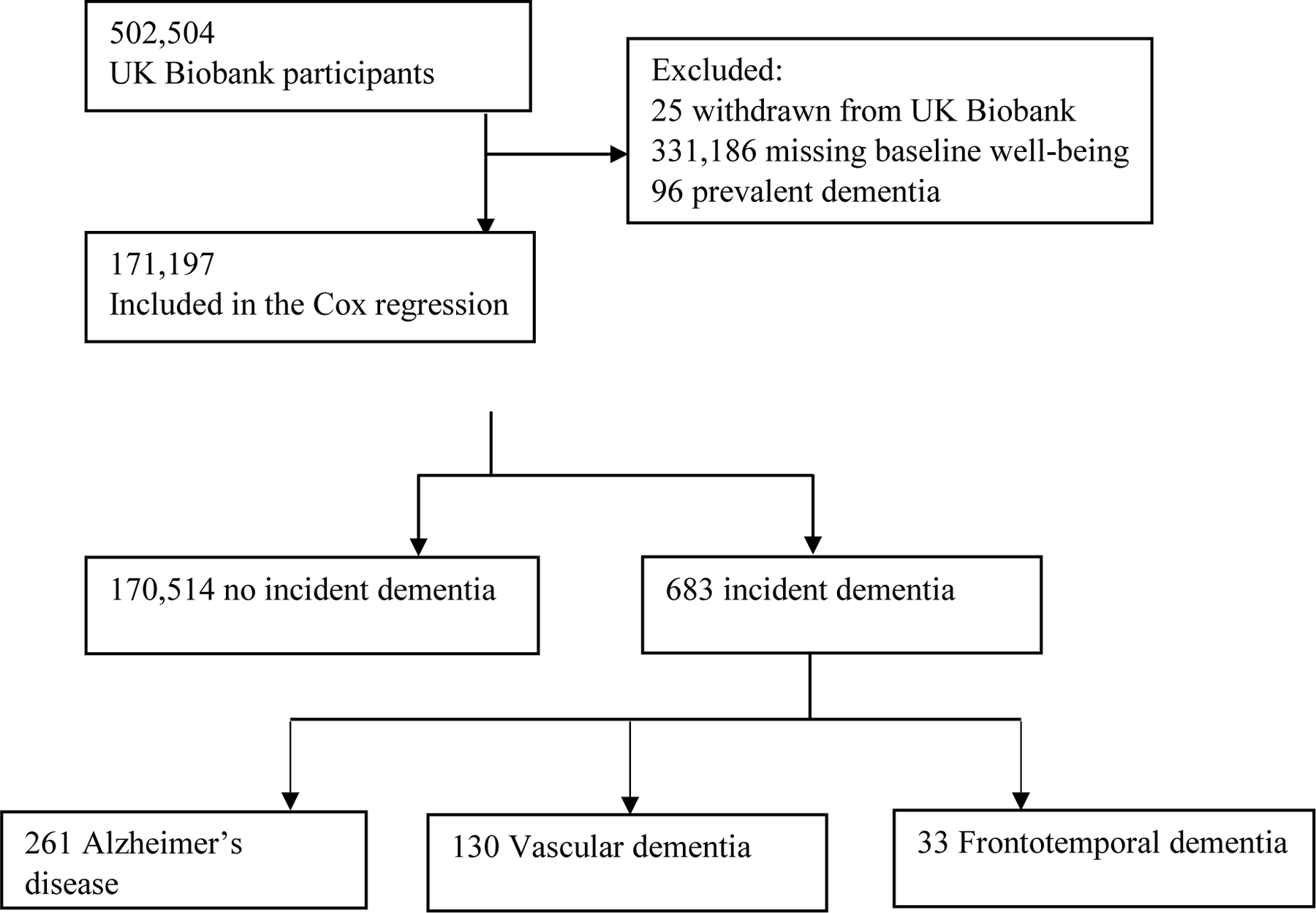
Flow chart of study sample
Measures
SWB.
The SWB indicators were measured through a touchscreen survey in the UK Biobank. Domain-general SWB was measured with the question “In general how happy are you?” Domain-specific SWB were asked with the items, “In general how satisfied are you with your HEALTH/your FAMILY RELATIONSHIPS/your FRIENDSHIPS/your FINANCIAL SITUATION/the WORK that you do?” (five separate items). These items were answered on a scale of 1 (Extremely happy/satisfied) to 6 (Extremely unhappy/dissatisfied) and reverse coded such that higher scores indicated higher levels of SWB. Responses of “Do not know” and “Prefer not to answer” (and “I am not employed” for work satisfaction) were coded as missing. Single well-being items are widely used in population-based surveys and demonstrate good psychometric properties (Cheung & Lucas, 2014). For example, using longitudinal data and multivariate latent state trait models, researchers have obtained estimates of reliability scores for single-item satisfaction measures, which ranged from .68 to .74 (Lucas & Donnellan, 2012). Scores on the single item were correlated with those on the well-validated 5-item Satisfaction with Life Scale (SWLS; Diener et al., 1985), r = .75 (Kobau et al., 2010). Both single-item and multi-item measures were similarly correlated with theoretically relevant constructs (Cheung & Lucas, 2014).
Breadth of SWB.
Satisfaction in each of the five domains above was coded as dissatisfied (0; for the responses of “extremely”, “very”, or “moderately” dissatisfied) or satisfied (1; for the responses of “extremely”, “very”, or “moderately” satisfied). The five binary scores were then summed to create a cumulative score (range=0–5; Saito et al., 2018), which is a count variable that indicates how many domains with which the participant felt satisfied. Higher scores therefore indicated satisfaction across more (compared to fewer) life domains, that is, greater breadth of satisfaction.
Dementia.
Dementia cases and dates were ascertained by the UK Biobank Outcome Adjudication Group (https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_dementia.pdf ). Specifically, incident dementia cases were the earliest known hospitalization with relevant International Classification of Diseases (ICD) code post-baseline, or cause-specific death based on death register records. Not every all-cause dementia case had a cause-specific ascertainment. All-cause dementia cases are inclusive of AD, VD, and frontotemporal dementia (FTD; Supplementary Table S3), as well as cases without a cause-specific code. In the current sample, 94.9% of all-cause dementia cases were from hospital records and 5.1% were from death records (Table 1). Individuals with a relevant ICD code prior to recruitment or self-reported diagnosis at recruitment were excluded from analyses (Fig. 1).
Table 1.
