Abstract
Background:
Hepatocellular carcinoma (HCC) accounts for more than 80% of primary liver cancers. Moreover, in the next 10 years, more than one million patients are expected to die from liver cancer as estimated by the World Health Organization. The aim of the present study is to evaluate the clinical utility of using Glypican (GPC3), Vascular endothelial growth factor (VEGF) and Golgi protein 73 (GP73) in serum by Enzyme-Linked Immunosorbent Assay (ELISA) and by Real-Time Polymerase Chain Reaction (RT-PCR), as diagnostic markers to differentiate HCC from cirrhotic liver disease.
Methods:
A total of 50 patients with histologically-proven HCC, 50 liver cirrhosis patients and 20 healthy volunteers as controls were enrolled in this study, blood samples were obtained from each patient. Expression of the studied biomarkers was evaluated by ELISA and Real-Time PCR.
Results:
Statistical analysis of RT-PCR results showed that the expression of GPC3, VEGF and GP73 in serum of patients with HCC was significant (P value < 0.001, 0.01, and < 0.001) respectively and increased when compared to the cirrhotic group. Furthermore, the serum protein levels of GPC3 and VEGF in HCC and cirrhotic patients were significant when compared to the control group. While no significance was found between HCC and cirrhotic group. The serum protein level of GP73 was significantly increased in HCC and cirrhosis groups compared to the control group (P value < 0.001). Moreover, a significant increase was evident in HCC group compared to cirrhotic group (P value < 0.001). The results of the present study showed that the combination of VEGF and GP73 could discriminate HCC from cirrhosis.
Conclusion:
GPC3, VEGF and GP73 are reliable biomarkers for diagnosis of HCC. The serum level of GP73 is a potential screening marker for discriminating HCC from liver cirrhosis.
Key Words: Glypican, VEGF, Golgi protein73, HCC
Introduction
Hepatocellular carcinoma (HCC) accounts for 70%–90% of primary liver cancers and is the second leading cause of cancer- related deaths for men and the fourth for women in 2020, in East Asia and sub-Saharan Africa and the sixth most common in Western countries (Mak, 2021). In Egypt, liver cancer forms 11.75% of the malignancies of all digestive organs and 1.68% of the total malignancies. HCC constitutes 70.48% of all liver tumors among Egyptians (El-Kady et al., 2021). In Egypt, the health authorities consider HCC as the most challenging health problem because it represents the fourth common cancer. The number of HCC patients increased twofold over a decade (Rashed et al., 2020).
Treatment is effective in the early stages of the disease. Unfortunately, HCC is diagnosed in most patients at a time when curative surgical resection or orthotopic liver transplantation (OLT) cannot be performed because of advanced disease or substantial impairment of liver function (Llovet et al., 2021).
A significant limitation in the HCC treatment is the absence of early diagnostic markers. Small tumoral masses with a diameter of less than 3 cm have over a 50% chance to be cured via surgical resection or thermal ablation. Nevertheless, the identification of these tumoral areas is still problematic due to the absence of pathognomonic symptoms in early stages of HCC. To overcome this problem, over the years, several laboratories have investigated molecules with a potential diagnostic impact as well as a possible therapeutic output when targeted (Greten et al., 2021). The identification of a biochemical marker with better sensitivity and/or specificity than αfetoprotein (AFP) could extremely be helpful in improving early diagnosis of HCC (Wang et al., 2020). Among the most interesting biomarkers are Glypican-3 (GPC3), Vascular endothelial growth factor (VEGF) and Golgi protein-73 (GP73).
Glypican-3 is an oncofetal protein located on the surface of liver cells. It is a heparan sulfate proteoglycan (HSPG) that is attached to the cell surface via a glycosyl-phosphatidylinositol (GPI) anchor (Kei et al., 2021). Glypican-3 is a star molecule in the GPCs family. The GPC3 gene is located on the long arm of the X chromosome at position 26, and contains 11 exons. The transcript is 2130 bp, encoding 580 amino acids, and the molecular weight of the protein is about 70 kDa. It is only expressed in the ovary and recently its diagnostic value in HCC has been gradually recognized (Meng et al., 2020).
Angiogenesis is believed to play a major role in the development and progression of HCC, a hyper vascular tumor (Michael et al., 2019).
Vascular endothelial growth factor is one of the most important angiogenesis regulators and has been suggested as a useful biological marker of tumor invasiveness and prognosis in HCC. It is the most potent angiogenic factor that promotes endothelial proliferation and increases vascular permeability by binding to specific receptors in endothelial cells (Atta et al., 2016). VEGF signaling is the main regulator of angiogenesis, which is impaired in most solid types of cancer as liver tumors’ growth needs high vascularity through new blood vessel formation to suffice its increased metabolic demands (El-Ghandour et al., 2021).
Golgi protein 73 is also known as Golgi membrane protein 1 (GOLM1) or Golgi phosphoprotein 2 (GOLPH2). The GP73gene has 3042 base pairs containing 9 introns and 10 exons and is localized in human chromosome 9q21.33. GP73 contains 401 amino acids with an estimated molecular weight of 45 kDa. However, due to protein modification such as glycosylation, GP73 has a molecular weight of 73 kDa based on denatured gel electrophoresis (Yanan et al., 2020).
Golgi Protein 73 type II Golgi membrane protein is expressed mostly in epithelial cells of many human tissues. In normal livers, GP73 is consistently present in biliary epithelial cells. Nevertheless, the expression of GP73 was notably increased in numerous liver diseases (Dala et al., 2021).
In this study we aim to evaluate the clinical importance of using GPC3, VEGF and GP73 in serum by ELISA and RT-PCR, as potential diagnostic markers to differentiate HCC from liver cirrhosis by non-invasive method, and to correlate positivity of these markers with different clinical prognostic factors of HCC.
Materials and Methods
Subjects and Methods
Selection of study population
The present study enrolled a total of 100 patients including 50 patients with single or multiple hepatic focal lesions in addition to 50 patients with liver cirrhosis who presented to the outpatient clinics of the National Cancer Institute, Cairo University (NCI-CU), and Theodor Bilharz Research Institute (TBRI), Giza, Egypt, during the period from June 2020 to January 2021.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The ethical committees of NCI-CU and TBRI have reviewed and approved the study protocol, and all participants gave a written informed consent, The work was performed at the Biochemistry and Molecular Biology Lab, TBRI.
