Abstract
Background:
The relevance of the study lies in the fact that although the role of polymorphism of some genes that are responsible for cell apoptosis and deoxyribonucleic acid repair in the development of acute leukemia has already been established, its relationship with the gender of patients has not been studied enough. This study was aimed at studying the relationship between the Arg399Gln polymorphism in the XRCC1 deoxyribonucleic acid repair gene and the Arg72Pro polymorphism in the TP53 tumor suppressor gene encoding the p53 protein with the gender of children with acute leukemia.
Material and methods:
The study included 100 newly diagnosed pediatric patients of Kyrgyz nationality (69 boys and 31 girls), among which there were 77 patients with acute lymphoblastic leukemia, 22 patients with acute myeloblastic leukemia and 1 patient with a biphenotypic variant. Determination of polymorphisms was carried out by PCR-RFLP analysis or polymerase chain reaction followed by an analysis of restriction fragment length polymorphism. The interrelation of the results obtained with the patients’ gender was assessed using statistical methods.
Results:
The study showed that there were no gender differences for all three genotypes of the Arg72Pro polymorphic marker of the tumor suppressor p53 (ТР53). Three Arg399Gln genotypes of the XRCC1 gene also did not depend on gender. However, with a separate analysis of each polymorphism, there was a tendency for a greater proportion of the Arg/Gln genotype in the group of boys compared to girls. The Gln/Gln polymorphism relationship requires further study due to insufficient data for analysis.
Conclusion:
The study has expanded the understanding of genetic changes and their relationship with gender, which have diagnostic, prognostic and therapeutic implications in acute leukemia. The conducted research of the relationship between individual phenotypes of acute lymphoblastic leukemia with risky polymorphisms in some genes contributes to the study of AL.
Key Words: Oncohematology, genotype, individual phenotypes, polymorphism, cancer
Introduction
Acute leukemia (AL) is one of the most urgent problems of oncohematology. Its main diagnostic criterion is the proliferation of immature leukocytes, which make up more than 20% of bone marrow cells or peripheral blood cells (Edlefsen, 2013). According to the modern classification, there are four main groups: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), (B-cell lymphoblastic leukemia (B-ALL) and T-cell lymphoblastic leukemia (T-ALL)), as well as biphenotypic leukemias – forms of acute leukemia of ambiguous origin (Brown, 2021). Acute lymphoblastic leukemias are more common in children (Malard and Mohty, 2020), while myeloid leukemias are considered the most common acute leukemias in adults (De Voeght et al., 2021; Ramilyeva et al., 2019).
The possibilities of modern medicine have made it possible to transfer AL from the category of fatal diseases to the category of curable diseases. The survival rate for acute leukemia depends on the leukemia type, general health, promptness of diagnosis establishing and initiation of treatment, and the patients’ age. For example, in the children’s population, the survival rate is on average 75-80%, while in the adult population – only 25-40% (Xie and Hossain, 2018; Pui, 2020). Every year, the survival rate for AL increases. For example, in adult patients with ALL, the 5-year survival rate increased from 31.6% to 39.0% from 1997 to 2008. As for adult patients with AML, the survival rate over the same period increased from 15.5% to 22.5% (Pulte et al., 2013). This is more due to an improved understanding of the importance of genetic variations in the pathogenesis, biology, diagnosis and treatment of these diseases, rather than because of the emergence of new methods of therapy.
Certain environmental factors may increase the risk of developing AL (Tebbi, 2021) such as exposure to ionizing radiation, a number of mutagens, alkylating agents, etc., but the genetic predisposition plays the main role, in particular, the presence of genetic variations that lead to neoplastic changes and clonal proliferation (Chesnokova et al., 2015; Ghosh et al., 2022). Damage to the deoxyribonucleic acid (DNA) of hematopoietic progenitor cells is a significant factor in the development of leukemia, since the host’s DNA repair system cannot correct errors in DNA (Chennamadhavuni et al., 2022). XRCC1 (X-Ray Repair Cross Complementing 1) is one of the most important genes involved in the repair of DNA damage that occurs as the result of the effects of metabolic synthesis of reactive oxygen species (ROS), alkylating agents or radiation. Usually, the cellular DNA damages are repaired by the interaction of XRCC1с with other DNA repair proteins, such as DNA-ligase 3, poly-ADP-ribose polymerase and DNA polymerase-β (London, 2020). However, the Arg399Gln polymorphism in the XRCC1 gene disrupts these processes, leading to an increased risk of developing hematological malignancies or leukemia, in particular in Asians (Yang et al., 2016).
Another important mechanism for the development of malignant neoplasms is a violation of the process of cell apoptosis. In half of all cases of human cancers, the p53 tumor suppressor protein is damaged as a result of a somatic mutation in tumor cells (Das et al., 2021). This protein, which is a product of the TP53 gene, is at the center of cellular regulatory pathways, by influencing the transcription and activity of several replication factors and transcription ones. Typically, when DNA is damaged, the p53 protein is activated, which leads to the launch of repair mechanisms, the induction of cell cycle arrest and the prevention of cancer growth through apoptosis. However, when this protein is damaged, cancer cells continue to multiply (Shah and Coleman, 2007). The Arg72Pro genomic substitution in the TP53 gene has an important effect on cell death through apoptosis (Khan et al., 2015). While these mutations are relatively rare in leukemia and occur predominantly in patients with acute myeloid leukemia with complex karyotype or acute lymphoblastic leukemia with complex karyotype, they are strongly associated with chemoresistance and a high risk of recurrence (Barbosa et al., 2019; Jafari Nedooshan et al., 2016).