Descriptive statistics of study variables for the full sample and by dementia outcome
| Full sample | No dementia | All-cause dementia | AD | VD | ||
|---|---|---|---|---|---|---|
|
| ||||||
| N | 171,197 | 170,514 | 683 | 261 | 130 | |
|
| ||||||
| Cases from hospital records | -- | -- | 648 | 244 | 121 | |
|
| ||||||
| Cases from death records | -- | -- | 35 | 17 | 9 | |
| Age, Mean (SD) | 56.78 (8.16) | 56.75 (8.15) | 63.96 (5.28) | 64.74 (4.02) | 64.04 (4.77) | |
| Age range | 39–72 | 39–72 | 42–70 | 48–70 | 42–70 | |
| Women, N (%) | 93,126 (54.4%) | 92,832 (54.4%) | 294 (43%) | 124 (47.5%) | 47 (36.2%) | |
| College, N (%) | 57,310 (33.5%) | 57,182 (33.5%) | 128 (18.7%) | 46 (17.6%) | 14 (10.8%) | |
| Deprivation, Mean (SD) | -1.10 (2.95) | -1.11 (2.95) | -0.72 (3.14) | -1.00 (3.00) | -0.35 (3.04) | |
| Diabetes, N (%) | 9,841 (5.7%) | 9,721 (5.7%) | 120 (17.6%) | 34 (13%) | 38 (29.2%) | |
| Vascular problems, N (%) | 51,162 (29.9%) | 50,814 (29.8%) | 348 (51%) | 127 (48.7%) | 83 (63.8%) | |
| Ever smokers, N (%) | 76,246 (44.5%) | 75,885 (44.5%) | 361 (52.9%) | 136 (52.1%) | 71 (54.6%) | |
| Physical activity (Minutes), Mean (SD) | 129.62 (102.24) | 129.66 (102.23) | 116.91 (103.67) | 118.53 (99.47) | 106.26 (103.99) | |
| Depressive symptoms (scores ≥3), N (%) | 10,035 (5.9%) | 9,962 (5.8%) | 73 (10.7%) | 24 (9.2%) | 13 (10%) | |
| Happiness (1–6), Mean (SD) | 4.43 (0.71) | 4.43 (0.71) | 4.35 (0.78) | 4.44 (0.73) | 4.30 (0.74) | |
| Health satisfaction (1–6), Mean (SD) | 4.23 (0.88) | 4.23 (0.88) | 3.80 (1.10) | 4.00 (1.07) | 3.58 (1.07) | |
| Family satisfaction (1–6), Mean (SD) | 4.76 (0.91) | 4.76 (0.91) | 4.69 (1.00) | 4.75 (0.91) | 4.60 (1.05) | |
| Friendship satisfaction (1–6), Mean (SD) | 4.74 (0.75) | 4.74 (0.75) | 4.70 (0.74) | 4.73 (0.68) | 4.65 (0.78) | |
| Financial satisfaction (1–6), Mean (SD) | 4.25 (0.98) | 4.25 (0.98) | 4.23 (1.01) | 4.29 (0.92) | 4.21 (0.94) | |
| Work satisfaction (1–6), Mean (SD) | 4.40 (0.87) | 4.40 (0.87) | 4.45 (0.84) | 4.50 (0.83) | 4.34 (1.05) | |
| Breadth of satisfaction (0–5), Mean (SD) | 4.22 (0.94) | 4.22 (0.93) | 3.72 (1.02) | 3.86 (0.95) | 3.58 (1.02) | |
| Follow-up years, Mean (SD) | 7.97 (0.81) | 7.99 (0.78) | 5.00 (1.82) | 5.25 (1.64) | 5.23 (1.76) | |
| Cases from hospital records | -- | -- | 4.91 (1.77) | 5.17 (1.59) | 5.15 (1.75) | |
| Cases from death records | -- | -- | 6.71 (1.97) | 6.45 (1.80) | 6.39 (1.53) | |
Note. Sample size is as indicated except for the following variables. Deprivation: NFull sample=170,918, NNo dementia=170,236, NAll-cause dementia=682, NAD=260, NVD=130; Vascular problems (participants who provided “yes/no” responses): NFull sample=170,766 (n=51,162 “yes” responses), NNo dementia=170,090, NAll-cause dementia=676, NAD=260, NVD=130; Physical activity: NFull sample=139,599, NNo dementia=139,092, NAll-cause dementia=507, NAD=198, NVD=99; Depressive symptoms (participants who provided responses on Patient Health Questionnaire): NFull sample=159,990 (n=10,035 scores ≥3 coded as likely depressed cases), NNo dementia=159,395, NAll-cause dementia=595, NAD=231, NVD=117; Health satisfaction: NFull sample=170,317, NNo dementia=169,641, NAll-cause dementia=676, NAD=257, NVD=130; Family satisfaction: NFull sample=169,404, NNo dementia=168,740, NAll-cause dementia=664, NAD=254, NVD=125; Friendship satisfaction: NFull sample= 168,980, NNo dementia=168,314, NAll-cause dementia=666, NAD=252, NVD=127; Financial satisfaction: NFull sample= 169,813, NNo dementia=169,149, NAll-cause dementia=664, NAD=255, NVD=125; Work satisfaction: NFull sample=119,755, NNo dementia=119,490, NAll-cause dementia=265, NAD=107, NVD=47; Breadth of satisfaction: NFull sample=171,159, NNo dementia=170,477, NAll-cause dementia=682, NAD=260, NVD=130.
Covariates.
Demographic covariates included baseline age (in years) and sex (0=women, 1=men). Health and behavioral covariates: Diabetes and vascular problems (heart attack, angina, stroke, and high blood pressure) were assessed by asking participants whether a doctor told them that they had these conditions (yes/no). Current (“Do you smoke tobacco now?”) and past (“In the past, how often have you smoked tobacco?”) tobacco smoking were also self-reported, and current or former smokers (1) were compared to never smokers (0). Physical activity was self-reported with the sum minutes of walking, moderate and vigorous activity based on the International Physical Activity Questionnaire (Craig et al., 2003). Depressive symptoms were self-reported using the two-item Patient Health Questionnaire-2 (Kroenke et al., 2003): “Over the past 2 weeks, how often have you had little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you felt down, depressed or hopeless?” (0=Not at all to 3=Nearly every day, sum score range=0–6). Scores ≥3 were used to indicate likely depressed cases (Kroenke et al., 2003). Socioeconomic covariates: Education level was coded as having a college/university degree or equivalent (yes/no). The Townsend deprivation index (Townsend et al., 1997) used in the UK Biobank was a proxy for socioeconomic status computed based on unemployment, non-home ownership, non-car ownership, and household overcrowding in a geographic area.
Statistical Analyses
Cox regression was conducted to examine the associations between SWB indicators and risk of incident dementia. Continuous variables were z-scored to facilitate interpretation and comparison of the associations. Survival time (or follow-up time) was computed in years from the date of SWB measurement (2006–2010) up to the date of earliest dementia incidence, death, or censoring (February 8, 2018). The proportional hazard assumption was met as indicated by the nondependence of the predictors’ effects on time. Multiple models were run for each predictor. Model 1 adjusted for age and sex. As sensitivity analyses or robustness checks, Model 1.1 excluded cases with more than one type of dementia. Model 1.2 excluded dementia cases within the first five years. Model 1.3 excluded cases from death records. Model 2 included the covariates of Model 1 plus physical health and behavioral covariates (diabetes, vascular problems, and smoking). Model 2.1 further added physical activity (due to a smaller sample size with non-missing data in the UK Biobank). Model 3 included covariates of Model 2 plus depressive symptoms. Model 3.1 added education and deprivation. Model 3.2 (for models with breadth of satisfaction) further included general happiness and health satisfaction.
3. Results
Descriptive statistics for the full sample and by dementia outcome are in Table 1. The full analytic sample had 171,197 participants aged 39–72 years (Mean=56.78, SD=8.16) at baseline. On average, participants reported higher-than-midpoint levels of SWB, and the scores were negatively skewed (Supplementary Table S1). During up to 8.78 years (Mean=7.97, SD=0.81) of follow-up and 1,365,154.50 person-years, there were 683 incident all-cause dementia, 261 AD, 130 VD, and 33 FTD cases. The maximum follow-up times were 7.59 years (Mean=4.91, SD=1.77) for all-cause dementia cases from hospital records and 8.45 years (Mean=6.71, SD=1.97) for cases from death records. As noted, the numbers of AD, VD, and FTD do not add up to the total number of all-cause dementia because not all cases had a cause-specific diagnostic code.