Venous blood samples were obtained from all subjects before any therapeutic intervention. After full investigations (laboratory tests and imaging studies), were commenced, 50 patients were diagnosed with HCC and fulfilled the Clinical Stage A and B inclusion criteria according to Barcelona Clinic Liver Cancer Staging System (BCLC). They included 42 males and 8 females; their age ranged from 41-73 years.
Other patients that did not fulfill the selection criteria included patients with metastatic or advanced HCC, cholangiocarcinoma, metastatic colorectal cancer, Non-Hodgkin’s Lymphoma, metastatic gastric cancer, liver hemangioma and metastatic pancreatic cancer patients.
Diagnosis of HCC was based on the Typical imaging features of HCC by Triphasic CT that includes arterial enhancement during the arterial phase and venous wash out in the delayed phase.
Also, this study included 50 patients with liver cirrhosis of whom 41 Males and 9 Females with age ranged from 23 to 58 years. They were diagnosed based on clinical, radiological and pathological evidence.
In addition, 20 apparently healthy volunteers of 12 Males and 8 Females their age ranged from 35 to 47 years were included as normal controls.
Blood sampling
Ten milliliters venous blood were withdrawn by venipuncture using serum separator tube vacutainers, blood was left to clot for 15 minutes then centrifuged at 1,000 xg for 10 minutes. The sera were divided into aliquots and stored at -80oC until used.
Assessment of Serum GPC-3, VEGF and GP73 by ELISA
All serum samples were tested for GPC-3, VEGF and GP73 by Enzyme-Linked Immunosorbent Assay (ELISA) method according to manufacturer’s instructions using Human assay kit, (Sun long Biotech Co., Ltd). Briefly, 100 μl of prepared standards and samples were added to appropriate wells of the ELISA plate and then assayed according to the manufacturer’s instructions. The absorbance was measured at 450 nm in a microwell plate reader spectrophotometer, (Abcam CA, USA) and the markers levels were quantified with a standard curve. Each standard or sample was assayed in duplicate.
Gene Expression for GPC-3, VEGF, GP73 and B-Actin mRNAs by RT-PCR
Total RNA extraction
Total RNA was extracted from 200 μl serum, using Abbott mSample preparation system kit (Abbott Molecular, Inc., Des Plaines, IL) according to manufacturer protocol and stored at -80oC until used.
Quantitative Real-Time Reverse-Transcription Assay (qRT-PCR)
Briefly, 5 μl of the extracted RNAs from serum samples were reversely transcribed using reverse transcription kit (Applied Biosystems, San Diego, CA, USA). Five μl of the cDNA were used for the real time PCR amplification step using GPC3, VEGF and GP73 specific primers and syber green master mix (Maxima SYBR Green/ROX qPCR Master Mix 2X, Thermo Fisher, UK). All reactions were run in duplicate. The ΔΔCT method was used for the relative quantification in all samples (Yilmaz et al., 2012).
Statistical analysis
The data were analyzed using Microsoft Excel 2016 and statistical package for social science ‘IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Continuous normally distributed variables were represented as mean ± SD. with 95% confidence interval, while non-normal variables were summarized as median with 25 and 75 percentiles, and using the frequencies and percentage for categorical variables; a P value < 0.05 was considered statistically significant. To compare the means of normally distributed variables between groups, the Student’s t test was performed, and Mann-Whitney U test was used in non-normal variables. χ2 test or Fisher’s exact test was used to determine the distribution of categorical variables between groups.
The diagnostic performances of GPC3, VEGF and GP73 were assessed by receiver operating characteristic (ROC) curves. The area under the ROC (AUC) was calculated as an accuracy index for prognostic performance of selected tests. The cut-off for the diagnosis of a group of the study was taken from the point of maximum combined sensitivity and specificity. Univariate analysis was conducted to determine the prognostic performance of each studied biomarker.
In addition, the STRING CONSORTIUM online database (Version 10.2.4) (2021) was used to determine the protein- protein interaction network.
Results
Patients’ characteristics: A total of 100 patients were recruited in this study, including 50 HCC patients, 50 patients with liver cirrhosis, in addition to, 20 healthy individuals with no history of liver disease or alcohol consumption who were included as a control group. Individual demographic and clinical data of the studied groups are shown in (Table 1).
Table 1.