Thus, the study of genetic changes associated with acute leukemia has important diagnostic, prognostic and even therapeutic implications. In particular, the relationship of polymorphisms of some genes with the patient gender is of great interest, which may explain the higher incidence of AL among men, as well as provide a better understanding of the poorer response to treatment observed in boys with ALL compared with girls with ALL (Bolufer et al., 2007). The relevance of gender as a risk modifier for AL requires further experimental study. The present study was aimed at identifying the relationship of the pediatric patients’ gender with the polymorphism of the XRCC1 Arg399Gln and TP53 Arg72Pro genes, whose influence on the risk of developing acute leukemia has already been confirmed (Usenova, 2018; Burkitbayev et al., 2017).
Materials and Methods
The study included 100 pediatric patients: 69 boys and 31 girls. All participants belonged to the Kyrgyz nationality and they were not linked to each other by ties of kinship. The group included 77 patients with acute lymphoblastic leukemia, 22 patients with acute myeloid leukemia and 1 patient with a biphenotypic variant. When studying the Arg399Gln polymorphism in the XRCC1 DNA repair gene, analysis was performed in 97 cases from 100 blood samples. It is important to note that all patients were under 18 years of age. All participants in the study were diagnosed with acute leukemia for the first time and none of them had other malignant neoplasms in anamnesis. All children subsequently received special treatment according to the established protocol.
The research plan was used on a case-by-case basis due to its greater statistical significance in detecting the interaction effect of gender and genotype compared to other traditional epidemiological plans (Clayton and McKeigue, 2001). The set of cases of a rare disease such as childrens’ AL is a complex task, and hence the preference to a statistically more powerful study design was more practical. To achieve this goal, the study was carried out in two stages:
1. Study of the Arg399Gln polymorphism in the XRCC1 DNA repair gene and the Arg72Pro polymorphism in the TP53 tumor suppressor gene in 100 patients with various types of acute leukemia, as well as with various phenotypes of acute lymphoblastic leukemia;
2. Analysis of the relationship between the obtained results of polymorphism and the belonging of patients to a certain gender.
Determination of Arg72Pro polymorphism in the XRCC1 gene and Arg72Pro in the TP53 gene was carried out by PCR-RFLP analysis or polymerase chain reaction followed by analysis of restriction fragment length polymorphism. The restriction fragment length polymorphism analyzes DNA fragments of different lengths that result from the digestion of a DNA sample with a restriction endonuclease. Then, they are hybridized with DNA probes that bind to a complementary DNA sequence in the sample. To analyze the relationship between the results obtained and the patients’ gender, the SPSS-16 program (Statistical Product and Service Solutions) was used, which included methods of descriptive statistics, in particular, distribution tables and comparison diagrams, as well as cross tabulation (contingency tables). SPSS-16 is a computer program for statistical data processing that allows the implementation a statistical analysis, data management and data documentation. Statistical calculation of the relationship between the patients’ gender and gene polymorphisms was carried out using the test called the “Value of difference between two independent proportions”, which uses the definition of z- criterion to compare proportions or percentages.
The study was conducted based on the children’s department of the National Center of Oncology and Hematology and the Osh Regional Children’s Clinical Hospital (Osh city, Kyrgyz Republic). Molecular genetic studies were carried out based on the Research Institute of Molecular Biology and Medicine at the National Center of Cardiology and Therapy named after Academician M. Mirrakhimov under the Ministry of Health of the Kyrgyz Republic.
Results
Study of the Arg399Gln polymorphism in the XRCC1 gene
When studying the Arg399Gln polymorphism in the XRCC1 DNA repair gene, analysis was performed in 97 cases from 100 blood samples. The results of its distribution among boys and girls are clearly presented in Table 1.
Table 1.
Genotypic Distribution of the Polymorphic Marker Arg399Gln of the XRCC1 Gene among Boys and Girls
| Gender of patients with AL | Number of examined and expected | Distribution of genotypes | Total | |||
| Gln/Gln | Arg/Gln | Arg/Arg | ||||
| Gender | girls | Number | 4 | 13 | 20 | 37 |
| Expected number | 3.1 | 16.4 | 17.5 | 37.0 | ||
| boys | Number | 4 | 30 | 26 | 60 | |
| Expected number | 4.9 | 26.6 | 28.5 | 60.0 | ||
| All patients, regardless of gender | Number | 8 | 43 | 46 | 97 | |
| Expected number | 8.0 | 43.0 | 46.0 | 97.0 | ||
The homozygous Gln/Gln genotype was detected in 8 patients with AL, that is, in 8.2% of cases; the heterozygous Arg/Gln genotype was found in 44 patients, i.e., in 44.9% of cases; the homozygous Arg/Arg genotype was detected in 46 patients, i.e., in 46.9% of cases. Analysis of the distribution features of the Arg399Gln polymorphism in the XRCC1 gene depending on the patients’ gender involved the determination of the Phi and Cramer coefficients. The symmetrical Phi measurement showed a statistical significance of 0.338. In other words, it was not found the statistical significance between the studied data (p>0.05). Thus, the analysis did not reveal any relationship with gender of all three Arg399Gln genotypes in the XRCC1 gene, that is, there were no statistically significant differences between the groups of boys and girls. However, upon further study of each genotype separately and statistical calculations of their relationship with the patients’ gender using the test called the “Value of difference between two independent proportions”, some differences were obtained, which are presented in Table 2, 3.