Results of Cox regression predicting all-cause dementia, AD, and VD are in Tables 2–4. The pattern of results remained similar when adjusting for racial/ethnic characteristics. Information for racial/ethnic characteristics and FTD is presented in Supplement Tables S2-S4.
Table 2.
Cox regression results of well-being indicators predicting all-cause dementia1
| General happiness | Health satisfaction | Family satisfaction | Friendship satisfaction | Financial satisfaction | Work satisfaction | Breadth of satisfaction | |
|---|---|---|---|---|---|---|---|
| Model 1, # dementia | 683/171,197 | 676/170,317 | 664/169,404 | 666/168,980 | 664/169,813 | 265/119,755 | 682/171,159 |
| Model 1, HR (95% CI) | 0.80 (0.74–0.86) | 0.62 (0.58–0.66) | 0.84 (0.78–0.91) | 0.89 (0.82–0.96) | 0.82 (0.75–0.89) | 0.84 (0.74–0.95) | 0.63 (0.59–0.67) |
| Sensitivity analyses | |||||||
| Model 1.1, # dementia | -- | -- | -- | -- | -- | -- | -- |
| Model 1.1, HR (95% CI) | -- | -- | -- | -- | -- | -- | -- |
| Model 1.2, # dementia | 396/170,910 | 392/170,033 | 384/169,124 | 385/168,699 | 386/169,535 | 150/119,640 | 395/170,872 |
| Model 1.2, HR (95% CI) | 0.84 (0.76–0.93) | 0.61 (0.56–0.67) | 0.90 (0.82–0.998) | 0.91 (0.82–1.01) | 0.87 (0.78–0.97) | 0.91 (0.77–1.09) | 0.66 (0.61–0.72) |
| Model 1.3, # dementia | 648/171,162 | 641/170,282 | 629/169,369 | 631/168,945 | 629/169,778 | 252/119,742 | 647/171,124 |
| Model 1.3, HR (95% CI) | 0.79 (0.73–0.86) | 0.61 (0.57–0.65) | 0.83 (0.77–0.90) | 0.88 (0.81–0.96) | 0.80 (0.74–0.87) | 0.84 (0.73–0.95) | 0.63 (0.59–0.67) |
| Health and behavioral covariates | |||||||
| Model 2, # dementia | 676/170,766 | 669/169,922 | 658/169,011 | 659/168,595 | 659/169,444 | 262/119,494 | 675/170,735 |
| Model 2, HR (95% CI) | 0.81 (0.75–0.88) | 0.64 (0.60–0.69) | 0.85 (0.79–0.92) | 0.90 (0.83–0.97) | 0.84 (0.78–0.92) | 0.85 (0.75–0.97) | 0.66 (0.62–0.70) |
| Model 2.1, # dementia | 505/139,340 | 502/138,913 | 491/138,175 | 495/137,893 | 496/138,683 | 200/99,190 | 505/139,331 |
| Model 2.1, HR (95% CI) | 0.83 (0.76–0.91) | 0.64 (0.59–0.69) | 0.87 (0.79–0.94) | 0.91 (0.83–1.00) | 0.84 (0.77–0.93) | 0.88 (0.76–1.02) | 0.65 (0.60–0.70) |
| Depression and socioeconomic covariates | |||||||
| Model 3, # dementia | 591/159,713 | 584/159,187 | 575/158,333 | 578/158,040 | 576/158,917 | 231/112,223 | 590/159,706 |
| Model 3, HR (95% CI) | 0.90 (0.83–0.98) | 0.68 (0.63–0.73) | 0.90 (0.83–0.97) | 0.95 (0.87–1.03) | 0.90 (0.82–0.98) | 0.91 (0.79–1.04) | 0.68 (0.63–0.73) |
| Model 3.1, # dementia | 590/159,448 | 583/158,924 | 574/158,072 | 577/157,780 | 575/158,653 | 230/112,030 | 589/159,441 |
| Model 3.1, HR (95% CI) | 0.90 (0.83–0.97) | 0.68 (0.63–0.74) | 0.90 (0.83–0.98) | 0.94 (0.87–1.02) | 0.93 (0.85–1.02) | 0.92 (0.80–1.05) | 0.69 (0.64–0.75) |
| Model 3.2, # dementia | -- | -- | -- | -- | -- | -- | 583/158,924 |
| Model 3.2, HR (95% CI) | -- | -- | -- | -- | -- | -- | 0.79 (0.72–0.86) |
. Model 1-Model 1.3 include the covariates age and sex. Model 1.1 excludes cases with more than one type of dementia. Model 1.2 excludes dementia cases within first five years. Model 1.3 excludes diagnosis from death records. Model 2 is Model 1 plus physical health covariates (self-reported diabetes, vascular diseases, and smoking). Model 2.1 is Model 2 and physical activity. Model 3 is Model 2 plus depressive symptoms. Model 3.1 is Model 3 plus education and deprivation. Model 3.2 further controls for general happiness and health satisfaction. The pattern of results remained similar when adjusting for racial background.
Table 4.