Demographic and Clinical Data of the Studied Groups
| Control N=20 | Cirrhosis N=50 | HCC N=50 | P. value | ||||
|---|---|---|---|---|---|---|---|
| Cirrhosis Vs Control |
HCC Vs Control |
HCC Vs Cirrhosis |
|||||
| Age | 40.3±3.6 | 40.3±8.7 | 58.4±8.1 | 0.9 | 0.001** | 0.001** | |
| Sex | Female | 8 (40.0%) | 9 (18.0%) | 8 (16.0%) | 0.01* | 0.01* | 0.6 |
| Male | 12 (60.0%) | 41 (82.0%) | 42 (84.0%) | ||||
| HBV | Normal | 20 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0.001** | 0.001** | N.A |
| HBV | 0 (0.0%) | 0 (0.0%) | 1 (2.0%) | N.A | 0.15 | 0.15 | |
| HCV | 0 (0.0%) | 50 (100.0%) | 26 (52.0%) | 0.001** | 0.001** | 0.01* | |
| Non B Non C | 0 (0.0%) | 0 (0.0%) | 23 (46.0%) | N.A | 0.01* | 0.01* | |
| Tumor grade | Normal | 20 (100.0%) | 50 (100.0%) | 0 (0.0%) | N.A | 0.001** | 0.001** |
| I | 0 (0.0%) | 0 (0.0%) | 31 (62.0%) | N.A | 0.01* | 0.01* | |
| II | 0 (0.0%) | 0 (0.0%) | 19 (38.0%) | N.A | 0.01* | 0.01* | |
| Number of Masses | Normal | 20 (100.0%) | 50 (100.0%) | 0 (0.0%) | N.A | 0.001** | 0.001** |
| 1 | 0 (0.0%) | 0 (0.0%) | 34 (68.0%) | N.A | 0.001** | 0.001** | |
| 2 | 0 (0.0%) | 0 (0.0%) | 9 (18.0%) | N.A | 0.02* | 0.02* | |
| 3 | 0 (0.0%) | 0 (0.0%) | 7 (14.0%) | N.A | 0.03* | 0.03* | |
| Size of the Mass | Normal | 20 (100.0%) | 50 (100.0%) | 0 (0.0%) | N.A | 0.001** | 0.001** |
| 1-3 cm | 0 (0.0%) | 0 (0.0%) | 17 (34.0%) | N.A | 0.01* | 0.01* | |
| >3cm | 0 (0.0%) | 0 (0.0%) | 33 (66.0%) | N.A | 0.001** | 0.001** | |
| AFP level | <400 | 20 (100.0%) | 50 (100.0%) | 37 (74.0%) | N.A | 0.01* | 0.01* |
| >400 ng/ml | 0 (0.0%) | 0 (0.0%) | 13 (26.0%) | ||||
| Hepatomegaly | Absent | 20 (100.0%) | 18 (36.0%) | 10 (20.0%) | 0.01* | 0.01* | 0.01* |
| Present | 0 (0.0%) | 32 (64.0%) | 40 (80.0%) | ||||
| Splenomegaly | Absent | 20 (100.0%) | 22 (44.0%) | 15 (30.0%) | 0.01* | 0.01* | 0.01* |
| Present | 0 (0.0%) | 28 (56.0%) | 35 (70.0%) | ||||
| Ascites | Absent | 20 (100.0%) | 23 (46.0%) | 11 (22.0%) | N.A | 0.04* | 0.01* |
| Present | 0 (0.0%) | 27 (54.0%) | 39 (78.0%) | ||||
| Oedema Lower Limbs | Absent | 20 (100.0%) | 50 (100.0%) | 32 (64.0%) | N.A | 0.01* | 0.01* |
| Present | 0 (0.0%) | 0 (0.0%) | 18 (36.0%) | ||||
| Portal Vein Thrombosis | Absent | 20 (100.0%) | 28 (56.0%) | 32 (64.0%) | N.A | 0.05* | 0.01* |
| Present | 0 (0.0%) | 22 (44.0%) | 18 (36.0%) | ||||
Age was represented as Mean± SD, the data were analyzed by t test. While HBV, CHILD grade, Number of masses, Size of the mass, AFP level, Hepatomegaly, Splenomegaly, Ascites, Oedema Lower Limbs, and Portal Vein Thrombosis were represented as frequency and percent, the data were analyzed by X2 test; * P value <0.05 is significant, ** P value <0.01 is highly significant, N.A, Not Applicable.
The HCC group included 42 (84%) males and 8 (16%) females with mean age (58.4±8.1 years). The liver cirrhosis group included 41 (82%) males and 9 (18%) females with mean age (40.3±8.7 years), and the control group included 12 (60%) males and 8 (40%) females with mean age (40.3±3.6 years).
By computed tomography, a single hepatic focal lesion was detected in 34 patients, 2 lesions in 9 patients, 3 lesions in 7 patients. Abdominal ultrasound detected ascites in 39 (78%) patients in the HCC group. It was noted that the frequency of patients with lower limbs oedema and portal vein thrombosis were more frequent in HCC group than cirrhosis group.
The demographic data, regarding HCV, showed a highly significant difference in HCC and cirrhotic groups vs control (P = 0.001), and also between HCC vs cirrhotic group (P = 0.01).
Regarding AFP level, hepatomegaly and splenomegaly, a significant difference was observed in HCC vs cirrhotic group (P= 0.01). For ascites, a significant difference was observed between HCC vs cirrhotic group (P= 0.1).
Regarding lower limbs oedema, it was significantly frequent in HCC group compared to cirrhotic group with P= 0.01, and finally for portal vein thrombosis, it was also statistically significant in HCC vs cirrhotic group with P= 0.01 (Table 1).
Expression of GPC3, VEGF and GP73 RNAs by qRT-PCR in the studied groups
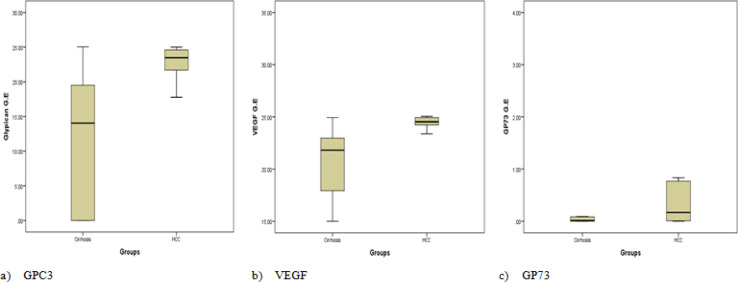
Statistical analysis showed that the expression of GPC3, VEGF and GP73 in patients with HCC is significantly increased, compared to the cirrhotic group (P value < 0.001, 0.01, and < 0.001) respectively (Table 2, Figure 1 a, b, and c).
Table 2.
Serum Expression Level of GPC3, VEGF and GP73 in the Studied Groups
| Control N=20 | Cirrhosis N=50 | HCC N=50 | P. value | |
|---|---|---|---|---|
| GPC3 | - | 14.04 (0.001- 19.64) | 23.48 (21.65- 24.64) | 0.001** |
| VEGF | - | 21.81 (17.64- 23.21) | 24.53 (24.20- 24.94) | 0.01* |
| GP73 | - | 0.03 (0.001- 0.19) | 0.51 (0.01- 14.52) | 0.001** |
The studied biomarkers were represented as Median with Interquartile range (25% -75%), the data were analyzed by Mann-Whitney U test; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 1.
Median of Serum Expression of GPC3, VEGF and GP73 in the Studied Groups
Quantitation of GPC3, VEGF and GP73 Proteins by ELISA in the studied groups
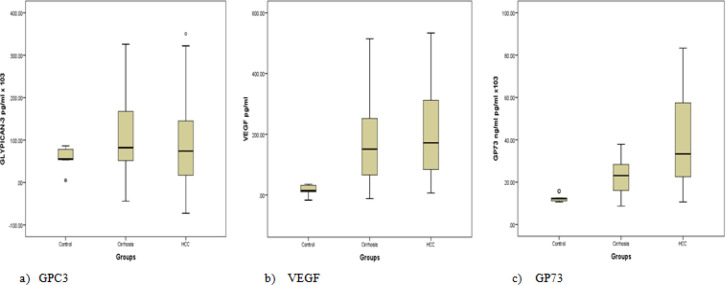
It was noticed that the serum protein levels of GPC3 in cirrhotic and HCC groups were significantly , increased compared to the control group (P = 0.05, 0.03) respectively. While no significant difference was observed in the HCC group compared to the cirrhotic group (P value < 0.9) (Table 3, Figure 2a).