Table 2.
Distribution of the Arg/Gln Genotype (Arg399Gln Polymorphism in the XRCC1 Gene) in Children with Acute Leukemia Depending on Gender
| Arg/Gln genotype, Girls | Arg/Gln genotype, Boys | Analysis results | |||
|---|---|---|---|---|---|
| ka | 13 | kb | 30 | ||
| na | 37 | nb | 60 | ||
| pa | 0.3514 | pb | 0.5 | pa-pb | 0.1486 |
| z | 1.432 | ||||
Note: ka, the number of patients with the studied genotype in one group (girls); kb, the number of patients with the studied genotype in the second group (boys); na, the total number of children in the first group (girls); nb, the total number of patients in the second group (boys); pa-pb, the ratio of the number of children with the studied genotype to the total number of children, respectively, in the first and second groups, z-z- criterion.
Table 3.
Distribution of the Arg/Arg Genotype (Arg399Gln Polymorphism in the XRCC1 Gene) in Children with Acute Leukemia Depending on Gender
| Arg/Arg genotype, girls | Arg/Arg genotype, boys | Analysis results | |||
|---|---|---|---|---|---|
| kb | 13 | kb | 30 | ||
| nb | 37 | nb | 60 | ||
| pb | 0.3514 | pb | 0.5 | pa-pb | 0.1072 |
| z | 1.027 | ||||
In this case, z- criterion was used to compare proportions. It was above one and approached the 1.5 value, which indicates that there was a tendency for a larger proportion of the Arg/Gln genotype in the group of boys compared to girls.
Results of analyzing the distribution of the homozygous Arg/Arg genotype depending on the gender of pediatric patients with AL indicate that the data obtained do not allow talking about the existence of a statistically significant relationship between these features. The calculation for the Gln/Gln genotype was not carried out, since the total number of examined patients in this group included only 4 people. This is not enough to carry out an analysis that would be of statistical significance.
Study of Arg72Pro polymorphism in the TP53 gene
When analyzing the relationship of the Arg72Pro polymorphism in the TP53 gene encoding the p53 protein with the patients’ gender was carried out among all 100 patients. The obtained results presented in Table 4.
Table 4.
Distribution of Genotypes of the Arg72Pro Polymorphic Marker of the ТP53 Gene among Boys and Girls
| Gender of patients with AL | Number of examined and expected | Distribution of genotypes | Total | |||
|---|---|---|---|---|---|---|
| Arg/Arg | Arg/Pro | Pro/Pro | ||||
| Gender | Girls | Number | 14 | 17 | 8 | 39 |
| Expected number | 16.8 | 13.6 | 8.6 | 39 | ||
| Boys | Number | 29 | 18 | 14 | 61 | |
| Expected number | 26.2 | 21.4 | 13.4 | 61 | ||
| All patients, regardless of gender | Number | 43 | 35 | 22 | 100 | |
| Expected number | 43 | 35 | 22 | 100 | ||
The homozygous Arg/Arg genotype was detected in 43 patients with AL, i.e. in 43% of cases, the heterozygous Arg/Pro genotype was detected in 35 patients, i.e. in 35% of cases, the homozygous Pro/Pro genotype was detected in 22 patients, i.e. in 22% of cases. The distribution analysis of the genotypes of the Arg72Pro polymorphic marker in the TP53 gene depending on the patients’ gender involved the determination of the Phi and Cramer coefficients. The symmetrical measurement by Phi and Cramer showed a statistical significance of 0.339.
Study of the relationship of Arg72Pro polymorphism in the TP53 gene and the Arg72Pro polymorphism in the TP53 gene with phenotypes of the acute lymphoblastic leukemia.
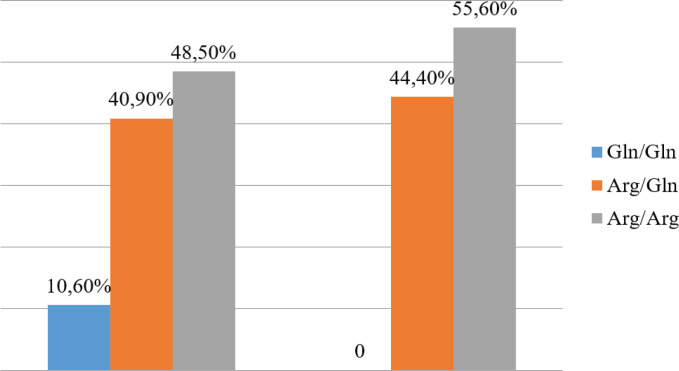
In the course of the study, the relationships of individual phenotypes of acute lymphoblastic leukemia with respect to Arg399Gln polymorphism in the XRCC1 gene and Arg72Pro polymorphism in the TP53 gene were studied. The results showed that the Gln/Gln genotype (Arg399Gln polymorphism in the XRCC1 gene) occurred in 7 cases (10.6%) in the group of 69 patients with B-ALL and 9 patients with T-ALL, however such combination in T-ALL was not found. The Arg/Gln genotype was determined in 27 cases (40.9%) with B-ALL and in 4 cases (44.4%) with T-ALL. The Arg/Arg genotype in the XRCC1 gene: 48.5% in B-ALL and 55.6% in T-ALL (Figure 1) was the most common genotype in both variants of ALL.