Cox regression results of well-being indicators predicting vascular dementia (VD)
| General happiness | Health satisfaction | Family satisfaction | Friendship satisfaction | Financial satisfaction | Work satisfaction | Breadth of satisfaction | |
|---|---|---|---|---|---|---|---|
| Model 1, # dementia | 130/171,197 | 130/170,317 | 125/169,404 | 127/168,980 | 125/169,813 | 47/119,755 | 130/171,159 |
| Model 1, HR (95% CI) | 0.74 (0.62–0.88) | 0.52 (0.45–0.59) | 0.77 (0.65–0.91) | 0.82 (0.69–0.98) | 0.80 (0.66–0.96) | 0.73 (0.55–0.98) | 0.58 (0.50–0.66) |
| Sensitivity analyses | |||||||
| Model 1.1, # dementia | 102/171,166 | 102/170,286 | 98/169,374 | 101/168,951 | 98/169,783 | 34/119,740 | 102/171,128 |
| Model 1.1, HR (95% CI) | 0.67 (0.55–0.80) | 0.50 (0.43–0.58) | 0.75 (0.62–0.90) | 0.80 (0.65–0.97) | 0.84 (0.68–1.04) | 0.77 (0.55–1.09) | 0.56 (0.48–0.66) |
| Model 1.2, # dementia | 82/171,149 | 82/170,269 | 78/169,357 | 80/168,933 | 80/169,768 | 28/119,736 | 82/171,120 |
| Model 1.2, HR (95% CI) | 0.82 (0.66–1.03) | 0.51 (0.43–0.61) | 0.82 (0.66–1.02) | 0.89 (0.71–1.12) | 0.86 (0.68–1.10) | 0.85 (0.58–1.25) | 0.59 (0.50–0.71) |
| Model 1.3, # dementia | 121/171,188 | 121/170,308 | 116/169,395 | 118/168,971 | 116/169,804 | 45/119,753 | 121/171,150 |
| Model 1.3, HR (95% CI) | 0.75 (0.63–0.89) | 0.52 (0.45–0.60) | 0.79 (0.66–0.94) | 0.85 (0.70–1.02) | 0.74 (0.62–0.89) | 0.72 (0.54–0.95) | 0.58 (0.50–0.67) |
| Health and behavioral covariates | |||||||
| Model 2, # dementia | 130/170,766 | 130/169,922 | 125/169,011 | 127/168,595 | 125/169,444 | 47/119,494 | 130/170,735 |
| Model 2, HR (95% CI) | 0.77 (0.65–0.91) | 0.58 (0.50–0.68) | 0.79 (0.68–0.94) | 0.84 (0.71–1.00) | 0.87 (0.72–1.04) | 0.75 (0.57–0.99) | 0.63 (0.55–0.73) |
| Model 2.1, # dementia | 99/139,340 | 99/138,913 | 95/138,175 | 97/137,893 | 95/138,683 | 39/99,190 | 99/139,331 |
| Model 2.1, HR (95% CI) | 0.78 (0.65–0.95) | 0.55 (0.47–0.65) | 0.79 (0.66–0.95) | 0.92 (0.75–1.13) | 0.91 (0.74–1.12) | 0.81 (0.60–1.11) | 0.62 (0.53–0.72) |
| Depression and socioeconomic covariates | |||||||
| Model 3, # dementia | 117/159,713 | 117/159,187 | 112/158,333 | 114/158,040 | 113/158,917 | 42/112,223 | 117/159,706 |
| Model 3, HR (95% CI) | 0.86 (0.72–1.04) | 0.58 (0.49–0.68) | 0.86 (0.72–1.02) | 0.88 (0.73–1.05) | 0.93 (0.76–1.12) | 0.84 (0.62–1.13) | 0.64 (0.54–0.75) |
| Model 3.1, # dementia | 117/159,448 | 117/158,924 | 112/158,072 | 114/157,780 | 113/158,653 | 42/112,030 | 117/159,441 |
| Model 3.1, HR (95% CI) | 0.86 (0.72–1.04) | 0.60 (0.51–0.70) | 0.86 (0.72–1.03) | 0.87 (0.72–1.04) | 0.99 (0.81–1.21) | 0.85 (0.63–1.15) | 0.66 (0.56–0.78) |
| Model 3.2, # dementia | -- | -- | -- | -- | -- | -- | 117/158,924 |
| Model 3.2, HR (95% CI) | -- | -- | -- | -- | -- | -- | 0.80 (0.66–0.99) |
. Model 1-Model 1.3 include the covariates age and sex. Model 1.1 excludes cases with more than one type of dementia. Model 1.2 excludes dementia cases within first five years. Model 1.3 excludes diagnosis from death records. Model 2 is Model 1 plus physical health covariates (self-reported diabetes, vascular diseases, and smoking). Model 2.1 is Model 2 and physical activity. Model 3 is Model 2 plus depressive symptoms. Model 3.1 is Model 3 plus education and deprivation. Model 3.2 further controls for general happiness and health satisfaction. The pattern of results remained similar when adjusting for racial background.
3.1. SWB and All-Cause Dementia
Table 2 presents the results for risk of all-cause dementia. Model 1 suggested significant associations between all seven SWB indicators and all-cause dementia adjusting for age and sex. One SD higher score on health satisfaction or breadth of satisfaction was associated with ~60% lower risk of all-cause dementia, followed by general happiness, financial, family, and work satisfaction (~20% lower risk) and friendship satisfaction (12% lower risk). Sensitivity analyses suggested that when dementia cases within five years of baseline (about one third of all cases) were excluded (Model 1.2), all predictors except friendship and work satisfaction remained significant. We also compared SWB scores between all-cause dementia cases within five years (Group 1) and cases later than five years (Group 2) using t-tests. Overall, both groups had similar SWB scores at baseline (Supplementary Table S1). When dementia cases determined through death records were excluded (Model 1.3), all predictors remained significant.
Controlling for physical health and smoking (Model 2), all seven SWB indicators remained significantly associated with all-cause dementia, although friendship and work satisfaction were nonsignificant when further controlling for physical activity (Model 2.1; note the smaller sample size). Health satisfaction, breadth of satisfaction, general happiness, family and financial satisfaction remained significant when depressive symptoms were included (Model 3). Financial satisfaction became nonsignificant when socioeconomic covariates were included (Model 3.1). However, when we removed depressive symptoms, financial satisfaction was significant (HR= 0.88, 95%CI: 0.81–0.96) over and above education (HR=0.63, 95% CI: 0.52–0.77) and deprivation (HR=1.15, 95% CI: 1.07–1.24). Satisfaction breadth remained significant after accounting for general happiness and health satisfaction (Model 3.2).
3.1.1. Post-hoc analyses
As a negative dimension of well-being, depression in dementia-free individuals is associated with higher risk of incident dementia (Diniz et al., 2013). Consistently, the current results also suggested an association between depressive symptoms and higher risk of all-cause dementia (HR=2.40, 95% CI: 1.83–3.14). As a post-hoc analysis, we tested whether there was an interaction between depressive symptoms and SWB in predicting all-cause dementia. The interaction was significant for the breadth of satisfaction but not for any other SWB variables. There was a stronger association between satisfaction breadth and risk of all-cause dementia in individuals who reported fewer depressive symptoms (PHQ<3, HRSatisfaction breadth=0.64, 95% CI: 0.59–0.70) compared to those who reported more symptoms (PHQ≥3, HRSatisfaction breadth=0.84, 95% CI: 0.71–0.99).
Because of the relatively young age of the sample, we also tested whether the associations between SWB measures and risk of all-cause dementia were moderated by age (i.e., age × SWB interaction). Significant interactions were found for happiness, family satisfaction, friendship satisfaction, and financial satisfaction. There was a pattern of stronger associations in younger (age <60) compared to older (age ≥60) participants (Supplementary Table S5).
3.2. SWB and AD
Table 3 presents the associations for AD. Controlling for age and sex, health and financial satisfaction and satisfaction breadth were associated with risk of AD. One SD higher score on the satisfaction breadth was associated with ~40% lower risk of AD, followed by health satisfaction (35% lower risk) and financial satisfaction (16% lower risk). Satisfaction breadth and health satisfaction remained significant across all sensitivity analyses (Models 1.1–1.3). Both variables remained significant after controlling for health, behavioral factors, depressive symptoms, and socioeconomic covariates (Model 2–3.1). Satisfaction breadth remained significant controlling for general happiness and health satisfaction (Model 3.2).
Table 3.