Table 3.
Protein Levels of GPC3, VEGF and GP73 in the Studied Groups
| Control N=20 | Cirrhosis N=50 | HCC N=50 | P. value | |||
|---|---|---|---|---|---|---|
| Cirrhosis Vs Control |
HCC Vs Control |
HCC Vs Cirrhosis |
||||
| GPC3 pg/ml x103 | 55.8 (53.8- 78.1) | 102.5 (48.7- 187.9) | 118.8 (20.2- 207.2) | 0.05* | 0.03* | 0.9 |
| VEGF pg/ml | 14.6 (10.4- 31.9) | 151.1 (65.6- 257.2) | 171.7 (83.7- 312.7) | 0.001** | 0.001** | 0.5 |
| GP73 pg/ml x103 | 12.2 (11.2- 12.6) | 23.1 (16.0- 28.4) | 33.3 (22.5- 57.5) | 0.001** | 0.001** | 0.001** |
The studied biomarkers were represented as Median with Interquartile range (25% -75%), the data were analyzed by Mann-Whitney U test; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 2.
Median of the Protein Levels of GPC3, VEGF and GP73 in the Studied Groups
Likewise, the serum protein levels of VEGF in the cirrhotic and HCC groups were significantly increased compared to the control group (P <0.001). While no significant difference was observed in the HCC compared to the cirrhotic group (P < 0.5) (Table 3, Figure 2b).
Interestingly, the serum protein levels of GP73 were significantly increased in both patient groups compared to the control group (P < 0.001). Also, a significant increase was observed in the HCC group compared to cirrhotic group which indicates that GP73 can differentiate between HCC and cirrhotic group (P < 0.001) (Table 3, Figure 2c).
The association between high levels of studied biomarkers and the tumor size
Statistical analysis of the association between the level of studied biomarkers and the tumor size, revealed that the increased level of GP73 is associated with the increase in the tumor size (P = 0.002). While GPC3 and VEGF elevations were not associated with increased tumor size (Table 4).
Table 4.
The association between Tumor Size with the Serum Level of the Studied Biomarkers
| Tumor size | P. value | ||
|---|---|---|---|
| 1-3 cm | >3cm | ||
| GPC3 pg/ml x103 | 133.0 (39.5- 196.0) | 70.0 (9.0- 139.1) | 0.1 |
| VEGF pg/ml | 168.3 (44.7- 245.2) | 178.9 (98.4- 326.5) | 0.2 |
| GP73 ng/ml pg/ml x103 | 22.5 (19.2- 39.8) | 45.6 (25.9- 59.9) | 0.002** |
The studied biomarkers were represented as Median with Interquartile range (25% -75%), the data were analyzed by Mann-Whitney U test. * P value <0.05 is significant, ** P value <0.01 is highly significant.
The Diagnostic performances of GPC3, VEGF and GP73 patients: in liver cirrhosis and HCC
Receiver Operating Curves (ROC) were established to show the diagnostic performances of the 3 studied biomarkers in liver cirrhosis and HCC patients.
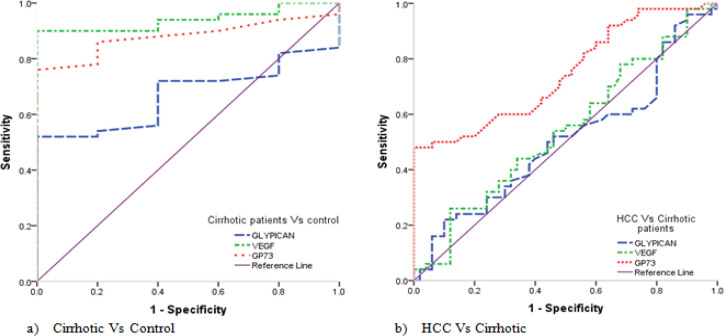
For discrimination of the cirrhotic group vs the control, it was found that, GPC3 at the cut-off value of >86.27, with sensitivity of 52.0% and specificity of 100.0%, with an area under curve (AUC) = 0.670 and accuracy of 52.0% (P = 0.0066). While VEGF at the cut-off value of >35.65, with sensitivity of 90.0% and specificity of 100.0%, with an AUC= 0.940 and accuracy of 90.0% (P <0.0001), and GP73 at the cut-off value of >15.75, with sensitivity of 76.0% and specificity of 100.0%, with an AUC= 0.880 and accuracy of 76.0% (P <0.0001) (Table 5, Figure 3a).
Table 5.
Diagnostic Performances of GPC3, VEGF and GP73 Regarding the Studied Groups
| Cut-off | Sn. | Sp. | PPT | NPV | Accuracy | AUC | 95% C.I | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cirrhosis Vs Control |
GPC3 | >86.27 | 52 | 100 | 100 | 45.5 | 52 | 0.67 | 0.547 - 0.778 | 0.0066 |
| VEGF | >35.65 | 90 | 100 | 100 | 80.0 | 90 | 0.94 | 0.856 - 0.983 | <0.0001 | |
| GP73 | >15.75 | 76 | 100 | 100 | 62.5 | 76 | 0.88 | 0.780 - 0.945 | <0.0001 | |
| HCC Vs Cirrhosis |
GPC3 | >45.62 | 62 | 24 | 44.9 | 38.7 | 14 | 0.506 | 0.404 - 0.607 | 0.9216 |
| VEGF | >293.85 | 26 | 88 | 68.4 | 54.3 | 14 | 0.541 | 0.438 - 0.641 | 0.4836 | |
| GP73 | >37.9 | 48 | 100 | 100 | 65.8 | 48 | 0.747 | 0.650 - 0.829 | <0.0001 |
Sn, Sensitivity; Sp, Specificity;PPT, Positive predictive value; NPV,Negative predictive value; AUC, Area under curve and C.I: 95% Confidence Interval; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 3.