Figure 1.
Comparative Frequency of Genotypes’ Distribution of Arg399Gln Polymorphic Markers of the XRCC1 Gene in B-ALL and T-ALL
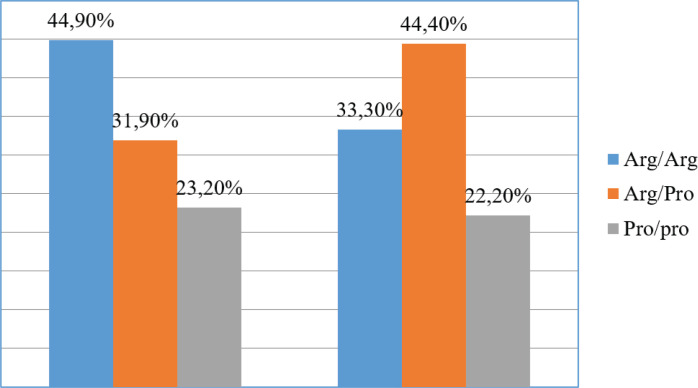
The study of Arg72Pro polymorphism of the TP53 gene in 69 patients with B-ALL and 9 patients with T-ALL showed the following results. The Arg/Arg genotype was found in 31 cases (44.9%) and 3 (33.3%) cases, respectively, in B and T-ALL. The Arg/Pro genotype – in 22 cases (31.9%) and 4 cases (44.4%), and the Pro/Pro genotype – in 46 cases (23.2%) and 2 cases (22.2%), respectively (Figure 2).
Figure 2.
Comparative Frequency of Genotypes’ Distribution of the Arg72Pro Polymorphic Markers of the TP53 Gene in B-ALL and T-ALL
A comparison of the results showed that there were statistically significant differences in the distribution frequency of the homozygous Arg/Arg genotype between B-ALL and T-ALL (p<0.05), as well as in the distribution frequency of the heterozygous Arg/Pro genotype. Figure 2 clearly shows that the Arg/Arg genotype was more often associated with B-cell ALL, and the Arg/Pro genotype was more often associated with T-cell ALL.
Discussion
Over the past three decades, there has been a qualitative breakthrough in the study and understanding of the nature of hematological malignant neoplasms. In many ways, it became possible due to the rapid development of genetic research methods, such as sequencing, genome-wide association search, FISH diagnostics, karyotyping, gene expression profiling, etc. As noted by Yue and Zheng (2013) in their work devoted to the study progress of acute leukemia genetics, these analyzes allowed redefining the molecular basis and clarifying the mechanisms underlying the leukemic transformation in some oncohematological diseases, including acute childhood leukemia. Acute lymphoblastic leukemia belongs to a family of genetically heterogeneous lymphoid neoplasms derived from B- and T-lymphoid progenitors (Pui, 2020). Current pediatric clinical trials have improved the 5-year childhood event-free survival above 85% and the 5-year overall survival above 90% for B-cell acute lymphoblastic leukemia (B-ALL), while results for T-cell ALL (T-ALL) are still 5-10% behind in most studies (Teachey and Pui, 2019). Several factors contribute to this conflicting result, including the features of patients’ genetic subtypes with different disease phenotypes. That is why their study is of great interest.
The researchers such as Vrooman and Silverman (2016) and Kayser and Levis (2018) also emphasize the growing role of genetic research to find new prognostic algorithms, ways to optimize patients’ treatment, identify specific molecular markers that can become potential targets for new methods of treatment developed in the framework of pharmacogenomics and personalized medicine. However, despite all the achievements of the genomic era, which made it possible to better understand the biological and genetic basis of oncohematological diseases, some of their features still require clarification. In particular, the reasons underlying the gender differences in susceptibility to cancer in general and acute leukemia still remain unclear. Studies of the gender influence on predisposition to malignant neoplasms have not yet become common practice, and the molecular mechanisms that determine these gender differences have not been studied enough to come to unambiguous conclusions. As a result, for example, as noted by Lopes-Ramos et al. (2020), when prescribing therapy, the effect of the patients’ gender is not taken into account.
Gender consideration as a biological variable has only recently begun to be introduced into the field of medical, biological, genetic and biopharmaceutical research, and so far, according to Cornelison and Clayton (2017), data on gender differences in predisposition to various diseases remain fragmented and contradictory even for those diseases for which the clear gender differences in incidence rates are well documented in the literature. These diseases include cancer. Gender differences in cancer susceptibility are one of the most consistent results in cancer epidemiology. All types of malignant neoplasms, including hematological malignancies, tend to be statistically more common in men than in women (Edgren et al., 2012).