Cox regression results of well-being indicators predicting Alzheimer’s disease (AD)1
| General happiness | Health satisfaction | Family satisfaction | Friendship satisfaction | Financial satisfaction | Work satisfaction | Breadth of satisfaction | |
|---|---|---|---|---|---|---|---|
| Model 1, # dementia | 261/171,197 | 257/170,317 | 254/169,404 | 252/168,980 | 255/169,813 | 107/119,755 | 260/171,159 |
| Model 1, HR (95% CI) | 0.90 (0.79–1.02) | 0.74 (0.66–0.83) | 0.90 (0.79–1.01) | 0.90 (0.79–1.03) | 0.86 (0.75–0.98) | 0.86 (0.70–1.06) | 0.71 (0.63–0.80) |
| Sensitivity analyses | |||||||
| Model 1.1, # dementia | 232/171,166 | 228/170,286 | 226/169,374 | 225/168,951 | 227/169,783 | 92/119,740 | 231/171,128 |
| Model 1.1, HR (95% CI) | 0.87 (0.76–0.99) | 0.74 (0.66–0.84) | 0.88 (0.78–1.01) | 0.90 (0.79–1.03) | 0.88 (0.76–1.02) | 0.90 (0.71–1.12) | 0.72 (0.63–0.81) |
| Model 1.2, # dementia | 161/171,097 | 159/170,219 | 155/169,305 | 156/168,884 | 159/169,717 | 68/119,716 | 160/171,059 |
| Model 1.2, HR (95% CI) | 0.91 (0.78–1.07) | 0.70 (0.60–0.81) | 0.98 (0.83–1.16) | 0.92 (0.78–1.09) | 0.98 (0.82–1.17) | 0.90 (0.70–1.17) | 0.74 (0.64–0.86) |
| Model 1.3, # dementia | 244/171,180 | 240/170,300 | 237/169,387 | 236/168,964 | 238/169,796 | 100/119,748 | 243/171,142 |
| Model 1.3, HR (95% CI) | 0.92 (0.80–1.04) | 0.73 (0.65–0.82) | 0.91 (0.80–1.03) | 0.91 (0.80–1.05) | 0.86 (0.75–0.99) | 0.91 (0.73–1.13) | 0.71 (0.63–0.80) |
| Health and behavioral covariates | |||||||
| Model 2, # dementia | 260/170,766 | 256/169,922 | 253/169,011 | 251/168,595 | 255/169,444 | 107/119,494 | 259/170,735 |
| Model 2, HR (95% CI) | 0.91 (0.80–1.03) | 0.75 (0.67–0.85) | 0.90 (0.79–1.02) | 0.91 (0.80–1.03) | 0.87 (0.76–1.00) | 0.87 (0.71–1.07) | 0.73 (0.65–0.82) |
| Model 2.1, # dementia | 198/139,340 | 197/138,913 | 194/138,175 | 193/137,893 | 196/138,683 | 84/99,190 | 198/139,331 |
| Model 2.1, HR (95% CI) | 0.92 (0.80–1.07) | 0.73 (0.64–0.84) | 0.92 (0.80–1.07) | 0.93 (0.80–1.07) | 0.84 (0.72–0.97) | 0.86 (0.68–1.08) | 0.72 (0.63–0.82) |
| Depression and socioeconomic covariates | |||||||
| Model 3, # dementia | 231/159,713 | 227/159,187 | 225/158,333 | 225/158,040 | 227/158,917 | 93/112,223 | 230/159,706 |
| Model 3, HR (95% CI) | 0.98 (0.85–1.12) | 0.81 (0.71–0.92) | 0.94 (0.82–1.07) | 0.95 (0.83–1.09) | 0.94 (0.81–1.08) | 0.94 (0.76–1.17) | 0.76 (0.67–0.86) |
| Model 3.1, # dementia | 230/159,448 | 226//158,924 | 224/158,072 | 224/157,780 | 226/158,653 | 92/112,030 | 229/159,441 |
| Model 3.1, HR (95% CI) | 0.97 (0.85–1.11) | 0.81 (0.71–0.92) | 0.94 (0.82–1.07) | 0.94 (0.82–1.08) | 0.97 (0.84–1.12) | 0.95 (0.76–1.19) | 0.77 (0.67–0.88) |
| Model 3.2, # dementia | -- | -- | -- | -- | -- | -- | 226/158,924 |
| Model 3.2, HR (95% CI) | -- | -- | -- | -- | -- | -- | 0.81 (0.70–0.95) |
. Model 1-Model 1.3 include the covariates age and sex. Model 1.1 excludes cases with more than one type of dementia. Model 1.2 excludes dementia cases within first five years. Model 1.3 excludes diagnosis from death records. Model 2 is Model 1 plus physical health covariates (self-reported diabetes, vascular diseases, and smoking). Model 2.1 is Model 2 and physical activity. Model 3 is Model 2 plus depressive symptoms. Model 3.1 is Model 3 plus education and deprivation. Model 3.2 further controls for general happiness and health satisfaction. The pattern of results remained similar when adjusting for racial background.
3.3. SWB and VD
Table 4 shows the associations for VD. Controlling for age and sex, all seven SWB indicators were associated with risk of VD (Model 1). One SD higher score on health satisfaction was associated with ~90% lower risk of VD, followed by satisfaction breadth (~70% lower risk), work satisfaction, general happiness, family and financial satisfaction (~30% lower risk), and friendship satisfaction (~20% lower risk). The association between satisfaction breadth and health satisfaction with VD remained significant across the sensitivity analyses (Models 1.1–1.3). General happiness and family satisfaction remained significant when individuals with more than one cause of dementia were excluded (Model 1.1) or when cases determined through death records were excluded (Model 1.3), but they became nonsignificant when VD cases within first five years were excluded (Model 1.2). Friendship, financial, and work satisfaction each failed two robustness checks (Models 1.1–1.3).
Controlling for physical health and smoking (Model 2), health satisfaction, satisfaction breadth, work satisfaction, general happiness, and family satisfaction were associated with risk of VD. Work satisfaction became nonsignificant when physical activity was included (Model 2.1). Further, only satisfaction breadth and health satisfaction remained significant when depressive symptoms and socioeconomic covariates were included (Model 3–3.1). Satisfaction breadth remained significant after controlling for general happiness and health satisfaction (Model 3.2).
4. Discussion
The study examined the associations between level (domain-general and domain-specific) and breadth of SWB with risk of all-cause dementia, AD, and VD. Using population-based data from the UK Biobank, we found that general happiness, health and family satisfaction, as well as the breadth of satisfaction across domains, were associated with lower risk of all-cause dementia. These associations were observed across multiple models that accounted for sociodemographic, economic, health and behavioral risk factors, and depressive symptoms. Health satisfaction and breadth of satisfaction were associated with lower risk of AD and VD. There was a pattern of slightly stronger associations between SWB with VD compared to AD. Satisfaction breadth was among the most robust predictors, remaining significant after accounting for general happiness and health satisfaction.
4.1. SWB and All-Cause Dementia
4.1.1. General Happiness
As a central component of SWB, higher levels of happiness and pleasant feelings are generally associated with better health outcomes. For example, individuals who are happier and more satisfied with their lives tend to have greater longevity and lower morbidity, better cognitive performance, less cognitive decline over time, and lower risk of dementia (Diener et al., 2018; Gerstorf et al., 2007; Hill et al., 2005; Rawtaer et al., 2017). The neuropsychological perspective suggests that positive affect facilitates cognitive function (e.g., attention, memory, and problem solving) by increasing dopamine release in associated brain areas such as anterior cingulate and prefrontal cortex (Ashby et al., 1999). At the behavioral level, the upward spiral theory of lifestyle change (Van Cappellen et al., 2018) underscores the role of positive affect in driving and sustaining health behaviors such as physical activity and healthy diet, which are in turn associated with cognitive health (Chou et al., 2019; Norton et al., 2014). In contrast, as noted, depression in dementia-free individuals at baseline is associated with higher risk of incident dementia (Diniz et al., 2013). Of note, we found that the association of general happiness was independent of depressive symptoms, which indicates the presence of happiness may function as an asset protective against dementia beyond the absence of distress, which is broadly in line with the positive health framework (Seligman, 2008).