ROC Curve of GPC3, VEGF and GP73 for the Studied Groups. a) ROC Curve for the studied biomarkers regarding Cirrhosis vs. control group, b) ROC Curve for the studied biomarkers regarding HCC vs. Cirrhosis group
For discrimination of the HCC vs the cirrhotic group, it was found that GP73 at the cut-off value of >37.9, with sensitivity of 48.0% and specificity of 100.0%, with an AUC = 0.747 and accuracy of 48.0% (P <0.0001). While there was no significant difference between the HCC group and the cirrhotic group regarding GPC3 and VEGF (Table 5, Figure 3b).
Diagnostic performances of GPC3, VEGF and GP73 after combination
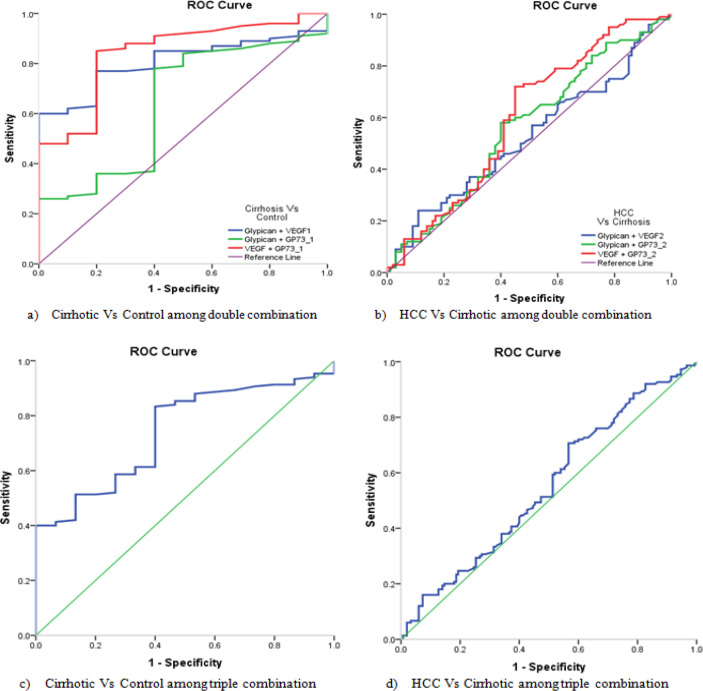
For discrimination of the cirrhotic group vs the control group, the double combination between the studied biomarkers, revealed that, the combination of GPC3 + VEGF, the sensitivity was 60% and specificity of 100%, with an AUC= 0.807 and accuracy of 60% (P < 0.0001). While for GPC3 + GP73, the sensitivity was 78.% and specificity of 60%, with an AUC= 0.641 and accuracy of 38.% (P = 0.0066). Also, for combination of VEGF + GP73, the sensitivity was 85% and specificity of 80%, with an AUC= 0.843 and accuracy of 65% (P < 0.0001) (Table 6, Figure 4a).
Table 6.
Diagnostic Performances of GPC3, VEGF and GP73 in the Studied Groups after Combination
| Sn. | Sp. | PPT | NPV | Accuracy | AUC | 95% C.I | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Double markers | |||||||||
| Cirrhosis Vs Control | GPC3 + VEGF | 60 | 100 | 100 | 50.0 | 60 | 0.807 | 0.731 - 0.868 | <0.0001** |
| GPC3 + GP73 | 78 | 60 | 83 | 52.2 | 38 | 0.641 | 0.556 - 0.721 | 0.0066** | |
| VEGF + GP73 | 85 | 80 | 91.4 | 68.1 | 65 | 0.843 | 0.772 - 0.899 | <0.0001** | |
| HCC Vs Cirrhosis | GPC3 + VEGF | 24 | 89 | 68.6 | 53.9 | 13 | 0.525 | 0.453 - 0.596 | 0.5466 |
| GPC3 + GP73 | 58 | 60 | 59.2 | 58.8 | 18 | 0.56 | 0.488 - 0.630 | 0.1416 | |
| VEGF + GP73 | 72 | 55 | 61.5 | 66.3 | 27 | 0.597 | 0.526 - 0.666 | 0.0163* | |
| Triple markers | |||||||||
| Cirrhosis Vs Control | 83.33 | 60 | 83.9 | 59.0 | 43.33 | 0.742 | 0.677 to 0.799 | <0.0001** | |
| HCC Vs Cirrhosis | 70.67 | 43.33 | 55.5 | 59.6 | 14 | 0.548 | 0.490 to 0.605 | 0.1478 | |
Sn, Sensitivity; Sp, Specificity; PPT, Positive predictive value; NPV, Negative predictive value; AUC, Area under curve and C.I, 95% Confidence Interval.; * P value <0.05 is significant, ** P value <0.01 is highly significant
Figure 4.
ROC Curve of GPC3, VEGF and GP73 for the Studied Groups after Combination
For discrimination of the HCC group vs the cirrhotic group, the double combination between the studied biomarkers, revealed that the combination of VEGF + GP73, the sensitivity was 72% and specificity of 55%, with an AUC= 0.597 and accuracy of 27% (P = 0.016). While there was no significant difference between the two patient groups regarding GPC3 + VEGF and GPC3 + GP73 (Table 6, Figure 4b).
The triple combination of the studied biomarkers for discrimination between the cirrhotic group vs the control group showed 83.33% sensitivity and 60% specificity, with an AUC= 0.742 and accuracy of 43.33% (P < 0.0001). While no significant difference was observed between the HCC group vs the cirrhotic group (Table 6, Figure 4c, d).
Prognostic performances of GPC3, VEGF and GP73 in HCC and liver cirrhosis patients
Univariate logistic regression analysis was performed to characterize the GPC3, VEGF and GP73 as a predictor and/or prognostic parameter for cirrhosis progression. An increase in 1 degree of VEGF increased the odds of liver cirrhosis diagnosis by a factor of 1.06 (P <0.001). Similarly, an increase in 1 degree of GP73 level increased the odds of liver cirrhosis diagnosis by a factor of 1.42 (P = 0.001), this means that VEGF and GP73 could be used as predictors and/or prognostic parameters for liver cirrhosis progression. While there was no significance for liver cirrhosis prediction concerning GPC3.