This trend continues in the pediatric population. Dorak and Karpuzoglu (2012) emphasize that gender differences in cancer incidence in children are well-known and constant throughout the world, and the work of Hjalgrim et al., (2003) shows that acute leukemias are no exception to this rule. In general, AL in the pediatric population is more common in boys. According to the study by Gupta et al., (2022), boys are more likely to have acute lymphoblastic leukemia, and girls are more likely to have acute myeloid leukemia. Males with ALL also have worse survival compared to girls, a higher risk of disease recurrence and the formation of chemoresistance during therapy.
An analysis by Holmes et al., (2012) shows that gender differences in cancer incidence cannot be explained by treatment received, prognostic or sociodemographic factors and apparently, are related to the biological characteristics of both genders. According to Pui (2020), gender differences in the incidence of AL and other oncological diseases also cannot be explained solely by hormonal and behavioral causes, since they are observed even at a very early age (the peak incidence occurs in children aged between 2 and 5 years old). An analysis of the literature by professors such as Dorak and Karpuzoglu (2012) from Robert Stempel College of Public Health & Social of Florida International University showed that there are several alternative hypotheses, according to which the gender differences in the risk of developing acute leukemia. These differences may be associated with the characteristics of the immune system of girls and boys, the effectiveness of mechanisms for monitoring the genome, the number of X chromosomes, the influence of various factors during fetal development, etc. However, none of the above hypotheses, according to the researcher, provides an exhaustive explanation of this phenomenon.
Today, most researchers agree that gender differences in oncological diseases arise due to a combination of environmental, genetic and epigenetic factors, as well as differences in gene regulation and expression in different genders (Rubin et al., 2020). Since the vast differences between men and women occur in the entire genome, they ultimately affect not only the cancer biology, but also its outcomes. In addition, the study of these differences provides very useful information that can be used to refine the causal hypothesis of the disease, identify subgroups with the highest risk, develop a set of preventive measures and develop treatment tactics. Thus, it makes sense to take into account gender differences in all genetic and non-genetic association studies. An integrative analysis of the interaction of gender and genomics in oncological diseases is of particular interest here.
In some previous studies, attempts have already been made to genomic characterize individual types of acute leukemia and link it to the patients’ gender. Thus, a group of Italian researchers under the leadership of S. Chiaretti et al. (2014) from the hematology department of the department of cellular biotechnology and hematology of University of Rome La Sapienza, several specific molecular markers for AML and ALL as B-cell types. Group of American scientists headed by S.K. Singh et al. (2016), in the course of a genome-wide association study of 236 children with ALL who were consecutively selected at the Texas Children’s Cancer Center (Houston, USA), identified some gender-dependent risk variants for this disease. In the current study, the problem was considered for the first time from another standpoint, which set the task of studying the relationship of the patients’ gender with acute leukemia with already known risky polymorphic variations in some genes.
Two polymorphisms Arg399Gln in the XRCC1 gene and Arg72Pro in the TP53 gene were selected for the study. Many researchers, in particular Floris et al. (2022), A.A. Usenova (2018), X. Yang et al. (2016), K. Barbosa et al. (2019) and others noted their role in the development of malignant neoplasms, and, in particular, AL. Previous studies have shown that several Arg399Gln polymorphisms in the X-ray repair cross-complementing group (XRCC1) gene are associated with a significant reduction in the ability to repair DNA single-strand breaks (Özgöz et al., 2019). In particular, it was noted in the work of G. Ginsberg et al. (2011) that the ability to remove DNA adducts and oxidized DNA damages is reduced by 3-4 times in people with a homozygous variant (Gln/Gln), which is a possible risk factor for oncological diseases.
In 2014, a group of Chinese researchers from the hematology department of the Guiyang College of Traditional Chinese Medicine Hospital under the leadership of Huang et al., (2014) conducted a meta-analysis of 17 “case-control” studies and found that the Arg399Gln polymorphism in the XRCC1 gene is a statistically significant risk factor of leukemia among Asians, which is important for this study. The connection of this polymorphism with oncological diseases was also established in persons of Kyrgyz nationality. Thus, Semetei kyzy et al., (2018) and co-authors showed its significance for breast cancer, and Usenova (2018) established its relationship with AL in the Kyrgyz pediatric population.
Some authors, in particular Bănescu et al., (2014), also point to the Arg399Gln polymorphism in the XRCC1 gene as a prognostic marker for certain types of acute leukemia, since it is significantly associated with overall survival. The second chosen polymorphic marker Arg72Pro in the TP53 gene is located in the domain with a high proline concentration, which is responsible for the apoptotic functioning of the p53 protein. After the mutation, the p53 protein is no longer able to activate the transcription of proapoptotic genes, which leads to disruption of the cell cycle and apoptosis. This causes a significant increase in the number of cells with various DNA changes, followed by cell proliferation (London, 2020).
Frequent mutations and differential expression of TP53 in various types of malignant neoplasms emphasize the important role of p53 in carcinogenesis and tumor progression. Researches by Isakova et al., (2017), Diakite et al., (2020),Usenova (2018) and Drokow et al., (2020) confirmed the relationship between the Arg72Pro polymorphism in the TP53 gene and the risk of developing oncological diseases, in particular, acute lymphoblastic leukemia. In addition, Isakova et al., (2017) and Usenova (2018) demonstrate in their work the key role of this polymorphism specifically for people of Kyrgyz nationality.