4.1.2. Health Satisfaction
Of the five domains examined, satisfaction with health had the strongest associations with all-cause dementia. Different indicators of subjective evaluations of health (e.g., self-rated health and health-related quality of life) have been associated with cognitive as well as general health outcomes. For example, individuals who perceive higher health-related quality of life were found to have better memory and executive function (Chamberlain et al., 2021; Ezzati et al., 2019). Self-rated health, the global evaluation of one’s own health status, is associated with risk of dementia, mortality, objective and subjective physical capacity, and health behaviors (Abuladze et al., 2017; Aschwanden et al., 2020; Bopp et al., 2012; Qazi et al., 2021; Stephan et al., 2021). In addition, people who have a lower evaluation of their own health also tend to score higher on neuroticism, a personality trait associated with a tendency towards distress, which is also a risk factor for dementia (Terracciano et al., 2021). The rich information possibly indicated by subjective evaluations of one’s health (Jylha, 2009) may be one explanation for the relatively stronger association of health satisfaction.
4.1.3. Family Satisfaction
Social relationships in general are associated with cognitive functioning and risk of dementia (Ge et al., 2017; Kelly et al., 2017; Kuiper et al., 2015), and family plays a vital role in providing social support that may in turn protect cognitive health. There is cross-sectional evidence that suggests a stronger association between social support from family (compared to friends and other sources) with self-efficacy for practicing health behavior and frequency of health behavior (Wu & Sheng, 2019), as well as cognitive functioning (Zhu et al., 2012). In addition, longitudinal evidence suggests that being married and perceiving high marital quality are associated with less cognitive decline and lower risk of dementia (Liu et al., 2020; Liu et al., 2021). In the current study, the association of family satisfaction was independent of socioeconomic and health covariates and was stronger than financial satisfaction. It is possible that emotional support, beyond instrumental or financial support, that plays a more important role in the association (Deng & Liu, 2021).
4.1.4. Financial, Friendship, and Work Satisfaction
Satisfaction with one’s financial situation was associated with lower risk of all-cause dementia independent of education and deprivation status, although the association became nonsignificant after accounting for all covariates combined. In previous studies, financial satisfaction was associated with cognitive function and risk of cognitive impairment and dementia (Boo et al., 2021; Foong et al., 2021). By accounting for multiple covariates, our analyses indicate that this association might be explained by the combination of socioeconomic status and physical and mental health. Among all SWB indicators, friendship and work satisfaction had the least robust associations with dementia. The sensitivity analyses suggested that their associations with dementia were driven by cases that occurred within five years of baseline assessment. This indicates that the associations may be attributed to individuals with prodromal symptoms of dementia experiencing lower friendship and work satisfaction (reverse causation; Andrade, 2020). The associations also became nonsignificant after accounting for depressive symptoms, indicating a possible explanatory role of depressive symptoms in the association between friendship and work satisfaction with risk of dementia.
4.1.5. Breadth of Satisfaction
In addition to level of SWB, we demonstrated the utility of examining breadth, the extent to which SWB spreads across domains. Being satisfied across multiple domains was associated with lower risk of all-cause dementia independent of health satisfaction and general happiness. In general, satisfaction is associated with the presence of resources that people can use to work towards their goals (Diener & Fujita, 1995). Whereas satisfaction in a single domain is valuable, the benefits it can confer may be limited (Diener et al., 2009; Sirgy & Wu, 2007). However, consistently available resources and satisfaction across multiple domains may meet a broader range of needs (Sirgy & Wu, 2007), possibly maximizing the benefits for health and cognition. In particular, resources and satisfaction across multiple domains may allow people to engage with life more actively and fully, especially in the form of participating in diverse activities, which is in turn associated with better cognitive functioning and lower risk of cognitive impairment (Carlson et al., 2012; Lee et al., 2020). Further, as the ecological systems model (Bronfenbrenner, 1977) suggests, different domains of one’s life and environment are interconnected. Through this lens, cross-domain well-being may therefore reflect not only healthy functioning of individual systems, but also the positive interactions between domains (e.g., a good economic environment may promote work and health satisfaction). Together, the breadth of satisfaction across life domains may indicate availability of various resources at one’s repertoire, which may in turn contribute to enhanced benefits for cognitive health.
Notably, for happiness, family satisfaction, friendship satisfaction, and financial satisfaction, there was a pattern of stronger associations with all-cause dementia in younger (age <60) compared to older (age ≥60) participants. This was in the opposite direction of what would be expected by reverse causality — the associations should get stronger at older ages if it was due entirely to the disease process because older people are more likely to have the neuropathology for dementia that presumably would cause the lower well-being.
4.2. SWB, AD, and VD
There was a pattern of slightly stronger associations of SWB with VD compared to AD. For example, health satisfaction and the breadth of satisfaction were associated with 70–90% lower risk of VD and ~40% lower risk of AD, accounting for age and sex. The associations for VD were attenuated by about 20% after accounting for vascular risk factors (diabetes, hypertension, stroke, heart attack, angina, and smoking), indicating a possible explanatory role of vascular health, whereas the associations remained largely unchanged for AD. This pattern is consistent with some previous findings. For example, neuroticism, a personality trait associated with the tendency towards distress, showed a slightly stronger association with VD compared to AD, and the association with VD was attenuated by one third after accounting for vascular risk factors (Terracciano et al., 2021). In addition, depression (the negative dimension of well-being) is bidirectionally linked with vascular diseases, and both depression and vascular diseases are associated with increased risk of dementia, especially VD (Alexopoulos, 2003; Barnes, 2021). Although common pathologies are associated with VD and AD, and both have been described as conditions along the same continuum (Bennett et al., 2009), cerebrovascular damage and disease remain to be considered primary features of VD, as amyloid deposition and neurofibrillary tangles are for AD (Kester & Scheltens, 2009). The current findings seem to indicate the relative importance of vascular pathologies as a possible pathway underlying the SWB-dementia association.
4.3. Strengths, Limitations, and Future Directions
This is the first study to our knowledge that examined the SWB-dementia association using a multidimensional approach that encompassed levels (domain-general and domain-specific) and breadth of SWB. The large sample size and rich data allowed us to examine the associations of SWB with dementia risk while accounting for a broad range of covariates. Another strength of the study was the examination of all-cause dementia as well as separate analyses for AD and VD.
Limitations and future directions should be noted. First, although we tested for the robustness of associations through sensitivity analyses, we cannot rule out reverse causation. There is still a possibility that individuals who were already experiencing preclinical and prodromal symptoms of dementia had lower SWB, especially given our relatively shorter follow-up compared to previous studies (e.g., Sundström et al., 2020). Longitudinal studies that track changes in well-being in the preclinical and clinical phases are needed to better evaluate whether reverse causality could explain the observed findings. Second, the proportion of individuals who developed incident dementia was relatively low given the relatively younger age of the participants and shorter follow-up period, which might also have impacted the power to detect the associations of some SWB indicators, especially for AD and VD. Another potential reason for the low proportion of dementia cases is the reliance on health and death records. This ascertainment method is likely to miss dementia cases, especially in the early stages of the disease, and may not provide a reliable measure for time of diagnosis. However, the passive follow-up that relies on linked health records has the advantage of reducing the biases associated with attrition in typical longitudinal studies. In addition, we capitalized on the available SWB measures and data in the UK Biobank but recognize the importance of including other constructs and measures. For example, general happiness was used as a domain-general, non-contextualized indicator of SWB, whereas the measure of satisfaction with life overall was not available but should be examined in the future. Likewise, while domain-specific as well as domain-general SWB were assessed, the focus was on global evaluations and feelings. Future work can conduct fine-grained assessment of each domain (e.g., spouses/partners and children under the family domain), as well as including other domains (e.g., physical environment). Furthermore, we focused on the predictive value of baseline SWB as a potential protective factor against risk of dementia, but we consider the association between changes in SWB over time with risk of dementia as an important future direction. Lastly, we note that the breadth of SWB is only one of many possible approaches to cross-domain SWB, and it has the merit of straightforward interpretation. Future research could also assess, for example, the balance of SWB that taps how evenly distributed levels of SWB are across domains (Sheldon et al., 2010), and how that is associated with dementia risk.