Regarding the prediction value of GPC3, VEGF and GP73 for HCC progression, an increase in 1 degree of GP73 increased the odds of HCC diagnosis by a factor of 1.10 P <0.001. While there was no significance for HCC prediction regarding GPC3 and VEGF (Table 7).
Table 7.
Prognostic Performances of GPC3, VEGF and GP73 Regarding the Studied Groups
| OR | 95% C.I | P. value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Cirrhosis Vs Control | GPC3 | 1.01 | 1 | 1.01 | 0.08 |
| VEGF | 1.06 | 1.02 | 1.1 | 0.001** | |
| GP73 | 1.42 | 1.15 | 1.76 | 0.001** | |
| HCC Vs Cirrhosis | GPC3 | 1 | 1 | 1 | 0.5 |
| VEGF | 1 | 1 | 1 | 0.5 | |
| GP73 | 1.1 | 1.05 | 1.15 | 0.001** | |
OR, Odd Ratio; CI, Confidence Interval; P value calculated depend on logistic regression analysis; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Protein-protein interaction
In molecular biology, STRING is a biological database and web resource of known and predicted protein–protein interactions. The STRING database contains information from numerous sources, including experimental data, computational prediction methods and public text collections (Szklarczyk et al., 2019).
Determining protein-protein interaction (PPI) in biological systems is of considerable importance, and prediction of PPI has become a popular research area. Although different classifiers have been developed for PPI prediction, no single classifier seems to be able to predict PPI with high confidence. It was postulated that by combining individual classifiers the accuracy of PPI prediction could be improved (Jianzhuang et al., 2015).
Protein-protein interaction networks play important roles in many cellular activities, including complex formation and metabolic pathways (Swamy et al., 2021), and identification of PPI pairs may provide important insights into the molecular basis of cellular processes (Bludau et al., 2020).
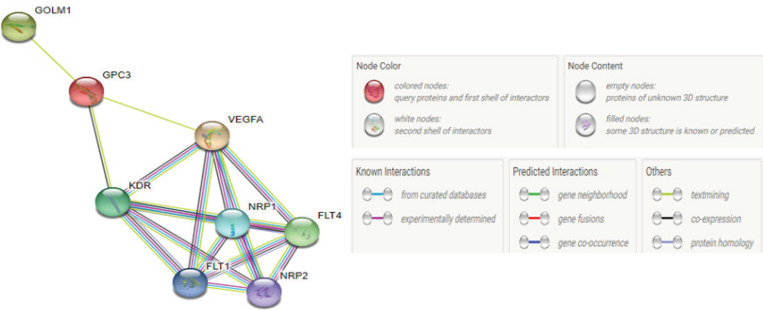
By determining the PPI in this study, it was found that GP73 binds to GPC3 by text mining interaction which interacts to VEGF also by text mining and co-expression interaction (Figure 5).
Figure 5.
Protein Protein Interaction Network of GPC3, VEGF and GP73. STRING network. Each different colored line that connects proteins indicates a separate evidence channel for the particular interaction, such as text mining (green), experiments (magenta), and databases (blue)
Also, it was found that VEGF interacts with receptor tyrosine kinase1(FLT1), receptor tyrosine kinase 4 (FLT4), kinase insert domain receptor (KDR), neuropilin 1(NRP1) and finally it interacts by neuropilin 2(NRP2) with a score of 0.999. The PPI showed an interaction between GPC3 protein and GP73, KDR and VEGF with a score of 0.652 and 0.465 respectively.
Discussion
Hepatocellular carcinoma (HCC) is one of the common and fatal malignancies, which is a significant global health problem (Niu et al., 2021).
The main problem physicians are facing in the management of HCC patients is that the available biomarkers are not sufficiently specific and sensitive. In recent years, considerable interest has been focused on the identification of new molecular biomarkers for prognosis of patient survival and/or malignant recurrence (Federico et al., 2020).
In the present study, we aimed to assess the diagnostic value of serum GPC3, VEGF and GP73 by ELISA and their mRNAs expression by real time PCR to differentiate HCC from cirrhotic liver disease by non-invasive method.
Glypian-3 (GPC3) is a heparan sulfate proteoglycan (HSPG). There are six glypican subtypes, namely, GPCs 1-6, with similar structures consisting of a 60-70 kDa protein connected to the cell membrane. GPC3 has been implicated in a variety of processes, including cell growth, differentiation, and migration (Zheng et al., 2022).
The specific expression of GPC3 in tumor cells has received widespread attention. Here, we discuss the relevance of GPC3 to HCC diagnosis and prognosis. Our results indicated that the expression of GPC3 in HCC group was significantly increased compared to the cirrhotic group and controls. Moreover, the serum protein levels of GPC3 in cirrhotic and HCC groups were significantly increased compared to the control group, while no significant difference was observed in HCC patients compared to cirrhotic patients.
These results are in agreement with that of Kandil and Cooper (2009) who revealed that GPC3 was expressed in 70-80% of HCC lesions while it was virtually absent in the normal liver. Furthermore, Yan et al., (2011) detected GPC3 mRNA in the peripheral blood of 76% of patients with HCC, while it was not detected in samples of healthy volunteers. The results of the current study concerning GPC3 expression goes with those of Youssef et al., (2010), who reported that GPC3 mRNA was detected in 100% of patients with HCC, 5% of liver cirrhosis patients and none in normal control subjects.
However, these results were on the contrary to those of Wang et al., (2011) who did not detect any significant difference in GPC3 mRNA expression in the peripheral blood of HCC patients and healthy volunteers.
Never the less, these results are similar to Jia et al., (2016) who assessed the clinical utility of GPC3 as serum marker for diagnosis of HCC using ELISA. The study included 283 patients with HCC, 445 liver cirrhosis patients and 162 normal controls. Their results indicated that serum GPC3 was elevated in patients with HCC and liver cirrhosis compared to the normal controls, but there was no difference between HCC and liver cirrhosis which is parallel to our results.