Having selected these polymorphisms, which are significant risk factors for the AL development within the framework of the study, they were compared with the gender of 100 pediatric patients (69 boys and 31 girls) with various types of acute leukemia, and a statistical analysis to find a significant relationship between the results obtained was carried out. In general, for both studied polymorphisms, it was not possible to identify this relationship with one exception. When studying various genotypes of the Arg399Gln polymorphism in the XRCC1 gene, it was observed a tendency of the presence of a larger proportion of the heterozygous Arg/Gln variant in the group of boys compared to the group of girls. It is worth noting that this study was limited to a relatively small sample (100 patients) of Kyrgyz ethnicity. This, in particular, did not allow us to analyze the relationship of gender with the homozygous Gln/Gln genotype (Arg399Gln polymorphism in the XRCC1 gene), since the total number of examined patients in this group was only equal to 4 people.
Additionally, within the framework of this study, it was also considered the relationship between the Arg399Gln polymorphism in the XRCC1 gene and the Arg72Pro polymorphism in the TP53 gene with individual phenotypes of acute lymphoblastic leukemia. Acute lymphoblastic leukemia is the most common oncological disease in children that make up 80% of all acute leukemias in non-adults, which in turn accounts for 34% of all cancers in this age group (Pui, 2020). There are 2 subtypes of this disease depending on the affected lymphoid progenitor: B-cell ALL and T-cell ALL.
Studies of different years by Du et al., (2022), C. Spix et al. (2008), Steliarova-Foucher et al., (2018) show that B-cell ALL remains the most common type, and its incidence among children is constantly increasing in the developed countries of the world. T-cell ALL is less common: according to statistical data collected by the researchers Karrman and Johansson (2017) of the department of clinical genetics of Lund University (Sweden) Karrman and Johansson (2017), it occurs in about 10-15% of cases. However, as the Swedish researchers note, this phenotype of acute lymphoblastic leukemia is more often associated with a poor prognosis, as it is the most complex and heterogeneous at the genetic level, and fewer new therapeutic alternatives are currently available for it.
Analysis conducted in USA by Raetz and Teachey (2016) showed that if outcomes gradually improve for debut T-cell ALL with the introduction of modern diagnosis methods and treatment, by approaching the 80-85% of 5-year event-free survival observed for B-cell ALL, then with relapses of this disease, the overall event-free survival is only 25%. Thus, further study of the genetics and biology of T-cell acute lymphoblastic leukemia is an urgent task for finding new approaches to its therapy and prevention of disease recurrence.
The study showed that the frequency distribution of Arg399Gln polymorphism genotypes in the XRCC1 gene did not differ significantly in patients with B-cell ALL and patients with T-cell ALL. The Arg/Arg genotype was the most common genotype in both variants (48.5% and 55.6%, respectively). The Arg/Gln genotype was found in 40.9% of patients with B-ALL and in 44.4% of patients with T-ALL. The Gln/Gln genotype was found in 10.6% of cases of B-ALL, while it was not identified the patients with this genotype and T-cell ALL, which is probably due to the small sample size: only 9 children with this phenotype of acute lymphoblastic leukemia were included in the study. An analysis of the distribution frequency of the Arg72Pro polymorphic variants in the TP53 gene showed different results. The homozygous Arg/Arg genotype was statistically significantly more often associated with B-cell ALL, and the heterozygous Arg/Pro genotype was associated with T-cell ALL, while the percentage of the Pro/Pro genotype was approximately the same in percentage terms for both groups.
Thus, in the course of the study, authors identified several previously unknown associations: the Arg/Gln genotype (Arg399Gln polymorphism in the XRCC1 gene) was more common in boys with ALL than in girls with ALL. The Arg/Arg genotype (Arg72Pro polymorphism in the TP53 gene) was associated with B-cell ALL, while the Arg/Pro genotype was associated with T-cell ALL. However, it should be noted that this study did not set the task of putting the biological variables on the clinical footing, therefore, both the prognostic and therapeutic value of data authors have received requires further study in larger samples of patients with acute lymphoblastic leukemia.
In conclusions, In the current study, it was conducted the search for correlations between some genetic variants which have been determined to be a variants that increase the risk of hematological malignancies with the gender of children with acute leukemia. There was not found statistically significant relationship with gender of all three Arg399Gln genotypes in the XRCC1 gene.
The relationship between the Arg399Gln polymorphisms in the XRCC1 gene and the Arg72Pro in the TP53 gene was also studied in patients with B-cell acute lymphoblastic leukemia and T-cell acute lymphoblastic leukemia. It was found that the Arg/Arg genotype in the XRCC1 gene was the most common genotype in both B-ALL and T-ALL; the Arg/Arg genotype in the TP53 gene was more often associated with B-ALL, while the Arg/Pro genotype in the TP53 gene was associated with T-ALL.