5. Conclusion
In a population-based longitudinal study, we found that domain-general and domain-specific SWB, as well as the extent to which SWB spread across domains, are associated with risk of incident dementia. A pattern of slightly stronger associations of SWB were observed for VD compared to AD. Some life domains (e.g., health) may be more fruitfully targeted in interventions to promote well-being and help protect against risk of dementia. It is also important to view multiple domains collectively, particularly the less satisfying domains, to enhance the breadth of well-being and the associated cognitive benefits.
Supplementary Material
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10902-022-00613-3.
Data Availability Data used in this study are publically available from the UK Biobank.
Conflict of interest The authors have no relevant financial ornon-financial interests to disclose.
Informed Consent All individual participantsof the UK Biobank signed informed consent on participation.
Ethical Approval This study used publicly available, de-identified datafrom the UK Biobank (http://www.ukbiobank.ac.uk), and was thus exempted fromadditional review by the Institutional Review Board at Florida State University.The UK Biobank obtained ethical approval from the North West Multicenter ResearchEthics Committee.
References
- Abuladze L, Kunder N, Lang K, & Vaask S (2017). Associations between self-rated health and health behaviour among older adults in Estonia: A cross-sectional analysis. BMJ Open, 7(6), e013257–e013257. 10.1136/bmjopen-2016-013257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS (2003). Vascular disease, depression, and dementia. Journal of the American Geriatrics Society (JAGS), 51(8), 1178–1180. 10.1046/j.1532-5415.2003.51373.x [DOI] [PubMed] [Google Scholar]
- Andrade C (2020). Reverse causation, physical inactivity, and dementia. Indian Journal of Psychological Medicine, 42(2), 205–206. 10.4103/IJPSYM.IJPSYM_45_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden D, Aichele S, Ghisletta P, Terracciano A, Kliegel M, Sutin AR, Brown J, & Allemand M (2020). Predicting cognitive impairment and dementia: A machine learning approach. Journal of Alzheimer’s Disease, 75(3), 717–728. 10.3233/JAD-190967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken, & U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106(3), 529–550. 10.1037/0033-295X.106.3.529 [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, & Whitmer RA (2012). Midlife vs late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Archives of General Psychiatry, 69(5), 493–498. 10.1001/archgenpsychiatry.2011.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S, Grant MM, & Aldred S (2009). Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. Journal of Alzheimer’s Disease, 17(2), 245–257. 10.3233/JAD-2009-1041 [DOI] [PubMed] [Google Scholar]
- Boo YY, Jutila OE, Cupp MA, Manikam L, & Cho SI (2021). The identification of established modifiable mid-life risk factors for cardiovascular disease which contribute to cognitive decline: Korean Longitudinal Study of Aging (KLoSA). Aging Clinical and Experimental Research, 33(9), 2573–2586. 10.1007/s40520-020-01783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp M, Braun J, Gutzwiller F, & Faeh D (2012). Health risk or resource? Gradual and independent association between self-rated health and mortality persists over 30 years. PloS One, 7(2), e30795–e30795. 10.1371/journal.pone.0030795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U (1977). Toward an experimental ecology of human development. American Psychologist, 32(7), 513–531. 10.1037/0003-066X.32.7.513 [DOI] [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q-L, Rebok GW, Bandeen-Roche K, & Fried LP (2012). Lifestyle activities and memory: Variety may be the spice of life. The Women’s Health and Aging Study II. Journal of the International Neuropsychological Society, 18(2), 286–294. 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JD, Sprague BN, & Ross LA (2021). Age- and time-varying associations between subjective health and episodic memory in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, gbab 10.1093/geronb/gbab142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung F & Lucas RE (2014). Assessing the validity of single-item life satisfaction measures: Results from three large samples. Quality of Life Research, 23(10), 2809–2818. 10.1007/s11136-014-0726-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YC, Lee MS, Chiou JM, Chen TF, Chen YC, & Chen JH (2019). Association of diet quality and vegetable variety with the risk of cognitive decline in chinese older adults. Nutrients 10.3390/nu11071666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, & Oja P (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35(8), 1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- Deng Q & Liu W (2021). Inequalities in cognitive impairment among older adults in China and the associated social determinants: a decomposition approach. International Journal for Equity in Health, 20(1), 82–82. 10.1186/s12939-021-01422-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Emmons R, Larsen J, & Griffin S (1985). The satisfaction with life scale. Journal of Personality Assessment, 49, 71–75. [DOI] [PubMed] [Google Scholar]
- Diener E & Fujita F (1995). Resources, personal strivings, and subjective well-being: A nomothetic and idiographic approach. Journal of Personality Social Psychology, 68(5), 926–35. 10.1037//0022-3514.68.5.926 [DOI] [PubMed] [Google Scholar]
- Diener E, Lucas RE, & Oishi S (2018). Advances and open questions in the science of subjective well-being. Collabra: Psychology, 4(1), 15. 10.1525/collabra.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Ng W, & Tov W (2009). Balance in life and declining marginal utility of diverse resources. Applied Research in Quality of Life, 3(4), 277–291. 10.1007/s11482-009-9062-1 [DOI] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, & Reynolds CF (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry, 202(5), 329–335. 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerwald F, Albrecht BM, Stalling I, & Bammann K (2021). Domain-specific life satisfaction among older adults with and without children: The role of intergenerational contact. PloS One, 16(9), e0257048. 10.1371/journal.pone.0257048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewelies J, Koffer RE, Ram N, Almeida DM, & Gerstorf D (2019). Control diversity: How across-domain control beliefs are associated with daily negative affect and differ with age. Psychology and Aging, 34(5), 625–639. 10.1037/pag0000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Zammit AR, Katz MJ, Derby CA, Zimmerman ME, & Lipton RB (2019). Health-related quality of life, cognitive performance, and incident dementia in a community-based elderly cohort. Alzheimer Disease and Associated Disorders, 33(3), 240–245. 10.1097/WAD.0000000000000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong HF, Haron SA, Koris R, Hamid TA, & Ibrahim R (2021). Relationship between financial well-being, life satisfaction, and cognitive function among low-income community-dwelling older adults: The moderating role of sex. Psychogeriatrics, 21(4), 586–595. 10.1111/psyg.12709 [DOI] [PubMed] [Google Scholar]
- Ge S, Wu B, Bailey DE, & Dong X (2017). Social support, social strain, and cognitive function among community-dwelling US Chinese older adults. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 72(suppl_1), S16–S21. 10.1093/gerona/glw221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, & Lindenberger U (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43(3), 705–718. 10.1037/0012-1649.43.3.705 [DOI] [PubMed] [Google Scholar]