Angiogenesis is a dynamic process of hypoxia and growth factors where it leads to the formation of new vessels. Liver angiogenesis is either physiological as in liver regeneration or pathological as in chronic liver diseases, HCC, and metastatic liver cancer (Elpek 2015). One of the most potent pro-angiogenic factors is VEGF, secreted primarily by cancer cells and has the highest specificity for endothelial cells (Han et al., 2022). By binding to its receptors, mainly VEGFR2, on the membrane of endothelial cells, VEGF-VEGFR2 exerts a central regulatory role in the formation of tumor blood vessels.
Statistical analysis of the current study showed that the expression of VEGF in patients with HCC was significantly increased compared to the cirrhotic group and controls. On the other hand, the ELISA results showed that the protein level of VEGF in cirrhotic and HCC groups is significantly increased compared to the control group. While no significant difference was observed in the HCC group compared to the cirrhotic group .
The above findings of our study are the exact opposite to the results of El-mezayen and Darwish (2014), who assayed VEGF by ELISA in 123 HCC patients, 210 liver cirrhosis patients and 50 individuals were included in his study as a control group, they concluded that VEGF could be used for discriminating HCC patients from liver cirrhosis with sensitivity of 91% and specificity of 82%.
The results of the present study are completely different from those of Alzamzamy et al., (2021) who determined the accuracy of serum VEGF as a tumor marker for the early detection of HCC by ELISA, the study included 40 patients with HCC, 30 cirrhotic and 30 healthy volunteers were included as controls, they found that HCC patients had significantly higher serum VEGF level than cirrhotic non-HCC group.
The results of the study of Sadik et al., (2019) who evaluated the serum VEGF levels in patients with HCC and liver cirrhosis by ELISA, were in accordance to our results as they reported that the serum VEGF was highly expressed in the HCC group and the liver cirrhosis group with no significant difference with P = 0.767.
The results of study by Atta et al., (2016) contradict our results, they evaluated the role of VEGF level and expression (measured in plasma and liver tissue) in patients with HCC to assess its significance in the diagnosis and prognosis of HCC, the authors reported that the plasma VEGF levels in the HCC group were significantly higher than those of the non-HCC cirrhotic group, and both groups had significantly higher plasma VEGF levels than did the control group. Liver tissue VEGF expression was significantly higher in the HCC group than in the non-HCC group and positively correlated with plasma VEGF in the HCC group.
The GP73 is expressed in the biliary epithelial cells and hepatocytes and its increase is always related to the degree of liver injury (Zekri et al., 2020). Our results showed that the expression of GP73 in patients with HCC was significant with P < 0.001 and increased when compared to the cirrhotic group and controls. For GP73 protein level, a significant (P < 0.001) increased in all diseased groups when compared to the control group. Also, significant increase in HCC group when compared to cirrhotic group (P < 0.001).
Similarly, the results of the current study are in total agreement to that of Dala et al., (2021) who investigated the serum expression of GP73 in patients with HCC and determined its efficacy as a screening test in early detection of HCC, they reported that GP73 level in the HCC group was significantly higher than the cirrhotic group (P = 0.001), the GP73 level in the HCC group was significantly higher than the control group (P = 0.001), and GP73 level in the cirrhotic group was significantly higher than the control group (P = 0.001).
The results of study by Ali et al., (2020) corroborate the results of the present study, they made a comparison between the potential biomarker GP73 versus the standard biomarker AFP in the diagnosis of HCC, 60 patients were included in the study divided into 30 patients with HCC, 30 with liver cirrhosis. In addition, 30 healthy volunteers were included as a control group. Statistically significant differences between groups in terms of serum AFP (P<0.001) and GP73 (P<0.001) were found. They reported that the HCC patients had significantly higher AFP and GP73 than cirrhotic patients, they also reported that GP73 had higher diagnostic performance than AFP.
There is a slight difference between our results and the results of Zekri et al., (2020) who reported that the serum levels of AFP and GP73 were significantly higher in HCC compared to the cirrhotic and control groups. Yet, in contrary to our results there was no significant difference in serum concentration of GP73 among cirrhotic HCV patients and the healthy control groups.
In this study, the diagnostic performances of the 3 studied biomarkers were examined, for discrimination of cirrhotic group vs control, the combination of GPC3 + VEGF was of sensitivity 60%, specificity 100% and P <0.0001, combination of GPC3 + GP73 was of sensitivity 78%, specificity 60% and P <0.0066, while the combination of VEGF + GP73 was of sensitivity 85%, specificity 80% and P <0.0001. Furthermore, For discrimination of HCC vs cirrhotic patients, the combination of VEGF + GP73 was of sensitivity 72%, specificity 55% and P <0.0163, on the other hand, there was no significant difference between the two groups by the combination of GPC3 + VEGF and GPC3 + GP73. The above findings showed that the combination of VEGF + GP73 could improve the diagnostic efficacy for discriminating HCC patients from cirrhotic patients by non-invasive method. This study also demonstrated that the triple combination between the studied biomarkers have shown a discriminatory ability between cirrhosis patients and control P <0.0001.
Protein-protein interactions play a fundamental role in various biological functions. PPI prediction is of particular relevance for the development of drugs employing targeted protein degradation, as their efficacy relies on the formation of a stable ternary complex involving two proteins. However, experimental methods to detect PPI sites are both costly and time-intensive. In recent years, computer-aided approaches have been developed as screening tools, but these tools are primarily based on sequence information and are therefore limited in their ability to address spatial requirements and have thus far not been applied to targeted protein degradation (Orasch et al., 2022).
In this study, the STRING CONSORTIUM online database (Version 10.2.4) (2021) was used to determine the protein-protein interaction network to find the type and score of interaction between the studied biomarkers.
In conclusion, the mRNAs expression and protein levels of GPC3, VEGF and GP73 in serum were significantly higher in patients with HCC compared to liver cirrhosis patients and healthy controls.
Statistical analysis of GP73 showed its high diagnostic and prognostic performances, high specificity in detecting HCC patients from controls. It may be used as a reliable serological marker for diagnosis of HCC and for discriminating HCC patients from others with liver cirrhosis.