The genetic research has played the key role in the diagnosis and treatment of acute leukemia, so this study allowed expanding the understanding of the biology and genetics of these diseases. The results obtained may be important for establishing the nature of persistent gender differences observed in AL and eliminating their influence on the prognosis of these diseases. The conducted research of the relationship between individual phenotypes of acute lymphoblastic leukemia with risky polymorphisms in some genes contributes to the study of AL. Additional studies will be needed to evaluate the specific practical role of the established associations.
Author Contribution Statement
AA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Acknowledgements
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- Bănescu C, Duicu C, Trifa AP, Dobreanu M. XRCC1 Arg194Trp and Arg399Gln polymorphisms are significantly associated with shorter survival in acute myeloid leukemia. Leuk Lymphoma. 2014;55:365–70. doi: 10.3109/10428194.2013.802781. [DOI] [PubMed] [Google Scholar]
- Bolufer P, Collado M, Barragán E, et al. The potential effect of gender in combination with common genetic polymorphisms of drug-metabolizing enzymes on the risk of developing acute leukemia. Haematologica. 2007;92:308–14. doi: 10.3324/haematol.10752. [DOI] [PubMed] [Google Scholar]
- Brown G. Introduction and classification of leukemias. Methods Mol Biol. 2021;2185:3–23. doi: 10.1007/978-1-0716-0810-4_1. [DOI] [PubMed] [Google Scholar]
- Burkitbayev ZK, Abdrakhmanova SA, Turganbekova AA, Ramilyeva IR, Baimukasheva DK. Detection of the HLA-DQB1 allele, DQB1*03:82, in a Kazakh patient with acute myeloid leukemia. HLA. 2017;90:181–182. doi: 10.1111/tan.13072. [DOI] [PubMed] [Google Scholar]
- Chennamadhavuni A, Lyengar V, Shimanovsky A. Leukemia. 2022 [PubMed] [Google Scholar]
- Chesnokova NP, Nevvazhay TA, Zhevak TN, Polutova NV, Bizenkova MN. Acute myelo- and lympholeukosis: classification principles, development stages General regularities of clinical manifestations. Int J Appl Fundam Res. 2015;7:163–6. [Google Scholar]
- Chiaretti S, Gianfelici V, Ceglie G, Foà R. Genomic characterization of acute leukemias. Med Princ Pract. 2014;23:487–506. doi: 10.1159/000362793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet. 2001;358:1356–60. doi: 10.1016/S0140-6736(01)06418-2. [DOI] [PubMed] [Google Scholar]
- Cornelison T, Clayton J. Considering Sex as a Biological Variable in Biomedical Research. Gend Genome. 2017;1:89–93. [Google Scholar]
- Das S, Shukla N, Singh SS, Kushwaha S, Shrivastava R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis. 2021;26:512–33. doi: 10.1007/s10495-021-01687-9. [DOI] [PubMed] [Google Scholar]
- De Voeght A, Jaspers A, Beguin Y, Baron F, De Prijck B. Overview of the general management of acute leukemia for adults. Rev Méd Liège. 2021;76:470–5. [PubMed] [Google Scholar]
- Diakite B, Kassogue Y, Dolo G, et al. p Arg72Pro polymorphism of P53 and breast cancer risk: a meta-analysis of case-control studies. BMC Med Genet. 2020;21:1–11. doi: 10.1186/s12881-020-01133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:1–11. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drokow EK, Chen Y, Waqas Ahmed HA, et al. The relationship between leukemia and TP53 gene codon Arg72Pro polymorphism: analysis in a multi-ethnic population. Future Oncol. 2020;16:923–37. doi: 10.2217/fon-2019-0792. [DOI] [PubMed] [Google Scholar]
- Du M, Chen W, Liu K, et al. The global burden of leukemia and its attributable factors in 204 countries and territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J Oncol. 2022;2022:1612702. doi: 10.1155/2022/1612702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2021;27:187–96. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- Floris M, Pira G, Castiglia P, et al. Impact on breast cancer susceptibility and clinicopathological traits of common genetic polymorphisms in TP53, MDM2 and ATM genes in Sardinian women. Oncol Lett. 2022;24:1–12. doi: 10.3892/ol.2022.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bhattacharjee M, Jana N. Gene regulation by p53 in human cancer system. Asian Pac J Cancer Biol. 2022;7:97–109. [Google Scholar]
- Ginsberg G, Angle K, Guyton K, Sonawane B. Polymorphism in the DNA repair enzyme XRCC1: utility of current database and implications for human health risk assessment. Mutat Res. 2011;727:1–15. doi: 10.1016/j.mrrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Gupta S, Teachey DT, Chen Z, et al. Sex-based disparities in outcome in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group report. Cancer. 2022;128:1863–70. doi: 10.1002/cncr.34150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim L, Rostgaard K, Schmiegelow K, et al. Age- and sex-specific incidence of childhood leukemia by immunophenotype in the Nordic countries. J Nat Cancer I. 2003;95:1539–44. doi: 10.1093/jnci/djg064. [DOI] [PubMed] [Google Scholar]