- Hill RD, van Boxtel MP, Ponds R, Houx PJ, & Jolles J (2005). Positive affect and its relationship to free recall memory performance in a sample of older Dutch adults from the Maastricht Aging Study. International Journal of Geriatric Psychiatry, 20(5), 429–35. 10.1002/gps.1300 [DOI] [PubMed] [Google Scholar]
- Jylha M (2009). What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine, 69(3), 307–316. 10.1016/j.socscimed.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Kaiser T, Hennecke M, & Luhmann M (2020). The interplay of domain-and life satisfaction in predicting life events. PloS one, 15(9), e0238992. 10.1371/journal.pone.0238992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Duff H, Kelly S, McHugh Power JE, Brennan S, Lawlor BA, & Loughrey DG (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MI & Scheltens P (2009). Dementia: The bare essentials. Practical Neurology, 9(4), 241–251. 10.1136/jnnp.2009.182477 [DOI] [PubMed] [Google Scholar]
- Kobau R, Sniezek J, Zack MM, Lucas RE, & Burns A (2010). Well‐being assessment: An evaluation of well‐being scales for public health and population estimates of well‐being among US adults. Applied Psychology: Health and Well‐Being, 2(3), 272–297. [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2003). The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care, 41(11), 1284–1292. 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- Kubzansky Huffman, J. C. Boehm, J. K. Hernandez, R. Kim, E. S. Koga, H. K. Feig, E. H. Lloyd-Jones, D. M. Seligman, P. ME, & Labarthe DR (2018). Positive psychological well-being and cardiovascular disease: JACC Health Promotion Series. Journal of the American College of Cardiology, 72(12), 1382–1396. 10.1016/j.jacc.2018.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, & Smidt N (2015). Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Research Reviews, 22, 39–57. 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Lee S, Charles ST, & Almeida DM (2020). Change is good for the brain: Activity diversity and cognitive functioning across adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(6), 1036–1048. 10.1093/geronb/gbaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Min DK, Thorpe L, & Lee CH (2016). Multidimensional construct of life satisfaction in older adults in Korea: A six-year follow-up study. BMC Geriatrics, 16(1), 197. 10.1186/s12877-016-0369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Z, & Zhang Y (2021). A national longitudinal study of marital quality and cognitive decline among older men and women. Social Science & Medicine, 282, 114151. 10.1016/j.socscimed.2021.114151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Z, Choi SW, & Langa KM (2020). Marital status and dementia: Evidence from the health and retirement study. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences, 75(8), 1783–1795. 10.1093/geronb/gbz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RE, & Donnellan MB (2012). Estimating the reliability of single-item life satisfaction measures: Results from four national panel studies. Social Indicators Research, 105(3), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, & Lepper HS (1999). A measure of subjective happiness: Preliminary reliability and construct validation. Social Indicators Research, 46(2), 137–155. 10.1023/A:1006824100041 [DOI] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaff K, & Brayne C (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurology, 13(8), 788–794. 10.1016/s1474-4422(14)70136-x [DOI] [PubMed] [Google Scholar]
- Peitsch L, Tyas SL, Menec VH, & St John PD (2016). General life satisfaction predicts dementia in community living older adults: A prospective cohort study. International Psychogeriatrics, 28(7), 1101–1109. 10.1017/S1041610215002422 [DOI] [PubMed] [Google Scholar]
- Qazi SL, Koivumaa-Honkanen H, Rikkonen T, Sund R, Kroeger H, Isanejad M, & Sirola J (2021). Physical capacity, subjective health, and life satisfaction in older women: A 10-year follow-up study. BMC Geriatrics, 21(1), 658. 10.1186/s12877-021-02605-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawtaer I, Gao Q, Nyunt MSZ, Feng L, Chong MS, Lim WS, Lee TS, Yap P, Yap KB, & Ng TP (2017). Psychosocial risk and protective factors and incident mild cognitive impairment and dementia in community dwelling elderly: Findings from the Singapore Longitudinal Ageing Study. Journal of Alzheimer’s Disease, 57(2), 603–611. 10.3233/JAD-160862 [DOI] [PubMed] [Google Scholar]
- Saito T, Murata C, Saito M, Takeda T, & Kondo K (2018). Influence of social relationship domains and their combinations on incident dementia: A prospective cohort study. Journal of Epidemiology and Community Health, 72(1), 7–12. 10.1136/jech-2017-209811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME (2008). Positive health. Applied Psychology, 57(s1), 3–18. 10.1111/j.1464-0597.2008.00351.x [DOI] [Google Scholar]
- Sheldon KM, Cummins R, & Kamble S (2010). Life balance and well-being: Testing a novel conceptual and measurement approach. Journal of Personality, 78(4), 1093–1134. 10.1111/j.1467-6494.2010.00644.x [DOI] [PubMed] [Google Scholar]
- Sirgy MJ, Kim MY, Joshanloo M, Lee DJ, & Bosnjak M (2020). The relationship between domain satisfaction and domain importance: The moderating role of depression. Journal of Happiness Studies, 21(6), 2007–2030. 10.1007/s10902-019-00168-w [DOI] [Google Scholar]
- Sirgy MJ & Wu J (2007). The pleasant life, the engaged life, and the meaningful life: What about the balanced life? Journal of Happiness Studies, 10(2), 183–196. 10.1007/s10902-007-9074-1 [DOI] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, Aschwanden D, & Terracciano A (2021). Self-rated health and incident dementia over two decades: Replication across two cohorts. Journal of Psychiatric Research, 143, 462–466. 10.1016/j.jpsychires.2021.06.036 [DOI] [PubMed] [Google Scholar]
- Sundström A, Adolfsson AN, Nordin M, & Adolfsson R (2020). Loneliness increases the risk of all-cause dementia and Alzheimer’s disease. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences, 75(5), 919–926. 10.1093/geronb/gbz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Aschwanden D, Passamonti L, Toschi N, Stephan Y, Luchetti M, Lee JH, Sesker A, O’Súilleabháin PS, & Sutin AR (2021). Is neuroticism differentially associated with risk of Alzheimer’s disease, vascular dementia, and frontotemporal dementia? Journal of Psychiatric Research, 138, 34–40. 10.1016/j.jpsychires.2021.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend P, Phillimore P, & Beattie A (1997). Health and deprivation: Inequality and the North. Revista cubana de higiene y epidemiología, 35(1), 48–50. [Google Scholar]
- Van Cappellen P, Rice EL, Catalino LI, & Fredrickson BL (2018). Positive affective processes underlie positive health behaviour change. Psychology and Health, 33(1), 77–97. 10.1080/08870446.2017.1320798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F & Sheng Y (2019). Social support network, social support, self-efficacy, health-promoting behavior and healthy aging among older adults: A pathway analysis. Archives of Gerontology and Geriatrics, 85, 103934. 10.1016/j.archger.2019.103934 [DOI] [PubMed] [Google Scholar]
- Zhu S, Hu J, & Efird JT (2012). Role of social support in cognitive function among elders. Journal of Clinical Nursing, 21(15–16), 2118–2125. 10.1111/j.1365-2702.2012.04178.x [DOI] [PubMed] [Google Scholar]
- Zhu X, Luchetti M, Aschwanden D, Sesker AA, Stephan Y, Sutin AR, & Terracciano A (2022). Satisfaction with life and risk of dementia: Findings from the Korean Longitudinal Study of Aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences Advance Online Publication. 10.1093/geronb/gbac064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