References
- Ali OM, El Amin HA, Sharkawy YL, et al. Golgi protein 73 versus alpha-fetoprotein as a new biomarker in early diagnosis of hepatocellular carcinoma. Int J General Med. 2020;13:193–200. doi: 10.2147/IJGM.S253622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzamzamy A, Elsayed H, Abd Elraouf M, et al. Serum vascular endothelial growth factor as a tumor marker for hepatocellular carcinoma in hepatitis C virus-related cirrhotic patients. World J Gastrointest Oncol. 2021;13:600–11. doi: 10.4251/wjgo.v13.i6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta MM, Atta H, Gad M, et al. Clinical significance of vascular endothelial growth factor in hepatitis C related hepatocellular carcinoma in Egyptian patients. J Hepatocell Carcinoma. 2016;3:19–24. doi: 10.2147/JHC.S86708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau I, Ruedi A. Author Correction: Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat Rev Mol Cell Biol. 2020;21:353. doi: 10.1038/s41580-020-0249-5. [DOI] [PubMed] [Google Scholar]
- Dala AG, ElArab A Ezz, Sonbol AA, Ibrahim AS. Study of Golgi protein 73 in patients with hepatitis C virusrelated hepatocellular carcinoma. Menoufia Med J. 2021;34:564–9. [Google Scholar]
- El-Ghandour AM, Bayoumy EM, Ibrahim WA, et al. Vascular endothelial growth factor in relation to the development of hepatocellular carcinoma in hepatitis C virus patients treated by direct-acting antivirals. Egypt Liver J. 2021;11:5. [Google Scholar]
- El-Kady MS, El-Toukhy NE, Abd El-Wahhab MS. Hepatocellular carcinoma in hepatitis C virus cirrhosis after treatment with direct acting antiviral therapy. Benha J Appl Sci. 2021;6:243–8. [Google Scholar]
- El-mezayen HA, Darwish H. Development of a novel score for early detection of hepatocellular carcinoma among high-risk hepatitis C virus patients. Tumor Biol. 2014;35:6501–9. doi: 10.1007/s13277-014-1858-4. [DOI] [PubMed] [Google Scholar]
- Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7:377–91. doi: 10.4254/wjh.v7.i3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P, Melisa D, Mário GP. Biomarkers in hepatocellular carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells. 2020;9:1370. doi: 10.3390/cells9061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF, Abou-Alfa GK, Cheng A-L, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J ImmunoTher Cancer. 2021;9:e002794. doi: 10.1136/jitc-2021-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Lin X, Yan Q, et al. PBLD inhibits angiogenesis via impeding VEGF/VEGFR2-mediated microenvironmental cross-talk between HCC cells and endothelial cells. Oncogene. 2022:2022. doi: 10.1038/s41388-022-02197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Gao Y, Zhai D, et al. Assessment of the Clinical Utility of Glypican 3 as a Serum Marker for Diagnosis of Hepatocellular Carcinoma. J Technol Cancer Res Treat. 2016;15:780–6. doi: 10.1177/1533034615605248. [DOI] [PubMed] [Google Scholar]
- Jianzhuang Y, Hong G, Xiaohan Y. PPCM: Combing Multiple Classifiers to Improve Protein-Protein Interaction Prediction. Int J Genomics. 2015;2015:608042–9. doi: 10.1155/2015/608042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil DH, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125–9. doi: 10.1097/PAP.0b013e3181992455. [DOI] [PubMed] [Google Scholar]
- Kei N, Akira K, Hisakazu I, et al. Association between microRNA527 and glypican3 in hepatocellular carcinoma. Oncol Lett. 2021;21:299. [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- Meng G, Hailing Z, Jianming Z, Yang FL. 2020), Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer. 11:2008–21. doi: 10.7150/jca.39972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AM, Weijing S, Richard K, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912–20. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- Niu M, Yi M, Li N, Wu K, Wu K. 2021) Advances of Targeted Therapyfor Hepatocellular Carcinoma. Front Oncol. 11:719896. doi: 10.3389/fonc.2021.719896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orasch O, Weber N, Müller M, et al. Protein-protein interaction prediction for targeted protein degradation. bioRxiv. 2022:481776. doi: 10.3390/ijms23137033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed WM, Kandeil MAM, Mahmoud MO, et al. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J Egyptian Nat Cancer Institut. 2020;32:5. doi: 10.1186/s43046-020-0016-x. [DOI] [PubMed] [Google Scholar]
- Sadik NA, Nagwa RA, Moataz FM, Ashoush OA. Serum Vascular Endothelial Growth Factor in Patients with Hepatocellular Carcinoma and its Validity as a Tumor Biomarker. Open Biomarkers J. 2019;9:84–94. [Google Scholar]
- Swamy KBS, Schuyler SC, Leu J-Y. Protein Complexes Form a Basis for Complex Hybrid Incompatibility. Front Genet. 2021;12:609766. doi: 10.3389/fgene.2021.609766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:607–13. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shen Z, Zhu Z, Han R, Huai M. Clinical Values of AFP, GPC3 m RNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3, mRNA for prediction of HCC. Hepat. 2011;11:195–9. [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang K-H. New Blood Biomarkers for the Diagnosis of AFP-Negative Hepatocellular Carcinoma. Front Oncol. 2020;10:1316. doi: 10.3389/fonc.2020.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, He Q, Chen Y, Wang L, Zhang X. Detection of α-fetoprotein and glypican-3 mRNAs in the peripheral blood of hepatocellular carcinoma patients by using multiple FQ-RT-PCR. J Clin Lab Anal. 2011;25:113–7. doi: 10.1002/jcla.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanan W, Yu-J , Yvonne W. Golgi protein 73, hepatocellular carcinoma and other types of cancers. Liver Res. 2020;4:161–7. doi: 10.1016/j.livres.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef MF, EL-Sharkawy SL, Abbas NF. Clinical utility of Glypican-3 in hepatocellular carcinoma. Int J Integ Biol. 2010;10:41–7. [Google Scholar]
- Zekri NA, EL Kassas M, El-Sayed TA, et al. The possible role of Dickkopf-1, Golgi protein- 73 and Midkine as predictors of hepatocarcinogenesis: a review and an Egyptian study. Sci Rep. 2020;10:5156. doi: 10.1038/s41598-020-62051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Liu X, Lei Y, Wang G, Liu M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front Oncol. 2022;12:824208. doi: 10.3389/fonc.2022.824208. [DOI] [PMC free article] [PubMed] [Google Scholar]