- Holmes L, Hossain J, des Vignes-Kendrick M, Opara F. Sex variability in pediatric leukemia survival: Large cohort evidence. Int Sch Res Notices. 2012;2012:439070. doi: 10.5402/2012/439070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xie D, Tang N, et al. XRCC1 Arg399Gln variation and leukemia susceptibility: evidence from 2647 cases and 5518 controls. Tumor Biol. 2014;35:799–808. doi: 10.1007/s13277-013-1110-7. [DOI] [PubMed] [Google Scholar]
- Isakova ZT, Kipen VN, Semetei KA, et al. Molecular histological characteristics of breast tumors for women from the Kyrgyz population – the contribution of the genes TP53, XRCC1 and MDM2. Med Genet. 2017;16:36–42. [Google Scholar]
- Jafari Nedooshan J, Yazdi MF, Neamatzadeh H, et al. Association between TP53 codon 72 G>C polymorphism and thyroid carcinoma risk: An up-to-date meta-analysis. Asian Pac J Cancer Biol. 2016;1:89–95. [Google Scholar]
- Karrman K, Johansson B. Pediatric T-cell acute lymphoblastic leukemia. Genes Chromosom Cancer. 2017;56:89–116. doi: 10.1002/gcc.22416. [DOI] [PubMed] [Google Scholar]
- Kayser S, Levis MJ. Clinical implications of molecular markers in acute myeloid leukemia. Eur J Haematol. 2018;102:20–35. doi: 10.1111/ejh.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MH, Khalil A, Rashid H. Evaluation of the p53 Arg72Pro polymorphism and its association with cancer risk: a HuGE review and meta-analysis. Genet Res. 2015;97:1–11. doi: 10.1017/S0016672315000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London RE. XRCC1 – Strategies for coordinating and assembling a versatile DNA damage response. DNA Repair. 2020;93:102917. doi: 10.1016/j.dnarep.2020.102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Ramos CM, Quackenbush J, DeMeo DL. Genome-wide sex and gender differences in cancer. Front Oncol. 2020;10:597788. doi: 10.3389/fonc.2020.597788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malard F, Mohty M. Acute lymphoblastic leukemia. Lancet. 2020;395:1146–62. doi: 10.1016/S0140-6736(19)33018-1. [DOI] [PubMed] [Google Scholar]
- Özgöz A, Hekimler Öztürk K, Yükseltürk A, et al. Genetic variations of DNA repair genes in breast cancer. Pathol Oncol Res. 2019;25:107–14. doi: 10.1007/s12253-017-0322-3. [DOI] [PubMed] [Google Scholar]
- Pui CH. Precision medicine in acute lymphoblastic leukemia. Fron Med. 2020;14:689–700. doi: 10.1007/s11684-020-0759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98:222–9. doi: 10.3324/haematol.2012.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. 2016;2016:580–8. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramilyeva IR, Burkitbaev ZhK, Abdrakhmanova SA, Turganbekova AA, Baimukasheva DK, Zhiburt EB. Distribution pattern for HLA specificities in the patients with acute myeloid leukemia. Medical Immunology (Russia) 2019;21:965–72. [Google Scholar]
- Rubin JB, Lagas JS, Broestl L, et al. Sex differences in cancer mechanisms. Biol Sex Differ. 2020;11:1–29. doi: 10.1186/s13293-020-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semetei kyzy A, Makimbetov EK, Isakova JT, Kudaibergenova IO, Kamarli ZP. Association of XRCC1, HMMR genes with breast cancer in the Kyrgyz ethnic group. Malignant Tumours. 2018;8:45–9. [Google Scholar]
- Shah A, Coleman MP. Increasing incidence of childhood leukemia: a controversy re-examined. Br J Cancer. 2007;97:1009–12. doi: 10.1038/sj.bjc.6603946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Lupo PhJ, Scheurer ME, et al. A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine. 2016;95:e5300. doi: 10.1097/MD.0000000000005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spix C, Eletr D, Blettner M, Kaatsch P. Temporal trends in the incidence rate of childhood cancer in Germany 1987-2004. Int J Cancer. 2008;122:1859–67. doi: 10.1002/ijc.23281. [DOI] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Fidler MM, Colombet M, et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): a population-based study. Lancet Oncol. 2018;19:1159–69. doi: 10.1016/S1470-2045(18)30423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019;20:142–54. doi: 10.1016/S1470-2045(19)30031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbi CK. Etiology of acute leukemia: A review. Cancers. 2021;13:2256. doi: 10.3390/cancers13092256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenova AA. Polymorphism of the XRCC1 and P53 genes in patients with acute leukemia. Vestnik KRSU. 2018;18:157–60. [Google Scholar]
- Vrooman LM, Silverman LB. Treatment of childhood acute lymphoblastic leukemia: Prognostic factors and clinical advances. Curr Hematol Malig R. 2016;11:385–94. doi: 10.1007/s11899-016-0337-y. [DOI] [PubMed] [Google Scholar]
- Xie S, Hossain MJ. Survival differences in childhood and young adult acute myeloid leukemia: A cross-national study using US and England data. Cancer Epidemiol. 2018;54:19–24. doi: 10.1016/j.canep.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ma L, Zhao X, Yang H, Ruan L. A meta-analysis study on XRCC1 Arg399Gln polymorphism and hematological malignancies. Int J Clin Exp Med. 2016;9:19244–55. [Google Scholar]
- Yue ZX, Zheng HY. Progress of studies on genetics of childhood acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:791–5. doi: 10.7534/j.issn.1009-2137.2013.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.